Anticancer Activity of Ethanolic Extract of Tabernaemontana catharinensis in Breast Cancer Lines MCF-7 and MDA-MB-231
Abstract
1. Introduction
2. Results
2.1. Compounds Identification of EET by Ultra-Performance Liquid Chromatography with Time-of-Flight Mass Spectrometry (UPLC-Q-TOF/MSE) and Quantification of Compounds in Crude Extract by HPLC
2.2. Two-Dimensional Cell Model Assays
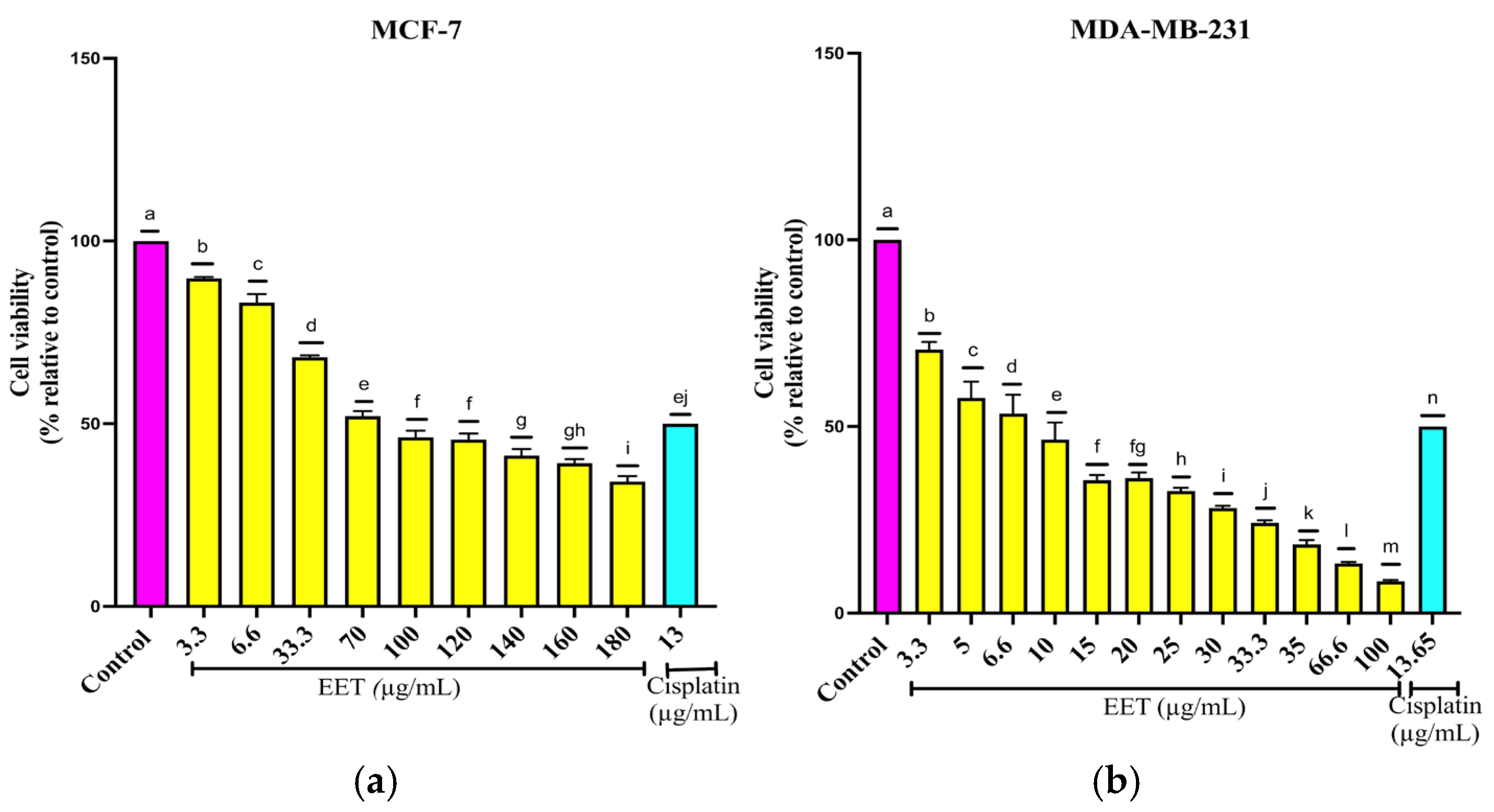
2.2.1. EET Decreases MCF-7 and MDA-MB-231 Cell Viability
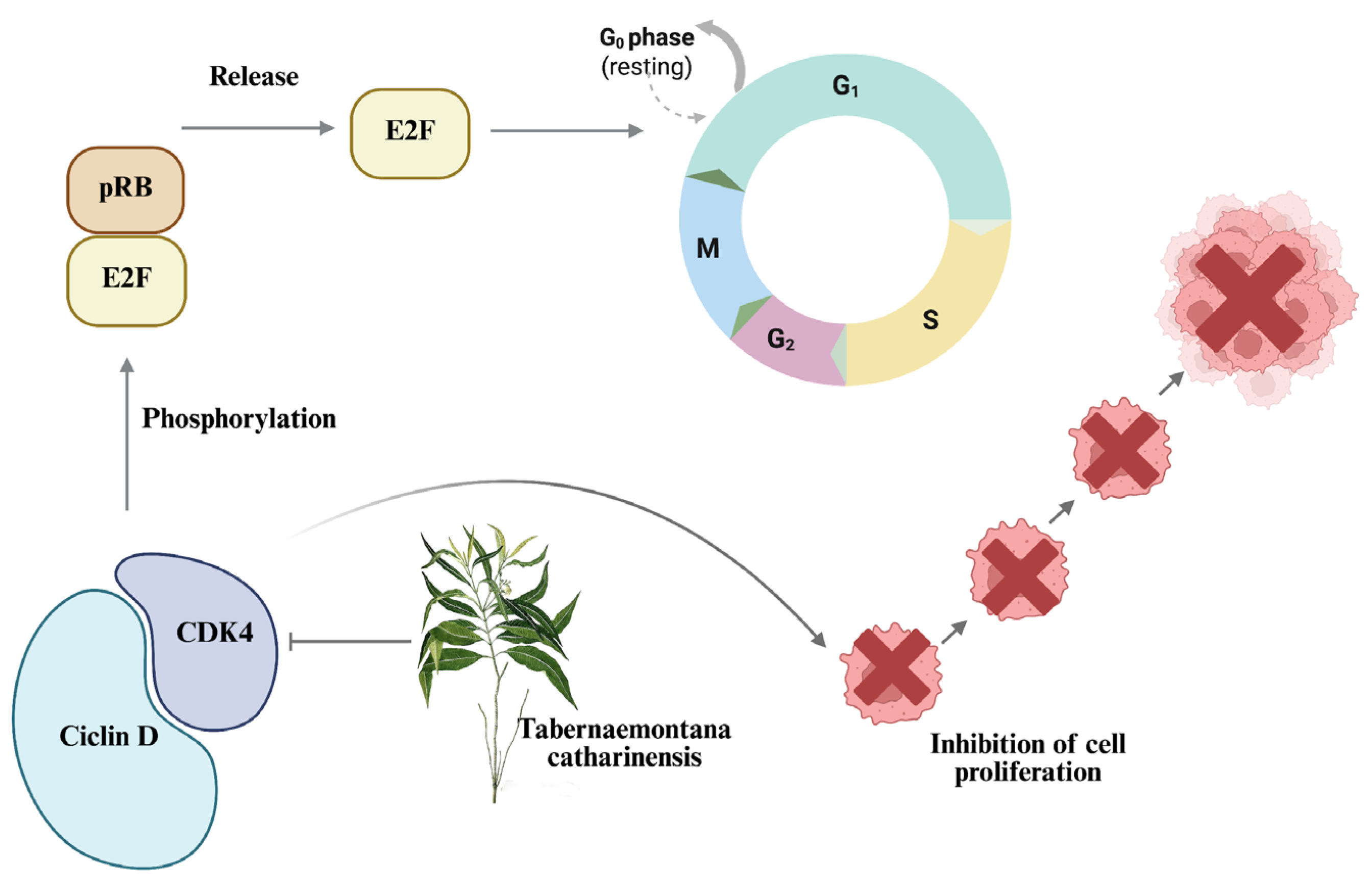
2.2.2. Cell Cycle Arrest in the G1 Phase by EET Treatment in a 2D Model of MCF-7 and MDA-MB-231 Cells
2.2.3. Decrease of Human Aldehyde Dehydrogenase 3A1 (ALDH3A1) Activity by EET Treatment
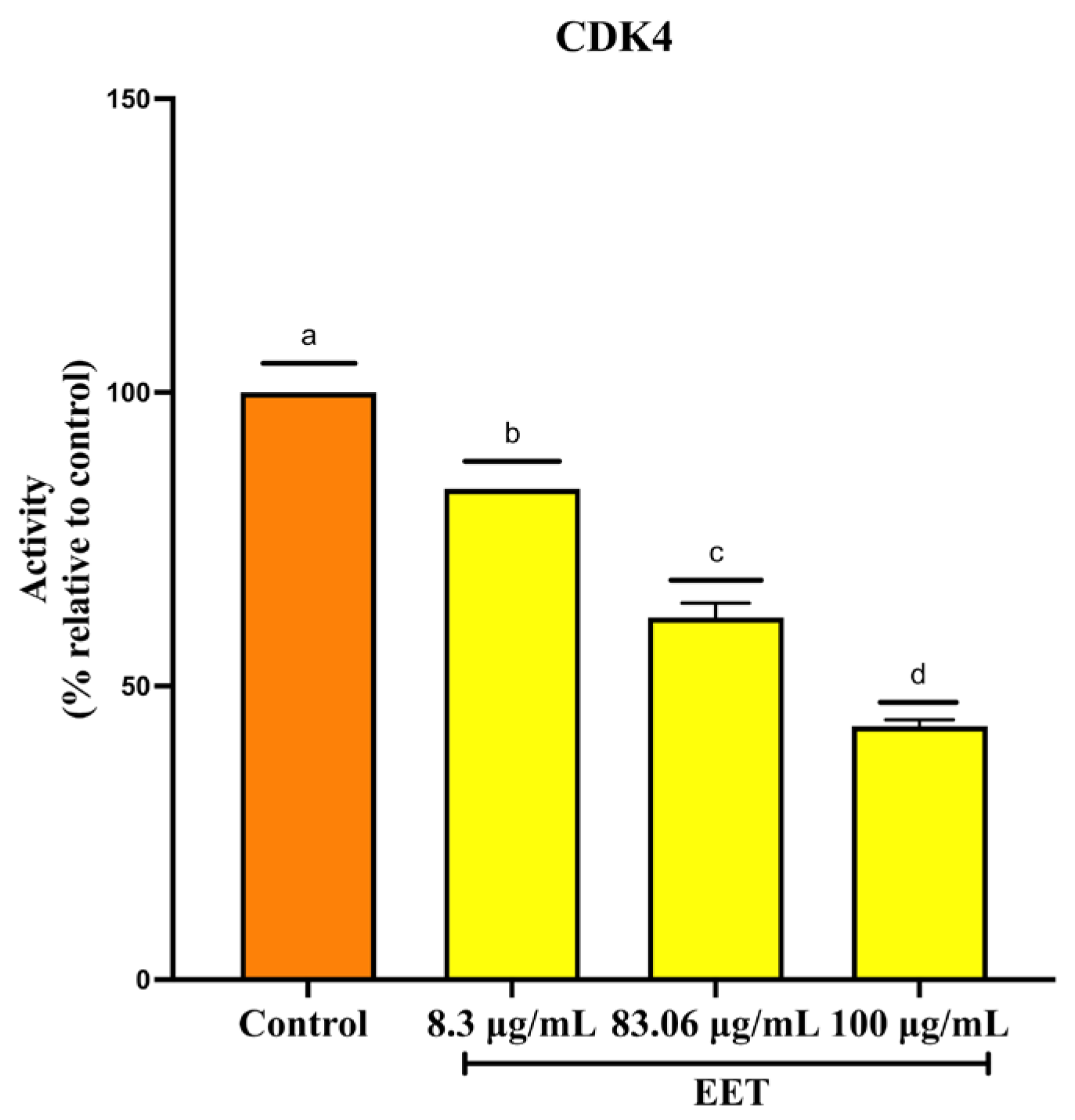
2.2.4. Decrease in Cyclin-Dependent Kinase 4 (CDK4) Activity by EET Treatment
2.2.5. Increase in p53 Protein Activity by EET Treatment
2.3. Three-Dimensional Cell Model Assays
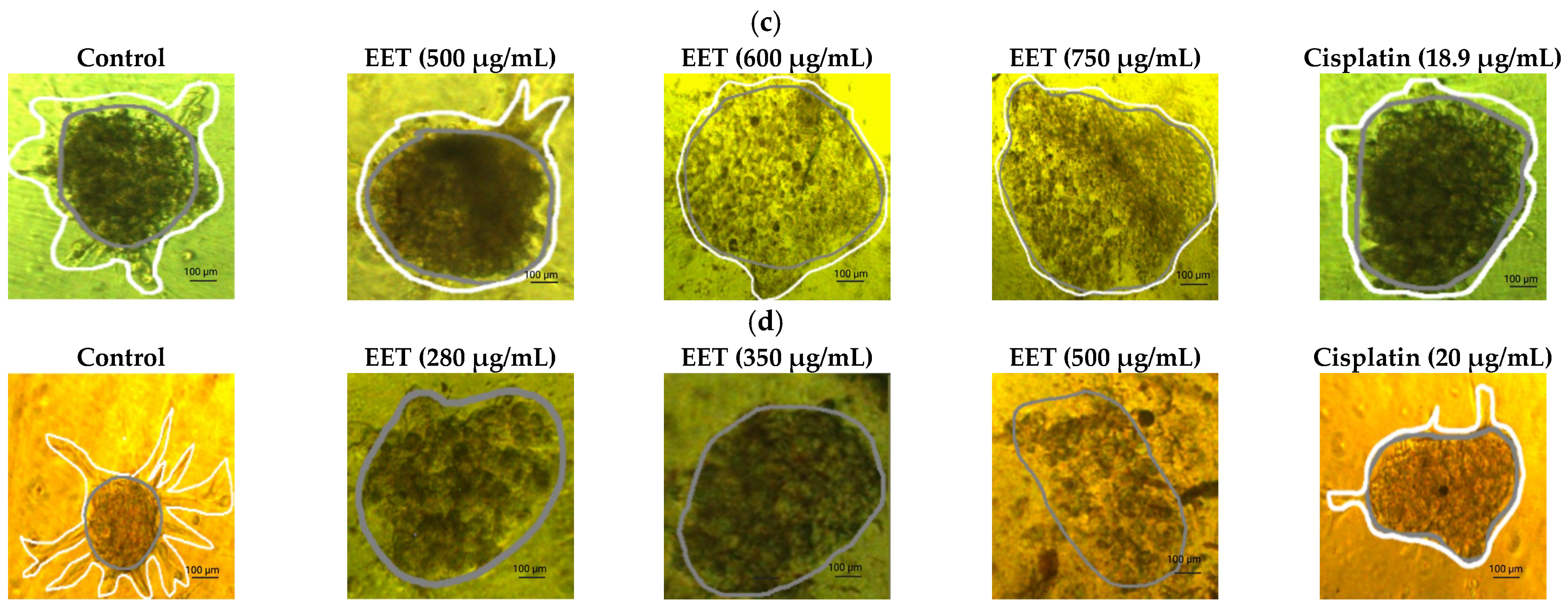
2.3.1. EET Decreases Cell Viability in 3D Models by Assessing Plasma Membrane Integrity in MCF-7 and MDA-MB-231 Cells
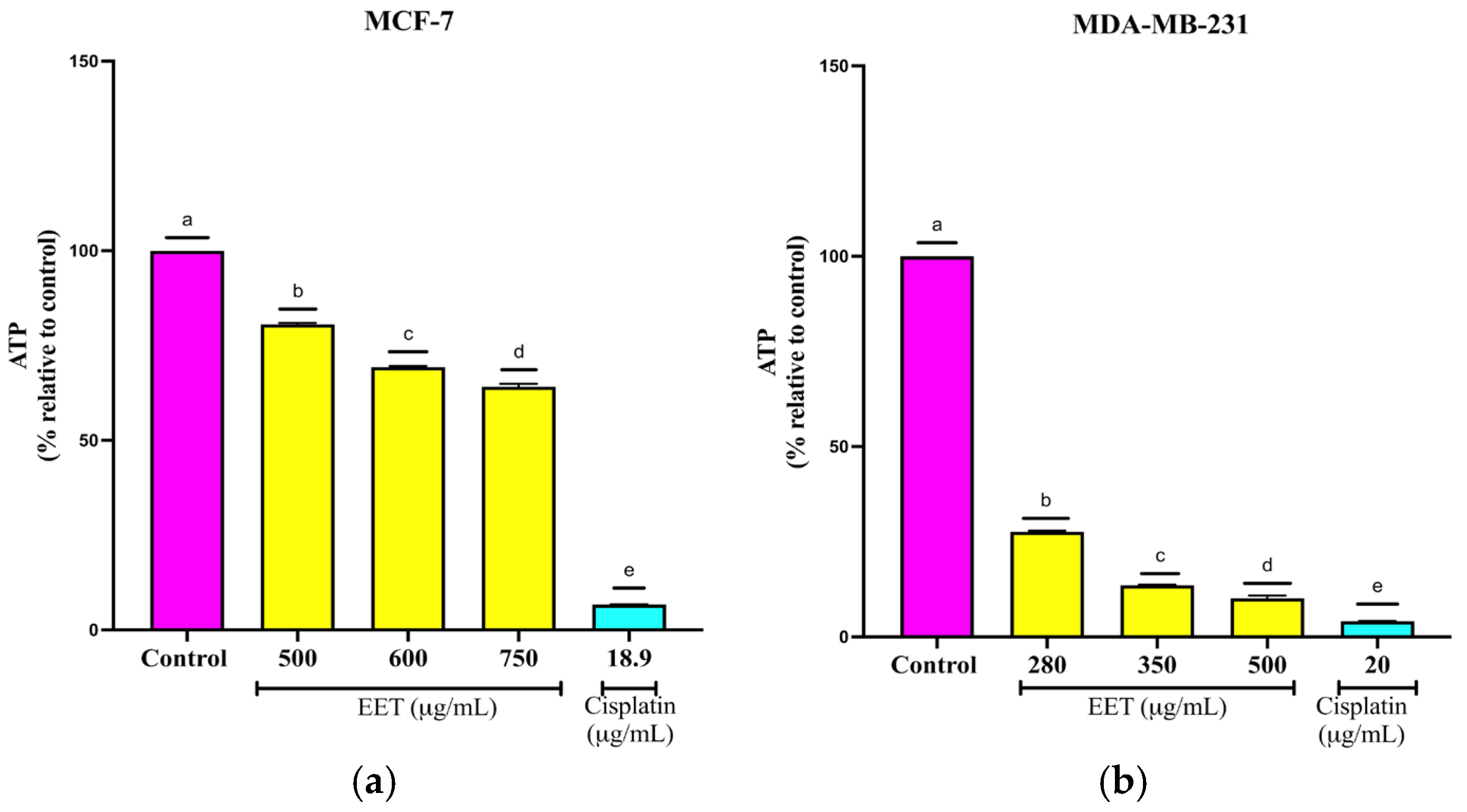
2.3.2. EET Decreases ATP Levels of MCF-7 and MDA-MB-231 Cells in the 3D Model
2.3.3. EET Activates Caspases -3/7, -8 and -9 in MCF-7 and MDA-MB-231 Cell Lines in a 3D Model
2.3.4. Inhibition of Cell Invasion by EET in MCF-7 and MDA-MB-231 Cells in a 3D Model
2.3.5. EET in Co3mbination with Cisplatin and Paclitaxel Decreases the Viability of MCF-7 and MDA-MB-231 in 2D and 3D Models
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of the Extracts
4.3. Ultra-Performance Liquid Chromatography with Time-of-Flight Mass Spectrometry (UPLC-Q-TOF/MSE) and Quantification of Compounds in Crude Extract by HPLC
4.4. Culture
4.5. Assays in 2D Cell Model
4.5.1. D Cell Viability by SRB (Sulforhodamine B)
4.5.2. Cell Cycle Assay in 2D Model in MCF-7 and MDA-MB-231 Cells, by Flow Cytometry
4.5.3. Human Aldehyde Dehydrogenase 3A1 (ALDH3A1) Activity Assay
4.5.4. Cyclin-Dependent Kinase 4 (CDK4) Activity Inhibition Assay
4.5.5. p53 Protein Transcription Assay
4.6. D Cell Model Assays
4.6.1. Cell Viability by Plasma Membrane Integrity in 3D Culture of MCF-7 and MDA-MB-231 Cells
4.6.2. Quantification of ATP Levels in the 3D Model
4.6.3. Caspases -3/7, -8, and -9 Activity of MCF-7 and MDA-MB-231 Cells in 3D Model
4.6.4. Tumor Invasion Assay in a 3D Model of MCF-7 and MDA-MB-231 Cells
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGrowder, D.A.; Miller, F.G.; Nwokocha, C.R.; Anderson, M.S.; Wilson-Clarke, C.; Vaz, K.; Anderson-Jackson, L.; Brown, J. Medicinal Herbs Used in Traditional Management of Breast Cancer: Mechanisms of Action. Medicines 2020, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Globocan. New Global Cancer Data. 2022. Available online: https://gco.iarc.fr/today/en/dataviz/pie?mode=cancer&group_populations=1 (accessed on 9 August 2025).
- Xiong, X.; Zheng, L.-W.; Ding, Y.; Chen, Y.-F.; Cai, Y.-W.; Wang, L.-P.; Huang, L.; Liu, C.-C.; Shao, Z.-M.; Yu, K.-D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Casciati, A.; Bakar, Z.A.; Hamzah, H.; Ahmad, T.A.T.; Noor, M.H.M. The Detection of DNA Damage Response in MCF7 and MDA-MB-231 Breast Cancer Cell Lines after X-ray Exposure. Genome Integr. 2023, 14, e20220001. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- Wang, H.; Guo, S.; Kim, S.-J.; Shao, F.; Ho, J.W.K.; Wong, K.U.; Miao, Z.; Hao, D.; Zhao, M.; Xu, J.; et al. Cisplatin prevents breast cancer metastasis through blocking early EMT and retards cancer growth together with paclitaxel. Theranostics 2021, 11, 2442–2459. [Google Scholar] [CrossRef]
- Rusia, K.; Madke, B.; Kashikar, Y.; Meghe, S. Paclitaxel-Induced Cutaneous Lupus Erythematosus and Raynaud’s Phenomenon. Cureus 2023, 15, e50974. [Google Scholar] [CrossRef]
- Abdelmaksoud, N.M.; Abulsoud, A.I.; Doghish, A.S.; Abdelghany, T.M. From resistance to resilience: Uncovering chemotherapeutic resistance mechanisms; insights from established models. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2023, 1878, 188993. [Google Scholar] [CrossRef]
- Iweala, E.E.J.; Amuji, D.N.; Nnaji, F.C. Protein biomarkers for diagnosis of breast cancer. Sci. Afr. 2024, 25, e02308. [Google Scholar] [CrossRef]
- Valachis, A.; Biganzoli, L.; Christopoulou, A.; Fjermeros, K.; Fountzila, E.; Geisler, J.; Gomez-Bravo, R.; Karihtala, P.; Kosmidis, P.; Koutras, A.; et al. Implementing geriatric assessment for dose optimization of CDK4/6 inhibitors in older breast cancer patients. Future Oncol. 2024, 20, 2937–2948. [Google Scholar] [CrossRef]
- Li, M.; Cescon, D.W. From Intractable to Treatable: Milestones and Horizons in the Management of HER2+ Breast Cancer. Can. Oncol. Today 2024, 1, 20–26. [Google Scholar] [CrossRef]
- Shalata, W.; Zolnoorian, J.; Migliozzi, G.; Jama, A.A.; Dudnik, Y.; Cohen, A.Y.; Meirovitz, A.; Yakobson, A. Long-Lasting Therapeutic Response following Treatment with Pembrolizumab in Patients with Non-Small Cell Lung Cancer: A Real-World Experience. Int. J. Mol. Sci. 2023, 24, 5938. [Google Scholar] [CrossRef] [PubMed]
- Hayatou, M.-U.; Tembe, E.A.; Herve, B.; Borgia, N.N.; Fokunang, C.N. Qualitative and Quantitative Phytochemical Characterization of Leaf Extracts of Mimosa pudica (Mimosaceae). J. Complement. Altern. Med. Res. 2023, 23, 1–13. [Google Scholar] [CrossRef]
- Higashi, B.; De Almeida, R.T.R.; Pilau, E.J.; Gonçalves, J.E.; Gonçalves, R.A.C.; de Oliveira, A.J.B. Metabolic profiling of monoterpenoid indole alkaloids from Tabernaemontana catharinensis (A. DC) latex by GC-MS. Phytochem. Lett. 2021, 41, 6–13. [Google Scholar] [CrossRef]
- Rizo, W.F.; Ferreira, L.E.; Colnaghi, V.; Martins, J.S.; Franchi, L.P.; Takahashi, C.S.; Beleboni, R.O.; Marins, M.; Pereira, P.S.; Fachin, A.L. Cytotoxicity and genotoxicity of coronaridine from Tabernaemontana catharinensis A.DC in a human laryngeal epithelial carcinoma cell line (Hep-2). Genet. Mol. Biol. 2013, 36, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Boligon, A.A.; Piana, M.; Schawnz, T.G.; Pereira, R.P.; Rocha, J.B.T.; Athayde, M.L. Chromatographic Analysis and Antioxidant Capacity of Tabernaemontana catharinensis. Nat. Prod. Commun. 2014, 9, 1934578X1400900. [Google Scholar] [CrossRef]
- Sari, R.; Conterno, P.; da Silva, L.D.; de Lima, V.A.; Oldoni, T.L.C.; Thomé, G.R.; Carpes, S.T. Extraction of Phenolic Compounds from Tabernaemontana catharinensis Leaves and Their Effect on Oxidative Stress Markers in Diabetic Rats. Molecules 2020, 25, 2391. [Google Scholar] [CrossRef]
- Mocanu, M.-M.; Nagy, P.; Szöllősi, J. Chemoprevention of Breast Cancer by Dietary Polyphenols. Molecules 2015, 20, 22578–22620. [Google Scholar] [CrossRef]
- Rezaei-Seresht, H.; Cheshomi, H.; Falanji, F.; Movahedi-Motlagh, F.; Hashemian, M.; Mireskandari, E. Cytotoxic activity of caffeic acid and gallic acid against MCF-7 human breast cancer cells: An in silico and in vitro study. Avicenna J. Phytomedicine 2019, 9, 574–586. [Google Scholar] [CrossRef]
- Guneidy, R.; Shokeer, A.; Abdel karim, G.S.A.; Saleh, N.; Zaki, E. Effect of Protocatechuic Acid on Tamoxifen Efficacy and Oxidative Stress in Breast Cancer Cells: Implications for Combination Therapy. Egypt. J. Chem. 2024, 67, 167–181. [Google Scholar] [CrossRef]
- Trujillo, L.; Bedoya, J.; Cortés, N.; Osorio, E.H.; Gallego, J.-C.; Leiva, H.; Castro, D.; Osorio, E. Cytotoxic Activity of Amaryllidaceae Plants against Cancer Cells: Biotechnological, In Vitro, and In Silico Approaches. Molecules 2023, 28, 2601. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic acid: A review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.-M.; Chen, C.-W.; Chen, C.-C.; Wang, H.-W.; Jhang, J.-H.; Huang, Y.-H.; Wen, K.-C. Role of Coffea arabica Extract and Related Compounds in Preventing Photoaging and Photodamage of the Skin. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 523–530. [Google Scholar] [CrossRef]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Alam, W.; Alsharif, K.F.; Aschner, M.; Pervez, S.; Saso, L. Alkaloids and Colon Cancer: Molecular Mechanisms and Therapeutic Implications for Cell Cycle Arrest. Molecules 2022, 27, 920. [Google Scholar] [CrossRef]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Jastrzębska-Stojko, Ż.; Stojko, R.; Wojtyczka, R.; Stojko, J. Comparison of Two Components of Propolis: Caffeic Acid (CA) and Caffeic Acid Phenethyl Ester (CAPE) Induce Apoptosis and Cell Cycle Arrest of Breast Cancer Cells MDA-MB-231. Molecules 2017, 22, 1554. [Google Scholar] [CrossRef]
- Almalki, S.G. The pathophysiology of the cell cycle in cancer and treatment strategies using various cell cycle checkpoint inhibitors. Pathol.-Res. Pract. 2023, 251, 154854. [Google Scholar] [CrossRef]
- Missihoun, T.D.; Kotchoni, S.O.; Bartels, D. Aldehyde Dehydrogenases Function in the Homeostasis of Pyridine Nucleotides in Arabidopsis thaliana. Sci. Rep. 2018, 8, 2936. [Google Scholar] [CrossRef]
- Gelardi, E.L.M.; Colombo, G.; Picarazzi, F.; Ferraris, D.M.; Mangione, A.; Petrarolo, G.; Aronica, E.; Rizzi, M.; Mori, M.; La Motta, C.; et al. A Selective Competitive Inhibitor of Aldehyde Dehydrogenase 1A3 Hinders Cancer Cell Growth, Invasiveness and Stemness In Vitro. Cancers 2021, 13, 356. [Google Scholar] [CrossRef]
- Duan, J.-J.; Cai, J.; Gao, L.; Yu, S.-C. ALDEFLUOR activity, ALDH isoforms, and their clinical significance in cancers. J. Enzym. Inhib. Med. Chem. 2023, 38, 2166035. [Google Scholar] [CrossRef]
- Qu, Y.; He, Y.; Yang, Y.; Li, S.; An, W.; Li, Z.; Wang, X.; Han, Z.; Qin, L. ALDH3A1 acts as a prognostic biomarker and inhibits the epithelial mesenchymal transition of oral squamous cell carcinoma through IL-6/STAT3 signaling pathway. J. Cancer 2020, 11, 2621–2631. [Google Scholar] [CrossRef]
- Baker, S.J.; Poulikakos, P.I.; Irie, H.Y.; Parekh, S.; Reddy, E.P. CDK4: A master regulator of the cell cycle and its role in cancer. Genes Cancer 2022, 13, 21–45. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Jang, H.; Nussinov, R. CDK2 and CDK4: Cell Cycle Functions Evolve Distinct, Catalysis-Competent Conformations, Offering Drug Targets. JACS Au 2024, 4, 1911–1927. [Google Scholar] [CrossRef] [PubMed]
- Hernández Borrero, L.J.; El-Deiry, W.S. Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef] [PubMed]
- Capuozzo, M.; Santorsola, M.; Bocchetti, M.; Perri, F.; Cascella, M.; Granata, V.; Celotto, V.; Gualillo, O.; Cossu, A.M.; Nasti, G.; et al. p53: From Fundamental Biology to Clinical Applications in Cancer. Biology 2022, 11, 1325. [Google Scholar] [CrossRef]
- Kepp, O.; Bezu, L.; Yamazaki, T.; Di Virgilio, F.; Smyth, M.J.; Kroemer, G.; Galluzzi, L. ATP and cancer immunosurveillance. EMBO J. 2021, 40, e108130. [Google Scholar] [CrossRef]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin; Hassan, M.I.; Habib, S.; et al. Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef]
- Gupta, G.P.; Massagué, J. Cancer Metastasis: Building a Framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef]
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef]
- Torborg, S.R.; Li, Z.; Chan, J.E.; Tammela, T. Cellular and molecular mechanisms of plasticity in cancer. Trends Cancer 2022, 8, 735–746. [Google Scholar] [CrossRef]
- Chou, T.-C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Camponogara, C.; Casoti, R.; Brusco, I.; Piana, M.; Boligon, A.A.; Cabrini, D.A.; Trevisan, G.; Ferreira, J.; Silva, C.R.; Oliveira, S.M. Tabernaemontana catharinensis leaves effectively reduce the irritant contact dermatitis by glucocorticoid receptor-dependent pathway in mice. Biomed. Pharmacother. 2019, 109, 646–657. [Google Scholar] [CrossRef]
- Rosales, P.F.; Marinho, F.F.; Gower, A.; Chiarello, M.; Canci, B.; Roesch-Ely, M.; Paula, F.R.; Moura, S. Bio-guided search of active indole alkaloids from Tabernaemontana catharinensis: Antitumour activity, toxicity in silico and molecular modelling studies. Bioorganic Chem. 2019, 85, 66–74. [Google Scholar] [CrossRef]
- Boligon, A.A.; Schwanz, T.G.; Piana, M.; Bandeira, R.V.; Frohlich, J.K.; de Brum, T.F.; Zadra, M.; Athayde, M.L. Chemical composition and antioxidant activity of the essential oil of Tabernaemontana catharinensis A. DC. leaves. Nat. Prod. Res. 2012, 27, 68–71. [Google Scholar] [CrossRef]
- Da Silva Menecucci, C.; Mucellini, K.L.; de Oliveira, M.M.; Higashi, B.; de Almeida, R.T.R.; Porto, C.; Pilau, E.J.; Gonçalves, J.E.; Correia Gonçalves, R.A.; de Oliveira, A.J.B. Latex from Tabernaemontana catharinensis (A. DC)—Apocynaceae: An alternative for the sustainable production of biologically active compounds. Ind. Crops Prod. 2019, 129, 74–84. [Google Scholar] [CrossRef]
- Piana, M.; Boligon, A.A.; Brum, T.F.D.; Zadra, M.; Belke, B.V.; Froeder, A.L.F.; Frohlich, J.K.; Nunes, L.T.; Pappis, L.; Boligon, A.A.; et al. Phytochemical analysis and antioxidant capacity of Tabernaemontana catharinensis A. DC. Fruits and branches. An. Acad. Bras. Ciências 2014, 86, 881–888. [Google Scholar] [CrossRef]
- Yin, M.-C.; Lin, C.-C.; Wu, H.-C.; Tsao, S.-M.; Hsu, C.-K. Apoptotic Effects of Protocatechuic Acid in Human Breast, Lung, Liver, Cervix, and Prostate Cancer Cells: Potential Mechanisms of Action. J. Agric. Food Chem. 2009, 57, 6468–6473. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.H.; Hunakova, L.; Chen, Y.; Bortner, C.; Van Houten, B. Cell Sorting Experiments Link Persistent Mitochondrial DNA Damage with Loss of Mitochondrial Membrane Potential and Apoptotic Cell Death. J. Biol. Chem. 2002, 278, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-H.; Chen, W.-H.; Juan-Lu, C.; Hsieh, S.-C.; Lin, S.-C.; Mai, R.-T.; Chen, S.-Y. Hesperidin and Chlorogenic Acid Synergistically Inhibit the Growth of Breast Cancer Cells via Estrogen Receptor/Mitochondrial Pathway. Life 2021, 11, 950. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Identifying Peach and Plum Polyphenols with Chemopreventive Potential against Estrogen-Independent Breast Cancer Cells. J. Agric. Food Chem. 2009, 57, 5219–5226. [Google Scholar] [CrossRef]
- Xie, C.; Chan, L.; Pang, Y.; Shang, Y.; Cao, W.; Tuohan, M.; Deng, Q.; Wang, Y.; Zhao, L.; Wang, W. Caffeic acid inhibits the tumorigenicity of triple-negative breast cancer cells through the FOXO1/FIS pathway. Biomed. Pharmacother. 2024, 178, 117158. [Google Scholar] [CrossRef]
- Yuan, D.; Wang, J.; Yan, M.; Xu, Y. SOX2 as a prognostic marker and a potential molecular target in cervical cancer: A meta-analysis. Int. J. Biol. Markers 2021, 36, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Tian, W.; Zheng, S.; Zou, Y.; Xie, J.; Zhang, J.; Li, X.; Sun, Y.; Lan, J.; Li, N.; et al. Dissection of FOXO1-induced LYPLAL1-DT Impeding Triple Negative Breast Cancer Progression via Mediating hnRNPK/β-catenin Complex. Research 2023, 6, 0289. [Google Scholar] [CrossRef] [PubMed]
- Agunloye, O.M.; Oboh, G. Hypercholesterolemia, angiotensin converting enzyme and ecto-enzymes of purinergic system: Ameliorative properties of caffeic and chlorogenic acid in hypercholesterolemic rats. J. Food Biochem. 2018, 42, e12604. [Google Scholar] [CrossRef]
- Yegutkin, G.G.; Boison, D. ATP and Adenosine Metabolism in Cancer: Exploitation for Therapeutic Gain. Pharmacol. Rev. 2022, 74, 797–822. [Google Scholar] [CrossRef]
- Rosendahl, A.H.; Perks, C.M.; Zeng, L.; Markkula, A.; Simonsson, M.; Rose, C.; Ingvar, C.; Holly, J.M.P.; Jernström, H. Caffeine and Caffeic Acid Inhibit Growth and Modify Estrogen Receptor and Insulin-like Growth Factor I Receptor Levels in Human Breast Cancer. Clin. Cancer Res. 2015, 21, 1877–1887. [Google Scholar] [CrossRef]
- Jaganathan, S.K. Growth Inhibition by Caffeic Acid, One of the Phenolic Constituents of Honey, in HCT 15 Colon Cancer Cells. Sci. World J. 2012, 2012, 372345. [Google Scholar] [CrossRef]
- Ranjbary, A.G.; Bagherzadeh, A.; Sabbaghi, S.S.; Faghihi, A.; Karimi, D.N.; Naji, S.; Kardani, M. Chlorogenic acid induces apoptosis and cell-cycle arrest in colorectal cancer cells. Mol. Biol. Rep. 2023, 50, 9845–9857. [Google Scholar] [CrossRef]
- Pumiputavon, K.; Chaowasku, T.; Saenjum, C.; Osathanunkul, M.; Wungsintaweekul, B.; Chawansuntati, K.; Wipasa, J.; Lithanatudom, P. Cell cycle arrest and apoptosis induction by methanolic leaves extracts of four Annonaceae plants. BMC Complement. Altern. Med. 2017, 17, 294. [Google Scholar] [CrossRef]
- Chinnasamy, A.; Jayaprakash, V.; Padmanaban, D.; Sekar, N.; Valayapathi, R.; Azhagudurai, A.; Ethiraj, S. Effect of crude ethanolic seed extract from Mucuna pruriens on proliferation, apoptosis, and cell cycle arrest in gastric adenocarcinoma (AGS) cells. Future J. Pharm. Sci. 2024, 10, 141. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, H.; Yan, L.; Wang, X.; Che, X.; Hou, K.; Yang, Y.; Li, X.; Li, Y.; Zhang, Y.; et al. Hypoxia-induced ALDH3A1 promotes the proliferation of non-small-cell lung cancer by regulating energy metabolism reprogramming. Cell Death Dis. 2023, 14, 617. [Google Scholar] [CrossRef]
- Sládek, N.; Kollander, R.; Sreerama, L.; Kiang, D. Cellular levels of aldehyde dehydrogenases (ALDH1A1 and ALDH3A1) as predictors of therapeutic responses to cyclophosphamide-based chemotherapy of breast cancer: A retrospective study. Cancer Chemother. Pharmacol. 2002, 49, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Dogra, S.; Dimri, K.; Pandey, A.K.; Jose, J.S.; Punia, R. Metaplastic breast cancer: Experience with ifosfamide based chemotherapy. Curr. Probl. Cancer 2024, 53, 101148. [Google Scholar] [CrossRef] [PubMed]
- Voulgaridou, G.-P.; Kiziridou, M.; Mantso, T.; Chlichlia, K.; Galanis, A.; Koukourakis, M.I.; Franco, R.; Panayiotidis, M.I.; Pappa, A. Aldehyde dehydrogenase 3A1 promotes multi-modality resistance and alters gene expression profile in human breast adenocarcinoma MCF-7 cells. Int. J. Biochem. Cell Biol. 2016, 77, 120–128. [Google Scholar] [CrossRef]
- Ye, F.; Qiu, Y.; Li, L.; Yang, L.; Cheng, F.; Zhang, H.; Wei, B.; Zhang, Z.; Sun, L.; Bu, H. The Presence of EpCAM-/CD49f+Cells in Breast Cancer Is Associated with a Poor Clinical Outcome. J. Breast Cancer 2015, 18, 242. [Google Scholar] [CrossRef] [PubMed]
- Stengel, K.R.; Thangavel, C.; Solomon, D.A.; Angus, S.P.; Zheng, Y.; Knudsen, E.S. Retinoblastoma/p107/p130 Pocket Proteins. J. Biol. Chem. 2009, 284, 19265–19271. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, L.; Sun, Z.; Li, J. CDK4/6 inhibitor resistance mechanisms and treatment strategies (Review). Int. J. Mol. Med. 2022, 50, 128. [Google Scholar] [CrossRef]
- Liang, Y.; Feng, G.; Wu, L.; Zhong, S.; Gao, X.; Tong, Y.; Cui, W.; Qin, Y.; Xu, W.; Xiao, X.; et al. Caffeic acid phenethyl ester suppressed growth and metastasis of nasopharyngeal carcinoma cells by inactivating the NF-κB pathway. Drug Des. Dev. Ther. 2019, 13, 1335–1345. [Google Scholar] [CrossRef]
- Wadhwa, R.; Nigam, N.; Bhargava, P.; Dhanjal, J.K.; Goyal, S.; Grover, A.; Sundar, D.; Ishida, Y.; Terao, K.; Kaul, S.C. Molecular Characterization and Enhancement of Anticancer Activity of Caffeic Acid Phenethyl Ester by γ Cyclodextrin. J. Cancer 2016, 7, 1755–1771. [Google Scholar] [CrossRef]
- Razak, N.A.; Abu, N.; Ho, W.Y.; Zamberi, N.R.; Tan, S.W.; Alitheen, N.B.; Long, K.; Yeap, S.K. Cytotoxicity of eupatorin in MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle arrest, anti-angiogenesis and induction of apoptosis. Sci. Rep. 2019, 9, 1514. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, Y.S.; Chao, M.; Yang, D.; Liu, C.; Tseng, J.; Chen, Y. An alleviative effect of Lonicerae japonicae flos water extract against liver fibrogenesis in vitro and in vivo. Environ. Toxicol. 2024, 39, 2881–2892. [Google Scholar] [CrossRef]
- Abdel-Razek, M.A.M.; Abdelwahab, M.F.; Mohamad, S.A.; Abou-Zied, H.A.; Abdelmohsen, U.R.; Hamed, A.N.E. Cytotoxic Potential and Metabolomic Profiling of Solanum lycopersicum Roots Extract and Their Nanocrystals: An In Silico Approach. Integr. Cancer Ther. 2025, 24, 15347354251335599. [Google Scholar] [CrossRef]
- Kabalan, Y.; Matulewicz, K.; Tylkowski, B.; Woźniak-Budych, M.; Staszak, K.; Montané, X.; Bajek, A. Investigation of Anti-Cancer Properties of Nano-Encapsulated Ciprofloxacin Using 3D Cancer Cell Spheroids as Tumour Models. Int. J. Mol. Sci. 2025, 26, 5530. [Google Scholar] [CrossRef]
- Cordeiro, S.; Oliveira, B.B.; Valente, R.; Ferreira, D.; Luz, A.; Baptista, P.V.; Fernandes, A.R. Breaking the mold: 3D cell cultures reshaping the future of cancer research. Front. Cell Dev. Biol. 2024, 12, 1507388. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Wiig, H. Tumor Interstitial Fluid Formation, Characterization, and Clinical Implications. Front. Oncol. 2015, 5, 115. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Sandra, F.; Putri, J.; Limen, H.; Sarizta, B. Caffeic Acid Inhibits RANKL and TNFa-induced Osteoclastogenesis by Targeting TAK1-p44/42 MAPK. Indones. Biomed. J. 2021, 13, 433–437. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Orlowski, R.Z.; Small, G.W.; Shi, Y.Y. Evidence That Inhibition of p44/42 Mitogen-activated Protein Kinase Signaling Is a Factor in Proteasome Inhibitor-mediated Apoptosis. J. Biol. Chem. 2002, 277, 27864–27871. [Google Scholar] [CrossRef]
- Lin, H.-H.; Chen, J.-H.; Chou, F.-P.; Wang, C.-J. Protocatechuic acid inhibits cancer cell metastasis involving the down-regulation of Ras/Akt/NF-κB pathway and MMP-2 production by targeting RhoB activation. Br. J. Pharmacol. 2010, 162, 237–254. [Google Scholar] [CrossRef]
- López-Bojorquez, L.N. La regulación del factor de transcripción NF-κB. Un mediador molecular en el proceso inflamatorio. Rev. Investig. Clínica 2004, 56, 83–92. [Google Scholar]
- Qiu, Z.; Wang, Y.; Zhang, Z.; Qin, R.; Peng, Y.; Tang, W.; Xi, Y.; Tian, G.; Zhang, Y. Roles of intercellular cell adhesion molecule-1 (ICAM-1) in colorectal cancer: Expression, functions, prognosis, tumorigenesis, polymorphisms and therapeutic implications. Front. Oncol. 2022, 12, 1052672. [Google Scholar] [CrossRef]
- Wainwright, C.L.; Teixeira, M.M.; Adelson, D.L.; Buenz, E.J.; David, B.; Glaser, K.B.; Harata-Lee, Y.; Howes, M.-J.R.; Izzo, A.A.; Maffia, P.; et al. Future directions for the discovery of natural product-derived immunomodulating drugs: An IUPHAR positional review. Pharmacol. Res. 2022, 177, 106076. [Google Scholar] [CrossRef]
- Sirota, R.; Gibson, D.; Kohen, R. The timing of caffeic acid treatment with cisplatin determines sensitization or resistance of ovarian carcinoma cell lines. Redox Biol. 2017, 11, 170–175. [Google Scholar] [CrossRef]
- LIU, S.; LI, X. Autophagy inhibition enhances sensitivity of endometrial carcinoma cells to paclitaxel. Int. J. Oncol. 2015, 46, 2399–2408. [Google Scholar] [CrossRef]
- Škubník, J.; Pavlíčková, V.S.; Ruml, T.; Rimpelová, S. Autophagy in cancer resistance to paclitaxel: Development of combination strategies. Biomed. Pharmacother. 2023, 161, 114458. [Google Scholar] [CrossRef] [PubMed]
- Macho-González, A.; Sánchez-Muniz, F.J. Autophagy, a key cellular cleansing system for health. A visit to the 2016 Nobel Prize in Physiology or Medicine. J. Negat. No Posit. Results 2022, 8, 417–439. [Google Scholar] [CrossRef]
- Koraneekit, A.; Limpaiboon, T.; Sangka, A.; Boonsiri, P.; Daduang, S.; Daduang, J. Synergistic effects of cisplatin-caffeic acid induce apoptosis in human cervical cancer cells via the mitochondrial pathways. Oncol. Lett. 2018, 15, 7397–7402. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jia, W.-W.; Yang, J.-L.; Cheng, C.; Olaleye, O.E. Multi-compound and drug-combination pharmacokinetic research on Chinese herbal medicines. Acta Pharmacol. Sin. 2022, 43, 3080–3095. [Google Scholar] [CrossRef]
- Lin, C.-L.; Chen, R.-F.; Chen, J.Y.-F.; Chu, Y.-C.; Wang, H.-M.; Chou, H.-L.; Chang, W.-C.; Fong, Y.; Chang, W.-T.; Wu, C.-Y.; et al. Protective Effect of Caffeic Acid on Paclitaxel Induced Anti-Proliferation and Apoptosis of Lung Cancer Cells Involves NF-κB Pathway. Int. J. Mol. Sci. 2012, 13, 6236–6245. [Google Scholar] [CrossRef]
- Hou, Y.-F.; Bai, L.; Guo, S.; Hu, J.-B.; Zhang, S.-S.; Liu, S.-J.; Zhang, Y.; Li, S.; Ho, C.-T.; Bai, N.-S. Nontargeted metabolomic analysis of four different parts of Actinidia arguta by UPLC-Q-TOF-MSE. Food Res. Int. 2023, 163, 112228. [Google Scholar] [CrossRef]
- LIU, P.-P.; SHAN, G.-S.; ZHANG, F.; CHEN, J.-N.; JIA, T.-Z. Metabolomics analysis and rapid identification of changes in chemical ingredients in crude and processed Astragali Radix by UPLC-QTOF-MS combined with novel informatics UNIFI platform. Chin. J. Nat. Med. 2018, 16, 714–720. [Google Scholar] [CrossRef]
- Vannabhum, M.; Ziangchin, N.; Thepnorarat, P.; Akarasereenont, P. Metabolomic analysis of Thai Herbal Analgesic Formula based on ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Heliyon 2023, 9, e18296. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Gomes, D.; Telles, C.; Costa, M.; Almeida-Lima, J.; Costa, L.; Keesen, T.; Rocha, H. Methanolic Extracts from Brown Seaweeds Dictyota cilliolata and Dictyota menstrualis Induce Apoptosis in Human Cervical Adenocarcinoma HeLa Cells. Molecules 2015, 20, 6573–6591. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, O.P.; González-Torres, A.; Álvarez-Salas, L.M.; Hernández-Sánchez, H.; García-Pérez, B.E.; Thompson-Bonilla, M.d.R.; Jaramillo-Flores, M.E. Effect of naringenin and its combination with cisplatin in cell death, proliferation and invasion of cervical cancer spheroids. RSC Adv. 2021, 11, 129–141. [Google Scholar] [CrossRef]






| Identification | Tr | Formula | Measured m/z | Mass Error (ppm) | Fragmentation |
|---|---|---|---|---|---|
| Caffeic acid 3-glucoside | 4.23 | C15H18O9 | 341.0884 | 1.8 | 135.0436, 179.0340 |
| Protocatechiuc acid | 3.94 | C7H6O4 | 153.0183 | 6.8 | 109.0237, 108.0195 |
| Protocatechiuc acid 4-glucoside | 2.55 | C13H16O9 | 315.0726 | 1.5 | 109.0275, 153.0181 |
| Chlorogenic acid | 4.02 | C16H18O9 | 353.0895 | 4.9 | 191.0555 |
| Caffeic acid | 4.84 | C9H8O4 | 179.0348 | −0.9 | 135.0438 |
| Coniferyl aldehyde | 5.67 | C10H10O3 | 177.0551 | −3.5 | 133.0280, 135.0432 |
| Compound | µg/g Dry Sample |
|---|---|
| Chlorogenic acid | 1248 |
| Caffeic acid | 118 |
| Protocatechiuc acid | 105 |
| Treatment/ Phases | MCF-7 | MDA-MB-231 | ||||
|---|---|---|---|---|---|---|
| G1 | S | G2 | G1 | S | G2 | |
| Control | 51.7 ± 3.04 | 3.7 ± 0.21 | 44.6 ± 1.30 | 56.7 ± 0.28 | 1.51 ± 0.12 | 41.75 ± 0.07 |
| Starvation | 64 ± 4.38 | 0.15 ± 0.11 | 35.85 ± 3.17 | 58 ± 0.49 | 5.65 ± 0.15 | 36.35 ± 3.87 |
| Cisplatin 13/13.65 μg/mL | 19.85 ± 3.46 | 79.4 ± 3.53 | 0.75 ± 0.07 | 16.2 ± 0.14 | 82.8 ± 0.14 | 1 ± 0 |
| EET 500 μg/mL | 70.3 ± 0.70 | 0.77 ± 0.04 | 28.2 ± 0.28 | 97.7 ± 3.25 | 2.3 ± 0.20 | 0 ± 0 |
| EET 1000 μg/mL | 86.1 ± 4.94 | 13.24 ± 0.88 | 0.66 ± 0.01 | 100 ± 0 | 0 ± 0 | 0 ± 0 |
| Extract or Cisplatin/ Combination | MCF-7 | MDA-MB-231 | Extract or Paclitaxel/ Combination | MCF-7 | MDA-MB-231 |
|---|---|---|---|---|---|
| Cell Viability (%) | Cell Viability (%) | ||||
| Control | 100 | 100 | Control | 100 | 100 |
| ½ IC50 EET | 72 | 72 | ½ IC50 EET | 73 | 73 |
| IC50 EET | 50 | 50 | IC50 EET | 51 | 51 |
| ½ IC50 Cisplatin | 72 | 73 | ½ IC50 Paclitaxel | 73 | 73 |
| IC50 Cisplatin | 50 | 50 | IC50 Paclitaxel | 50 | 50 |
| ½ IC50 EET + ½ IC50 Cisplatin | 62 | 55 | ½ IC50 EET + ½ IC50 Paclitaxel | 52 | 44 |
| ½ IC50 EET + IC50 Cisplatin | 39 | 30 | ½ IC50 EET + IC50 Paclitaxel | 29 | 34 |
| IC50 EET + ½ IC50 Cisplatin | 45 | 42 | IC50 EET + ½ IC50 Paclitaxel | 45 | 37 |
| IC50 EET + IC50 Cisplatin | 30 | 28 | IC50 EET + IC50 Paclitaxel | 19 | 28 |
| Extract or Cisplatin/ Combination | MCF-7 | MDA-MB-231 | Extract or Paclitaxel/ Combination | MCF-7 | MDA-MB-231 |
|---|---|---|---|---|---|
| Cell Viability (%) | Cell Viability (%) | ||||
| Control | 100 | 100 | Control | 100 | 100 |
| ½ IC50 EET | 72 | 73 | ½ IC50 EET | 73 | 73 |
| IC50 EET | 50 | 51 | IC50 EET | 50 | 51 |
| ½ IC50 Cisplatin | 74 | 75 | ½ IC50 Paclitaxel | 74 | 74 |
| IC50 Cisplatin | 50 | 50 | IC50 Paclitaxel | 50 | 50 |
| ½ IC50 EET + ½ IC50 Cisplatin | 67 | 66 | ½ IC50 EET + ½ IC50 Paclitaxel | 62 | 67 |
| ½ IC50 EET + IC50 Cisplatin | 39 | 42 | ½ IC50 EET + IC50 Paclitaxel | 37 | 40 |
| IC50 EET + ½ IC50 Cisplatin | 42 | 45 | IC50 EET + ½ IC50 Paclitaxel | 42 | 43 |
| IC50 EET + IC50 Cisplatin | 37 | 38 | IC50 EET + IC50 Paclitaxel | 32 | 39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Méndez, D.d.C.; Sánchez-Mundo, M.d.l.L.; Thompson-Bonilla, M.d.R.; Álvarez-Salas, L.M.; Rosales-García, V.H.; Rodríguez-Campos, J.; Jaramillo-Flores, M.E. Anticancer Activity of Ethanolic Extract of Tabernaemontana catharinensis in Breast Cancer Lines MCF-7 and MDA-MB-231. Int. J. Mol. Sci. 2025, 26, 8111. https://doi.org/10.3390/ijms26168111
Martínez-Méndez DdC, Sánchez-Mundo MdlL, Thompson-Bonilla MdR, Álvarez-Salas LM, Rosales-García VH, Rodríguez-Campos J, Jaramillo-Flores ME. Anticancer Activity of Ethanolic Extract of Tabernaemontana catharinensis in Breast Cancer Lines MCF-7 and MDA-MB-231. International Journal of Molecular Sciences. 2025; 26(16):8111. https://doi.org/10.3390/ijms26168111
Chicago/Turabian StyleMartínez-Méndez, Diana del Carmen, María de la Luz Sánchez-Mundo, María del Rocío Thompson-Bonilla, Luis Marat Álvarez-Salas, Víctor Hugo Rosales-García, Jacobo Rodríguez-Campos, and María Eugenia Jaramillo-Flores. 2025. "Anticancer Activity of Ethanolic Extract of Tabernaemontana catharinensis in Breast Cancer Lines MCF-7 and MDA-MB-231" International Journal of Molecular Sciences 26, no. 16: 8111. https://doi.org/10.3390/ijms26168111
APA StyleMartínez-Méndez, D. d. C., Sánchez-Mundo, M. d. l. L., Thompson-Bonilla, M. d. R., Álvarez-Salas, L. M., Rosales-García, V. H., Rodríguez-Campos, J., & Jaramillo-Flores, M. E. (2025). Anticancer Activity of Ethanolic Extract of Tabernaemontana catharinensis in Breast Cancer Lines MCF-7 and MDA-MB-231. International Journal of Molecular Sciences, 26(16), 8111. https://doi.org/10.3390/ijms26168111









