Alkali Lignin-Based Biopolymer Formulations for Electro-Assisted Drug Delivery of Natural Antioxidants in Breast Cancer Cells—A Preliminary Study
Abstract
1. Introduction
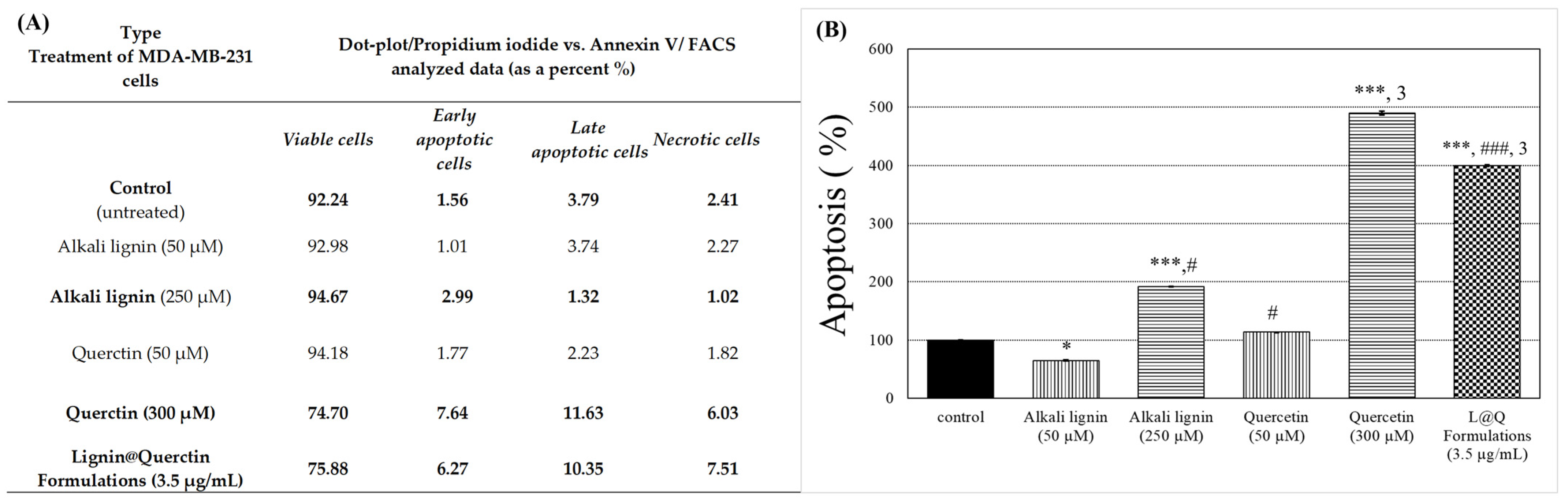
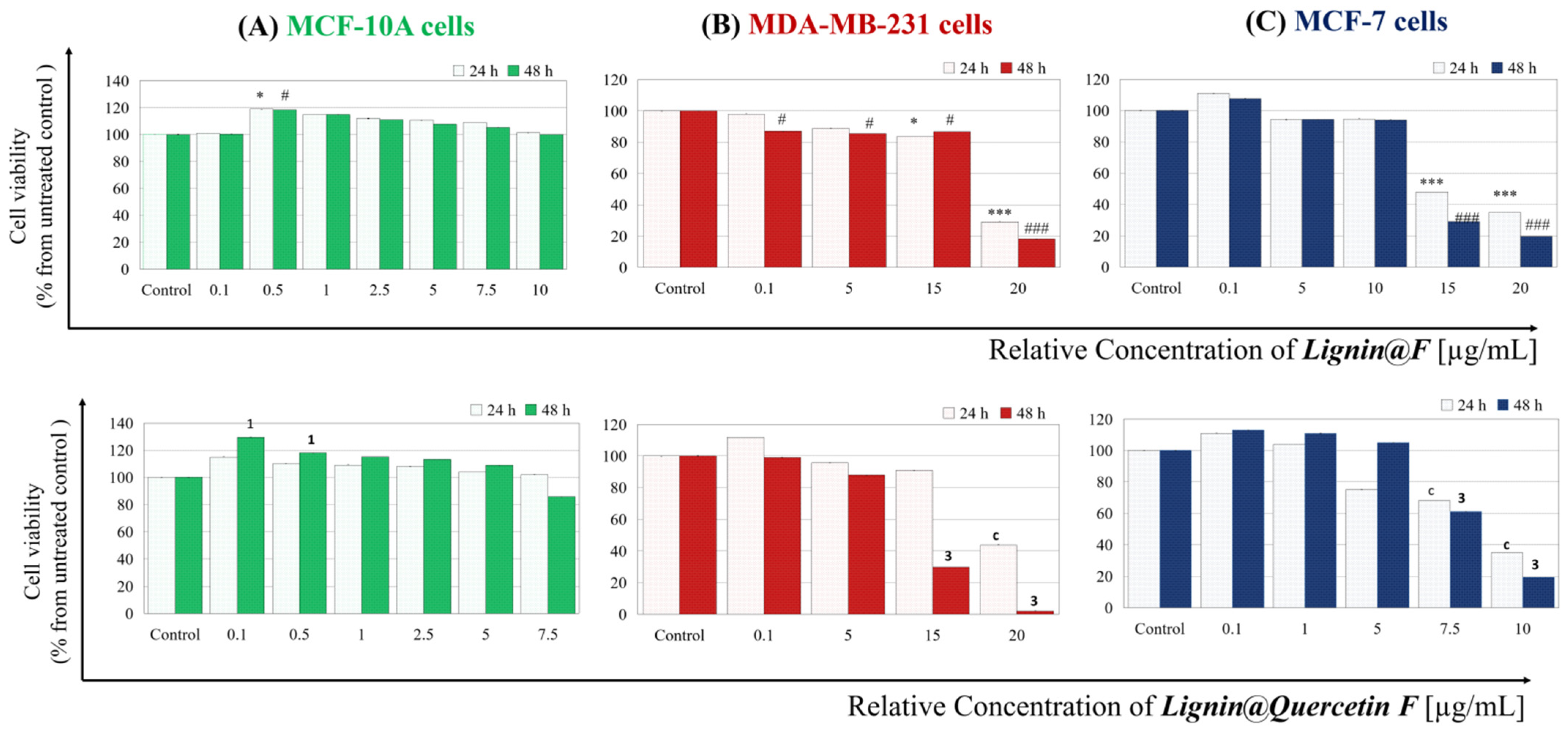
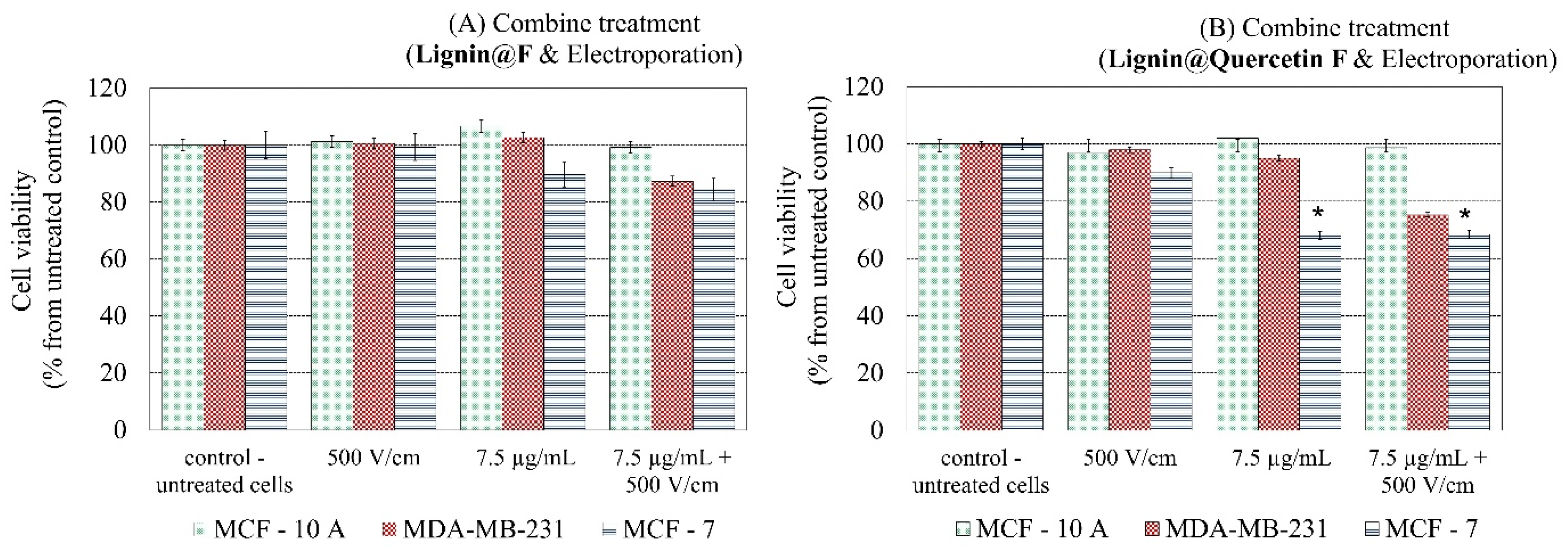
2. Results and Discussion
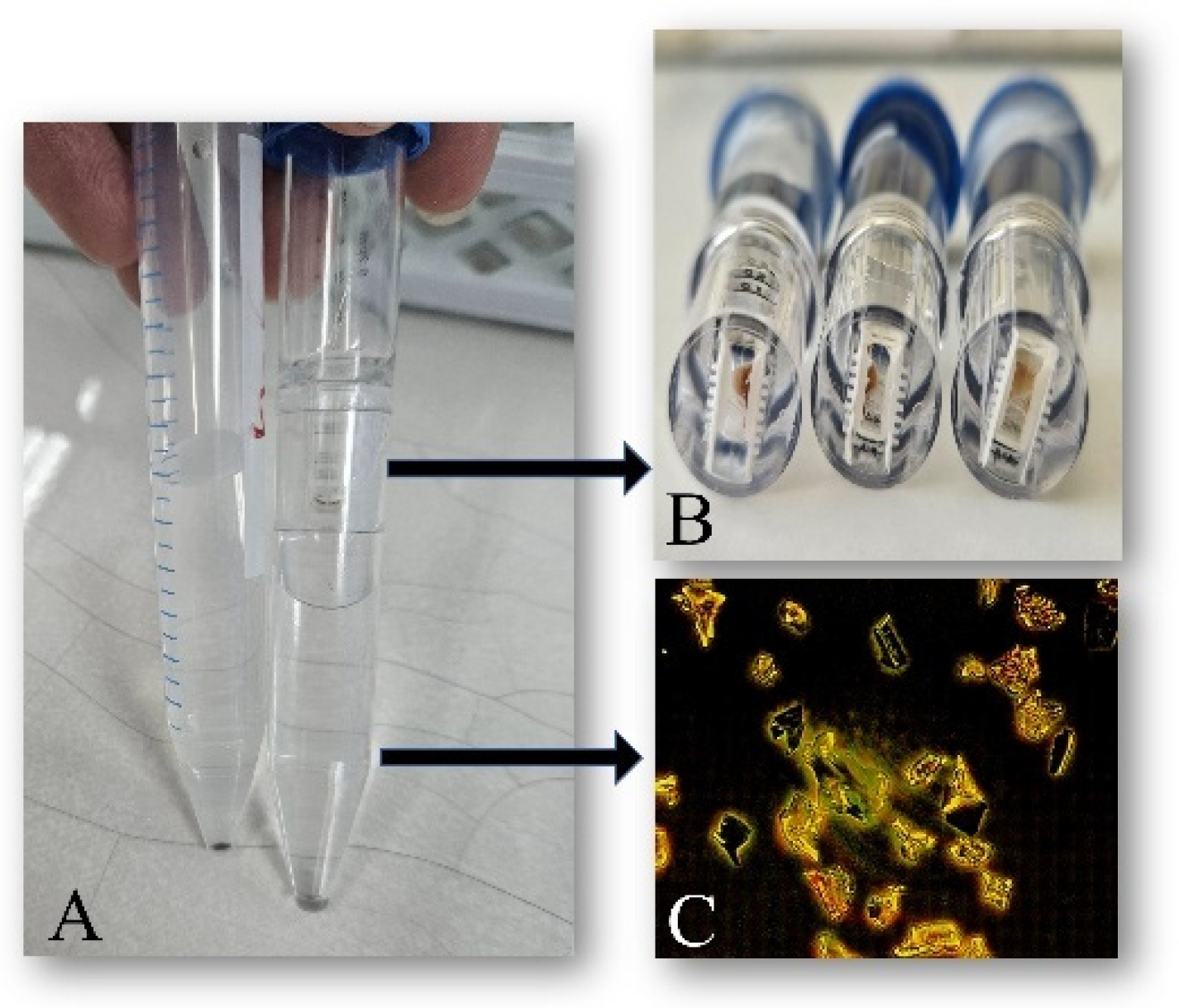
2.1. Synthesis of Alkali Lignin-Based Submicron Formulations
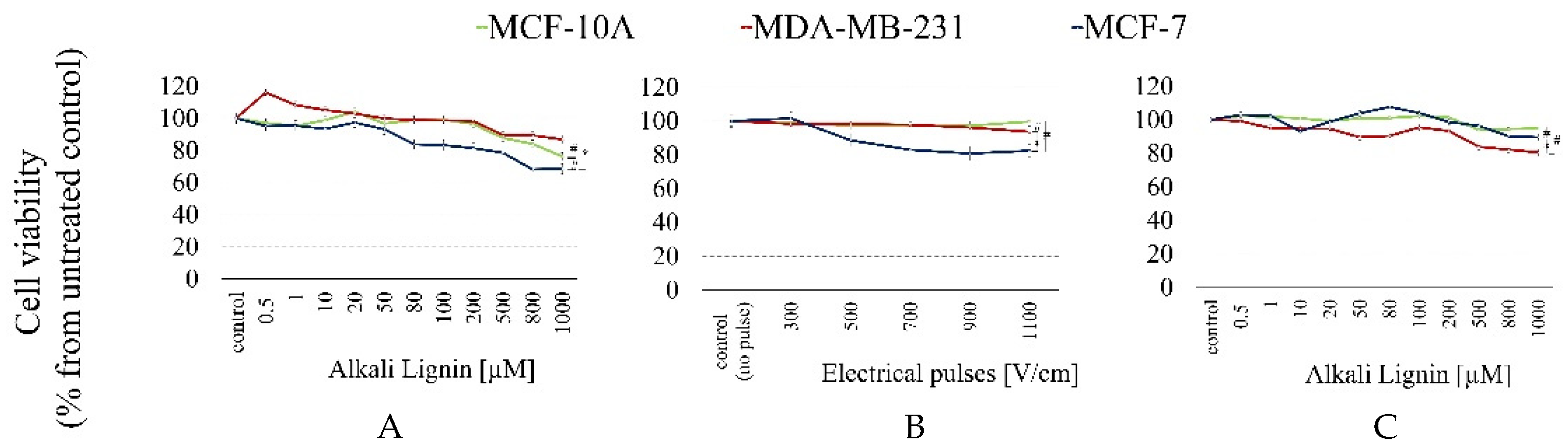
2.2. DLS Data and Electroporation Influence on Formulation’s Structure
3. Materials and Methods
3.1. Synthesis of Alkali Lignin-Based Submicron Formulations (Lignin@Formulations)
3.2. Improved Separation Procedure
3.3. Dynamic Light Scattering (DLS) Analysis
3.4. Calculation of Relative Concentration
3.5. Cell Lines and Culture Conditions
3.6. Cytotoxicity Assessment
3.7. Electroporation (EP) Protocol
3.8. Cell Proliferation Analysis via Lens-Free Microscopy
3.9. Flow Cytometry Analysis (FACS Assay)
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lammers, T. Nanomedicine Tumor Targeting. Adv. Mater. 2024, 36, 2312169. [Google Scholar] [CrossRef]
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and limitations in traditional anti-cancer therapies: A comprehensive review of surgery, chemotherapy, radiation therapy, and hormonal therapy. Discov. Oncol. 2025, 16, 607. [Google Scholar] [CrossRef]
- Ivanova, D.; Zhelev, Z.; Semkova, S.; Aoki, I.; Bakalova, R. Pesveratrol modulates the redox-status and cytotoxicity of anticancer drugs by sensitizing leukemic lymphocytes and protecting normal lymphocytes. Anticancer Res. 2019, 39, 3745–3755. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.; Zhelev, Z.; Lazarova, D.; Getsov, P.; Bakalova, R.; Aoki, I. Vitamins C and K3: A powerful redox system for sensitizing leukemia lymphocytes to everolimus and barasertib. Anticancer Res. 2018, 38, 1407–1414. [Google Scholar] [PubMed]
- Ivanova, D.; Zhelev, Z.; Zlateva, G.; Lazarova, D.; Yaneva, Z.; Panovska, R.; Aoki, I.; Bakalova, R. Effect of alpha-tocopheryl succinate on the cytotoxicity of anticancer drugs towards leukemia lymphocytes. Anticancer Res. 2022, 42, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Semkova, S.; Ivanova, D.; Nikolova, B.; Zlateva, G.; Bakalova, R.; Zhelev, Z.; Aoki, I. Inhibition of ATP-synthase potentiates cytotoxicity of combination drug menadione/ascorbate in leukaemia lymphocytes. Biotechnol. Biotechnol. Equip. 2021, 35, 1738–1744. [Google Scholar] [CrossRef]
- Ivanova, D.; Semkova, S.; Grigorov, B.; Tzanova, M.; Georgieva, A.; Danchev, D.; Nikolova, B.; Yaneva, Z. The general principle of the Warburg effect as a possible approach for cancer immunotherapy: The regulatory effect of plant extracts could change the game. Molecules 2025, 30, 393. [Google Scholar] [CrossRef]
- Markman, J.L.; Rekechenetskiy, A.; Holler, E.; Ljubimova, J.Y. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1866–1879. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, properties, and regulatory issues. Front. Chem. 2018, 6, 36. [Google Scholar] [CrossRef]
- Tang, Q.; Qian, Y.; Yang, D.; Qiu, X.; Qin, Y.; Zhou, M. Lignin-based nanoparticles: A review on their preparations and applications. Polymers 2020, 12, 2471. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in green polymer composites from lignin for multifunctional applications: A review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Liu, R.; Dai, L.; Xu, C.; Wang, K.; Zheng, C.; Si, C. Lignin-based micro- and nanomaterials and their composites in biomedical applications. Chem. Sus. Chem. 2020, 13, 4266–4283. [Google Scholar] [CrossRef]
- Korányi, T.I.; Fridrich, B.; Pineda, A.; Barta, K. Development of ‘lignin-first’ approaches for the valorization of lignocellulosic biomass. Molecules 2020, 25, 2815. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Si, C.; Wang, G.; Sui, W.; Tao, Z. Enhancing the solubility and antioxidant activity of high-molecular-weight lignin by moderate depolymerization via in situ ethanol/acid catalysis. Ind. Crops Prod. 2019, 128, 177–185. [Google Scholar] [CrossRef]
- Huang, D.; Li, R.; Xu, P.; Li, T.; Deng, R.; Chen, S.; Zhang, Q. The cornerstone of realizing lignin value-addition: Exploiting the native structure and properties of lignin by extraction methods. Chem. Eng. J. 2020, 402, 126237. [Google Scholar] [CrossRef]
- Grossman, A.; Vermerris, W. Lignin-based polymers and nanomaterials. Curr. Opin. Biotechnol. 2019, 56, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and folin-ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Wei, X.; Liu, Y.; Luo, Y.; Shen, Z.; Wang, S.; Li, M.; Zhang, L. Effect of organosolv extraction on the structure and antioxidant activity of eucalyptus kraft lignin. Int. J. Biol. Macromol. 2021, 187, 462–470. [Google Scholar] [CrossRef]
- Li, K.; Zhong, W.; Li, P.; Ren, J.; Jiang, K.; Wu, W. Recent advances in lignin antioxidant: Antioxidant mechanism, evaluation methods, influence factors and various applications. Int. J. Biol. Macromol. 2023, 251, 125992. [Google Scholar] [CrossRef]
- Barapatre, A.; Meena, A.S.; Mekala, S.; Das, A.; Jha, H. In vitro evaluation of antioxidant and cytotoxic activities of lignin fractions extracted from acacia nilotica. Int. J. Biol. Macromol. 2016, 86, 443–453. [Google Scholar] [CrossRef]
- Ivanova, D.; Nikolova, G.; Karamalakova, Y.; Semkova, S.; Marutsova, V.; Yaneva, Z. Water-soluble alkali lignin as a natural radical scavenger and anticancer alternative. Int. J. Mol. Sci. 2023, 24, 12705. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, T.; Kai, J.; Sugiarto, S.; Yu, Y.; Soo, D.X.Y.; Zhu, Q.; Merzaban, J.; Kai, D. Nano-strategies for lignin biomaterials toward cancer therapy. Adv. Healthc. Mater. 2023, 12, 2300024. [Google Scholar] [CrossRef] [PubMed]
- Witzler, M.; Alzagameem, A.; Bergs, M.; Khaldi-Hansen, B.E.; Klein, S.E.; Hielscher, D.; Kamm, B.; Kreyenschmidt, J.; Tobiasch, E.; Schulze, M. Lignin-derived biomaterials for drug release and tissue engineering. Molecules 2018, 23, 1885. [Google Scholar] [CrossRef] [PubMed]
- Abraham, B.; Syamnath, V.L.; Arun, K.B.; Fathima Zahra, P.M.; Anjusha, P.; Kothakotta, A.; Chen, Y.-H.; Ponnusamy, V.K.; Nisha, P. Lignin-based nanomaterials for food and pharmaceutical applications: Recent trends and future outlook. Sci. Total Environ. 2023, 881, 163316. [Google Scholar] [CrossRef]
- Yaneva, Z.; Ivanova, D.; Toneva, M. Green Synthesis, Characterization, encapsulation, and measurement of the release potential of novel alkali lignin micro-/submicron particles. J. Vis. Exp. 2024, 66216. [Google Scholar] [CrossRef]
- Kumar, R.; Butreddy, A.; Kommineni, N.; Reddy, P.G.; Bunekar, N.; Sarkar, C.; Dutt, S.; Mishra, V.K.; Aadil, K.R.; Mishra, Y.K.; et al. Lignin: Drug/gene delivery and tissue engineering applications. Int. J. Nanomed. 2021, 16, 2419–2441. [Google Scholar] [CrossRef]
- Yaneva, Z.; Ivanova, D.; Toneva, M.; Tzanova, M.; Marutsova, V.; Grozeva, N. Menadione contribution to the in vitro radical scavenging potential of phytochemicals naringenin and lignin. Int. J. Mol. Sci. 2023, 24, 16268. [Google Scholar] [CrossRef]
- Ivanova, D.; Toneva, M.; Simeonov, E.; Nikolova, B.; Semkova, S.; Antov, G.; Yaneva, Z. Newly synthesized lignin microparticles as bioinspired oral drug-delivery vehicles: Flavonoid-carrier potential and in vitro radical-scavenging activity. Pharmaceutics 2023, 15, 1067. [Google Scholar] [CrossRef]
- Fernandes, A.S. Redox-active molecules as therapeutic agents. Antioxidants 2022, 11, 1004. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Nice, E.C.; Dong, J.; Du, Z.; Huang, C. Redox regulation: Mechanisms, biology and therapeutic targets in diseases. Signal Transduct. Target. Ther. 2025, 10, 72. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Rlavonoids a bioactive compound from medicinal plants and its therapeutic applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Hasibuan, P.A.Z.; Simanjuntak, Y.; Hey-Hawkins, E.; Lubis, M.F.; Rohani, A.S.; Park, M.N.; Kim, B.; Syahputra, R.A. Unlocking the potential of flavonoids: Natural solutions in the fight against colon cancer. Biomed. Pharmacother. 2024, 176, 116827. [Google Scholar] [CrossRef]
- Gonfa, Y.H.; Tessema, F.B.; Bachheti, A.; Rai, N.; Tadesse, M.G.; Nasser Singab, A.; Chaubey, K.K.; Bachheti, R.K. Anti-inflammatory activity of phytochemicals from medicinal plants and their nanoparticles: A review. Curr. Res. Biotechnol. 2023, 6, 100152. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Hazafa, A.; Rehman, K.-U.; Jahan, N.; Jabeen, Z. The role of polyphenol (flavonoids) compounds in the treatment of cancer cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Veiga, F.; Ribeiro, A.J. New delivery systems to improve the bioavailability of resveratrol. Expert Opin. Drug Deliv. 2011, 8, 973–990. [Google Scholar] [CrossRef]
- Zverev, Y.F.; Rykunova, A.Y. Modern nanocarriers as a factor in increasing the bioavailability and pharmacological activity of flavonoids. Appl. Biochem. Microbiol. 2022, 58, 1002–1020. [Google Scholar] [CrossRef]
- Ranjbar, S.; Emamjomeh, A.; Sharifi, F.; Zarepour, A.; Aghaabbasi, K.; Dehshahri, A.; Sepahvand, A.M.; Zarrabi, A.; Beyzaei, H.; Zahedi, M.M.; et al. Lipid-based delivery systems for flavonoids and flavonolignans: Liposomes, nanoemulsions, and solid lipid nanoparticles. Pharmaceutics 2023, 15, 1944. [Google Scholar] [CrossRef]
- Mukheja, Y.; Kaur, J.; Pathania, K.; Sah, S.P.; Salunke, D.B.; Sangamwar, A.T.; Pawar, S.V. Recent advances in pharmaceutical and biotechnological applications of lignin-based materials. Int. J. Biol. Macromol. 2023, 241, 124601. [Google Scholar] [CrossRef]
- Zheng, L.; Lu, G.; Pei, W.; Yan, W.; Li, Y.; Zhang, L.; Huang, C.; Jiang, Q. Understanding the relationship between the structural properties of lignin and their biological activities. Int. J. Biol. Macromol. 2021, 190, 291–300. [Google Scholar] [CrossRef]
- Morena, A.G.; Bassegoda, A.; Natan, M.; Jacobi, G.; Banin, E.; Tzanov, T. Antibacterial properties and mechanisms of action of sonoenzymatically synthesized lignin-based nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 37270–37279. [Google Scholar] [CrossRef]
- Belyy, V.; Kuzivanov, I.; Istomina, E.; Mikhaylov, V.; Tropnikov, E.; Karmanov, A.; Bogdanovich, N. Water stable colloidal lignin-pvp particles prepared by electrospray. Int. J. Biol. Macromol. 2021, 190, 533–542. [Google Scholar] [CrossRef]
- Jędrzejczak, P.; Puszka, A.; Kubiak, A.; Podkościelna, B.; Klapiszewski, Ł. New lignin-based hybrid materials as functional additives for polymer biocomposites: From design to application. Int. J. Biol. Macromol. 2021, 190, 624–635. [Google Scholar] [CrossRef]
- Santos, H.A.; Figueiredo, P. Lignin-Based Materials for Biomedical Applications: Preparation, Characterization, and Implementation; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-12-820303-3. [Google Scholar]
- Zhao, W.; Simmons, B.; Singh, S.; Ragauskas, A.; Cheng, G. From lignin association to nano-/micro-particle preparation: Extracting higher value of lignin. Green Chem. 2016, 18, 5693–5700. [Google Scholar] [CrossRef]
- Yan, Z.; Liao, G.; Zou, X.; Zhao, M.; Wu, T.; Chen, Y.; Fang, G. Size-controlled and super long-term stable lignin nanospheres through a facile self-assembly strategy from kraft lignin. J. Agric. Food Chem. 2020, 68, 8341–8349. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B.; et al. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Dempere, L.A.; Tong, Z. Synthesis of ph-responsive lignin-based nanocapsules for controlled release of hydrophobic molecules. ACS Sustain. Chem. Eng. 2016, 4, 5204–5211. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.-L.; Hult, E.-L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A simple process for lignin nanoparticle preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and formation mechanism of size-controlled lignin nanospheres by self-assembly. Ind. Crops Prod. 2017, 100, 146–152. [Google Scholar] [CrossRef]
- Yearla, S.R.; Padmasree, K. Preparation and characterisation of lignin nanoparticles: Evaluation of their potential as antioxidants and uv protectants. J. Exp. Nanosci. 2016, 11, 289–302. [Google Scholar] [CrossRef]
- Tan, S.; Wu, T.; Zhang, D.; Zhang, Z. Cell or cell membrane-based drug delivery systems. Theranostics 2015, 5, 863–881. [Google Scholar] [CrossRef]
- Direct Permeation of Nanoparticles Across Cell Membrane: A Review. Available online: https://www.jstage.jst.go.jp/article/kona/35/0/35_2018011/_article/-char/ja/ (accessed on 1 June 2025).
- Tsong, T.Y. Electroporation of cell membranes. Biophys. J. 1991, 60, 297–306. [Google Scholar] [CrossRef]
- Aadil, K.R.; Barapatre, A.; Sahu, S.; Jha, H.; Tiwary, B.N. Free radical scavenging activity and reducing power of acacia nilotica wood lignin. Int. J. Biol. Macromol. 2014, 67, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, L.; Orazizadeh, M.; Niazvand, F.; Abbaspour, M.R.; Mansouri, E.; Khodadadi, A. Quercetin induces apoptosis and necroptosis in mcf-7 breast cancer cells. Bratisl. Med. J. 2017, 118, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A review on anti-cancer properties of quercetin in breast cancer. Life Sci. 2020, 248, 117463. [Google Scholar] [CrossRef] [PubMed]
- Mawalizadeh, F.; Mohammadzadeh, G.; Khedri, A.; Rashidi, M. Quercetin potentiates the chemosensitivity of mcf-7 breast cancer cells to 5-fluorouracil. Mol. Biol. Rep. 2021, 48, 7733–7742. [Google Scholar] [CrossRef]
- Phansalkar, N.; More, S.; Sabale, A.; Joshi, M. Adaptive local thresholding for detection of nuclei in diversity stained cytology images. In Proceedings of the 2011 International Conference on Communications and Signal Processing, Kerala, India, 10–12 February 2011; pp. 218–220. [Google Scholar] [CrossRef]
- Sakao, K.; Hamamoto, S.; Urakawa, D.; He, Z.; Hou, D.-X. Anticancer activity and molecular mechanisms of acetylated and methylated quercetin in human breast cancer cells. Molecules 2024, 29, 2408. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.-B.; Jung, G.O.; Oh, J.T.; Park, D.E.; Chae, K.M. Effect of quercetin on apoptosis of PANC-1 Cells. J. Korean Surg. Soc. 2013, 85, 249. [Google Scholar] [CrossRef]
- Cheng, S.; Gao, N.; Zhang, Z.; Chen, G.; Budhraja, A.; Ke, Z.; Son, Y.; Wang, X.; Luo, J.; Shi, X. Quercetin induces tumor-selective apoptosis through downregulation of Mcl-1 and activation of Bax. Clin. Cancer Res. 2010, 16, 5679–5691. [Google Scholar] [CrossRef]
- Mutlu Altundağ, E.; Kasacı, T.; Yılmaz, A.M.; Karademir, B.; Koçtürk, S.; Taga, Y.; Yalçın, A.S. Quercetin-induced cell death in human papillary thyroid cancer (B-CPAP) cells. J. Thyroid Res. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]


| Type Alkali Lignin@Formulations | Size (nm) | ζ-Potential (mV) | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Lignin@F (empty) | 310.70 (74.66%) | 79.88 (46.78%) | −31.48 | −25.35 |
| Lignin@Naringenin F | 488.70 (50.43%) | 125.60 (46.39%) | −33.78 | −19.37 |
| Lignin@Morin F | 361.30 (41.45%) | 171.20 (42.33%) | −40.69 | −32.58 |
| Lignin@Quercetin F | 413.43 (98%) | 109.56 (41.97%) | −41.09 | −19.89 |
| Type Electroporation Treatment | Lignin@F (Empty) | Lignin@Quercetin F | ||
|---|---|---|---|---|
| Size (nm) | ζ-Potential (mV) | Size (nm) | ζ-Potential (mV) | |
| w/o Electroporation | 410.6 | −36.28 | 458.8 | −33.19 |
| After 300 V/cm | 313.1 | −34.56 | 325.4 | −31.20 |
| After 500 V/cm | 379.9 | −37.29 | 289.0 | −31.66 |
| After 700 V/cm | 287.2 | −37.10 | 252.4 | −27.83 |
| After 1000 V/cm | 235.4 | −26.79 | 269.4 | −28.19 |
| Formula for Calculation of Relative Concentration CR = (U − Z)/(Y − Z) × X | ||||
|---|---|---|---|---|
| Parameter | Type Alkali Lignin@Formulations | |||
| Lignin@ (Empty) | Lignin@Naringenin | Lignin@Morin | Lignin@Quercetin | |
| 1Y | −31.48 | −33.78 | −40.69 | −41.09 |
| 2U | −25.35 | −19.37 | −32.58 | −19.89 |
| 3X | 0.05 mg/mL | |||
| 4Z | −10.34 | −10.34 | −5.06 | −5.06 |
| 5CR | 0.035 mg/mL | 0.0247 mg/mL | 0.0386 mg/mL | 0.018 mg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semkova, S.; Deneva, R.; Antov, G.; Ivanova, D.; Nikolova, B. Alkali Lignin-Based Biopolymer Formulations for Electro-Assisted Drug Delivery of Natural Antioxidants in Breast Cancer Cells—A Preliminary Study. Int. J. Mol. Sci. 2025, 26, 7481. https://doi.org/10.3390/ijms26157481
Semkova S, Deneva R, Antov G, Ivanova D, Nikolova B. Alkali Lignin-Based Biopolymer Formulations for Electro-Assisted Drug Delivery of Natural Antioxidants in Breast Cancer Cells—A Preliminary Study. International Journal of Molecular Sciences. 2025; 26(15):7481. https://doi.org/10.3390/ijms26157481
Chicago/Turabian StyleSemkova, Severina, Radina Deneva, Georgi Antov, Donika Ivanova, and Biliana Nikolova. 2025. "Alkali Lignin-Based Biopolymer Formulations for Electro-Assisted Drug Delivery of Natural Antioxidants in Breast Cancer Cells—A Preliminary Study" International Journal of Molecular Sciences 26, no. 15: 7481. https://doi.org/10.3390/ijms26157481
APA StyleSemkova, S., Deneva, R., Antov, G., Ivanova, D., & Nikolova, B. (2025). Alkali Lignin-Based Biopolymer Formulations for Electro-Assisted Drug Delivery of Natural Antioxidants in Breast Cancer Cells—A Preliminary Study. International Journal of Molecular Sciences, 26(15), 7481. https://doi.org/10.3390/ijms26157481








