The β-1,4 GalT-V Interactome—Potential Therapeutic Targets and a Network of Pathways Driving Cancer and Cardiovascular and Inflammatory Diseases
Abstract
1. Introduction
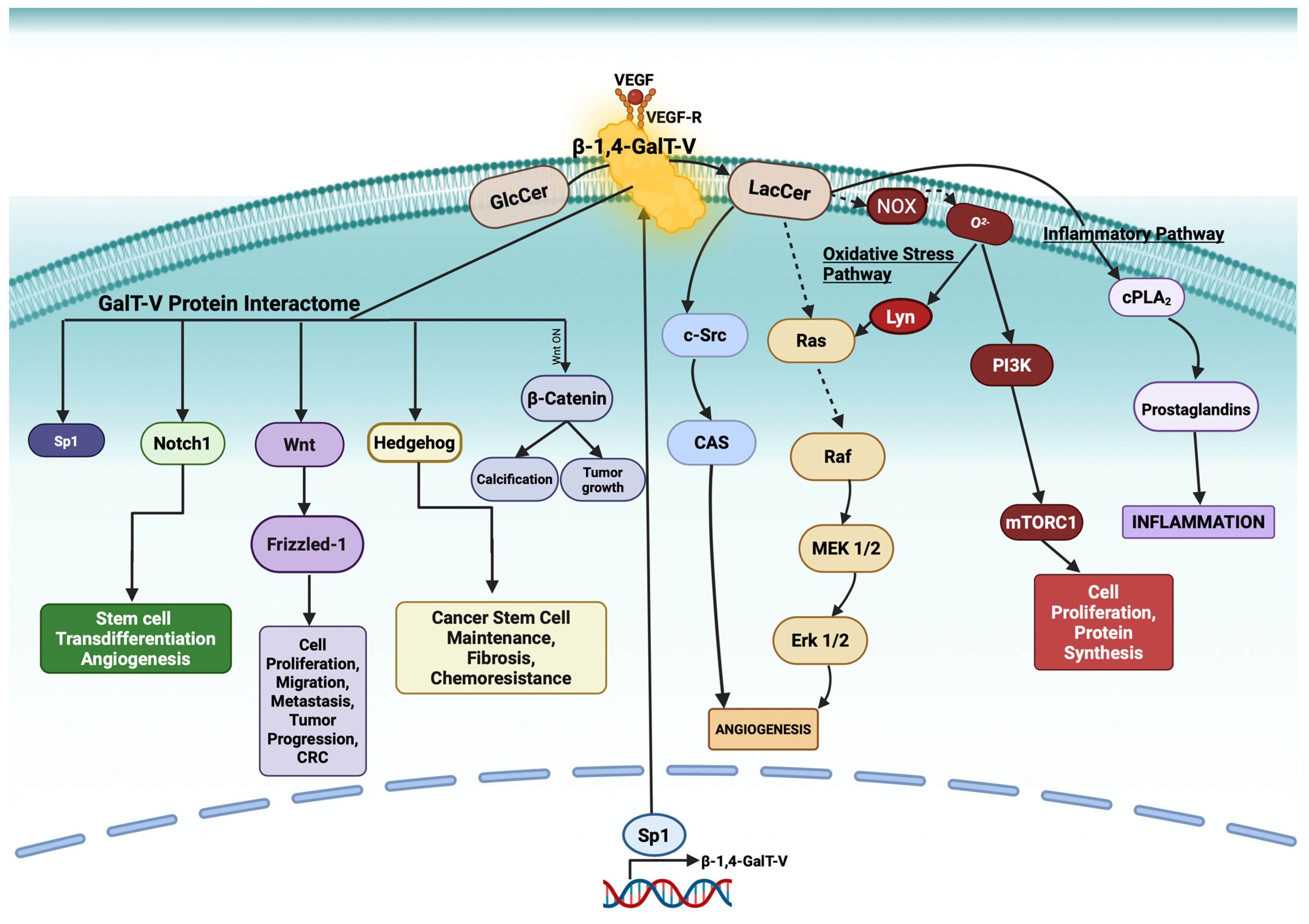
2. β-1,4-GalT-V and Glucosylceramide: Formation of LacCer
- The oxidative stress pathway via superoxide (O2−) production.
- The inflammatory pathway by activating cytosolic phospholipase A2 (cPLA2), which releases arachidonic acid from phosphatidylcholine—an upstream precursor of proinflammatory prostaglandins.
3. NOX Activation by LacCer and Superoxide Production
4. β-1,4-GalT-V Inhibitors
5. β-1,4-GalT-V-Protein Interactome
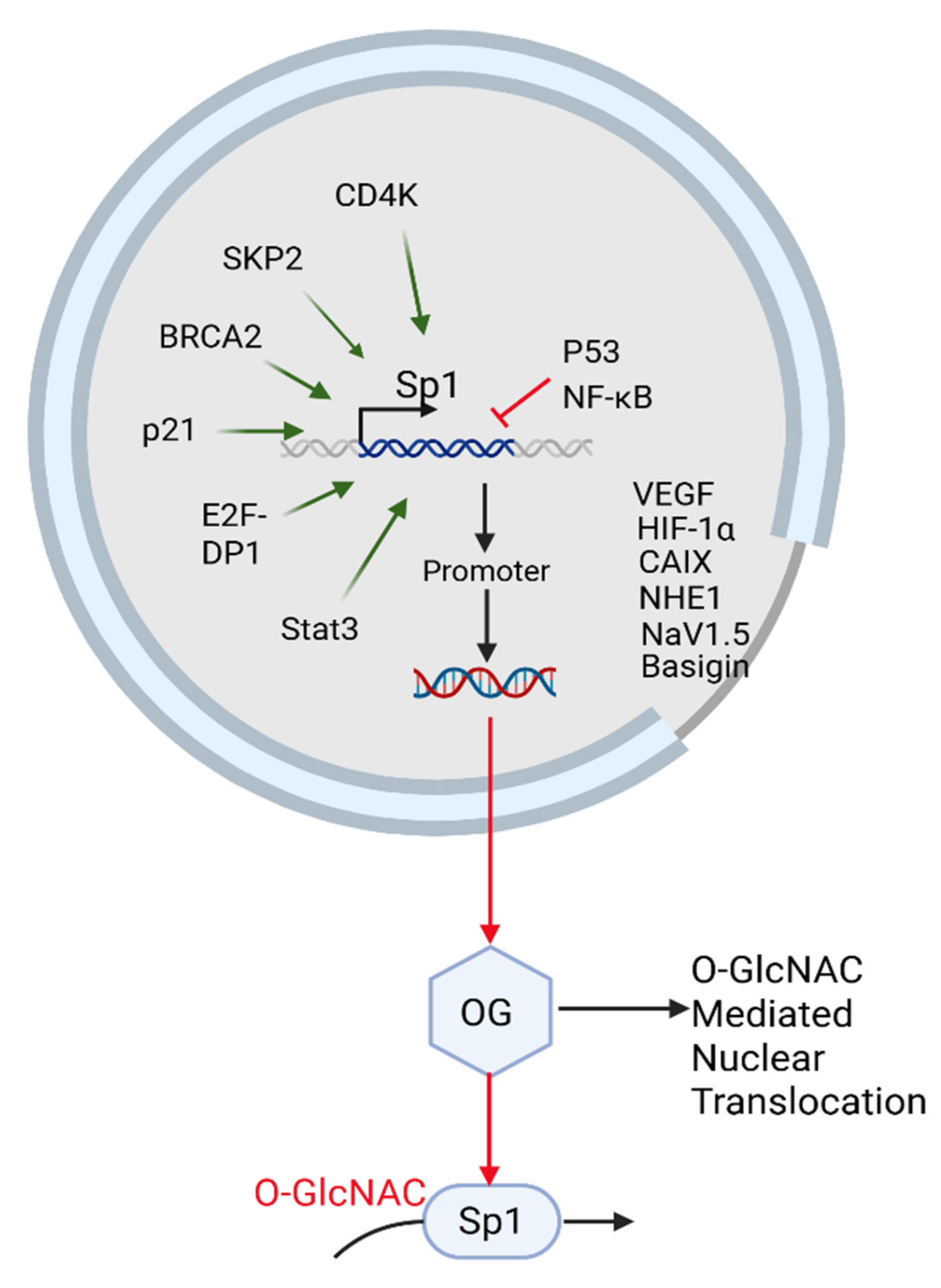
5.1. β-1,4-GalT-V Interaction with Sp-1
5.1.1. Sp1 Regulates β-1,4-GalT-V Gene and Protein Expression
5.1.2. Background
5.1.3. Glycosylation-Dependent Regulation of Sp1: Linking Nuclear Signaling, Apoptosis, and Metastatic Potential
5.1.4. Sp1 in the Tumor Microenvironment and Immune Modulation
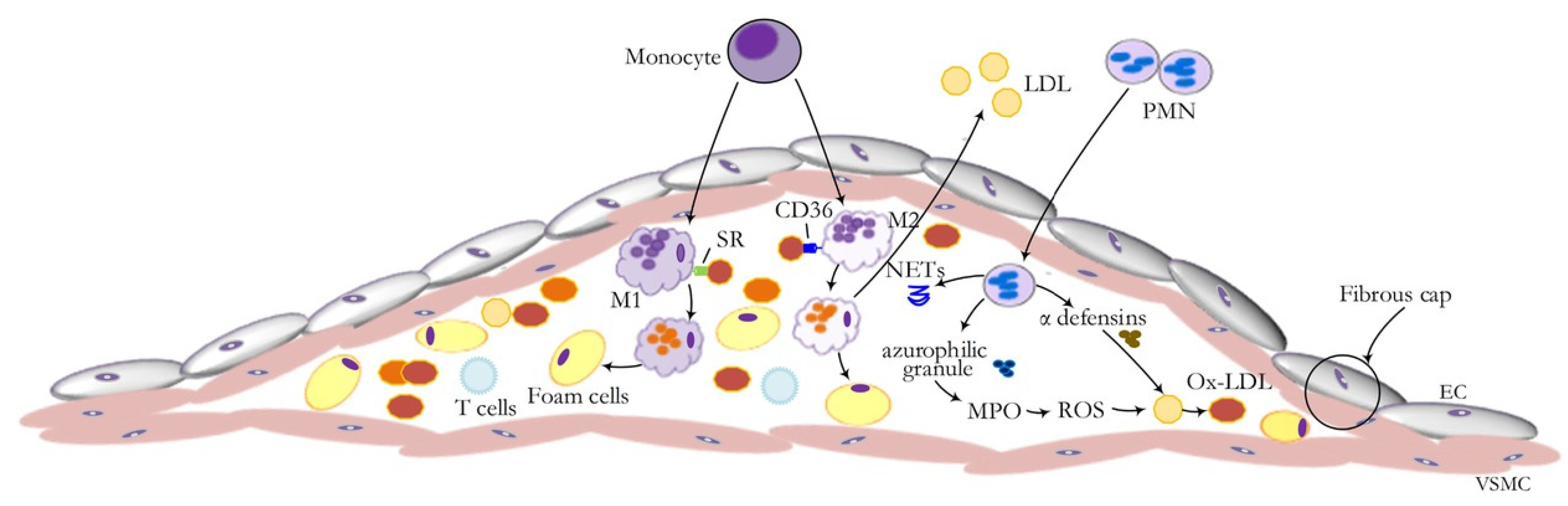
5.1.5. Sp1 and Low-Density Lipoprotein (LDL) in Atherosclerosis
5.1.6. β-1,4-GalT-V, Clathrin-Coated Pits, and LDL Receptor Interactome
5.1.7. Cancer Stem Cells and the Sp1 Pathway
5.1.8. Sp1 Interaction with PD-L1
5.1.9. Sp1 as a Therapeutic Target
5.1.10. Sp1 and the Cell Cycle
5.1.11. Sp1 and the pH-Tome
5.1.12. Role of SP1 in CRC Growth, Progression, and Metastasis
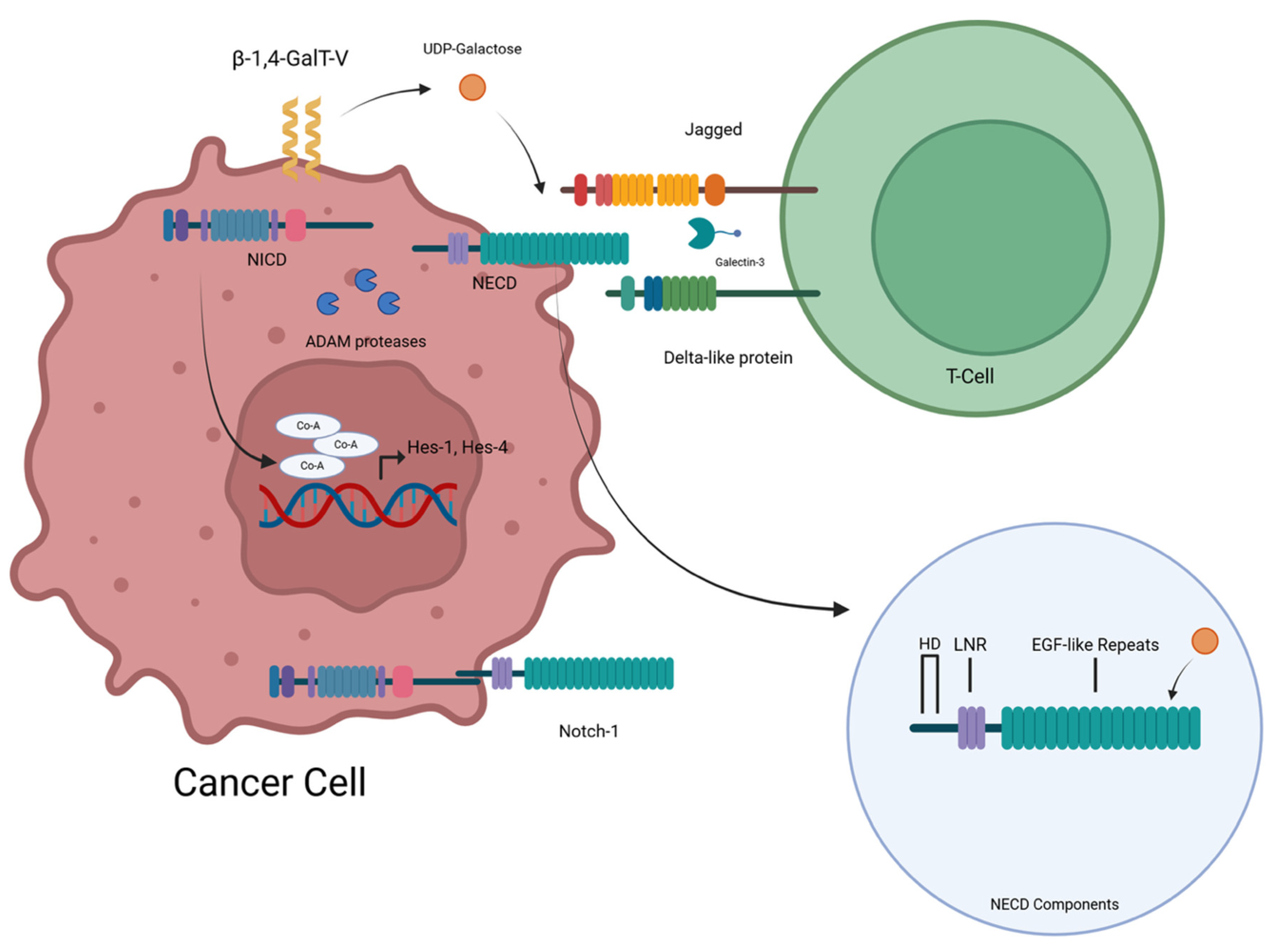
5.2. β-1,4-GalT-V Interaction with Notch-1
5.2.1. β-1,4-GalT-V Regulates Notch-1 Functions
Background
5.2.2. Relationship Between Notch-1 and T-Cell Development
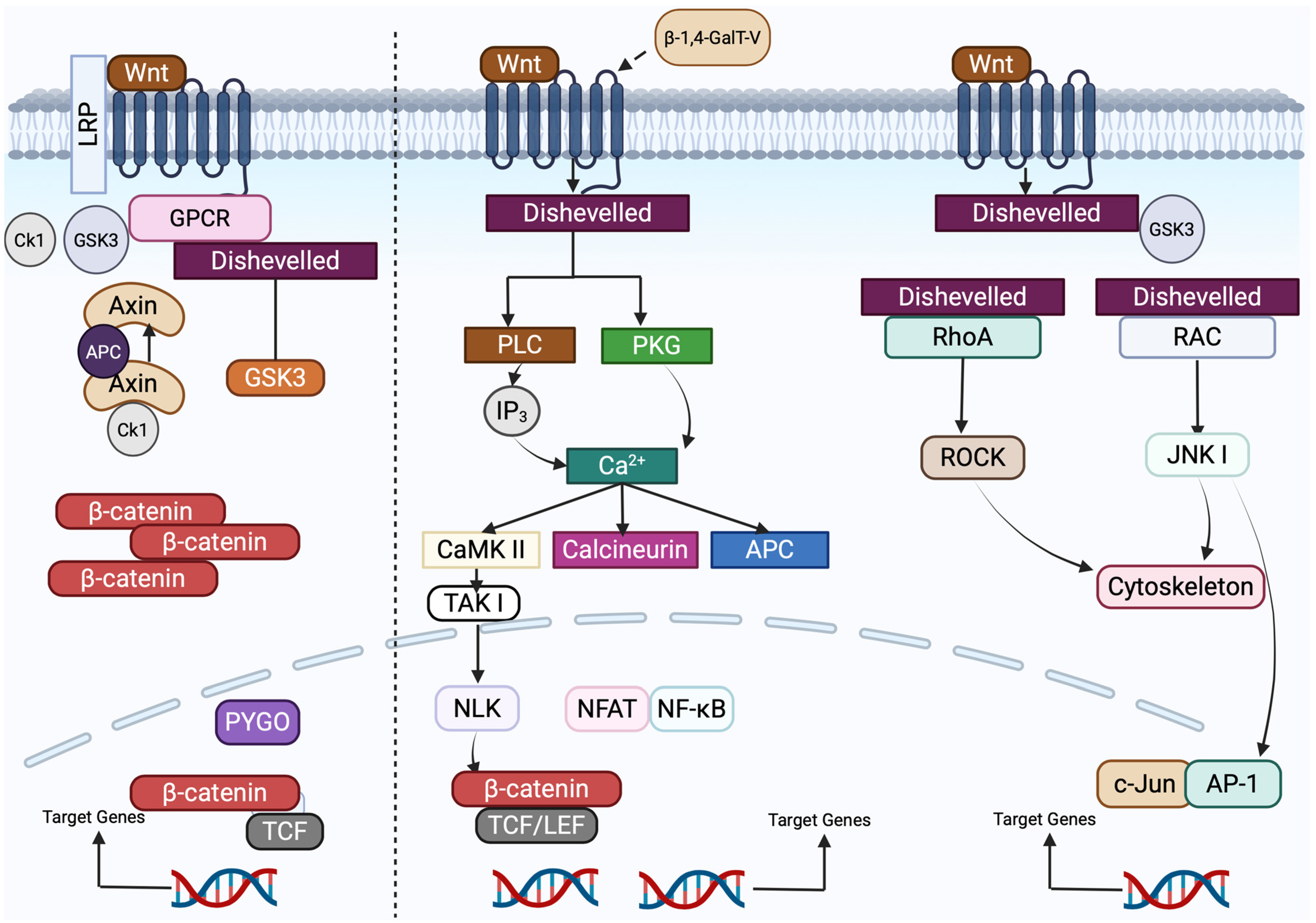
5.3. β-1,4-GalT-V Interaction with Wnt
5.3.1. The Canonical Wnt/FZD Signaling Pathway
5.3.2. Non-Canonical Wnt/FZD Signaling
5.3.3. Relationship Between β-1,4-GalT-V and Wnt-1
5.3.4. Relationship Between Wnt-1 and T-Cells
5.3.5. Relationship between Wnt-1 and Notch-1
5.3.6. Wnt-1 and Colorectal Cancer
5.3.7. Interaction Between β-1,4-GalT-V, Wnt-1, and Colorectal Cancer
5.3.8. Therapeutic Approaches Targeting Wnt-1
5.4. β-1,4-GalT-V Interaction with Frizzled
5.4.1. Background
5.4.2. Frizzled Receptors Orchestrate Canonical and Non-Canonical Wnt Signaling in Colorectal Cancer Progression
5.4.3. β-1,4-GalT-V Promotes Breast Cancer Stemness and Tumor Progression
5.4.4. FZD-Based Targeted Therapy
5.5. Hedgehog Pathway
- (1)
- A non-canonical, ligand-independent pathway;
- (2)
- A canonical, ligand-dependent pathway.
5.5.1. β-1,4-GalT-V and Other Glycotransferases in Hedgehog Signaling Pathways
5.5.2. Hedgehog-Focused Targeted Therapies
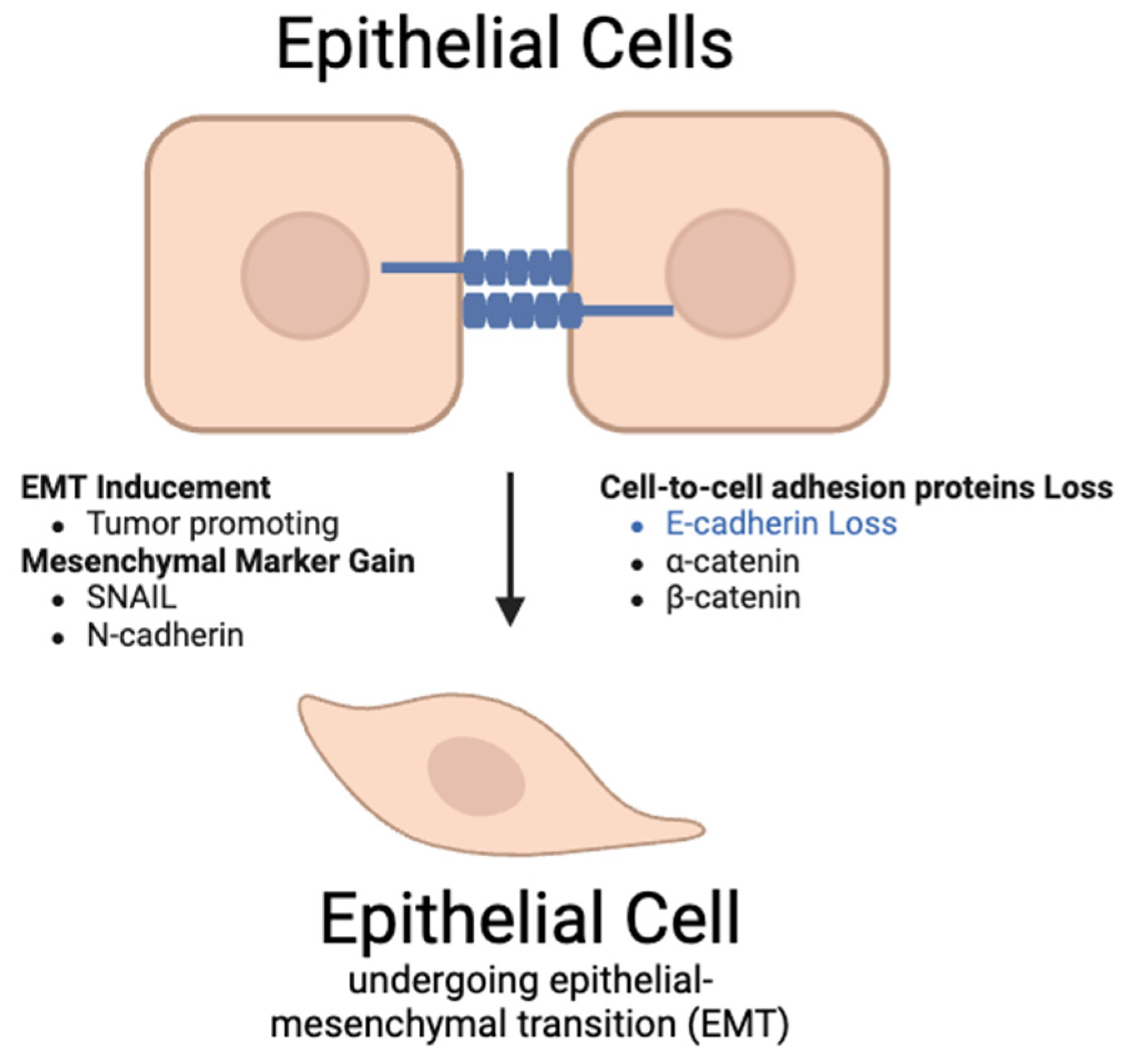
5.6. β-1,4-GalT-V Interaction with Snail-1
5.6.1. Background
5.6.2. Relationship Between Snail-1 and β-1,4-GalT-V
5.6.3. Relationship Between Snail-1 and CRC
5.6.4. Snail-1 Prospects
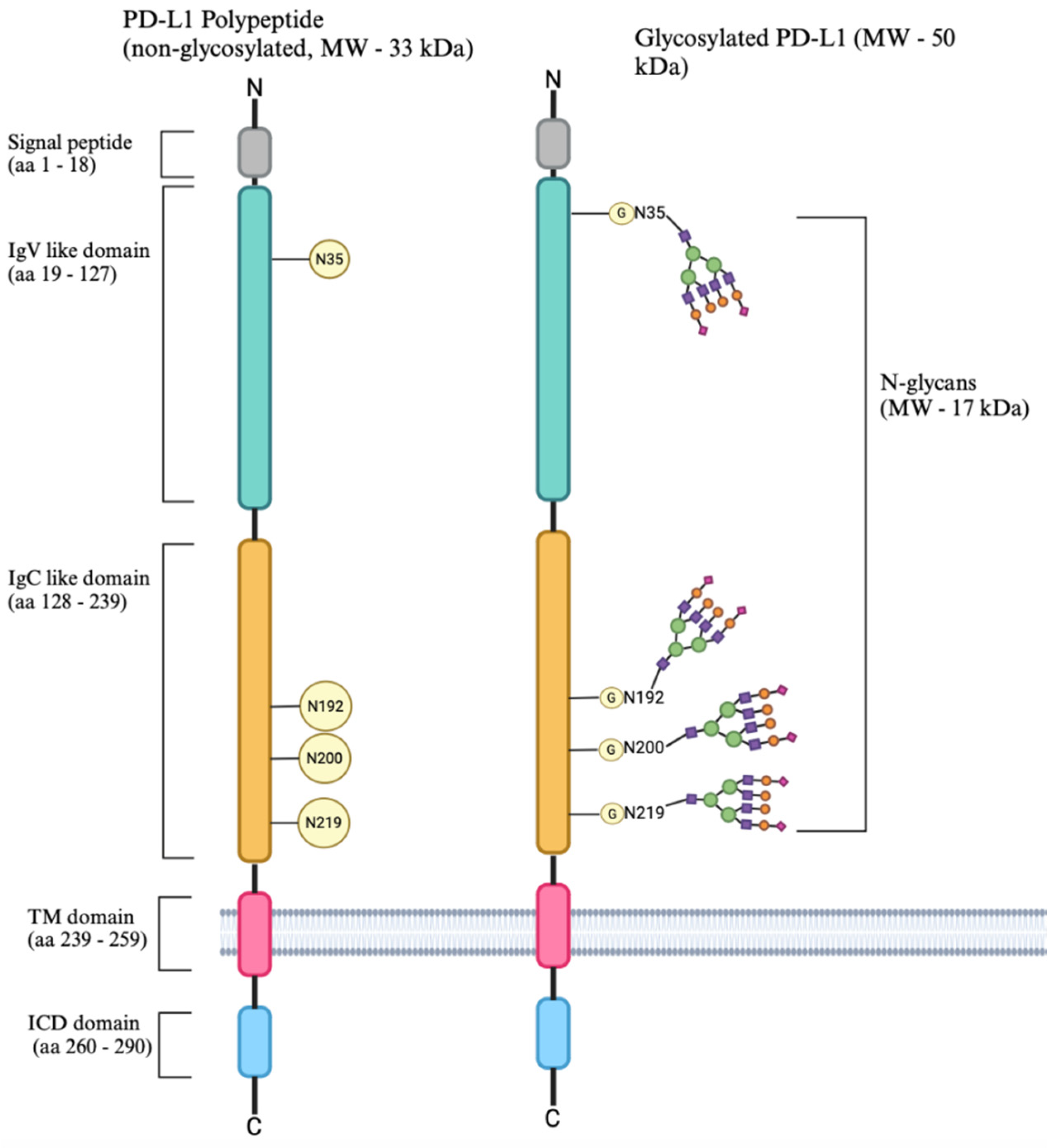
5.7. Is β-1,4-GalT-V Engaged with PD-L1 Glycosylation?
5.7.1. Background
5.7.2. PD-1-PD-L1 Immune Checkpoint Pathway
5.7.3. The Regulatory Mechanism of PD-L1 Expression
5.7.4. Immunotherapy by Targeting PD-1/PD-L1 Immune Checkpoint Pathway
5.8. β-1,4-GalT-V Interaction with β-Catenin
Background
5.9. β-1,4-GalT-V Interaction with P62 and Autophagy
| Drug | Phase | Cancer | Target Pathway |
|---|---|---|---|
| Vantictumab [114] | Phase 1 | Breast cancer | Frizzled |
| Ipafricept [116] | Phase 1 | Ovarian cancer | |
| BMS-833923 (XL-139) [149] | Phase 1 and 2 | Chronic myeloid leukemia | Hedgehog |
| Vismodegib (GDC-0449) [145] | Phase 2 | Multiple tumors including colorectal cancer | |
| Phase 2 | mCRC | ||
| Phase 1/2 | First line mCRC | ||
| Itraconazole [147] | Phase 2 | Basal cell carcinoma | |
| Taladegib (LY2940680) [149] | Phase 1 and 2 | Small cell lung carcinoma | |
| Phase 1 and 2 | Esophageal junction cancer | ||
| Phase 1 | Idiopathic pulmonary fibrosis | ||
| Sonidegib (Erismodegib, LDE-225, NVP-LDE-225) [149] | Phase 1 and 2 | Medulloblastoma | |
| Phase 2 | Basal cell carcinoma | ||
| Phase 2 | Multiple myeloma | ||
| WNT974 [101,102] | Phase 1, 1b, and 2 | Melanoma, breast cancer, CRC, and pancreatic adenocarcinoma | Wnt-1 pathway |
| Polyethylene Glycol 3350 [171] | Phase 1 and 2 | Colorectal cancer | Snail-1 |
| Nivolumab/Ipilimumab [164] | Phase 1 and 2 | NSCLC | |
| Phase 1 and 2 | Advanced solid tumors | PD-1/PD-L1 pathway | |
| Pembrolizumab [164] | Phase 1 | Squamous NSCLC | |
| Phase 1 and 2 | NSCLC | ||
| Phase 1 | Advanced/metastatic non-squamous NSCLC | ||
| Atezolizumab [164] | Phase 3 | Metastatic NSCLC | |
| Phase 2 | NSCLC stage 3 and 4 | ||
| Sintilimab [164] | Phase 2 | Advanced NSCLC | |
| Avelumab [164] | Phase 1 and 2 | Metastatic NSCLC | |
| Toripalimab [164] | Phase 2 | NSCLC |
5.10. Perspectives
6. Conclusions
- Sp1 is a nuclear factor and a transcriptional regulator of β-1,4-GalT-V.
- β-1,4-GalT-V regulates VEGF-independent angiogenesis by generating LacCer and galactosylation of Notch-1, regulating production of glioma-like stem cell differentiation into endothelial cells and promoting tumorigenesis. These observations suggest a VEGF-independent pathway contributing to angiogenesis, a phenotype critical in tumor metastasis and atherosclerotic plaque growth, and plaque stability via inducing mature neo-vessels and monocyte/neutrophil infiltration.
- Oxidative stress increases LDL oxidation to form oxidized LDL that enters cells via an LDL-receptor-independent pathway/scavenger pathway. Oxidized LDL phosphorylates serine, threonine, and tryptophan in β-1,4-GalT-V, thus generating LacCer, causing downstream activation of critical phenotypes in cultured vascular cells and blood vessels in ApoE-/- mice fed a Western diet. In turn, this leads to atherosclerosis, cardiac hypertrophy, and atherosclerotic plaque development. Conversely, feeding D-PDMP or a biopolymer-encapsulated D-PDMP (inhibitor of GlcCer synthase and LacCer synthase) reverses atherosclerosis and cardiac hypertrophy, and improves vascular and cardiac functions.
- The Snail-1 protein plays a vital role in inducing EMT and tumor progression by inhibiting cell-adhesion proteins, promoting immunosuppressive tumor microenvironment, and influencing PD-L1 accumulation. This inhibits the p38-MAPK pathway that degrades Snail-1. The Ras signaling pathway is activated by superoxides, a downstream upregulation of β-1,4-GalT-V. This pathway involves ERK, which is involved in the promotion of EMT, a process regulated by Snail-1.
- Cigarette smoke increases LacCer accumulation in bronchial epithelial cells and macrophages as well as in the lungs in patients with COPD. This is accompanied by the increased expression of defective-autophagy marker p62. Conversely, treatment with D-PDMP reversed the pathology in mice subject to cigarette smoke. Additionally, blocking GlcCer and LacCer synthesis using D-PDMP or Eliglustat alters autophagy in osteoclasts, improving myeloma bone disease.
- β-1,4-GalT-V protects FZD1 from degradation, possibly via N-linked glycosylation, activating the Wnt/b-Catenin pathway. PD-L1 also activates the β-catenin signaling pathway, causing β-catenin to bind to TCF4/β-catenin binding sites on the PD-L1 promoter. Thus, PD-L1 and β-catenin form a positive feedback loop in regulating the expression of target genes such as stem cell markers and calcification. β-1,4-GalT-V also promotes cell proliferation via the Hedgehog pathway.
- β-1,4-GalT-V’s influence on these pathways posits it as a promising therapeutic agent as well as a diagnostic marker in colorectal cancer and many other cancers. Our future research aims are to define mechanisms fundamental to β-1,4-GalT-V’s regulatory effects and to further investigate therapeutics that target β-1,4-GalT-V in treating cancer, cardiovascular diseases, and inflammatory diseases.
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| β-1,4-GalT-V | β-1,4-Galactosyl Transferase-V |
| TF | Transcription factors |
| CRC | Colorectal cancer |
| PROM 1 | Prominin 1 |
| RNF 128 | RING finger protein 128 |
| FOXO4 | Fork head box O4 |
| EMT | Epithelial mesenchymal transition |
| NB | Neuroblastoma |
| HCC | Hepatocellular carcinoma |
| CSCC | Cutaneous squamous cell carcinoma |
| TNBC | Triple-negative breast cancer |
| EA | Endometrial adenocarcinoma |
| GB | Glioblastoma |
| GC | Gastric cancer |
| BCSC | Breast cancer stem cells |
| PKC | Protein Kinase C |
| CaMKII | Calcium/calmodulin-dependent kinase II |
| CaN | Calcineurin and NFAT (Nuclear Factor of Activated T-cells) |
| mAb | Monoclonal antibody |
| PCP | Planar cell polarity |
| APC | Adenomatous Polyposis Coli |
| CTNNB | Catenin Beta 1 |
| MCF-7 | Michigan Cancer Foundation 7 |
| TCF4 | Transcription factor 4 |
| HL-60 | Human leukemia |
| AML | Acute myeloid leukemia |
| shRNA | Short hairpin RNA |
| GALNT1 | Polypeptide N-acetylgalactosaminyltransferase 1 |
| ALDH1A1 | Aldehyde dehydrogenase 1A1 |
| PD-L1 | Programmed Death Ligand –1 |
| TNM | Tumor, node, metastasis |
| MCP-1 | Human monocyte chemoattractant protein 1 |
| COX-2 | Cyclooxygenases-2 |
| IL-10 | Interleukin-10 |
| Tregs | Regulatory T-cells |
| TGF-β1 | Transforming growth factor beta 1 |
| PD 1 | Programmed cell Death protein 1 |
| PTM | Post-translational modifications |
| MYC | Proto-oncogene |
| MAPK | Mitogen-activated Protein Kinases |
| PI3K/Akt | Phosphoinositide 3-Kinase/Protein kinase B (Akt) |
| INF | Interferon |
| ITIM | Immuno Receptor Tyrosine based Inhibitory Motif |
| ITSM | Immuno Receptor Tyrosine based Switch Motif |
| SHP2 | Src homology region 2-containing protein tyrosine phosphatase-2 |
| NXS/T motif | Asparagine (N)-X-serine-Threonine(T) motif |
| GATA3 | GATA binding protein 3 |
| T-bet | T box protein expressed in T-cells |
| FOX P3 | Fork head box protein P3 |
| TCF/LEF | T-cell factor/lymphoid enhancer factor |
| LacCer | Lactosylceramide |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| PDGF | Platelet derived growth factor |
| MAPK | Mitogen-activated Protein Kinase |
| GlcCer | Glucosylceramide |
| D-PDMP | D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol |
| EGF | Epidermal growth factor |
| PECAM-1 | Platelet-endothelial cell adhesion molecule-1 |
| ICAM-1 | Intra cellular adhesion molecule-1 |
| cPLA-2 | Cytosolic phospholipase-C |
| GSL | Glycosphingolipid |
| GCS | Glucosylceramide synthase |
| CSCs | Cancer stem cells |
| HPV | Human papilloma virus |
| ICIs | Immune checkpoint inhibitors |
| NF-kB | Nuclear Factor Kappa-light-chain-enhancer of Activated B cells |
| LPS | Lipopolysaccharides |
| FGF-BP10 | Fibroblast growth factor binding protein |
| SGCE | Epsilon-sarcoglycan |
| CDKs | Cyclin dependent kinases |
| TBP | TATA-binding protein |
| GTFs | General transcription factors |
| HIF-1α | Hypoxia inducible factor 1 alpha |
| CAIX | Carbonic anhydrase 9 |
| HBP | Hexosamine biosynthetic pathway |
| TGF-β1 | Transforming growth factor β1 |
| VPF/VEGF | Vascular permeability factor/vascular endothelial growth factor |
| O-GlcNAc | β-O-linked N-acetylglucosamine |
| OGT | O-GlcNAc Transferase |
| NSCLC | Non-small-cell lung cancer |
| AXIN2 | Axis Inhibition Protein 2 |
| NOX | NADPH oxidase |
| ROS | Reactive oxygen species |
| PI3K | Phosphoinositide 3-kinase |
| mTORC | Mechanistic target of rapamycin complex |
| cPLA2 | Cytosolic phospholipase A2 |
| CAS | Crk-associated substrate |
| MEK | MAPK/ERK kinase |
| ERK | Extracellular signal-regulated kinase |
| Sp1 | Specificity protein 1 |
References
- Arango, J.; Pierce, M. Comparison of N-acetylglucosaminyltransferase V activities in Rous sarcoma-transformed baby hamster kidney (RS-BHK) and BHK cells. J. Cell Biochem. 1988, 37, 225–231. [Google Scholar] [CrossRef]
- Shirane, K.; Sato, T.; Segawa, K.; Furukawa, K. Involvement of β-1,4-galactosyltransferase V in malignant transformation-associated changes in glycosylation. Biochem. Biophys. Res. Commun. 1999, 265, 434–438. [Google Scholar] [CrossRef]
- Sato, T.; Furukawa, K. β-1,4-Galactosyltransferase and cancer metastasis. Yakugaku Zasshi 2012, 132, 691–697. [Google Scholar] [CrossRef][Green Version]
- Balram, A.; Thapa, S.; Chatterjee, S. Glycosphingolipids in diabetes, oxidative stress, and cardiovascular disease: Prevention in experimental animal models. Int. J. Mol. Sci. 2022, 23, 15442. [Google Scholar] [CrossRef]
- Chatterjee, S.; Yuan, R.; Thapa, S.; Talwar, R. Central role of β-1,4-GalT-V in cancer signaling, inflammation, and other disease-centric pathways. Int. J. Mol. Sci. 2023, 25, 483. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, X.; Wang, J.; Chen, H.; Chen, L. Exploration of Simiao-Yongan Decoction on knee osteoarthritis based on network pharmacology and molecular docking. Medicine 2023, 102, e35193. [Google Scholar] [CrossRef]
- Rodeheffer, C.; Shur, B.D. Targeted mutations in beta1,4-galactosyltransferase I reveal its multiple cellular functions. Biochim. Biophys. Acta 2002, 1573, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Okajima, T.; Takeuchi, H. Significant roles of Notch O-glycosylation in cancer. Molecules 2022, 27, 1783. [Google Scholar] [CrossRef]
- Chatterjee, S.B.; Hou, J.; Bandaru, V.V.R.; Pezhouh, M.K.; Mannan, A.A.S.R.; Sharma, R. Lactosylceramide synthase β-1,4-GalT-V: A novel target for the diagnosis and therapy of human colorectal cancer. Biochem. Biophys. Res. Commun. 2019, 508, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Balram, A.; Li, W. Convergence: Lactosylceramide-centric signaling pathways induce inflammation, oxidative stress, and other phenotypic outcomes. Int. J. Mol. Sci. 2021, 22, 1816. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bedja, D.; Mishra, S.; Amuzie, C.; Avolio, A.; Kass, D.A.; Berkowitz, D.; Renehan, M. Inhibition of glycosphingolipid synthesis ameliorates atherosclerosis and arterial stiffness in apolipoprotein E–/–mice and rabbits fed a high-fat and -cholesterol diet. Circulation 2014, 129, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.F.; Williams, N.; Chatterjee, S. Lactosylceramide is required in apoptosis induced by N-Smase. Glycoconj. J. 2006, 23, 147–157. [Google Scholar] [CrossRef]
- Bhunia, A.; Arai, T.; Bhunia, A.K.; Chatterjee, S.; Bulkley, G.B. Lactosylceramide stimulates human neutrophils to upregulate Mac-1, adhere to endothelium, and generate reactive oxygen metabolites in vitro. Circ. Res. 1998, 82, 540–547. [Google Scholar] [CrossRef]
- Fiorelli, S.; Anesi, A.; Porro, B.; Cosentino, N.; Werba, J.P.; Di Minno, A.; Manega, C.M.; Barbieri, S.; Colombo, G.I.; Marenzi, G.; et al. Lipidomics analysis of monocytes from patients with acute myocardial infarction reveals lactosylceramide as a new player in monocyte migration. FASEB J. 2021, 35, e21504. [Google Scholar] [CrossRef]
- Kolmakova, A.; Rajesh, M.; Zang, D.; Pili, R.; Chatterjee, S. VEGF recruits lactosylceramide to induce endothelial cell adhesion molecule expression and angiogenesis in vitro and in vivo. Glycoconj. J. 2009, 26, 547–558. [Google Scholar] [CrossRef]
- Iwabuchi, K.; Nagaoka, I. Lactosylceramide-enriched glycosphingolipid signaling domain mediates superoxide generation from human neutrophils. Blood 2002, 100, 1454–1464. [Google Scholar] [CrossRef]
- Bodas, M.; Min, T.; Vij, N. Lactosylceramide-accumulation in lipid rafts mediates aberrant autophagy, inflammation, and apoptosis in cigarette smoke-induced emphysema. Apoptosis 2015, 20, 725–739. [Google Scholar] [CrossRef]
- Mishra, S.; Bedja, D.; Amuzie, C.; Foss, C.A.; Pomper, M.G.; Bhattacharya, R.; Yarema, K.J.; Chatterjee, S. Improved intervention of atherosclerosis and cardiac hypertrophy through biodegradable polymer-encapsulated delivery of glycosphingolipid inhibitor. Biomaterials 2015, 64, 125–135. [Google Scholar] [CrossRef]
- Rajesh, M.; Kolmakova, A.; Chatterjee, S. Novel role of lactosylceramide in vascular endothelial growth factor-mediated angiogenesis in human endothelial cells. Circ. Res. 2005, 97, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Furukawa, K. Transcriptional regulation of the human β-1,4-galactosyltransferase V gene in cancer cells. J. Biol. Chem. 2004, 279, 39574–39583. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Colorectal Cancer Statistics: How Common Is Colorectal Cancer? Available online: https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html (accessed on 29 January 2024).
- American Cancer Society. Detecting Colorectal Cancer: Can Colorectal Cancer Be Found Early? Available online: https://www.cancer.org/cancer/types/colon-rectal-cancer/detection-diagnosis-staging/detection.html#:~:text=When%20colorectal%20cancer%20is%20found,rectum%2C%20survival%20rates%20are%20lower (accessed on 29 January 2024).
- Anderson, K.L.; Snyder, K.M.; Ito, D.; Lins, D.C.; Mills, L.J.; Weiskopf, K.; Modiano, J.F. Evolutionarily conserved resistance to phagocytosis observed in melanoma cells is insensitive to upregulation of pro-phagocytic signals and to CD47 blockade. Melanoma Res. 2020, 30, 147–158. [Google Scholar] [CrossRef]
- Vellingiri, B.; Iyer, M.; Devi Subramaniam, M.; Jayaramayya, K.; Siama, Z.; Giridharan, B.; Narayanasamy, A.; Abdal Dayem, A.; Cho, S.-G. Understanding the role of the transcription factor SP1 in ovarian cancer: From theory to practice. Int. J. Mol. Sci. 2020, 21, 1153. [Google Scholar] [CrossRef]
- Vizcaíno, C.; Mansilla, S.; Portugal, J. Sp1 transcription factor: A long-standing target in cancer chemotherapy. Pharmacol. Ther. 2015, 152, 111–124. [Google Scholar] [CrossRef]
- Han, I.; Kudlow, J.E. Reduced O glycosylation of Sp1 is associated with increased proteasome susceptibility. Mol. Cell Biol. 1997, 17, 2550–2558. [Google Scholar] [CrossRef]
- Roos, M.D.; Su, K.; Baker, J.R.; Kudlow, J.E. O glycosylation of an Sp1-derived peptide blocks known Sp1 protein interactions. Mol. Cell Biol. 1997, 17, 6472–6480. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Su, K.; Roos, M.D.; Chang, Q.; Paterson, A.J.; Kudlow, J.E. O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proc. Natl. Acad. Sci. USA 2001, 98, 6611–6616. [Google Scholar] [CrossRef] [PubMed]
- Han, I.; Roos, M.D.; Kudlow, J.E. Interaction of the transcription factor Sp1 with the nuclear pore protein p62 requires the C-terminal domain of p62. J. Cell Biochem. 1998, 68, 50–61. [Google Scholar] [CrossRef]
- Slawson, C.; Hart, G.W. Dynamic interplay between O-GlcNAc and O-phosphate: The sweet side of protein regulation. Curr. Opin. Struct. Biol. 2003, 13, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Edelstein, D.; Dimmeler, S.; Ju, Q.; Sui, C.; Brownlee, M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J. Clin. Investig. 2001, 108, 1341–1348. [Google Scholar] [CrossRef]
- Federici, M.; Menghini, R.; Mauriello, A.; Hribal, M.L.; Ferrelli, F.; Lauro, D.; Sbraccia, P.; Spagnoli, L.G.; Sesti, G.; Lauro, R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 2002, 106, 466–472. [Google Scholar] [CrossRef]
- Fiordaliso, F.; Leri, A.; Cesselli, D.; Limana, F.; Safai, B.; Nadal-Ginard, B.; Anversa, P.; Kajstura, J. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes 2001, 50, 2363–2375. [Google Scholar] [CrossRef]
- James, L.R.; Tang, D.; Ingram, A.; Ly, H.; Thai, K.; Cai, L.; Scholey, J.W. Flux through the hexosamine pathway is a determinant of nuclear factor kappaB-dependent promoter activation. Diabetes 2002, 51, 1146–1156. [Google Scholar] [CrossRef]
- Cawley, S.; Bekiranov, S.; Ng, H.H.; Kapranov, P.; Sekinger, E.A.; Kampa, D.; Piccolboni, A.; Sementchenko, V.; Cheng, J.; Williams, A.J.; et al. Unbiased mapping of transcription factor binding sites along human chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. Cell 2004, 116, 499–509. [Google Scholar] [CrossRef]
- Jackson, S.P.; Tjian, R. O-glycosylation of eukaryotic transcription factors: Implications for mechanisms of transcriptional regulation. Cell 1988, 55, 125–133. [Google Scholar] [CrossRef]
- Lagger, G.; Doetzlhofer, A.; Schuettengruber, B.; Haidweger, E.; Simboeck, E.; Tischler, J.; Chiocca, S.; Suske, G.; Rotheneder, H.; Wintersberger, E.; et al. The tumor suppressor p53 and histone deacetylase 1 are antagonistic regulators of the cyclin-dependent kinase inhibitor p21/WAF1/CIP1 gene. Mol. Cell Biol. 2003, 23, 2669–2679. [Google Scholar] [CrossRef]
- Yuan, L.; Ahn, I.S.; Davis, P.F. Inhibition of tyrosine phosphorylation of vascular endothelial growth factor receptors in human umbilical vein endothelial cells: A potent anti-angiogenic lipid-rich extract from shark. J. Med. Food 2007, 10, 657–661. [Google Scholar] [CrossRef]
- Kavurma, M.M.; Khachigian, L.M. Sp1 inhibits proliferation and induces apoptosis in vascular smooth muscle cells by repressing p21WAF1/Cip1 transcription and cyclin D1-Cdk4-p21WAF1/Cip1 complex formation. J. Biol. Chem. 2003, 278, 32537–32543. [Google Scholar] [CrossRef]
- Ganapathy, M.; Ghosh, R.; Xie, J.; Zhang, X.; Bedolla, R.; Schoolfield, J.; Yeh, I.T.; Troyer, D.A.; Olumi, A.F.; Kumar, A.P. Involvement of FLIP in 2-methoxyestradiol-induced tumor regression in transgenic adenocarcinoma of mouse prostate model. Clin. Cancer Res. 2009, 15, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Suske, G. The Sp-family of transcription factors. Gene 1999, 238, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Bilsland, A.E.; Stevenson, K.; Atkinson, S.; Kolch, W.; Keith, W.N. Transcriptional repression of telomerase RNA gene expression by c-Jun-NH2-kinase and Sp1/Sp3. Cancer Res. 2006, 66, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Higgins, K.J.; Liu, S.; Abdelrahim, M.; Yoon, K.; Vanderlaag, K.; Porter, W.; Metz, R.P.; Safe, S. Vascular endothelial growth factor receptor-2 expression is induced by 17beta-estradiol in ZR-75 breast cancer cells by estrogen receptor alpha/Sp proteins. Endocrinology 2006, 147, 3285–3295. [Google Scholar] [CrossRef]
- Lee, V.H.; Chow, B.K.; Lo, K.W.; Chow, L.S.; Man, C.; Tsao, S.W.; Lee, L.T. Regulation of RASSF1A in nasopharyngeal cells and its response to UV irradiation. Gene 2009, 443, 55–63. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sun, J.M.; Li, L.; Davie, J.R. Differential intranuclear organization of transcription factors Sp1 and Sp3. Mol. Biol. Cell 2005, 16, 4073–4083. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Lin, Y.-J.; Chen, Y.-C.; Liu, I.-L.; You, S.-L.; Hu, J.-M.; Lin, T.-C.; Chang, P.-K.; Chen, C.-Y.; Chou, Y.-C.; et al. Human papillomavirus and risk of colorectal cancer: An analysis of nationwide claims data. Medicina 2022, 58, 1461. [Google Scholar] [CrossRef]
- Rönsch, K.; Jägle, S.; Rose, K.; Seidl, M.; Baumgartner, F.; Freihen, V.; Yousaf, A.; Metzger, E.; Lassmann, S.; Schüle, R.; et al. SNAIL1 combines competitive displacement of ASCL2 and epigenetic mechanisms to rapidly silence the EPHB3 tumor suppressor in colorectal cancer. Mol. Oncol. 2015, 9, 335–354. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Lao, Y.; Yu, W.; Zhang, X.; Jiang, M.; Zhu, C. Progress in the application of immune checkpoint inhibitor-based immunotherapy for targeting different types of colorectal cancer. Front. Oncol. 2021, 11, 764618. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Kim, S.; Koh, J.; Kim, M.Y.; Kwon, D.; Go, H.; Kim, Y.A.; Chung, D.H. PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Hum. Pathol. 2016, 58, 7–14. [Google Scholar] [CrossRef]
- Wu, X.; Qin, L.; Fako, V.; Zhang, J.T. Molecular mechanisms of fatty acid synthase (FASN)-mediated resistance to anti-cancer treatments. Adv. Biol. Regul. 2014, 54, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, J.; Ni, X.; He, Y.; Wang, J.; Deng, Z.; Zhang, G.; Shi, T.; Chen, W. FASN promotes lipid metabolism and progression in colorectal cancer via the SP1/PLA2G4B axis. Cell Death Discov. 2025, 11, 122. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kwiterovich, P.O.; Gupta, P.; Erozan, Y.S.; Alving, C.R.; Richards, R.L. Localization of urinary lactosylceramide in cytoplasmic vesicles of renal tubular cells in homozygous familial hypercholesterolemia. Proc. Natl. Acad. Sci. USA 1983, 80, 1313–1317. [Google Scholar] [CrossRef]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The role of lipids and lipoproteins in atherosclerosis. In Endotext; Feingold, K.R., Ed.; MDText.com, Inc.: South Dartmouth, MA, USA, 2019. [Google Scholar]
- Sun, X.; Zhou, Q.; Xiao, C.; Mao, C.; Liu, Y.; Chen, G.; Song, Y. Role of post-translational modifications of SP1 in cardiovascular diseases. Front. Cell Dev. Biol. 2024, 12, 1453901. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Bie, Y.; Feng, H.; Xie, B.; Liu, M.; Zhao, F. Inflammatory factors driving atherosclerotic plaque progression: New insights. J. Transl. Intern. Med. 2022, 10, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Ghosh, N. Oxidized low density lipoprotein stimulates aortic smooth muscle cell proliferation. Glycobiology 1996, 6, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Garris, C.S.; Blaho, V.A.; Hla, T.; Han, M.H. Sphingosine-1-phosphate receptor 1 signaling in T cells: Trafficking and beyond. Immunology 2014, 142, 347–353. [Google Scholar] [CrossRef]
- Wu, X.; Dong, Z.; Wang, C.J.; Barlow, L.J.; Fako, V.; Serrano, M.A.; Zou, Y.; Liu, J.-Y.; Zhang, J.-T. FASN regulates cellular response to genotoxic treatments by increasing PARP-1 expression and DNA repair activity via NF-κB and SP1. Proc. Natl. Acad. Sci. USA 2016, 113, 12608–12613. [Google Scholar] [CrossRef]
- Ölmez, T. Is there an aesthetics in golden ratio as regards to the common cis-regulatory elements versus to atomic numbers of elements with respect to Quantum Perspective Model? Neurol. Neurosci. Rep. 2020, 3, 1–4. [Google Scholar] [CrossRef]
- Fessele, K.L.; Wright, F. Primer in genetics and genomics, Article 6: Basics of epigenetic control. Biol. Res. Nurs. 2018, 20, 103–110. [Google Scholar] [CrossRef]
- Tao, L.H.; Zhou, X.R.; Li, F.C.; Chen, Q.; Meng, F.Y.; Mao, Y.; Li, R.; Hua, D.; Zhang, H.J.; Wang, W.P.; et al. A polymorphism in the promoter region of PD-L1 serves as a binding-site for SP1 and is associated with PD-L1 overexpression and increased occurrence of gastric cancer. Cancer Immunol. Immunother. 2017, 66, 309–318. [Google Scholar] [CrossRef]
- Gao, Y.; Gan, K.; Liu, K.; Xu, B.; Chen, M. SP1 expression and the clinicopathological features of tumors: A meta-analysis and bioinformatics analysis. Pathol. Oncol. Res. 2021, 27, 581998. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, S.; Sun, J.M.; Davie, J.R. Gene regulation by Sp1 and Sp3. Biochem. Cell Biol. 2004, 82, 460–471. [Google Scholar] [CrossRef]
- Spengler, M.L.; Brattain, M.G. Sumoylation inhibits cleavage of Sp1 N-terminal negative regulatory domain and inhibits Sp1-dependent transcription. J. Biol. Chem. 2006, 281, 5567–5574. [Google Scholar] [CrossRef] [PubMed]
- Tapias, A.; Ciudad, C.J.; Roninson, I.B.; Noé, V. Regulation of Sp1 by cell cycle related proteins. Cell Cycle 2008, 7, 2856–2867. [Google Scholar] [CrossRef]
- Koltai, T.; Reshkin, S.J.; Harguindey, S. The Specificity protein 1 (Sp1) transcription factor. In An Innovative Approach to Understanding and Treating Cancer: Targeting pH; Academic Press: Cambridge, MA, USA, 2020; pp. 271–285. [Google Scholar]
- Pal, S.; Claffey, K.P.; Cohen, H.T.; Mukhopadhyay, D. Activation of Sp1-mediated vascular permeability factor/vascular endothelial growth factor transcription requires specific interaction with protein kinase C zeta. J. Biol. Chem. 1998, 273, 26277–26280. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Okuda, K.; Ohno, S.; Shirai, A.; Igarashi, T.; Matsunaga, K.; Fukushima, J.; Kawamoto, S.; Ishigatsubo, Y.; Okubo, T. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J. Immunol. 1994, 153, 2052–2063. [Google Scholar] [CrossRef]
- Karlseder, J.; Rotheneder, H.; Wintersberger, E. Interaction of Sp1 with the growth- and cell cycle-regulated transcription factor E2F. Mol. Cell Biol. 1996, 16, 1659–1667. [Google Scholar] [CrossRef]
- Xu, K.; Shu, H.K. EGFR activation results in enhanced cyclooxygenase-2 expression through p38 mitogen-activated protein kinase-dependent activation of the Sp1/Sp3 transcription factors in human gliomas. Cancer Res. 2007, 67, 6121–6129. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Lim, W.; Gee, K.; Aucoin, S.; Nandan, D.; Kozlowski, M.; Diaz-Mitoma, F.; Kumar, A. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J. Biol. Chem. 2001, 276, 13664–13674. [Google Scholar] [CrossRef]
- Zhang, H.W.; Wang, E.W.; Li, L.X.; Yi, S.H.; Li, L.C.; Xu, F.L.; Wang, D.L.; Wu, Y.Z.; Nian, W.Q. A regulatory loop involving miR-29c and Sp1 elevates the TGF-β1 mediated epithelial-to-mesenchymal transition in lung cancer. Oncotarget 2016, 7, 85905–85916. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Guo, Z.; Ma, F.; Wu, Y.; Bai, Y.; Gong, W.; Chen, Y.; Cheng, T.; Zhi, F.; et al. Inhibition of the transcription factor Sp1 suppresses colon cancer stem cell growth and induces apoptosis in vitro and in nude mouse xenografts. Oncol. Rep. 2013, 30, 1782–1792. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, M.Y.; Mo, J.S.; Park, H.S. Notch1 intracellular domain suppresses APP intracellular domain–Tip60–Fe65 complex mediated signaling through physical interaction. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 736–746. [Google Scholar] [CrossRef]
- Xing, Y.; Ge, Y.; Liu, C.; Zhang, X.; Jiang, J.; Wei, Y. ER stress inducer tunicamycin suppresses the self-renewal of glioma-initiating cell partly through inhibiting Sox2 translation. Oncotarget 2016, 7, 36395–36406. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kwiterovich, P.O., Jr.; Sekerke, C.S. Effects of tunicamycin on the binding and degradation of low density lipoproteins and glycoprotein synthesis in cultured human fibroblasts. J. Biol. Chem. 1979, 254, 3704–3707. [Google Scholar] [CrossRef]
- Shojaei, F.; Ferrara, N. Refractoriness to antivascular endothelial growth factor treatment: Role of myeloid cells. Cancer Res. 2008, 68, 5501–5504. [Google Scholar] [CrossRef]
- Cui, C.; Chen, X.; Liu, Y.; Cao, B.; Xing, Y.; Liu, C.; Yang, F.; Li, Y.; Yang, T.; Hua, L.; et al. β1,4-Galactosyltransferase V activates Notch1 signaling in glioma stem-like cells and promotes their transdifferentiation into endothelial cells. J. Biol. Chem. 2018, 293, 2219–2230. [Google Scholar] [CrossRef]
- Lo, N.W.; Shaper, J.H.; Pevsner, J.; Shaper, N.L. The expanding beta 4-galactosyltransferase gene family: Messages from the databanks. Glycobiology 1998, 8, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Hovinga, K.E.; Shimizu, F.; Wang, R.; Panagiotakos, G.; Van Der Heijden, M.; Moayedpardazi, H.; Correia, A.S.; Soulet, D.; Major, T.; Menon, J.; et al. Inhibition of Notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells 2010, 28, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Asano, N.; Watanabe, T.; Kitani, A.; Fuss, I.J.; Strober, W. Notch1 signaling and regulatory T cell function. J. Immunol. 2008, 180, 2796–2804. [Google Scholar] [CrossRef]
- Koduru, S.; Kumar, R.; Srinivasan, S.; Evers, M.B.; Damodaran, C. Notch-1 inhibition by Withaferin-A: A therapeutic target against colon carcinogenesis. Mol. Cancer Ther. 2010, 9, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Groot, A.J.; Vooijs, M.A. The role of Adams in Notch signaling. Adv. Exp. Med. Biol. 2012, 727, 15–36. [Google Scholar]
- Ye, S.; Wang, C.; Xu, Z.; Lin, H.; Wan, X.; Yu, Y.; Adhicary, S.; Zhang, J.Z.; Zhou, Y.; Liu, C.; et al. Impaired human cardiac cell development due to NOTCH1 deficiency. Circ. Res. 2023, 132, 187–204. [Google Scholar] [CrossRef]
- Zhao, H.; Ming, T.; Tang, S.; Ren, S.; Yang, H.; Liu, M.; Tao, Q.; Xu, H. Wnt signaling in colorectal cancer: Pathogenic role and therapeutic target. Mol. Cancer 2022, 21, 144. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X. Advances of Wnt Signalling Pathway in Colorectal Cancer. Cells 2023, 12, 447. [Google Scholar] [CrossRef]
- Castelo-Branco, G.; Wagner, J.; Rodriguez, F.J.; Kele, J.; Sousa, K.; Rawal, N.; Pasolli, H.A.; Fuchs, E.; Kitajewski, J.; Arenas, E. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc. Natl. Acad. Sci. USA 2003, 100, 12747–12752. [Google Scholar] [CrossRef]
- Albrecht, L.V.; Tejeda-Muñoz, N.; De Robertis, E.M. Cell Biology of Canonical Wnt Signaling. Annu. Rev. Cell Dev. Biol. 2021, 37, 369–389. [Google Scholar] [CrossRef]
- Rim, E.Y.; Clevers, H.; Nusse, R. The Wnt Pathway: From Signaling Mechanisms to Synthetic Modulators. Annu. Rev. Biochem. 2022, 91, 571–598. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.T.; Wallingford, J.B. Planar cell polarity in development and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Schulte, G.; Wright, S.C. Frizzleds as GPCRs—More Conventional Than We Thought! Trends Pharmacol. Sci. 2018, 39, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Chen, Z.; Jin, X.; Mao, R.; Chen, Z. The many postures of noncanonical Wnt signaling in development and diseases. Biomed. Pharmacother. 2017, 93, 359–369. [Google Scholar] [CrossRef]
- Gujral, T.S.; Chan, M.; Peshkin, L.; Sorger, P.K.; Kirschner, M.W.; MacBeath, G. A noncanonical Frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Cell 2014, 159, 844–856. [Google Scholar] [CrossRef]
- Li, X.; Xiang, Y.; Li, F.; Yin, C.; Li, B.; Ke, X. WNT/β-Catenin Signaling Pathway Regulating T Cell-Inflammation in the Tumor Microenvironment. Front. Immunol. 2019, 10, 2293. [Google Scholar] [CrossRef] [PubMed]
- Speiser, J.; Foreman, K.; Drinka, E.; Godellas, C.; Perez, C.; Salhadar, A.; Erşahin, Ç.; Rajan, P. Notch-1 and Notch-4 biomarker expression in triple-negative breast cancer. Int. J. Surg. Pathol. 2012, 20, 139–145. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Ishiguro, H.; Okubo, T.; Kuwabara, Y.; Kimura, M.; Mitsui, A.; Sugito, N.; Ogawa, R.; Katada, T.; Tanaka, T.; Shiozaki, M.; et al. NOTCH1 activates the Wnt/β-catenin signaling pathway in colon cancer. Oncotarget 2017, 8, 60378–60389. [Google Scholar] [CrossRef]
- Meng, F.; Liu, X.; Lin, C.; Xu, L.; Liu, J.; Zhang, P.; Zhang, X.; Song, J.; Yan, Y.; Ren, Z.; et al. SMYD2 suppresses APC2 expression to activate the Wnt/β-catenin pathway and promotes epithelial-mesenchymal transition in colorectal cancer. Am. J. Cancer Res. 2020, 10, 997–1011. [Google Scholar]
- Rodon, J.; Argilés, G.; Connolly, R.M.; Vaishampayan, U.; de Jonge, M.; Garralda, E.; Giannakis, M.; Smith, D.C.; Dobson, J.R.; McLaughlin, M.E.; et al. Phase 1 study of single-agent WNT974, a first-in-class Porcupine inhibitor, in patients with advanced solid tumours. Br. J. Cancer 2021, 125, 28–37. [Google Scholar] [CrossRef]
- Tabernero, J.; Van Cutsem, E.; Garralda, E.; Tai, D.; De Braud, F.; Geva, R.; van Bussel, M.T.J.; Dotti, K.F.; Elez, E.; de Miguel, M.J.; et al. A Phase Ib/II Study of WNT974 + Encorafenib + Cetuximab in Patients with BRAF V600E-Mutant KRAS Wild-Type Metastatic Colorectal Cancer. Oncologist 2023, 28, 230–238. [Google Scholar] [CrossRef]
- Liu, H.Y.; Sun, X.J.; Xiu, S.Y.; Zhang, X.Y.; Wang, Z.Q.; Gu, Y.L.; Yi, C.X.; Liu, J.Y.; Dai, Y.S.; Yuan, X.; et al. Frizzled receptors (FZDs) in Wnt signaling: Potential therapeutic targets for human cancers. Acta Pharmacol. Sin. 2024, 45, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, H.; Rattner, A.; Nathans, J. Frizzled Receptors in Development and Disease. Curr. Top. Dev. Biol. 2016, 117, 113–139. [Google Scholar]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Kirikoshi, H.; Sagara, N.; Koike, J.; Tanaka, K.; Sekihara, H.; Hirai, M.; Katoh, M. Molecular cloning and characterization of human Frizzled-4 on chromosome 11q14-q21. Biochem. Biophys. Res. Commun. 1999, 264, 955–961. [Google Scholar] [CrossRef]
- Chung, D.C. The genetic basis of colorectal cancer: Insights into critical pathways of tumorigenesis. Gastroenterology 2000, 119, 854–865. [Google Scholar] [CrossRef]
- Ueno, K.; Hiura, M.; Suehiro, Y.; Hazama, S.; Hirata, H.; Oka, M.; Imai, K.; Dahiya, R.; Hinoda, Y. Frizzled-7 as a potential therapeutic target in colorectal cancer. Neoplasia 2008, 10, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Vincan, E.; Darcy, P.K.; Smyth, M.J.; Thompson, E.W.; Thomas, R.J.; Phillips, W.A.; Ramsay, R.G. Frizzled-7 receptor ectodomain expression in a colon cancer cell line induces morphological change and attenuates tumor growth. Differentiation 2005, 73, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Hazama, S.; Mitomori, S.; Nishioka, M.; Suehiro, Y.; Hirata, H.; Oka, M.; Imai, K.; Dahiya, R.; Hinoda, Y. Down-regulation of frizzled-7 expression decreases survival, invasion and metastatic capabilities of colon cancer cells. Br. J. Cancer 2009, 101, 1374–1381. [Google Scholar] [CrossRef]
- Nagayama, S.; Yamada, E.; Kohno, Y.; Aoyama, T.; Fukukawa, C.; Kubo, H.; Watanabe, G.; Katagiri, T.; Nakamura, Y.; Sakai, Y.; et al. Inverse correlation of the up-regulation of FZD10 expression and the activation of beta-catenin in synchronous colorectal tumors. Cancer Sci. 2009, 100, 405–412. [Google Scholar] [CrossRef]
- Fu, L.; Fan, J.; Maity, S.; McFadden, G.; Shi, Y.; Kong, W. PD-L1 interacts with Frizzled 6 to activate β-catenin and form a positive feedback loop to promote cancer stem cell expansion. Oncogene 2022, 41, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Li, M.; Qi, X.; Li, J. β1,4-Galactosyltransferase V Modulates Breast Cancer Stem Cells through Wnt/β-catenin Signaling Pathway. Cancer Res. Treat. 2020, 52, 1084–1102. [Google Scholar] [CrossRef]
- Larasati, Y.; Boudou, C.; Koval, A.; Katanaev, V.L. Unlocking the Wnt pathway: Therapeutic potential of selective targeting FZD7 in cancer. Drug Discov. Today 2022, 27, 777–792. [Google Scholar] [CrossRef]
- Diamond, J.R.; Becerra, C.; Richards, D.; Mita, A.; Osborne, C.; O’Shaughnessy, J.; Zhang, C.; Henner, R.; Kapoun, A.M.; Xu, L.; et al. Phase Ib clinical trial of the anti-frizzled antibody vantictumab (OMP-18R5) plus paclitaxel in patients with locally advanced or metastatic HER2-negative breast cancer. Breast Cancer Res. Treat. 2020, 184, 53–62. [Google Scholar] [CrossRef]
- Le, P.N.; McDermott, J.D.; Jimeno, A. Targeting the Wnt pathway in human cancers: Therapeutic targeting with a focus on OMP-54F28. Pharmacol. Ther. 2015, 146, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Gunderson, C.C.; Sabbatini, P.; McMeekin, D.S.; Mantia-Smaldone, G.; Burger, R.A.; Morgan, M.A.; Kapoun, A.M.; Brachmann, R.K.; Stagg, R.; et al. A phase 1b dose escalation study of ipafricept (OMP54F28) in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Gynecol. Oncol. 2019, 154, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhu, X.; Liu, W.; Ruan, T.; Tao, K. Hedgehog signaling pathway in colorectal cancer: Function, mechanism, and therapy. OncoTargets Ther. 2017, 10, 3249–3259. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulosr, V.; Tsapakidis, K.; Riobo Del Galdo, N.A.; Papandreou, C.N.; Del Galdo, F.; Anthoney, A.; Sakellaridis, N.; Dimas, K.; Kamposioras, K. The prognostic significance of the Hedgehog signaling pathway in colorectal cancer. Clin. Color. Cancer 2016, 15, 116–127. [Google Scholar] [CrossRef]
- Taipale, J.; Beachy, P.A. The Hedgehog and Wnt signaling pathways in cancer. Nature 2001, 411, 349–354. [Google Scholar] [CrossRef]
- Ingham, P.W.; McMahon, A.P. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001, 15, 3059–3087. [Google Scholar] [CrossRef]
- Sugito, H.; Shibukawa, Y.; Kinumatsu, T.; Yasuda, T.; Nagayama, M.; Yamada, S.; Minugh-Purvis, N.; Pacifici, M.; Koyama, E. Ihh signaling regulates mandibular symphysis development and growth. J. Dent. Res. 2011, 90, 625–631. [Google Scholar] [CrossRef]
- Kong, J.H.; Siebold, C.; Rohatgi, R. Biochemical mechanisms of vertebrate hedgehog signaling. Development 2019, 146, dev166892. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Z.; Qu, X.; Bai, X.; Liu, X.; Tao, W.; Zhou, L.; Wang, D.; Wei, J. Desert hedgehog mediates the proliferation of medaka spermatogonia through Smoothened signaling. Reproduction 2022, 163, 209–218. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Y.; Cai, Q.; Wei, L.; Zhan, Y.; Shen, A.; Sferra, T.J.; Peng, J. Scutellaria Barbata D Don inhibits colorectal cancer growth via suppression of multiple signaling pathways. Integr. Cancer Ther. 2014, 13, 240–248. [Google Scholar] [CrossRef]
- Watt, F.M. Unexpected Hedgehog-Wnt interactions in epithelial differentiation. Trends Mol. Med. 2004, 10, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Katoh, M. Hedgehog signaling pathway and gastrointestinal stem cell signaling network (review). Int. J. Mol. Med. 2006, 18, 1019–1023. [Google Scholar] [CrossRef]
- Van den Brink, G.R.; Hardwick, J.C. Hedgehog Wnteraction in colorectal cancer. Gut 2006, 55, 912–914. [Google Scholar] [CrossRef]
- Lees, C.W.; Satsangi, J. Hedgehog, Paneth cells, and colon cancer: A cautionary note for the use of systemic agonists/antagonists. Gastroenterology 2006, 131, 1657–1658. [Google Scholar] [CrossRef]
- Chowdhury, S.; Pradhan, R.N.; Sarkar, R.R. Structural and logical analysis of a comprehensive hedgehog signaling pathway to identify alternative drug targets for glioma, colon and pancreatic cancer. PLoS ONE 2013, 8, e69132. [Google Scholar] [CrossRef]
- Ciucci, A.; De Stefano, I.; Vellone, V.G.; Lisi, L.; Bottoni, C.; Scambia, G.; Zannoni, G.F.; Gallo, D. Expression of the glioma-associated oncogene homolog 1 (Gli1) in advanced serous ovarian cancer is associated with unfavorable overall survival. PLoS ONE 2013, 8, e60145. [Google Scholar] [CrossRef] [PubMed]
- Ruiz i Altaba, A. Hedgehog signaling and the Gli code in stem cells, cancer, and metastases. Sci. Signal. 2011, 4, pt9. [Google Scholar] [CrossRef] [PubMed]
- Van den Brink, G.R.; Bleuming, S.A.; Hardwick, J.C.; Schepman, B.L.; Offerhaus, G.J.; Keller, J.J.; Nielsen, C.; Gaffield, W.; van Deventer, S.J.; Roberts, D.J.; et al. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat. Genet. 2004, 36, 277–282. [Google Scholar] [CrossRef]
- Fu, R.X.; Deng, H.; Zhao, L.; Li, J.; Zhou, Y.; Zhang, Y. Distinct expression patterns of hedgehog ligands between cultured and primary colorectal cancers are associated with aberrant methylation of their promoters. Mol. Cell Biochem. 2010, 337, 185–192. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.Y.; Wu, Y.Y.; Nie, Y.Q. Expression and clinical significance of hedgehog signaling pathway related components in colorectal cancer. Asian Pac. J. Cancer Prev. 2012, 13, 2319–2324. [Google Scholar] [CrossRef]
- Fu, X.; Yang, X.; Li, J.; Tian, X.; Cai, J.; Zhang, Y. Opposite expression patterns of Sonic hedgehog and Indian hedgehog are associated with aberrant methylation status of their promoters in colorectal cancers. Pathology 2010, 42, 553–559. [Google Scholar] [CrossRef]
- Chung, J.H.; Larsen, A.R.; Chen, E.; Bunz, F. A PTCH1 homolog transcriptionally activated by p53 suppresses Hedgehog signaling. J. Biol. Chem. 2014, 289, 33020–33031. [Google Scholar] [CrossRef]
- Ichimura, Y.; Komatsu, M. Selective degradation of p62 by autophagy. Semin. Immunopathol. 2010, 32, 431–436. [Google Scholar] [CrossRef]
- Song, L.; Liu, J.; Jin, X.; Li, Z.; Zhao, M.; Liu, W. p,p′-Dichlorodiphenyldichloroethylene induces colorectal adenocarcinoma cell proliferation through oxidative stress. PLoS ONE 2014, 9, e112700. [Google Scholar] [CrossRef]
- Bertrand, F.E.; Angus, C.W.; Partis, W.J.; Sigounas, G. Developmental pathways in colon cancer: Crosstalk between WNT, BMP, Hedgehog and Notch. Cell Cycle 2012, 11, 4344–4351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Z.; Liu, C.; Jin, C.; Zhang, J.; Miao, X.; Jia, L. B4GALT1 gene knockdown inhibits the hedgehog pathway and reverses multidrug resistance in the human leukemia K562/adriamycin-resistant cell line. IUBMB Life 2012, 64, 889–900. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, H.; Wei, W.; Ji, D.; Song, X.; Sun, J.; Zhang, J.; Jia, L. B4GALT family mediates the multidrug resistance of human leukemia cells by regulating the hedgehog pathway and the expression of p-glycoprotein and multidrug resistance-associated protein 1. Cell Death Dis. 2013, 4, e654. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Du, Y.; Yang, Z.; He, L.; Wang, Y.; Hao, L.; Ding, M.; Yan, R.; Wang, J.; Fan, Z. Activation of Sonic Hedgehog signaling maintains the self-renewal and tumor-initiating capacity of bladder cancer stem cells. Cancer Res. 2016, 76, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.T.; Zhang, R.; Boohaker, R.J. GLI1: A therapeutic target for cancer. Front. Oncol. 2021, 11, 673154. [Google Scholar] [CrossRef]
- Massimino, M.; Stella, S.; Tirrò, E.; Romano, C.; Pennisi, M.S.; Puma, A.; Manzella, L.; Zanghì, A.; Stagno, F.; Di Raimondo, F.; et al. Non ABL-directed inhibitors as alternative treatment strategies for chronic myeloid leukemia. Mol. Cancer 2018, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Berlin, J.; Bendell, J.C.; Hart, L.L.; Firdaus, I.; Gore, I.; Hermann, R.C.; Mulcahy, M.F.; Zalupski, M.M.; Mackey, H.M.; Yauch, R.L.; et al. A randomized phase II trial of vismodegib versus placebo with FOLFOX or FOLFIRI and bevacizumab in patients with previously untreated metastatic colorectal cancer. Clin. Cancer Res. 2013, 19, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Bendell, J.; Andre, V.; Ho, A.; Kudchadkar, R.; Migden, M.; Infante, J.; Tiu, R.V.; Pitou, C.; Tucker, T.; Brail, L.; et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin. Cancer Res. 2018, 24, 2082–2091. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Kim, J.; Spaunhurst, K.; Montoya, J.; Khodosh, R.; Chandra, K.; Fu, T.; Gilliam, A.; Molgo, M.; Beachy, P.A.; et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J. Clin. Oncol. 2014, 32, 745–751. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Cho, J. Hedgehog pathway inhibitors as targeted cancer therapy and strategies to overcome drug resistance. Int. J. Mol. Sci. 2022, 23, 1733. [Google Scholar] [CrossRef]
- Kieran, M.W.; Chisholm, J.; Casanova, M.; Brandes, A.A.; Aerts, I.; Bouffet, E.; Bailey, S.; Leary, S.; MacDonald, T.J.; Mechinaud, F.; et al. Phase I study of oral sonidegib (LDE225) in pediatric brain and solid tumors and a phase II study in children and adults with relapsed medulloblastoma. Neuro-Oncology 2017, 19, 1542–1552. [Google Scholar] [CrossRef]
- Brzozowa-Zasada, M. Immunohistochemical expression of Snail1 protein in colorectal adenocarcinoma samples and its prognostic activity in Caucasian patients. Prz. Gastroenterol. 2021, 16, 339–345. [Google Scholar] [CrossRef]
- Villarejo, A.; Cortés-Cabrera, A.; Molina-Ortíz, P.; Portillo, F.; Cano, A. Differential role of Snail1 and Snail2 zinc fingers in E-cadherin repression and epithelial to mesenchymal transition. J. Biol. Chem. 2014, 289, 930–941. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Tai, S.K.; Yang, M.H. Snail-overexpressing cancer cells promote M2-like polarization of tumor-associated macrophages by delivering miR-21-abundant exosomes. Neoplasia 2018, 20, 775–788. [Google Scholar] [CrossRef]
- Huang, S.; Hua, C.; Ding, B.; Chen, J.; Zheng, S.; Ding, C. Advances in improving the efficacy of anti-PD-1/PD-L1 therapy in intrahepatic cholangiocarcinoma. Crit. Rev. Oncol. Hematol. 2025, 213, 104784. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Lee, H.-H.; Hsu, J.-L.; Yu, D.; Hung, M.-C. The impact of PD-L1 N-linked glycosylation on cancer therapy and clinical diagnosis. J. Biomed. Sci. 2020, 27, 77. [Google Scholar] [CrossRef] [PubMed]
- Escors, D.; Gato-Cañas, M.; Zuazo, M.; Arasanz, H.; García-Granda, M.J.; Vera, R.; Kochan, G. The intracellular signalosome of PD-L1 in cancer cells. Signal Transduct. Target. Ther. 2018, 3, 26. [Google Scholar] [CrossRef]
- Li, C.W.; Lim, S.O.; Xia, W.; Lee, H.-H.; Chan, L.-C.; Kuo, C.-W.; Khoo, K.-H.; Chang, S.-S.; Cha, J.-H.; Kim, T.; et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016, 7, 12632. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, L.; Li, S.C.; He, Q.J.; Yang, B.; Cao, J. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharmacol. Sin. 2021, 42, 1–9. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Ju, X.; Zhang, H.; Zhou, Z.; Wang, Q. Regulation of PD-L1 expression in cancer and clinical implications in immunotherapy. Am. J. Cancer Res. 2020, 10, 1–11. [Google Scholar]
- Wang, D.; Lin, J.; Yang, X.; Long, J.; Bai, Y.; Yang, X.; Mao, Y.; Sang, X.; Seery, S.; Zhao, H. Combination regimens with PD-1/PD-L1 immune checkpoint inhibitors for gastrointestinal malignancies. J. Hematol. Oncol. 2019, 12, 42. [Google Scholar] [CrossRef]
- Kilaru, S.; Panda, S.S.; Moharana, L.; Mohapatra, D.; Mohapatra, S.S.G.; Panda, A.; Kolluri, S.; Devaraj, S.; Biswas, G. PD-L1 expression and its significance in advanced NSCLC: Real-world experience from a tertiary care center. J. Egypt. Natl. Canc. Inst. 2024, 36, 3. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Shen, X.; Pan, Y.; Zheng, Q.; Chen, H.; Hu, H.; Li, Y. Correlation between PD-L1 expression and clinicopathological characteristics of non-small cell lung cancer: A real-world study of a large Chinese cohort. J. Thorac. Dis. 2019, 11, 4591–4601. [Google Scholar] [CrossRef] [PubMed]
- Mussafir, O.; Mei, J.; Mao, W.; Wan, Y. Immune checkpoint inhibitors for PD-1/PD-L1 axis in combination with other immunotherapies and targeted therapies for non-small cell lung cancer. Front. Oncol. 2022, 12, 948405. [Google Scholar]
- Cadigan, K.M.; Waterman, M.L. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb. Perspect. Biol. 2012, 4, a007906. [Google Scholar] [CrossRef]
- Albanese, I.; Khan, K.; Barratt, B.; Al-Kindi, H.; Schwertani, A. Atherosclerotic calcification: Wnt is the hint. J. Am. Heart Assoc. 2018, 7, e007356. [Google Scholar] [CrossRef]
- Leng, H.; Zhang, H.; Li, L.; Zhang, S.; Wang, Y.; Chavda, S.J.; Galas-Filipowicz, D.; Lou, H.; Ersek, A.; Morris, E.V.; et al. Modulating glycosphingolipid metabolism and autophagy improves outcomes in preclinical models of myeloma bone disease. Nat. Commun. 2022, 13, 7868. [Google Scholar] [CrossRef]
- Leng, H.; Simon, A.K.; Horwood, N.J. Blocking glycosphingolipid production alters autophagy in osteoclasts and improves myeloma bone disease. Autophagy 2024, 20, 930–932. [Google Scholar] [CrossRef]
- Li, H.M.; Bi, Y.R.; Li, Y.; Fu, R.; Lv, W.C.; Jiang, N.; Xu, Y.; Ren, B.X.; Chen, Y.D.; Xie, H.; et al. A potent CBP/p300-Snail interaction inhibitor suppresses tumor growth and metastasis in wild-type p53-expressing cancer. Sci. Adv. 2020, 6, eaaw8500. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, S.; Kapila, D.; Dubey, P.; Pasunooti, S.; Tatavarthi, S.; Park, C.; Ramdat, C. The β-1,4 GalT-V Interactome—Potential Therapeutic Targets and a Network of Pathways Driving Cancer and Cardiovascular and Inflammatory Diseases. Int. J. Mol. Sci. 2025, 26, 8088. https://doi.org/10.3390/ijms26168088
Chatterjee S, Kapila D, Dubey P, Pasunooti S, Tatavarthi S, Park C, Ramdat C. The β-1,4 GalT-V Interactome—Potential Therapeutic Targets and a Network of Pathways Driving Cancer and Cardiovascular and Inflammatory Diseases. International Journal of Molecular Sciences. 2025; 26(16):8088. https://doi.org/10.3390/ijms26168088
Chicago/Turabian StyleChatterjee, Subroto, Dhruv Kapila, Priya Dubey, Swathi Pasunooti, Sruthi Tatavarthi, Claire Park, and Caitlyn Ramdat. 2025. "The β-1,4 GalT-V Interactome—Potential Therapeutic Targets and a Network of Pathways Driving Cancer and Cardiovascular and Inflammatory Diseases" International Journal of Molecular Sciences 26, no. 16: 8088. https://doi.org/10.3390/ijms26168088
APA StyleChatterjee, S., Kapila, D., Dubey, P., Pasunooti, S., Tatavarthi, S., Park, C., & Ramdat, C. (2025). The β-1,4 GalT-V Interactome—Potential Therapeutic Targets and a Network of Pathways Driving Cancer and Cardiovascular and Inflammatory Diseases. International Journal of Molecular Sciences, 26(16), 8088. https://doi.org/10.3390/ijms26168088






