Evaluation of Selected Serum Adipocytokines in Patients with Relapsing–Remitting Multiple Sclerosis Treated with Immunomodulatory Second-Line Drugs
Abstract
1. Introduction
Adipocytokines in MS
2. Results
3. Discussion
3.1. Fingolimod and Adipocytokines
3.2. Natalizumab and Adipocytokines
3.3. Clinical and Radiological Parameters and Adipocytokines
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sommer, A.J.; Leray, E.; Lee, Y.; Bind, M.C. Assessing environmental epidemiology questions in practice with a causal inference pipeline: An investigation of the air pollution-multiple sclerosis relapses relationship. Stat. Med. 2021, 40, 1321–1335. [Google Scholar] [CrossRef]
- Wnuk, M.; Maluchnik, M.; Perwieniec, J.; Podwojcic, K.; Szelag, M.; Walkiewicz, D.; Zakrzewski, M.; Kulakowska, A.; Brola, W.; Rejdak, K.; et al. Multiple sclerosis incidence and prevalence in Poland: Data from administrative health claims. Mult. Scler. Relat. Disord. 2021, 55, 103162. [Google Scholar] [CrossRef]
- Ascherio, A. Environmental factors in multiple sclerosis. Expert Rev. Neurother. 2013, 13, 3–9. [Google Scholar] [CrossRef]
- Wu, G.F.; Alvarez, E. The Immunopathophysiology of Multiple Sclerosis. Neurol. Clin. 2011, 29, 257–278. [Google Scholar] [CrossRef]
- Frohman, E.M.; Racke, M.K.; Raine, C.S. Multiple Sclerosis—The Plaque and Its Pathogenesis. N. Engl. J. Med. 2006, 354, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Sredni-Kenigsbuch, D. Th1/Th2 Cytokines in the Central Nervous System. Int. J. Neurosci. 2002, 112, 665–703. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, B.; Morawiec, N.; Arendarczyk, M.; Baran, M.; Wierzbicki, K.; Sowa, P.; Adamczyk-Sowa, M. Multiple sclerosis immunomodulatory therapies tested for effectiveness in COVID-19. Neurol. I Neurochir. Pol. 2021, 55, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, B.; Adamczyk-Sowa, M. New Insights into the Role of Oxidative Stress Mechanisms in the Pathophysiology and Treatment of Multiple Sclerosis. Oxidative Med. Cell. Longev. 2016, 2016, 1973834. [Google Scholar] [CrossRef]
- Mokry, L.E.; Ross, S.; Timpson, N.J.; Sawcer, S.; Davey Smith, G.; Richards, J.B. Obesity and Multiple Sclerosis: A Mendelian Randomization Study. PLoS Med. 2016, 13, e1002053. [Google Scholar] [CrossRef]
- Rhead, B.; Bäärnhielm, M.; Gianfrancesco, M.; Mok, A.; Shao, X.; Quach, H.; Shen, L.; Schaefer, C.; Link, J.; Gyllenberg, A.; et al. Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol. Genet. 2016, 2, e97. [Google Scholar] [CrossRef]
- Würtz, P.; Wang, Q.; Kangas, A.J.; Richmond, R.C.; Skarp, J.; Tiainen, M.; Tynkkynen, T.; Soininen, P.; Havulinna, A.S.; Kaakinen, M.; et al. Metabolic Signatures of Adiposity in Young Adults: Mendelian Randomization Analysis and Effects of Weight Change. PLoS Med. 2014, 11, e1001765. [Google Scholar] [CrossRef]
- Matarese, G.; Carrieri, P.B.; La Cava, A.; Perna, F.; Sanna, V.; De Rosa, V.; Aufiero, D.; Fontana, S.; Zappacosta, S. Leptin increase in multiple sclerosis associates with reduced number of CD4+ CD25+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5150–5155. [Google Scholar] [CrossRef]
- Orton, S.-M.; Herrera, B.M.; Yee, I.M.; Valdar, W.; Ramagopalan, S.V.; Sadovnick, A.D.; Ebers, G.C. Sex ratio of multiple sclerosis in Canada: A longitudinal study. Lancet Neurol. 2006, 5, 932–936. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.; Yao-Borengasser, A.; Rasouli, N.; Bodles, A.M.; Phanavanh, B.; Lee, M.-J.; Starks, T.; Kern, L.M.; Spencer, H.J.; McGehee, R.E.; et al. Human Visfatin Expression: Relationship to Insulin Sensitivity, Intramyocellular Lipids, and Inflammation. J. Clin. Endocrinol. Metab. 2007, 92, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Versini, M.; Jeandel, P.-Y.; Rosenthal, E.; Shoenfeld, Y. Obesity in autoimmune diseases: Not a passive bystander. Autoimmun. Rev. 2014, 13, 981–1000. [Google Scholar] [CrossRef]
- Emamgholipour, S.; Eshaghi, S.M.; Hossein-nezhad, A.; Mirzaei, K.; Maghbooli, Z.; Sahraian, M.A. Adipocytokine Profile, Cytokine Levels and Foxp3 Expression in Multiple Sclerosis: A Possible Link to Susceptibility and Clinical Course of Disease. PLoS ONE 2013, 8, e76555, Correction in PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Dakroub, A.; ANasser, S.; Younis, N.; Bhagani, H.; Al-Dhaheri, Y.; Pintus, G.; Eid, A.A.; El-Yazbi, A.F.; Eid, A.H. Visfatin: A Possible Role in Cardiovasculo-Metabolic Disorders. Cells 2020, 9, 2444. [Google Scholar] [CrossRef]
- Ahmed, H.H.; Shousha, W.G.; El-mezayen, H.A.; Emara, I.A.; Hassan, M.E. New Biomarkers as Prognostic Factors for Cardiovascular Complications in Type 2 Diabetic Patients. Indian J. Clin. Biochem. 2020, 35, 54–62. [Google Scholar] [CrossRef]
- Romacho, T.; Sánchez-Ferrer, C.F.; Peiró, C. Visfatin/Nampt: An Adipokine with Cardiovascular Impact. Mediat. Inflamm. 2013, 2013, 946427. [Google Scholar] [CrossRef]
- Bruzzone, S.; Fruscione, F.; Morando, S.; Ferrando, T.; Poggi, A.; Garuti, A.; D’URso, A.; Selmo, M.; Benvenuto, F.; Cea, M.; et al. Catastrophic NAD+ Depletion in Activated T Lymphocytes through Nampt Inhibition Reduces Demyelination and Disability in EAE. PLoS ONE 2009, 4, e7897. [Google Scholar] [CrossRef] [PubMed]
- Filková, M.; Haluzík, M.; Gay, S.; Šenolt, L. The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin. Immunol. 2009, 133, 157–170. [Google Scholar] [CrossRef]
- Tripathi, D.; Kant, S.; Pandey, S.; Ehtesham, N.Z. Resistin in metabolism, inflammation, and disease. FEBS J. 2020, 287, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.P.; Lehrke, M.; Wolfe, M.L.; Rohatgi, A.; Lazar, M.A.; Rader, D.J. Resistin Is an Inflammatory Marker of Atherosclerosis in Humans. Circulation 2005, 111, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Kamil, M.A.; Peeran, S.W.; Basheer, S.N.; Elhassan, A.; Alam, M.N.; Thiruneervannan, M. Role of Resistin in Various Diseases with Special Emphasis on Periodontal and Periapical Inflammation—A Review. J. Pharm. Bioallied Sci. 2023, 15, S31–S35. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Cai, D.; Guo, H.; Fang, J.; Cui, H.; Gou, L.; Deng, J.; Wang, Z.; Zuo, Z. Resistin, a Novel Host Defense Peptide of Innate Immunity. Front. Immunol. 2021, 12, 699807. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Ramakrishnan, N.; Auger, K.; Rahimi, N.; Jialal, I. Biochemistry, Adiponectin. In StatPearls; StatPearls Publishing: Petersburg, FL, USA, 2025. [Google Scholar]
- Signoriello, E.; Mallardo, M.; Nigro, E.; Polito, R.; Casertano, S.; Di Pietro, A.; Coletta, M.; Monaco, M.L.; Rossi, F.; Lus, G.; et al. Adiponectin in Cerebrospinal Fluid from Patients Affected by Multiple Sclerosis Is Correlated with the Progression and Severity of Disease. Mol. Neurobiol. 2021, 58, 2663–2670. [Google Scholar] [CrossRef]
- Mallardo, M.; Signoriello, E.; Lus, G.; Daniele, A.; Nigro, E. Adiponectin Alleviates Cell Injury due to Cerebrospinal Fluid from Multiple Sclerosis Patients by Inhibiting Oxidative Stress and Proinflammatory Response. Biomedicines 2023, 11, 1692. [Google Scholar] [CrossRef]
- Guerrero-García, J.d.J.; Carrera-Quintanar, L.; López-Roa, R.I.; Márquez-Aguirre, A.L.; Rojas-Mayorquín, A.E.; Ortuño-Sahagún, D. Multiple Sclerosis and Obesity: Possible Roles of Adipokines. Mediat. Inflamm. 2016, 2016, 4036232. [Google Scholar] [CrossRef] [PubMed]
- Hietaharju, A.; Kuusisto, H.; Nieminen, R.; Vuolteenaho, K.; Elovaara, I.; Moilanen, E. Elevated cerebrospinal fluid adiponectin and adipsin levels in patients with multiple sclerosis: A Finnish co-twin study. Eur. J. Neurol. 2010, 17, 332–334. [Google Scholar] [CrossRef]
- Milo, R.; Miller, A. Revised diagnostic criteria of multiple sclerosis. Autoimmun. Rev. 2014, 13, 518–524. [Google Scholar] [CrossRef]
- Overs, S.; Hughes, C.M.; Haselkorn, J.K.; Turner, A.P. Modifiable Comorbidities and Disability in Multiple Sclerosis. Curr. Neurol. Neurosci. Rep. 2012, 12, 610–617. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L. Weighing Evidence from Mendelian Randomization—Early-Life Obesity as a Causal Factor in Multiple Sclerosis? PLoS Med. 2016, 13, e1002054. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Novo, A.M.; Batista, S. Multiple Sclerosis: Implications of Obesity in Neuroinflammation. Obes. Brain Funct. 2017, 19, 191–210. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, T.-T.; Yu, J.; Liu, Y.-L.; Qi, S.-F.; Zhao, J.-J.; Liu, D.-W.; Tian, Q.-B. Excess Body Weight during Childhood and Adolescence Is Associated with the Risk of Multiple Sclerosis: A Meta-Analysis. Neuroepidemiology 2016, 47, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Penesova, A.; Vlcek, M.; Imrich, R.; Vernerova, L.; Marko, A.; Meskova, M.; Grunnerova, L.; Turcani, P.; Jezova, D.; Kollar, B. Hyperinsulinemia in newly diagnosed patients with multiple sclerosis. Metab. Brain Dis. 2015, 30, 895–901. [Google Scholar] [CrossRef]
- McGinley, M.P.; Cohen, J.A. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet 2021, 398, 1184–1194, Erratum in Lancet 2021, 398, 1132. [Google Scholar]
- Astapova, O.; Leff, T. Adiponectin and PPARγ. Vitam. Horm. 2012, 90, 143–162. [Google Scholar] [CrossRef]
- Yevgi, R.; Demir, R. Oxidative stress activity of fingolimod in multiple sclerosis. Clin. Neurol. Neurosurg. 2021, 202, 106500. [Google Scholar] [CrossRef]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Mosheimer, B.; Theurl, M.; Niederegger, H.; Tilg, H. Visfatin, an Adipocytokine with Proinflammatory and Immunomodulating Properties. J. Immunol. 2007, 178, 1748–1758. [Google Scholar] [CrossRef]
- Ahmed, M.; Gaffen, S.L. IL-17 inhibits adipogenesis in part via C/EBPα, PPARγ and Krüppel-like factors. Cytokine 2013, 61, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.P.; Choi, Y.; Wang, P.; Belt Davis, D.; Rabaglia, M.E.; Oler, A.T.; Stapleton, D.S.; Argmann, C.; Schueler, K.L.; Edwards, S.; et al. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res. 2008, 18, 706–716. [Google Scholar] [CrossRef]
- Jung, H.; Park, K.; Cho, Y.; Chung, S.; Cho, H.; Cho, S.; Kim, S.; Lee, H. Resistin is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovasc. Res. 2006, 69, 76–85. [Google Scholar] [CrossRef]
- So, W.-Y.; Kalron, A. The Association between Body Mass Index and Leisure-Time Physical Activity in Adults with Multiple Sclerosis. Int. J. Environ. Res. Public Health 2020, 17, 920. [Google Scholar] [CrossRef]
- Munger, K.L.; Bentzen, J.; Laursen, B.; Stenager, E.; Koch-Henriksen, N.; Sørensen, T.I.; Baker, J.L. Childhood body mass index and multiple sclerosis risk: A long-term cohort study. Mult. Scler. J. 2013, 19, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Nezhad, A.; Varzaneh, F.N.; Mirzaei, K.; Emamgholipour, S.; Varzaneh, F.N.; Sahraian, M.A. A polymorphism in the resistin gene promoter and the risk of multiple sclerosis. Minerva Med. 2013, 104, 431–438. [Google Scholar] [PubMed]
- Park, H.K.; Ahima, R.S. Resistin in Rodents and Humans. Diabetes Metab. J. 2013, 37, 404. [Google Scholar] [CrossRef]
- Babaesfahani, A.; Khanna, N.R.; Patel, P.; Kuns, B. Natalizumab. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534201/ (accessed on 17 August 2025).
- Rogue, A.; Spire, C.; Brun, M.; Claude, N.; Guillouzo, A. Gene Expression Changes Induced by PPAR Gamma Agonists in Animal and Human Liver. PPAR Res. 2010, 2010, 325183. [Google Scholar] [CrossRef]
- Duvanel, C.B.; Honegger, P.; Pershadsingh, H.; Feinstein, D.; Matthieu, J. Inhibition of glial cell proinflammatory activities by peroxisome proliferator-activated receptor gamma agonist confers partial protection during antimyelin oligodendrocyte glycoprotein demyelination in vitro. J. Neurosci. Res. 2003, 71, 246–255. [Google Scholar] [CrossRef]
- Shamsi, B.H.; Ma, C.; Naqvi, S.; Xiao, Y. Effects of Pioglitazone Mediated Activation of PPAR-γ on CIDEC and Obesity Related Changes in Mice. PLoS ONE 2014, 9, e106992. [Google Scholar] [CrossRef]
- Tasset, I.; Bahamonde, C.; Agüera, E.; Conde, C.; Cruz, A.H.; Pérez-Herrera, A.; Gascón, F.; Giraldo, A.I.; Ruiz, M.C.; Lillo, R.; et al. Effect of natalizumab on oxidative damage biomarkers in relapsing-remitting multiple sclerosis. Pharmacol. Rep. 2013, 65, 624–631. [Google Scholar] [CrossRef]
- Tasset Cuevas, I.; Agüera, E.; Gascón, F.; Giraldo, A.I.; Salcedo, M.; Cruz, A.H.; López, F.S.; Fiñana, I.T. Natalizumab y reducción de los niveles de proteínas carboniladas en pacientes con esclerosis múltiple. Rev. De Neurol. 2012, 54, 449. [Google Scholar] [CrossRef]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017, 13, 25–36. [Google Scholar] [CrossRef]
- Esposito, S.; Bonavita, S.; Sparaco, M.; Gallo, A.; Tedeschi, G. The role of diet in multiple sclerosis: A review. Nutr. Neurosci. 2018, 21, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Ghaffary, E.M.; Bjørklund, G.; Bhat, R.S.; Mirmosayyeb, O. Adipokines in multiple sclerosis: Immune dysregulation, neuroinflammation, and therapeutic opportunities. Autoimmun. Rev. 2025, 24, 103825. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Marrodan, M. Multiple sclerosis and obesity: The role of adipokines. Front. Immunol. 2022, 13, 1038393. [Google Scholar] [CrossRef]
- Rijnsburger, M.; Djuric, N.; Mulder, I.A.; de Vries, H.E. Adipokines as Immune Cell Modulators in Multiple Sclerosis. Int. J. Mol. Sci. 2021, 22, 10845. [Google Scholar] [CrossRef]


| RRMS | Control | p | |
|---|---|---|---|
| N | 49 | 38 | 0.32 |
| Age (years) | 36.11 ± 11.18 | 39.94 ± 12.03 | 0.14 |
| Sex (% women) | 65.31 | 76.31 | 0.23 |
| FG | NT | p | |
|---|---|---|---|
| Disease duration (years) | 7.46 ± 4.34 | 5.52 ± 3.23 | 0.10 |
| BMI (kg/m2) | 23.47 ± 2.41 | 23.93 ± 3.82 | 0.61 |
| EDSS (points) | 3.28 ± 1.03 | 3.11 ± 1.03 | 0.92 |
| ARR (N) | 0.34 ± 0.61 | 0.32 ± 0.53 | 0.21 |
| Mean number of Gd(+) lesions on brain MRI (N) | 0.11 ± 0.42 | 0.17 ± 0.71 | 0.49 |
| Mean number of T2 lesions on brain MRI (N) | 19.30 ± 1.65 | 18.52 ± 2.27 | 0.32 |
| NT | FG | Control | p | |
|---|---|---|---|---|
| N | 19 | 30 | 38 | |
| Visfatin (pg/mL) | 54.58 ± 40.26 | 56.75 ± 41.72 | 48.80 ± 31.52 | 0.961 |
| Adiponectin (ng/mL) | 9372.02 ± 4003.78 | 5141.65 ± 3362.47 | 5145.54 ± 2959.85 | 0.001 |
| Resistin (ng/mL) | 10.34 ± 6.64 | 7.68 ± 3.69 | 7.43 ± 3.08 | 0.253 |
| Group | FG | NT | Control |
|---|---|---|---|
| FG | x | p = 0.000 | NS |
| NT | p = 0.000 | x | p = 0.000 |
| Control | NS | p = 0.000 | x |
| Women with RRMS | Men with RRMS | Women —Control Group | Men —Control Group | p | |
|---|---|---|---|---|---|
| N | 32 | 17 | 29 | 9 | |
| Visfatin (pg/mL) | 54.11 ± 40.01 | 59.29 ± 43.16 | 48.68 ± 33.34 | 49.19 ± 26.52 | 0.733 |
| Adiponectin (ng/mL) | 6587.80 ± 3866.66 | 6983.77 ± 4686.89 | 5303.22 ± 2980.45 | 4637.46 ± 3007.82 | 0.397 |
| Resistin (ng/mL) | 8.46 ± 5.37 | 9.18 ± 4.82 | 7.52 ± 3.27 | 7.14 ± 2.57 | 0.447 |
| Treatment Duration (Months) | <18 | >18 | p |
|---|---|---|---|
| Visfatin (pg/mL) | 51.27 ± 43.49 | 59.12 ± 39.22 | 0.031 |
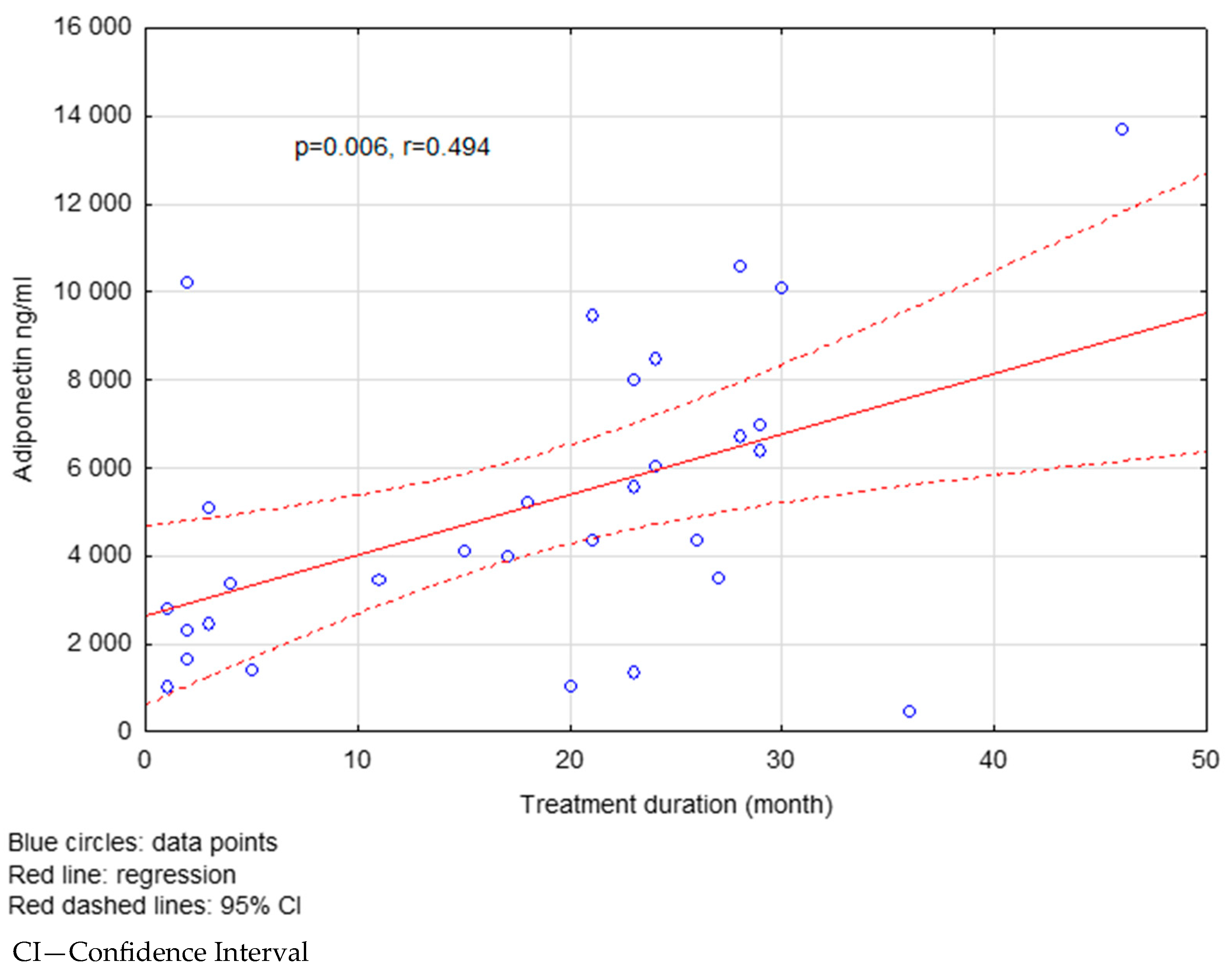
| Adiponectin (ng/mL) | 5916.72 ± 4306.89 | 7307.55 ± 3975.48 | 0.022 |
| Resistin (ng/mL) | 9.37 ± 6.50 | 8.25 ± 4.03 | 0.734 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk, B.; Morawiec, N.; Kwinta, R.; Rakoca, M.; Wawrzyniak, S.; Zalejska-Fiolka, J.; Sowa, A.; Sawa, K.; Adamczyk-Sowa, M. Evaluation of Selected Serum Adipocytokines in Patients with Relapsing–Remitting Multiple Sclerosis Treated with Immunomodulatory Second-Line Drugs. Int. J. Mol. Sci. 2025, 26, 8070. https://doi.org/10.3390/ijms26168070
Adamczyk B, Morawiec N, Kwinta R, Rakoca M, Wawrzyniak S, Zalejska-Fiolka J, Sowa A, Sawa K, Adamczyk-Sowa M. Evaluation of Selected Serum Adipocytokines in Patients with Relapsing–Remitting Multiple Sclerosis Treated with Immunomodulatory Second-Line Drugs. International Journal of Molecular Sciences. 2025; 26(16):8070. https://doi.org/10.3390/ijms26168070
Chicago/Turabian StyleAdamczyk, Bożena, Natalia Morawiec, Robert Kwinta, Michał Rakoca, Sławomir Wawrzyniak, Jolanta Zalejska-Fiolka, Agata Sowa, Ksawier Sawa, and Monika Adamczyk-Sowa. 2025. "Evaluation of Selected Serum Adipocytokines in Patients with Relapsing–Remitting Multiple Sclerosis Treated with Immunomodulatory Second-Line Drugs" International Journal of Molecular Sciences 26, no. 16: 8070. https://doi.org/10.3390/ijms26168070
APA StyleAdamczyk, B., Morawiec, N., Kwinta, R., Rakoca, M., Wawrzyniak, S., Zalejska-Fiolka, J., Sowa, A., Sawa, K., & Adamczyk-Sowa, M. (2025). Evaluation of Selected Serum Adipocytokines in Patients with Relapsing–Remitting Multiple Sclerosis Treated with Immunomodulatory Second-Line Drugs. International Journal of Molecular Sciences, 26(16), 8070. https://doi.org/10.3390/ijms26168070







