Identification of Bioactive Compounds in Warburgia salutaris Leaf Extracts and Their Pro-Apoptotic Effects on MCF-7 Breast Cancer Cells
Abstract
1. Introduction
2. Results
2.1. GC-MS Analysis
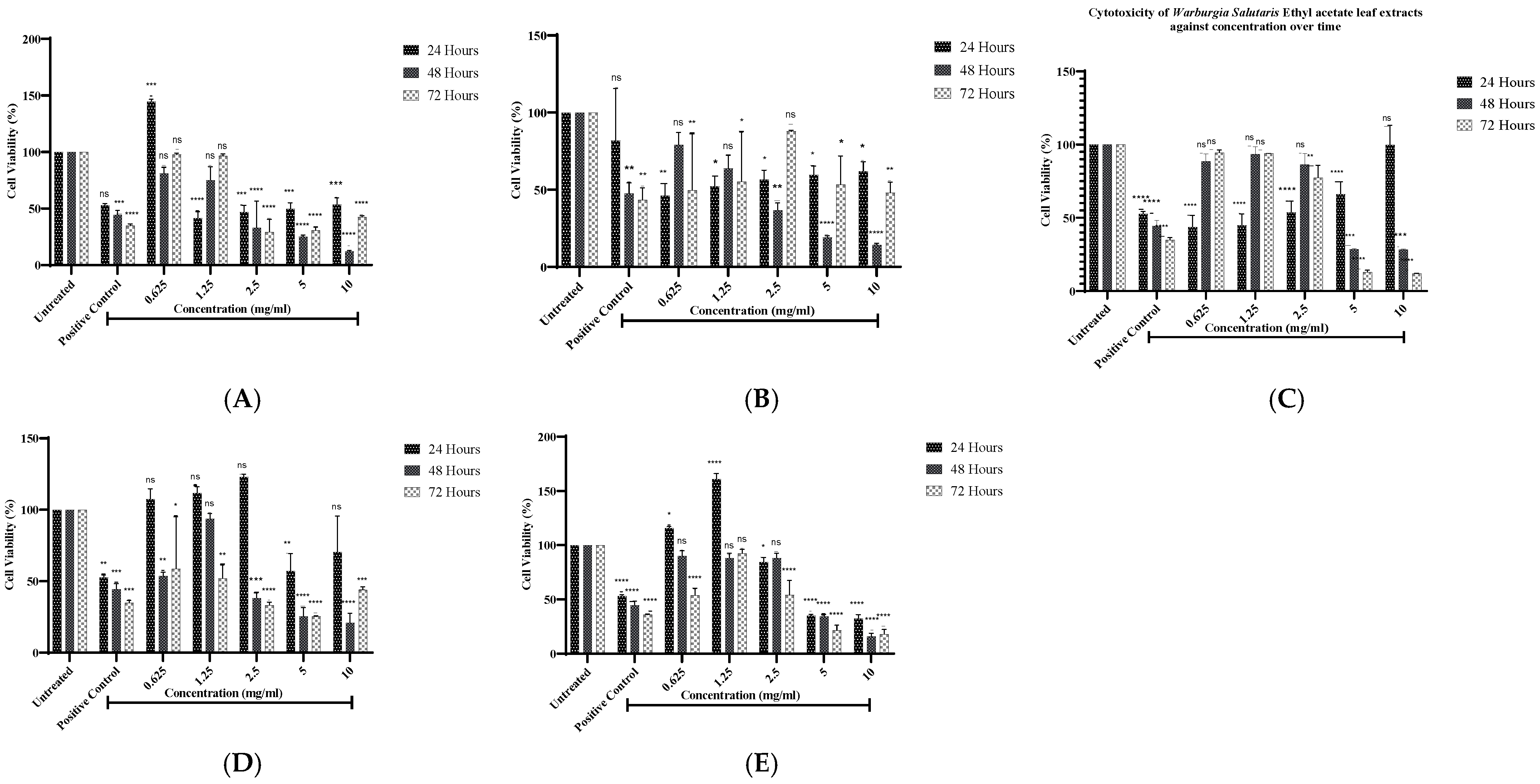
2.2. Cell Viability Analysis of Warburgia salutaris Leaf Extracts of Mcf-7 Cells
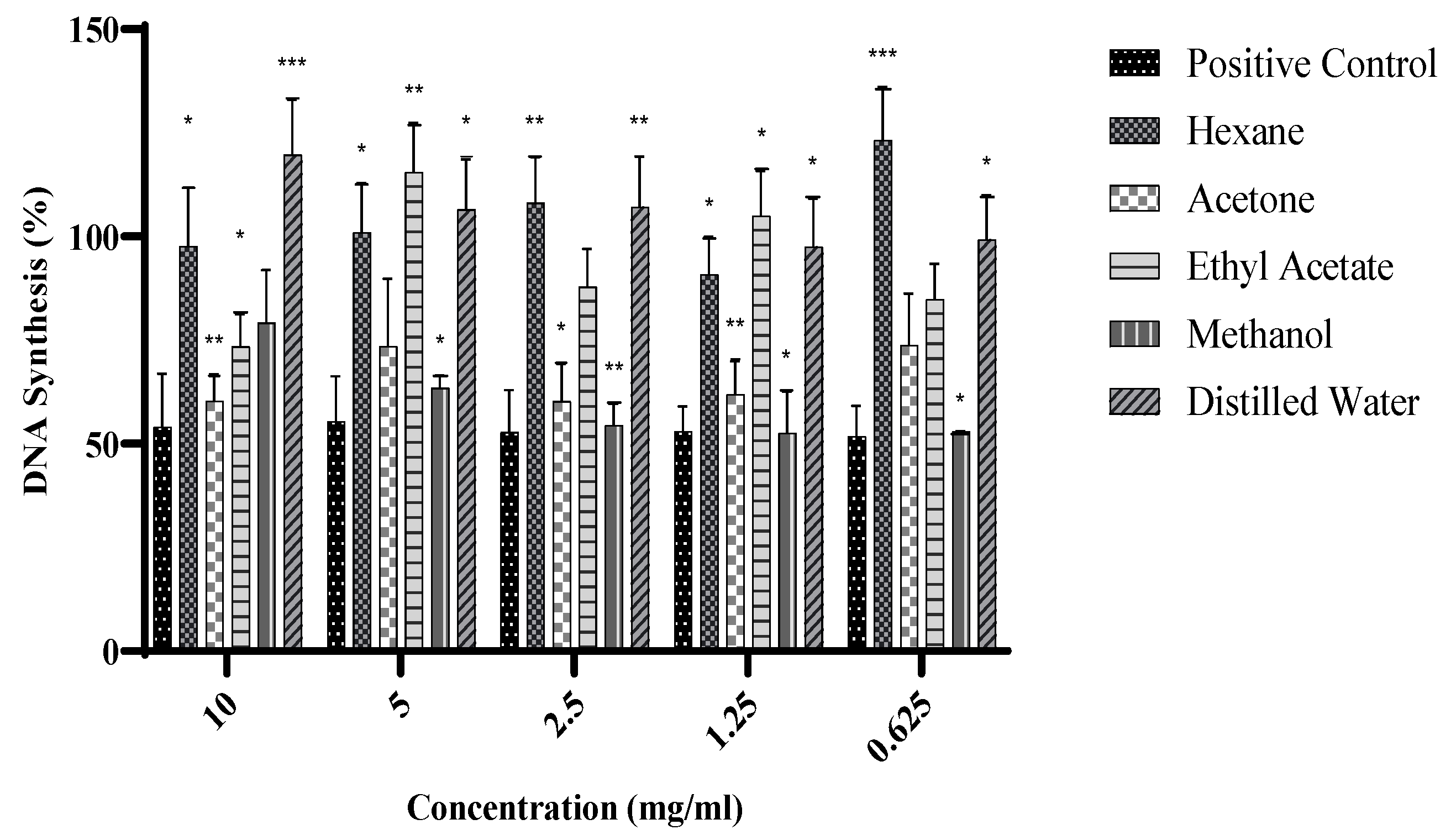
2.3. DNA Synthesis of Warburgia salutaris Leaf Extracts on MCF-7 Cells
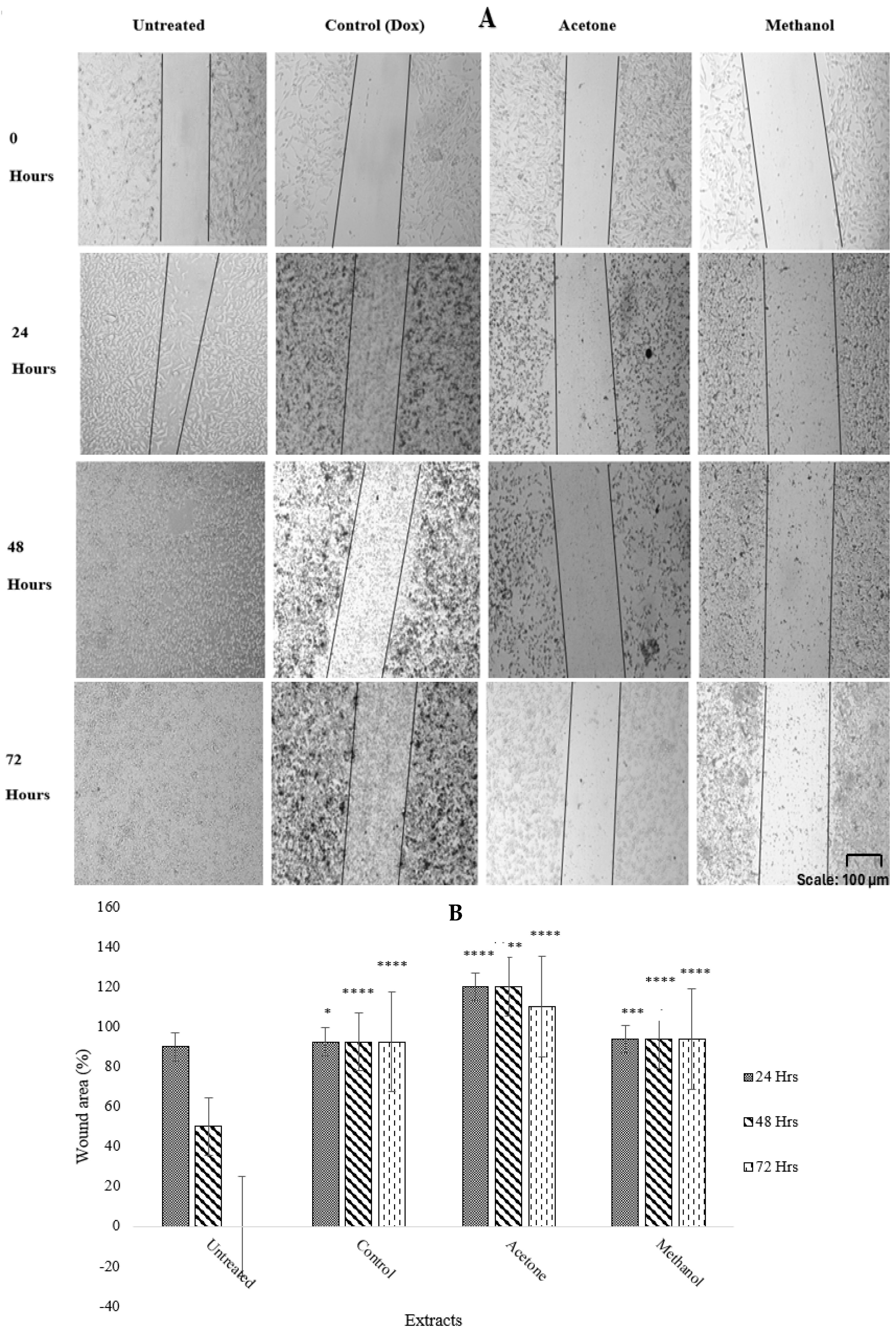
2.4. Antimetastatic Effects of Warburgia salutaris Leaf Extracts on MCF-7 Cells
2.5. Morphological Analysis of MCF-7 Cells Treated with W. salutaris
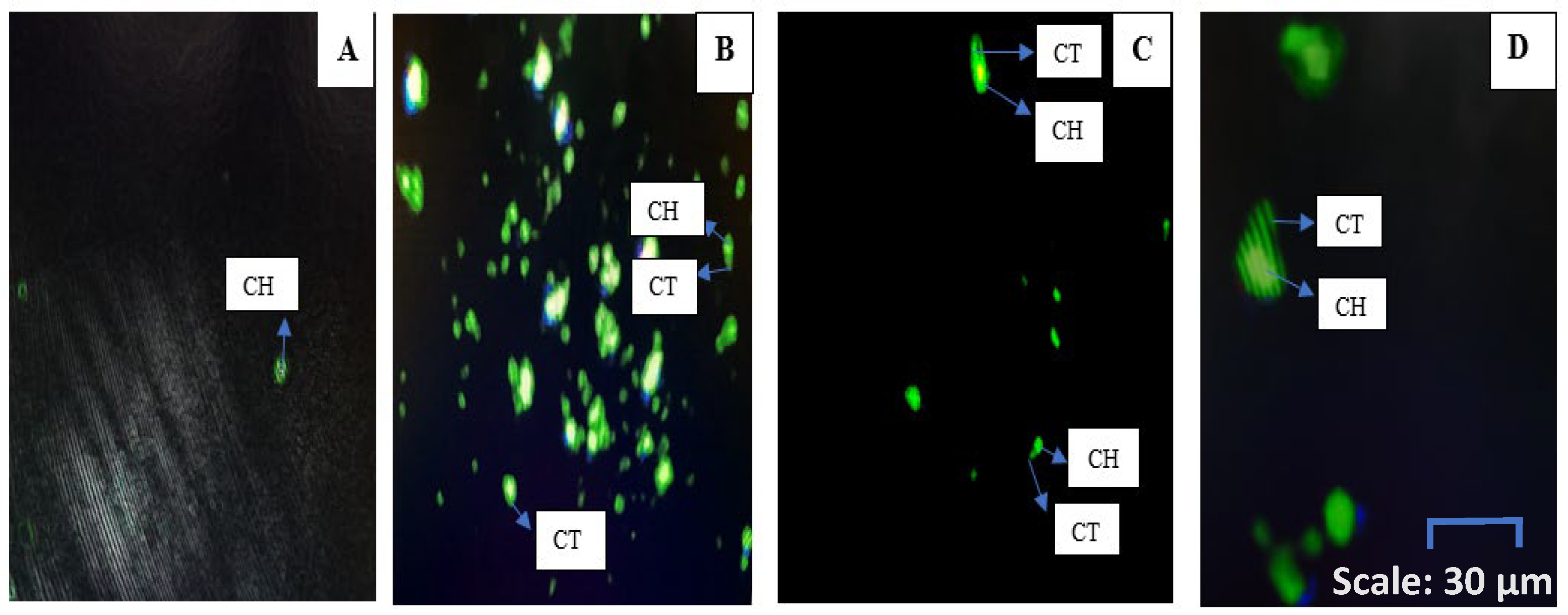
2.6. DNA Damage Analysis of MCF-7 Cells Treated with Warburgia salutaris Leaf Extracts
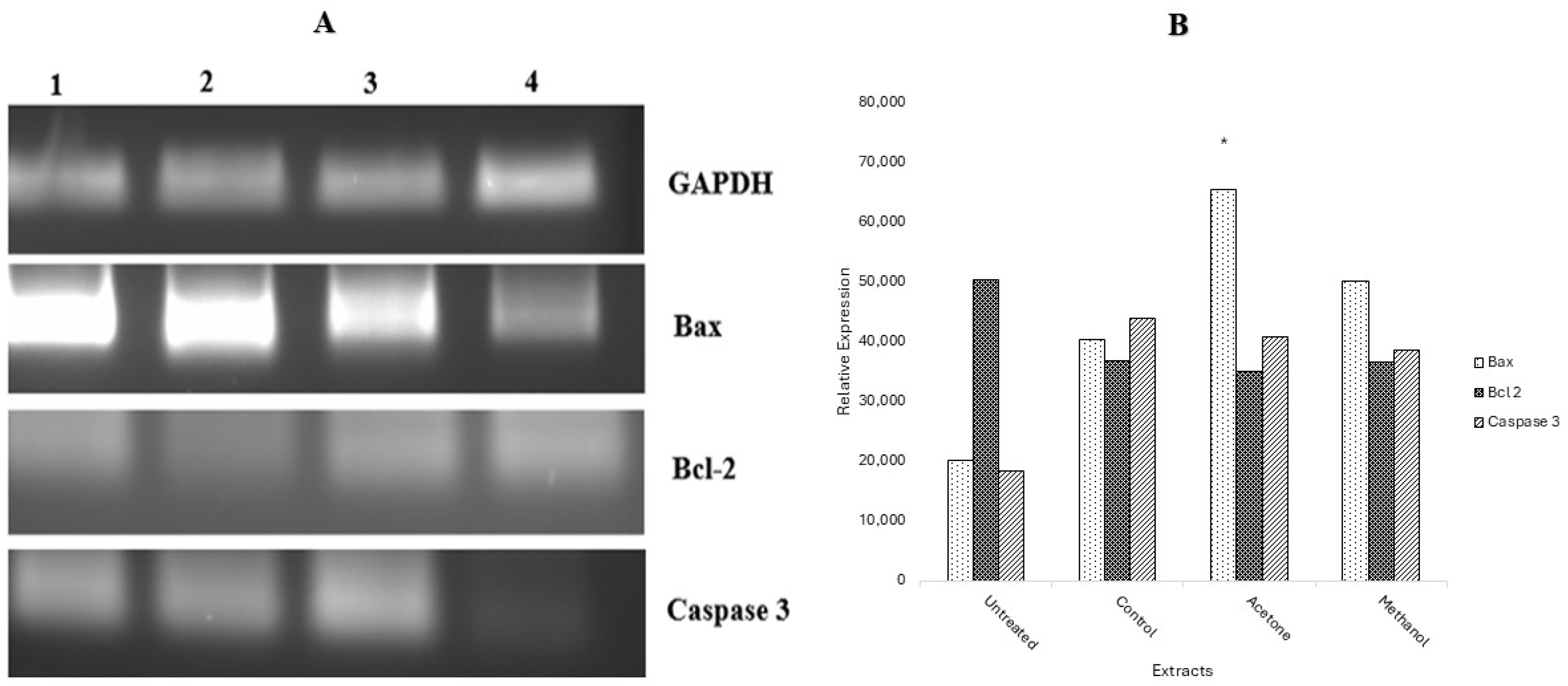
2.7. Gene Expression by Reverse Transcription PCR (RT-PCR)
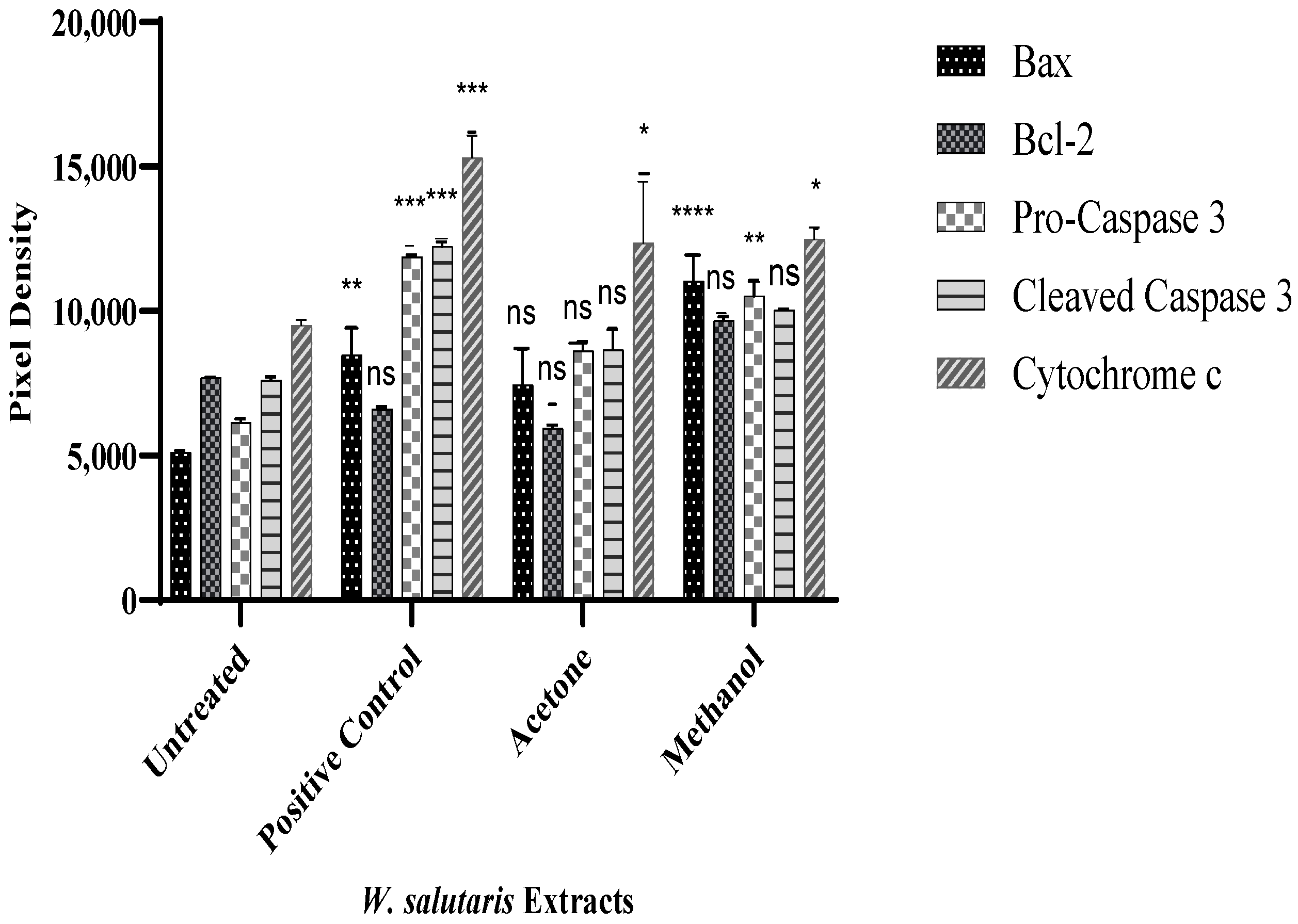
2.8. Protein Expression by Human Apoptotic Profiler Assay
3. Discussion
4. Materials and Methods
4.1. Gas Chromatography-Mass Spectrometry Analysis
4.2. Cell Culture
4.2.1. Cell Viability Analysis
4.2.2. DNA Synthesis Analysis Using BrdU Assay Kit
4.2.3. Antimetastatic Analysis Using Scratch Assay
4.2.4. Apoptotic Morphological Analysis Using DAPI/PI Staining
4.2.5. DNA Damage Analysis by Comet Assay
4.2.6. Gene Expression by Polymerase Chain Reaction (PCR)
RNA Isolation
First Strand cDNA Synthesis
Polymerase Chain Reaction (PCR)
4.2.7. Protein Expression by Human Apoptotic Proteome Profiler
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFRO | African Regional Office |
| BrdU | 5-Bromo-2′-Deoxyuridine |
| Bax | B-cell Lymphoma 2-Associated X |
| Bcl-2 | B-cell Lymphoma 2 |
| CANSA | South African Cancer Association |
| CASPASE | Cysteine-Aspartic Proteases |
| cDNA | Complementary Deoxyribonucleic Acid |
| DAPI | 4′, 6 Diamidino-2-phenylindoleDD: Death Domain |
| DMSO | Dimethyl Sulfoxide |
| DNA | Deoxyribonucleic Acid |
| FBS | Foetal Bovine Serum |
| GLOBOCAN | Global Burden of Diseases and Cancer Collaboration |
| IARC | International Agency for Research on Cancer |
| IC50 | Half-maxima Inhibitory Concentration |
| MTT | 3-(4,5- dimethylthiazol-2-yl)-2,5 diphenyl-2H-tetrazolium bromide |
| NCI | National Cancer Institute |
| PBS | Phosphate-Buffered Saline |
| PI | Propidium Iodide |
| RT-PCR | Reverse-Transcription Polymerase Chain Reaction |
| SANBI | South African National Biodiversity Institute |
| TBE | Tris-Borate-EDTA |
| TMB | 3,3′,5,5′- Tetramethylbenzidine |
| WHO | World Health Organization |
| WHO-AFRO | World Health Organization African Regional Office |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Twilley, D.; Rademan, S.; Lall, N. A review on traditionally used South African medicinal plants, their secondary metabolites and their potential development into anticancer agents. J. Ethnopharmacol. 2020, 261, 113101. [Google Scholar] [CrossRef]
- Soyingbe, O.S.; Mongalo, N.I.; Makhafola, T.J. In vitro antibacterial and cytotoxic activity of leaf extracts of Centella asiatica (L.) Urb, Warburgia salutaris (Bertol. F.) Chiov and Curtisia dentata (Burm. F.) CA Sm-medicinal plants used in South Africa. BMC Complement. Altern. Med. 2018, 18, 315. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Raphela-Choma, P.P.; Simelane, M.B.; Choene, M.S. Evaluation of the antiproliferative effect of Iso-mukaadial acetate on breast and ovarian cancer cells. Adv. Tradit. Med. 2023, 23, 251–260. [Google Scholar] [CrossRef]
- Luo, H.; Zhao, Q.; Wei, W.; Zheng, L.; Yi, S.; Li, G.; Wang, W.; Sheng, H.; Pu, H.; Mo, H.; et al. Circulating tumor DNA methylation profiles enable early diagnosis, prognosis prediction, and screening for colorectal cancer. Sci. Transl. Med. 2020, 12, eaax7533, Erratum in Sci. Transl. Med. 2020, 12, eabc1078. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 1941–1953. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603. [Google Scholar] [CrossRef]
- Abaza, A.; Vasavada, A.M.; Sadhu, A.; Valencia, C.; Fatima, H.; Nwankwo, I.; Anam, M.; Maharjan, S.; Amjad, Z.; Khan, S. A Systematic Review of Apoptosis in Correlation with Cancer: Should Apoptosis Be the Ultimate Target for Cancer Treatment? Cureus 2022, 14, e28496. [Google Scholar] [CrossRef] [PubMed]
- Drewes, S.E.; Crouch, N.R.; Mashimbye, M.J.; De Leeuw, B.M.; Horn, M.M. A phytochemical basis for the potential use of Warburgia salutaris (pepper-bark tree) leaves in the place of bark. S. Afr. J. Sci. 2001, 97, 383–386. [Google Scholar]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Ashok, K.; Babu, M. Improved micro propagation of Vigna radiata and anticancer activity of in vitro raised plant extract against human breast cancer cell line (MCF-7). Malaya J. Mat. 2020, S, 4501–4508. [Google Scholar]
- Mou, L.; Liang, B.; Liu, G.; Jiang, J.; Liu, J.; Zhou, B.; Huang, J.; Zang, N.; Liao, Y.; Ye, L.; et al. Berbamine exerts anticancer effects on human colon cancer cells via induction of autophagy and apoptosis, inhibition of cell migration and MEK/ERK signalling pathway. J BUON 2019, 24, 1870–1875. Available online: https://pubmed.ncbi.nlm.nih.gov/31786849/ (accessed on 11 February 2023).
- Pender, G.C.; Guyah, B.; Mwitari, P.G.; Jepkorir, M.; Lagu, I.J.L.; Ombaka, J. In silico and in vitro evaluation of compounds from Methanol whole extracts of Solanum aculeastrum Dunal berries against benign prostatic hyperplasia and prostate cancer. Int. J. Sci. Res. Arch. 2024, 13, 193–217. [Google Scholar] [CrossRef]
- Sul‘ain, M.D.; Zakaria, F.; Johan, M.F. Anti-proliferative effects of methanol and water extracts of Pyrrosia piloselloides on the hela human cervical carcinoma cell line. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, D.H.; Han, S.I.; Go, B.; Oh, U.H.; Kim, C.S.; Jung, Y.H.; Lee, J.; Kim, J.H. 2-methoxy-4-vinylphenol attenuates migration of human pancreatic cancer cells via blockade of FAK and AKT signaling. Anticancer Res. 2019, 39, 6685–6691. [Google Scholar] [CrossRef] [PubMed]
- Sayed, E.M.; Bakhite, E.A.; Hassanien, R.; Farhan, N.; Aly, H.F.; Morsy, S.G.; Hassan, N.A. Novel tetrahydroisoquinolines as DHFR and CDK2 inhibitors: Synthesis, characterization, anticancer activity and antioxidant properties. BMC Chem. 2024, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Vlad, P.; Gorincioi, E.; Aricu, A.; Barba, A.; Manzocchi, A.; Santaniello, E. Asymmetric dihydroxylation of drim-7-en-11-ol: Synthesis of diastereomerically pure driman-7α, 8α, 11-triol and its elaboration into novel chlorinated norlabdanic compounds. Tetrahedron Asymmetry 2010, 21, 2108–2116. [Google Scholar] [CrossRef]
- Chandan, G.; Kumar, C.; Verma, M.K.; Satti, N.K.; Saini, A.K.; Saini, R.V. Datura stramonium essential oil composition and it’s immunostimulatory potential against colon cancer cells. 3 Biotech 2020, 10, 451. [Google Scholar] [CrossRef]
- Raina, S.; Sharma, V.; Sheikh, Z.N.; Kour, N.; Singh, S.K.; Zari, A.; Zari, T.A.; Alharby, H.F.; Hakeem, K.R. Anticancer activity of cordia dichotoma against a panel of human cancer cell lines and their phytochemical profiling via HPLC and GCMS. Molecules 2022, 27, 2185. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.J.; De Groot, A. Occurrence, biological activity and synthesis of drimane sesquiterpenoids. Nat. Prod. Rep. 2004, 21, 449–477. [Google Scholar] [CrossRef]
- Bayala, B.; Bassole, I.H.; Maqdasy, S.; Baron, S.; Simpore, J.; Lobaccaro, J.M. Cymbopogon citratus and Cymbopogon giganteus essential oils have cytotoxic effects on tumor cell cultures. Identification of citral as a new putative anti-proliferative molecule. Biochimie 2018, 153, 162–170. [Google Scholar] [CrossRef]
- Lagos-Cabré, R.; Moreno, R.D. Contribution of environmental pollutants to male infertily: A working model of germ cell apoptosis induced by plasticizers. Biol. Res. 2012, 45, 5–14. [Google Scholar] [CrossRef]
- Maione, F.; Russo, R.; Khan, H.; Mascolo, N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2016, 30, 1343–1352. [Google Scholar] [CrossRef]
- Smolarek, A.K.; Suh, N. Chemopreventive activity of vitamin E in breast cancer: A focus on γ-and δ-tocopherol. Nutrients 2011, 3, 962–986. [Google Scholar] [CrossRef]
- Shiozaki, M.; Tashiro, T.; Koshino, H.; Nakagawa, R.; Inoue, S.; Shigeura, T.; Watarai, H.; Taniguchi, M.; Mori, K. Synthesis and biological activity of ester and ether analogues of α-galactosylceramide (KRN7000). Carbohydr. Res. 2010, 345, 1663–1684. [Google Scholar] [CrossRef]
- Nkqenkqa, V.; Mundembe, R. Warburgia salutaris Metabolites of Medicinal Value—A Review. Malays. J. Sci. Adv. Technol. 2023, 3, 244–254. [Google Scholar] [CrossRef]
- Moustafa, M.F.; Alrumman, S.A. First report about pharmaceutical properties and phytochemicals analysis of Rosa abyssinica R. Br. ex Lindl. (Rosaceae). Pak. J. Pharm. Sci. 2015, 28, 2009. Available online: https://research.ebsco.com/linkprocessor/plink?id=2cf3ab09-42ba-36ce-99fb-5a3f597739c8 (accessed on 20 September 2023). [PubMed]
- Potikha, L.; Brovarets, V.; Zhirnov, V. Biological Evaluation of 3-Aminoisoquinolin-1 (2H)-one Derivatives as Potential Anticancer agents. Fr.-Ukr. J. Chem. 2021, 9, 52–63. [Google Scholar] [CrossRef]
- Mohareb, R.M.; Al Farouk, F.O.; Wardakhan, W.W. Uses of dimedone for the synthesis of new heterocyclic derivatives with anti-tumor, c-Met, tyrosine, and Pim-1 kinases inhibitions. Med. Chem. Res. 2018, 27, 1984–2003. [Google Scholar] [CrossRef]
- Ramadhani, N.T.; Handayani, W.; Yasman, Y.; Putrika, A. Induction of in vitro shoots in Liverwort Acrolejeunea fertilis (Reinw., Blume & Nees) Schiffn. Gametophyte explants and their comparative metabolite and Bioactivity Analysis. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 158, 10. [Google Scholar] [CrossRef]
- Sajitha Menon, K.; Thoppil, J.E. Metabolite Profiling of Bioactive Compounds in Selected Species of Gomphostemma Wall. ex Benth. from the Western Ghats by GC-MS Analysis. In Natural Product Experiments in Drug Discovery; Springer: New York, NY, USA, 2022; pp. 97–107. [Google Scholar]
- Sangpairoj, K.; Settacomkul, R.; Siangcham, T.; Meemon, K.; Niamnont, N.; Sornkaew, N.; Tamtin, M.; Sobhon, P.; Vivithanaporn, P. Hexadecanoic acid-enriched extract of Halymenia durvillei induces apoptotic and autophagic death of human triple-negative breast cancer cells by upregulating ER stress. Asian Pac. J. Trop. Biomed. 2022, 12, 132–140. [Google Scholar] [CrossRef]
- Khan, A.Y.F.; Wahab, R.A.; Ahmed, Q.U.; Khatib, A.; Ibrahim, Z.; Nipun, T.S.; Nawawi, H.; Azmi, S.N.H.; Sarker, Z.I.; Zakaria, Z.A.; et al. Potential anticancer agents identification of Hystrix brachyura bezoar through gas chromatography-mass spectrometry-based metabolomics and protein-ligand interaction with molecular docking analyses. J. King Saud Univ.-Sci. 2023, 35, 102727. [Google Scholar] [CrossRef]
- Yang, Z.; Chan, K.W.; Abu Bakar, Z.; Deng, X. Unveiling drimenol: A phytochemical with multifaceted bioactivities. Plants 2024, 13, 2492. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K. Anticancer effect of lemongrass oil and citral on cervical cancer cell lines. Pharmacogn. Commun. 2013, 3, 41–48. [Google Scholar]
- Javed, M.R.; Salman, M.; Tariq, A.; Tawab, A.; Zahoor, M.K.; Naheed, S.; Shahid, M.; Ijaz, A.; Ali, H. The antibacterial and larvicidal potential of bis-(2-ethylhexyl) phthalate from Lactiplantibacillus plantarum. Molecules 2022, 27, 7220. [Google Scholar] [CrossRef]
- El-Naggar, N.E.; Soliman, H.M.; El-Shweihy, N.M. Extracellular cholesterol oxidase production by Streptomyces aegyptia, in vitro anticancer activities against rhabdomyosarcoma, breast cancer cell-lines and in vivo apoptosis. Sci. Rep. 2018, 8, 2706. [Google Scholar] [CrossRef]
- Zulkapli, R.; Razak, F.A.; Zain, R.B. Vitamin E (α-Tocopherol) exhibits antitumour activity on Oral squamous carcinoma cells ORL-48. Integr. Cancer Ther. 2017, 16, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Pani, S.; Mohapatra, S.; Sahoo, A.; Baral, B.; Debata, P.R. Shifting of cell cycle arrest from the S-phase to G2/M phase and downregulation of EGFR expression by phytochemical combinations in HeLa cervical cancer cells. J. Biochem. Mol. Toxicol. 2022, 36, e22947. [Google Scholar] [CrossRef]
- Wang, C.; Kurzer, M.S. Phytoestrogen concentration determines effects on DNA synthesis in human breast cancer cells. Nutr. Cancer 1997, 28, 236–247. [Google Scholar] [CrossRef]
- Serala, K.; Steenkamp, P.; Mampuru, L.; Prince, S.; Poopedi, K.; Mbazima, V. In vitro antimetastatic activity of Momordica balsamina crude acetone extract in HT-29 human colon cancer cells. Environ. Toxicol. 2021, 36, 2196–2205. [Google Scholar] [CrossRef]
- Rönnberg, H. Signal transduction inhibitors. In Therapeutic Strategies in Veterinary Oncology; CABI: Wallingford, UK, 2023; pp. 89–110. [Google Scholar] [CrossRef]
- Sultan, M.H.; Zuwaiel, A.A.; Moni, S.S.; Alshahrani, S.; Alqahtani, S.S.; Madkhali, O.; Elmobark, M.E. Bioactive Principles and Potentiality of Hot Methanolic Extract of the Leaves from Artemisia absinthium L. “in vitro Cytotoxicity Against Human MCF-7 Breast Cancer Cells, Antibacterial Study and Wound Healing Activity”. Curr. Pharm. Biotechnol. 2020, 21, 1711–1721. [Google Scholar] [CrossRef]
- Mabasa, R.; Malemela, K.; Serala, K.; Kgakishe, M.; Matsebatlela, T.; Mokgotho, M.; Mbazima, V. Ricinus communis Butanol Fraction Inhibits MCF-7 Breast Cancer Cell Migration, Adhesion, and Invasiveness. Integr. Cancer Ther. 2021, 20, 1534735420977684. [Google Scholar] [CrossRef]
- Abdul, W.M.; Hajrah, N.H.; Sabir, J.S.; Al-Garni, S.M.; Sabir, M.J.; Kabli, S.; Saini, K.S.; Bora, R.S. Therapeutic role of Ricinus communis L. and its bioactive compounds in disease prevention and treatment. Asian Pac. J. Trop. Med. 2018, 11, 177–185. [Google Scholar] [CrossRef]
- Meirson, T.; Gil-Henn, H.; Samson, A.O. Invasion and metastasis: The elusive hallmark of cancer. Oncogene 2020, 39, 2024–2026. [Google Scholar] [CrossRef] [PubMed]
- Ulukaya, E.; Acilan, C.; Ari, F.; Ikitimur, E.; Yilmaz, Y. A Glance at the methods for detection of apoptosis qualitatively and quantitatively. Turk. J. Biochem. Turk Biyokim. Derg. 2011, 36, 261–269. Available online: https://www.academia.edu/download/70234763/Technical_Review_Metod_Derlemesi20210925-26817-3mrpza.pdf (accessed on 20 February 2024).
- Kari, S.; Subramanian, K.; Altomonte, I.A.; Murugesan, A.; Yli-Harja, O.; Kandhavelu, M. Programmed cell death detection methods: A systematic review and a categorical comparison. Apoptosis 2022, 27, 482–508. [Google Scholar] [CrossRef] [PubMed]
- Kntayya, S.B.; Ibrahim, M.D.; Ain, N.M.; Iori, R.; Ioannides, C.; Abdull Razis, A.F. Induction of apoptosis and cytotoxicity by isothiocyanate sulforaphene in human hepatocarcinoma HepG2 cells. Nutrients 2018, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Khumalo, G.P.; Sadgrove, N.J.; Van Vuuren, S.F.; Van Wyk, B.E. South Africa’s best bark medicines prescribed at the Johannesburg muthi markets for skin, gut, and lung infections: Mic’s and brine shrimp lethality. Antibiotics 2021, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef]
- Khumalo, G.P.; Van Wyk, B.E.; Feng, Y.; Cock, I.E. Immunomodulatory and cytotoxicity properties of selected southern African medicinal plants traditionally used to treat pain and inflammation. S. Afr. J. Bot. 2023, 159, 146–154. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Yang, C. Evaluating in vitro DNA damage using comet assay. JoVE (J. Vis. Exp.) 2017, 128, e56450. [Google Scholar]
- Cordelli, E.; Bignami, M.; Pacchierotti, F. Comet assay: A versatile but complex tool in genotoxicity testing. Toxicol. Res. 2021, 10, 68–78. [Google Scholar] [CrossRef]
- Maruma, L.N.; Somboro, A.M.; Amoako, D.G.; Khumalo, H.M.; Khan, R.B. Molecular mechanisms underlying Warburgia salutaris effects on 1 oxidative stress and apoptotic parameters in Human Hepatoma Cells. BioRxiv 2022. [Google Scholar] [CrossRef]
- Papaliagkas, V.; Anogianaki, A.; Anogianakis, G.; Ilonidis, G. The proteins and the mechanisms of apoptosis: A mini review of the fundamentals. Hippokratia 2007, 11, 108. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2658792/ (accessed on 10 March 2024).
- Maroyi, A. The genus Warburgia: A review of its traditional uses and pharmacology. Pharm. Biol. 2014, 52, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. In Cancer Cell Culture: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2011; pp. 237–245. [Google Scholar] [CrossRef]
- Ghasemi, A.; Khanzadeh, T.; Heydarabad, M.Z.; Khorrami, A.; Esfahlan, A.J.; Ghavipanjeh, S.; Belverdi, M.G.; Fikouhi, S.D.; Darbin, A.; Najafpour, M.; et al. Evaluation of BAX and BCL-2 gene expression and apoptosis induction in acute lymphoblastic leukemia cell line CCRF-CEM after high-dose prednisolone treatment. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 2319. [Google Scholar] [PubMed]
- Khanzadeh, T.; Hagh, M.F.; Talebi, M.; Yousefi, B.; Azimi, A.; Baradaran, B. Investigation of BAX and BCL2 expression and apoptosis in a resveratrol- and prednisolone-treated human T-ALL cell line, CCRF-CEM. Blood Res. 2018, 53, 53. [Google Scholar] [CrossRef] [PubMed]
- Sangour, M.H.; Ali, I.M.; Atwan, Z.W.; Al Ali, A.A. Effect of Ag nanoparticles on viability of MCF-7 and Vero cell lines and gene expression of apoptotic genes. Egypt. J. Med. Hum. Genet. 2021, 22, 9. [Google Scholar] [CrossRef]

| Peak No. | Retention Time (min) | Compound Name | Molecular Weight (g/mol) | Molecular Formula | Peak Area (%) |
|---|---|---|---|---|---|
| 1 | 1.649 | Carbon dioxide | 44.009 | CO2 | 0.36 |
| 2 | 2.072 | Methylene chloride | 84.93 | CH2Cl2 | 22.40 |
| 3 | 3.670 | 1-Nitro-2-acetamido-1,2-dideoxy-D-glucitol | 252.22 | C8H16O7 | 0.08 |
| 4 | 4.004 | N-Methoxy-N-methylacetamide | 103.12 | C4H9NO2 | 0.06 |
| 5 | 4.331 | 6,6,7,7,8,8,8-Heptafluoro-5,5-bis(trifluoromethyl)oct-1-ene | 374.15 | C10H7F13 | 0.06 |
| 6 | 5.197 | 2-Chloropent-4-ene carboxylic acid, methyl ester | 148.6 | C6H9ClO2 | 0.22 |
| 7 | 5.753 | Dimethyl sulfoxide | 78.13 | C2H6OS | 0.11 |
| 8 | 6.119 | Cyclopentane, 2-ethylidene-1,1-dimethyl- | 124.23 | C9H16 | 0.15 |
| 9 | 6.549 | Butanoic acid, 2,2-dimethyl-3-oxo-, methyl ester | 130.14 | C6H10O3 | 0.12 |
| 10 | 6.903 | 2-Pentanone, 4-hydroxy- | 102.13 | C5H10O2 | 0.19 |
| 11 | 7.494 | Oxepine, 2,7-dimethyl- | 122.17 | C8H10O | 0.13 |
| 12 | 7.820 | 2(5H)-Furanone, 5,5-dimethyl- | 112.13 | C6H8O2 | 0.07 |
| 13 | 8.342 | Hexanoic acid, 1-methylethyl ester | 158.24 | C9H18O2 | 0.23 |
| 14 | 8.564 | 2-Cyclopropyl-2-propanol | 100.16 | C6H12O | 0.11 |
| 15 | 8.994 | Butanoic acid, 3-hydroxy- | 104.11 | C4H8O3 | 0.25 |
| 16 | 9.282 | Hexanal dimethyl acetal | 146.23 | C8H18O2 | 0.41 |
| 17 | 9.858 | D-Arabinitol | 152.15 | C5H12O5 | 0.64 |
| 18 | 10.289 | Octadecyl propyl ether | 312.58 | C21H44O | 0.29 |
| 19 | 10.537 | 4-Bromo-2-methylpent-2-enoic acid, methyl ester | 193.04 | C6H9BrO2 | 0.32 |
| 20 | 11.043 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 144.13 | C6H8O4 | 0.39 |
| … | … | … | … | … | … |
| 41 | 21.117 | n-Hexadecanoic acid (Palmitic acid) | 256.43 | C16H32O2 | 9.95 |
| 42 | 21.846 | Isolongifolol | 222.37 | C15H26O | 1.79 |
| 43 | 22.211 | 2,4-Pentanedione, 3-(phenylmethyl)- | 190.24 | C12H14O2 | 3.34 |
| 44 | 22.665 | Cyclopropane, 1,1-dimethyl-2-(2-methyl-1-propenyl)- | 124.22 | C9H16 | 4.31 |
| 45 | 23.266 | Cycloisolongifolene, 9,10-dehydro- | 202.33 | C15H22 | 2.26 |
| 46 | 23.592 | 2,4-Heptadiene, 2,6-dimethyl- | 124.23 | C9H16 | 1.83 |
| 47 | 24.104 | Cyclohexane, 3,3,5-trimethyl- | 126.24 | C9H18 | 1.05 |
| 48 | 24.503 | 2-Dodecen-1-yl (-) succinic anhydride | 266.38 | C16H26O3 | 1.10 |
| 49 | 25.051 | Neral | 152.24 | C10H16O | 1.68 |
| 50 | 25.731 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 330.51 | C19H38O4 | 1.18 |
| 51 | 26.040 | Bis(2-ethylhexyl) phthalate | 390.56 | C24H38O4 | 0.68 |
| 52 | 26.319 | Docosane | 310.61 | C22H46 | 0.59 |
| 53 | 26.684 | 4-(2,2-Dimethyl-6-methylenecyclohexyl) butanal | 194.32 | C13H22O | 0.88 |
| 54 | 27.195 | Heptacosane | 380.75 | C27H56 | 0.51 |
| 55 | 27.475 | (E)-15,16-Dinorlabda-8(17),11-dien-13-one | 288.47 | C20H32O | 0.49 |
| … | … | … | … | … | … |
| 70 | 35.637 | Campesterol | 400.07 | C28H48O | 0.05 |
| Peak No. | Retention Time (min) | Compound Name | Molecular Weight | Molecular Formula | Peak Area (%) | Reported Activity | References |
|---|---|---|---|---|---|---|---|
| 17 | 9.858 | D-Arabinitol | 152.15 | C5H12O5 | 0.64 | Anti-proliferative (via oxidative stress induction in cancer cells) | [15] |
| 20 | 11.043 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 144.13 | C6H8O4 | 0.39 | Cytotoxic to tumour cells (analogues inhibit cell cycle progression) | [16] |
| 26 | 13.476 | 2-Methoxy-4-vinylphenol | 150.18 | C9H10O2 | 1.35 | Antitumor (induces apoptosis in leukaemia cells) | [17] |
| 34 | 17.409 | 3(2H)-Isoquinolinone, octahydro- | 153.23 | C9H15NO | 4.37 | Antiproliferative (binds to DNA in cancer cells) | [18] |
| 37 | 18.869 | Drim-7-en-11-ol | 222.37 | C15H26O | 2.40 | Cytotoxic to breast cancer cells (MCF-7) | [19] |
| 39 | 19.667 | Neophytadiene | 278.05 | C20H38 | 3.10 | Anti-proliferative (induces apoptosis in colon cancer) | [20] |
| 41 | 21.117 | n-Hexadecanoic acid (Palmitic acid) | 256.43 | C16H32O2 | 9.95 | Anti-tumour (modulates lipid metabolism in cancer cells) | [21] |
| 42 | 21.846 | Isolongifolol | 222.37 | C15H26O | 1.79 | Suppresses metastasis in melanoma | [16] |
| 44 | 22.665 | Drimenin (from Drim-derivatives) | 234.33 | C15H22O2 | 4.31 | Potent cytotoxic activity against HeLa cells | [22] |
| 49 | 25.051 | Neral (Citral isomer) | 152.24 | C10H16O | 1.68 | Anti-proliferative (via ROS generation) | [23] |
| 51 | 26.040 | Bis(2-ethylhexyl) phthalate | 390.56 | C24H38O4 | 0.68 | Controversial (some studies show anticancer effects at low doses) | [24] |
| 62 | 31.635 | Cholesterol epoxide | 402.66 | C27H46O2 | 0.21 | Anti-angiogenic (blocks tumour vascularization) | [25] |
| 66 | 33.458 | Vitamin E (α-Tocopherol) | 430.72 | C29H50O2 | 0.30 | Chemo preventive (reduces oxidative stress in tumours) | [26] |
| Template | 2 µL |
| Random Hexamer Primer | 1 µL |
| Nuclease-free Water | 9 µL |
| 5× Reaction Buffer | 4 µL |
| RiboLock RNase Inhibitor | 1 µL |
| 10 mM dNTP | 2 µL |
| RevertAid M-MuLV RT (200 U/µL) | 1 µL |
| Total Volume | 20 µL |
| Gene | Primer Sequences |
|---|---|
| Bax | Forward: 5’TCCCCCCAGAGGTCTTTT 3′ Reverse: 5’ CGGCCCCAGTTGAAGTTG 3′ |
| Bcl-2 | Forward: 5’CTGCACCTGACGCCCTTCACC 3′ Reverse: 5’CACATGACCCCACCGAACTCAAAGA 3′ |
| Caspase-3 | Forward: 5’CCATGGGTAGCAGCCTCCTTC 3′ Reverse: 5’TGCGCTGCTCTGCCTTCT 3′ |
| GAPDH | Forward: 5’TGCGCTGCTGCTCTGCCTTCT 3′ Reverse: 5’CCATGGGTAGCAGCTCCTTC 3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monama, L.V.; Tswaledi, D.L.; Hadzhi, T.M.; Mphahlele, M.S.; Madihlaba, M.B.; Mokgotho, M.P.; Shai, L.J.; Mathe, E.H. Identification of Bioactive Compounds in Warburgia salutaris Leaf Extracts and Their Pro-Apoptotic Effects on MCF-7 Breast Cancer Cells. Int. J. Mol. Sci. 2025, 26, 8065. https://doi.org/10.3390/ijms26168065
Monama LV, Tswaledi DL, Hadzhi TM, Mphahlele MS, Madihlaba MB, Mokgotho MP, Shai LJ, Mathe EH. Identification of Bioactive Compounds in Warburgia salutaris Leaf Extracts and Their Pro-Apoptotic Effects on MCF-7 Breast Cancer Cells. International Journal of Molecular Sciences. 2025; 26(16):8065. https://doi.org/10.3390/ijms26168065
Chicago/Turabian StyleMonama, Lebogang Valentia, Daniel Lefa Tswaledi, Tshisikhawe Masala Hadzhi, Makgwale Sharon Mphahlele, Mopeledi Blandina Madihlaba, Matlou Phineas Mokgotho, Leshweni Jeremia Shai, and Emelinah Hluphekile Mathe. 2025. "Identification of Bioactive Compounds in Warburgia salutaris Leaf Extracts and Their Pro-Apoptotic Effects on MCF-7 Breast Cancer Cells" International Journal of Molecular Sciences 26, no. 16: 8065. https://doi.org/10.3390/ijms26168065
APA StyleMonama, L. V., Tswaledi, D. L., Hadzhi, T. M., Mphahlele, M. S., Madihlaba, M. B., Mokgotho, M. P., Shai, L. J., & Mathe, E. H. (2025). Identification of Bioactive Compounds in Warburgia salutaris Leaf Extracts and Their Pro-Apoptotic Effects on MCF-7 Breast Cancer Cells. International Journal of Molecular Sciences, 26(16), 8065. https://doi.org/10.3390/ijms26168065





