Long Non-Coding RNA 74687 Regulates Meiotic Progression and Gonadal Development in Rainbow Trout (Oncorhynchus mykiss) via the miR-15a-5p–ccne1 Regulatory Axis
Abstract
1. Introduction
2. Results
2.1. Defining the Target Genes of miR-15a-5p
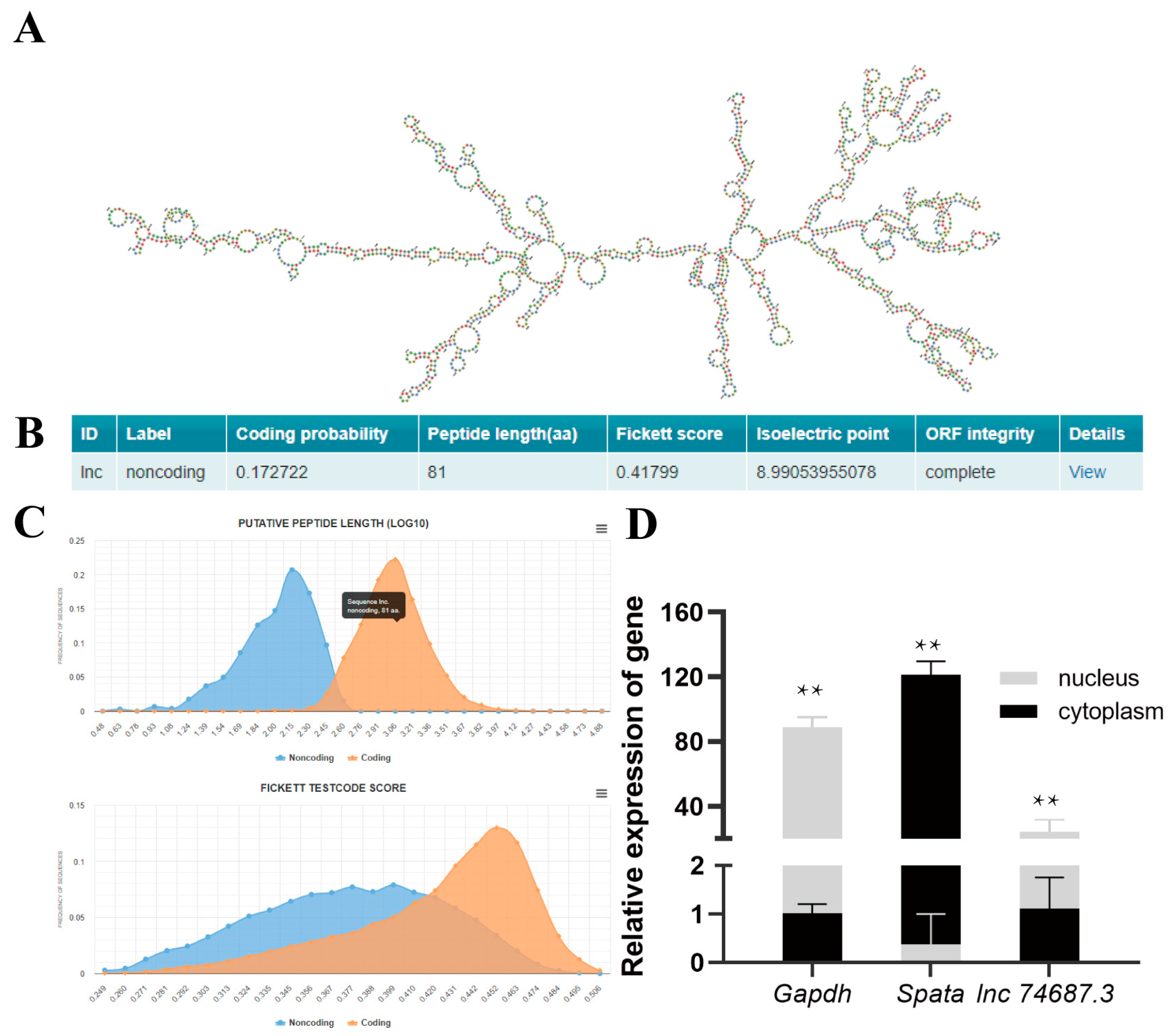
2.2. Characterisation of lnc74687.3
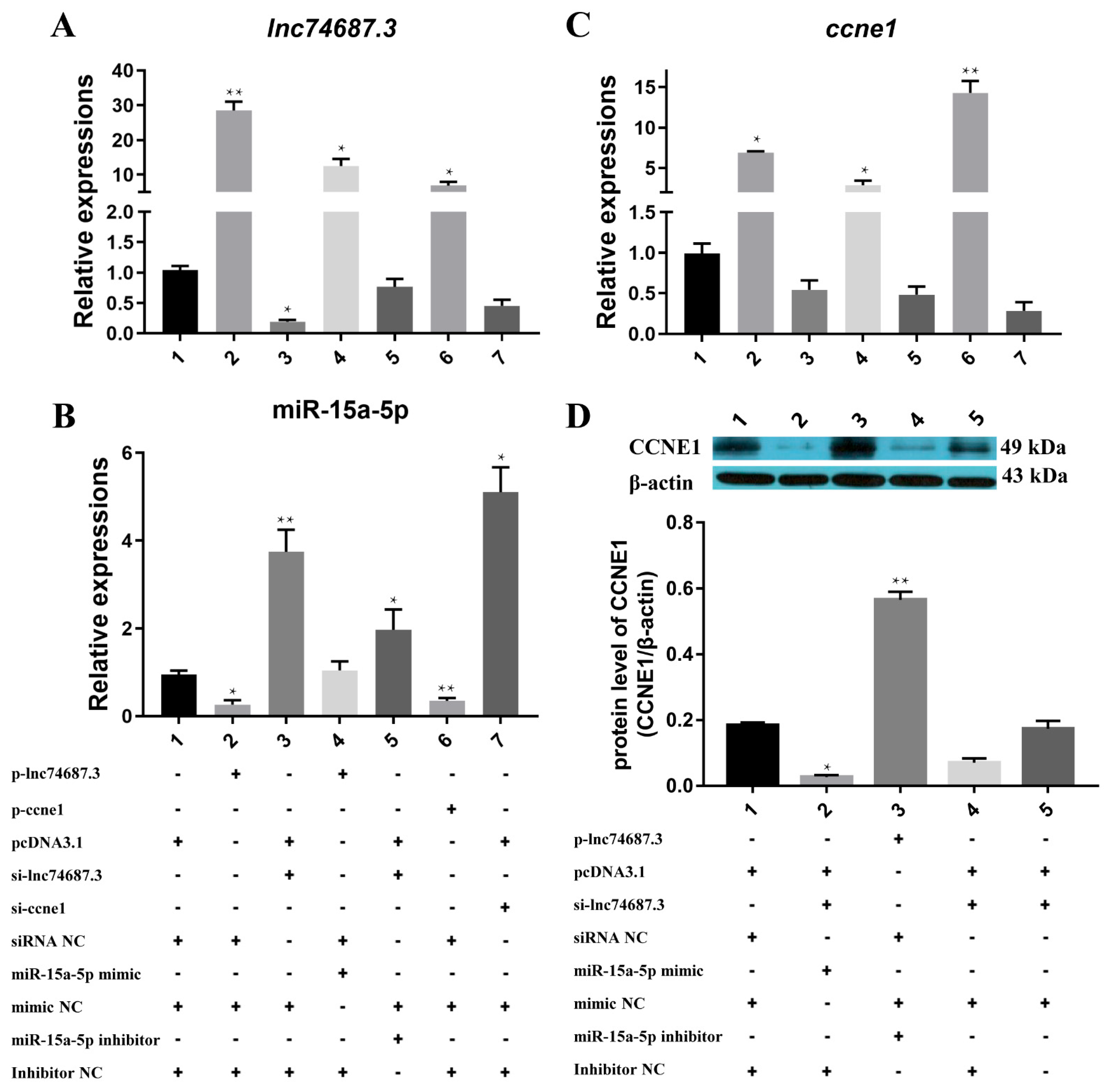
2.3. Co-Expression of miR-15a-5p, Ccne1, and lncRNA74687.3
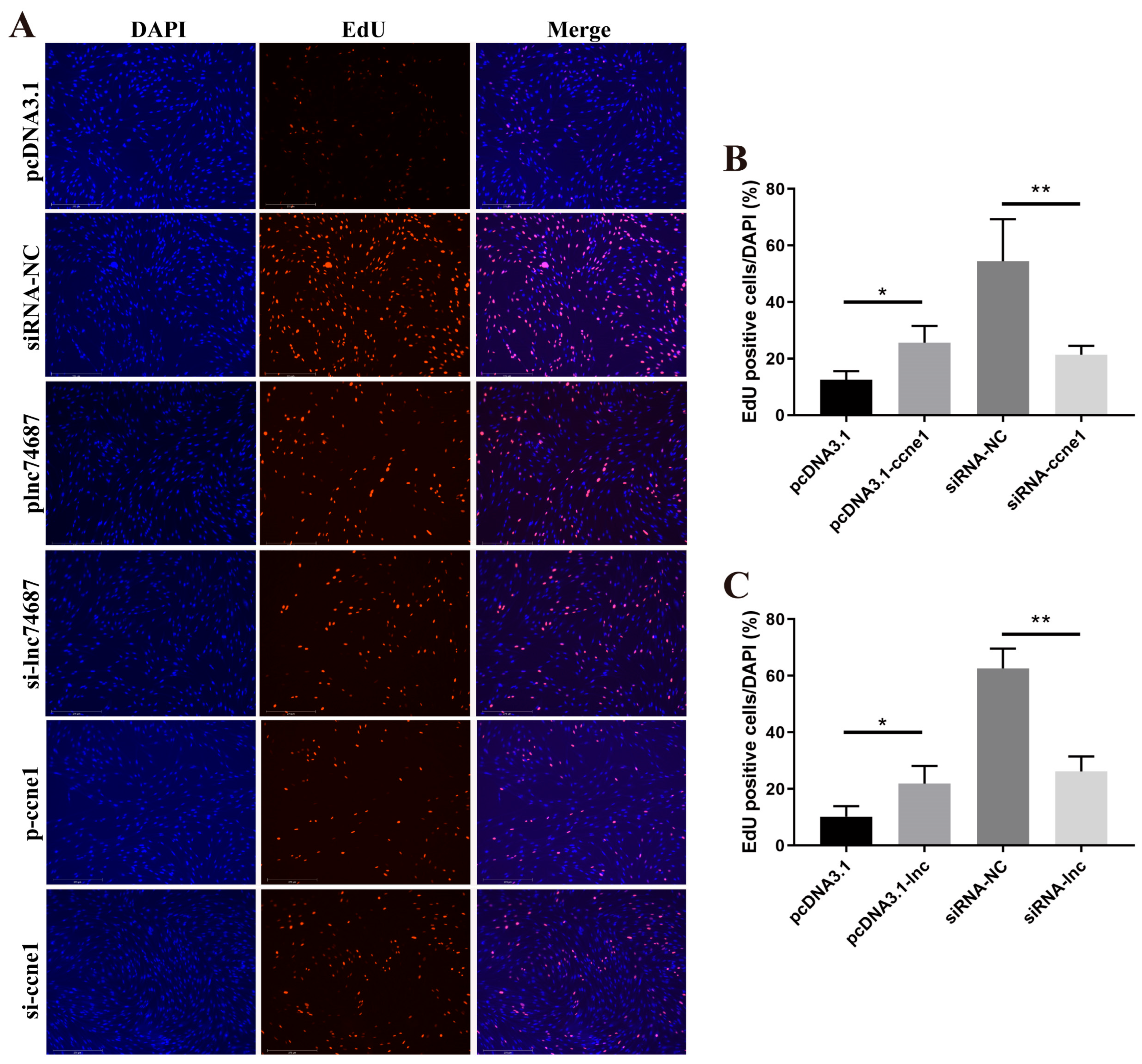
2.4. lnc74687.3 and Ccne1 Promote Gonocyte Proliferation in Rainbow Trout
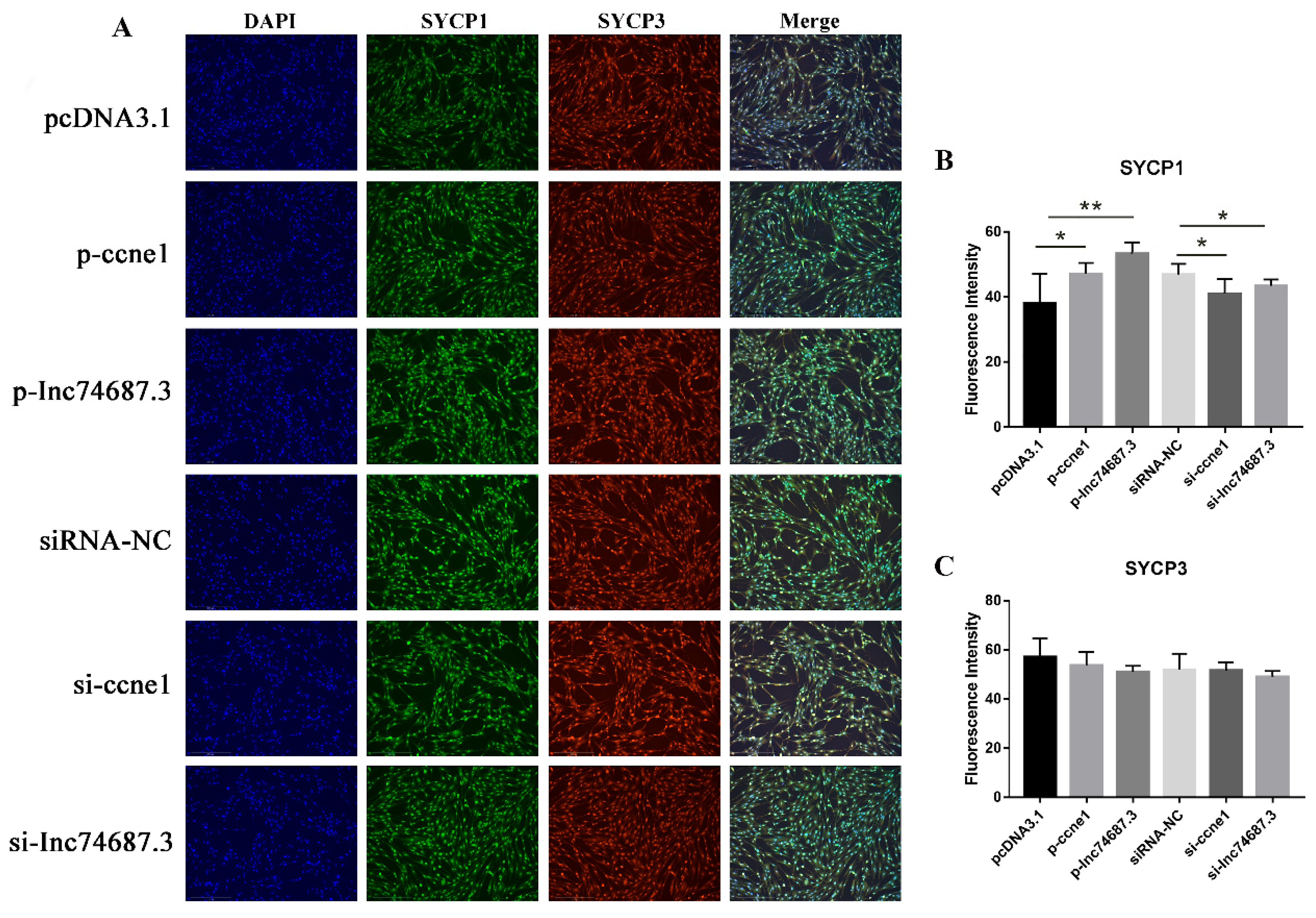
2.5. lnc74687.3 Affects the Expression of Synaptonemal Complex Protein 1 (SYCP1)
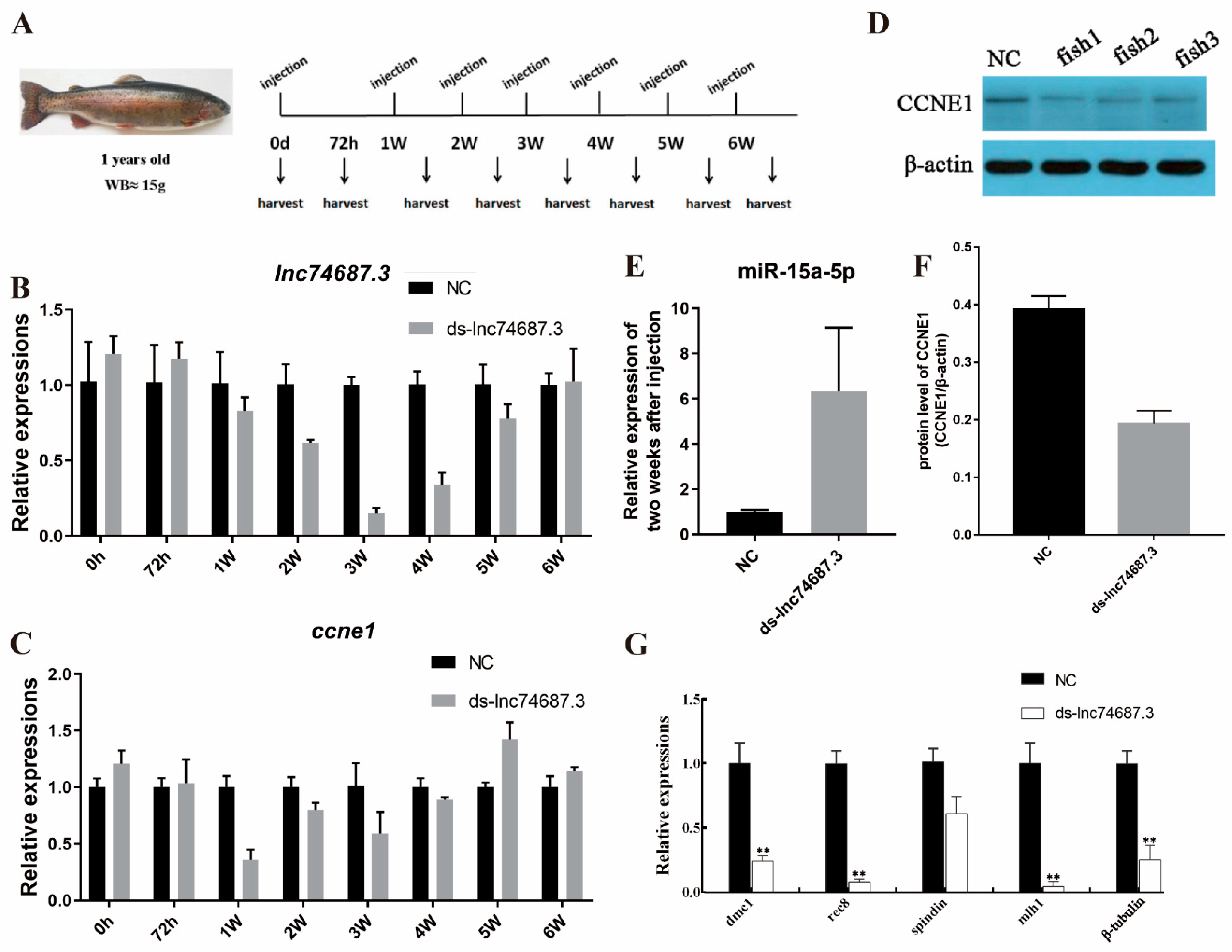
2.6. lnc74687.3 Regulates Ccne1 Gene Expression In Vivo by Targeting miR-15a-5p
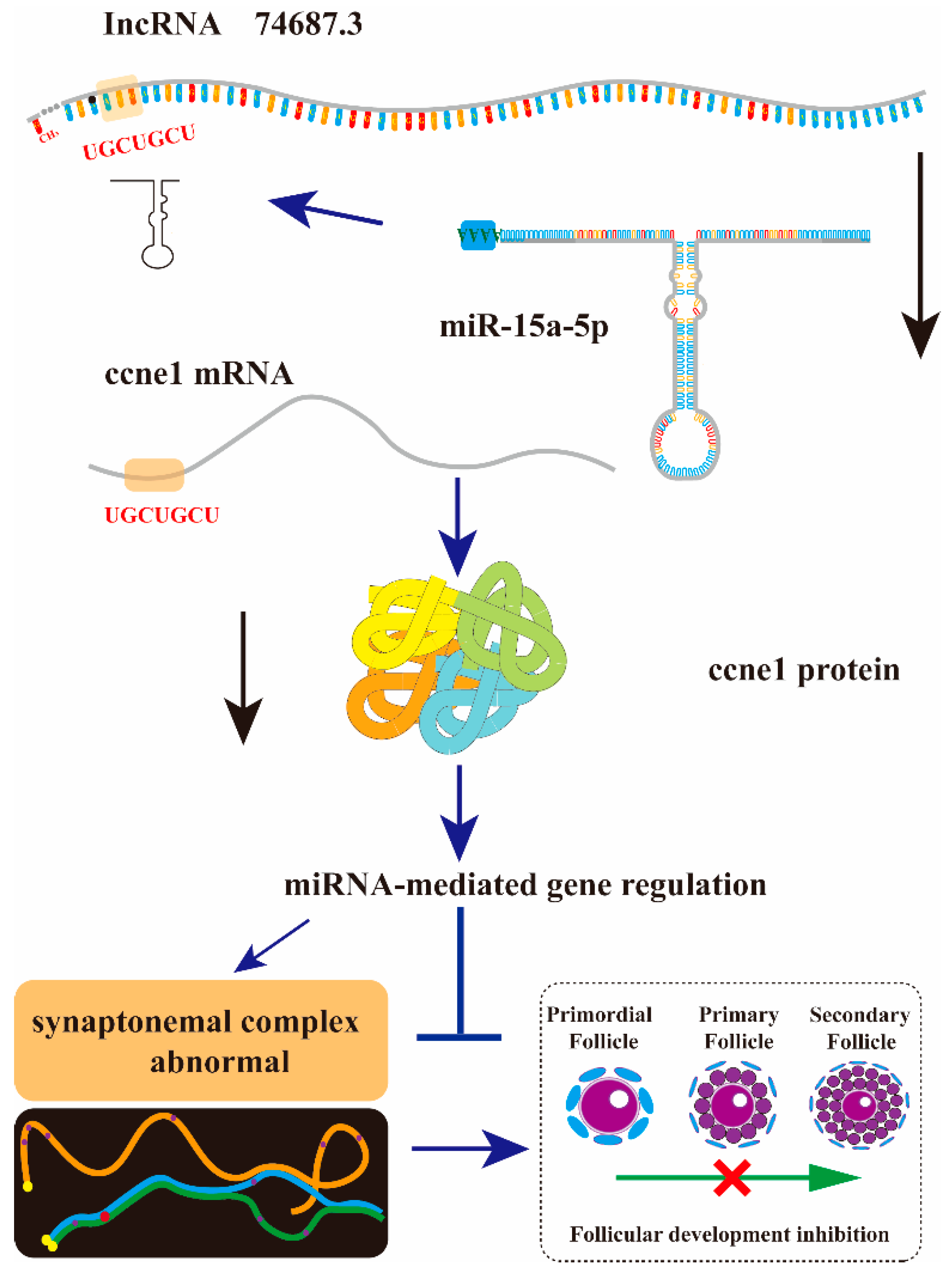
3. Discussion
3.1. lnc74687.3 and Ccne1 Are Target Genes of miR-15a-5p
3.2. lnc74687.3 as a Nuclear Non-Coding RNA May Be Involved in Transcriptional Regulation
3.3. lnc74687.3 Affects Germ-Cell Meiotic Progression by Regulating Cell Cycle- and Meiosis-Related Protein Expression in Gonadal Cells
3.4. lnc74687.3 Affects Gamete Production by Regulating the miR-15a-5p/ccne1/CCNE1 Pathway
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals
4.3. Cell Culture
4.4. Dual-Luciferase Reporter Assays
4.5. Subcellular Fractionation
4.6. RNA Extraction and Real-Time PCR Analysis
4.7. Western Blot Analysis
4.8. Cell Proliferation Assay
4.9. Bioinformatics Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.F.; Liang, L.Q.; Liew, H.J.; Chang, Y.M.; Sun, B.; Wang, S.Y.; Mi, B.H.; Zhang, L.M. Identification and analysis of long non-coding RNAs in Leuciscus waleckii adapted to highly alkaline conditions. Front. Physiol. 2021, 12, 665268. [Google Scholar] [CrossRef]
- Ning, X.; Sun, L. Identification and characterization of immune-related lncRNAs and lncRNA-miRNA-mRNA networks of Paralichthys olivaceus involved in Vibrio anguillarum infection. BMC Genom. 2021, 22, 447. [Google Scholar]
- Hao, R.; Zhu, X.; Tian, C.; Jiang, M.; Huang, Y.; Li, G.; Zhu, C. LncRNA–miRNA–mRNA ceRNA network of different body colors in Plectropomus leopardus. Front. Mar. Sci. 2023, 10, 1170762. [Google Scholar]
- Li, L.; Jia, X.; Liu, Y.; He, Y.; Pang, Y.; Shen, Y.; Xu, X.; Li, J. LncRNA-SUMO3 and lncRNA-HDMO13 modulate the inflammatory response by binding miR-21 and miR-142a-3p in grass carp. Dev. Comp. Immunol. 2021, 121, 104082. [Google Scholar] [PubMed]
- Deng, Q.; Zhao, N.; Zhu, C.; Zhang, B. Long non-coding RNAs in the physiology of aquaculture animals: A perspective update. Rev. Fish Biol. Fish. 2022, 32, 1103–1122. [Google Scholar] [CrossRef]
- Mahajan, A.; Hong, J.; Krukovets, I.; Shin, J.; Tkachenko, S.; Espinosa-Diez, C.; Owens, G.K.; Cherepanova, O.A. Integrative analysis of the lncRNA-miRNA-mRNA interactions in smooth muscle cell phenotypic transitions. Front. Genet. 2024, 15, 1356558. [Google Scholar]
- He, Y.; Zhou, H.; Wang, W.; Xu, H.; Cheng, H. Construction of a circRNA-miRNA-mRNA Regulatory Network Reveals Potential Mechanism and Treatment Options for Osteosarcoma. Front. Genet. 2021, 12, 632359. [Google Scholar]
- Qin, Q.; Zhou, Y.; Wang, C.; Zhang, M.; Qin, H.; Zhao, C.; Liu, S. Analysis on the meiosis-related gene (Dmc1, Ph1) expression in autotriploid Carassius auratus. Mar. Biotechnol. 2019, 21, 753–761. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Li, Q.; Liu, S. Transcription analysis for core networks of lncRNAs–mRNAs: Implication for potential role in sterility of Crassostrea gigas. Biology 2022, 11, 378. [Google Scholar]
- Zhang, C.; Li, Q.; Zhu, L.; He, W.; Yang, C.; Zhang, H.; Sun, Y.; Zhou, L.; Sun, Y.; Zhu, S. Abnormal meiosis in fertile and sterile triploid cyprinid fish. Sci. China Life Sci. 2021, 64, 1917–1928. [Google Scholar] [CrossRef]
- Honda, R.; Tanaka, H.; Yasuda, H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997, 420, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.; Limzerwala, J.F.; Sturmlechner, I.; Hurley, E.; Zhang, C.; Jeganathan, K.B.; Nelson, G.; Bronk, S.; Velasco, R.O.F.; van Deursen, E.; et al. Ccne1 Overexpression Causes Chromosome Instability in Liver Cells and Liver Tumor Development in Mice. Gastroenterology 2019, 157, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pang, Z.; Gao, J.; Dai, Q.; Liu, X.; Shen, Y.; Baloch, W.A.; Noonari, S.; Wang, P.; Gao, H. Functional analysis of the cell cycle protein E gene (ccne) in ovarian development of the white ridgetail prawn, Exopalaemon carinicauda. Aquac. Rep. 2023, 32, 101716. [Google Scholar] [CrossRef]
- Chen, L.; Miao, X.; Si, C.; Qin, A.; Zhang, Y.; Chu, C.; Li, Z.; Wang, T.; Liu, X. Long non-coding RNA SENP3-eif4a1 functions as a sponge of miR-195-5p to drive triple-negative breast cancer progress by overexpressing CCNE1. Front. Cell Dev. Biol. 2021, 9, 647527. [Google Scholar]
- Bao, S.; Zhuo, L.; Qi, D.; Tian, H.; Wang, D.; Zhu, B.; Meng, Y.; Ma, R. Comparative study on the fillet nutritional quality of diploid and triploid rainbow trout (Oncorhynchus mykiss). Aquac. Rep. 2023, 28, 101431. [Google Scholar]
- Nynca, J.; Malinowska, A.; Świderska, B.; Wiśniewska, J.; Dobosz, S.; Ciereszko, A. Triploidization of rainbow trout affects proteins related to ovary development and reproductive activity. Aquaculture 2023, 565, 739145. [Google Scholar]
- Huang, T.; Gu, W.; Liu, E.; Shi, X.; Wang, B.; Wu, W.; Dong, F.; Xu, G. Comprehensive analysis of miRNA-mRNA/lncRNA during gonadal development of triploid female rainbow trout (Oncorhynchus mykiss). Genomics 2021, 113, 3533–3543. [Google Scholar]
- Iwai, T.; Yoshii, A.; Yokota, T.; Sakai, C.; Hori, H.; Kanamori, A.; Yamashita, M. Structural components of the synaptonemal complex, SYCP1 and SYCP3, in the medaka fish Oryzias latipes. Exp. Cell Res. 2006, 312, 2528–2537. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Duan, C.; Li, M.; Gao, L. Novel Long Non-Coding RNA (lncRNA) Transcript AL137782. 1 Promotes the Migration of Normal Lung Epithelial Cells through Positively Regulating LMO7. Int. J. Mol. Sci. 2023, 24, 13904. [Google Scholar]
- Liebermann, J.; Tucker, M.J. Effect of carrier system on the yield of human oocytes and embryos as assessed by survival and developmental potential after vitrification. Reproduction 2002, 124, 483–489. [Google Scholar] [CrossRef]
- Brown, M.S.; Evans, B.S.; Afonso, L.O. Developmental changes in gene expression and gonad morphology during sex differentiation in Atlantic salmon (Salmo salar). Gene 2022, 823, 146393. [Google Scholar] [CrossRef]
- Zhang, X.; Min, Q.; Li, M.; Liu, X.; Li, M.; Wang, D. Mutation of cyp19a1b results in sterile males due to efferent duct obstruction in Nile tilapia. Mol. Reprod. Dev. 2019, 86, 1224–1235. [Google Scholar]
- Qi, S.; Dai, S.; Zhou, X.; Wei, X.; Chen, P.; He, Y.; Kocher, T.D.; Wang, D.; Li, M. Dmrt1 is the only male pathway gene tested indispensable for sex determination and functional testis development in tilapia. PLoS Genet. 2024, 20, e1011210. [Google Scholar]
- Yan, D.; Long, X.; Zhang, X.; Dong, X.; Wang, Z.; Jiang, H.; An, M.; Chen, J.; Gan, L. Identification and characterization of long non-coding RNAs in intestinal immune regulation of largemouth bass, Micropterus salmoides, under acute heat stress. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 48, 101132. [Google Scholar]
- Li, Y.; Yang, B.; Shi, C.; Tan, Y.; Ren, L.; Mokrani, A.; Li, Q.; Liu, S. Integrated analysis of mRNAs and lncRNAs reveals candidate marker genes and potential hub lncRNAs associated with growth regulation of the Pacific Oyster, Crassostrea gigas. BMC Genom. 2023, 24, 453. [Google Scholar]
- Fan, K.; Shen, Y.; Xu, X.; Tao, L.; Bao, T.; Li, J. LncRNA-WAS and lncRNA-C8807 interact with miR-142a-3p to regulate the inflammatory response in grass carp. Fish Shellfish Immunol. 2021, 111, 201–207. [Google Scholar]
- Dykes, I.M.; Emanueli, C. Transcriptional and post-transcriptional gene regulation by long non-coding RNA, Genomics. Proteom. Bioinform. 2017, 15, 177–186. [Google Scholar]
- Statello, L.; Guo, C.; Chen, L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118, Correction in Nat. Rev. Mol. Cell Biol. 2021, 22, 159. [Google Scholar] [CrossRef]
- Padilla, L.F.A.; Castañeda-Cortés, D.C.; Rosa, I.F.; Acosta, O.D.M.; Hattori, R.S.; Nóbrega, R.H.; Fernandino, J.I. Cystic proliferation of germline stem cells is necessary to reproductive success and normal mating behavior in medaka. eLife 2021, 10, e62757. [Google Scholar]
- Marinović, Z.; Lujić, J.; Bajec, S.S.; Djurdjevič, I.; Snoj, A.; Hoitsy, G.; Urbányi, B.; Horváth, Á. Evaluation of triploid rainbow trout Oncorhynchus mykiss as a surrogate parent for brown trout Salmo trutta m. fario and grayling Thymallus thymallus. Aquac. Rep. 2022, 24, 101163. [Google Scholar] [CrossRef]
- Imai, Y.; Saito, K.; Takemoto, K.; Velilla, F.; Kawasaki, T.; Ishiguro, K.; Sakai, N. Sycp1 is not required for subtelomeric DNA double-strand breaks but is required for homologous alignment in zebrafish spermatocytes. Front. Cell Dev. Biol. 2021, 9, 664377. [Google Scholar] [CrossRef]
- Liang, M.; Wang, H.; He, C.; Zhang, K.; Hu, K. LncRNA-Gm2044 is transcriptionally activated by a-MYB and regulates Sycp1 expression as a miR-335-3p sponge in mouse spermatocyte-derived GC-2spd (ts) cells. Differentiation 2020, 114, 49–57. [Google Scholar]
- Garcia-Alonso, L.; Lorenzi, V.; Mazzeo, C.I.; Alves-Lopes, J.P.; Roberts, K.; Sancho-Serra, C.; Engelbert, J.; Marečková, M.; Gruhn, W.H.; Botting, R.A. Single-cell roadmap of human gonadal development. Nature 2022, 607, 540–547. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, F.; He, Q.; Huang, Q.; Duan, X.; Liu, X.; Xiao, S.; Yang, H.; Zhao, H. Cloning and characterization of rec8 gene in orange-spotted grouper (Epinephelus coioides) and Dmrt1 regulation of rec8 promoter activity. Fish Physiol. Biochem. 2021, 47, 393–407. [Google Scholar] [CrossRef]
- Lin, F.; Tong, F.; He, Q.; Xiao, S.; Liu, X.; Yang, H.; Guo, Y.; Wang, Q.; Zhao, H. In vitro effects of androgen on testicular development by the AR-foxl3-rec8/fbxo47 axis in orange-spotted grouper (Epinephelus coioides). Gen. Comp. Endocrinol. 2020, 292, 113435. [Google Scholar] [PubMed]
- Leal, M.C.; Feitsma, H.; Cuppen, E.; França, L.R.; Schulz, R.W. Completion of meiosis in male zebrafish (Danio rerio) despite lack of DNA mismatch repair gene mlh1. Cell Tissue Res. 2008, 332, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, F.; Ma, W. Abnormal expression of miR-1388-5p and its target spindlin-1 in female triploid rainbow trout (Oncorhynchus mykiss). Aquac. Rep. 2020, 18, 100420. [Google Scholar]
- Needleman, D. Developing cell biology. eLife 2013, 2, e00571. [Google Scholar]
- Liu, E.; Song, H.; Gu, W.; Wang, G.; Fan, P.; Ge, K.; Sun, Y.; Li, D.; Xu, G.; Huang, T. Functional Analysis of the Cyclin E Gene in the Reproductive Development of Rainbow Trout (Oncorhynchus mykiss). Biology 2025, 14, 862. [Google Scholar] [CrossRef]

| Primer Name | Nucleotide Sequence (5′-3′) | Accession Number |
|---|---|---|
| dmc1-F | GCACGAGCGTCAAACATTCC | XM 021575970.2 |
| dmc1-R | CGACCTCATCCTCCACAACT | |
| rec8b-F | GCAGTATGGGGTGGTTAT | XM 021562658.2 |
| rec8b-R | CTCGTCATGGAATTGGTC | |
| mlh1-F | CAGGATTGTGGAGGTGGTGA | XM 021606940.2 |
| mlh1-R | ATGGGTGAGAGTTCTTGGGA | |
| β-tubulin-F | CGACCCCACTGGCACATA | XM 021601756.2 |
| β-tubulin-R | CCTCACCACATCCAAAAC | |
| spindling-F | CCATACCAGGCGGTGATACA | XM 021588921.2 |
| spindling-R | CAACCACAGGAGGCTGAAGA | |
| ccne1-F | AACGGCAACGTCTGATTTTC | LOC 110486705 |
| ccne1-R | CGTGGGATATAATGCCAAGC | |
| gapdh-F | CATTGAGGGTCTGATGAGCA | NM 001124209.1 |
| gapdh -R | CCTCCACAGCTTTCCAGAAG | |
| spata4-F | ATTTTGTAAGCGTGTGTG | NM 001124526.2 |
| spata4-R | TTTGGTTGGAAGTGATGT | |
| lnc74687.3-F | CAGGCCTCCATTCCAAATTA | MSTRG. 74687 |
| lnc74687.3-R | ACGTGAGTTGAACACGCAAC | |
| β-actin-F | CTCACCGACTACCTGATGAAGATC | LOC 100136352 |
| β-actin-R | GTAGCACAGCTTCTCCTTGATGTC | |
| miR-15a-5p | TAGCAGCACAGAATGGTTTGTGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.; Cao, B.; Liu, E.; Gu, W.; Sun, Y.; Ge, K.; Wang, G.; Li, D.; Fan, P.; Xing, R.; et al. Long Non-Coding RNA 74687 Regulates Meiotic Progression and Gonadal Development in Rainbow Trout (Oncorhynchus mykiss) via the miR-15a-5p–ccne1 Regulatory Axis. Int. J. Mol. Sci. 2025, 26, 8036. https://doi.org/10.3390/ijms26168036
Huang T, Cao B, Liu E, Gu W, Sun Y, Ge K, Wang G, Li D, Fan P, Xing R, et al. Long Non-Coding RNA 74687 Regulates Meiotic Progression and Gonadal Development in Rainbow Trout (Oncorhynchus mykiss) via the miR-15a-5p–ccne1 Regulatory Axis. International Journal of Molecular Sciences. 2025; 26(16):8036. https://doi.org/10.3390/ijms26168036
Chicago/Turabian StyleHuang, Tianqing, Baorui Cao, Enhui Liu, Wei Gu, Yunchao Sun, Kaibo Ge, Gaochao Wang, Datian Li, Peng Fan, Ruiyan Xing, and et al. 2025. "Long Non-Coding RNA 74687 Regulates Meiotic Progression and Gonadal Development in Rainbow Trout (Oncorhynchus mykiss) via the miR-15a-5p–ccne1 Regulatory Axis" International Journal of Molecular Sciences 26, no. 16: 8036. https://doi.org/10.3390/ijms26168036
APA StyleHuang, T., Cao, B., Liu, E., Gu, W., Sun, Y., Ge, K., Wang, G., Li, D., Fan, P., Xing, R., & Xu, G. (2025). Long Non-Coding RNA 74687 Regulates Meiotic Progression and Gonadal Development in Rainbow Trout (Oncorhynchus mykiss) via the miR-15a-5p–ccne1 Regulatory Axis. International Journal of Molecular Sciences, 26(16), 8036. https://doi.org/10.3390/ijms26168036






