Microbial Enhancement of Plant Tolerance to Waterlogging: Mechanisms and Interplay with Biological Control of Pathogens
Abstract
1. Introduction
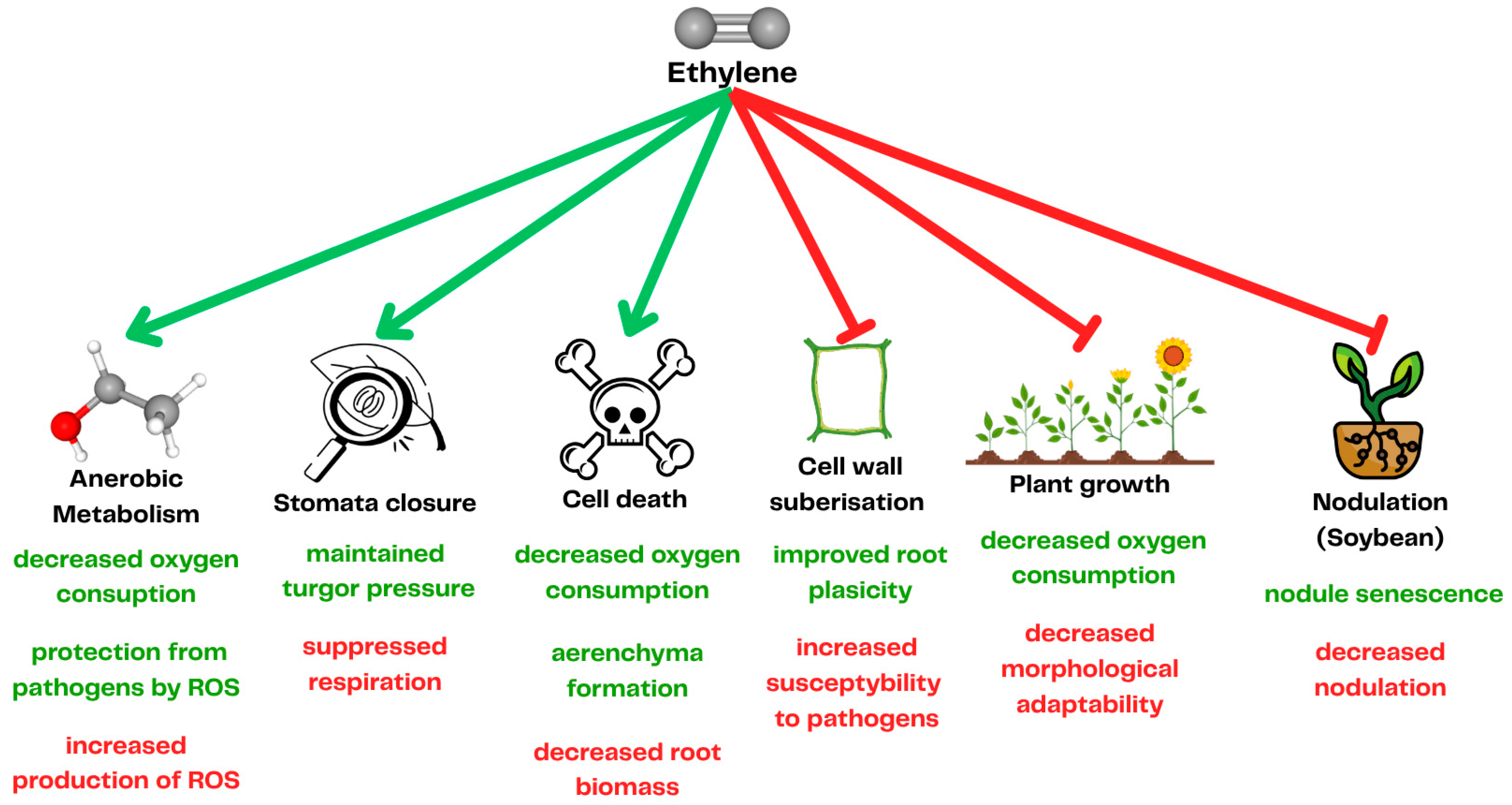
2. Plant Response to Waterlogging
3. Microbial Mechanisms Enhancing Plant Tolerance to Waterlogging
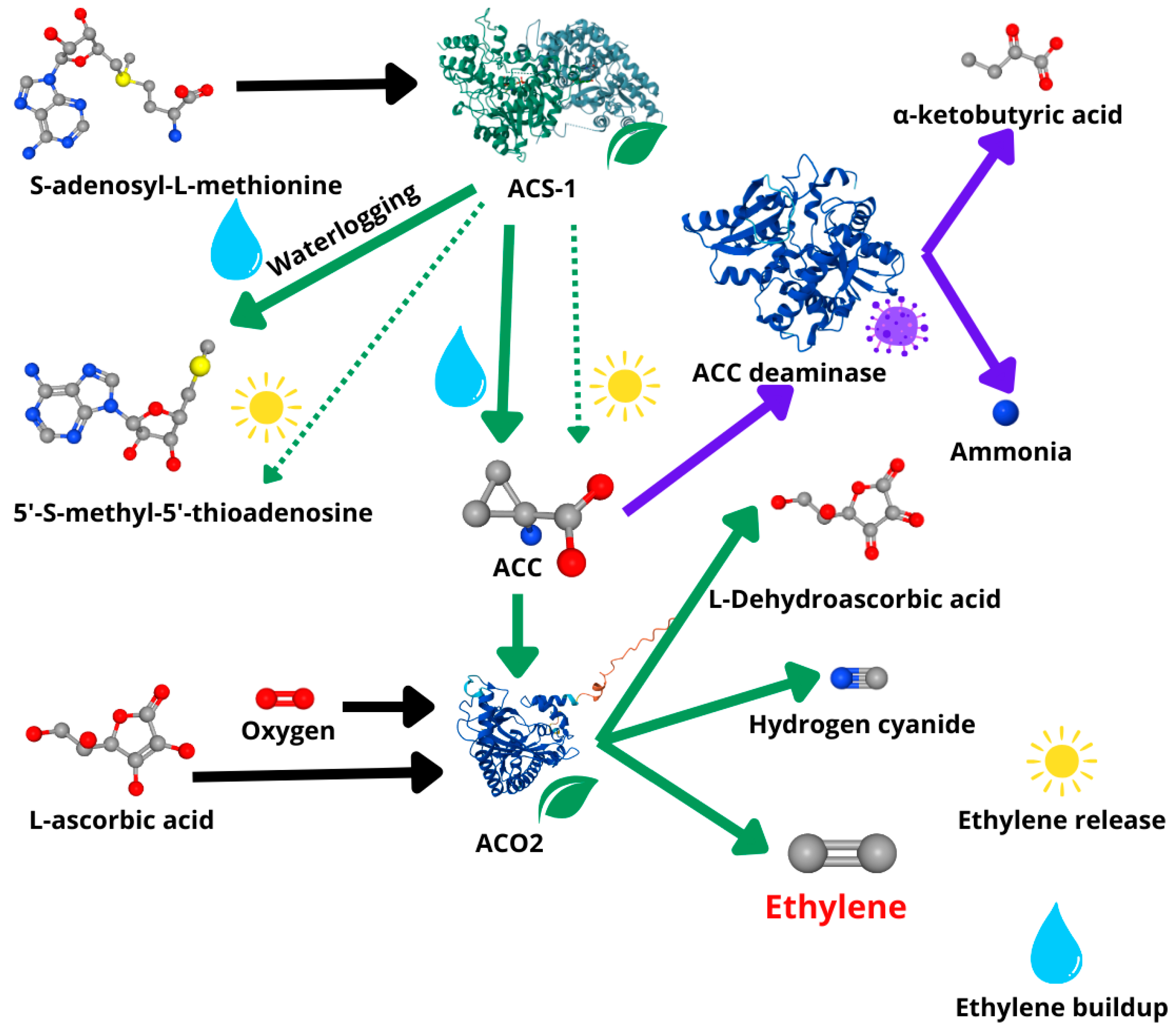
3.1. Targeting Ethylene Synthesis
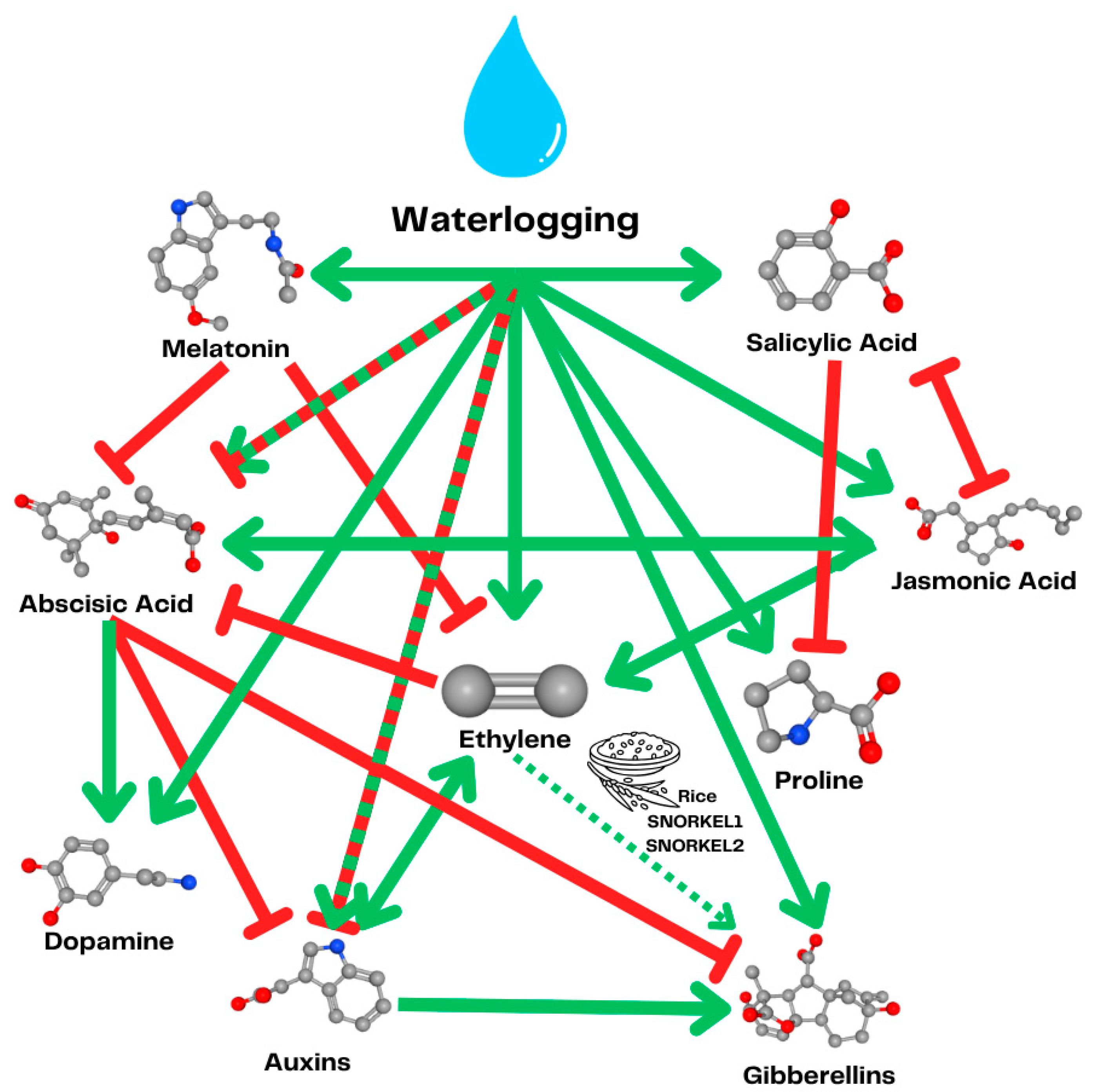
3.2. Targeting Hormonal Signaling
3.3. Targeting Metabolism and Reactive Oxygen Species
3.4. Targeting the Plant Anatomical Changes
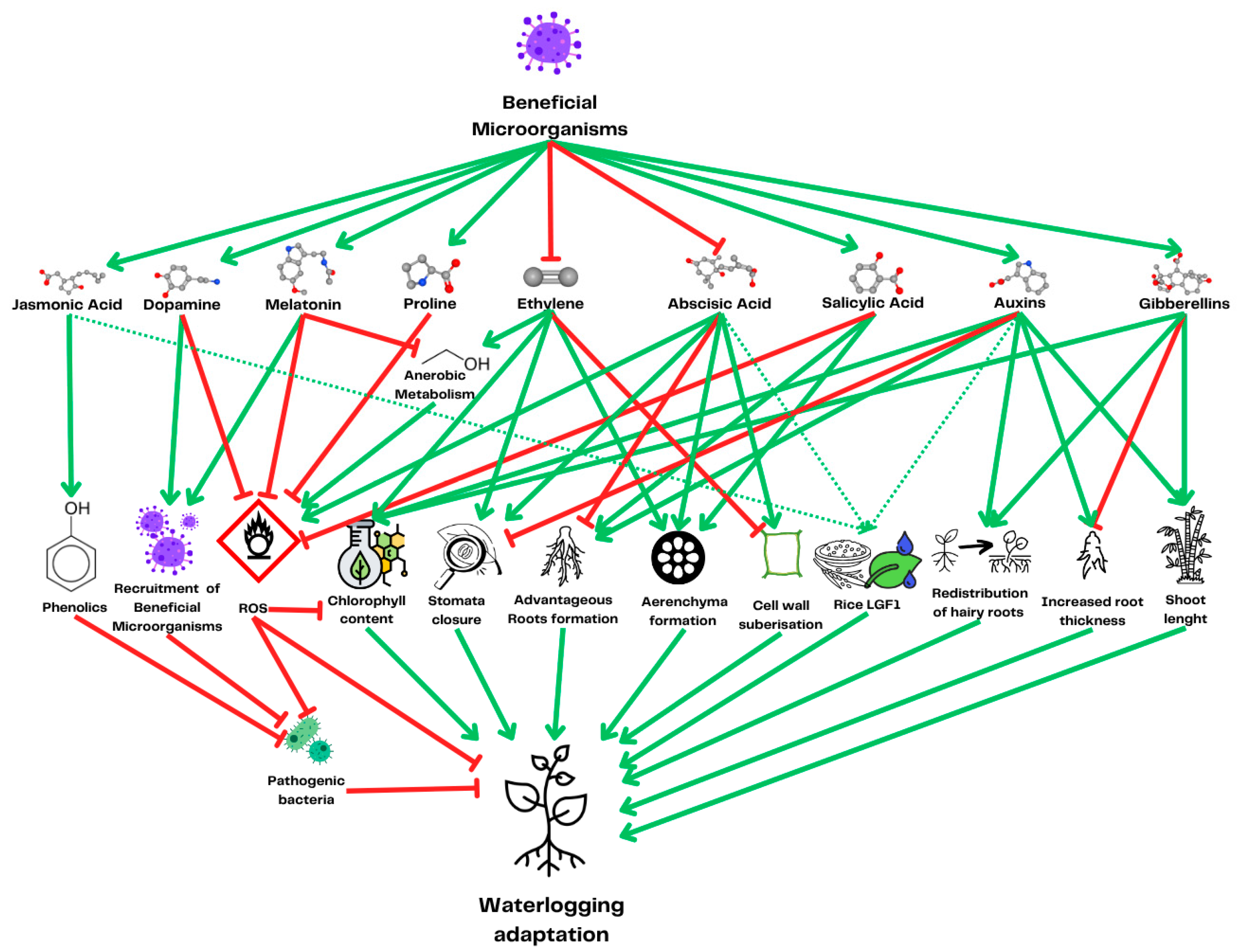
3.5. Targeting the Plant Microbiome
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- Spiertz, J.H.J.; Ewert, F. Crop Production and Resource Use to Meet the Growing Demand for Food, Feed and Fuel: Opportunities and Constraints. NJAS—Wagening. J. Life Sci. 2009, 56, 281–300. [Google Scholar] [CrossRef]
- The Guardian, Global Development. Nobel Prize Winners Call for Urgent ‘Moonshot’ Effort to Avert Global Hunger Catastrophe. Available online: https://www.theguardian.com/global-development/2025/jan/14/nobel-world-food-prize-laureates-global-hunger-open-letter-food-production?utm_source=chatgpt.com (accessed on 17 March 2025).
- Haggag, W.M.; F, A.H.; Habbasha, E.S. Agriculture Biotechnology for Management of Multiple Biotic and Abiotic Environmental Stress in Crops. J. Chem. Pharm. Res. 2015, 7, 882–889. [Google Scholar]
- Jin, X.; Jin, Y.; Zhai, J.; Fu, D.; Mao, X. Identification and Prediction of Crop Waterlogging Risk Areas under the Impact of Climate Change. Water 2022, 14, 1956. [Google Scholar] [CrossRef]
- Yang, R.; Wang, C.; Yang, Y.; Harrison, M.T.; Zhou, M.; Liu, K. Implications of Soil Waterlogging for Crop Quality: A Meta-Analysis. Eur. J. Agron. 2024, 161, 127395. [Google Scholar] [CrossRef]
- Rodriguez, R.; Durán, P. Natural Holobiome Engineering by Using Native Extreme Microbiome to Counteract the Climate Change Effects. Front. Bioeng. Biotechnol. 2020, 8, 568. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Tumpa, F.H.; Sultana, A.; Akter, M.A.; Chakraborty, A. Plant Microbiome–an Account of the Factors That Shape Community Composition and Diversity. Curr. Plant Biol. 2020, 23, 100161. [Google Scholar] [CrossRef]
- Abhilash, P.C.; Dubey, R.K.; Tripathi, V.; Gupta, V.K.; Singh, H.B. Plant Growth-Promoting Microorganisms for Environmental Sustainability. Trends Biotechnol. 2016, 34, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents against Plant Diseases: Relevance beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kloepper, J.W.; Ryu, C.-M. Rhizosphere Bacteria Help Plants Tolerate Abiotic Stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ogorek, L.L.P.; de la Cruz Jiménez, J.; Visser, E.J.W.; Takahashi, H.; Nakazono, M.; Shabala, S.; Pedersen, O. Outer Apoplastic Barriers in Roots: Prospects for Abiotic Stress Tolerance. Funct. Plant Biol. 2023, 51, FP23133. [Google Scholar] [CrossRef]
- Verma, S.; Nizam, S.; Verma, P.K. Biotic and Abiotic Stress Signaling in Plants. In Stress Signaling in Plants: Genomics and Proteomics Perspective; Springer: New York, NY, USA, 2013; Volume 1, pp. 25–50. [Google Scholar] [CrossRef]
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant Secondary Metabolites Synthesis and Their Regulations under Biotic and Abiotic Constraints. J. Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Nawaz, M.; Sun, J.; Shabbir, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A Review of Plants Strategies to Resist Biotic and Abiotic Environmental Stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar] [CrossRef]
- Van Der Biezen, E.A.; Jones, J.D.G. Plant Disease-Resistance Proteins and the Gene-for-Gene Concept. Trends Biochem. Sci. 1998, 23, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Barna, B.; Fodor, J.; Harrach, B.D.; Pogány, M.; Király, Z. The Janus Face of Reactive Oxygen Species in Resistance and Susceptibility of Plants to Necrotrophic and Biotrophic Pathogens. Plant Physiol. Biochem. 2012, 59, 37–43. [Google Scholar] [CrossRef] [PubMed]
- McMillan, G.P.; Hedley, D.; Fyffe, L.; Pérombelon, M.C.M. Potato Resistance to Soft-Rot Erwinias Is Related to Cell Wall Pectin Esterification. Physiol. Mol. Plant Pathol. 1993, 42, 279–289. [Google Scholar] [CrossRef]
- Wegener, C.B.; Jansen, G. Soft-Rot Resistance of Coloured Potato Cultivars (Solanum tuberosum L.): The Role of Anthocyanins. Potato Res. 2007, 50, 31–44. [Google Scholar] [CrossRef]
- Wolters, P.J.; Collins, W.W. Estimation of Genetic Parameters for Resistance to Erwinia Soft Rot, Specific Gravity, and Calcium Concentration in Diploid Potatoes. Crop Sci. 1995, 35, 1346–1352. [Google Scholar] [CrossRef]
- Maciag, T.; Kozieł, E.; Otulak-Kozieł, K.; Jafra, S.; Czajkowski, R. Looking for Resistance to Soft Rot Disease of Potatoes Facing Environmental Hypoxia. Int. J. Mol. Sci. 2024, 25, 3757. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Francl, L.J. The Disease Triangle: A Plant Pathological Paradigm Revisited. Plant Health Instr. 2001, 1. [Google Scholar] [CrossRef]
- Roeters, S.J.; Golbek, T.W.; Bregnhøj, M.; Drace, T.; Alamdari, S.; Roseboom, W.; Kramer, G.; Šantl-Temkiv, T.; Finster, K.; Pfaendtner, J.; et al. Ice-Nucleating Proteins Are Activated by Low Temperatures to Control the Structure of Interfacial Water. Nat. Commun. 2021, 12, 1183. [Google Scholar] [CrossRef]
- Agyemang, P.A.; Kabir, M.N.; Kersey, C.M.; Dumenyo, C.K. The Bacterial Soft Rot Pathogens, Pectobacterium Carotovorum and P. Atrosepticum, Respond to Different Classes of Virulence-Inducing Host Chemical Signals. Horticulturae 2020, 6, 13. [Google Scholar] [CrossRef]
- Narváez-Barragán, D.A.; de Sandozequi, A.; Rodríguez, M.; Estrada, K.; Tovar-Herrera, O.E.; Martínez-Anaya, C. Analysis of Two Mexican Pectobacterium Brasiliense Strains Reveals an Inverted Relationship between C-Di-GMP Levels with Exopolysaccharide Production and Swarming Motility. Microbiol. Res. 2020, 235, 126427. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and Biotic Stress Combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Saijo, Y.; Loo, E.P. Plant Immunity in Signal Integration between Biotic and Abiotic Stress Responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef]
- van Loon, L.C.; Geraats, B.P.J.; Linthorst, H.J.M. Ethylene as a Modulator of Disease Resistance in Plants. Trends Plant Sci. 2006, 11, 184–191. [Google Scholar] [CrossRef]
- Ali, S.; Kim, W.-C. Plant Growth Promotion under Water: Decrease of Waterlogging-Induced ACC and Ethylene Levels by ACC Deaminase-Producing Bacteria. Front. Microbiol. 2018, 9, 1096. [Google Scholar] [CrossRef]
- Tian, L.-X.; Zhang, Y.-C.; Chen, P.-L.; Zhang, F.-F.; Li, J.; Yan, F.; Dong, Y.; Feng, B.-L. How Does the Waterlogging Regime Affect Crop Yield? A Global Meta-Analysis. Front. Plant Sci. 2021, 12, 634898. [Google Scholar] [CrossRef]
- Fukao, T.; Barrera-Figueroa, B.E.; Juntawong, P.; Peña-Castro, J.M. Submergence and Waterlogging Stress in Plants: A Review Highlighting Research Opportunities and Understudied Aspects. Front. Plant Sci. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Shivani; Kaur, J.; Sharma, P.; Shubham; Kaushal, S. Cultivating Resilience: Exploring Root Systems in Hydroponic Agriculture. J. Exp. Agric. Int. 2024, 46, 915–925. [Google Scholar] [CrossRef]
- Nitu, O.A.; Ivan, E.Ş; Tronac, A.S.; Arshad, A. Optimizing Lettuce Growth in Nutrient Film Technique Hydroponics: Evaluating the Impact of Elevated Oxygen Concentrations in the Root Zone under LED Illumination. Agronomy 2024, 14, 1896. [Google Scholar] [CrossRef]
- Zabalza, A.; Van Dongen, J.T.; Froehlich, A.; Oliver, S.N.; Faix, B.; Gupta, K.J.; Schmälzlin, E.; Igal, M.; Orcaray, L.; Royuela, M.; et al. Regulation of Respiration and Fermentation to Control the Plant Internal Oxygen Concentration. Plant Physiol. 2009, 149, 1087–1098. [Google Scholar] [CrossRef]
- Mustroph, A.; Albrecht, G. Tolerance of Crop Plants to Oxygen Deficiency Stress: Fermentative Activity and Photosynthetic Capacity of Entire Seedlings under Hypoxia and Anoxia. Physiol. Plant 2003, 117, 508–520. [Google Scholar] [CrossRef]
- Yan, B.; Dai, Q.; Liu, X.; Huang, S.; Wang, Z. Flooding-Induced Membrane Damage, Lipid Oxidation and Activated Oxygen Generation in Corn Leaves. Plant Soil. 1996, 179, 261–268. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Vayda, M.E.; Shewmaker, C.K.; Morelli, J.K. Translational Arrest in Hypoxic Potato Tubers Is Correlated with the Aberrant Association of Elongation Factor EF-1α with Polysomes. Plant Mol. Biol. 1995, 28, 751–757. [Google Scholar] [CrossRef]
- Steffens, B.; Rasmussen, A. The Physiology of Adventitious Roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, O.; Sauter, M.; Colmer, T.D.; Nakazono, M. Regulation of Root Adaptive Anatomical and Morphological Traits during Low Soil Oxygen. New Phytol. 2021, 229, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Björn, L.O.; Middleton, B.A.; Germ, M.; Gaberščik, A. Ventilation Systems in Wetland Plant Species. Diversity 2022, 14, 517. [Google Scholar] [CrossRef]
- Ejiri, M.; Fukao, T.; Miyashita, T.; Shiono, K. A Barrier to Radial Oxygen Loss Helps the Root System Cope with Waterlogging-Induced Hypoxia. Breed. Sci. 2021, 71, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Nagai, K.; Huan, P.D.; Shimazaki, K.; Qu, H.; Mori, Y.; Toda, Y.; Kuroha, T.; Hayashi, N.; Aiga, S.; et al. Rice Leaf Hydrophobicity and Gas Films Are Conferred by a Wax Synthesis Gene (LGF1) and Contribute to Flood Tolerance. New Phytol. 2018, 218, 1298–1300. [Google Scholar] [CrossRef] [PubMed]
- Kuroha, T.; Nagai, K.; Gamuyao, R.; Wang, D.R.; Furuta, T.; Nakamori, M.; Kitaoka, T.; Adachi, K.; Minami, A.; Mori, Y.; et al. Ethylene-Gibberellin Signaling Underlies Adaptation of Rice to Periodic Flooding. Science 2018, 361, 181–186. [Google Scholar] [CrossRef]
- Daniel, K.; Hartman, S.; Vissenberg, K. How Plant Roots Respond to Waterlogging. J. Exp. Bot. 2024, 75, 511–525. [Google Scholar] [CrossRef]
- Pattyn, J.; Vaughan-Hirsch, J.; Van de Poel, B. The Regulation of Ethylene Biosynthesis: A Complex Multilevel Control Circuitry. New Phytol. 2021, 229, 770–782. [Google Scholar] [CrossRef]
- Hartman, S.; Sasidharan, R.; Voesenek, L.A.C.J. The Role of Ethylene in Metabolic Acclimations to Low Oxygen. New Phytol. 2019, 229, 64–70. [Google Scholar] [CrossRef]
- PubChem Compound Summary for CID 34755, Ademetionine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ademetionine (accessed on 20 April 2025).
- Barnawal, D.; Bharti, N.; Maji, D.; Chanotiya, C.S.; Kalra, A. 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Deaminase-Containing Rhizobacteria Protect Ocimum Sanctum Plants during Waterlogging Stress via Reduced Ethylene Generation. Plant Physiol. Biochem. 2012, 58, 227–235. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Vicente, C.S.L.; Barbosa, P.; Espada, M.; Glick, B.R.; Mota, M.; Oliveira, S. Evidence for the Involvement of ACC Deaminase from Pseudomonas Putida UW4 in the Biocontrol of Pine Wilt Disease Caused by Bursaphelenchus Xylophilus. BioControl 2013, 58, 427–433. [Google Scholar] [CrossRef]
- PubChem PubChem Compound Summary for CID 222, Ammonia. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ammonia (accessed on 20 April 2025).
- PubChem Compound Summary for CID 58, 2-Oxobutanoic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2-oxobutanoate (accessed on 20 April 2025).
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant Growth-Promoting Bacteria Confer Resistance in Tomato Plants to Salt Stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef]
- Berman, H.M.; Kleywegt, G.J.; Nakamura, H.; Markley, J.L. The Protein Data Bank Archive as an Open Data Resource. J. Comput. Mol. Des. 2014, 28, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Capitani, G.; Hohenester, E.; Feng, L.; Storici, P.; Kirsch, J.F.; Jansonius, J.N. Structure of 1-Aminocyclopropane-1-Carboxylate Synthase, a Key Enzyme in the Biosynthesis of the Plant Hormone Ethylene. J. Mol. Biol. 1999, 294, 745–756. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Drew, M.C. Oxygen Deficiency and Root Metabolism: Injury and Acclimation under Hypoxia and Anoxia. Annu. Rev. Plant Biol. 1997, 48, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Shiono, K.; Takahashi, H.; Colmer, T.D.; Nakazono, M. Role of Ethylene in Acclimations to Promote Oxygen Transport in Roots of Plants in Waterlogged Soils. Plant Sci. 2008, 175, 52–58. [Google Scholar] [CrossRef]
- Qi, X.; Li, Q.; Ma, X.; Qian, C.; Wang, H.; Ren, N.; Shen, C.; Huang, S.; Xu, X.; Xu, Q.; et al. Waterlogging-Induced Adventitious Root Formation in Cucumber Is Regulated by Ethylene and Auxin through Reactive Oxygen Species Signalling. Plant Cell Environ. 2019, 42, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.V.; Iyer, S.; Amerhauser, C.; Lehmann, M.; van Dongen, J.T.; Geigenberger, P. Oxygen Sensing via the Ethylene Response Transcription Factor RAP2.12 Affects Plant Metabolism and Performance under Both Normoxia and Hypoxia. Plant Physiol. 2016, 172, 141–153. [Google Scholar] [CrossRef]
- Feng, K.; Wang, X.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D.; Cai, J. Waterlogging Priming Enhances Hypoxia Stress Tolerance of Wheat Offspring Plants by Regulating Root Phenotypic and Physiological Adaption. Plants 2022, 11, 1969. [Google Scholar] [CrossRef]
- Naing, A.H.; Maung, T.T.; Kim, C.K. The ACC Deaminase-Producing Plant Growth-Promoting Bacteria: Influences of Bacterial Strains and ACC Deaminase Activities in Plant Tolerance to Abiotic Stress. Physiol. Plant 2021, 173, 1992–2012. [Google Scholar] [CrossRef]
- Jaemsaeng, R.; Jantasuriyarat, C.; Thamchaipenet, A. Molecular Interaction of 1-Aminocyclopropane-1-Carboxylate Deaminase (ACCD)-Producing Endophytic Streptomyces Sp. GMKU 336 towards Salt-Stress Resistance of Oryza Sativa L. Cv. KDML105. Sci. Rep. 2018, 8, 1950. [Google Scholar] [CrossRef] [PubMed]
- Jaemsaeng, R.; Jantasuriyarat, C.; Thamchaipenet, A. Positive Role of 1-Aminocyclopropane-1-Carboxylate Deaminase-Producing Endophytic Streptomyces Sp. GMKU 336 on Flooding Resistance of Mung Bean. Agric. Nat. Resour. 2018, 52, 330–334. [Google Scholar] [CrossRef]
- Chandra, D.; Srivastava, R.; Gupta, V.V.S.R.; Franco, C.M.M.; Sharma, A.K. Evaluation of Acc-Deaminase-Producing Rhizobacteria to Alleviate Water-Stress Impacts in Wheat (Triticum aestivum L.) Plants. Can. J. Microbiol. 2019, 65, 387–403. [Google Scholar] [CrossRef]
- Etesami, H.; Hosseini, H.M.; Alikhani, H.A. Bacterial Biosynthesis of 1-Aminocyclopropane-1-Caboxylate (ACC) Deaminase, a Useful Trait to Elongation and Endophytic Colonization of the Roots of Rice under Constant Flooded Conditions. Physiol. Mol. Biol. Plants 2014, 20, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Woody, O.Z.; McConkey, B.J.; Glick, B.R. Combined Effects of the Plant Growth-Promoting Bacterium Pseudomonas Putida UW4 and Salinity Stress on the Brassica Napus Proteome. Appl. Soil. Ecol. 2012, 61, 255–263. [Google Scholar] [CrossRef]
- Ravanbakhsh, M.; Sasidharan, R.; Voesenek, L.A.C.J.; Kowalchuk, G.A.; Jousset, A. ACC Deaminase-Producing Rhizosphere Bacteria Modulate Plant Responses to Flooding. J. Ecol. 2017, 105, 979–986. [Google Scholar] [CrossRef]
- Ali, S.; Khan, M.A.; Kim, W.-C. Pseudomonas Veronii KJ Mitigates Flood Stress-Associated Damage in Sesamum Indicum L. Appl. Biol. Chem. 2018, 61, 575–585. [Google Scholar] [CrossRef]
- Wang, C.; Knill, E.; Glick, B.R.; Defago, G. Effect of Transferring 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Deaminase Genes into Pseudomonas Fluorescens Strain CHA0 and Its GacA Derivative CHA96 on Their Growth-Promoting and Disease-Suppressive Capacities. Can. J. Microbiol. 2000, 46, 898–907. [Google Scholar] [CrossRef]
- Grichko, V.P.; Glick, B.R. Amelioration of Flooding Stress by ACC Deaminase-Containingplant Growth-Promoting Bacteria. Plant Physiol. Biochem. 2001, 39, 11–17. [Google Scholar] [CrossRef]
- Ueckert, J.; Hurek, T.; Fendrik, I.; Niemann, E.-G. Radial Gas Diffusion from Roots of Rice (Oryza sativa L.) and Kallar Grass (Leptochloa Fusca L. Kunth), and Effects of Inoculation with Azospirillum Brasilense Cd. Plant Soil. 1990, 122, 59–65. [Google Scholar] [CrossRef]
- Neuer, G.; Kronenberg, A.; Bothe, H. Denitrification and Nitrogen Fixation by Azospirillum—III. Properties of a Wheat-Azospirillum brasilense Association. Arch. Microbiol. 1985, 141, 364–370. [Google Scholar] [CrossRef]
- Salazar-Garcia, G.; Balaguera-Lopez, H.E.; Hernandez, J.P. Effect of Plant Growth-Promoting Bacteria Azospirillum Brasilense on the Physiology of Radish (Raphanus sativus L.) under Waterlogging Stress. Agronomy 2022, 12, 726. [Google Scholar] [CrossRef]
- Rauf, M.; Awais, M.; Ud-Din, A.; Ali, K.; Gul, H.; Rahman, M.M.; Hamayun, M.; Arif, M. Molecular Mechanisms of the 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Deaminase Producing Trichoderma asperellum MAP1 in Enhancing Wheat Tolerance to Waterlogging Stress. Front. Plant Sci. 2021, 11, 614971. [Google Scholar] [CrossRef]
- Smith, A.M.; Cook, R.J. Implications of Ethylene Production by Bacteria for Biological Balance of Soil. Nature 1974, 252, 703–705. [Google Scholar] [CrossRef]
- Arshad, M.; Frankenberger, W.T. Ethylene in Pathogenesis. In Ethylene; Springer: Boston, MA, USA, 2002; pp. 241–288. [Google Scholar] [CrossRef]
- Chagué, V.; Danit, L.V.; Siewers, V.; Gronover, C.S.; Tudzynski, P.; Tudzynski, B.; Sharon, A. Ethylene Sensing and Gene Activation in Botrytis Cinerea: A Missing Link in Ethylene Regulation of Fungus-Plant Interactions? Mol. Plant-Microbe Interact. 2006, 19, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Hasnain, S. Auxins as One of the Factors of Plant Growth Improvement by Plant Growth Promoting Rhizobacteria. Pol. J. Microbiol. 2014, 63, 251–263. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Bacterial Biosynthesis of Indole-3-Acetic Acid. Can. J. Microbiol. 1996, 42, 207–220. [Google Scholar] [CrossRef]
- Prinsen, E. Azospirillum brasilense Indole-3-Acetic Acid Biosynthesis: Evidence for a Non-Tryptophan Dependent Pathway. Mol. Plant-Microbe Interact. 1993, 6, 609–615. [Google Scholar] [CrossRef]
- Ahmad, E.; Sharma, S.K.; Sharma, P.K. Deciphering Operation of Tryptophan-Independent Pathway in High Indole-3-Acetic Acid (IAA) Producing Micrococcus Aloeverae DCB-20. FEMS Microbiol. Lett. 2021, 367, 190. [Google Scholar] [CrossRef]
- Kim, A.-Y.; Shahzad, R.; Kang, S.-M.; Seo, C.-W.; Park, Y.-G.; Park, H.-J.; Lee, I.-J. IAA-Producing Klebsiella Variicola AY13 Reprograms Soybean Growth during Flooding Stress. J. Crop Sci. Biotechnol. 2017, 20, 235–242. [Google Scholar] [CrossRef]
- Ratnaningsih, H.R.; Noviana, Z.; Dewi, T.K.; Loekito, S.; Wiyono, S.; Gafur, A.; Antonius, S. IAA and ACC Deaminase Producing-Bacteria Isolated from the Rhizosphere of Pineapple Plants Grown under Different Abiotic and Biotic Stresses. Heliyon 2023, 9, e16306. [Google Scholar] [CrossRef]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of Waterlogging Tolerance in Plants: Research Progress and Prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef]
- Lu, C.; Chen, M.-X.; Liu, R.; Zhang, L.; Hou, X.; Liu, S.; Ding, X.; Jiang, Y.; Xu, J.; Zhang, J.; et al. Abscisic Acid Regulates Auxin Distribution to Mediate Maize Lateral Root Development under Salt Stress. Front. Plant Sci. 2019, 10, 716. [Google Scholar] [CrossRef]
- Kang, S.-M.; Adhikari, A.; Khan, M.A.; Kwon, E.-H.; Park, Y.-S.; Lee, I.-J. Influence of the Rhizobacterium Rhodobacter Sphaeroides Ke149 and Biochar on Waterlogging Stress Tolerance in Glycine max L. Environments 2021, 8, 94. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Piater, L.A.; Dubery, I.A.; Tugizimana, F.; Mhlongo, M.I. Rhizosphere Tripartite Interactions and PGPR-Mediated Metabolic Reprogramming towards ISR and Plant Priming: A Metabolomics Review. Biology 2022, 11, 346. [Google Scholar] [CrossRef]
- Ouli-Jun; Chao-Hui, Z.; Zhou-Bin, L.; Ge, W.; Bo-Zhi, Y.; Xue-Xiao, Z. Mitigation of Waterlogging-Induced Damages to Pepper by Exogenous MeJA. Pak. J. Bot. 2017, 49, 1127–1135. [Google Scholar]
- Bhavanam, S.; Stout, M. Seed Treatment With Jasmonic Acid and Methyl Jasmonate Induces Resistance to Insects but Reduces Plant Growth and Yield in Rice, Oryza Sativa. Front. Plant Sci. 2021, 12, 691768. [Google Scholar] [CrossRef] [PubMed]
- Puentes, A.; Zhao, T.; Lundborg, L.; Björklund, N.; Borg-Karlson, A.-K. Variation in Methyl Jasmonate-Induced Defense Among Norway Spruce Clones and Trade-Offs in Resistance Against a Fungal and an Insect Pest. Front. Plant Sci. 2021, 12, 678959. [Google Scholar] [CrossRef]
- Cole, S.J.; Yoon, A.J.; Faull, K.F.; Diener, A.C. Host Perception of Jasmonates Promotes Infection by Fusarium Oxysporum Formae Speciales That Produce Isoleucine- and Leucine-Conjugated Jasmonates. Mol. Plant Pathol. 2014, 15, 589–600. [Google Scholar] [CrossRef]
- Verma, I.; Soni, S.K.; Singh, P.C. Trichoderma Produces Methyl Jasmonate-Rich Metabolites in the Presence of Fusarium, Showing Biostimulant Activity and Wilt Resistance in Tomatoes. Plant Physiol. Biochem. 2024, 215, 108953. [Google Scholar] [CrossRef]
- Ghorbel, M.; Brini, F.; Sharma, A.; Landi, M. Role of Jasmonic Acid in Plants: The Molecular Point of View. Plant Cell Rep. 2021, 40, 1471–1494. [Google Scholar] [CrossRef]
- Caarls, L.; Pieterse, C.M.J.; Van Wees, S.C.M. How Salicylic Acid Takes Transcriptional Control over Jasmonic Acid Signaling. Front. Plant Sci. 2015, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Baek, K.-H. Salicylic Acid Biosynthesis and Metabolism: A Divergent Pathway for Plants and Bacteria. Biomolecules 2021, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Lindow, S.E.; Wildermuth, M.C. Salicylic Acid, Yersiniabactin, and Pyoverdin Production by the Model Phytopathogen Pseudomonas Syringae Pv. Tomato DC3000: Synthesis, Regulation, and Impact on Tomato and Arabidopsis Host Plants. J. Bacteriol. 2007, 189. [Google Scholar] [CrossRef] [PubMed]
- Koramutla, M.K.; Tuan, P.A.; Ayele, B.T. Salicylic Acid Enhances Adventitious Root and Aerenchyma Formation in Wheat under Waterlogged Conditions. Int. J. Mol. Sci. 2022, 23, 1243. [Google Scholar] [CrossRef]
- Islam, M.R.; Rahman, M.M.; Mohi-Ud-Din, M.; Akter, M.; Zaman, E.; Keya, S.S.; Hasan, M.; Hasanuzzaman, M. Cytokinin and Gibberellic Acid-Mediated Waterlogging Tolerance of Mungbean (Vigna radiata L. Wilczek). PeerJ 2022, 10, e12862. [Google Scholar] [CrossRef]
- Malonek, S.; Bömke, C.; Bornberg-Bauer, E.; Rojas, M.C.; Hedden, P.; Hopkins, P.; Tudzynski, B. Distribution of Gibberellin Biosynthetic Genes and Gibberellin Production in the Gibberella fujikuroi Species Complex. Phytochemistry 2005, 66, 1296–1311. [Google Scholar] [CrossRef]
- Lu, X.; Hershey, D.M.; Wang, L.; Bogdanove, A.J.; Peters, R.J. An Ent-Kaurene-Derived Diterpenoid Virulence Factor from Xanthomonas oryzae Pv. oryzicola. New Phytol. 2015, 206, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Nett, R.S.; Bender, K.S.; Peters, R.J. Production of the Plant Hormone Gibberellin by Rhizobia Increases Host Legume Nodule Size. ISME J. 2022, 16, 1809–1817. [Google Scholar] [CrossRef]
- Woo, J.-I.; Hoque, I.-U.; Zainurin, N.; Shaffique, S.; Kwon, E.H.; Gam, H.J.; Jeon, J.R.; Lee, I.J.; Joo, G.J.; Kang, S.M. Gibberellin-Producing Bacteria Isolated from Coastal Soil Enhance Seed Germination of Mallow and Broccoli Plants under Saline Conditions. BioTech 2023, 12, 66. [Google Scholar] [CrossRef]
- Nett, R.S.; Nguyen, H.; Nagel, R.; Marcassa, A.; Charles, T.C.; Friedberg, I.; Peters, R.J. Unraveling a Tangled Skein: Evolutionary Analysis of the Bacterial Gibberellin Biosynthetic Operon. mSphere 2020, 5, e00292-20. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.B.; Sheteiwy, M.S.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-Induced Water Stress Tolerance in Plants: Recent Advances. Antioxidants 2020, 9, 809. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, J.; Tan, D.-X.; Wang, N.; Wang, L.; Shan, D.; Kong, J. Melatonin Improves Waterlogging Tolerance of Malus baccata (Linn.) Borkh. Seedlings by Maintaining Aerobic Respiration, Photosynthesis and ROS Migration. Front. Plant Sci. 2017, 8, 483. [Google Scholar] [CrossRef]
- Cao, Y.; Du, P.-H.; Shang, Y.-W.; Ji, J.-H.; Tan, L.-Q.; Zhang, X.; Xu, J.; Liang, B. Melatonin and Dopamine Alleviate Waterlogging Stress in Apples by Recruiting Beneficial Endophytes to Enhance Physiological Resilience. J. Integr. Agric. 2024, 23, 2270–2291. [Google Scholar] [CrossRef]
- Cao, Y.; Du, P.; Yin, B.; Zhou, S.; Li, Z.; Zhang, X.; Xu, J.; Liang, B. Melatonin and Dopamine Enhance Waterlogging Tolerance by Modulating ROS Scavenging, Nitrogen Uptake, and the Rhizosphere Microbial Community in Malus hupehensis. Plant Soil. 2023, 483, 475–493. [Google Scholar] [CrossRef]
- Parent, C.; Capelli, N.; Berger, A.; Crèvecoeur, M.; Dat, J.F. An Overview of Plant Responses to Soil Waterlogging. Plant Stress 2008, 2, 20–22. [Google Scholar]
- Irfan, M.; Hayat, S.; Hayat, Q.; Afroz, S.; Ahmad, A. Physiological and Biochemical Changes in Plants under Waterlogging. Protoplasma 2010, 241. [Google Scholar] [CrossRef]
- Ashraf, M.A. Waterlogging Stress in Plants: A Review. Afr. J. Agric. Res. 2012, 7, 1976–1981. [Google Scholar] [CrossRef]
- Noctor, G.; De Paepe, R.; Foyer, C.H. Mitochondrial Redox Biology and Homeostasis in Plants. Trends Plant Sci. 2007, 12, 125–134. [Google Scholar] [CrossRef]
- Dzikovski, B.G.; Livshits, V.A.; Marsh, D. Oxygen Permeation Profile in Lipid Membranes: Comparison with Transmembrane Polarity Profile. Biophys. J. 2003, 85, 1005–1012. [Google Scholar] [CrossRef]
- Jeyanthi, V.; Kanimozhi, S. Plant Growth Promoting Rhizobacteria (PGPR)-Prospective and Mechanisms: A Review. J. Pure Appl. Microbiol. 2018, 12, 733–749. [Google Scholar] [CrossRef]
- Luo, Q.; Ma, Y.; Xie, H.; Chang, F.; Guan, C.; Yang, B.; Ma, Y. Proline Metabolism in Response to Climate Extremes in Hairgrass. Plants 2024, 13, 1408. [Google Scholar] [CrossRef] [PubMed]
- Barickman, T.C.; Simpson, C.R.; Sams, C.E. Waterlogging Causes Early Modification in the Physiological Performance, Carotenoids, Chlorophylls, Proline, and Soluble Sugars of Cucumber Plants. Plants 2019, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a Multifaceted Signalling Molecule in Plant Responses to Abiotic Stress: Understanding the Physiological Mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Tuo, X.-Q.; Li, S.; Wu, Q.-S.; Zou, Y.-N. Alleviation of Waterlogged Stress in Peach Seedlings Inoculated with Funneliformis Mosseae: Changes in Chlorophyll and Proline Metabolism. Sci. Hortic. 2015, 197, 130–134. [Google Scholar] [CrossRef]
- Ahmad, I.; Song, X.; Ibrahim, M.E.H.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Ali, A.Y.A. The Role of Melatonin in Plant Growth and Metabolism, and Its Interplay with Nitric Oxide and Auxin in Plants under Different Types of Abiotic Stress. Front. Plant Sci. 2023, 14, 1108507. [Google Scholar] [CrossRef]
- Jiao, J.; Ma, Y.; Chen, S.; Liu, C.; Song, Y.; Qin, Y.; Yuan, C.; Liu, Y. Melatonin-Producing Endophytic Bacteria from Grapevine Roots Promote the Abiotic Stress-Induced Production of Endogenous Melatonin in Their Hosts. Front. Plant Sci. 2016, 7, 1387. [Google Scholar] [CrossRef] [PubMed]
- Iqrar, I.; Numan, M.; Khan, T.; Shinwari, Z.K.; Ali, G.S. LC–MS/MS-Based Profiling of Bioactive Metabolites of Endophytic Bacteria from Cannabis Sativa and Their Anti-Phytophthora Activity. Antonie van Leeuwenhoek 2021, 114, 1165–1179. [Google Scholar] [CrossRef]
- Nakamura, T.; Nakamura, M. Root Respiratory Costs of Ion Uptake, Root Growth, and Root Maintenance in Wetland Plants: Efficiency and Strategy of O2 Use for Adaptation to Hypoxia. Oecologia 2016, 182, 667–678. [Google Scholar] [CrossRef]
- De Simone, O.; Haase, K.; Müller, E.; Junk, W.J.; Hartmann, K.; Schreiber, L.; Schmidt, W. Apoplasmic Barriers and Oxygen Transport Properties of Hypodermal Cell Walls in Roots from Four Amazonian Tree Species. Plant Physiol. 2003, 132, 206–217. [Google Scholar] [CrossRef]
- Ganie, S.A.; Ahammed, G.J.; Wani, S.H. Vascular Plant One Zinc-Finger (VOZ) Transcription Factors: Novel Regulators of Abiotic Stress Tolerance in Rice (Oryza sativa L.). Genet. Resour. Crop Evol. 2020, 67, 799–807. [Google Scholar] [CrossRef]
- Grover, M.; Bodhankar, S.; Sharma, A.; Sharma, P.; Singh, J.; Nain, L. PGPR Mediated Alterations in Root Traits: Way Toward Sustainable Crop Production. Front. Sustain. Food Syst. 2021, 4, 618230. [Google Scholar] [CrossRef]
- Akhtyamova, Z.; Martynenko, E.; Arkhipova, T.; Seldimirova, O.; Galin, I.; Belimov, A.; Vysotskaya, L.; Kudoyarova, G. Influence of Plant Growth-Promoting Rhizobacteria on the Formation of Apoplastic Barriers and Uptake of Water and Potassium by Wheat Plants. Microorganisms 2023, 11, 1227. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.C.; Bougoure, J.; Ryan, M.H.; Bennett, W.W.; Colmer, T.D.; Joyce, N.K.; Olsen, Y.S.; Kendrick, G.A. Oxygen Loss from Seagrass Roots Coincides with Colonisation of Sulphide-Oxidising Cable Bacteria and Reduces Sulphide Stress. ISME J. 2019, 13, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Ambreetha, S.; Chinnadurai, C.; Marimuthu, P.; Balachandar, D. Plant-Associated Bacillus Modulates the Expression of Auxin-Responsive Genes of Rice and Modifies the Root Architecture. Rhizosphere 2018, 5, 57–66. [Google Scholar] [CrossRef]
- Zhen, B.; Li, H.; Niu, Q.; Qiu, H.; Tian, G.; Lu, H.; Zhou, X. Effects of Combined High Temperature and Waterlogging Stress at Booting Stage on Root Anatomy of Rice (Oryza sativa L.). Water 2020, 12, 2524. [Google Scholar] [CrossRef]
- Singh, T.; Bisht, N.; Ansari, M.M.; Mishra, S.K.; Chauhan, P.S. Paenibacillus lentimorbus Alleviates Nutrient Deficiency-Induced Stress in Zea mays by Modulating Root System Architecture, Auxin Signaling, and Metabolic Pathways. Plant Cell Rep. 2024, 43, 49. [Google Scholar] [CrossRef]
- Erturk, Y.; Ercisli, S.; Haznedar, A.; Cakmakci, R. Effects of Plant Growth Promoting Rhizobacteria (PGPR) on Rooting and Root Growth of Kiwifruit (Actinidia deliciosa) Stem Cuttings. Biol. Res. 2010, 43, 91–98. [Google Scholar] [CrossRef]
- Tuheteru, F.D.; Wu, Q.S. Arbuscular Mycorrhizal Fungi and Tolerance of Waterlogging Stress in Plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Springer: Singapore, 2017; pp. 43–66. [Google Scholar] [CrossRef]
- Xiong, B.-J.; Kleinsteuber, S.; Sträuber, H.; Dusny, C.; Harms, H.; Wick, L.Y. Impact of Fungal Hyphae on Growth and Dispersal of Obligate Anaerobic Bacteria in Aerated Habitats. mBio 2022, 13. [Google Scholar] [CrossRef]
- Cheng, X.-F.; Wu, H.-H.; Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Mycorrhizal Response Strategies of Trifoliate Orange under Well-Watered, Salt Stress, and Waterlogging Stress by Regulating Leaf Aquaporin Expression. Plant Physiol. Biochem. 2021, 162, 27–35. [Google Scholar] [CrossRef]
- Yang, M.; Song, Y.; Ma, H.; Li, Z.; Ding, J.; Yin, T.; Niu, K.; Sun, S.; Qi, J.; Lu, G.; et al. Unveiling the Hidden World: How Arbuscular Mycorrhizal Fungi and Its Regulated Core Fungi Modify the Composition and Metabolism of Soybean Rhizosphere Microbiome. Environ. Microbiome 2024, 19, 78. [Google Scholar] [CrossRef]
- Chung, H.; Lee, Y.-H. Hypoxia: A Double-Edged Sword During Fungal Pathogenesis? Front. Microbiol. 2020, 11, 1920. [Google Scholar] [CrossRef]
- Senko, H.; Kajić, S.; Huđ, A.; Palijan, G.; Petek, M.; Rajnović, I.; Šamec, D.; Udiković-Kolić, N.; Mešić, A.; Brkljačić, L.; et al. Will the Beneficial Properties of Plant-Growth Promoting Bacteria Be Affected by Waterlogging Predicted in the Wake of Climate Change: A Model Study. Appl. Soil. Ecol. 2024, 198, 105379. [Google Scholar] [CrossRef]
- Ferrando, L.; Cavino, A.F. Strong Shift in the Diazotrophic Endophytic Bacterial Community Inhabiting Rice (Oryza sativa) Plants after Flooding. FEMS Microbiol. Ecol. 2015, 91, 104. [Google Scholar] [CrossRef][Green Version]
- Du, B.; Haensch, R.; Alfarraj, S.; Rennenberg, H. Strategies of Plants to Overcome Abiotic and Biotic Stresses. Biol. Rev. 2024, 99, 1524–1536. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Carvalhais, L.C.; Percy, C.D.; Verma, J.P.; Schenk, P.M.; Singh, B.K. Evidence for the Plant Recruitment of Beneficial Microbes to Suppress Soil-Borne Pathogens. New Phytol. 2021, 229, 2873–2885. [Google Scholar] [CrossRef] [PubMed]
- Gschwend, F.; Aregger, K.; Gramlich, A.; Walter, T.; Widmer, F. Periodic Waterlogging Consistently Shapes Agricultural Soil Microbiomes by Promoting Specific Taxa. Appl. Soil. Ecol. 2020, 155, 103623. [Google Scholar] [CrossRef]
- Tyagi, A.; Ali, S.; Mir, R.A.; Sharma, S.; Arpita, K.; Almalki, M.A.; Mir, Z.A. Uncovering the Effect of Waterlogging Stress on Plant Microbiome and Disease Development: Current Knowledge and Future Perspectives. Front. Plant Sci. 2024, 15, 1407789. [Google Scholar] [CrossRef] [PubMed]
- Scheuring, I.; Yu, D.W. How to Assemble a Beneficial Microbiome in Three Easy Steps. Ecol. Lett. 2012, 15, 1300–1307. [Google Scholar] [CrossRef]
- Durán, P.; Thiergart, T.; Garrido-Oter, R.; Agler, M.; Kemen, E.; Schulze-Lefert, P.; Hacquard, S. Microbial Interkingdom Interactions in Roots Promote Arabidopsis Survival. Cell 2018, 175, 973–983.e14. [Google Scholar] [CrossRef]
- Maciag, T.; Kozieł, E.; Rusin, P.; Otulak-Kozieł, K.; Jafra, S.; Czajkowski, R. Microbial Consortia for Plant Protection against Diseases: More than the Sum of Its Parts. Int. J. Mol. Sci. 2023, 24, 12227. [Google Scholar] [CrossRef]
- Niu, B.; Wang, W.; Yuan, Z.; Sederoff, R.R.; Sederoff, H.; Chiang, V.L.; Borriss, R. Microbial Interactions Within Multiple-Strain Biological Control Agents Impact Soil-Borne Plant Disease. Front. Microbiol. 2020, 11, 585404. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Hossain, M.S.; Akter, M. Challenges Faced by Plant Growth-Promoting Bacteria in Field-Level Applications and Suggestions to Overcome the Barriers. Physiol. Mol. Plant Pathol. 2023, 126, 102029. [Google Scholar] [CrossRef]
- Carlström, C.I.; Field, C.M.; Bortfeld-Miller, M.; Müller, B.; Sunagawa, S.; Vorholt, J.A. Synthetic Microbiota Reveal Priority Effects and Keystone Strains in the Arabidopsis Phyllosphere. Nat. Ecol. Evol. 2019, 3, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Sun, Z.; Jia, H.; Michel-Mata, S.; Angulo, M.T.; Dai, L.; He, X.; Weiss, S.T.; Liu, Y.Y. Identifying Keystone Species in Microbial Communities Using Deep Learning. Nat. Ecol. Evol. 2024, 8, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Han, X.; Zhao, D.; Wei, K.; Yuan, Y.; Li, Y.; Liu, M.; Zhang, C.S. Exploring Biocontrol Agents From Microbial Keystone Taxa Associated to Suppressive Soil: A New Attempt for a Biocontrol Strategy. Front. Plant Sci. 2021, 12, 655673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, X.; Shen, Z.; Zhu, C.; Jiao, Z.; Li, R.; Shen, Q. Pre-Colonization of PGPR Triggers Rhizosphere Microbiota Succession Associated with Crop Yield Enhancement. Plant Soil. 2019, 439, 553–567. [Google Scholar] [CrossRef]
- Chihaoui, S.-A.; Trabelsi, D.; Jdey, A.; Mhadhbi, H.; Mhamdi, R. Inoculation of Phaseolus vulgaris with the Nodule-Endophyte Agrobacterium Sp. 10C2 Affects Richness and Structure of Rhizosphere Bacterial Communities and Enhances Nodulation and Growth. Arch. Microbiol. 2015, 197, 805–813. [Google Scholar] [CrossRef]
- Karmakar, B.; Thakuria, D.; Begum, R.H.; Joga, R.J. Recent Advances in Experimental Design of Synthetic Microbial Communities for Biocontrol Application. BioControl 2025, 70, 229–244. [Google Scholar] [CrossRef]
- Nuti, M.; Ercoli, L.; Pellegrino, E. The Bacterial-Fungal Consortia: Farmer’s Needs, Legal and Scientific Opportunities, and Constraints. Microorg. Sustain. 2023, 43, 109–125. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]

| Species | Model Plant | Observed Response | Additional Properties | Pathogen Antagonism | Reference |
|---|---|---|---|---|---|
| Chromobacter spp. | wheat (Triticum aestivum L.) | SL, SDW, RDW, CH, Apx, N, P, K | Sid, IAA, NH4 | not studied | Chandra et al., 2019 [66] |
| Pseudomonas spp. | SL, SDW, RDW, CH, PH, Apx, N, P, K | PS, Sid, IAA, NH4, NF | |||
| Variovorax paradoxus | SL, SDW, RDW, CH, PH, N, P, K | Sid, NF | |||
| Ochrobactrum anthropi | SL, SDW, RDW, CH, PH, Apx, N, P, K | PS, Sid, IAA, NH4, NF | |||
| Pseudomonas fluorescens | rice (Oryza sativa L., Cv, Gohar) | RL | Sid, IAA, HCN | not studied | Etasami et al., 2014 [67] |
| Achromobacter xylosoxidans | holy basil (Ocimum tenuiflorum L.) | SFW, RFW, PL, LN, CH, N, P, Pro, MDA | Sid, IAA, NR, Nod | not studied | Barnawal et al., 2012 [50] |
| Serratia ureilytica | SFW, PL, LN, CH, P | PS, Sid, NR, Nod | |||
| Herbaspirillum seropedicae | PL, LN, Pro, N, P, | Sid, IAA, NR | |||
| Ochrobactrum rhizosphaerae | SFW, PL, LN, P, | Sid, IAA, NR | |||
| Pseudomonas putida | marsh dock (Rumex palustris Sm.) | SDW *, SFW, RDW *, RFW * | control of pine wilt disease [51] and drought stress in rapeseed [68] | not studied | Ravanbakhsh et al., 2016 [69] |
| Streptomyces sp. | mung bean (Vigna radiata L.) | SL, RL, SFW, RFW, SDW, RDW, CH, LA, SR, | salt stress alleviation in rice [64] | not studied | Jaemsaeng et al., 2018 [65] |
| Pseudomonas veronii | sesame (Sesamum indicum L.) | SL, RL, FB, DB, CH, | not studied | not studied | Ali et al., 2018 [70] |
| Enterobacter cloacae | tomato (Lycopersicon esculentum L.) | SL, SFW, SDW, CH | not studied | Against Pythium ultimum; Rhizoctonia solani; Fusarium oxysporum and Thielaviopsis basicola; [71] | Grichko and Glick 2001 [72] |
| Pseudomonas putida | SL, SFW, SDW, CH | not studied | not studied | ||
| Azospirillum brasilense | radish (Raphanus sativus L.) | LN, LA, FB, DB, SC, TRD, | Increased gas exchange in rice roots [73], nitrogen fixation, and denitrification [74] | not studied | Salazar-Garcia et al., 2022 [75] |
| Trichoderma asperellum | wheat (Triticum aestivum L.) | CH, SFW, Pro, MDA, SC, | IAA, Pro, Phe, Flav, ROSs | not studied | Rauf et al., 2021 [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciag, T.; Krzyżanowska, D.M. Microbial Enhancement of Plant Tolerance to Waterlogging: Mechanisms and Interplay with Biological Control of Pathogens. Int. J. Mol. Sci. 2025, 26, 8034. https://doi.org/10.3390/ijms26168034
Maciag T, Krzyżanowska DM. Microbial Enhancement of Plant Tolerance to Waterlogging: Mechanisms and Interplay with Biological Control of Pathogens. International Journal of Molecular Sciences. 2025; 26(16):8034. https://doi.org/10.3390/ijms26168034
Chicago/Turabian StyleMaciag, Tomasz, and Dorota M. Krzyżanowska. 2025. "Microbial Enhancement of Plant Tolerance to Waterlogging: Mechanisms and Interplay with Biological Control of Pathogens" International Journal of Molecular Sciences 26, no. 16: 8034. https://doi.org/10.3390/ijms26168034
APA StyleMaciag, T., & Krzyżanowska, D. M. (2025). Microbial Enhancement of Plant Tolerance to Waterlogging: Mechanisms and Interplay with Biological Control of Pathogens. International Journal of Molecular Sciences, 26(16), 8034. https://doi.org/10.3390/ijms26168034







