MXene-Based Electrocatalysts for Water Splitting: Material Design, Surface Modulation, and Catalytic Performance
Abstract
1. Introduction
2. Fundamentals of Water Splitting
2.1. Thermodynamics and Kinetics of the HER and OER
- Acidic medium HER:
- Alkaline medium HER:
- Acidic medium OER:
- Alkaline medium OER:
2.2. Electrocatalytic Performance Metrics
2.3. Role of Electrocatalyst Surface and Interface in Reaction Pathways
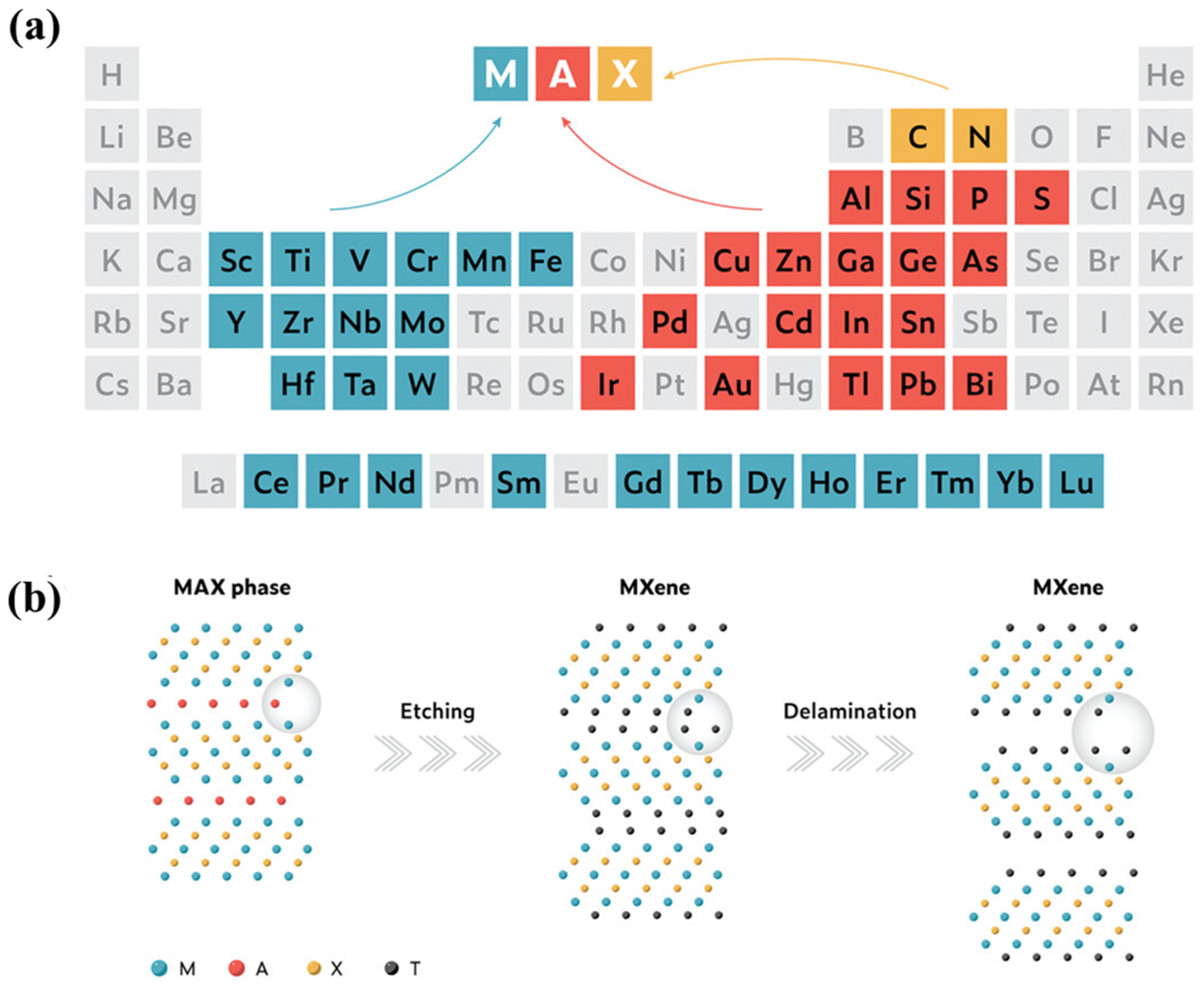
3. MXene: Synthesis, Structures, and Properties
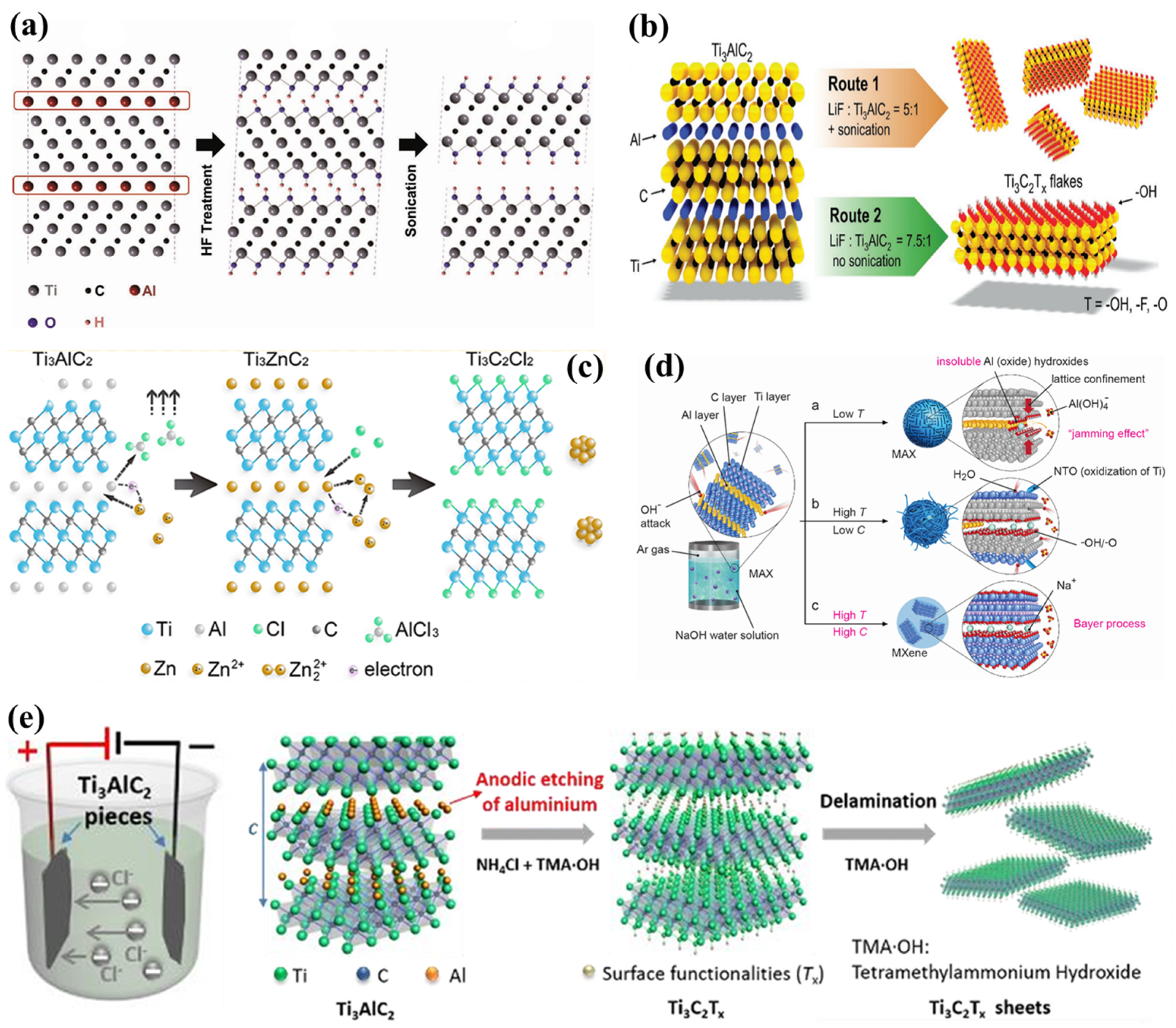
3.1. Synthetic Strategies of MXenes
3.2. Physical Properties and Structural Engineering of MXene-Based Materials
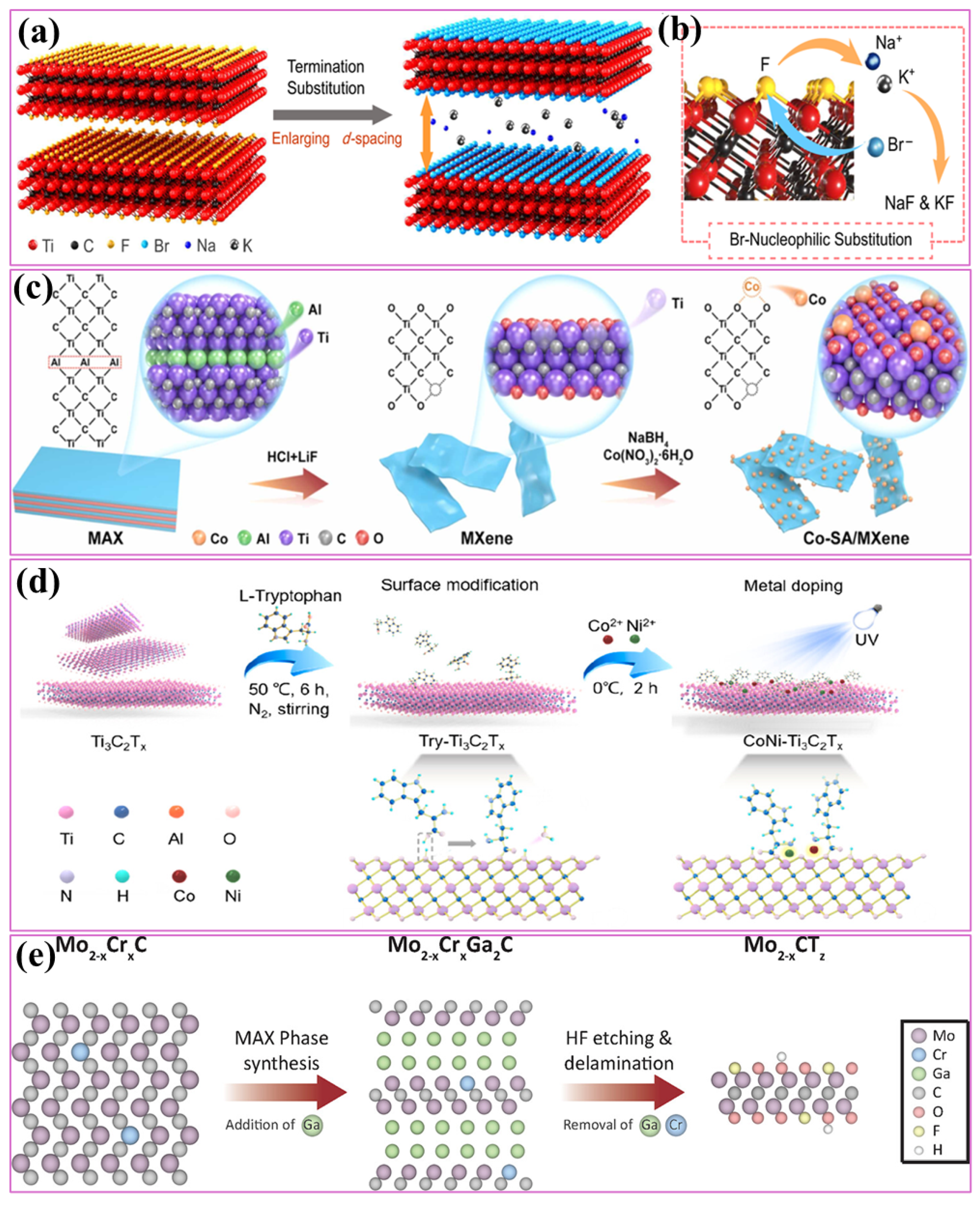
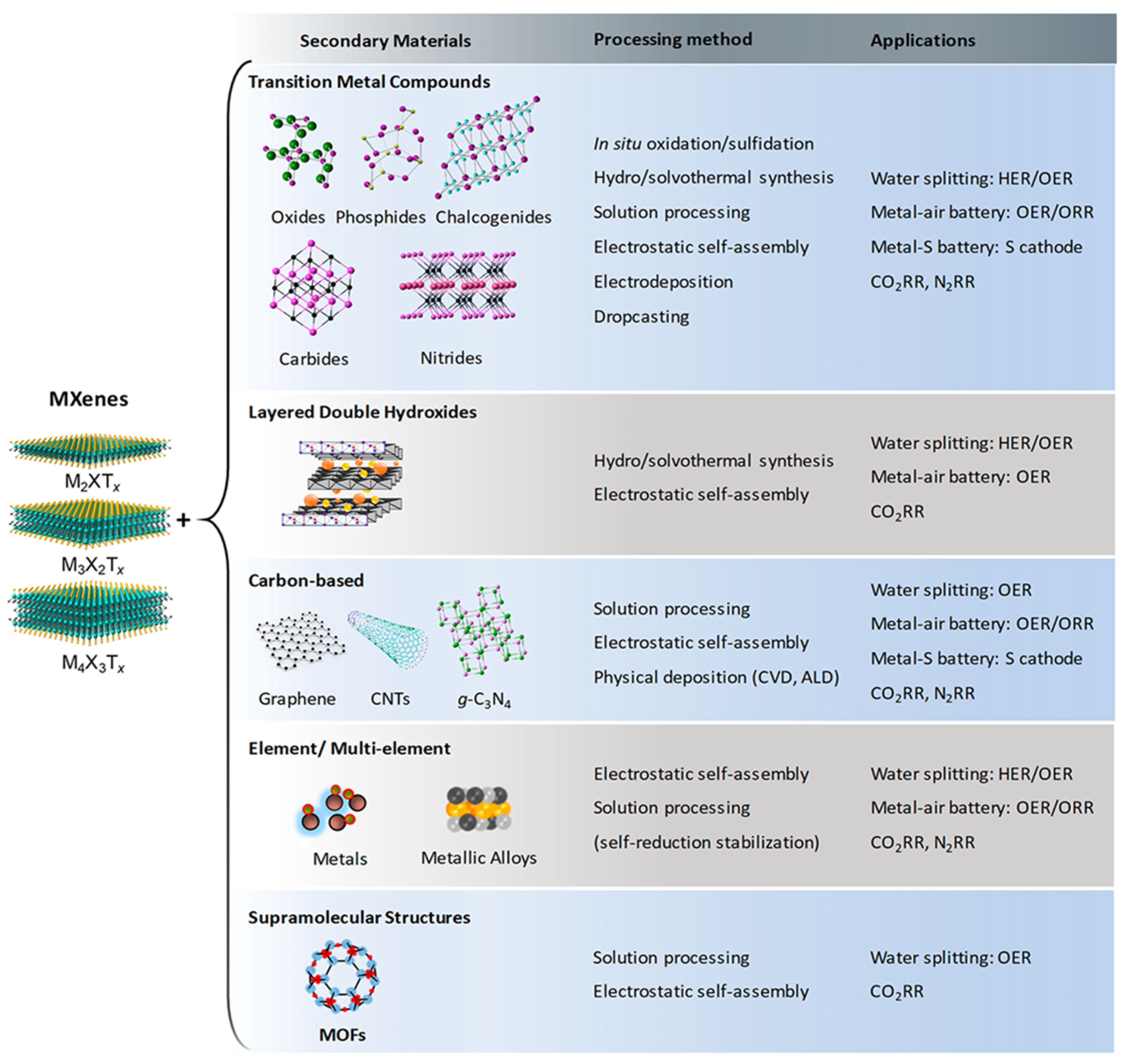
4. Electrocatalytic Activity of MXene-Based Materials
4.1. MXene-Based Electrocatalysts for the HER
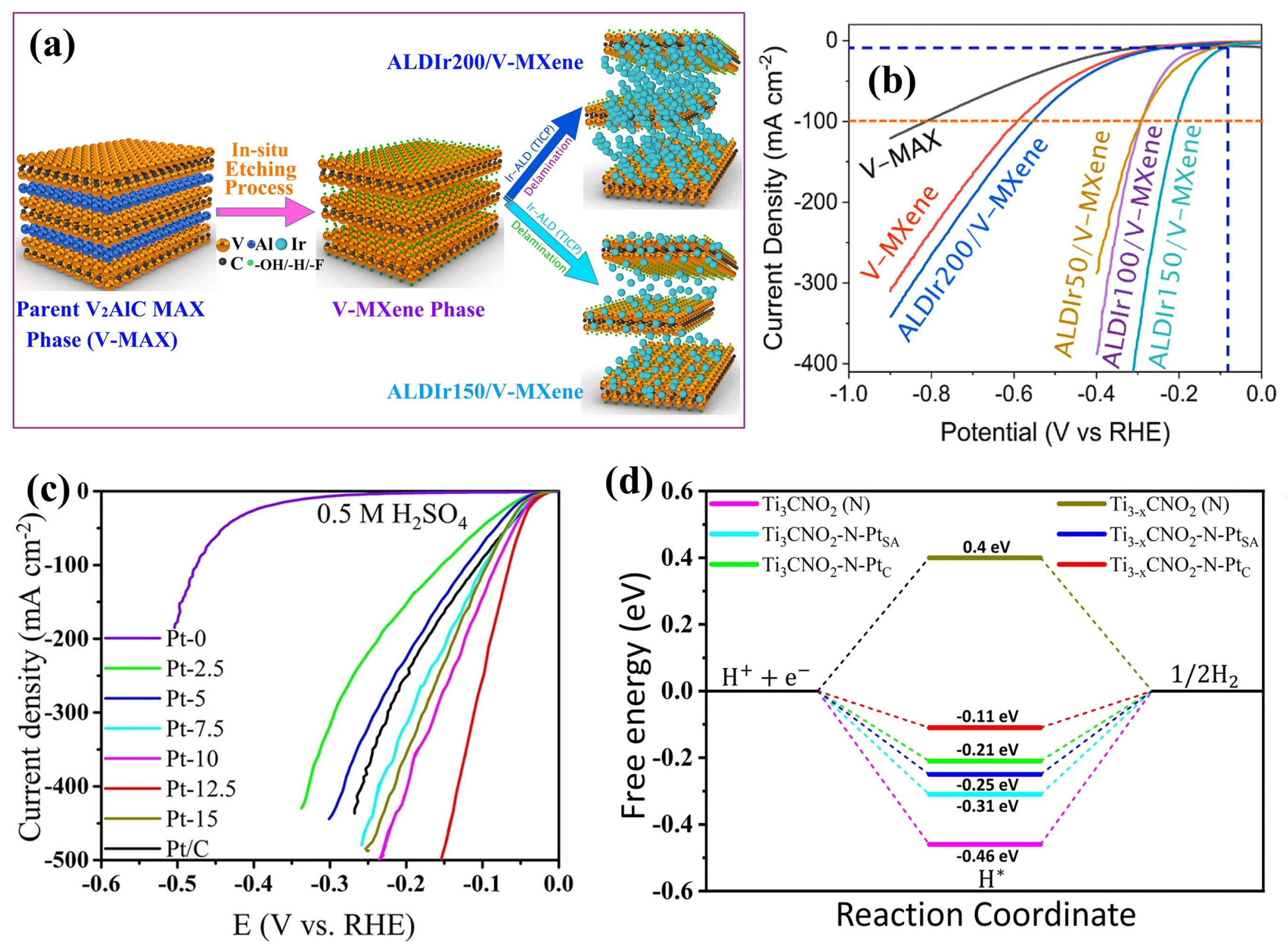
4.1.1. Pristine MXene
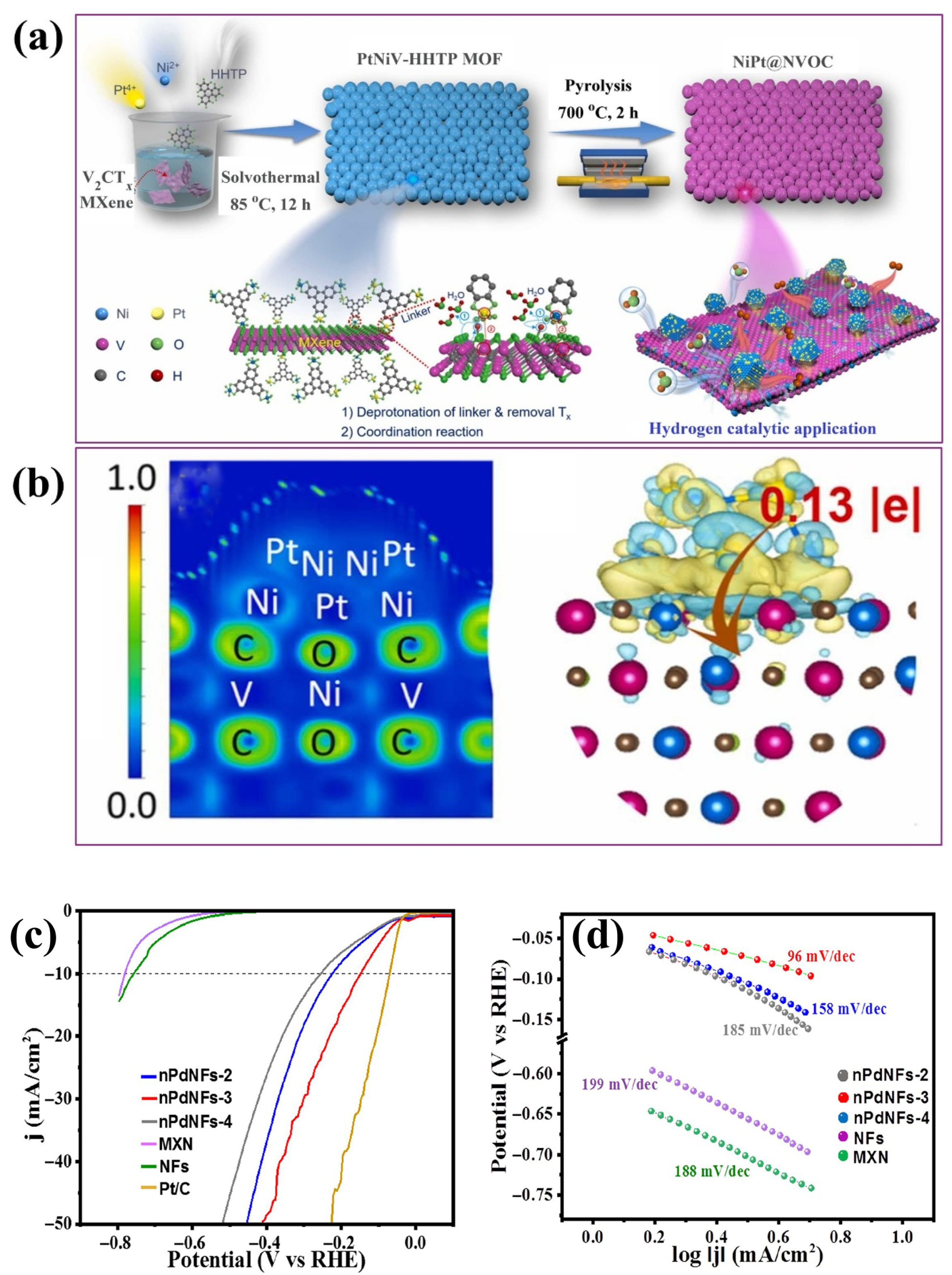
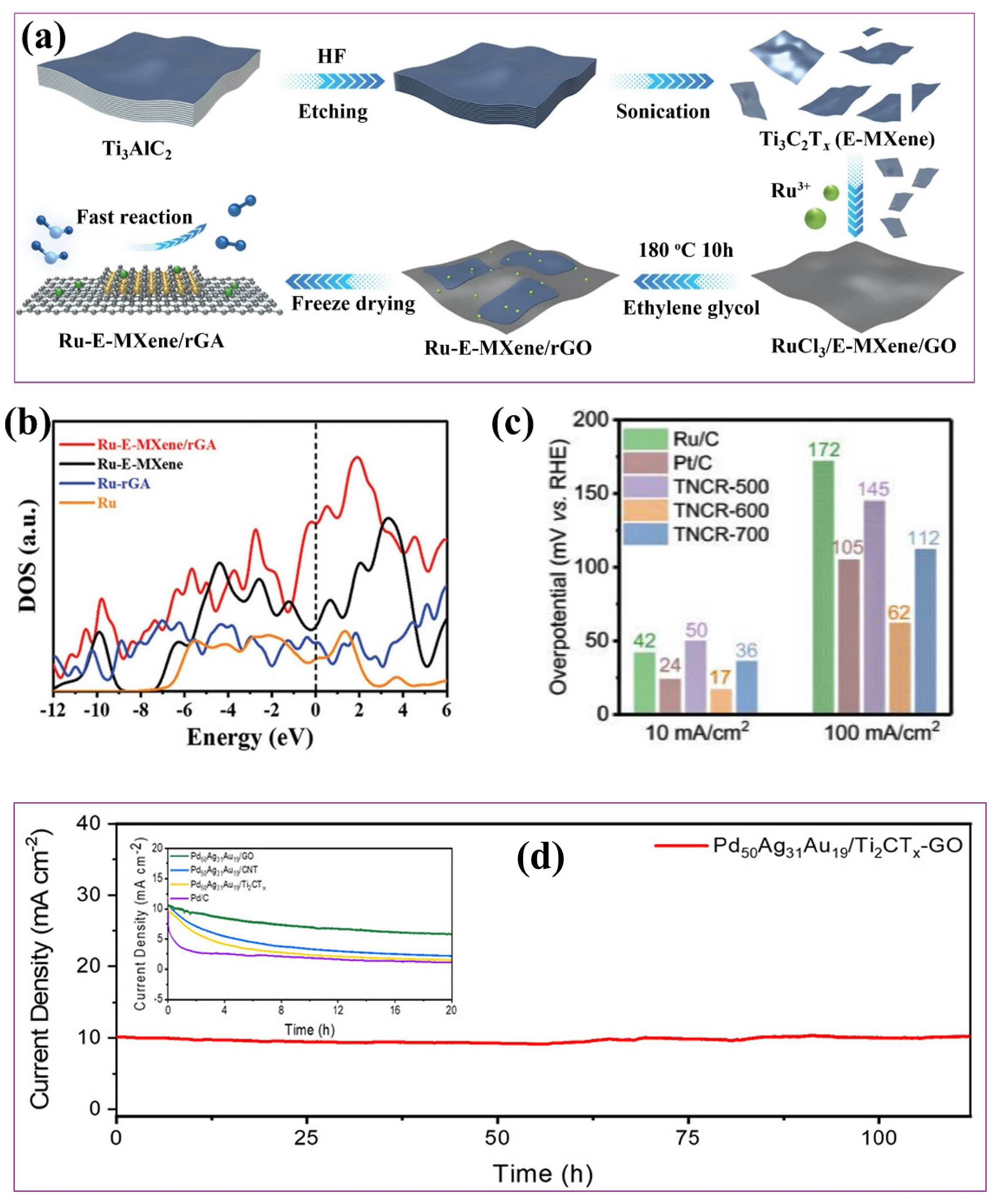
4.1.2. Metals Incorporated into MXene
4.1.3. Metal-Incorporated MXene/Carbon Hybrids
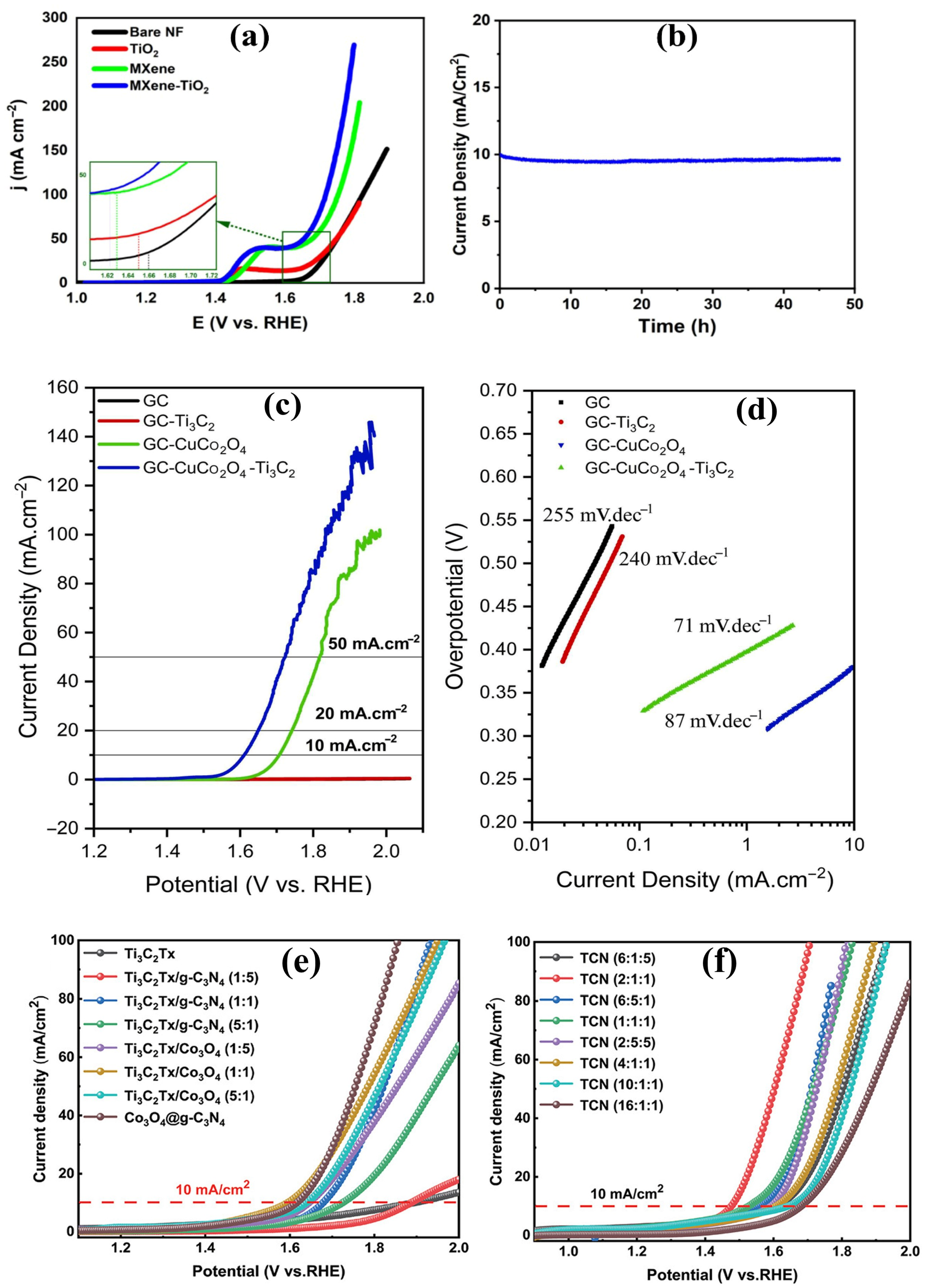
4.1.4. Metal Oxide–MXene Composites
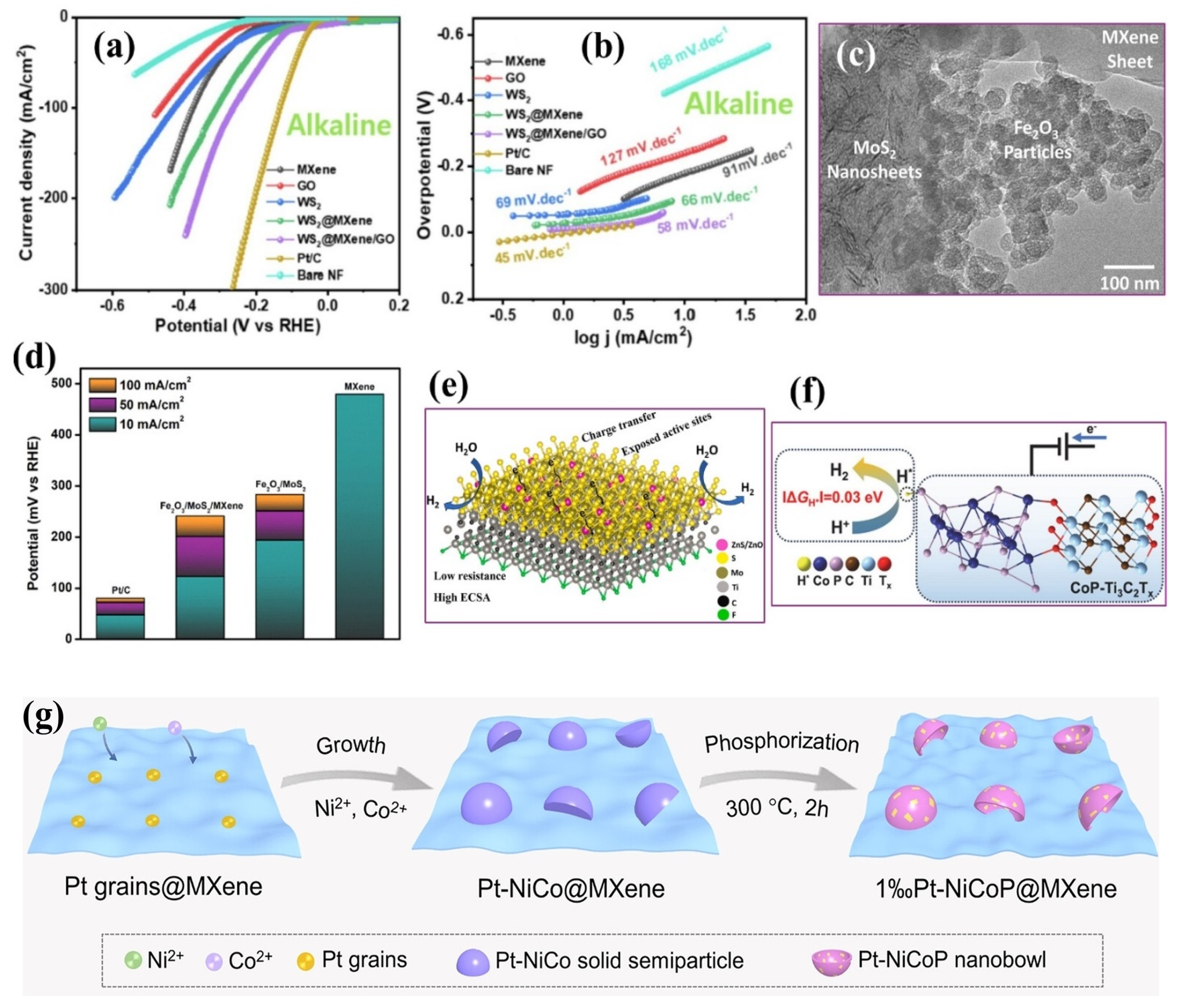
4.1.5. Metal Sulfide–MXene Composites
4.1.6. Metal Phosphide–MXene Composites
4.2. MXene-Based Electrocatalysts for the OER
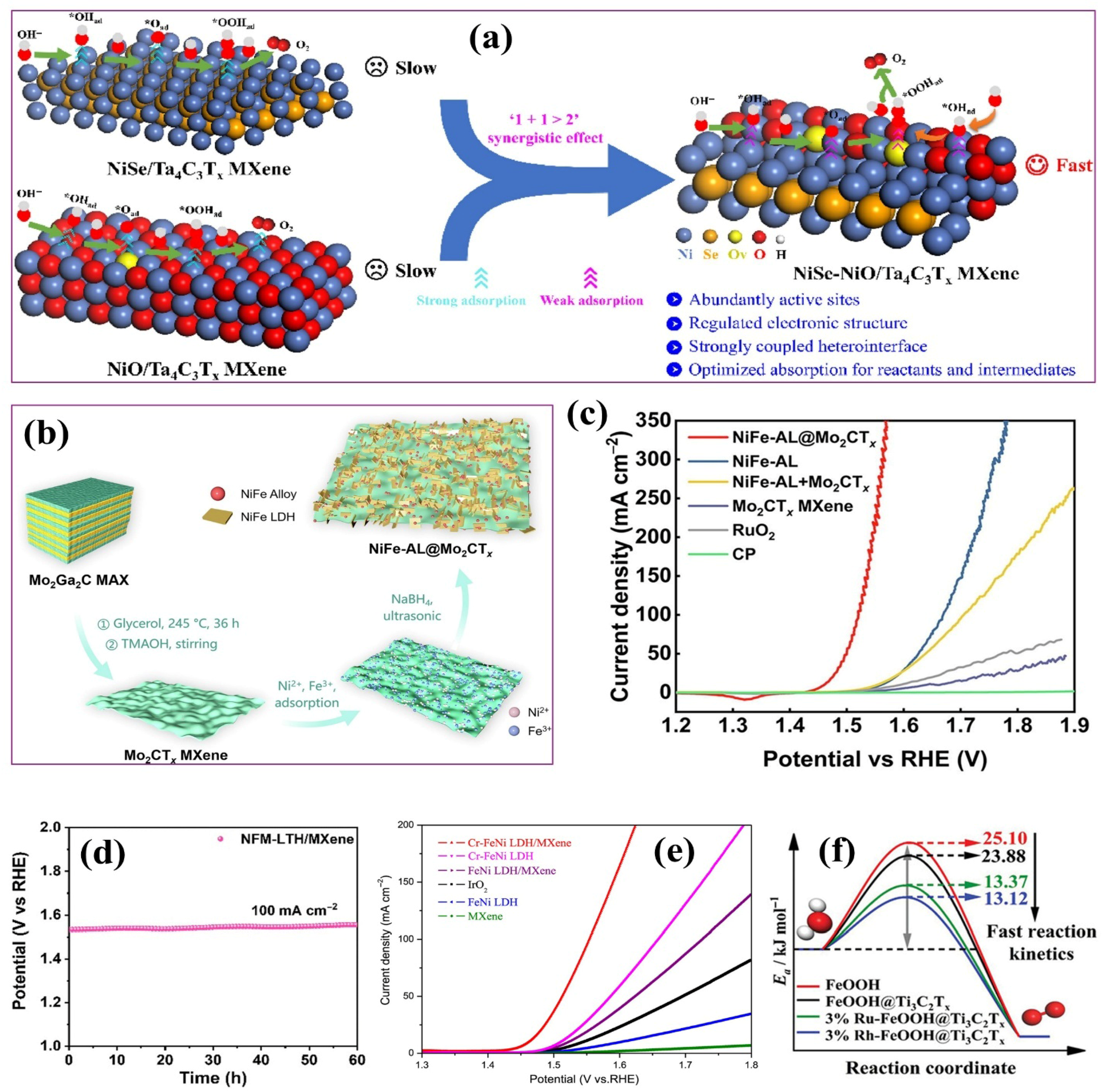
4.2.1. Metal Oxide–MXene Composites
4.2.2. LDH–MXene Composites
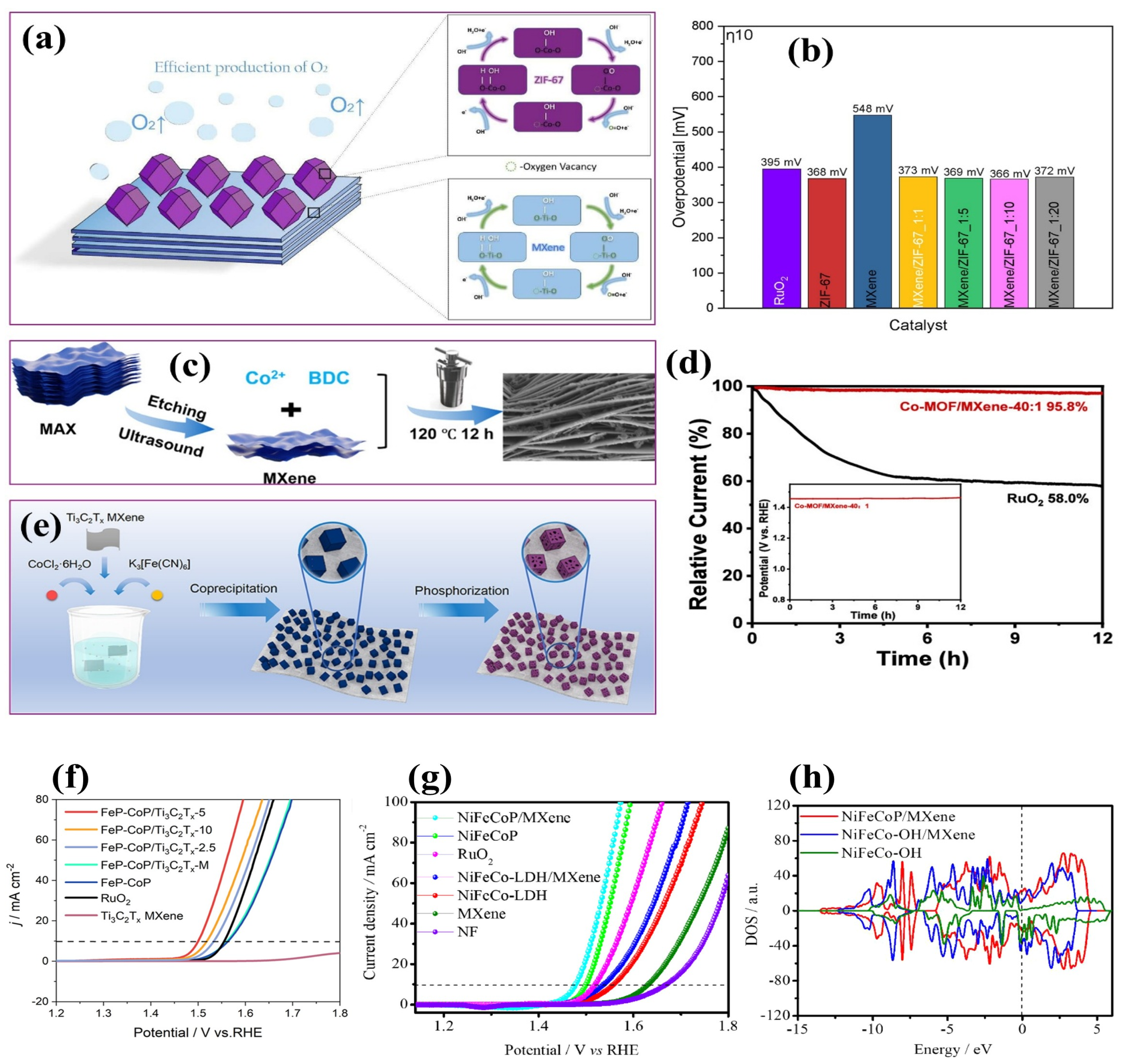
4.2.3. MOF–MXene Composites
4.2.4. Metal Phosphide–MXene Composites
5. Challenges and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OER | Oxygen evolution reaction |
| HER | Hydrogen evolution reaction |
| RHE | Reversible hydrogen electrode |
| MOFs | Metal–organic frameworks |
| 2D | Two-dimensional |
| LDHs | Layered double hydroxides |
| ΔGH* | Gibbs free energy of hydrogen adsorption |
| η | Overpotential |
| jo | Exchange current density |
| TOF | Turnover frequency |
| ECSA | Electrochemically active surface area |
| CA | Chronoamperometry |
| CP | Chronopotentiometry |
| CV | Cyclic voltammetry |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| TEM | Transmission electron microscopy |
| EIS | Electrochemical impedance spectroscopy |
| HF | Hydrofluoric acid |
| DES | Deep eutectic solvents |
| TMAOH | Tetramethylammonium hydroxide |
| TMDs | Transition metal dichalcogenides |
| GQDs | Graphene quantum dots |
| TDOS | Total density of states |
| DFT | Density functional theory |
| LTH | Layered triple hydroxide |
| Ea | Activation energy |
| ZIF-67 | Zeolitic imidazolate framework |
| STEM | Scanning transmission electron microscopy |
| EDX | Energy-dispersive X-ray spectroscopy |
| EELS | Electron energy loss spectroscopy |
| FT-IR | Fourier-transform infrared spectroscopy |
| DEMS | Differential electrochemical mass spectrometry |
| PEC | Photoelectrochemical |
References
- Durairaj, S.; Annamalai, P.; Dhanalakshmi, R.; Ghosh, D. Advances and outlook of Ti3C2-based catalysts for electrocatalytic hydrogen production: A comprehensive overview. Energy Fuels 2024, 38, 20258–20284. [Google Scholar] [CrossRef]
- Lakhan, M.N.; Hanan, A.; Wang, Y.; Lee, H.K.; Arandiyan, H. Integrated MXene and metal oxide electrocatalysts for the oxygen evolution reaction: Synthesis, mechanisms, and advances. Chem. Sci. 2024, 15, 15540–15564. [Google Scholar] [CrossRef]
- Kulkarni, R.; Lingamdinne, L.P.; Koduru, J.R.; Karri, R.R.; Chang, Y.-Y.; Kailasa, S.K.; Mubarak, N.M. Recent advanced developments and prospects of surface functionalized MXenes-based hybrid composites toward electrochemical water splitting applications. ACS Mater. Lett. 2024, 6, 2660–2686. [Google Scholar] [CrossRef]
- Mohapatra, D.; Ansari, M.Z.; Son, Y.; Lee, S.; Kang, Y.; Kim, S.-H. Precious metal Ir-ALD process engineered 2D V-MXene advanced heterostructures for next-generation hydrogen evolution electrocatalyst. Mater. Today Nano 2025, 29, 100557. [Google Scholar] [CrossRef]
- Zhao, Z.; Murad, M.; Pei, C.; Park, H.S.; Yu, X. Rational design of heterostructured MXene-based nanomaterials in electrocatalytic water splitting. ChemCatChem 2024, 17, e202401261. [Google Scholar] [CrossRef]
- Sundarraj, S.; Vadivel, N.; Murthy, A.P.; Theerthagiri, J.; Choi, M.Y. MXene electrocatalysts: Transformative approaches in hydrogen production with alternative anode reactions. Small 2025, 21, e2407120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, G.; Jiao, Y.; Yan, H.; Fu, H. Subtle 2D/2D MXene-based heterostructures for high-performance electrocatalytic water splitting. Small Methods 2024, 9, 2301602. [Google Scholar] [CrossRef]
- Shi, L.-N.; Cui, L.-T.; Ji, Y.-R.; Xie, Y.; Zhu, Y.-R.; Yi, T.-F. Towards high-performance electrocatalysts: Activity optimization strategy of 2D MXenes-based nanomaterials for water-splitting. Coord. Chem. Rev. 2022, 469, 214668. [Google Scholar] [CrossRef]
- Murtaza, M.; Farooq, K.; Shah, W.A.; Waseem, A. CoBDC MOF derived CoP/C couple with 2D Nb2CTx MXene as an efficient bifunctional catalyst for water splitting. Fuel 2025, 394, 135095. [Google Scholar] [CrossRef]
- Soni, K.; Ameta, R.K. Advancements in MXene-based electrocatalysts for hydrogen evolution reaction processes: A comprehensive review. J. Alloys Compd. Commun. 2024, 3, 100016. [Google Scholar] [CrossRef]
- Haider, Z.; Fatima, S.; Zahra, S.A.; Li, H.; Jafri, S.H.M.; Amin, F.; Rizwan, S. Ag nanoparticle-decorated V2CTx MXene nanosheets as catalysts for water splitting. ACS Appl. Nano Mater. 2023, 6, 2374–2384. [Google Scholar] [CrossRef]
- Mguni, L.L.; Mmelesi, O.K.; Tetteh, E.K.; Sijadu, N.G.; Yao, Y.; Rathilal, S. Pristine metal–organic framework electrocatalysts for hydrogen production: Role of electrocatalyst properties in basic media. Clean. Chem. Eng. 2025, 11, 100170. [Google Scholar] [CrossRef]
- Xiao, L.; Wen, J.; Wu, G.; Chen, P.; Wang, M.; Jiang, H.; Zhou, X.; Yan, J. FeCo-MOF-74/Mn-MOF-74 nanocomposite as a electrocatalyst for improved oxygen evolution reaction catalytic activity. Fuel 2025, 381, 133516. [Google Scholar] [CrossRef]
- Yue, C.; Bao, W.; Liu, Y.; Chao, X.; Liu, N.; Hao, H.; Sun, F.; Zhang, C.; Yan, D.; Bi, J.; et al. Ultrafine Co-MoC particles in porous carbon derived from polyoxometalate-based metal organic framework for efficient hydrogen evolution reaction. J. Colloid. Interface Sci. 2024, 667, 184–191. [Google Scholar] [CrossRef]
- Huang, M.; Zhou, S.; Ma, D.-D.; Wei, W.; Zhu, Q.-L.; Huang, Z. MOF-derived MoC-Fe heterojunctions encapsulated in N-doped carbon nanotubes for water splitting. Chem. Eng. J. 2023, 473, 145170. [Google Scholar] [CrossRef]
- Han, W.; Hu, J.; Su, H.; Zhang, Q.; Sun, B.; Fan, L. Self-supported Ni2P nanoarrays as efficient electrocatalysts for oxygen evolution reaction and hydrogen evolution reaction. Mater. Lett. 2023, 351, 134998. [Google Scholar] [CrossRef]
- Sun, W.-H.; Hua, Y.-Q.; Zhang, X. Assembly of amorphous CoP electrocatalysts on flexible polyester textile for alkaline hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 63, 28–35. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Ma, Y.; Guo, J.; Xin, B.; Wang, C.; Wang, C.; Tong, J. S-vacancies and iron-doped nickel sulfide nanosheets constructed by a solvothermal method as efficient catalysts for electrocatalytic oxygen evolution. J. Energy Chem. 2025, 105, 872–884. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, J.; Yang, Q.; Wang, S.; Chen, X.; Li, B.; Zou, B. Synthesis of hierarchically structured nickel–iron sulfide on nickel foam as a catalyst for efficient oxygen evolution reaction. Int. J. Electrochem. Sci. 2025, 20, 101051. [Google Scholar] [CrossRef]
- Lakshmanan, K.; Palanisamy, V.; Rojviroon, T.; Rojviroon, O.; Sirivithayapakorn, S. Carbon-wrapped iron nitride nanoparticles: A cost-effective strategy for bifunctional electrocatalysts with enhanced HER and OER performance. Diam. Relat. Mater. 2025, 156, 112414. [Google Scholar] [CrossRef]
- Alhakemy, A.Z.; Elsayed, M.H.; Algethami, F.K.; Abdelhamid, H.N. Metal-organic framework (MOF)-derived bimetallic (Ni, Cu) Oxide@C electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2025, 115, 289–298. [Google Scholar] [CrossRef]
- Lee, S.H.; Jo, S.; Jeon, J.I.; Sohn, J.I.; Hong, J. A heterostructured ternary transition metal oxide composite as an efficient electrocatalyst for the hydrogen evolution reaction. J. Environ. Chem. Eng. 2024, 12, 112796. [Google Scholar] [CrossRef]
- Subha, N.; Sankar, A.R. Bifunctional metal oxide-infused SWCNT/Nickel phosphides for efficient electrocatalytic water splitting. Colloids Surf. A Physicochem. Eng. Asp. 2025, 708, 135998. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Y.; Cui, Z.; Huang, H.; Cai, D.; Zhang, J.; Zhou, Y.; Xu, M.; Tong, R. Nickel sulfide-based electrocatalysts for overall water splitting. Int. J. Hydrogen Energy 2023, 48, 27992–28017. [Google Scholar] [CrossRef]
- Sahoo, K.; Kumar, D.; Kushwaha, V.K.; Verma, V.K.; Basu, S.; Indra, A.; Sonawane, S.H. Bimetallic copper-cobalt oxide/graphene nano-composite: Potential as a pseudocapacitive electrode and OER-HER electrocatalyst. Mater. Res. Bull. 2025, 188, 113417. [Google Scholar] [CrossRef]
- Song, X.-L.; Chen, L.; Ren, J.-T.; Gao, L.-J.; Yuan, Z.-Y. Engineering of g-C3N4-based composites for photocatalytic and electrocatalytic water splitting: Recent progress, challenges and perspective. Coord. Chem. Rev. 2024, 507, 215752. [Google Scholar] [CrossRef]
- Yu, Z.-R.; Ji, M.-X.; Zhang, Z.-H.; Zhang, Y.; Barras, A.; Addad, A.; Tang, L.-C.; Chi, Y.-H.; Roussel, P.; Szunerits, S.; et al. Electrocatalytic upcycling of polyethylene terephthalate to formic acid and hydrogen fuels using CoCuO/MXene catalyst. J. Energy Chem. 2025, 104, 91–100. [Google Scholar] [CrossRef]
- Mahmoudi, F.; Park, C.M.; Shim, J.-J. Ultrasound-assisted heterogeneous Fenton-like process for efficient degradation of tetracycline over SmFeO3/Ti3C2Tx catalyst. J. Water Process Eng. 2022, 50, 103235. [Google Scholar] [CrossRef]
- He, L.; Zhuang, H.; Fan, Q.; Yu, P.; Wang, S.; Pang, Y.; Chen, K.; Liang, K. Advances and challenges in MXene-based electrocatalysts: Unlocking the potential for sustainable energy conversion. Mater. Horiz. 2024, 11, 4239–4255. [Google Scholar] [CrossRef]
- Kumar, D.R.; Kanagaraj, I.; Sukanya, R.; Karthik, R.; Hasan, M.; Thalji, M.R.; Dhakal, G.; Milton, A.; Prakash, A.S.; Shim, J.-J. Ti3C2Tx filled in EMIMBF4 semi-solid polymer electrolytes for the zinc-metal battery. ACS Appl. Mater. Interfaces 2024, 16, 33294–33306. [Google Scholar] [CrossRef]
- Alli, Y.A.; Bamisaye, A.; Nancy, P.; Zachariah, S.M.; Oladoye, P.O.; Bankole, O.M.; Akamo, D.O.; Chkirida, S.; Anuar, H.; Thomas, S. MXene composites: Properties, synthesis and its emerging application in rechargeable batteries. J. Energy Storage 2024, 77, 109954. [Google Scholar] [CrossRef]
- Saju, D.M.; Sapna, R.; Deka, U.; Hareesh, K. MXene material for supercapacitor applications: A comprehensive review on properties, synthesis and machine learning for supercapacitance performance prediction. J. Power Sources 2025, 647, 237302. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, K.; Mendhe, A.C.; Thalji, M.R.; Mandal, S.; Ali, I.; El-Mahdy, A.F.M.; Rosaiah, P.; Aslam, M.K.; Tamang, T.L.; et al. Integration of non-Ti3C2 MXene with carbon-based materials for energy storage devices: Recent advancements and future aspects. Prog. Solid. State Chem. 2025, 78, 100523. [Google Scholar] [CrossRef]
- Zubair, M.; Ul Hassan, M.M.; Mehran, M.T.; Baig, M.M.; Hussain, S.; Shahzad, F. 2D MXenes and their heterostructures for HER, OER and overall water splitting: A review. Int. J. Hydrogen Energy 2022, 47, 2794–2818. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.; Li, Y.; Fu, R. 2D Ti3C2Tx MXene-supported graphitic carbon-nitride-decorated Co3O4 nanoparticles as efficient catalysts for oxygen evolution reaction. ACS Appl. Energy Mater. 2023, 6, 5774–5786. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, X.; Li, Z.; Beyene, T.T.; Zheng, T.; Liu, Y.; Zhu, K. Review and perspectives on preparation strategies and applications of Ti3C2 MXene for Li metal batteries/Li–S batteries. Energy Fuels 2024, 38, 14866–14890. [Google Scholar] [CrossRef]
- Das, H.T.; Balaji, T.E.; Dutta, S.; Das, N.; Maiyalagan, T. Recent advances in MXene as electrocatalysts for sustainable energy generation: A review on surface engineering and compositing of MXene. Int. J. Energy Res. 2022, 46, 8625–8656. [Google Scholar] [CrossRef]
- Yang, C.; Huang, H.; He, H.; Yang, L.; Jiang, Q.; Li, W. Recent advances in MXene-based nanoarchitectures as electrode materials for future energy generation and conversion applications. Coord. Chem. Rev. 2021, 435, 213806. [Google Scholar] [CrossRef]
- Al Mahmud, A.; Thalji, M.R.; Dhakal, G.; Haldorai, Y.; Kim, W.K.; Shim, J.-J. Bifunctional MoC/NiC@N-doped reduced graphene oxide nano electrocatalyst for simultaneous production of hydrogen and oxygen through efficient overall electrochemical water splitting. Mater. Today Nano 2024, 27, 100489. [Google Scholar] [CrossRef]
- Hao, L.P.; Hanan, A.; Walvekar, R.; Khalid, M.; Bibi, F.; Wong, W.Y.; Prakash, C. Synergistic integration of MXene and metal-organic frameworks for enhanced electrocatalytic hydrogen evolution in an alkaline environment. Catalysts 2023, 13, 802. [Google Scholar] [CrossRef]
- Mahmud, A.A.; Alshatteri, A.H.; Alhasan, H.S.; Zoubi, W.A.; Omer, K.M.; Thalji, M.R. Copper-doped strontium metal-organic framework: Dual-function active material for supercapacitor and oxygen evolution reaction. Electrochim. Acta 2024, 503, 144857. [Google Scholar] [CrossRef]
- Wu, Y.; Bo, T.; Tu, H.; Wang, L.; Shi, W. U and Co dual single-atom doped MXene for accelerating electrocatalytic hydrogen evolution activity. Small 2024, 20, e2402847. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Oh, Y.; Ghimire, M.; Son, J.W.; Lee, M.; Jun, Y. Value addition of MXenes as photo-/electrocatalysts in water splitting for sustainable hydrogen production. Chem. Commun. 2024, 60, 8789–8805. [Google Scholar] [CrossRef] [PubMed]
- Reghunath, B.S.; KR, S.D.; Rajasekaran, S.; Saravanakumar, B.; William, J.J.; Pinheiro, D. Hierarchical BiFeO3/Cr2CTx MXene composite as a multifunctional catalyst for hydrogen evolution reaction and as an electrode material for energy storage devices. Electrochim. Acta 2023, 461, 142685. [Google Scholar] [CrossRef]
- Anne, B.R.; Kundu, J.; Kabiraz, M.K.; Kim, J.; Cho, D.; Choi, S.I. A review on MXene as promising support materials for oxygen evolution reaction catalysts. Adv. Funct. Mater. 2023, 33, 202306100. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Guo, M.; Li, M.; Hao, H.; Wang, C.; Jin, L.; Zhao, C.; Shao, X.; Yu, X. Advances in atomically dispersed catalysts for water splitting. Adv. Funct. Mater. 2025, 35, e2503228. [Google Scholar] [CrossRef]
- Mane, R.S.; Zaroliwalla, D.; Periyasamy, G.; Jha, N. Leafy ZIF-derived bi-metallic phosphate-Mxene nanocomposites for overall water splitting. Small 2025, 21, e2503228. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Boakye, F.O.; Zaman, F.u.; Zhang, H.; Saeed, A.; Dajan, F.T.; Iqbal, S.; Harrath, K. Functional interface optimization strategy for Fe3Se4/NiSe2 anchored on MXene for ultrastable seawater splitting at industrial-level current density. Adv. Funct. Mater. 2025, 35, 2424718. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Al Mahmud, A.; Hazmatulhaq, F.; Thalji, M.R.; Leoni, S.; Kang, J.H.; Ko, Y.G. Origin of the synergistic effects of bimetallic nanoparticles coupled with a metal oxide heterostructure for accelerating catalytic performance. SusMat 2024, 4, e216. [Google Scholar] [CrossRef]
- Vazhayil, A.; Vazhayal, L.; Thomas, J.; Ashok, C.S.; Thomas, N. A comprehensive review on the recent developments in transition metal-based electrocatalysts for oxygen evolution reaction. Appl. Surf. Sci. Adv. 2021, 6, 100184. [Google Scholar] [CrossRef]
- Sajid, I.H.; Iqbal, M.Z.; Rizwan, S. Recent advances in the role of MXene based hybrid architectures as electrocatalysts for water splitting. RSC Adv. 2024, 14, 6823–6847. [Google Scholar] [CrossRef] [PubMed]
- Uwadiunor, E.; Johnson, D.; Hansen, K.; Djire, A. Controlling the surface reactivity of hybrid Ti3CN MXene via in-situ electrocatalysis. ChemCatChem 2022, 14, 202200702. [Google Scholar] [CrossRef]
- Bhattacharyya, H.P.; Sarma, M. MaxKinEff: A collision theory-based approach for analyzing turnover frequency and turnover number in catalytic processes. Chem. Asian J. 2024, 19, e202400674. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Guo, X.; Cao, X.; Wang, S.; Liu, H.; Wang, G. Engineering strategies and active site identification of MXene-based catalysts for electrochemical conversion reactions. Chem. Soc. Rev. 2023, 52, 3215–3264. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Wu, Z.S. Recent advancement and key opportunities of MXenes for electrocatalysis. iScience 2024, 27, 108906. [Google Scholar] [CrossRef]
- Ooka, H.; Huang, J.; Exner, K.S. The sabatier principle in electrocatalysis: Basics, limitations, and extensions. Front. Energy Res. 2021, 9, 654460. [Google Scholar] [CrossRef]
- Feng, Z.; Dai, C.; Shi, P.; Lei, X.; Guo, R.; Wang, B.; Liu, X.; You, J. Seven mechanisms of oxygen evolution reaction proposed recently: A mini review. Chem. Eng. J. 2024, 485, 149992. [Google Scholar] [CrossRef]
- Qian, Z.X.; Peng, C.K.; Yue, M.F.; Hsu, L.C.; Zeng, J.S.; Wei, D.Y.; Du, Z.Y.; Xu, G.Y.; Zhang, H.; Tian, J.H.; et al. Direct capturing and regulating key intermediates for high-efficiency oxygen evolution reactions. Small Methods 2024, 8, e2301504. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Faridi, S.; Razzaq, S.; Singh, D.; Meng, L.; Viñes, F.; Illas, F.; Exner, K.S. Trends in competing oxygen and chlorine evolution reactions over electrochemically formed single-atom centers of MXenes. J. Mater. Chem. A 2025, 13, 16481–16490. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y.; Huang, Q. MXenes: Two-dimensional building blocks for future materials and devices. ACS Nano 2021, 15, 5775–5780. [Google Scholar] [CrossRef]
- Fei, L.; Lei, L.; Xu, H.; Guo, X.; Chen, B.; Han, X.; Chen, X.; Huang, Q.; Wang, D. Ion transport behaviors in MXenes for electrochemical energy storage and conversion. Carbon Energy 2025, 7, e678. [Google Scholar] [CrossRef]
- Paul, T.K.; Khaleque, M.A.; Ali, M.R.; Aly Saad Aly, M.; Bacchu, M.S.; Rahman, S.; Khan, M.Z.H. MXenes from MAX phases: Synthesis, hybridization, and advances in supercapacitor applications. RSC Adv. 2025, 15, 8948–8976. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, T.; Tian, Z.; Bibi, K.; Zhang, Y.Z.; Alshareef, H.N. MXenes for energy harvesting. Adv. Mater. 2022, 34, e2108560. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Jin, S.; Xia, Q.; Chang, Y.; Wang, L.; Zhou, A. Synthesis of Mo2C MXene with high electrochemical performance by alkali hydrothermal etching. J. Adv. Ceram. 2023, 12, 1889–1901. [Google Scholar] [CrossRef]
- Xue, N.; Li, X.; Zhang, M.; Han, L.; Liu, Y.; Tao, X. Chemical-combined ball-milling synthesis of fluorine-free porous MXene for high-performance lithium ion batteries. ACS Appl. Energy Mater. 2020, 3, 10234–10241. [Google Scholar] [CrossRef]
- Lipatov, A.; Alhabeb, M.; Lukatskaya, M.R.; Boson, A.; Gogotsi, Y.; Sinitskii, A. Effect of synthesis on quality, electronic properties and environmental stability of individual monolayer Ti3C2 MXene flakes. Adv. Electron. Mater. 2016, 2, 201600255. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P.; et al. Element replacement approach by reaction with lewis acidic molten salts to synthesize nanolaminated MAX phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B.; et al. Fluorine-free synthesis of high-purity Ti3C2Tx (T=OH, O) via alkali treatment. Angew. Chem. Int. Ed. 2018, 57, 6115–6119. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, P.; Wang, F.; Ricciardulli, A.G.; Lohe, M.R.; Blom, P.W.M.; Feng, X. Fluoride-free synthesis of two-dimensional titanium carbide (MXene) using a binary aqueous system. Angew. Chem. Int. Ed. 2018, 57, 15491–15495. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y.; Zhang, Y.; Meng, H.; Xu, Y.; Han, Y.; Wang, Z.; Dong, Y.; Zhang, X. Highly safe and ionothermal synthesis of Ti3C2 MXene with expanded interlayer spacing for enhanced lithium storage. J. Energy Chem. 2020, 47, 203–209. [Google Scholar] [CrossRef]
- Avinashi, S.K.; Mishra, R.K.; Singh, R.; Shweta; Rakhi; Fatima, Z.; Gautam, C.R. Fabrication methods, structural, surface morphology and biomedical applications of MXene: A review. ACS Appl. Mater. Interfaces 2024, 16, 47003–47049. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, M.; Zhou, W.; Xu, X.; Liu, B.; Zhang, W.; Wong, C. Simplified synthesis of fluoride-free Ti3C2Tx via electrochemical etching toward high-performance electrochemical capacitors. ACS Nano 2022, 16, 2461–2470. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, M.; Sharma, A. Review of Ti3C2Tx MXene nanosheets and their applications. ACS Appl. Nano Mater. 2024, 7, 9847–9867. [Google Scholar] [CrossRef]
- Ronchi, R.M.; Arantes, J.T.; Santos, S.F. Synthesis, structure, properties and applications of MXenes: Current status and perspectives. Ceram. Int. 2019, 45, 18167–18188. [Google Scholar] [CrossRef]
- Oyehan, T.A.; Salami, B.A.; Abdulrasheed, A.A.; Hambali, H.U.; Gbadamosi, A.; Valsami-Jones, E.; Saleh, T.A. MXenes: Synthesis, properties, and applications for sustainable energy and environment. Appl. Mater. Today 2023, 35, 101993. [Google Scholar] [CrossRef]
- Murray, S.L.; Serajian, S.; Gnani Peer Mohamed, S.I.; Robinson, S.; Krishnamoorthy, R.; Das, S.R.; Bavarian, M.; Nejati, S.; Kilic, U.; Schubert, M.; et al. Ultrabroadband optical properties of 2D titanium carbide MXene. ACS Appl. Mater. Interfaces 2024, 16, 70763–70773. [Google Scholar] [CrossRef]
- Ahouei, M.A.; Syed, T.H.; Bishop, V.; Halacoglu, S.; Wang, H.; Wei, W. Ti3C2Tx MXene framework materials: Preparation, properties and applications in energy and environment. Catal. Today 2023, 409, 162–172. [Google Scholar] [CrossRef]
- Fu, B.; Sun, J.; Wang, C.; Shang, C.; Xu, L.; Li, J.; Zhang, H. MXenes: Synthesis, optical properties, and applications in ultrafast photonics. Small 2021, 17, e2006054. [Google Scholar] [CrossRef]
- Gautam, J.; Lee, S.-Y.; Park, S.-J. Strategic structural design of transition metal electrocatalysts for efficient water splitting: A comprehensive review. Nano Today 2024, 59, 102487. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2013, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chang, L.; Zhang, X.; Wan, H.; Liu, N.; Zhou, L.; Xiao, X. Simultaneously tuning interlayer spacing and termination of MXenes by Lewis-basic halides. Nat. Commun. 2022, 13, 6731. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Liu, J.; Zhao, W.; Du, J.; Zhong, W. Molybdenum carbide MXene embedded with nickel sulfide clusters as an efficient electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 17526–17535. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, H.; Xue, H.; Li, Q.; Li, H.; Zhang, Y.; Dong, Y.; Xie, H.; Ma, Y. Ti3C2-based MXene anchoring single-atom Co as a long-lasting peroxymonosulfate activator enabling efficient water decontamination: Deciphering the critical role of titanium vacancies. Appl. Catal. B Environ. Energy 2025, 365, 124966. [Google Scholar] [CrossRef]
- Zhao, X.; Li, W.P.; Cao, Y.; Portniagin, A.; Tang, B.; Wang, S.; Liu, Q.; Yu, D.Y.W.; Zhong, X.; Zheng, X.; et al. Dual-atom Co/Ni electrocatalyst anchored at the surface-modified Ti3C2Tx MXene enables efficient hydrogen and oxygen evolution reactions. ACS Nano 2024, 18, 4256–4268. [Google Scholar] [CrossRef]
- Li, H.; Wei, S.; Wang, H.; Cai, Q.; Zhao, J. Enhanced catalytic activity of MXene for nitrogen electoreduction reaction by carbon doping. J. Colloid Interface Sci. 2021, 588, 1–8. [Google Scholar] [CrossRef]
- Ronchi, R.M.; Chen, N.; Halim, J.; Persson, P.O.A.; Rosen, J. Defect engineering of Mo(2-x) CT(z) MXenes through precursor alloying and effects on electrochemical properties. Chem. Mater. 2025, 37, 4005–4015. [Google Scholar] [CrossRef]
- Fatima, S.; Hakim, M.W.; Zheng, X.; Sun, Y.; Li, Z.; Han, N.; Li, M.; Wang, Z.; Han, L.; Wang, L.; et al. Constructing nitrogen-doped graphene quantum dots/tantalum carbide MXene heterojunctions as bifunctional catalysts for efficient water splitting. Int. J. Hydrogen Energy 2025, 117, 420–429. [Google Scholar] [CrossRef]
- Mathew, S.; Madhushree, R.; KR, S.D. CoFe2O4/g-C3N4 intercalated Ti3C2 MXene for efficient electrocatalytic hydrogen evolution reaction. Sustain. Energy Fuels 2023, 7, 2601–2612. [Google Scholar] [CrossRef]
- Yan, L.; Du, Z.; Lai, X.; Lan, J.; Liu, X.; Liao, J.; Feng, Y.; Li, H. Synergistically modulating the electronic structure of Cr-doped FeNi LDH nanoarrays by O-vacancy and coupling of MXene for enhanced oxygen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 1892–1903. [Google Scholar] [CrossRef]
- Ghaemmaghami, M.; Yamini, Y. Three-dimensional network of highly uniform cobalt oxide microspheres/MXene composite as a high-performance electrocatalyst in hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2024, 16, 18782–18789. [Google Scholar] [CrossRef] [PubMed]
- Farooq, K.; Murtaza, M.; Yang, Z.; Waseem, A.; Zhu, Y.; Xia, Y. MXene boosted MOF-derived cobalt sulfide/carbon nanocomposites as efficient bifunctional electrocatalysts for OER and HER. Nanoscale Adv. 2024, 6, 3169–3180. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Ahmed, W.; Zeeshan, M.; Hu, S.; Zhang, X. Integral 2D/3D structured CoSnO3@MXene/NF as a highly active and stable bifunctional electrocatalyst for alkaline water splitting. Int. J. Hydrogen Energy 2024, 70, 448–460. [Google Scholar] [CrossRef]
- Fang, T.; Ma, J.; Dai, Y.; Gou, J.; Li, X.; Li, C.; Tong, J. 2D Sandwich-like Co-MOF/MXene composites as self-reconstructive oxygen evolution electrocatalysts. ACS Appl. Nano Mater. 2025, 8, 3519–3526. [Google Scholar] [CrossRef]
- Hanan, A.; Lakhan, M.N.; Bibi, F.; Khan, A.; Soomro, I.A.; Hussain, A.; Aftab, U. MOFs coupled transition metals, graphene, and MXenes: Emerging electrocatalysts for hydrogen evolution reaction. Chem. Eng. J. 2024, 482, 148776. [Google Scholar] [CrossRef]
- Ma, S.; Xu, Z.; Jia, Z.; Chen, L.; Zhu, H.; Chen, Y.; Guo, X.; Du, M. Facile fabrication of carbon fiber skeleton structure of MoS2 supported on 2D MXene composite with highly efficient and stable hydrogen evolution reaction. Compos. Sci. Technol. 2022, 222, 109380. [Google Scholar] [CrossRef]
- Patra, A.; Samal, R.; Rout, C.S. Promising water splitting applications of synergistically assembled robust orthorhombic CoSe2 and 2D Ti3C2Tx MXene hybrid. Catal. Today 2023, 424, 113853. [Google Scholar] [CrossRef]
- Rasool, F.; Pirzada, B.M.; Talib, S.H.; Alkhidir, T.; Anjum, D.H.; Mohamed, S.; Qurashi, A. In situ growth of interfacially nanoengineered 2D-2D WS2/Ti3C2Tx MXene for the enhanced performance of hydrogen evolution reactions. ACS Appl. Mater. Interfaces 2024, 16, 14229–14242. [Google Scholar] [CrossRef]
- Lim, K.R.G.; Handoko, A.D.; Nemani, S.K.; Wyatt, B.; Jiang, H.Y.; Tang, J.; Anasori, B.; Seh, Z.W. Rational design of two-dimensional transition metal carbide/nitride (MXene) hybrids and nanocomposites for catalytic energy storage and conversion. ACS Nano 2020, 14, 10834–10864. [Google Scholar] [CrossRef]
- Kumar, N.; Aepuru, R.; Lee, S.Y.; Park, S.J. Advances in catalysts for hydrogen production: A comprehensive review of materials and mechanisms. Nanomaterials 2025, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Fredrickson, K.D.; Anasori, B.; Kibsgaard, J.; Strickler, A.L.; Lukatskaya, M.R.; Gogotsi, Y.; Jaramillo, T.F.; Vojvodic, A. Two-dimensional molybdenum carbide (MXene) as an efficient electrocatalyst for hydrogen evolution. ACS Energy Lett. 2016, 1, 589–594. [Google Scholar] [CrossRef]
- Luxa, J.; Kupka, P.; Lipilin, F.; Sturala, J.; Subramani, A.; Lazar, P.; Sofer, Z. Hydrogen evolution reaction activity in Mo2TiC2Tx MXene derived from Mo2TiAlC2 MAX phase: Insights from compositional transformations. ACS Catal. 2024, 14, 15336–15347. [Google Scholar] [CrossRef]
- Snyder, R.M.; Nguyen, T.; Bhatt, P.; Riaz, A.A.; Thakur, P.K.; Lee, T.-L.; Regoutz, A.; Jones, A.K.; Birkel, C.S. (V1–yMoy)2CTx MXene Nanosheets as electrocatalysts for hydrogen evolution. ACS Appl. Nano Mater. 2025, 8, 1137–1146. [Google Scholar] [CrossRef]
- Lei, Z.; Ali, S.; Sathish, C.I.; Ahmed, M.; Qu, J.; Zheng, R.; Xi, S.; Yu, X.; Breese, M.B.H.; Liu, C.; et al. Transition metal carbonitride MXenes anchored with Pt sub-nanometer clusters to achieve high-performance hydrogen evolution reaction at all pH range. Nanomicro Lett. 2025, 17, 123. [Google Scholar] [CrossRef]
- Tran, P.K.L.; Nguyen, T.H.; Tran, D.T.; Dinh, V.A.; Nga Ta, T.T.; Dong, C.-L.; Kim, N.H.; Lee, J.H. Tunable charge Pt sites-dominated alloy confined by mxene-derived oxycarbide enables ultra-stable ampere-level hydrogen production. Appl. Catal. B Environ. Energy 2025, 363, 124801. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Kang, J.; Wang, X.; Yu, H.; Du, C.-F. Ni nanoparticles anchoring on vacuum treated Mo2TiC2Tx MXene for enhanced hydrogen evolution activity. Chin. J. Struct. Chem. 2023, 42, 100159. [Google Scholar] [CrossRef]
- Raj, S.K.; Patel, K.B.; Sharma, V.; Srivastava, D.N.; Kulshrestha, V. Nanopalladium-anchored MXene nanoflowers for boosting electrocatalytic hydrogen evolution reaction. Energy Fuels 2023, 37, 16856–16865. [Google Scholar] [CrossRef]
- Xue, Y.; Xie, Y.; Xu, C.; He, H.; Jiang, Q.; Ying, G.; Huang, H. 0D/2D heterojunction of graphene quantum dots/MXene nanosheets for boosted hydrogen evolution reaction. Surf. Interfaces 2022, 30, 101907. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Pei, C.; Lu, Y.; Kim, J.K.; Park, H.S.; Pang, H. Interfacial Design of Ti3C2Tx MXene/graphene heterostructures boosted Ru nanoclusters with high activity toward hydrogen evolution reaction. Adv. Sci. 2024, 11, e2310013. [Google Scholar] [CrossRef]
- Wu, M.; Fan, X.; Zhang, W.; Chen, B.; Ye, T.; Zhang, Q.; Fang, Y.; Wang, Y.; Tang, Y. Highly dispersed Ru nanospecies on N-doped carbon/MXene composite for highly efficient alkaline hydrogen evolution. Chin. Chem. Lett. 2024, 35, 109258. [Google Scholar] [CrossRef]
- Sun, C.; Tan, Y.; Huang, Y.; Guo, F.; Deng, L.; Cheng, S. Construction of hierarchical PdAgAu nanorings/MXene–GO electrocatalysts for efficient and ultrastable hydrogen evolution reaction. Adv. Energy Sustain. Res. 2023, 4, 202200194. [Google Scholar] [CrossRef]
- Yin, J.; Wei, K.; Bai, Y.; Liu, Y.; Zhang, Q.; Wang, J.; Qin, Z.; Jiao, T. Integration of amorphous CoSnO3 onto wrinkled MXene nanosheets as efficient electrocatalysts for alkaline hydrogen evolution. Sep. Purif. Technol. 2023, 308, 122947. [Google Scholar] [CrossRef]
- Khalil, M.; Lesa, M.; Juandito, A.G.; Sanjaya, A.R.; Ivandini, T.A.; Kadja, G.T.M.; Mahyuddin, M.H.; Sookhakian, M.; Alias, Y. A SBA-15-templated mesoporous NiFe2O4/MXene nanocomposite for the alkaline hydrogen evolution reaction. Mater. Adv. 2023, 4, 3853–3862. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, S.; Shen, B.; He, H.; Huang, H. Confining tungsten disulfide quantum dots on MXene nanosheets enables efficient hydrogen evolution reaction. ACS Appl. Energy Mater. 2025, 8, 2747–2754. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, Y.; Zhang, X.; Xing, F.; Zhang, Y.; Feng, Y.; Zhou, T. VS2 nanoflowers supported on Ti3C2 MXene as electrocatalyst for the hydrogen evolution reaction. ACS Appl. Nano Mater. 2024, 7, 25880–25890. [Google Scholar] [CrossRef]
- Hussain, S.; Vikraman, D.; Ali Sheikh, Z.; Taqi Mehran, M.; Shahzad, F.; Mujasam Batoo, K.; Kim, H.-S.; Kim, D.-K.; Ali, M.; Jung, J. WS2-embedded MXene/GO hybrid nanosheets as electrodes for asymmetric supercapacitors and hydrogen evolution reactions. Chem. Eng. J. 2023, 452, 139523. [Google Scholar] [CrossRef]
- Dongre, S.S.; Iqbal, A.; Thapa, R.; Pratheeksha, M.; Ramu, S.; Balakrishna, R.G. Synergistic catalyst design for enhanced electrochemical hydrogen evolution: Fe2O3/MoS2/Ti3C2Tx MXene ternary composite. ACS Appl. Eng. Mater. 2024, 2, 943–954. [Google Scholar] [CrossRef]
- Rasool, F.; Pirzada, B.M.; Misbah Uddin, M.; Mohideen, M.I.H.; Yildiz, I.; Elkadi, M.; Qurashi, A. Interfacial engineering of ZnS–ZnO decorated MoS2 supported on 2D Ti3C2Tx MXene sheets for enhanced hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 59, 63–73. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Y.; Xiang, K.; Bai, S.; Wang, H.; Zou, J.; Arramel; Jiang, J. CoP decorated on Ti3C2Tx MXene nanocomposites as robust electrocatalyst for hydrogen evolution reaction. Acta Phys.-Chim. Sin. 2024, 40, 2308015. [Google Scholar] [CrossRef]
- Niu, H.J.; Huang, C.; Sun, T.; Fang, Z.; Ke, X.; Zhang, R.; Ran, N.; Wu, J.; Liu, J.; Zhou, W. Enhancing Ni/Co activity by neighboring Pt atoms in NiCoP/MXene electrocatalyst for alkaline hydrogen evolution. Angew. Chem. Int. Ed. 2024, 63, e202401819. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Tang, Q. Surface termination (-O, -F or -OH) and metal doping on the HER activity of Mo2CTx MXene. Chemphyschem 2024, 25, e202400255. [Google Scholar] [CrossRef]
- Shen, Y.; Bai, J.; Wei, H.; Gu, J.; Cao, Q. Recent strategies for Ni3S2-based electrocatalysts with enhanced hydrogen evolution performance: A Tutorial Review. Int. J. Mol. Sci. 2025, 26, 3771. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Yuan, X.; Yang, W.; Zhang, H.; Lu, Y.; Zhang, R. Fe horizontal line N4 and Fe7Co3 nanoalloy dual-site modulation by skeleton defect in N-doped graphene aerogel for enhanced bifunctional oxygen electrocatalyst in zinc-air battery. Small 2025, 21, e2410264. [Google Scholar] [CrossRef] [PubMed]
- Rong, C.; Huang, X.; Arandiyan, H.; Shao, Z.; Wang, Y.; Chen, Y. Advances in Oxygen evolution reaction electrocatalysts via direct oxygen-oxygen radical coupling pathway. Adv. Mater. 2025, 37, e2416362. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wang, F.; Jin, R.; Guo, B.; Huo, H.; Dai, Y.; Liu, Z.; Liu, J.; Li, S. Dual doping in precious metal oxides: Accelerating acidic oxygen evolution reaction. Int. J. Mol. Sci. 2025, 26, 1582. [Google Scholar] [CrossRef]
- Liu, H.-J.; Dong, B. Recent advances and prospects of MXene-based materials for electrocatalysis and energy storage. Mater. Today Phys. 2021, 20, 100469. [Google Scholar] [CrossRef]
- Du, X.; Zhang, X.; Zhu, S.; Xu, Y.; Wang, Y. Modulated interfacial electron transfer of MXene-Tx@CoS for the oxygen evolution reaction. Sustain. Energy Fuels 2023, 7, 4977–4983. [Google Scholar] [CrossRef]
- Raj, R.; Iqbal, M.; Ram, M.; Kumar, G.; Patil, R.A.; Ma, Y.-R.; Prasad Singh, G.; Kanta Haldar, K. Enhanced electrochemical performance of Nickel Oxide-doped MXene heterostructures for advanced energy storage and conversion applications. Fuel 2025, 385, 133916. [Google Scholar] [CrossRef]
- Zaka, A.; Mansoor, M.A.; Asghar, M.A.; Haider, A.; Iqbal, M. V2C MXene-TiO2 nanocomposite as an efficient electrode material for oxygen evolution reaction (OER). Int. J. Hydrogen Energy 2023, 48, 34599–34609. [Google Scholar] [CrossRef]
- Ghorbanzadeh, S.; Hosseini, S.A.; Alishahi, M. CuCo2O4/Ti3C2Tx MXene hybrid electrocatalysts for oxygen evolution reaction of water splitting. J. Alloys Compd. 2022, 920, 165811. [Google Scholar] [CrossRef]
- Yan, L.; Liang, J.; Li, H. In situ construction of heterostructure NiSe–NiO nanoarrays with rich oxygen vacancy on MXene for efficient oxygen evolution. Int. J. Hydrogen Energy 2023, 48, 13159–13169. [Google Scholar] [CrossRef]
- Li, C.; Jing, H.; Wu, Z.; Jiang, D. Layered double hydroxides for photo(electro)catalytic applications: A mini review. Nanomaterials 2022, 12, 3525. [Google Scholar] [CrossRef]
- Chen, B.Q.; Xia, H.Y.; Mende, L.K.; Lee, C.H.; Wang, S.B.; Chen, A.Z.; Xu, Z.P.; Kankala, R.K. Trends in layered double hydroxides-based advanced nanocomposites: Recent progress and latest advancements. Adv. Mater. Interfaces 2022, 9, 2200373. [Google Scholar] [CrossRef]
- Yang, H.-X.; Xu, W.; Zhong, P.-W.; Zhang, D.; Yu, Z.; Li, B.; Wang, H.; Cao, Y.; Wang, H.-F.; Yu, H. Coupling NiFe alloy/LDH and Mo2CT MXene for enhanced oxygen evolution. J. Energy Chem. 2025, 105, 121–129. [Google Scholar] [CrossRef]
- Pal, S.; Chaturvedi, E.; Das, C.; Sinha, N.; Ahmed, T.; Roy, P. NiFeMo layered triple hydroxide and MXene heterostructure for boosted oxygen evolution reaction in anion exchange membrane water electrolysis. Nanoscale 2025, 17, 12094–12107. [Google Scholar] [CrossRef]
- Zhang, B.; Shan, J.; Wang, X.; Hu, Y.; Li, Y. Ru/Rh cation doping and oxygen-vacancy engineering of FeOOH nanoarrays@Ti3 C2Tx MXene heterojunction for highly efficient and stable electrocatalytic oxygen evolution. Small 2022, 18, e2200173. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, X.; Jia, X. From low- to high-crystallinity bimetal–organic framework nanosheet with highly exposed boundaries: An efficient and stable electrocatalyst for oxygen evolution reaction. ACS Sustain. Chem. Eng. 2019, 7, 16629–16639. [Google Scholar] [CrossRef]
- Środa, B.; Dymerska, A.G.; Zielińska, B.; Mijowska, E. Promotion of MXene (Ti3C2Tx) as a robust electrocatalyst for oxygen evolution reaction via active sites of ZIF-67- in situ mechanism investigations. Int. J. Hydrogen Energy 2023, 48, 18696–18707. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Y.; Wang, B.; Wang, J.; Dai, Y.; Hu, L.; Lv, X.; Dang, J. Significantly enhanced OER and HER performance of NiCo-LDH and NiCoP under industrial water splitting conditions through Ru and Mn bimetallic co-doping strategy. Chem. Eng. J. 2024, 494, 153212. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, Q.; Cao, S.; Lin, X.; Chen, X.; Wang, Z.; Wei, S.; Liu, S.; Dai, F.; Lu, X. Research status, opportunities, and challenges of cobalt phosphate based materials as OER electrocatalysts. Green Chem. 2023, 25, 7883–7903. [Google Scholar] [CrossRef]
- Mei, P.; Kim, J.; Kumar, N.A.; Pramanik, M.; Kobayashi, N.; Sugahara, Y.; Yamauchi, Y. Phosphorus-based mesoporous materials for energy storage and conversion. Joule 2018, 2, 2289–2306. [Google Scholar] [CrossRef]
- Guo, J.; Ouyang, C.; Zhan, Z.; Lei, T.; Yin, P. Facile synthesis of tubular CoP as a high efficient electrocatalyst for pH-universal hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 181–196. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, T.; Chen, Q.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. FeP-CoP nanocubes in situ grown on Ti3C2Tx MXene as efficient electrocatalysts for the oxygen evolution reaction. Ind. Eng. Chem. Res. 2022, 61, 10837–10845. [Google Scholar] [CrossRef]
- Li, N.; Han, J.; Yao, K.; Han, M.; Wang, Z.; Liu, Y.; Liu, L.; Liang, H. Synergistic phosphorized NiFeCo and MXene interaction inspired the formation of high-valence metal sites for efficient oxygen evolution. J. Mater. Sci. Technol. 2022, 106, 90–97. [Google Scholar] [CrossRef]
- John Jeya Kamaraj, J.J.; Kunka Ravindran, A.; Muthu, S.P.; Perumalsamy, R. MXene-supported 2D bimetallic chalcogenide electrocatalyst: Enhanced electrochemical seawater splitting. J. Power Sources 2025, 629, 235951. [Google Scholar] [CrossRef]
- Lee, D.J.; Sekar, S.; Jeon, H.C.; Lee, Y.; Lee, S.; Kim, D.Y.; Ilanchezhiyan, P. Co modified ReS2 nanospheres coupled with Ti3C2Tx MXene nanohybrid heterostructures as bifunctional electrocatalyst for highly efficient water splitting applications. Appl. Surf. Sci. 2025, 696, 162823. [Google Scholar] [CrossRef]
- Manchuri, A.R.; Devarayapalli, K.C.; Kim, B.; Lim, Y.; Lee, D.S. Ti3C2 MXene nanosheets integrated cobalt-doped nickel hydroxide heterostructured composite: An efficient electrocatalyst for overall water-splitting. Green Energy Environ. 2025, 10, 854–868. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, D.; Chen, G.; An, P.; Zhang, J.; Li, Y.; Liu, S.F.; Yan, J. Novel Gel method MXene-supported dual-site PtNi-NiO for electrocatalytic water reduction and urea oxidation. Small 2025, 21, e2409461. [Google Scholar] [CrossRef]
- Chauhan, S.V.; Joshi, K.K.; Pataniya, P.M.; Sahatiya, P.; Bhadu, G.; Sumesh, C.K. Fe2B/MXene@NF electrocatalyst for efficient water splitting and green hydrogen production at high current densities. Renew. Energy 2025, 241, 122370. [Google Scholar] [CrossRef]
- Farooq, K.; Yang, Z.; Murtaza, M.; Naseeb, M.A.; Waseem, A.; Zhu, Y.; Xia, Y. MXene-enhanced metal-organic framework-derived CoP nanocomposites as highly efficient trifunctional electrocatalysts for OER, HER, and ORR. Adv. Energy Sustain. Res. 2025, 6, 2400400. [Google Scholar] [CrossRef]
- Sharifi, R.; Ashoori, A.; Dolati, A.; Seif, A. Hierarchical micro-nano 3D structures of metal selenides on 2D NiCo–MXene nanosheets as a dual-layer electrocatalyst for effective seawater electrolysis and hydrazine degradation. Mater. Today Phys. 2025, 57, 101816. [Google Scholar] [CrossRef]
- Pan, Y.-H.; Perumal, S.; Sakthivel, M.; Lin, S.-N.; Yeh, M.-H.; Yu, W.-Y.; Ho, K.-C. Reconstructed vanadium carbide MXene supported cerium based trimetallic phosphide as an efficient electrocatalyst for alkaline water splitting. Chem. Eng. J. 2025, 515, 163888. [Google Scholar] [CrossRef]

| Performance Metric | Definition/ Significance | Evaluation Method | MXene-Specific Considerations |
|---|---|---|---|
| Overpotential | Additional potential required beyond the thermodynamic value to drive HER/OER | Linear sweep voltammetry (LSV) | Reduced via heteroatom doping, interlayer spacing, and hybridization |
| Tafel Slope | Indicates reaction kinetics and charge transfer efficiency | Tafel analysis from LSV | Improved by structural tuning, defects, and atomic-scale doping |
| Exchange Current Density (jo) | Intrinsic rate of electron transfer at the equilibrium potential | Extrapolated from Tafel plots | Sensitive to surface termination; enhanced with conductive additives |
| Turnover Frequency (TOF) | Reactant molecules converted per active site per unit time | Estimated via ECSA | Depending on accurate site quantification, reflects intrinsic catalytic activity |
| Durability/Stability | Catalyst’s ability to retain performance over time | Chronoamperometry, chronopotentiometry, and cycling voltammetry | MXenes show good stability due to a robust structure and modifiable surfaces |
| Technique | Information Provided | Application in Electrocatalysis | MXene-Specific Insights |
|---|---|---|---|
| TEM/HR-TEM | Morphology, particle size, layer thickness, lattice fringes | Reveals nanoscale structural features, defects, and stacking | Identifies exfoliation quality, interlayer spacing, and hybrid structures |
| XPS | Surface elemental composition, oxidation states, bonding environments | Tracks surface chemistry evolution, oxidation, and doping effects | Confirms terminations (–O, –F, –OH), dopant incorporation, and post-reaction changes |
| Raman Spectroscopy | Vibrational modes of chemical bonds and structural defects | Detects phase changes, disorder, and oxidation | Monitors degradation and structural disorder |
| XRD | Crystalline structure, interlayer spacing | Detects structural changes pre- and post-reaction | Tracks restacking, interlayer expansion, and hybrid phase formation |
| EIS | Charge transfer resistance, ion diffusion, capacitance | Evaluates charge transport kinetics | Assesses conductivity enhancements via doping/composites |
| CV Cycling | Reversibility and stability over cycles | Used for long-term durability tests | Tracks degradation in HER/OER cycles |
| CA CP | Current or potential retention over time | Tests for long-term operational durability | Quantifies time-dependent stability under HER/OER |
| Catalyst | Gibbs Free Energy (ΔGH*, eV) | Overpotential (ƞ10, mV) | [Ref.] |
|---|---|---|---|
| Ti3CNTx MXene | +0.8 | 329 | [106] |
| Pt/Ti3CNTx MXene | –0.11 | 28 | |
| V2CTx MXene | +1.23 | 450.8 | [107] |
| NiPt/V2CTx MXene | −0.67 | 11.9 | |
| Ru/rGO | −0.36 | 70 | [111] |
| Ru/Ti3C2Tx MXene/rGO | −0.18 | 42 | |
| RuSA + RuNP-N/C | ~−0.65 | 42 | [112] |
| RuSA + RuNP-N/C-MXene | ~0.00 | 17 | |
| Ti3C2Tx MXene | −0.312 | 155 | [114] |
| CoSnO3/Ti3C2Tx MXene | +0.015 | 45 | |
| Ti3C2Tx MXene | −0.18 | 449 | [121] |
| CoP/Ti3C2Tx MXene | −0.03 | 135 |
| Catalyst | Application | Electrolyte | Current Density (mA cm−2) | Overpotential (mV) | Tafel Slope (mV dec−1) | [Ref.] |
|---|---|---|---|---|---|---|
| CoMoSe2@ Ti3C2Tx | HER | 1.0 M KOH | 10 | 82 | 124 | [147] |
| Co-ReS2@Ti3C2Tx | HER | 1.0 M KOH | 10 | 65 | 92 | [148] |
| CoNi(OH)2@ Ti3C2Tx | HER | 1.0 M KOH | 10 | 73 | 85 | [149] |
| PtNi-NiOx/Ti3C2Tx | HER | 1.0 M KOH | 10 | 24 | 56.4 | [150] |
| Fe2B/Ti3C2Tx | HER | 1.0 M KOH | 100 | 294 | 92.0 | [151] |
| Ni1.5Co1.5(PO4)2@Ti3C2Tx | OER | 1.0 M KOH | 100 | 286 | 84.0 | [47] |
| NiFeMo/Ti3C2Tx | OER | 1.0 M KOH | 100 | 280 | 56.0 | [137] |
| CoP@C/Ti3C2Tx | OER | 1.0 M KOH | 10 | 235 | 54.0 | [152] |
| NiCoSe/Ti3C2Tx | OER | 1.0 M KOH | 10 | 170 | 66.7 | [153] |
| CeCoFePLDH@ Ti3C2Tx | OER | 1.0 M KOH | 10 | 266 | 35.1 | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thalji, M.R.; Mahmoudi, F.; Bachas, L.G.; Park, C. MXene-Based Electrocatalysts for Water Splitting: Material Design, Surface Modulation, and Catalytic Performance. Int. J. Mol. Sci. 2025, 26, 8019. https://doi.org/10.3390/ijms26168019
Thalji MR, Mahmoudi F, Bachas LG, Park C. MXene-Based Electrocatalysts for Water Splitting: Material Design, Surface Modulation, and Catalytic Performance. International Journal of Molecular Sciences. 2025; 26(16):8019. https://doi.org/10.3390/ijms26168019
Chicago/Turabian StyleThalji, Mohammad R., Farzaneh Mahmoudi, Leonidas G. Bachas, and Chinho Park. 2025. "MXene-Based Electrocatalysts for Water Splitting: Material Design, Surface Modulation, and Catalytic Performance" International Journal of Molecular Sciences 26, no. 16: 8019. https://doi.org/10.3390/ijms26168019
APA StyleThalji, M. R., Mahmoudi, F., Bachas, L. G., & Park, C. (2025). MXene-Based Electrocatalysts for Water Splitting: Material Design, Surface Modulation, and Catalytic Performance. International Journal of Molecular Sciences, 26(16), 8019. https://doi.org/10.3390/ijms26168019









