Preparturient Oral Selenitetriglycerides Supplementation Elevates Erythrocyte Glutathione Peroxidase Activity and Modulates Hepatic TNF-α, PPAR-α, and PPAR-δ mRNA in Postparturient Holstein–Friesian Cows
Abstract
1. Introduction
2. Results
2.1. Biochemical Parameters
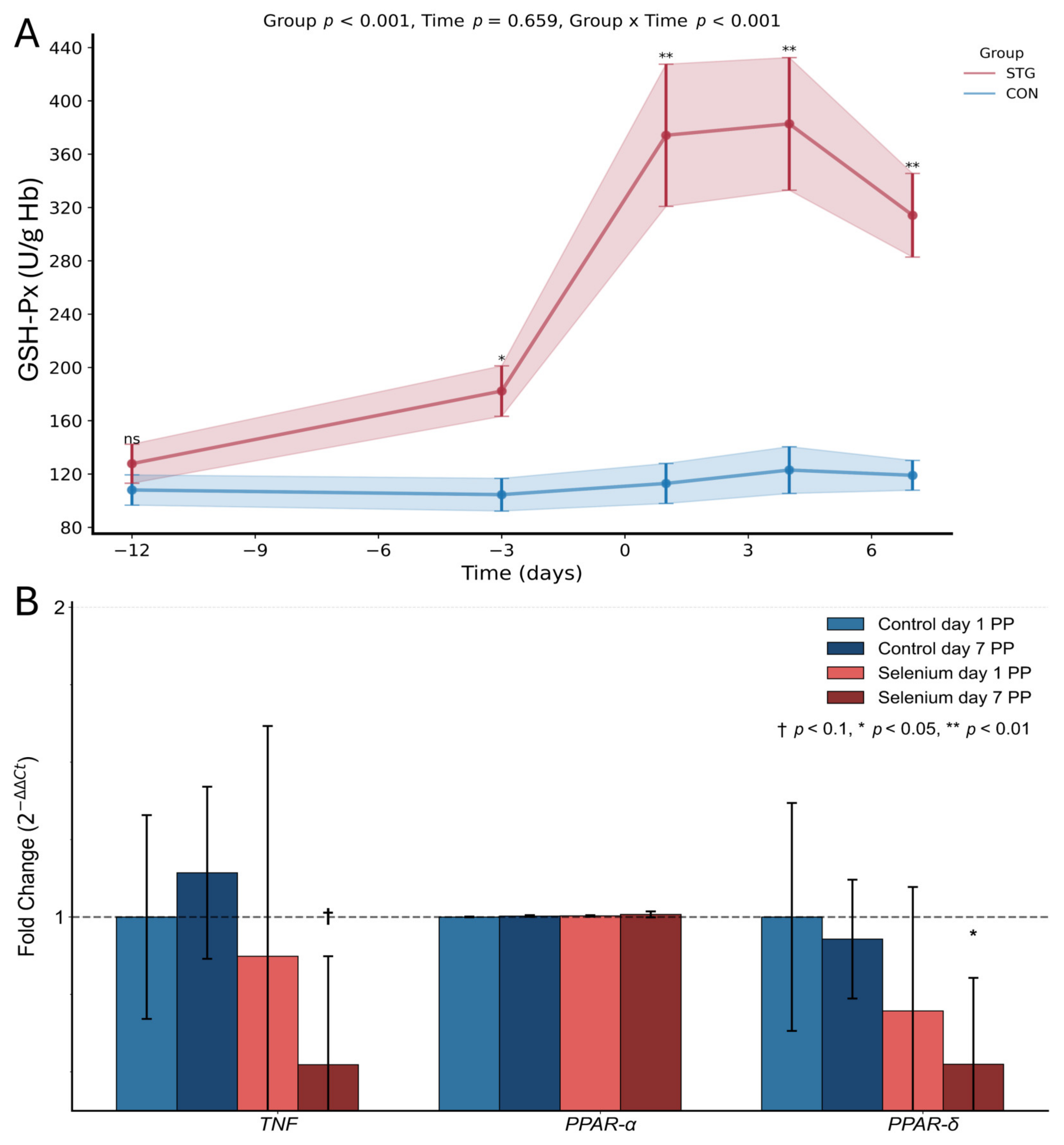
2.2. Antioxidant Parameters
2.3. Gene Expression of TNF, PPAR-α, and PPAR-δ
3. Discussion
4. Materials and Methods
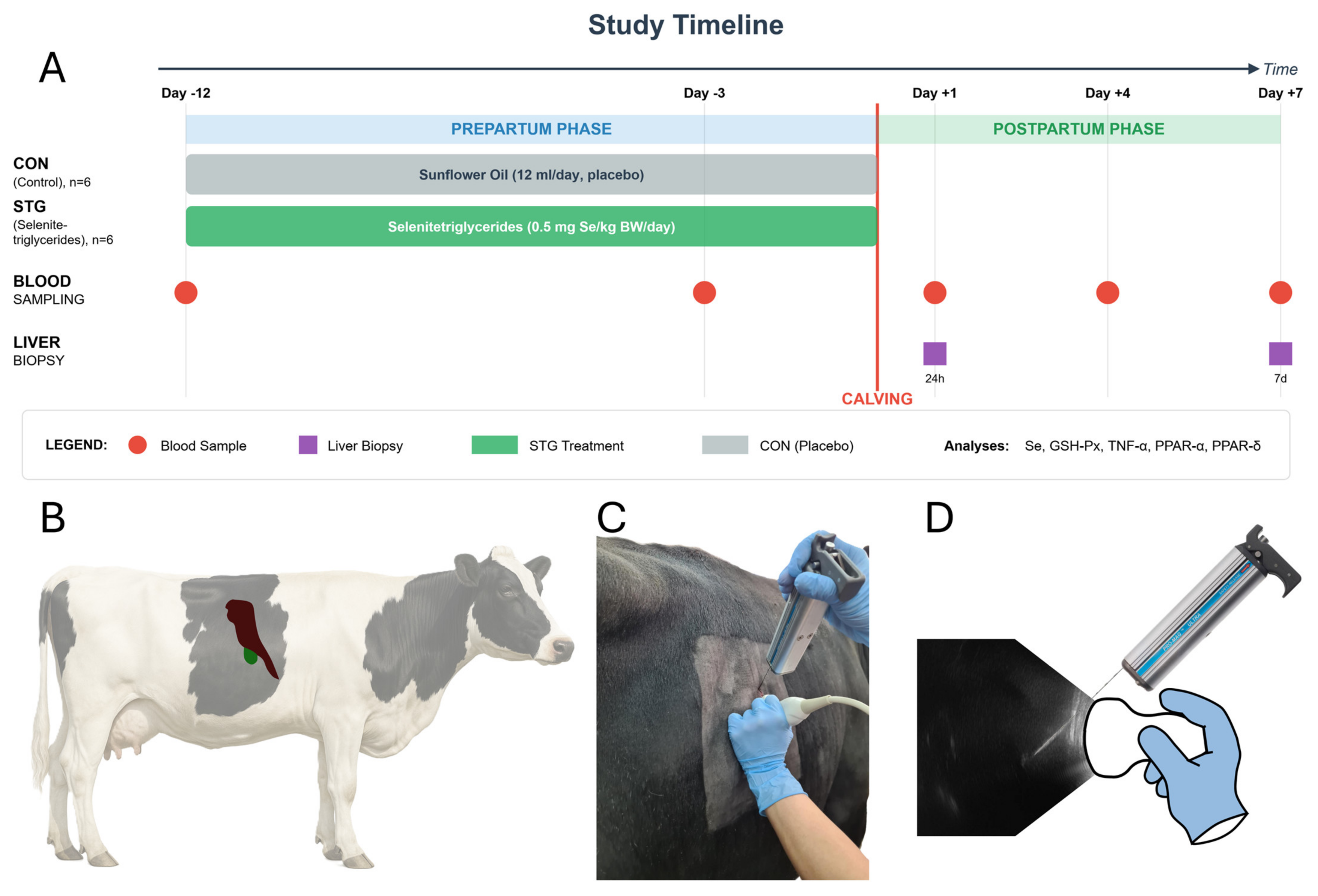
4.1. Animals and Methodology
4.2. Blood Collection and Analyses
4.3. Liver Sampling and RNA Techniques
4.4. cDNA Synthesis and RT-qPCR Details
4.5. The cDNA Synthesis and RT-qPCR Details
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- LeBlanc, S. Monitoring metabolic health of dairy cattle in the transition period. J. Reprod. Dev. 2010, 56, S29–S35. [Google Scholar] [CrossRef]
- Pascottini, O.B.; Leroy, J.; Opsomer, G. Metabolic Stress in the Transition Period of Dairy Cows: Focusing on the Prepartum Period. Animals 2020, 10, 1419. [Google Scholar] [CrossRef]
- Qiao, K.; Jiang, R.; Contreras, G.A.; Xie, L.; Pascottini, O.B.; Opsomer, G.; Dong, Q. The Complex Interplay of Insulin Resistance and Metabolic Inflammation in Transition Dairy Cows. Animals 2024, 14, 832. [Google Scholar] [CrossRef]
- Soares, R.A.N.; Vargas, G.; Muniz, M.M.M.; Soares, M.A.M.; Canovas, A.; Schenkel, F.; Squires, E.J. Differential gene expression in dairy cows under negative energy balance and ketosis: A systematic review and meta-analysis. J. Dairy. Sci. 2021, 104, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Farney, J.K.; Mamedova, L.K.; Sordillo, L.M.; Bradford, B.J. TNFalpha altered inflammatory responses, impaired health and productivity, but did not affect glucose or lipid metabolism in early-lactation dairy cows. PLoS ONE 2013, 8, e80316. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.J.; Mamedova, L.K.; Minton, J.E.; Drouillard, J.S.; Johnson, B.J. Daily injection of tumor necrosis factor-alpha increases hepatic triglycerides and alters transcript abundance of metabolic genes in lactating dairy cattle. J. Nutr. 2009, 139, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, H.; Koiwa, M.; Hatsugaya, A.; Kudo, K.; Hoshi, F.; Itoh, N.; Yokota, H.; Okada, H.; Kawamura, S. Relationship between serum TNF activity and insulin resistance in dairy cows affected with naturally occurring fatty liver. J. Vet. Med. Sci. 2001, 63, 1021–1025. [Google Scholar] [CrossRef]
- Hansen, P.J.; Soto, P.; Natzke, R.P. Mastitis and fertility in cattle—possible involvement of inflammation or immune activation in embryonic mortality. Am. J. Reprod. Immunol. 2004, 51, 294–301. [Google Scholar] [CrossRef]
- Loor, J.J. Genomics of metabolic adaptations in the peripartal cow. Animal 2010, 4, 1110–1139. [Google Scholar] [CrossRef]
- Bionaz, M.; Thering, B.J.; Loor, J.J. Fine metabolic regulation in ruminants via nutrient-gene interactions: Saturated long-chain fatty acids increase expression of genes involved in lipid metabolism and immune response partly through PPAR-alpha activation. Br. J. Nutr. 2012, 107, 179–191. [Google Scholar] [CrossRef]
- Hassan, F.U.; Nadeem, A.; Li, Z.; Javed, M.; Liu, Q.; Azhar, J.; Rehman, M.S.; Cui, K.; Rehman, S.U. Role of Peroxisome Proliferator-Activated Receptors (PPARs) in Energy Homeostasis of Dairy Animals: Exploiting Their Modulation through Nutrigenomic Interventions. Int. J. Mol. Sci. 2021, 22, 12463. [Google Scholar] [CrossRef]
- Suwik, K.; Sinderewicz, E.; Boruszewska, D.; Kowalczyk-Zięba, I.; Staszkiewicz-Chodor, J.; Łukaszuk, K.; Wocławek-Potocka, I. mRNA Expression and Role of PPAR? and PPAR? in Bovine Preimplantation Embryos Depending on the Quality and Developmental Stage. Animals 2020, 10, 2358. [Google Scholar] [CrossRef]
- Zarczynska, K.; Brym, P.; Tobolski, D. The Role of Selenitetriglycerides in Enhancing Antioxidant Defense Mechanisms in Peripartum Holstein-Friesian Cows. Animals 2024, 14, 610. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Chen, B.; Zhao, B.; Gao, X.J. Selenium Deficiency Promotes Oxidative Stress-Induced Mastitis via Activating the NF-kappaB and MAPK Pathways in Dairy Cow. Biol. Trace Elem. Res. 2022, 200, 2716–2726. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, Y.; Dufrasne, I. Selenium in Cattle: A Review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, A.; Alves-Nores, V.; Hernandez, J.; Muino, R.; Benedito, J.L.; Castillo, C. Effect of Parenteral Antioxidant Supplementation During the Dry Period on Postpartum Glucose Tolerance in Dairy Cows. J. Vet. Intern. Med. 2016, 30, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Khan, R.U.; Tufarelli, V.; Laudadio, V. Selenium: An Essential Micronutrient for Sustainable Dairy Cows Production. Sustainability 2020, 12, 10693. [Google Scholar] [CrossRef]
- Cerri, R.L.; Rutigliano, H.M.; Lima, F.S.; Araujo, D.B.; Santos, J.E. Effect of source of supplemental selenium on uterine health and embryo quality in high-producing dairy cows. Theriogenology 2009, 71, 1127–1137. [Google Scholar] [CrossRef]
- Todisco, S.; Santarsiero, A.; Convertini, P.; De Stefano, G.; Gilio, M.; Iacobazzi, V.; Infantino, V. PPAR Alpha as a Metabolic Modulator of the Liver: Role in the Pathogenesis of Nonalcoholic Steatohepatitis (NASH). Biology 2022, 11, 792. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Bionaz, M.; Chen, S.; Khan, M.J.; Loor, J.J. Functional Role of PPARs in Ruminants: Potential Targets for Fine-Tuning Metabolism during Growth and Lactation. PPAR Res. 2013, 2013, 684159. [Google Scholar] [CrossRef]
- Xiao, J.; Khan, M.Z.; Ma, Y.; Alugongo, G.M.; Ma, J.; Chen, T.; Khan, A.; Cao, Z. The Antioxidant Properties of Selenium and Vitamin E.; Their Role in Periparturient Dairy Cattle Health Regulation. Antioxidants 2021, 10, 1555. [Google Scholar] [CrossRef] [PubMed]
- Zarczynska, K.; Sobiech, P.; Tobolski, D.; Mee, J.F.; Illek, J. Effect of a single, oral administration of selenitetriglycerides, at two dose rates, on blood selenium status and haematological and biochemical parameters in Holstein-Friesian calves. Ir. Vet. J. 2021, 74, 11. [Google Scholar] [CrossRef] [PubMed]
- Jastrzebski, Z.; Czyzewska-Szafran, H.; Remiszewska, M.; Fijalek, Z.; Fitak, B.A.; Suchocki, P. Pharmacokinetics of selol, a new agent containing selenium, in rats. Drugs Exp. Clin. Res. 1997, 23, 7–11. [Google Scholar]
- Zarczynska, K.; Sobiech, P.; Mee, J.; Illek, J. The influence of short-term selenitetriglycerides supplementation on blood selenium, and hepatic, renal, metabolic and hematological parameters in dairy cows. Pol. J. Vet. Sci. 2020, 23, 637–646. [Google Scholar] [CrossRef]
- Swierczynski, G.; Tobolski, D.; Zarczynska, K. Effect of selenitetriglyceride supplementation in pregnant sows on hematological and biochemical profiles, Se concentration and transfer to offspring. J. Elem. 2024, 29, 21–35. [Google Scholar]
- Takano, H.; Nagai, T.; Asakawa, M.; Toyozaki, T.; Oka, T.; Komuro, I.; Saito, T.; Masuda, Y. Peroxisome proliferator-activated receptor activators inhibit lipopolysaccharide-induced tumor necrosis factor-alpha expression in neonatal rat cardiac myocytes. Circ. Res. 2000, 87, 596–602. [Google Scholar] [CrossRef]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular actions of PPAR α in lipid metabolism and inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef]
- Szostek, A.Z.; Lukasik, K.; Majewska, M.; Bah, M.M.; Znaniecki, R.; Okuda, K.; Skarzynski, D.J. Tumor necrosis factor-alpha inhibits the stimulatory effect of luteinizing hormone and prostaglandin E(2) on progesterone secretion by the bovine corpus luteum. Domest. Anim. Endocrinol. 2011, 40, 183–191. [Google Scholar] [CrossRef]
- Tobolski, D.; Lukasik, K.; Baclawska, A.; Skarzynski, D.J.; Hostens, M.; Baranski, W. Prediction of Calving to Conception Interval Length Using Algorithmic Analysis of Endometrial mRNA Expression in Bovine. Animals 2021, 11, 236. [Google Scholar] [CrossRef]
- Bogado Pascottini, O.; LeBlanc, S.J.; Gnemi, G.; Leroy, J.; Opsomer, G. Genesis of clinical and subclinical endometritis in dairy cows. Reproduction 2023, 166, R15–R24. [Google Scholar] [CrossRef]
- Wilde, D. Influence of macro and micro minerals in the peri-parturient period on fertility in dairy cattle. Anim. Reprod. Sci. 2006, 96, 240–249. [Google Scholar] [CrossRef]
- Harrison, J.H.; Conrad, H.R. Effect of selenium intake on selenium utilization by the nonlactating dairy cow. J. Dairy. Sci. 1984, 67, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Zhao, R.; Song, G.; Zhong, W. Selenite reactivates silenced genes by modifying DNA methylation and histones in prostate cancer cells. Carcinogenesis 2008, 29, 2175–2181. [Google Scholar] [CrossRef] [PubMed]
- Adimulam, T.; Arumugam, T.; Foolchand, A.; Ghazi, T.; Chuturgoon, A.A. The Effect of Organoselenium Compounds on Histone Deacetylase Inhibition and Their Potential for Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 12952. [Google Scholar] [CrossRef]
- Bradford, B.J.; Yuan, K.; Farney, J.K.; Mamedova, L.K.; Carpenter, A.J. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. Dairy. Sci. 2015, 98, 6631–6650. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
| Gene Symbol | Primer Sequences | Annealing Temp | Tm | Amplicon Size | E | Error |
|---|---|---|---|---|---|---|
| (F-forward/R-reverse) (5′-3′) | (°C) | (°C) | bp | 10−1/slope | MSE | |
| TNF | F—CTGGTTCAGACACTCAGGTCC R—GAGGTAAAGCCCGTCAGCA | 58 | 87.7 | 183 | 1.99 | 0.005 |
| PPAR-α | F—GGATGTCCCATAACGCGATT R—GGTCATGCTCACACGTAAGGATT | 60 | 79.3 | 90 | 1.97 | 0.011 |
| PPAR-δ | F—TGTGGCAGCCTCAATATGGA R—GACGGAAGAAGCCCTTGCA | 60 | 86.6 | 100 | 1.99 | 0.007 |
| GAPDH reference gene | F—GTCTTCACTACCATGGAGAAGG R—TCATGGATGACCTTGGCCAG | 60 | 86.1 | 197 | 2.03 | 0.011 |
| RPL32 reference gene | F—AAAGAGGACCAAGAAGTTCATTAG R—CGCCAGTTCCGCTTGATTT | 60 | 78.1 | 66 | 1.98 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żarczyńska, K.; Różańska, K.; Brym, P.; Tobolski, D. Preparturient Oral Selenitetriglycerides Supplementation Elevates Erythrocyte Glutathione Peroxidase Activity and Modulates Hepatic TNF-α, PPAR-α, and PPAR-δ mRNA in Postparturient Holstein–Friesian Cows. Int. J. Mol. Sci. 2025, 26, 8018. https://doi.org/10.3390/ijms26168018
Żarczyńska K, Różańska K, Brym P, Tobolski D. Preparturient Oral Selenitetriglycerides Supplementation Elevates Erythrocyte Glutathione Peroxidase Activity and Modulates Hepatic TNF-α, PPAR-α, and PPAR-δ mRNA in Postparturient Holstein–Friesian Cows. International Journal of Molecular Sciences. 2025; 26(16):8018. https://doi.org/10.3390/ijms26168018
Chicago/Turabian StyleŻarczyńska, Katarzyna, Katarzyna Różańska, Paweł Brym, and Dawid Tobolski. 2025. "Preparturient Oral Selenitetriglycerides Supplementation Elevates Erythrocyte Glutathione Peroxidase Activity and Modulates Hepatic TNF-α, PPAR-α, and PPAR-δ mRNA in Postparturient Holstein–Friesian Cows" International Journal of Molecular Sciences 26, no. 16: 8018. https://doi.org/10.3390/ijms26168018
APA StyleŻarczyńska, K., Różańska, K., Brym, P., & Tobolski, D. (2025). Preparturient Oral Selenitetriglycerides Supplementation Elevates Erythrocyte Glutathione Peroxidase Activity and Modulates Hepatic TNF-α, PPAR-α, and PPAR-δ mRNA in Postparturient Holstein–Friesian Cows. International Journal of Molecular Sciences, 26(16), 8018. https://doi.org/10.3390/ijms26168018






