Bioactive Properties of Selected European Phellinus Species: A Comprehensive Study
Abstract
1. Introduction
1.1. Chemical Composition
1.2. Bioactivity
2. Results and Discussion
2.1. Antimicrobial Activity—Preliminary Studies
2.2. Elemental Analysis
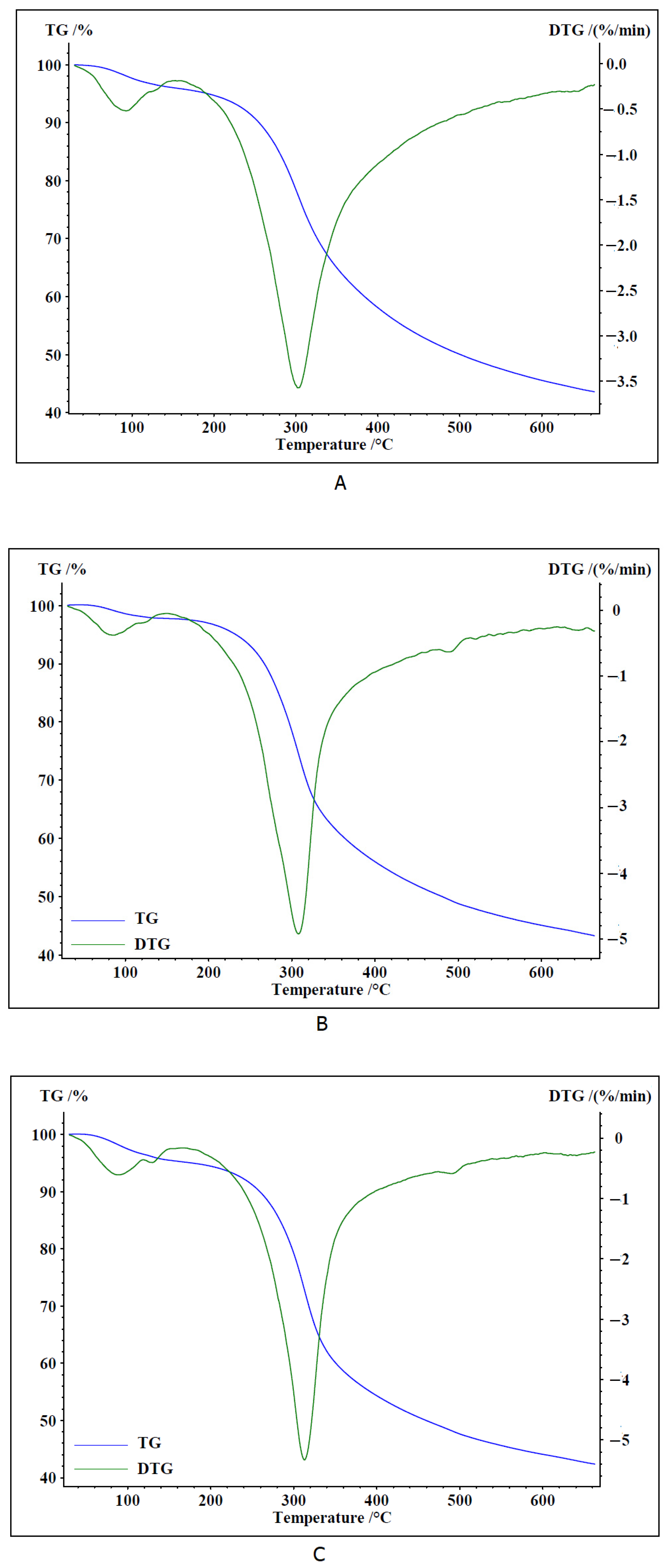
2.3. Thermal Analysis
2.4. FTIR Spectroscopy
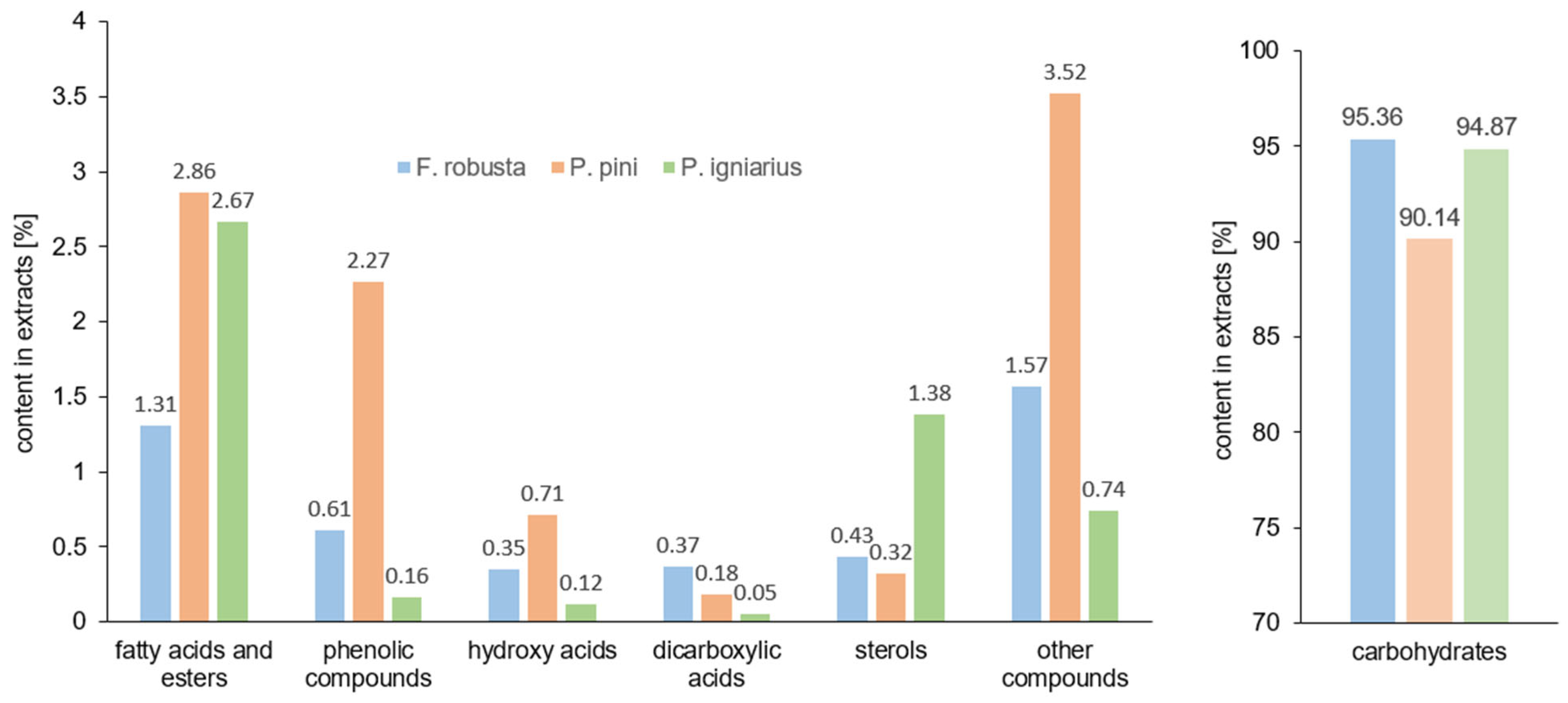
2.5. GC/MS Analysis
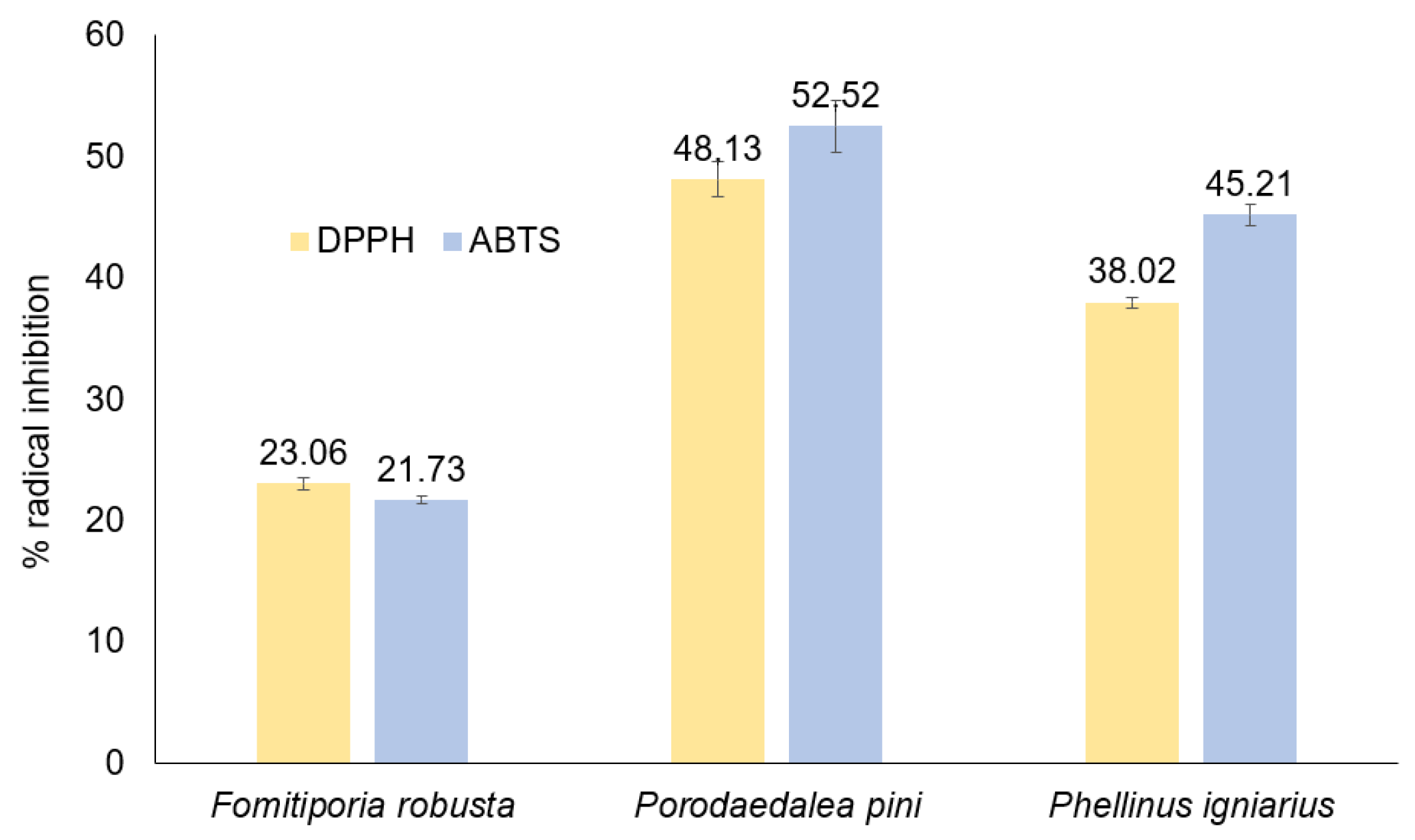
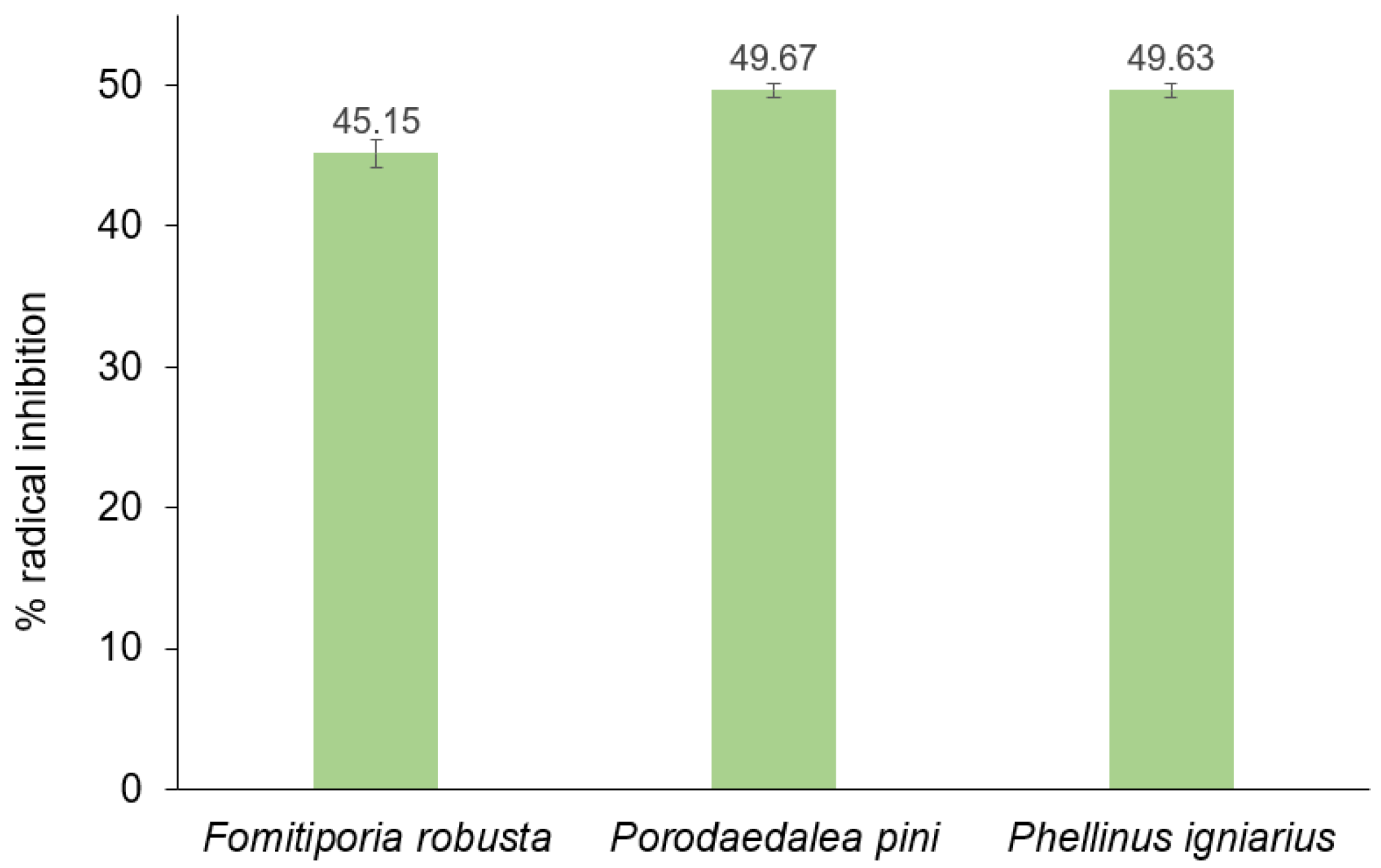
2.6. Antioxidant Study
2.7. Antimicrobial Study
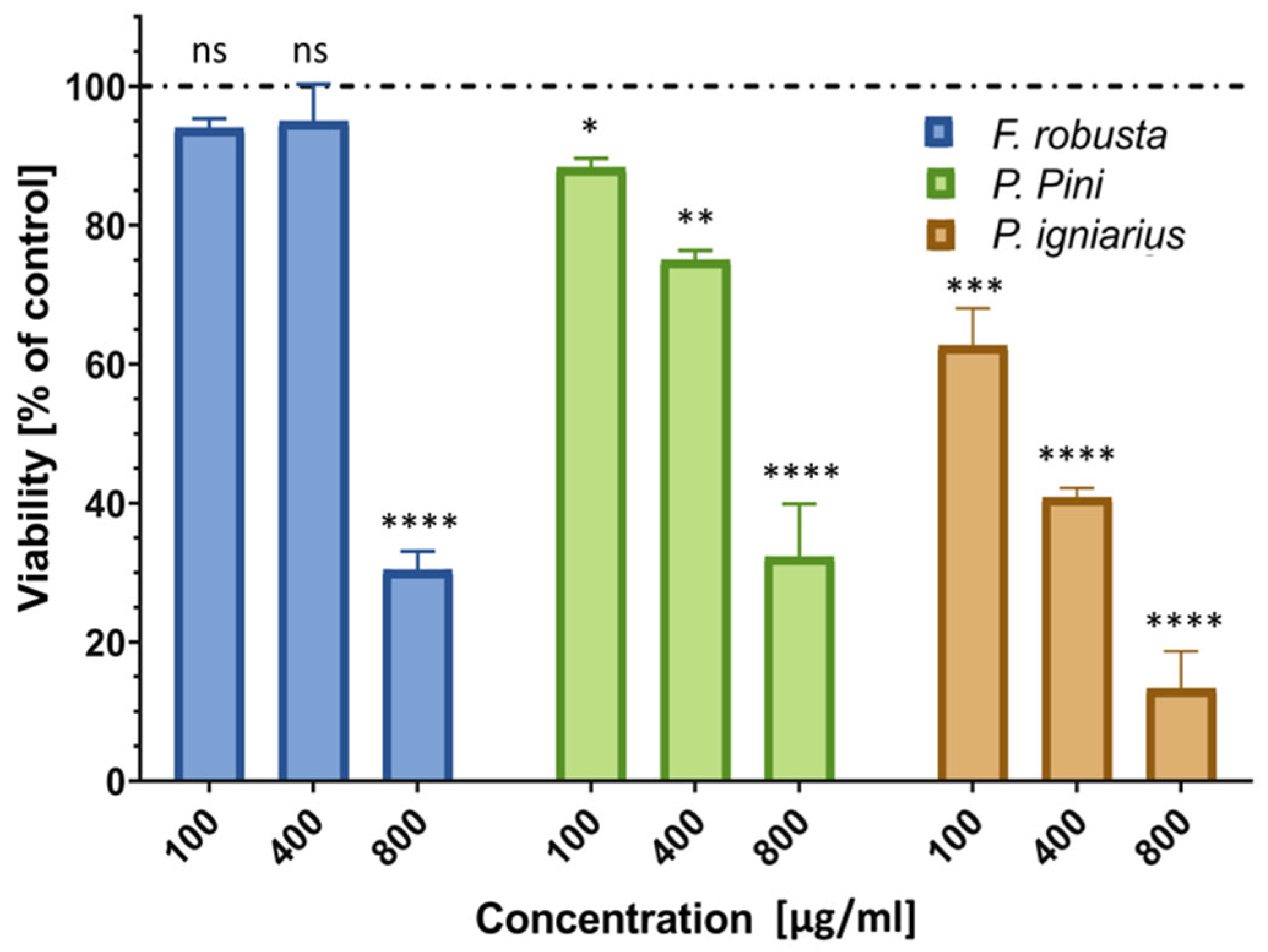
2.8. Cytotoxicity
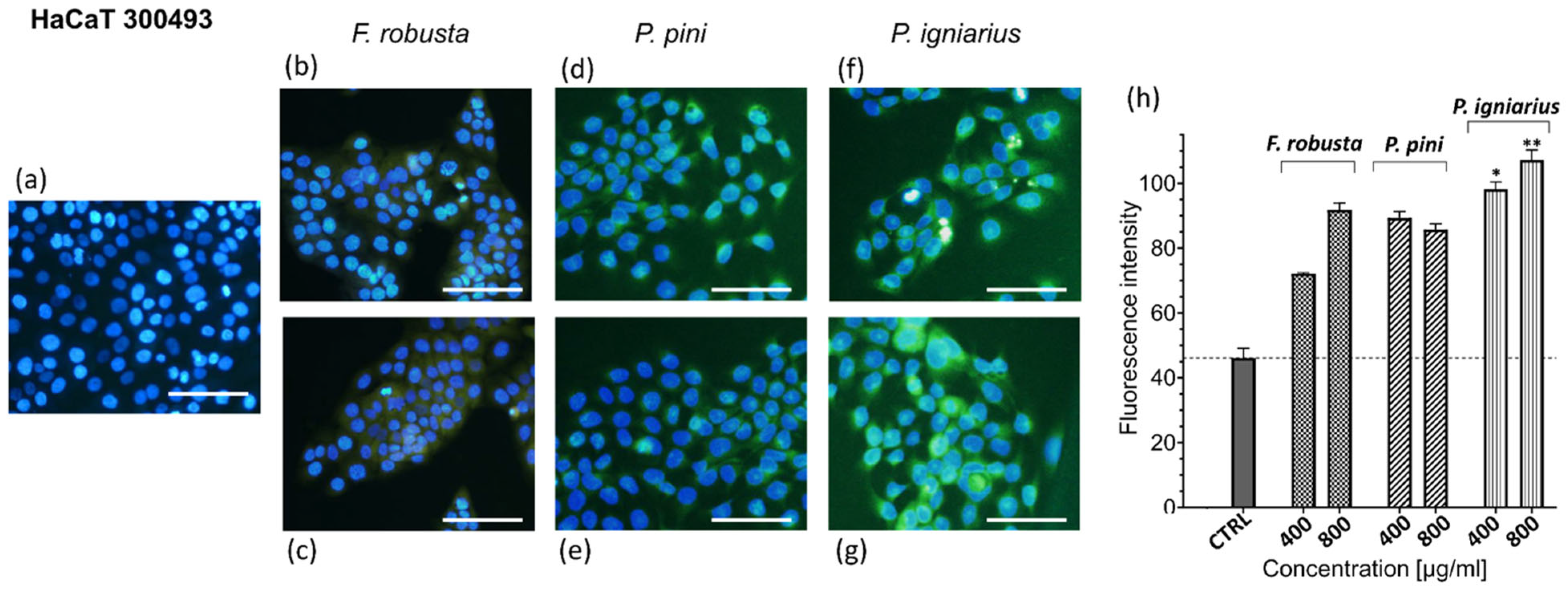
2.9. Reactive Oxygen Species
3. Materials and Methods
3.1. Collection of Fungal Samples in the Field
3.2. Morphological Identification
3.3. Genetic Identification
3.4. Preparation of Extracts
3.5. Antimicrobial Activity—Preliminary Studies
3.6. Elemental Analysis
3.7. Thermal Analysis
3.8. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
3.9. Spectroscopy
3.10. Antioxidant Properties and Lipid Peroxidation Analysis
3.11. Antibacterial Activity
3.12. Cytotoxic Study
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- He, P.; Zhang, Y.; Li, N. The phytochemistry and pharmacology of medicinal fungi of the genus: Phellinus: A review. Food Funct. 2021, 12, 1856–1881. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, F.; Li, L.; Liu, X.; Yan, J.-K. Recent advances in the bioactive polysaccharides and other key components from Phellinus spp. and their pharmacological effects: A review. Int. J. Biol. Macromol. 2022, 222, 3108–3128. [Google Scholar] [CrossRef] [PubMed]
- Pošta, A.; Matočec, N.; Kušan, I.; Tkalčec, Z.; Mešić, A. The Lignicolous Genus Entonaema: Its Phylogenetic–Taxonomic Position within Hypoxylaceae (Xylariales, Fungi) and an Overview of Its Species, Biogeography, and Ecology. Forests 2023, 14, 1764. [Google Scholar] [CrossRef]
- Nankone, S.; Sanon, E.; Sawadogo, B.R.; Dabire, K.; Guissou, M.-L.K. Ecology and morphological characterization of the genus Phellinus sensu-lato (Basidiomycetes, Hymenochaetaceae) in Burkina Faso. Afr. J. Plant Sci. 2020, 14, 451–460. [Google Scholar]
- Sułkowska-Ziaja, K.; Balik, M.; Muszyńska, B. Selected Species of the Genus Phellinus–Chemical Composition, Biological Activity, and Medicinal Applications. Chem. Biodivers. 2021, 18, e2100609. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Montoya, C.A.; Elias, S.G.; Popoff, O.F.; Robledo, G.L.; Urcelay, C.; Góes-Neto, A.; Martínez, S.; Drechsler-Santos, E.R. Neotropical Studies on Hymenochaetaceae: Unveiling the Diversity and Endemicity of Phellinotus. J. Fungi 2022, 8, 216. [Google Scholar] [CrossRef]
- Zhou, L.W.; Vlasák, J.; Dai, Y.C. Taxonomy and phylogeny of Phellinidium (Hymenochaetales, Basidiomycota): A redefinition and the segregation of Coniferiporia gen. nov. for forest pathogens. Fungal Biol. 2016, 120, 988–1001. [Google Scholar] [CrossRef]
- Luan, F.; Peng, X.; Zhao, G.; Zeng, J.; Zou, J.; Rao, Z.; Liu, Y.; Zhang, X.; Ma, H.; Zeng, N. Structural diversity and bioactivity of polysaccharides from medicinal mushroom Phellinus spp.: A review. Food Chem. 2022, 397, 133731. [Google Scholar] [CrossRef]
- Yan, J.K.; Pei, J.J.; Ma, H.L.; Wang, Z.-B.; Liu, Y.-S. Advances in antitumor polysaccharides from phellinus sensu lato: Production, isolation, structure, antitumor activity, and mechanisms. Crit. Rev. Food Sci. Nutr. 2017, 57, 1256–1269. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Dong, X.Y.; Ning, J.L.; Jin, Y.-X.; Wang, G.-K.; Feng, T. Chemical constituents from the fungus Phellinus baumii and their anti-inflammatory activity. Phytochem. Lett. 2023, 54, 18–22. [Google Scholar] [CrossRef]
- Azeem, U. Antioxidant efficacy of a wood degrading fungus Phellinus gilvus (Schwein.) Pat., from Indian fungal flora. J. Pharmacogn. Phytochem. 2018, 7, 1017–1021. [Google Scholar]
- Kim, G.Y.; Park, H.S.; Nam, B.H.; Lee, S.-J.; Lee, J.-D. Purification and characterization of acidic proteo-heteroglycan from the fruiting body of Phellinus linteus (Berk. & M.A. Curtis) Teng. Bioresour. Technol. 2003, 89, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shang, X.Y.; Wang, S.J.; Mo, S.-Y.; Li, S.; Yang, Y.-C.; Ye, F.; Shi, J.-G.; He, L. Structures, biogenesis, and biological activities of pyrano [4,3-c] isochromen-4-one derivatives from the fungus Phellinus igniarius. J. Nat. Prod. 2007, 70, 296–299. [Google Scholar] [CrossRef]
- Song, A.R.; Sun, X.L.; Kong, C.; Zhao, C.; Qin, D.; Huang, F.; Yang, S. Discovery of a new sesquiterpenoid from Phellinus ignarius with antiviral activity against influenza virus. Arch. Virol. 2014, 159, 753–760. [Google Scholar] [CrossRef]
- Gao, M.; Huang, Y.; Hu, C.; Hu, J.; Wang, Y.; Chen, Y.; Huang, Y.; Song, G.; Song, Z.; Wang, Z. Selective Anticancer Effect of Phellinus Linteus on Epidermoid Cell Lines Studied by Atomic Force Microscopy: Anticancer Activity on A431 Cancer Cells and Low Toxicity on HaCat Normal Cells. IEEE Nanotechnol. Mag. 2021, 15, 4–16. [Google Scholar] [CrossRef]
- Li, X.; Jiao, L.L.; Zhang, X.; Tian, W.-M.; Chen, S.; Zhang, L.-P. Anti-tumor and immunomodulating activities of proteoglycans from mycelium of Phellinus nigricans and culture medium. Int. Immunopharmacol. 2008, 8, 909–915. [Google Scholar] [CrossRef]
- Chen, W.; He, F.Y.; Li, Y.Q. The apoptosis effect of hispolon from Phellinus linteus (Berkeley & Curtis) Teng on human epidermoid KB cells. J. Ethnopharmacol. 2006, 105, 280–285. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Khan, I.N.; Shaw, S.; Uddin, S.J.; Rouf, R.; Dev, S.; Saravi, S.S.; Das, N.; Tripathi, S.; et al. Anticancer Perspectives on the Fungal-Derived Polyphenolic Hispolon. Anti-Cancer Agents Med. Chem. 2020, 20, 1636–1647. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, R.; Su, X.; Shang, K.; Tan, C.; Ma, J.; Zhang, Y.; Lin, D.; Ma, Y.; Zhou, M.; et al. Immune-enhancing activity of polysaccharides and flavonoids derived from Phellinus igniarius YASH1. Front. Pharmacol. 2023, 14, 1124607. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Yoon, D.H.; Lee, W.H.; Han, S.K.; Shrestha, B.; Kim, C.H.; Lim, M.H.; Chang, W.; Lim, S.; Choi, S.; et al. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-κB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophage. J. Ethnopharmacol. 2007, 114, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, Y.; Huang, J.; Qian, N.; Shen, G.; Chen, L. Anti-inflammatory activity of polysaccharides from Phellinus linteus by regulating the NF-κB translocation in LPS-stimulated RAW264.7 macrophages. Int. J. Biol. Macromol. 2019, 129, 61–67. [Google Scholar] [CrossRef]
- Shin, M.R.; Lee, J.H.; Lee, J.A.; Kim, M.J.; Park, H.-J.; Park, B.W.; Seo, S.B.; Roh, S.-S. Immunomodulatory and anti-inflammatory effects of Phellinus linteus mycelium. BMC Complement. Med. Ther. 2021, 21, 269. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Lin, Q.; Guo, T.; Yang, T.; Zhou, W.; Deng, X.; Yan, J.-K.; Luo, Y.; Ju, M.; Luo, F. Polysaccharide isolated from Phellinus linteus mycelia exerts anti-inflammatory effects via MAPK and PPAR signaling pathways. Carbohydr. Polym. 2018, 200, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Lee, Y.S.; Ku, S.K.; Lee, H.J. Phellinus baumii enhances the immune response in cyclophosphamide-induced immunosuppressed mice. Nutr. Res. 2020, 75, 15–31. [Google Scholar] [CrossRef]
- Sun, Y.; Huo, J.; Zhong, S.; Zhu, J.; Li, Y.; Li, X. Chemical structure and anti-inflammatory activity of a branched polysaccharide isolated from Phellinus baumii. Carbohydr. Polym. 2021, 268, 118214. [Google Scholar] [CrossRef]
- Zhu, F.; Lu, W.; Feng, W.; Song, Z.; Wang, C.; Chen, X. Preliminary Investigation on the Chemical Constituents and Antioxidant Activities of Two Phellinus Mushrooms Collected in Foshan. Int. J. Org. Chem. 2017, 7, 25–33. [Google Scholar] [CrossRef]
- Dimitrova-Shumkovska, J.; Kosharkoska-Spasovska, F.; Krstanoski, L.; Karadelev, M. Antioxidant properties of fortified yogurt with medicinal mushrooms from Phellinus species. J. Food Biochem. 2022, 46, e14364. [Google Scholar] [CrossRef]
- Shon, M.Y.; Kim, T.H.; Sung, N.J. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem. 2003, 82, 593–597. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.; Liu, Y.; Wang, W.; Feng, N.; Wu, D. Antioxidant activities of extracts from the genus Phellinus species. In Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products (ICMBMP7), Arcachon, France, 4–7 October 2011. [Google Scholar]
- Ajith, T.A.; Janardhanan, K.K. Antioxidant and antihepatotoxic activities of Phellinus rimosus (Berk) Pilat. J. Ethnopharmacol. 2002, 81, 387–391. [Google Scholar] [CrossRef]
- Song, Y.S.; Kim, S.H.; Sa, J.H.; Jin, C.; Lim, C.-J.; Park, E.-H. Anti-angiogenic, antioxidant and xanthine oxidase inhibition activities of the mushroom Phellinus linteus. J. Ethnopharmacol. 2003, 88, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Seephonkai, P.; Samchai, S.; Thongsom, A.; Sunaart, S.; Kiemsanmuang, B.; Chakuton, K. DPPH radical scavenging activity and total phenolics of Phellinus mushroom extracts collected from northeast of Thailand. Chin. J. Nat. Med. 2011, 9, 441–445. [Google Scholar] [CrossRef]
- Laovachirasuwan, P.; Judprakob, C.; Sinaphet, B.; Phadungkit, M. In vitro antioxidant and antimutagenic activities of different solvent extracts of Phellinus spp. Int. Food Res. J. 2016, 23, 2608. [Google Scholar]
- Kim, Y.H.; Choi, D.C.; Ki, D.W.; Seo, W.-G.; Song, J.-H.; Ko, H.-J.; Lee, I.-K.; Yun, B.-S. Three undescribed dimeric ferulates from the culture broth of Phellinus linteus and their dengue virus type-2 inhibition activity. Phytochem. Lett. 2024, 60, 229–233. [Google Scholar] [CrossRef]
- Li, T.J.; Lin, T.W.; Lu, T.Y.; Tseng, C.-K.; Lin, C.-K.; Chu, H.-T.; Li, I.-C.; Chen, C.-C. Phellinus linteus mycelia extract in COVID-19 prevention and identification of its key metabolic compounds profiling using UPLC-QTOF-MS/MS spectrometry. Fitoterapia 2023, 171, 105695. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, S.; Wan, J.M.F.; Gui, L.; Ruan, M.; Li, N.; Zhang, H.; Liu, Z.; Wang, H. Polysaccharides extracted from Phellinus linteus ameliorate high-fat high-fructose diet induced insulin resistance in mice. Carbohydr. Polym. 2018, 200, 144–153. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, S.W.; Lim, J.M.; Joo, J.-H.; Kim, H.-O.; Kim, H.-M.; Yun, J.-W. Hypoglycemic effect of crude exopolysaccharides produced by a medicinal mushroom Phellinus baumii in streptozotocin-induced diabetic rats. Life Sci. 2005, 76, 3069–3080. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Deng, S.; Huang, Y.; Huang, M.; Zhao, P.; Ma, X.; Wen, Y.; Wang, Q.; Yang, X. Anti-diabetic activity of a polyphenol-rich extract from: Phellinus igniarius in KK-Ay mice with spontaneous type 2 diabetes mellitus. Food Funct. 2018, 9, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wang, L.; Sun, Y.; Pi, X.; Zhang, W.; Gao, P.; Lu, S.; Liu, W. Hypoglycemic effect of the Phellinus baumii extract with α-glucosidase-inhibited activity and its modulation to gut microbiota in diabetic patients. Biomed. Pharmacother. 2023, 158, 114130. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Wu, J.; Georgiev, M.I.; Xu, B.; Wong, K.-H.; Bai, W.; Tian, L. Isolation, Structural Properties, and Bioactivities of Polysaccharides from Mushrooms Termitomyces: A Review. J. Agric. Food Chem. 2022, 70, 21–33. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Xu, Z.; Ding, Z. Bioactive mushroom polysaccharides: A review on monosaccharide composition, biosynthesis and regulation. Molecules 2017, 22, 955. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hicks, K.B.; Moreau, R. Antioxidant activity of phytosterols, oryzanol, and other phytosterol conjugates. J. Am. Oil Chem. Soc. 2002, 79, 1201–1206. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E. Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef]
- Zapała, L.; Ciszkowicz, E.; Kosińska-Pezda, M.; Maciołek, U.; Kozioł, A.E.; Miłoś, A.; Woźnicka, E.; Bocian, A.; Zapała, W.; Rydel-Ciszek, K.; et al. Novel silver(I) complexes with fenamates: Insights into synthesis, spectral characterization, and bioactivity. J. Inorg. Biochem. 2025, 266, 112846. [Google Scholar] [CrossRef] [PubMed]
- Thieme, L.; Hartung, A.; Tramm, K.; Klinger-Strobel, M.; Jandt, K.D.; Makarewicz, O.; Pletz, M.W. MBEC Versus MBIC: The Lack of Differentiation between Biofilm Reducing and Inhibitory Effects as a Current Problem in Biofilm Methodology. Biol. Proced. Online 2019, 21, 18. [Google Scholar] [CrossRef]
- Bocian, A.; Ciszkowicz, E.; Hus, K.K.; Buczkowicz, J.; Lecka-Szlachta, K.; Pietrowska, M.; Petrilla, V.; Petrillova, M.; Legáth, Ľ.; Legáth, J. Antimicrobial activity of protein fraction from Naja ashei venom against Staphylococcus epidermidis. Molecules 2020, 25, 293. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Geddo, F.; Antoniotti, S.; Gallo, M.P.; Querio, G. Indole-3-Propionic Acid, a Gut Microbiota-Derived Tryptophan Metabolite, Promotes Endothelial Dysfunction Impairing Purinergic-Induced Nitric Oxide Release in Endothelial Cells. Int. J. Mol. Sci. 2024, 25, 3389. [Google Scholar] [CrossRef]
- Domański, S.; Orłaś, H.; Skirgiełło, A. Fungi. Polyporaceae II (pileatae) Mucronoporaceae II (pileatae) Ganodermataceae, Bon darzewiaceae, Boletopsidaceae, Fistulinaceae (Grzyby), revised ed.; Translated from Polish; Foreign Scientific Publications Department of the National Center for Scientific, Technical, and Economic Information: Warsaw, Poland, 1973.
- Domański, S.; Orłoś, H.; Skirgiełło, A. Podstawczaki (Basidiomycetes), bezblaszkowe (Aphyllophorales), żagwiowate II (Polyporaceae pileatae), szczecinkowate II (Mucroporonaceae pileatae), lakownicowate (Ganodermataceae), bondarcewowate (Bondarzewiaceae), boletkowate (Boletopsidaceae), ozorkowate (Fistulinaceae); Flora Polska. Rośliny zarodnikowe Polski i ziem ościennych. Grzyby (Mycota); Polska Akademia Nauk, Instytut Botaniki, Państwowe Wydawnictwo Naukowe: Warsaw, Poland, 1973; Volume 3. [Google Scholar]
- Ryvarden, L.; Gilbertson, R.L. European Polypores 1. Synop. Fungorum 1993, 6, 1–387. [Google Scholar]
- Ryvarden, L.; Gilbertson, R.L. European Polypores 2. Synop. Fungorum 1994, 7, 394–743. [Google Scholar]
- Larsen, M.J.; Cobb Poulle, L.A. Phellinus (Hymenochaetaceae).: A survey of the world taxa. Synop. Fungorum 1990, 3, 1. [Google Scholar]
- Sell, I. Taxonomy of the species in the Phellinus igniarius group. Mycotaxon 2008, 104, 337–347. [Google Scholar]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Ainsworth and Bisby’s Dictionary of the Fungi; CAB International: Wallingford, UK, 2008. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Rašeta, M.; Mišković, J.; Čapelja, E.; Zapora, E.; Fabijan, A.P.; Knežević, P.; Karaman, M. Do Ganoderma Species Represent Novel Sources of Phenolic Based Antimicrobial Agents? Molecules 2023, 28, 3264. [Google Scholar] [CrossRef]
- Isidorov, V.A. GC-MS of Biologically and Environmentally Significant Organic Compounds; TMS Derivatives: Hoboken, NJ, USA, 2020. [Google Scholar]
- Stephen, E.S. NIST/EPA/NIH Mass Spectral Library; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020.
- Stenhagen, E.A.; McLafferty, F.; Abrahamsson, S. Wiley Registry of Mass Spectral Data, 12th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Amel, B.; Seddik, K.; Shtaywy, A.; Saliha, D.; Mussa, A.Z.; Assia, B.; Saliha, D.; Abderahmane, B.; Smain, A. Phytochemical analysis, antioxidant activity and hypotensive effect of algerian azarole (Crataegus azarolus L.) leaves extracts. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 286–305. [Google Scholar]
- Karcz, D.; Starzak, K.; Ciszkowicz, E.; Lecka-Szlachta, K.; Kamiński, D.; Creaven, B.; Miłoś, A.; Jenkins, H.; Ślusarczyk, L.; Matwijczuk, A. Design, Spectroscopy, and Assessment of Cholinesterase Inhibition and Antimicrobial Activities of Novel Coumarin–Thiadiazole Hybrids. Int. J. Mol. Sci. 2022, 23, 6314. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ; National Institutes of Health: Bethesda, MD, USA, 2015. Available online: https://imagej.net/ij/ (accessed on 18 March 2025).


| Sample No. | Species Name | C. albicans [mg/mL] | P. aeruginosa [mg/mL] | S. aureus [mg/mL] | S. epidermidis [mg/mL] | K. pneumoniae [mg/mL] | K. rhizophila [mg/mL] |
|---|---|---|---|---|---|---|---|
| 16.D | Porodaedalea chrysoloma | >50 | >50 | 50 | >50 | 50 | >50 |
| 17.D | Porodaedalea pini | >50 | 50 | 25 | 50 | 50 | 50 |
| 18.D | Phellinus tremulae | >50 | 50 | 12,5 | 50 | 50 | 50 |
| 19.D | Phellinus laevigatus | >50 | >50 | 50 | >50 | >50 | >50 |
| 21.D | Phellinus tuberculosus | >50 | >50 | 25 | >50 | 50 | >50 |
| 22.D | Fomitiporia robusta | >50 | 50 | 25 | 25 | 50 | 25 |
| 25.D | Phellinopsis conchata | 25 | >50 | 25 | >50 | 50 | 50 |
| 38.D | Phellinus igniarius | >50 | >50 | 25 | >50 | >50 | 50 |
| 118.D | Phellinus alni | 25 | >50 | 25 | >50 | >50 | 50 |
| 160.D | Fuscoporia ferruginosa | 50 | 50 | 25 | 50 | 50 | 25 |
| 254.D | Phellinus alni | 25 | >50 | 25 | >50 | >50 | >50 |
| 255.D | Phellinus alni | 50 | >50 | >50 | 50 | >50 | 50 |
| 256.D | Phellinidium ferrugineofuscum | 25 | >50 | 50 | >50 | >50 | 50 |
| 257.D | Fomitiporia punctata | >50 | 50 | 50 | 50 | 50 | 50 |
| 258.D | Fomitiporia punctata | >50 | 50 | >50 | 50 | 25 | 25 |
| 261.D | Phellinus nigricans | >50 | >50 | 25 | 50 | >50 | >50 |
| 262.D | Phellinus populicola | >50 | 50 | 12.5 | >50 | 50 | 50 |
| 266.D | Fomitiporia robusta | >50 | >50 | 25 | 50 | 50 | 25 |
| 267.D | Phellinus nigricans | >50 | >50 | 12.5 | 50 | 50 | 50 |
| 268.D | Fomitiporia hartigii | >50 | 50 | 25 | 25 | 50 | 25 |
| 279.D | Phellinus tuberculosus | 50 | >50 | 25 | >50 | >50 | >50 |
| 280.D | Fomitiporia robusta | 25 | 50 | 25 | 50 | 50 | 25 |
| 298.D | Porodaedalea pini | 12.5 | 50 | 25 | 25 | 50 | 25 |
| 300.D | Phellinus nigricans | 25 | >50 | 25 | 50 | >50 | >50 |
| 300.Da | Phellinus nigricans | >50 | >50 | 6.3 | 50 | >50 | 50 |
| 301.D | Phellinus igniarius | 25 | >50 | 6.3 | 25 | 50 | 50 |
| 319.D | Fuscoporia torulosa | 50 | >50 | 50 | 50 | 50 | >50 |
| 320.D | Fuscoporia torulosa | 25 | >50 | >50 | 50 | 50 | 50 |
| 322.D | Fomitiporia mediterranea | >50 | 50 | 50 | 50 | 50 | 50 |
| 329.D | Porodaedalea chrysoloma | 50 | >50 | 12.5 | 50 | 50 | 50 |
| Element | Content of Elements [mg/kg of Extract] | |||||
|---|---|---|---|---|---|---|
| Fomitiporia robusta | * SD | Porodaedalea pini | * SD | Phellinus igniarius | * SD | |
| Cu | 17.50 | 0.75 | 23.18 | 0.83 | 6.01 | 0.22 |
| Fe | 10.84 | 0.69 | 5.35 | 0.22 | 5.04 | 0.88 |
| Mn | 10.86 | 0.79 | 1.69 | 0.10 | - | - |
| Ni | - | - | 0.41 | 0.14 | 0.57 | 0.14 |
| Zn | 18.52 | 0.27 | 16.15 | 0.79 | 6.01 | 0.22 |
| Ti | 5.76 | 0.28 | 4.92 | 0.27 | 8.09 | 1.02 |
| Rb | 12.81 | 0.55 | 11.47 | 0.70 | 18.27 | 0.72 |
| K | 3218.03 | 0.50 | 2349.73 | 5.62 | 2287.27 | 2.91 |
| Ca | 324.89 | 1.44 | 128.02 | 2.91 | 146.06 | 1.70 |
| Mg | - | - | - | - | 1051.27 | 1.90 |
| P | 2930.88 | 1.87 | 1224.77 | 2.40 | 2117.81 | 0.94 |
| S | 710.19 | 0.64 | 581.22 | 0.94 | 252.63 | 1.13 |
| Cl | 139.01 | 0.35 | 3194.66 | 1.00 | 3240.80 | 0.62 |
| Si | 202.47 | 0.89 | 88.55 | 1.90 | 70.70 | 2.40 |
| As | 1.78 | 0.48 | 1.41 | 0.09 | 0.99 | 0.11 |
| Br | 3.80 | 0.23 | 21.54 | 2.22 | 4.18 | 0.38 |
| Fomitiporia robusta | Porodaedalea pini | Phellinus igniarius | Type of Vibration | Groups of Chemical Compounds | |||
|---|---|---|---|---|---|---|---|
| Dried Fruiting Body | Extract | Dried Fruiting Body | Extract | Dried Fruiting Body | Extract | ||
| 1645 s | 1733 s | 1647 s | 1711 vs | 1647 s | 1735 s | νC = O | Carboxylic acid, esters, fatty acids, and carbohydrates |
| 1675 m | |||||||
| 1556 m | 1554 m | 1555 m | 1552 m | 1549 m | 1553 w | νCCring | Aromatic/phenolic compounds |
| 1515 m | 1520 m | 1514 m | 1517 m | 1515 m | 1519 w | νCCring | Aromatic/phenolic compounds |
| - | 1454 m | - | 1451 s | - | 1453 m | δCH2, δCH3 | Aromatic and aliphatic compounds |
| - | - | 1415 m | 1410 m | 1416 m | - | δCH2, δCH3, δOH | Aromatic and aliphatic compounds, and carbohydrates |
| 1372 m | 1374 m | 1365 m | 1374 m | 1366 m | 1374 m | γCHring | Aromatic/phenolic compounds |
| 1301 m | - | 1310 w | 1280 m | 1306 m | δCH2OH | Carbohydrates | |
| 1242 w | 1245 m | 1243 m | δCH2OH | Carbohydrates | |||
| 1206 w | 1202 m | - | 1202 m | 1205 m | 1205 m | νCO, νCC, δCOH | Carbohydrates |
| 1168 m | 1151 m | - | 1155 m | - | δCHring | Aromatic/phenolic compounds | |
| 1113 m | 1116 m | 1107 m | - | 1123 m | δCHring | Aromatic/phenolic compounds | |
| - | 1075 m | - | 1075 m | - | 1088 s | δCHring | Aromatic/phenolic compounds |
| 1054 vs, 1037 vs | 1029 vs | 1038 s | 1024 s | 1041 s | 1055 s | νC-O | Carboxylic acids, fatty acids, and carbohydrates |
| - | 993 m | - | - | - | 1000 s | ||
| 913 w | 946 w | 915 w | - | 899 m | - | δCOH, δCCH, δOCH | Carbohydrates |
| 862 m | 871 m | 860 m | 874 m | 858 m | 865 m | δCOH, δCCH, δOCH | Carbohydrates |
| 779 m | 779 m | - | 809 m | - | 800 m | δCOH, δCCH, δOCH | Carbohydrates |
| 691 m | 691 s | 697 s | - | - | 695 m | De-fring | Aromatic/phenolic compounds |
| 632 s | 657 s | 624 m | 633 m | 627 s | 635 s m | De-fring | Aromatic/phenolic compounds |
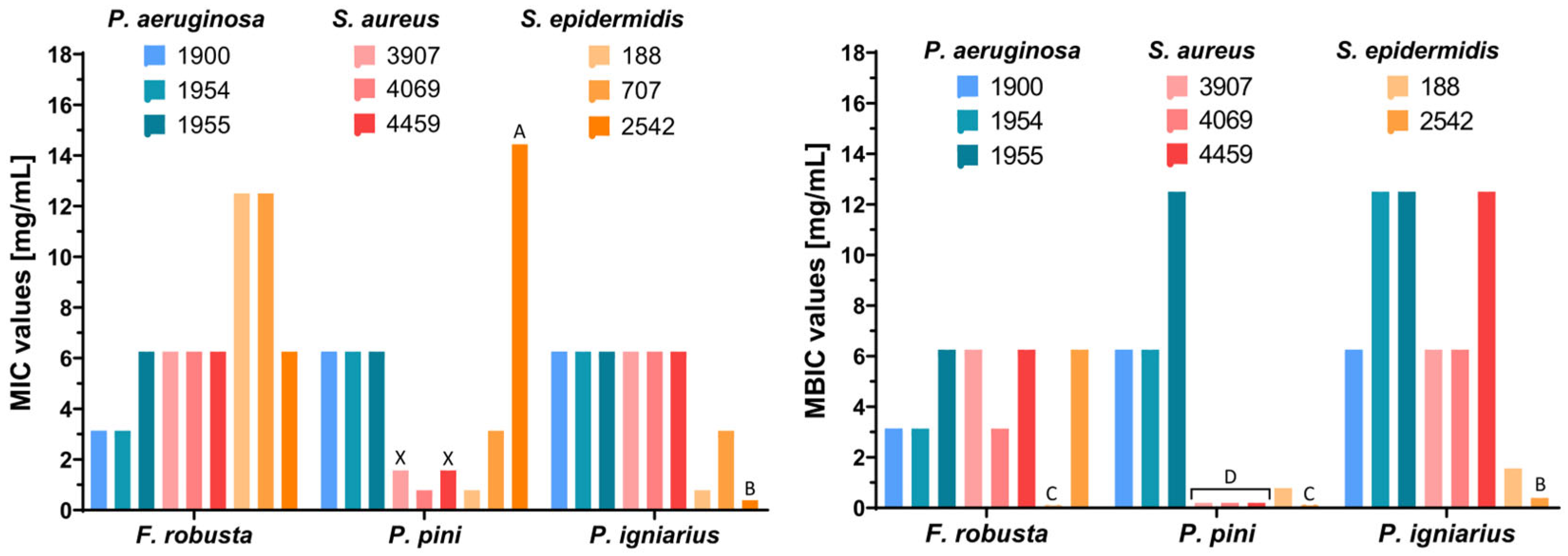
| Methanolic Extract | Concentration [mg/mL] | Clinical Strains | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa | Staphylococcus aureus | Staphylococcus epidermidis | ||||||||
| 1900 | 1954 | 1955 | 3907 | 4069 | 4459 | 188 | 707 | 2542 | ||
| F. robusta | MIC | 3.13 | 3.13 | 6.25 | 6.25 | 6.25 | 6.25 | >6.25 | >6.25 | 6.25 |
| MBC | BS a | BS a | BS a | BS a | BS a | BS a | BS a | BS a | BS a | |
| MBIC | 3.13 | 3.13 | 6.25 | 6.25 | 3.13 | 6.25 | - c | - b | 6.25 | |
| P. pini | MIC | 6.25 | 6.25 | 6.25 | 1.56 | 0.78 | 1.56 | 0.78 | 3.13 | >12.5 |
| MBC | BS a | BS a | BS a | 6.25 | BS a | 6.25 | BS a | BS a | BS a | |
| MBIC | 6.25 | 6.25 | 12.50 | <0.20 | <0.20 | <0.20 | 0.78 | - b | - c | |
| P. igniarius | MIC | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 | 0.78 | 3.13 | <0.78 |
| MBC | BS a | BS a | BS a | BS a | BS a | BS a | BS a | BS a | BS a | |
| MBIC | 6.25 | 12.50 | 12.50 | 6.25 | 6.25 | 12.50 | 1.56 | - b | <0.78 | |
| ERT | MIC | 0.06 | 0.06 | 0.06 | 0.50 | 2 × 10−4 | >0.50 | 2 × 10−4 | 2 × 10−3 | 2 × 10−4 |
| MBC | BS a | BS a | BS a | BS a | BS a | >0.50 | 4 × 10−3 | 2 × 10−2 | 4 × 10−3 | |
| MBIC | 0.06 | 0.06 | 0.06 | 0.50 | 2 × 10−4 | - c | 2 × 10−4 | - b | 2 × 10−4 | |
| Sample No. | Evidence Specimen Number | Species Name | Substrate/Host (Species Name) | Collection Site | Collected and Identified |
|---|---|---|---|---|---|
| 16.D | BLS-M-05505 | Porodaedalea chrysoloma (Fries) Fiasson & Niemelä [syn. Phellinus chrysoloma (Fr.) Donk] | Picea abies | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 17.D | BLS-M-05498 | Porodaedalea pini (Brot.) Murrill [syn. Phellinus pini (Brot.) A. Ames] | Pinus sylvestris | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 18.D | BLS-M-05506 | Phellinus tremulae (Bondartsev) Bondartsev & P.N. Borissov | Populus tremula | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 19.D | BLS-M-05512 | Phellinus laevigatus (Fries) Bourdot & Galzin | Betula pendula | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 21.D | BLS-M-05493 | Phellinus tuberculosus (Baumgarten) Niemelä [syn. Phellinus pomaceus (Pers.) Maire] | Prunus domestica | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 22.D | BLS-M-05497 | Fomitiporia robusta (P. Karst.) Fiasson & Niemelä [syn. Phellinus robustus (P. Karst.) Bourdot & Galzin] | Quercus robur | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 25.D | BLS-M-02596 | Phellinopsis conchata (Persoon) Dai [syn. Phellinus conchatus (Pers.: Fr.) Quél] | Salix caprea | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 38.D | BLS-M-01027 | Phellinus igniarius (L.) Quél. | Salix fragilis | Poland, Sokolskie Hill, Losiniany | Leg. et det. Marek Wołkowycki |
| 118.D | BLS-M-05609 | Phellinus alni (Bondartsev) Parmasto | Alnus glutinosa | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 160.D | BLS-M-05495 | Fuscoporia ferruginosa (Schrad ex J.F. Gmelin) Murill [syn. Phellinus ferruginosus (Schrad.: Fr.) Pat.] | Fraxinus excelsior | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 254.D | BLS-M-05492 | Phellinus alni (Bondartsev) Parmasto | Malus domestica | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 255.D | BLS-M-05490 | Phellinus alni (Bondartsev) Parmasto | Carpinus betulus | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 256.D | BLS-M-05448 | Phellinidium ferrugineofuscum (P. Karst) Fiasson & Niemelä [syn. Phellinus ferrugineofuscus (P. Karst.) Bourdot] | Picea abies | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 257.D | BLS-M-05494 | Fomitiporia punctata (Fries ex P. Karsten) Murill [syn. Phellinus punctatus (P.Karst.) Pilát] | Corylus avellana | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 258.D | BLS-M-05499 | Fomitiporia punctata (Fries ex P. Karsten) Murill [syn. Phellinus punctatus (P.Karst.) Pilát] | Sorbus aucuparia | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 261.D | BLS-M-05509 | Phellinus nigricans (Fries) P. Karsten | Betula pendula | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 262.D | BLS-M-05507 | Phellinus populicola Niemelä | Populus tremula | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 266.D | BLS-M-02977 | Fomitiporia robusta (P. Karst.) Fiasson & Niemelä [syn. Phellinus robustus (P. Karst.) Bourdot & Galzin] | Robinia pseudoacacia | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 267.D | BLS-M-04016 | Phellinus nigricans (Fries) P. Karsten | Betula pubescens | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 268.D | BLS-M-05496 | Fomitiporia hartigii (Allesch. & Schnabl) Fiasson & Niemelä [syn. Phellinus hartigii (Allesch. & Schnabl) Pat] | Abies alba | Poland, Beskid Niski | Leg. et det. Anna Hreczka rev. Marek Wołkowycki |
| 279.D | BLS-M-05599 | Phellinus tuberculosus (Baumgarten) Niemelä [syn. Phellinus pomaceus (Pers.) Maire] | Cerasus avium | Poland, Kuraszewo, wild fruit orchard | Leg. Konrad Wilamowski det. Marek Wołkowycki |
| 280.D | BLS-M-05610 | Fomitiporia robusta (P. Karst.) Fiasson & Niemelä [syn. Phellinus robustus (P. Karst.) Bourdot & Galzin] | Quercus rubra | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
| 298.D | BLS-M-10167 | Porodaedalea pini (Brot.) Murrill (syn. Porodaedalea pini (Brot.) A. Ames) | Pinus pinea | Portugal, Estremadura, Sesimbra, Lagoa de Albufeira | Leg. et det. Mitko Karadelev |
| 300.D | BLS-M-10094 | Phellinus nigricans (Fries) P. Karsten | Fagus sylvatica | Poland, Białowieża Forest | Leg. Konrad Wilamowski det. Marek Wołkowycki |
| 300.Da | BLS-M-10164 | Phellinus nigricans (Fries) P. Karsten | Fagus sylvatica | Poland, Baltic Coast, “Zagorska Struga” Valley | Leg. et det. Mirosław Wantoch-Rekowski rev. Marek Wołkowycki |
| 301.D | BLS-M-10093 | Phellinus igniarius (L.) Quél. | Sorbus intermedia | Poland, Romnicka Forest | Leg. Konrad Wilamowski det. Marek Wołkowycki |
| 319.D | BLS-M-10412 | Fuscoporia torulosa (Pers.) T. Wagner & M. Fisch [syn. Phellinus torulosus (Pers.) Bourdot & Galzin] | Quercus ilex | Italy, Perugia, Sacro Bosco di Monteluco, Spoleto | Leg. et det. Carolina Girometta |
| 320.D | BLS-M-10413 | Fuscoporia torulosa (Pers.) T. Wagner & M. Fisch [syn. Phellinus torulosus (Pers.) Bourdot & Galzin] | Robinia pseudoacacia | Italy, Pavia, “Bosco Siro Negri” State Nature Reserve | Leg. et det. Carolina Girometta |
| 322.D | BLS-M-10414 | Fomitiporia mediterranea M. Fisch. [syn. Phellinus punctatus (P. Karst.) Pilát] | Robinia pseudoacacia | Italy, Pavia, “Bosco Siro Negri” State Nature Reserve | Leg. et det. Carolina Girometta |
| 329.D | BLS-M-05505 | Porodaedalea chrysoloma (Fr.) [syn. Porodaedalea abietis] [syn. Phellinus chrysoloma (Fr.) Donk] | Picea abies | Poland, Białowieża Forest | Leg. et det. Marek Wołkowycki |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świderski, G.; Kalinowska, M.; Zapora, E.; Wołkowycki, M.; Stocki, M.; Ciszkowicz, E.; Bocian, A.; Jaromin, M.; Tyrka, M.; Lecka-Szlachta, K.; et al. Bioactive Properties of Selected European Phellinus Species: A Comprehensive Study. Int. J. Mol. Sci. 2025, 26, 8013. https://doi.org/10.3390/ijms26168013
Świderski G, Kalinowska M, Zapora E, Wołkowycki M, Stocki M, Ciszkowicz E, Bocian A, Jaromin M, Tyrka M, Lecka-Szlachta K, et al. Bioactive Properties of Selected European Phellinus Species: A Comprehensive Study. International Journal of Molecular Sciences. 2025; 26(16):8013. https://doi.org/10.3390/ijms26168013
Chicago/Turabian StyleŚwiderski, Grzegorz, Monika Kalinowska, Ewa Zapora, Marek Wołkowycki, Marcin Stocki, Ewa Ciszkowicz, Aleksandra Bocian, Marcin Jaromin, Mirosław Tyrka, Katarzyna Lecka-Szlachta, and et al. 2025. "Bioactive Properties of Selected European Phellinus Species: A Comprehensive Study" International Journal of Molecular Sciences 26, no. 16: 8013. https://doi.org/10.3390/ijms26168013
APA StyleŚwiderski, G., Kalinowska, M., Zapora, E., Wołkowycki, M., Stocki, M., Ciszkowicz, E., Bocian, A., Jaromin, M., Tyrka, M., Lecka-Szlachta, K., Wołejko, E., Wydro, U., Pawłowska, M., Golianek, P., Zawadzka, M., Ramshaj, Q., Girometta, C. E., & Karadelev, M. (2025). Bioactive Properties of Selected European Phellinus Species: A Comprehensive Study. International Journal of Molecular Sciences, 26(16), 8013. https://doi.org/10.3390/ijms26168013













