25-Hydroxycholesterol Induces Intrinsic Apoptosis via Mitochondrial Pathway in BE(2)-C Human Neuroblastoma Cells
Abstract
1. Introduction
2. Results
2.1. Viability of BE(2)-C Cells Following Oxysterol Treatment
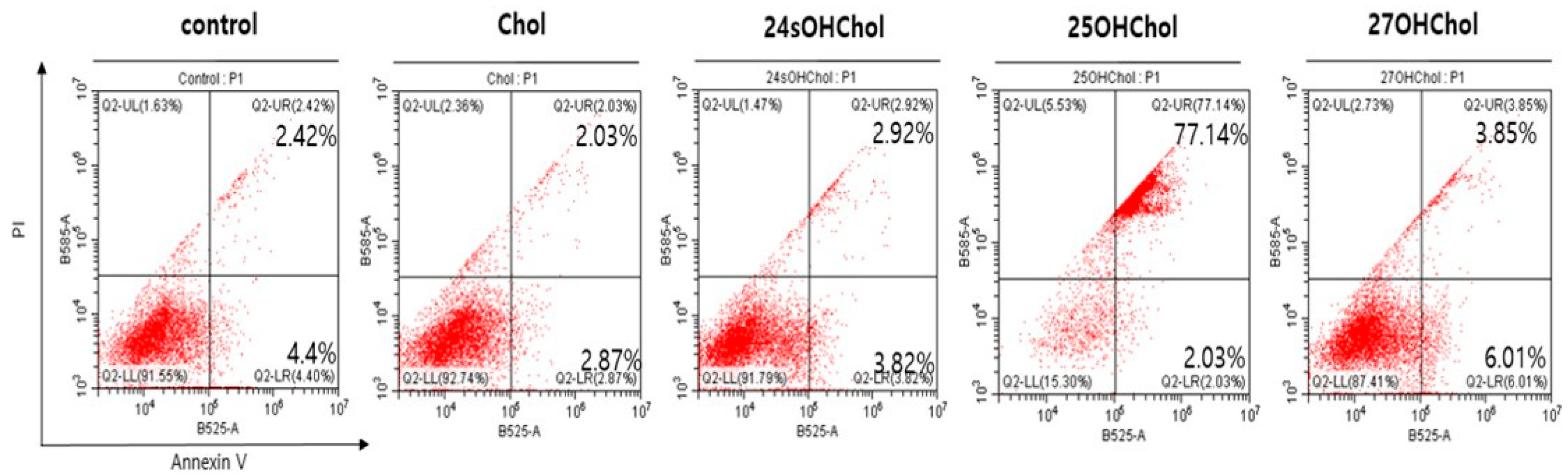
2.2. Induction of Apoptosis in BE(2)-C Cells by 25OHChol
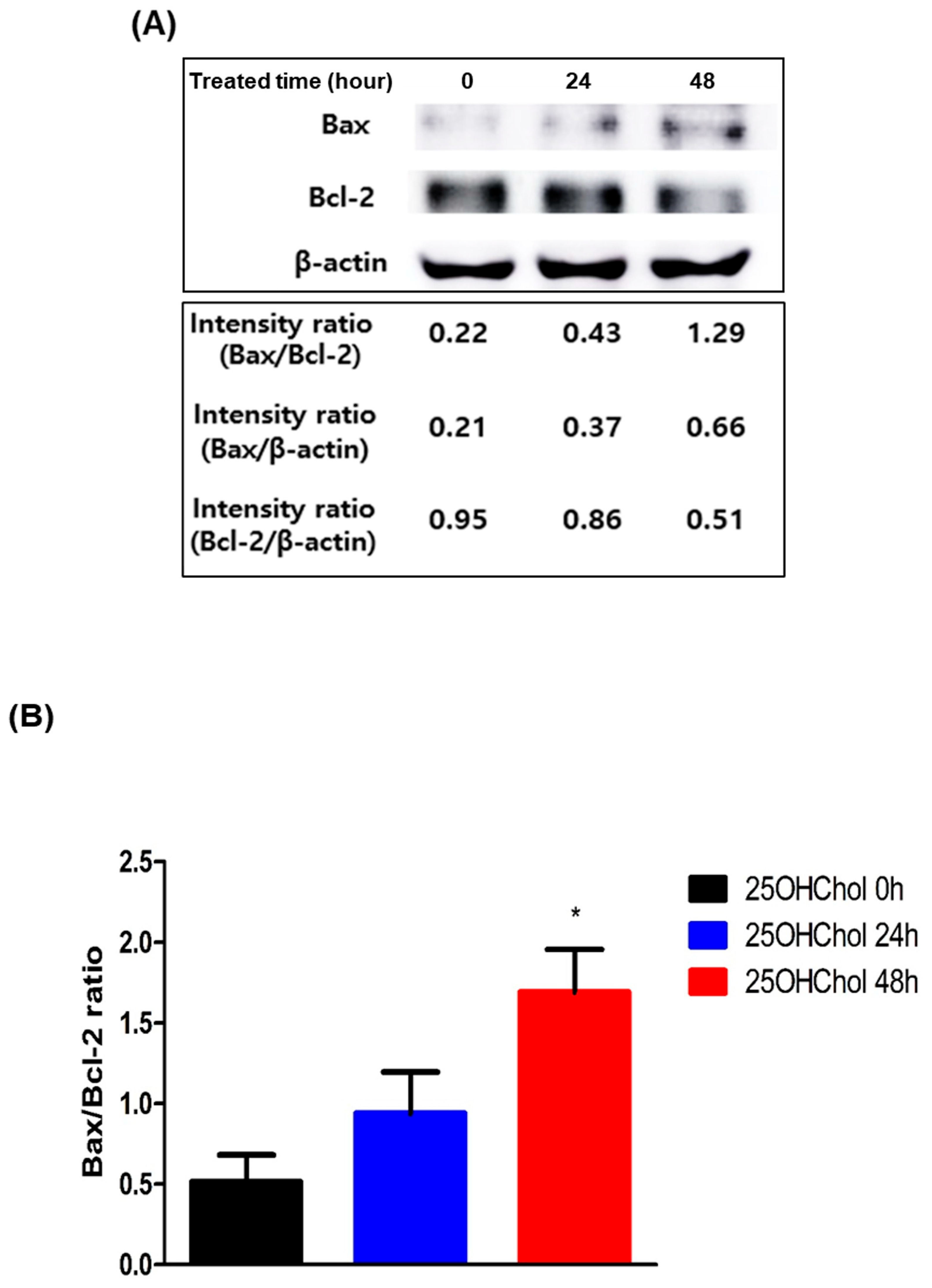
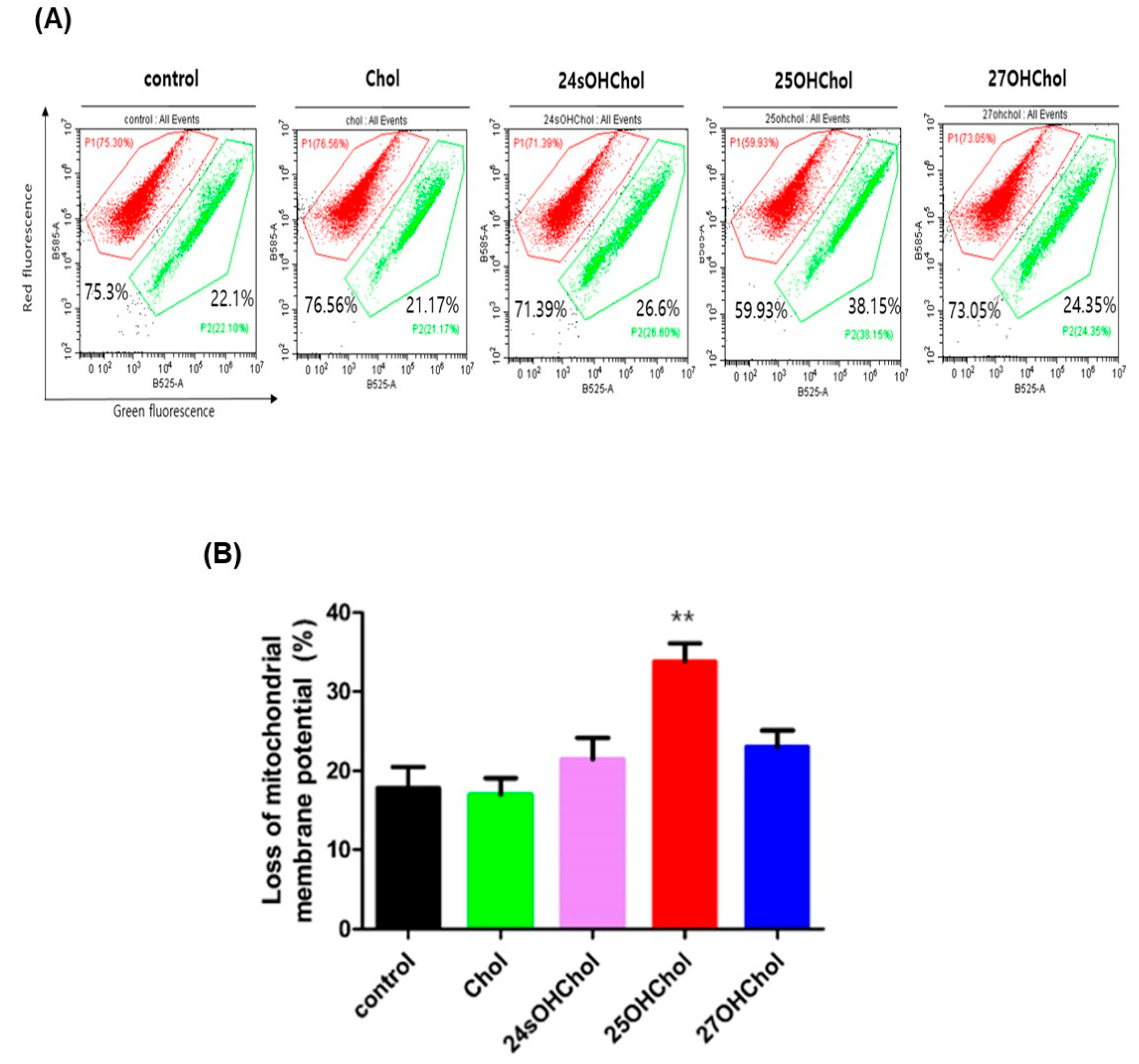
2.3. Role of 25OHChol in Apoptosis Through Mitochondrial Apoptotic Regulators in BE(2)-C Cells
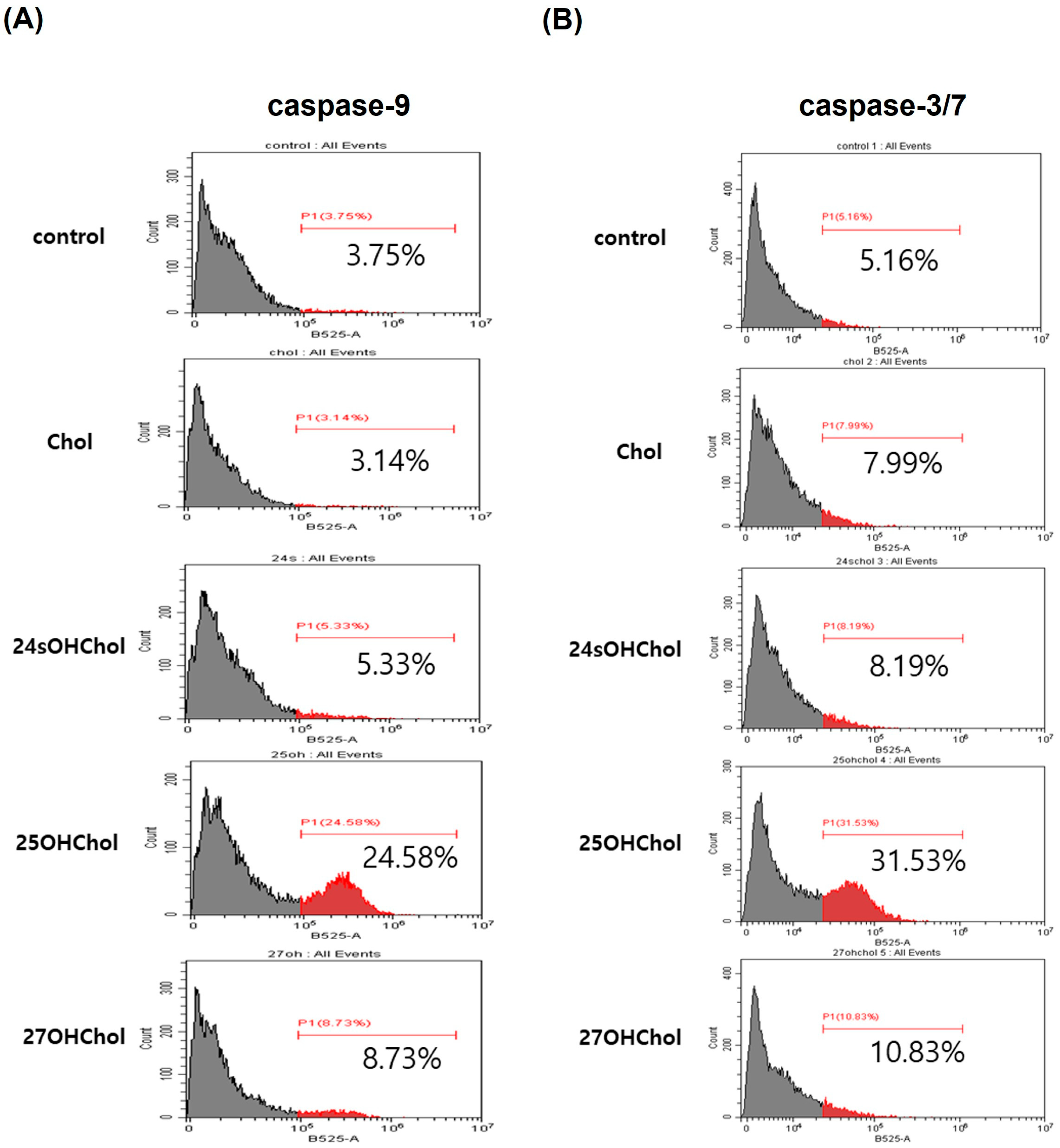
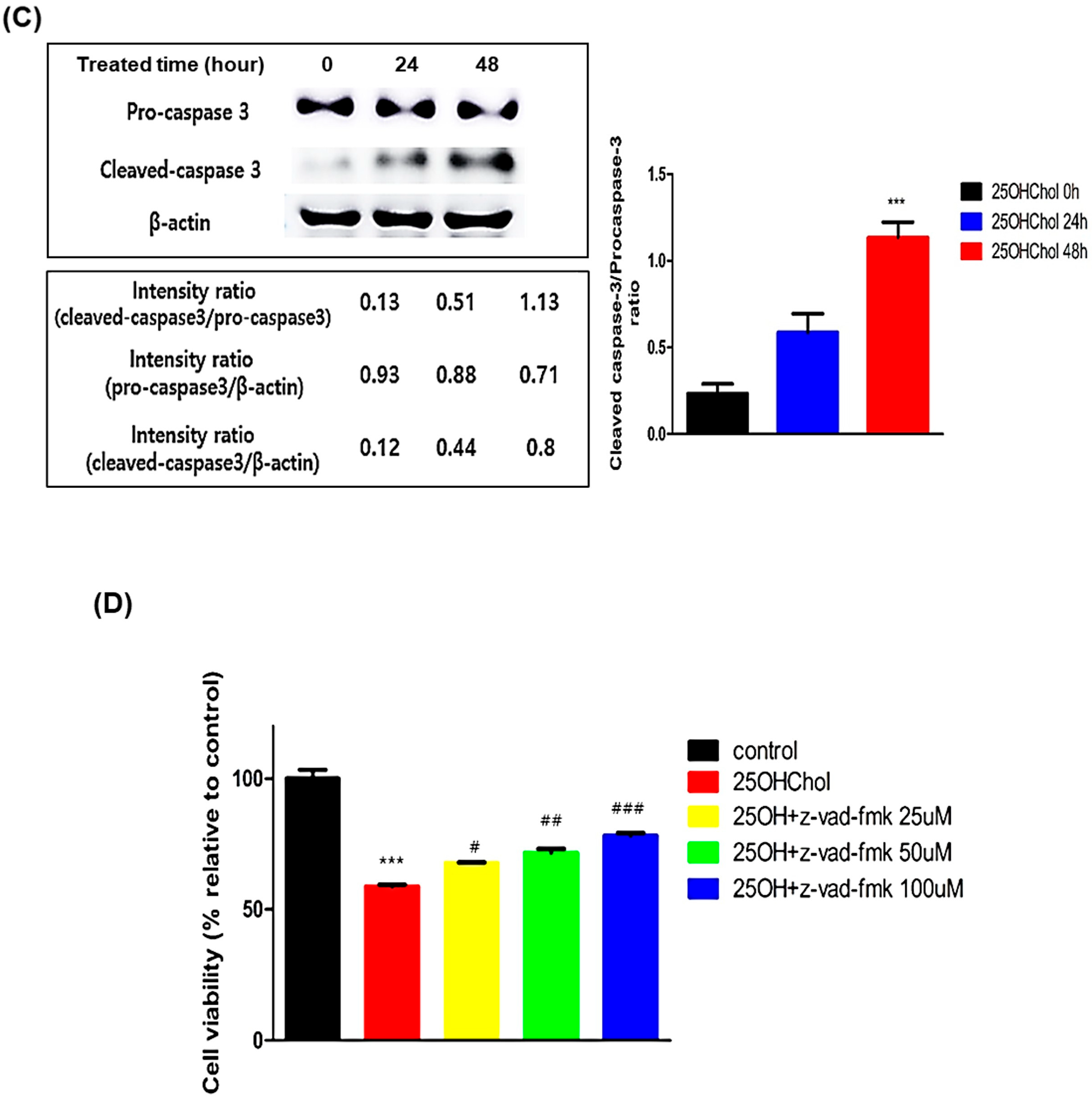
2.4. Caspase-Dependent Apoptotic Effects Induced by 25OHChol in BE(2)-C Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Treatment
4.3. Cell Viability Assay
4.4. Nuclear Staining
4.5. Assay of Annexin V/PI
4.6. Assessment of Mitochondrial Membrane Potential (MMP, ΔΨm)
4.7. Assay of Caspase Activity
4.8. Western Blot Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schulte, J.; Schulte, S.; Heukamp, L.; Astrahantseff, K.; Stephan, H.; Fischer, M.; Schramm, A.; Eggert, A. Targeted therapy for neuroblastoma: ALK inhibitors. Klin. Pädiatrie 2013, 225, 303–308. [Google Scholar] [CrossRef]
- Esposito, M.R.; Aveic, S.; Seydel, A.; Tonini, G.P. Neuroblastoma treatment in the post-genomic era. J. Biomed. Sci. 2017, 24, 14. [Google Scholar] [CrossRef]
- Ahmed, S.; Goel, S.; Khandwala, M.; Agrawal, A.; Chang, B.; Simmons, I. Neuroblastoma with orbital metastasis: Ophthalmic presentation and role of ophthalmologists. Eye 2006, 20, 466–470. [Google Scholar] [CrossRef]
- Costa, A.D.; Zerbini, M.C.N.; Cristofani, L. Metastatic congenital neuroblastoma associated with in situ neuroblastoma: Case report and review of literature. Autops. Case Rep. 2014, 4, 27. [Google Scholar] [CrossRef]
- Maris, J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, B.; Kremer, L.C.; Caron, H.N.; van Dalen, E.C. High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Peinemann, F.; van Dalen, E.C.; Enk, H.; Berthold, F. Retinoic acid postconsolidation therapy for high-risk neuroblastoma patients treated with autologous haematopoietic stem cell transplantation. Cochrane Database Syst. Rev. 2017, 2017, CD010685. [Google Scholar] [CrossRef] [PubMed]
- Van Ginkel, P.R.; Sareen, D.; Subramanian, L.; Walker, Q.; Darjatmoko, S.R.; Lindstrom, M.J.; Kulkarni, A.; Albert, D.M.; Polans, A.S. Resveratrol inhibits tumor growth of human neuroblastoma and mediates apoptosis by directly targeting mitochondria. Clin. Cancer Res. 2007, 13, 5162–5169. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Christgen, S.; Tweedell, R.E.; Kanneganti, T.-D. Programming inflammatory cell death for therapy. Pharmacol. Ther. 2022, 232, 108010. [Google Scholar] [CrossRef] [PubMed]
- Ameisen, J.C. On the origin, evolution, and nature of programmed cell death: A timeline of four billion years. Cell Death Differ. 2002, 9, 367–393. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wideranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Thompson, C.B. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.K.; Strasser, A. The essentials of developmental apoptosis. F1000Research 2020, 9, 148. [Google Scholar] [CrossRef]
- Strasser, A.; Harris, A.W.; Huang, D.; Krammer, P.H.; Cory, S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995, 14, 6136–6147. [Google Scholar] [CrossRef]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Henzel, W.J.; Liu, X.; Lutschg, A.; Wang, X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c–dependent activation of caspase-3. Cell 1997, 90, 405–413. [Google Scholar] [CrossRef]
- Fernández, C.; María del Val, T.L.; Gómez-Coronado, D.; Lasunción, M.A. Cholesterol is essential for mitosis progression and its deficiency induces polyploid cell formation. Exp. Cell Res. 2004, 300, 109–120. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Wang, Y. An update on oxysterol biochemistry: New discoveries in lipidomics. Biochem. Biophys. Res. Commun. 2018, 504, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Mutemberezi, V.; Guillemot-Legris, O.; Muccioli, G.G. Oxysterols: From cholesterol metabolites to key mediators. Prog. Lipid Res. 2016, 64, 152–169. [Google Scholar] [CrossRef]
- Duc, D.; Vigne, S.; Pot, C. Oxysterols in autoimmunity. Int. J. Mol. Sci. 2019, 20, 4522. [Google Scholar] [CrossRef]
- Crosignani, A.; Zuin, M.; Allocca, M.; Del Puppo, M. Oxysterols in bile acid metabolism. Clin. Chim. Acta 2011, 412, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Cyster, J.G.; Dang, E.V.; Reboldi, A.; Yi, T. 25-Hydroxycholesterols in innate and adaptive immunity. Nat. Rev. Immunol. 2014, 14, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, M.; Nunes, M.J.; Rodrigues, E. Cholesterol 24-hydroxylase: Brain cholesterol metabolism and beyond. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2016, 1861, 1911–1920. [Google Scholar] [CrossRef]
- Nakazawa, T.; Miyanoki, Y.; Urano, Y.; Uehara, M.; Saito, Y.; Noguchi, N. Effect of vitamin E on 24 (S)-hydroxycholesterol-induced necroptosis-like cell death and apoptosis. J. Steroid Biochem. Mol. Biol. 2017, 169, 69–76. [Google Scholar] [CrossRef]
- Rao, M.L.; Lutjohann, D.; Ludwig, M.; Kolsch, H. Induction of apoptosis and necrosis in human neuroblastoma cells by cholesterol oxides. Ann. N. Y. Acad. Sci. 1999, 893, 379–381. [Google Scholar] [CrossRef]
- Yamanaka, K.; Urano, Y.; Takabe, W.; Saito, Y.; Noguchi, N. Induction of apoptosis and necroptosis by 24 (S)-hydroxycholesterol is dependent on activity of acyl-CoA: Cholesterol acyltransferase 1. Cell Death Dis. 2014, 5, e990. [Google Scholar] [CrossRef]
- Woo, S.-Y.; Lee, H.; Park, S.M.; Choi, H.-S.; Kim, J.; Kwon, M.; Sohn, J.; Nam, J.H.; Kim, H.-S.; Song, P.; et al. Role of reactive oxygen species in regulating 27-hydroxycholesterol-induced apoptosis of hematopoietic progenitor cells and myeloid cell lines. Cell Death Dis. 2022, 13, 916. [Google Scholar] [CrossRef] [PubMed]
- Aupeix, K.; Weltin, D.; Mejia, J.E.; Christ, M.; Marchal, J.; Freyssinet, J.-M.; Bischoff, P. Oxysterol-induced apoptosis in human monocytic cell lines. Immunobiology 1995, 194, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Torres, S.; Moller, P.C.; Johnson, B.H.; Thompson, E.B. Characteristics of 25-hydroxycholesterol-induced apoptosis in the human leukemic cell line CEM. Exp. Cell Res. 1997, 235, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, Y.; Choi, I.; Kim, S.-W.; Kim, W.-K. 25-Hydroxycholesterol induces mitochondria-dependent apoptosis via activation of glycogen synthase kinase-3 β in PC12 cells. Free Radic. Res. 2008, 42, 544–553. [Google Scholar] [CrossRef]
- You, J.-S.; Lim, H.; Kim, T.-H.; Oh, J.-S.; Lee, G.-J.; Seo, Y.-S.; Kim, D.K.; Yu, S.-K.; Kim, H.-J.; Kim, C.S.; et al. 25-hydroxycholesterol induces death receptor-mediated extrinsic and mitochondria-dependent intrinsic apoptosis in head and neck squamous cell carcinoma cells. Anticancer Res. 2020, 40, 779–788. [Google Scholar] [CrossRef]
- Levy, D.; de Melo, T.C.; Ohira, B.Y.; Fidelis, M.L.; Ruiz, J.L.; Rodrigues, A.; Bydlowski, S.P. Oxysterols selectively promote short-term apoptosis in tumor cell lines. Biochem. Biophys. Res. Commun. 2018, 505, 1043–1049. [Google Scholar] [CrossRef]
- Schroepfer, G.J., Jr. Oxysterols: Modulators of cholesterol metabolism and other processes. Physiol. Rev. 2000, 80, 361–554. [Google Scholar] [CrossRef]
- Bischoff, P.L.; Holl, V.; Coelho, D.; Dufour, P.; Luu, B.; Weltin, D. Apoptosis at the interface of immunosuppressive and anticancer activities the examples of two classes of chemical inducers, oxysterols and alkylating agents. Curr. Med. Chem. 2000, 7, 693–713. [Google Scholar] [CrossRef]
- Whittle, S.B.; Smith, V.; Doherty, E.; Zhao, S.; McCarty, S.; Zage, P.E. Overview and recent advances in the treatment of neuroblastoma. Expert Rev. Anticancer Ther. 2017, 17, 369–386. [Google Scholar] [CrossRef]
- Tuli, H.S.; Kaur, J.; Vashishth, K.; Sak, K.; Sharma, U.; Choudhary, R.; Behl, T.; Singh, T.; Sharma, S.; Saini, A.K.; et al. Molecular mechanisms behind ROS regulation in cancer: A balancing act between augmented tumorigenesis and cell apoptosis. Arch. Toxicol. 2023, 97, 103–120. [Google Scholar] [CrossRef]
- Arur, S.; Uche, U.E.; Rezaul, K.; Fong, M.; Scranton, V.; Cowan, A.E.; Mohler, W.; Han, D.K. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev. Cell 2003, 4, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Bray, D.; Lewis, J.; Raff, M.; Roberts, K.; Watson, J.D. Molecular Biology of the Cell; Garland: New York, NY, USA, 1994. [Google Scholar]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.-I.; Jones, D.P.; Wang, X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Kuribayashi, K.; Mayes, P.A.; El-Deiry, W.S. What are caspases 3 and 7 doing upstream of the mitochondria? Cancer Biol. Ther. 2006, 5, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Slee, E.; Adrain, C.; Martin, S. Serial killers: Ordering caspase activation events in apoptosis. Cell Death Differ. 1999, 6, 1067–1074. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, K.; Park, D.; Eo, S.-K.; Lee, B.-A.; Son, Y. 25-Hydroxycholesterol Induces Intrinsic Apoptosis via Mitochondrial Pathway in BE(2)-C Human Neuroblastoma Cells. Int. J. Mol. Sci. 2025, 26, 8012. https://doi.org/10.3390/ijms26168012
Kim J, Kim K, Park D, Eo S-K, Lee B-A, Son Y. 25-Hydroxycholesterol Induces Intrinsic Apoptosis via Mitochondrial Pathway in BE(2)-C Human Neuroblastoma Cells. International Journal of Molecular Sciences. 2025; 26(16):8012. https://doi.org/10.3390/ijms26168012
Chicago/Turabian StyleKim, Jaesung, Koanhoi Kim, Dongha Park, Seong-Kug Eo, Bo-Ae Lee, and Yonghae Son. 2025. "25-Hydroxycholesterol Induces Intrinsic Apoptosis via Mitochondrial Pathway in BE(2)-C Human Neuroblastoma Cells" International Journal of Molecular Sciences 26, no. 16: 8012. https://doi.org/10.3390/ijms26168012
APA StyleKim, J., Kim, K., Park, D., Eo, S.-K., Lee, B.-A., & Son, Y. (2025). 25-Hydroxycholesterol Induces Intrinsic Apoptosis via Mitochondrial Pathway in BE(2)-C Human Neuroblastoma Cells. International Journal of Molecular Sciences, 26(16), 8012. https://doi.org/10.3390/ijms26168012






