Recent Advances in the Application of Cucurbitacin B as an Anticancer Agent
Abstract
1. Introduction
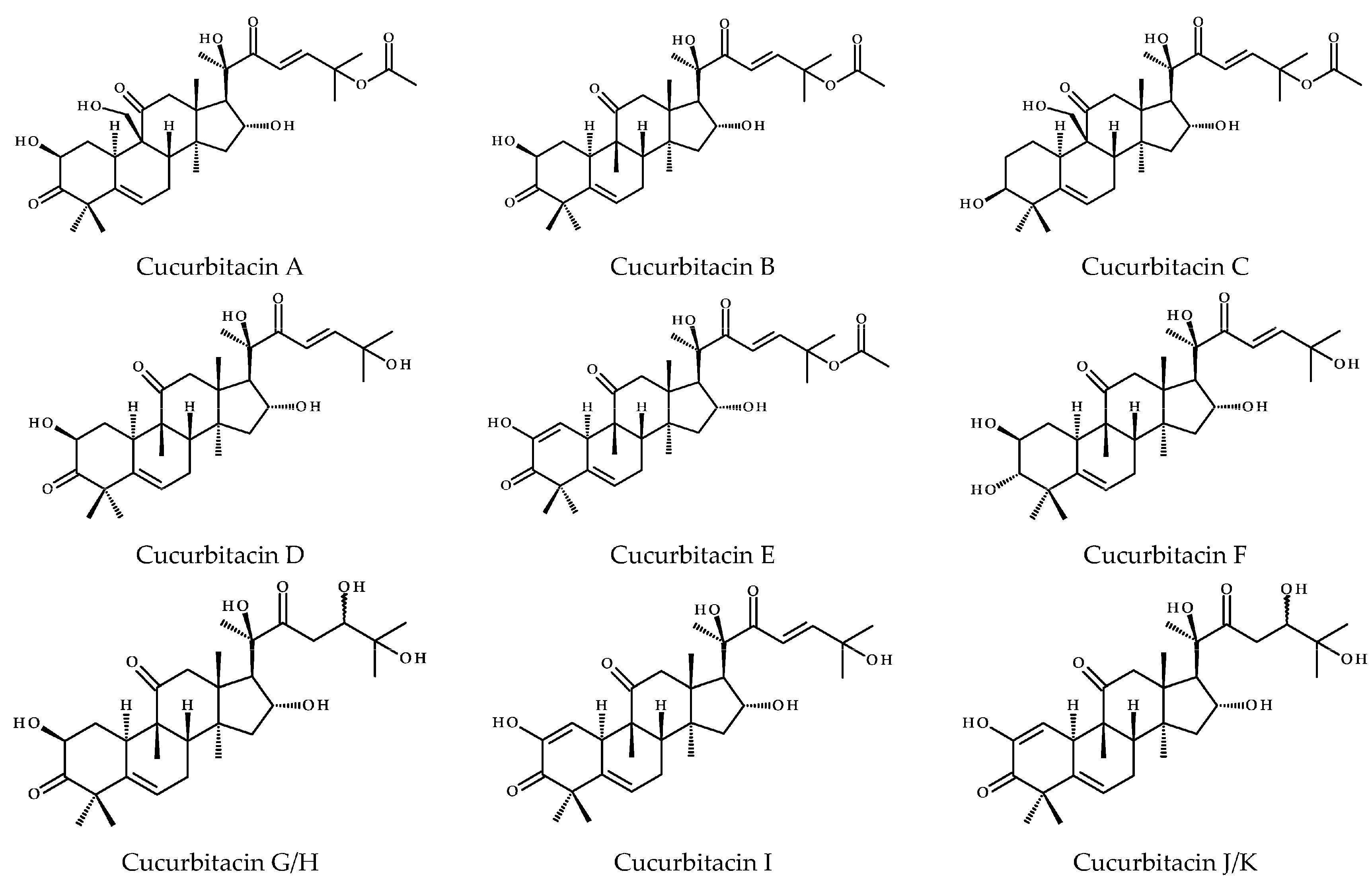
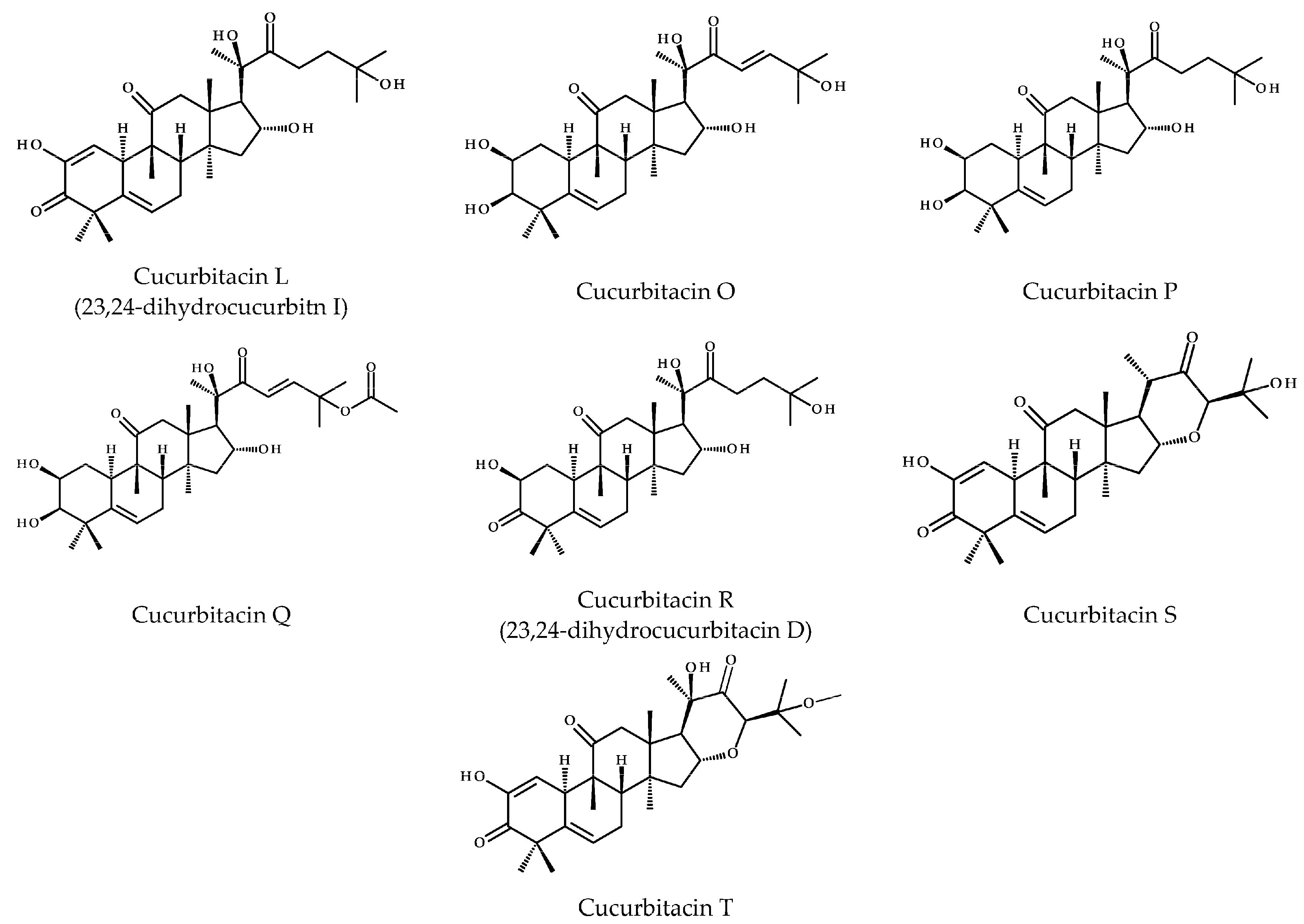
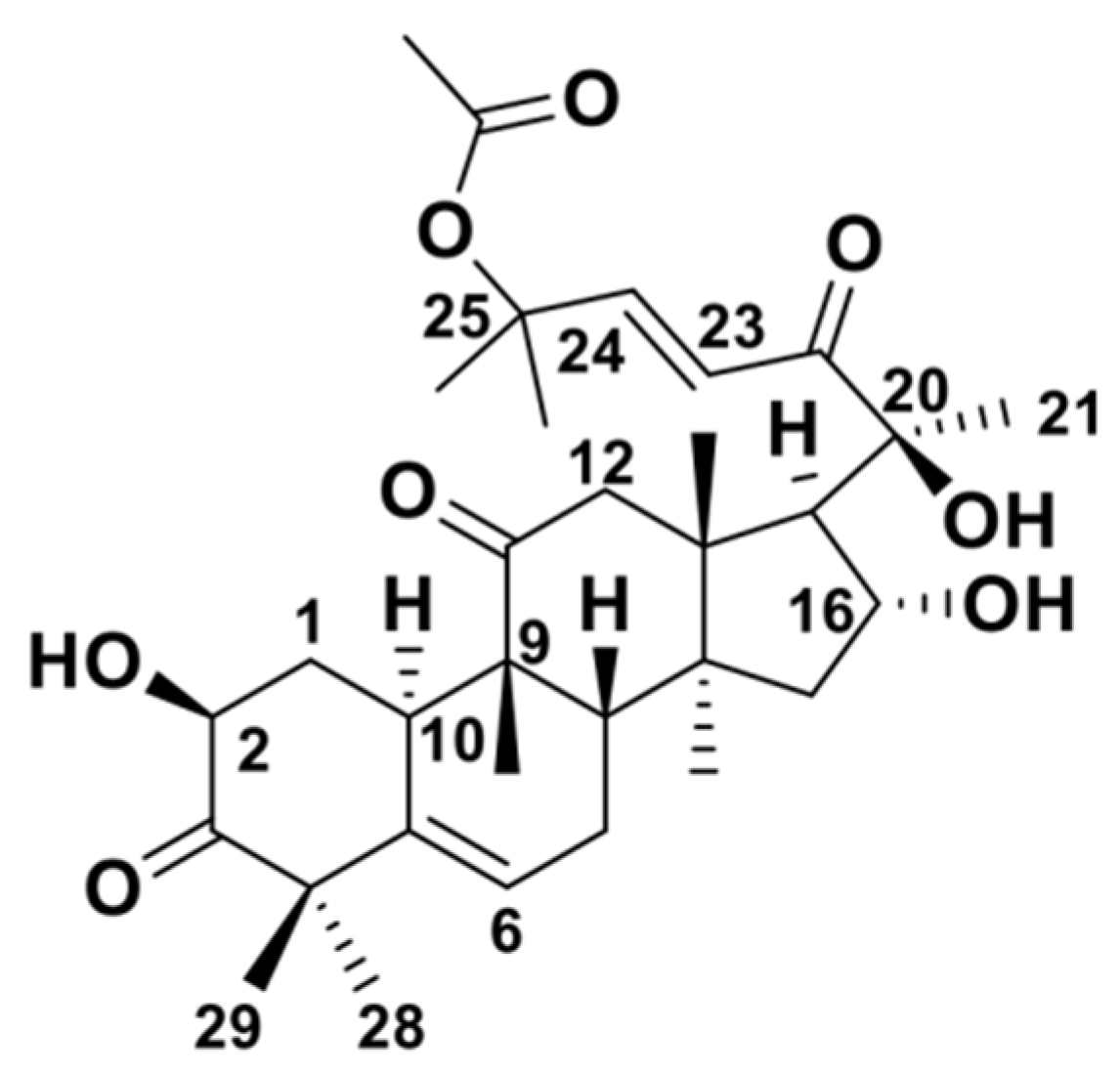
2. Structure and Physicochemical Properties of CuB
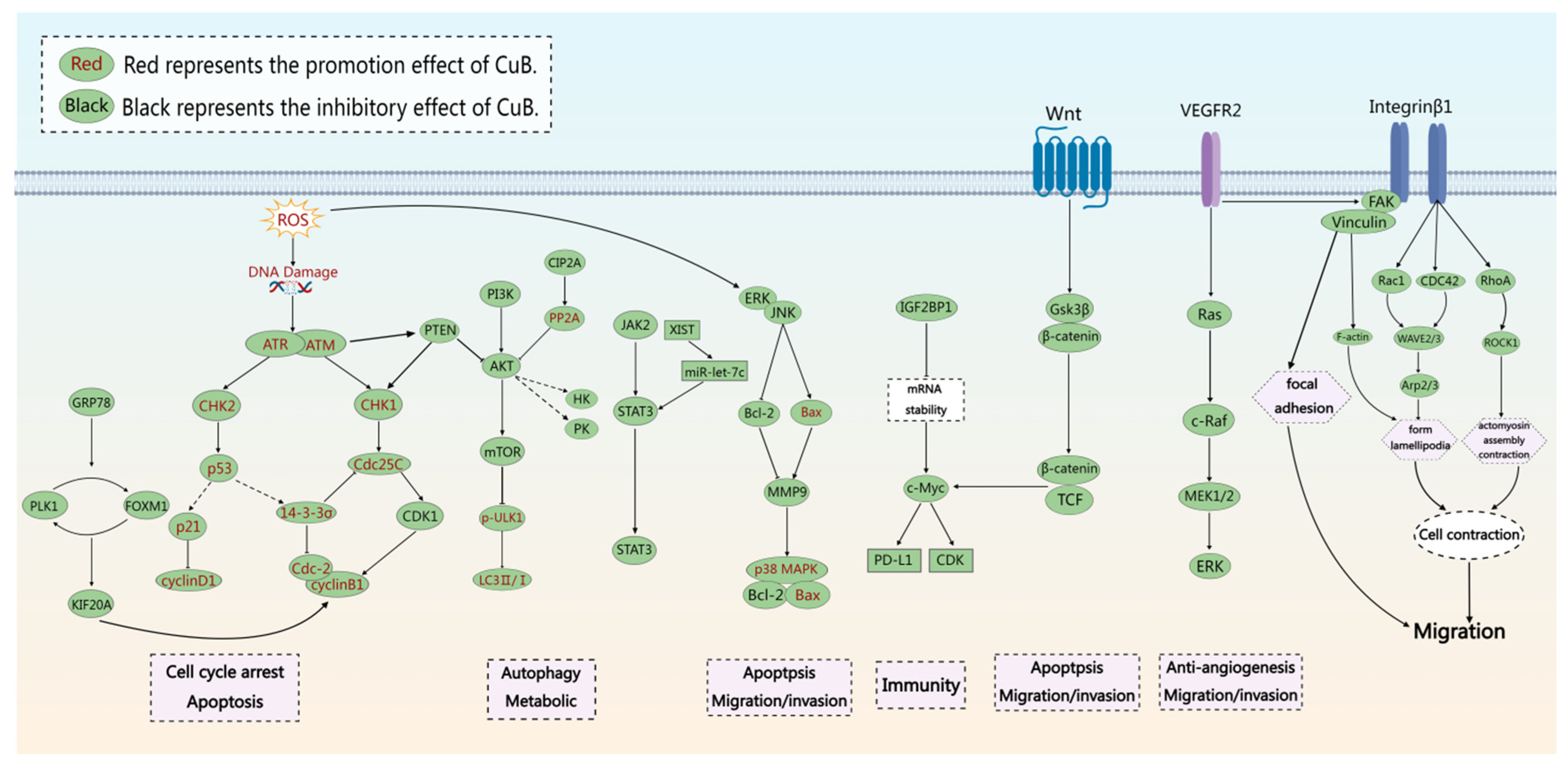
3. Pharmacological Properties of CuB as an Antitumor Agent
4. Inhibit Cell Growth and Proliferation
4.1. Promotion of Cell Apoptosis
4.2. Induction of Cycle Arrest
4.3. Induction of Cellular Autophagy
4.4. Cytoskeleton Alterations
5. Inhibit Cancer Cell Migration and Invasion
5.1. Anti-Angiogenesis
5.2. Improve Metabolic Reprogramming
5.3. The Impact on Immunity
5.4. Other Mechanisms
6. The Application of Cucurbitacin B
6.1. Individual Treatment
6.2. Combination Therapy
6.3. Nanomaterial Delivery
7. Summary and Outlook
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chan, K.T.; Meng, F.Y.; Li, Q.; Ho, C.Y.; Lam, T.S.; To, Y.; Lee, W.H.; Li, M.; Chu, K.H.; Toh, M. Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL-7402 human hepatocellular carcinoma cells and is effective via oral administration. Cancer Lett. 2010, 294, 118–124. [Google Scholar] [CrossRef]
- Thoennissen, N.H.; Iwanski, G.B.; Doan, N.B.; Okamoto, R.; Lin, P.; Abbassi, S.; Song, J.H.; Yin, D.; Toh, M.; Xie, W.D.; et al. Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT pathway and potentiates antiproliferative effects of gemcitabine on pancreatic cancer cells. Cancer Res. 2009, 69, 5876–5884. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, U.; Aeri, V.; Mir, S.R. Cucurbitacins-An insight into medicinal leads from nature. Pharmacogn. Rev. 2015, 9, 12–18. [Google Scholar]
- Teuscher, E.; Lindequist, U. Triterpene. In Biogene Gifte-Biologie, Chemie, Pharmakologie, 2nd ed.; Gustav Fischer Verlag: Stuttgart/Jena, Germany; New York, NY, USA, 1994; pp. 159–175. [Google Scholar]
- Cárdenas, P.D.; Almeida, A.; Bak, S. Evolution of Structural Diversity of Triterpenoids. Front. Plant Sci. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Zieniuk, B.; Pawełkowicz, M. Recent advances in the application of cucurbitacins as anticancer agents. Metabolites 2023, 13, 1081. [Google Scholar] [CrossRef]
- Ma, J.; Zi Jiang, Y.; Shi, H.; Mi, C.; Li, J.; Xing Nan, J.; Wu, X.; Joon Lee, J.; Jin, X. Cucurbitacin B inhibits the translational expression of hypoxia-inducible factor-1α. Eur. J. Pharmacol. 2014, 723, 46–54. [Google Scholar] [CrossRef]
- Gupta, P.; Srivastava, S.K. Inhibition of integrin-HER2 signaling by cucurbitacin B leads to in vitro and in vivo breast tumor growth suppression. Oncotarget 2014, 5, 1812–1828. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Sha, T.; Guo, J.; Li, W.; Lu, J.; Chen, X. Cucurbitacin B induces DNA damage and autophagy mediated by reactive oxygen species (ROS) in MCF-7 breast cancer cells. J. Nat. Med. 2015, 69, 522–530. [Google Scholar] [CrossRef]
- Sinha, S.; Khan, S.; Shukla, S.; Lakra, A.D.; Kumar, S.; Das, G.; Maurya, R.; Meeran, S.M. Cucurbitacin B inhibits breast cancer metastasis and angiogenesis through VEGF-mediated suppression of FAK/MMP-9 signaling axis. Int. J. Biochem. Cell Biol. 2016, 77, 41–56. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, X.L.; Yuan, J.W.; Zhang, H.R.; Liu, D.; Hao, J.; Ji, W.; Wu, X.Z.; Chen, D. Cucurbitacin B inhibits the migration and invasion of breast cancer cells by altering the biomechanical properties of cells. Phytother. Res. 2019, 33, 618–630. [Google Scholar] [CrossRef]
- Wang, X.; Tanaka, M.; Peixoto, H.S.; Wink, M. Cucurbitacins: Elucidation of their interactions with the cytoskeleton. PeerJ 2017, 5, e3357. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ma, T.; He, M.; Xie, W.; Wang, X.; Lu, L.; Wang, H.; Cui, Y. Cucurbitacin B modulates M2 macrophage differentiation and attenuates osteosarcoma progression via PI3K/AKT pathway. Phytother. Res. 2024, 38, 2215–2233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Xu, L.H.; Lu, Q.; Liu, K.P.; Liu, P.Y.; Ji, F.; Liu, X.M.; Ouyang, D.Y.; He, X.H. VASP activation via the Gα13/RhoA/PKA pathway mediates cucurbitacin-B-induced actin aggregation and cofilin-actin rod formation. PLoS ONE 2014, 9, e93547. [Google Scholar] [CrossRef]
- Wei, J.; Chen, X.; Li, Y.; Li, R.; Bao, K.; Liao, L.; Xie, Y.; Yang, T.; Zhu, J.; Mao, F.; et al. Cucurbitacin B-induced G2/M cell cycle arrest of conjunctival melanoma cells mediated by GRP78-FOXM1-KIF20A pathway. Acta Pharm. Sin. B 2022, 12, 3861–3876. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, Y.; Liu, W.; Ma, F.; Zhou, Y.; Chen, M.; Chang, J.; Wang, Y.; Yang, G.; He, G. Cucurbitacin B inhibits growth and induces apoptosis through the JAK2/STAT3 and MAPK pathways in SH-SY5Y human neuroblastoma cells. Mol. Med. Rep. 2014, 10, 89–94. [Google Scholar] [CrossRef]
- Gao, Y.; Islam, M.S.; Tian, J.; Lui, V.W.; Xiao, D. Inactivation of ATP citrate lyase by cucurbitacin B: A bioactive compound from cucumber, inhibits prostate cancer growth. Cancer Lett. 2014, 349, 15–25. [Google Scholar] [CrossRef]
- Zhou, P.; Huang, S.; Shao, C.; Huang, D.; Hu, Y.; Su, X.; Yang, R.; Jiang, J.; Wu, J. The antiproliferative and proapoptotic effects of cucurbitacin B on BPH-1 cells via the p53/MDM2 axis. Int. J. Mol. Sci. 2023, 25, 442. [Google Scholar] [CrossRef]
- Guo, J.; Wu, G.; Bao, J.; Hao, W.; Lu, J.; Chen, X. Cucurbitacin B induced ATM-mediated DNA damage causes G2/M cell cycle arrest in a ROS-dependent manner. PLoS ONE 2014, 9, e88140. [Google Scholar] [CrossRef]
- Shukla, S.; Khan, S.; Kumar, S.; Sinha, S.; Farhan, M.; Bora, H.K.; Maurya, R.; Meeran, S.M. Cucurbitacin B alters the expression of tumor-related genes by epigenetic modifications in NSCLC and inhibits NNK-induced lung tumorigenesis. Cancer Prev. Res. 2015, 8, 552–562. [Google Scholar] [CrossRef]
- Shukla, S.; Sinha, S.; Khan, S.; Kumar, S.; Singh, K.; Mitra, K.; Maurya, R.; Meeran, S.M. Cucurbitacin B inhibits the stemness and metastatic abilities of NSCLC via downregulation of canonical Wnt/β-catenin signaling axis. Sci. Rep. 2016, 6, 21860. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zheng, L.; Tang, H.; Wang, W.; Lin, Y. Cucurbitacin B enhances apoptosis in gefitinib-resistant non-small cell lung cancer by modulating the miR-17-5p/STAT3 axis. Mol. Med. Rep. 2021, 24, 710. [Google Scholar] [CrossRef]
- Alafnan, A.; Alamri, A.; Hussain, T.; Rizvi, S.M.D. Cucurbitacin-B exerts anticancer effects through instigation of apoptosis and cell cycle arrest within human prostate cancer PC3 cells via downregulating JAK/STAT signaling cascade. Pharmaceuticals 2022, 15, 1229. [Google Scholar] [CrossRef]
- Yuan, R.; Fan, Q.; Liang, X.; Han, S.; He, J.; Wang, Q.Q.; Gao, H.; Feng, Y.; Yang, S. Cucurbitacin B inhibits TGF-β1-induced epithelial-mesenchymal transition (EMT) in NSCLC through regulating ROS and PI3K/Akt/mTOR pathways. Chin. Med. 2022, 17, 24. [Google Scholar] [CrossRef]
- Liu, J.H.; Li, C.; Cao, L.; Zhang, C.H.; Zhang, Z.H. Cucurbitacin B regulates lung cancer cell proliferation and apoptosis via inhibiting the IL-6/STAT3 pathway through the lncRNA XIST/miR-let-7c axis. Pharm. Biol. 2022, 60, 154–162. [Google Scholar] [CrossRef]
- Liu, P.; Xiang, Y.; Liu, X.; Zhang, T.; Yang, R.; Chen, S.; Xu, L.; Yu, Q.; Zhao, H.; Zhang, L.; et al. Cucurbitacin B induces the lysosomal degradation of EGFR and suppresses the CIP2A/PP2A/Akt signaling axis in gefitinib-resistant non-small cell lung cancer. Molecules 2019, 24, 647. [Google Scholar] [CrossRef]
- Yuan, R.; Zhao, W.; Wang, Q.Q.; He, J.; Han, S.; Gao, H.; Feng, Y.; Yang, S. Cucurbitacin B inhibits non-small cell lung cancer in vivo and in vitro by triggering TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharmacol. Res. 2021, 170, 105748. [Google Scholar] [CrossRef]
- Zeng, Z.; Hu, Y.; Xiang, J.; Su, J.; Tan, H.; Lai, T.; Chen, X.; Fang, G.; Li, L.; Luo, L. Cucurbitacin B targets STAT3 to induce ferroptosis in non-small cell lung cancer. Eur. J. Pharmacol. 2024, 978, 176805. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Sun, W.; Lu, J.J.; Ma, D.L.; Leung, C.H.; Pei, L.; Chen, X. PTEN activation by DNA damage induces protective autophagy in response to cucurbitacin B in hepatocellular carcinoma cells. Oxid. Med. Cell Longev. 2016, 2016, 4313204. [Google Scholar] [CrossRef]
- Wang, X.; Bai, Y.; Yan, X.; Li, J.; Lin, B.; Dai, L.; Xu, C.; Li, H.; Li, D.; Yang, T.; et al. Cucurbitacin B exhibits antitumor effects on CD133+ HepG2 liver cancer stem cells by inhibiting JAK2/STAT3 signaling pathway. Anticancer Drugs 2021, 32, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, Q.; Yang, H.; Zhang, X.W.; Feng, N.; Wang, J.K.; Liu, T.T.; Zeng, K.W.; Tu, P.F. Allosteric regulation of IGF2BP1 as a novel strategy for the activation of tumor immune microenvironment. ACS Cent. Sci. 2022, 8, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.M.; Gao, F.; Zhu, J.X.; Wang, L.J.; Zhao, X.; Li, X.; Sheng, M.M.; Zhang, Y. Cucurbitacin B inhibits tumor angiogenesis by triggering the mitochondrial signaling pathway in endothelial cells. Int. J. Mol. Med. 2018, 42, 1018–1025. [Google Scholar] [CrossRef]
- Ji, X.; Chen, X.; Sheng, L.; Deng, D.; Wang, Q.; Meng, Y.; Qiu, Z.; Zhang, B.; Zheng, G.; Hu, J. Metabolomics profiling of AKT/c-Met-induced hepatocellular carcinogenesis and the inhibitory effect of Cucurbitacin B in mice. Front. Pharmacol. 2022, 13, 1009767. [Google Scholar] [CrossRef]
- Li, Q.Z.; Chen, Y.Y.; Liu, Q.P.; Feng, Z.H.; Zhang, L.; Zhang, H. Cucurbitacin B suppresses hepatocellular carcinoma progression through inducing DNA damage-dependent cell cycle arrest. Phytomedicine 2024, 26, 155177. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Duan, C.; Ji, J.; Zhang, T.; Yuan, X.; Zhang, Y.; Ma, W.; Yang, J.; Yang, L.; Jiang, Z.; et al. Cucurbitacin B induces autophagy and apoptosis by suppressing CIP2A/PP2A/mTORC1 signaling axis in human cisplatin resistant gastric cancer cells. Oncol. Rep. 2017, 38, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Liu, A.H.; Wang, Z.P.; Zhang, X.W.; Lu, H. Cucurbitacin B inhibits cell proliferation and induces cell apoptosis in colorectal cancer by modulating methylation status of BTG3. Neoplasma 2019, 66, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, B.; Wei, H.; Zeng, H.; Sheng, D.; Zhang, Y. Cucurbitacin B controls M2 macrophage polarization to suppresses metastasis via targeting JAK-2/STAT3 signalling pathway in colorectal cancer. J. Ethnopharmacol. 2022, 287, 114915. [Google Scholar] [CrossRef]
- Klungsaeng, S.; Kukongviriyapan, V.; Prawan, A.; Kongpetch, S.; Senggunprai, L. Cucurbitacin B induces mitochondrial-mediated apoptosis pathway in cholangiocarcinoma cells via suppressing focal adhesion kinase signaling. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 39, 271–278. [Google Scholar] [CrossRef]
- Ueno, M.; Kariya, R.; Sittithumcharee, G.; Okada, S. Cucurbitacin B induces apoptosis of primary effusion lymphoma via disruption of cytoskeletal organization. Phytomedicine 2021, 85, 153545. [Google Scholar] [CrossRef]
- Huang, S.; Cao, B.; Zhang, J.; Feng, Y.; Wang, L.; Chen, X.; Su, H.; Liao, S.; Liu, J.; Yan, J.; et al. Induction of ferroptosis in human nasopharyngeal cancer cells by cucurbitacin B: Molecular mechanism and therapeutic potential. Cell Death Dis. 2021, 12, 237. [Google Scholar] [CrossRef]
- Yin, S.; Mai, Z.; Liu, C.; Xu, L.; Xia, C. Label-free-based quantitative proteomic analysis of the inhibition of cisplatin-resistant ovarian cancer cell proliferation by cucurbitacin B. Phytomedicine 2023, 111, 154669. [Google Scholar] [CrossRef]
- Yang, M.; Chen, X.; Cheng, C. Cucurbitacin B induces ferroptosis in oral leukoplakia via the SLC7A11/mitochondrial oxidative stress pathway. Phytomedicine 2024, 129, 155548. [Google Scholar] [CrossRef]
- Liu, C.; Ji, J.; Li, C. Cucurbitacin B inhibits the malignancy of esophageal carcinoma through the KIF20A/JAK/STAT3 signaling pathway. Am. J. Chin. Med. 2024, 52, 275–289. [Google Scholar] [CrossRef]
- Lan, C.; Liu, C.C.; Nie, X.C.; Lei, L.; Xiao, Z.X.; Li, M.X.; Tang, X.N.; Jia, M.Y.; Xu, H.T. FAM83A Promotes the Proliferative and Invasive Abilities of Cervical Cancer Cells via Epithelial-Mesenchymal Transition and the Wnt Signaling Pathway. J. Cancer 2021, 12, 6320–6329. [Google Scholar] [CrossRef]
- Duangmano, S.; Sae-Lim, P.; Suksamrarn, A.; Domann, F.E.; Patmasiriwat, P. Cucurbitacin B inhibits human breast cancer cell proliferation through disruption of microtubule polymerization and nucleophosmin/B23 translocation. BMC Complement. Altern. Med. 2012, 12, 185. [Google Scholar]
- Zhang, S.; Hu, R.; Geng, Y.; Chen, K.; Wang, L.; Imam, M.U. The regulatory effects and the signaling pathways of natural bioactive compounds on ferroptosis. Foods 2021, 10, 2952. [Google Scholar] [CrossRef]
- Geiger, A.; Bossard, G.; Sereno, D.; Pissarra, J.; Lemesre, J.L.; Vincendeau, P.; Holzmuller, P. Escaping deleterious immune response in their hosts: Lessons from trypanosomatids. Front. Immunol. 2016, 7, 212. [Google Scholar] [CrossRef]
- Liu, H.; Wei, Q.; Wang, J.; Huang, X.; Li, C.; Zheng, Q.; Cao, J.; Jia, Z. DNA polymerases as targets for gene therapy of hepatocellular carcinoma. BMC Cancer 2015, 15, 325. [Google Scholar] [CrossRef]
- Khan, K.H.; Blanco-Codesido, M.; Molife, L.R. Cancer therapeutics: Targeting the apoptotic pathway. Crit. Rev. Oncol. Hematol. 2014, 90, 200–219. [Google Scholar] [CrossRef]
- Wang, L.; Yukselten, Y.; Nuwagaba, J.; Sutton, R.E. JAK/STAT signaling pathway affects CCR5 expression in human CD4+ T cells. Sci. Adv. 2024, 10, eadl0368. [Google Scholar] [CrossRef]
- Guo, X.; He, L.; Xu, W.; Wang, W.; Feng, X.; Fu, Y.; Zhang, X.; Ding, R.B.; Qi, X.; Bao, J.; et al. αO-Conotoxin GeXIVA [1,2] suppresses in vivo tumor growth of triple-negative breast cancer by inhibiting AKT-mTOR, STAT3 and NF-κB signaling mediated proliferation and inducing apoptosis. Mar. Drugs 2024, 22, 252. [Google Scholar] [CrossRef]
- Martínez-Abarca Millán, A.; Martín-Bermudo, M.D. Integrins can act as suppressors of Ras-mediated oncogenesis in the Drosophila wing disc epithelium. Cancers 2023, 15, 5432. [Google Scholar] [CrossRef]
- Liew, Y.X.; Karen-Ng, L.P.; Vincent-Chong, V.K. A comprehensive review of natural products as therapeutic or chemopreventive agents against head and neck squamous cell carcinoma cells using preclinical models. Biomedicines 2023, 11, 2359. [Google Scholar] [CrossRef]
- Wang, H.; Fan, Y.; Chen, W.; Lv, Z.; Wu, S.; Xuan, Y.; Wang, C.; Lu, Y.; Guo, T.; Shen, D.; et al. Loss of CMTM6 promotes DNA damage-induced cellular senescence and antitumor immunity. Oncoimmunology 2022, 11, 2011673. [Google Scholar] [CrossRef]
- Yang, Y.L.; Zhang, K.; Zhou, Z.T.; Jiang, Z.L.; Liu, Y.; Zhang, Y.X.; Liu, Z.H.; Ji, X.Y.; Wu, D.D. The Role of Hydrogen Sulfide in the Development and Progression of Lung Cancer. Molecules 2022, 27, 9005. [Google Scholar] [CrossRef]
- Guo, S.; Ramar, V.; Guo, A.A.; Saafir, T.; Akpobiyeri, H.; Hudson, B.; Li, J.; Liu, M. TRPM7 transactivates the FOSL1 gene through STAT3 and enhances glioma stemness. Cell Mol. Life Sci. 2023, 80, 270. [Google Scholar] [CrossRef]
- Wehbe, N.; Badran, A.; Baydoun, S.; Al-Sawalmih, A.; Maresca, M.; Baydoun, E.; Mesmar, J.E. The Antioxidant Potential and Anticancer Activity of Halodule uninervis Ethanolic Extract against Triple—Negative Breast Cancer Cells. Antioxidants 2024, 13, 726. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiang, K.; Liu, X.; Li, J.; Zhou, W.; Wang, C.; Cui, J.; Liang, T. FBXW7 and human tumors: Mechanisms of drug resistance and potential therapeutic strategies. Front. Pharmacol. 2023, 14, 1278056. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.; Zhang, J.; Zhang, L.; Chen, L. Inhibiting cytoprotective autophagy in cancer therapy: An update on pharmacological small-molecule compounds. Front. Pharmacol. 2022, 13, 966012. [Google Scholar] [CrossRef]
- Law, B.Y.; Chan, W.K.; Xu, S.W.; Wang, J.R.; Bai, L.P.; Liu, L.; Wong, V.K. Natural small-molecule enhancers of autophagy induce autophagic cell death in apoptosis-defective cells. Sci. Rep. 2014, 4, 5510. [Google Scholar] [CrossRef] [PubMed]
- Mrakovcic, M.; Bohner, L.; Hanisch, M.; Fröhlich, L.F. Epigenetic Targeting of Autophagy via HDAC Inhibition in Tumor Cells: Role of p53. Int. J. Mol. Sci. 2018, 19, 3952. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Park, K.A.; Byun, H.S.; Won, M.; Jeon, J.; Lee, Y.; Seok, J.H.; Choi, S.W.; Lee, S.H.; et al. Cucurbitacin induces autophagy through mitochondrial ROS production which counteracts to limit caspase-dependent apoptosis. Autophagy 2012, 8, 559–576. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, C.; Cai, S.; Feng, Y.; Shan, J.; Di, L. Aconiti Lateralis Radix Praeparata as potential anticancer herb: Bioactive compounds and molecular mechanisms. Front. Pharmacol. 2022, 13, 870282. [Google Scholar] [CrossRef]
- Haimov, E.; Windoffer, R.; Leube, R.E.; Urbakh, M.; Kozlov, M.M. Model for bundling of keratin intermediate filaments. Biophys. J. 2020, 119, 65–74. [Google Scholar] [CrossRef]
- Hall, A. The cytoskeleton and cancer. Cancer Metastasis Rev. 2009, 28, 5–14. [Google Scholar] [CrossRef]
- Li, X.; Wang, J. Mechanical tumor microenvironment and transduction: Cytoskeleton mediates cancer cell invasion and metastasis. Int. J. Biol. Sci. 2020, 16, 2014–2028. [Google Scholar] [CrossRef]
- Ruggiero, C.; Lalli, E. Targeting the cytoskeleton against metastatic dissemination. Cancer Metastasis Rev. 2021, 40, 89–140. [Google Scholar] [CrossRef]
- Yin, D.; Wakimoto, N.; Xing, H.; Lu, D.; Huynh, T.; Wang, X.; Black, K.L.; Koeffler, H.P. Cucurbitacin B markedly inhibits growth and rapidly affects the cytoskeleton in glioblastoma multiforme. Int. J. Cancer 2008, 123, 1364–1375. [Google Scholar] [CrossRef]
- Duan, J.; Jin, M.; Yang, D.; Shi, J.; Gao, J.; Guo, D.; Tang, H.; Zhang, S.; Qiao, B. Ubiquitin-specific peptidase 2 inhibits epithelial-mesenchymal transition in clear cell renal cell carcinoma metastasis by downregulating the NF-κB pathway. Bioengineered 2022, 13, 4455–4467. [Google Scholar] [CrossRef]
- Novikov, N.M.; Zolotaryova, S.Y.; Gautreau, A.M.; Denisov, E.V. Mutational drivers of cancer cell migration and invasion. Br. J. Cancer 2021, 124, 102–114. [Google Scholar] [CrossRef]
- Deng, L.; Wang, D.; Chen, S.; Hu, W.; Zhang, R. Epiphycan predicts poor outcomes and promotes metastasis in ovarian cancer. Front. Oncol. 2021, 11, 653782. [Google Scholar] [CrossRef]
- Li, J.; Wei, Q.; Song, K.; Wang, Y.; Yang, Y.; Li, M.; Yu, J.; Su, G.; Peng, L.; Fu, B.; et al. Tangeretin attenuates bleomycin-induced pulmonary fibrosis by inhibiting epithelial-mesenchymal transition via the PI3K/Akt pathway. Front. Pharmacol. 2023, 14, 1247800. [Google Scholar] [CrossRef]
- Liu, S.; Dai, W.; Jin, B.; Jiang, F.; Huang, H.; Hou, W.; Lan, J.; Jin, Y.; Peng, W.; Pan, J. Effects of super-enhancers in cancer metastasis: Mechanisms and therapeutic targets. Mol. Cancer 2024, 23, 122. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, X.J.; Xing, J. Signal transduction pathways of EMT induced by TGF-β, SHH, and WNT and their crosstalks. J. Clin. Med. 2016, 5, 41. [Google Scholar] [CrossRef]
- Papadimitriou, E.; Chatzellis, E.; Dimitriadi, A.; Kaltsas, G.A.; Theocharis, S.; Alexandraki, K.I. Prognostic Biomarkers in Pituitary Tumours: A Systematic Review. TouchREV Endocrinol. 2023, 19, 42–53. [Google Scholar] [CrossRef]
- Bi, Z.; Cui, E.; Yao, Y.; Chang, X.; Wang, X.; Zhang, Y.; Xu, G.X.; Zhuang, H.; Hua, Z.C. Recombinant Bifidobacterium longum carrying endostatin protein alleviates dextran sodium sulfate-induced colitis and colon cancer in rats. Front. Microbiol. 2022, 13, 927277. [Google Scholar] [CrossRef]
- He, Z.; Zhou, H.; Wang, J.; Li, D.; Zhang, X.; Wang, P.; Ma, T.; Zhang, Y.; Tian, C.; Chen, Y.; et al. Apatinib with etoposide capsules as a third-or further-line therapy for extensive-stage small cell lung cancer: An open-label, multicenter, single-arm phase II trial. Transl. Lung Cancer Res. 2021, 10, 889–899. [Google Scholar] [CrossRef]
- Osuka, K.; Ohmichi, Y.; Ohmichi, M.; Honma, S.; Suzuki, C.; Aoyama, M.; Iwami, K.; Watanabe, Y.; Miyachi, S. Angiogenesis in the Outer Membrane of Chronic Subdural Hematomas through Thrombin-Cleaved Osteopontin and the Integrin α9 and Integrin β1 Signaling Pathways. Biomedicines 2023, 11, 1440. [Google Scholar] [CrossRef]
- Peng, Z.; Pang, H.; Wu, H.; Peng, X.; Tan, Q.; Lin, S.; Wei, B. CCL2 promotes proliferation, migration and angiogenesis through the MAPK/ERK1/2/MMP9, PI3K/AKT, Wnt/β-catenin signaling pathways in HUVECs. Exp. Ther. Med. 2022, 25, 77. [Google Scholar] [CrossRef]
- Aghayants, S.; Zhu, J.; Yu, J.; Tao, R.; Li, S.; Zhou, S.; Zhou, Y.; Zhu, Z. The emerging modulators of non-coding RNAs in diabetic wound healing. Front. Endocrinol. 2024, 15, 1465975. [Google Scholar] [CrossRef]
- Forma, A.; Tyczyńska, M.; Kędzierawski, P.; Gietka, K.; Sitarz, M. Gastric carcinogenesis: A comprehensive review of the angiogenic pathways. Clin. J. Gastroenterol. 2021, 14, 14–25. [Google Scholar] [CrossRef]
- Panda, A.; Falasca, M.; Ragunath, K. Extracellular vesicles in pancreatic cancer: A new era in precision medicine. Transl. Gastroenterol. Hepatol. 2024, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zeng, F.; Liao, S.; Cao, L.; Zhou, Y. Effects of glycolysis on the polarization and function of tumor-associated macrophages (Review). Int. J. Oncol. 2023, 62, 70. [Google Scholar] [CrossRef]
- Cui, H.; Ren, X.; Dai, L.; Chang, L.; Liu, D.; Zhai, Z.; Kang, H.; Ma, X. Comprehensive analysis of nicotinamide metabolism-related signature for predicting prognosis and immunotherapy response in breast cancer. Front. Immunol. 2023, 14, 1145552. [Google Scholar] [CrossRef]
- Xiong, W.; Xiong, Z.; Song, A.; Lei, C.; Ye, C.; Zhang, C. Relieving lipid accumulation through UCP1 suppresses the progression of acute kidney injury by promoting the AMPK/ULK1/autophagy pathway. Theranostics 2021, 11, 4637–4654. [Google Scholar] [CrossRef]
- Tao, J.; Yang, G.; Zhou, W.; Qiu, J.; Chen, G.; Luo, W.; Zhao, F.; You, L.; Zheng, L.; Zhang, T.; et al. Targeting hypoxic tumor microenvironment in pancreatic cancer. J. Hematol. Oncol. 2021, 14, 14. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Szwed, A.; Kim, E.; Jacinto, E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol. Rev. 2021, 101, 1371–1426. [Google Scholar] [CrossRef]
- Gordan, J.D.; Thompson, C.B.; Simon, M.C. HIF and c-Myc: Sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell 2007, 12, 108–113. [Google Scholar] [CrossRef]
- van der Poel, H.G.; Hanrahan, C.; Zhong, H.; Simons, J.W. Rapamycin induces Smad activity in prostate cancer cell lines. Urol. Res. 2003, 30, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Deng, D.J.; Tan, X.Y.; Sun, Y.; Wang, C.T.; Zheng, G.H.; Hu, J.J. Mechanism of cucurbitacin B in inhibiting the proliferation of HuCCT1 cells by regulating glycolysis. Chin. J. Exp. Tradit. Med. Formul. 2022, 28, 74–81. [Google Scholar]
- Tang, L.; Zhang, Z.; Fan, J.; Xu, J.; Xiong, J.; Tang, L.; Jiang, Y.; Zhang, S.; Zhang, G.; Luo, W.; et al. Comprehensive analysis of immunophenotyping signature in triple-negative breast cancer patients based on machine learning. Front. Pharmacol. 2023, 14, 1195864. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Q.; Yu, X.; Hou, Y.; Yan, L.; Gao, Y.; Ji, L.; Zhang, X.; Fang, M.; Huang, L.; et al. Integrating bulk and single-cell RNA sequencing data to establish necroptosis-related lncRNA risk model and analyze the immune microenvironment in hepatocellular carcinoma. Heliyon 2023, 9, e22083. [Google Scholar] [CrossRef]
- Hegde, P.S.; Chen, D.S. Top 10 challenges in cancer immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef]
- Gao, D. Compound-therapy based on cancer-immunity cycle: Promising prospects for antitumor regimens. Am. J. Cancer Res. 2019, 9, 212–218. [Google Scholar]
- Pio, R.; Ajona, D.; Ortiz-Espinosa, S.; Mantovani, A.; Lambris, J.D. Complementing the Cancer-Immunity Cycle. Front. Immunol. 2019, 10, 774. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef]
- Silvestre, G.F.G.; de Lucena, R.P.; da Silva Alves, H. Cucurbitacins and the immune system: Update in research on anti-inflammatory, antioxidant, and immunomodulatory mechanisms. Curr. Med. Chem. 2022, 29, 3774–3789. [Google Scholar] [CrossRef]
- Kim, M.; Park, S.Y.; Jin, M.L.; Park, G.; Son, H.J. Cucurbitacin B inhibits immunomodulatory function and the inflammatory response in macrophages. Immunopharmacol. Immunotoxicol. 2015, 37, 473–480. [Google Scholar] [CrossRef]
- Lu, P.; Yu, B.; Xu, J. Cucurbitacin B regulates immature myeloid cell differentiation and enhances antitumor immunity in patients with lung cancer. Cancer Biother. Radiopharm. 2012, 27, 495–503. [Google Scholar] [CrossRef]
- Aditi, D.; Downing, S.M.; Schreiner, P.A.; Kwak, Y.D.; Li, Y.; Shaw, T.I.; Russell, H.R.; McKinnon, P.J. Genome instability independent of type I interferon signaling drives neuropathology caused by impaired ribonucleotide excision repair. Neuron 2021, 109, 3962–3979.e6. [Google Scholar] [CrossRef]
- Emam, A.; Wu, X.; Xu, S.; Wang, L.; Liu, S.; Wang, B. Stalled replication fork protection limits cGAS-STING and P-body-dependent innate immune signalling. Nat. Cell Biol. 2022, 24, 1154–1164. [Google Scholar] [CrossRef]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef]
- Feng, J.; Wang, J.; Wang, Y.; Huang, X.; Shao, T.; Deng, X.; Cao, Y.; Zhou, M.; Zhao, C. Oxidative stress and lipid peroxidation: Prospective associations between ferroptosis and delayed wound healing in diabetic ulcers. Front. Cell Dev. Biol. 2022, 10, 898657. [Google Scholar] [CrossRef]
- Shinjo, K.; Hara, K.; Nagae, G.; Umeda, T.; Katsushima, K.; Suzuki, M.; Murofushi, Y.; Umezu, Y.; Takeuchi, I.; Takahashi, S.; et al. A novel sensitive detection method for DNA methylation in circulating free DNA of pancreatic cancer. PLoS ONE 2020, 15, e0233782. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Liu, L.; Yang, T.; Song, J. Circular RNAs: New biomarkers of chemoresistance in cancer. Cancer Biol. Med. 2021, 18, 637–648. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Zhou, J.; Huang, Z.; Hu, H.; Qiao, M.; Zhao, X.; Chen, D. Synergistic effect of cucurbitacin B in combination with curcumin via enhancing apoptosis induction and reversing multidrug resistance in human hepatoma cells. Eur. J. Pharmacol. 2015, 768, 28–40. [Google Scholar] [CrossRef]
- Qu, Y.; Cong, P.; Lin, C.; Deng, Y.; Li-Ling, J.; Zhang, M. Inhibition of paclitaxel resistance and apoptosis induction by cucurbitacin B in ovarian carcinoma cells. Oncol. Lett. 2017, 14, 145–152. [Google Scholar] [CrossRef]
- Chen, Y.X. Cucurbitacin tablets, a new drug for treating hepatitis and liver cancer. Chin. Herb. Med. 1987, 18, 21. [Google Scholar]
- Sha, J.S.; Mao, H.K. Cucurbitacin Tablets. Chin. J. Pharm. 1986, 6, 357. [Google Scholar]
- Tan, D.; He, M.G.; Shen, N.Y.; Han, Z.; Liang, G.; Zheng, K.; Wang, Z.X. Clinical efficacy and safety of cucurbitacin tablets combined with thalidomide in TACE treatment of elderly unresectable primary liver cancer. Chin. J. Liver Dis. 2017, 9, 36–40. [Google Scholar]
- Li, Y.; Li, Y.; Yao, Y.; Li, H.; Gao, C.; Sun, C.; Zhuang, J. Potential of cucurbitacin as an anticancer drug. Biomed. Pharmacother. 2023, 168, 115707. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Chen, X.; Han, F.; Liu, Z.; Wang, T.; Wang, M.; Chen, Y.; Ding, Y.; Zhang, Q. Synthesis of Cucurbitacin B Derivatives as Potential Anti-Hepatocellular Carcinoma Agents. Molecules 2018, 23, 3345. [Google Scholar] [CrossRef] [PubMed]
- Suebsakwong, P.; Wang, J.; Khetkam, P.; Weerapreeyakul, N.; Wu, J.; Du, Y.; Yao, Z.J.; Li, J.X.; Suksamrarn, A. A bioreductive prodrug of cucurbitacin B significantly inhibits tumor growth in the 4T1 xenograft mice model. ACS Med. Chem. Lett. 2019, 10, 1400–1406. [Google Scholar] [CrossRef]
- Aribi, A.; Gery, S.; Lee, D.H.; Thoennissen, N.H.; Thoennissen, G.B.; Alvarez, R.; Ho, Q.; Lee, K.; Doan, N.B.; Chan, K.T.; et al. The triterpenoid cucurbitacin B augments the antiproliferative activity of chemotherapy in human breast cancer. Int. J. Cancer 2013, 132, 2730–2737. [Google Scholar] [CrossRef]
- El-Senduny, F.F.; Badria, F.A.; El-Waseef, A.M.; Chauhan, S.C.; Halaweish, F. Approach for chemosensitization of cisplatin-resistant ovarian cancer by cucurbitacin B. Tumour Biol. 2016, 37, 685–698. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, X.; Hu, H.; Chen, D.; Gu, J.; Deng, Y.; Sun, J. Galactosylated solid lipid nanoparticles with cucurbitacin B improves the liver targetability. Drug Deliv. 2010, 17, 114–122. [Google Scholar] [CrossRef]
- Wang, X.; Yang, B.; Luo, C.; Zhao, Z.; Zhao, J.; Lu, Q.; Zhang, H.; Kan, Q.; Wang, Y.; He, Z.; et al. An exosome-like programmable-bioactivating paclitaxel prodrug nanoplatform for enhanced breast cancer metastasis inhibition. Biomaterials 2020, 257, 120224. [Google Scholar] [CrossRef]
- Qi, R.; Zhang, Y.; Yan, F. Exosomes e.nriched by miR-429-3p derived from ITGB1-modified Telocytes alleviate hypoxia-induced pulmonary arterial hypertension through regulating Rac1 expression. Cell Biol. Toxicol. 2024, 40, 32. [Google Scholar] [CrossRef]
- Chen, T.; Ma, B.; Lu, S.; Zeng, L.; Wang, H.; Shi, W.; Zhou, L.; Xia, Y.; Zhang, X.; Zhang, J.; et al. Cucumber-derived nanovesicles containing cucurbitacin B for non-small cell lung cancer therapy. Int. J. Nanomed. 2022, 17, 3583–3599. [Google Scholar] [CrossRef]
- Peng, S.; Wang, W.; Zhang, R.; Wu, C.; Pan, X.; Huang, Z. Nano-Formulations for Pulmonary Delivery: Past, Present, and Future Perspectives. Pharmaceutics 2024, 16, 161. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bai, L.; Guo, K.; Jia, Y.; Zhang, K.; Liu, Q.; Wang, P.; Wang, X. Focused ultrasound-augmented targeting delivery of nanosonosensitizers from homogenous exosomes for enhanced sonodynamic cancer therapy. Theranostics 2019, 9, 5261–5281. [Google Scholar] [CrossRef] [PubMed]
- Ferrisse, T.M.; Dias, L.M.; de Oliveira, A.B.; Jordão, C.C.; Mima, E.G.O.; Pavarina, A.C. Efficacy of Antimicrobial Photodynamic Therapy Mediated by Photosensitizers Conjugated with Inorganic Nanoparticles: Systematic Review and Meta-Analysis. Pharmaceutics 2022, 14, 2050. [Google Scholar] [CrossRef]
- Xu, L.; Bai, E.; Zhu, Y.; Qin, J.; Du, X.; Huang, H. pH-Responsive Hydrogel as a Potential Oral Delivery System of Baicalin for Prolonging Gastroprotective Activity. Pharmaceutics 2023, 15, 257. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Yao, Y.; Yin, D.; Zhu, R.; Fu, T.; Kong, J.; Wang, K.; Liu, J.; Yao, A.; Ruan, Y.; et al. Enhanced lysosomal escape of cell penetrating peptide-functionalized metal-organic frameworks for co-delivery of survivin siRNA and oridonin. J. Colloid Interface Sci. 2023, 646, 370–380. [Google Scholar] [CrossRef]
- Wu, J.; Shaidani, S.; Theodossiou, S.K.; Hartzell, E.J.; Kaplan, D.L. Localized, on-demand, sustained drug delivery from biopolymer-based materials. Expert Opin. Drug Del. 2022, 19, 1317–1335. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, M.; Rani, R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics. Semin. Cancer Biol. 2022, 87, 184–195. [Google Scholar] [CrossRef]
- Chen, D.; Liu, P.; Lu, X.; Li, J.; Qi, D.; Zang, L.; Lin, J.; Liu, Y.; Zhai, S.; Fu, D.; et al. Pan-cancer analysis implicates novel insights of lactate metabolism into immunotherapy response prediction and survival prognostication. J. Exp. Clin. Cancer Res. 2024, 43, 125. [Google Scholar] [CrossRef]
- Kahveci, K.; Düzgün, M.B.; Atis, A.E.; Yılmaz, A.; Shahraki, A.; Coskun, B.; Durdagi, S.; Birgul Iyison, N. Discovering allatostatin type-C receptor specific agonists. Nat. Commun. 2024, 15, 3965. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR Effect: Combined Strategies to Improve the EPR Effect in the Tumor Microenvironment. Theranostics 2019, 9, 8073–8090. [Google Scholar] [CrossRef] [PubMed]
| Name | CuB |
|---|---|
| Molecular Formula | C32H46O8 |
| Molecular Weight | 558.7029 |
| Form | Powder |
| Colour | White |
| Density | 1.0953 |
| Melting Point | 180–183 °C |
| Boiling Point | 699.3 ± 55.0 °C (760 mmHg) |
| Refractive Index | 1.4900 |
| Solubility | DMSO:10 mg/ml |
| Flash Point | 218.7 °C |
| Vapor Pressure | 1.3 × 10−22 mmHg (25 °C) |
| Polar Surface Area | 138.20000 |
| LogP | 3.49930 |
| pKa | 12.60 ± 0.29 |
| Maximum Wavelength | 290 (in ethanol solution) |
| Optical Rotation | D25 + 88° (c = 1.55, in ethanol solution) |
| Storage Conditions | Powder: −20 °C (3 years), 4 °C (2 years); Solution: −80 °C (6 months), −20 °C (1 month) |
| Cancer | Subjects | In Vitro Dosage | In Vivo Dosage | Mechanisms | Ref. |
|---|---|---|---|---|---|
| Breast cancer | Hela cell | 3 μM | Inhibit the mTOR/p70S6k/4EBP1 and MEK/ERK signaling pathways by suppressing HIF-1 activation. | [7] | |
| BALB/c nude | 1 and 5 mg/kg; oral | ||||
| mice | |||||
| MDA, MB-231, SKBR3, MCF-7 and 4T-1 cell | 18–50 nM | Inhibit the expression of ITGA6B4 and induce the expression of ITGB1 and ITGB3, promoting the apoptosis of breast cancer cells by inhibiting the HER2-integrin signaling pathway. | [8] | ||
| MCF-7 cell | 0–200 nM | Induce DNA damage and autophagy by increasing the level of ROS. | [9] | ||
| MDA-MB-231 and 4T1 cell | 12.01 μM, 80 nM | Downregulate the VEGF/FAK/MMP signaling pathway to inhibit metastasis and angiogenesis. | [10] | ||
| Balb/c mice | 0.1 and 0.25 mg/kg; i.p. | ||||
| MDA-MB-231 and SKBR-3 cell | 0–125 μM | Mediate the biomechanical properties of breast cancer through the RAC1/CDC42/RhoA signaling pathway, thereby inhibiting cell migration and invasion. | [11] | ||
| Balb/c nude mice | 0.5 mg/kg; i.p. | ||||
| Breast cancer and osteosarcoma | Hela, MCF-7 and U2OS cell | 12.2, 22.93, 17.07 nM | Interact with the cytoskeleton by affecting actin filaments through depolymerization and aggregation, and induce cell cycle arrest. | [12] | |
| Osteosarcoma | HOS, and 143B | 0–140 nM | Inhibit M2 macrophage differentiation by inhibiting the PI3K/Akt pathway. | [13] | |
| Balb/c nude mice | 1 mg/kg; i.p. | ||||
| Melanoma | A375 and B16F10 cell | 0–1 μM | Induce the aggregation of actin and the formation of filamin-actin rods via the Gα13/RhoA/PKA/VASP pathway. | [14] | |
| CRMM2, CM-AS16, CRMM1 and CM2005.1 cell | 0.15, 0.08, 0.24 and 0.38 μM | Induce cell cycle arrest by inhibiting the GRP78/eFOXM1/eKIF20A pathway. | [15] | ||
| NCG mice | 1 mg/kg; oral | ||||
| Neuroblastoma | SH-SY5Y cell | 0–128 μM | Inhibit the growth and proliferation of SHSY5Y human neuroblastoma cells by inhibiting the JAK2/STAT3 pathway and activating the MAPK pathway. | [16] | |
| Prostate cancer | LNCaP and PC-3 cells | 0–0.3 μM | Induce apoptosis of prostate cancer cells through the ROS-dependent ACLY signaling pathway. | [17] | |
| nude mice | 0.1 μmol; oral | ||||
| BPH-1 cell | 0–200 nM | Inhibit prostate cell proliferation by activating p53/MDM2 signaling cascade and downregulating COX-2 expression. | [18] | ||
| Lung cancer | A549 | 0–200 nM | Induce cell DNA damage by increasing the formation of ROS, and then induce cell-cycle arrest. | [19] | |
| A549, H1299 and H1650 cell | 0–860 nM | Cause the upregulation of TSGs and the downregulation of TPG through changes in histone modification and promoter methylation, thereby inhibiting the growth and inducing apoptosis of NSCLC cells. | [20] | ||
| A549, H1299 and H23 cell | 0–200 nM | Inhibit the metastatic ability of NSCLC by suppressing the Wnt/β-catenin signaling axis. | [21] | ||
| PC9 cell | 0–50 μM | Improve the resistance of NSCLC to gefitinib by regulating the miR175p/STAT3 axis to reduce the protein level and phosphorylation of STAT3, inhibit proliferation, and promote cell apoptosis | [22] | ||
| PC3 cell | 0–25 µM | Induce apoptosis and cell cycle arrest in PC3 cells by downregulating of JAK/STAT signaling cascade. | [23] | ||
| A549 cell | 0–20 nM | Inhibit TGF-β1-induced EMT in A549 cells and gefitinib-resistant A549 cells, and inhibit cell migration and invasion by reducing the production of ROS and the PI3K/Akt/mTOR signaling pathway. | [24] | ||
| C57BL/6J mice | 0.25 and 0.5 mg/kg | ||||
| A549 cell | 0–0.9 μM | Suppress the proliferation and induce the apoptosis of lung cancer cells by inhibiting the IL-6/STAT3 pathway through the lncRNA XIST/miR-let-7c axis. | [25] | ||
| A549, H1299, H1975, H820 and 16-HBE cell | 0–100 nM | Suppress the growth and invasion of GR NSCLC cells by inducing the lysosomal degradation of EGFR and downregulating the CIP2A/PP2A/Akt signaling axis. | [26] | ||
| Balb/c nude mice | 0.5 mg/kg | ||||
| A549 cell | 0–1000 nM | Induce pyroptosis by binding to TLR4, thereby inhibiting tumor growth. | [27] | ||
| C57BL/6 mice | 0.25, 0.5, and 0.75 mg/kg; i.p. | ||||
| H358, A549, H23, H1650 and PC9 cell | 0–100 μM | Induce ferroptosis and the proliferation of non-small cell lung cancer by inhibiting the activation of STAT3. | [28] | ||
| Liver Cancer | BEL-7402 cell | 0–100 nM | Induce DNA damage mediated by ROS, and then activate PTEN to promote protective autophagy. | [29] | |
| HepG2 cell | 0–500 nM | Inhibit the growth and proliferation of CD133+ HepG2 cells by inhibiting the JAK2/signal transducer and activator of transcription-3 signaling pathway to induce cell cycle arrest. | [30] | ||
| Balb/c nude mice | 0.75 mg/kg; oral | ||||
| Huh7 cell | 0–100 μM | Block the m6A mRNA connection of IGF2BP1, thereby activating tumor immune microenvironment (TIME). | [31] | ||
| Balb/c mice | 1 and 5 mg/kg; i.p. | ||||
| HepG2 cell and HUVEC cell | 0–100 nM | Induce the mitochondrial apoptosis pathway to trigger apoptosis in HUVEC cells and inhibit the activity of VEGFR2, thereby suppressing angiogenesis. | [32] | ||
| FVB/N mice | 2 mg/kg; oral | Affect lipid metabolism, amino acid metabolism, and glucose metabolism by changing the Akt/mTORC1 signaling pathway, thereby reducing tumor progression. | [33] | ||
| Huh7, Hep3B and Hepa1/6 | 0–30 μM | Activate the ATM-dependent p53-p21-CDK1/CHK1/CDC25C signaling pathway, induce DNA damage, and consequently lead to cell cycle arrest. | [34] | ||
| Balb/c nude mice | 0.5 and 1 mg/kg; i.p. | ||||
| Gastric cancer | SGC7901 cell | 0–600 nM | Induce autophagy and apoptosis in human cisplatin-resistant gastric cancer cells by inhibiting the CIP2A/PP2A/mTORC1 signaling axis. | [35] | |
| Colorectal cancer | SW480 and Caco-2 cell | 0–50 μM | Inhibit the proliferation of colorectal cancer cells and induce apoptosis by regulating the demethylation of the BTG3 promoter. | [36] | |
| CT-26 and HCT116 cell | 0–2400 nM | Regulate TAMs through JAK2/STAT3 signaling pathway to inhibit the growth and metastasis of colon cancer cells. | [37] | ||
| C57BL/6 and Balb/c mice | 0.5 and 1 mg/kg; i.p. | ||||
| Cholangiocarcinoma | KKU-100 cell | 0.1–40 μM | Induce the intrinsic mitochondrial apoptosis pathway in CCA cells by inhibiting Fak-mediated PI3K/Akt. | [38] | |
| Lymphoma | BCBL-1, BC-1, GTO and TY-1 cell | 0–50 nM | Induce apoptosis of BCBL-1 cells by activating caspases, and cause cell cycle arrest of BCBL-1 cells by inducing actin aggregation and inhibiting the level of p-filamin. | [39] | |
| Balb/c mice | 0.5 mg/kg; i.p. | ||||
| Nasopharyngeal carcinoma | CNE1 cell | 16 nM | Induce ferroptosis to cause cell death by increasing lipid peroxidation and decreasing the expression of GPX4. | [40] | |
| Balb/c mice | 0.5 and 1 mg/kg; i.p. | ||||
| Ovarian cancer | A2780, OV2008, C13, and A2780-DDP cell | 0–1 μM | Inhibit PI3K/Akt/mTOR signaling pathway, thereby inhibiting the proliferation of cisplatin-resistant ovarian cancer cells, inducing DNA damage, activating cGASA, and activating immune regulation. | [41] | |
| Balb/c nude mice | 0.25, 0.5 and 1 mg/kg; i.p. | ||||
| Oral cancer | HOK and DOK cell | 0–120 nM | By activating the SLC7A11/mitochondrial oxidative stress pathway to induce ferroptosis to prevent the progression of malignant tumors. | [42] | |
| C57BL/6 mice | 0.5 and 1 mg/kg; i.p. | ||||
| Esophageal cancer | Het1A, TE-1, KYSE410, ECA109 and KYSE150 cell | 0–0.4 μM | Weakening the JAK/STAT3 pathway by inhibiting KIF20A expression to inhibit the progression of ESCA. | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, D.; Chen, H.; Lin, S.; Sun, Y.; Jing, X.; Chang, R.; Feng, Y.; Dong, X.; Qu, C.; Ni, J.; et al. Recent Advances in the Application of Cucurbitacin B as an Anticancer Agent. Int. J. Mol. Sci. 2025, 26, 8003. https://doi.org/10.3390/ijms26168003
Yin D, Chen H, Lin S, Sun Y, Jing X, Chang R, Feng Y, Dong X, Qu C, Ni J, et al. Recent Advances in the Application of Cucurbitacin B as an Anticancer Agent. International Journal of Molecular Sciences. 2025; 26(16):8003. https://doi.org/10.3390/ijms26168003
Chicago/Turabian StyleYin, Dongge, Hongyue Chen, Shuting Lin, Yufei Sun, Xiaohong Jing, Rongrong Chang, Yang Feng, Xiaoxv Dong, Changhai Qu, Jian Ni, and et al. 2025. "Recent Advances in the Application of Cucurbitacin B as an Anticancer Agent" International Journal of Molecular Sciences 26, no. 16: 8003. https://doi.org/10.3390/ijms26168003
APA StyleYin, D., Chen, H., Lin, S., Sun, Y., Jing, X., Chang, R., Feng, Y., Dong, X., Qu, C., Ni, J., & Yin, X. (2025). Recent Advances in the Application of Cucurbitacin B as an Anticancer Agent. International Journal of Molecular Sciences, 26(16), 8003. https://doi.org/10.3390/ijms26168003







