The Roles of RNA-Binding Proteins in Vasculogenic Mimicry Regulation in Glioblastoma
Abstract
1. Introduction
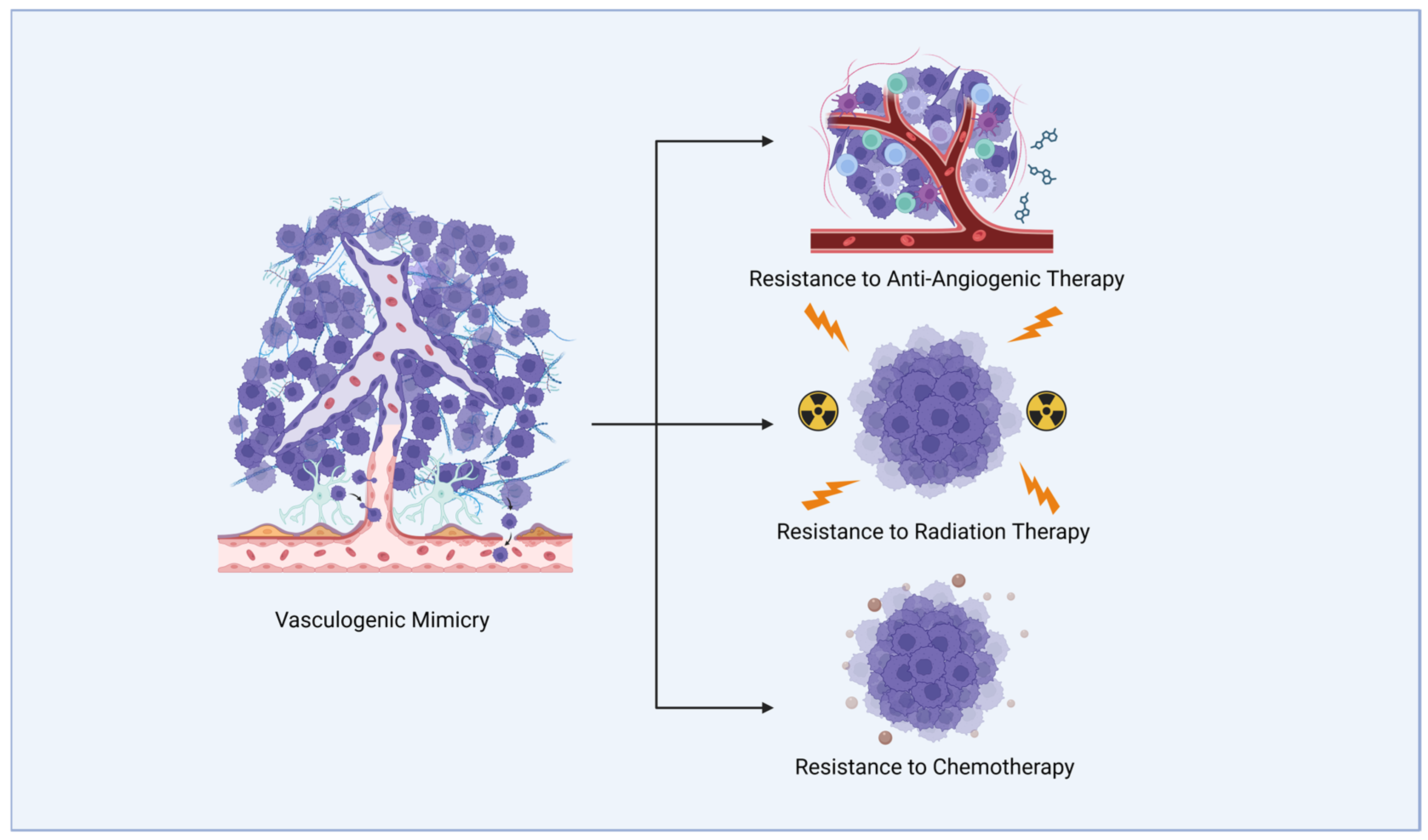
2. Vasculogenic Mimicry in Glioblastoma Treatment Resistance
2.1. Anti-Angiogenic Therapy Resistance
2.2. Radiotherapy and Chemotherapy Resistance
3. Mechanisms of Glioblastoma Vasculogenic Mimicry
3.1. The PI3K/Akt Pathway
3.2. The VE-Cadherin Pathway
3.3. The TGF-β Pathway
3.4. Hippo/YAP Pathway
3.5. Matrix Metalloproteinases and Laminin Pathway
3.6. RNAs in Glioblastoma Vasculogenic Mimicry Formation
4. RNA-Binding Proteins in Vasculogenic Mimicry in Glioblastoma
4.1. Messenger RNA-Binding Proteins
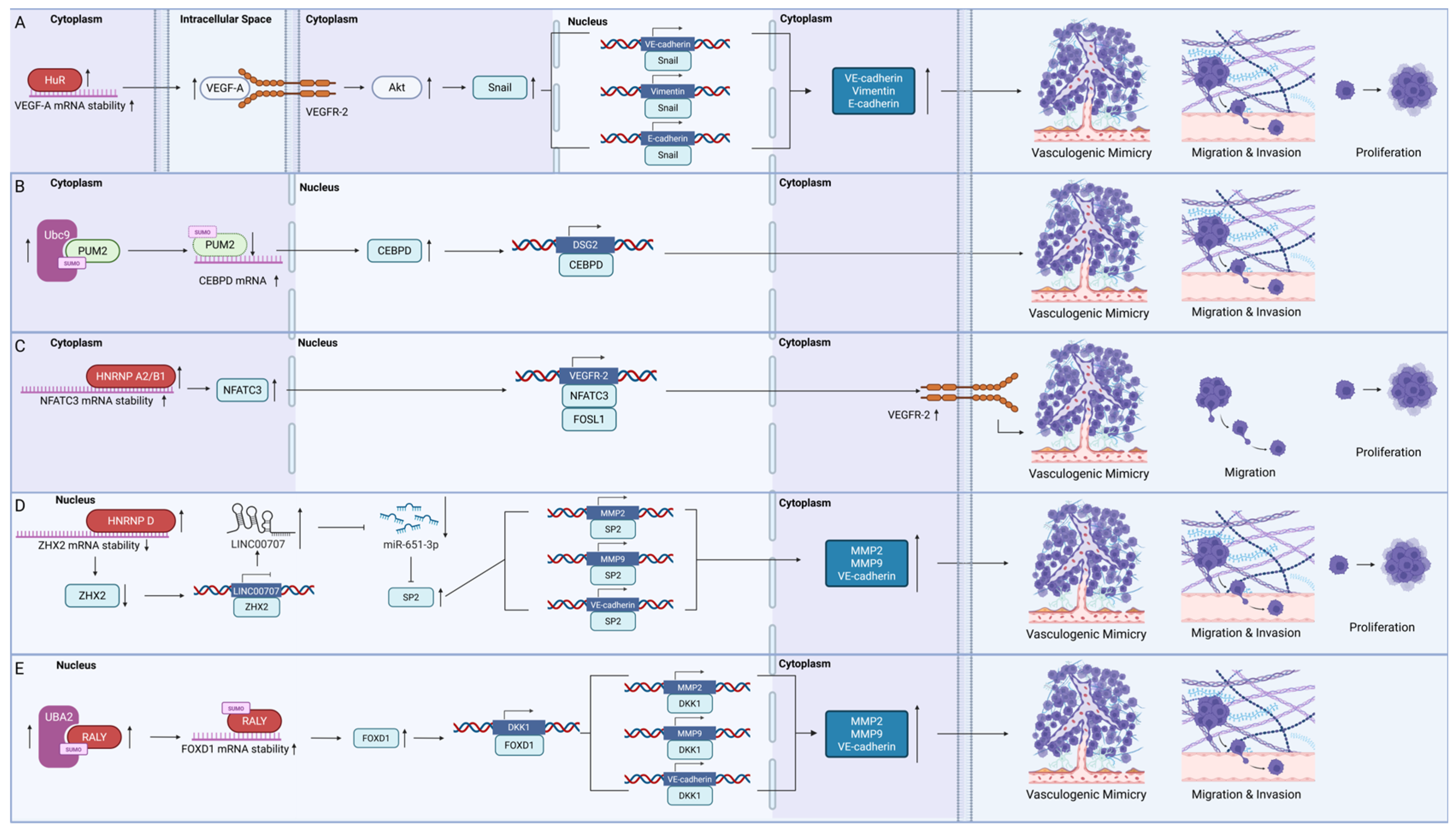
4.1.1. HuR
4.1.2. Pumilio Homolog 2 (PUM2)
4.1.3. Heterogeneous Nuclear Ribonucleoproteins (HNRNPs)
4.2. Circular RNA-Binding Proteins
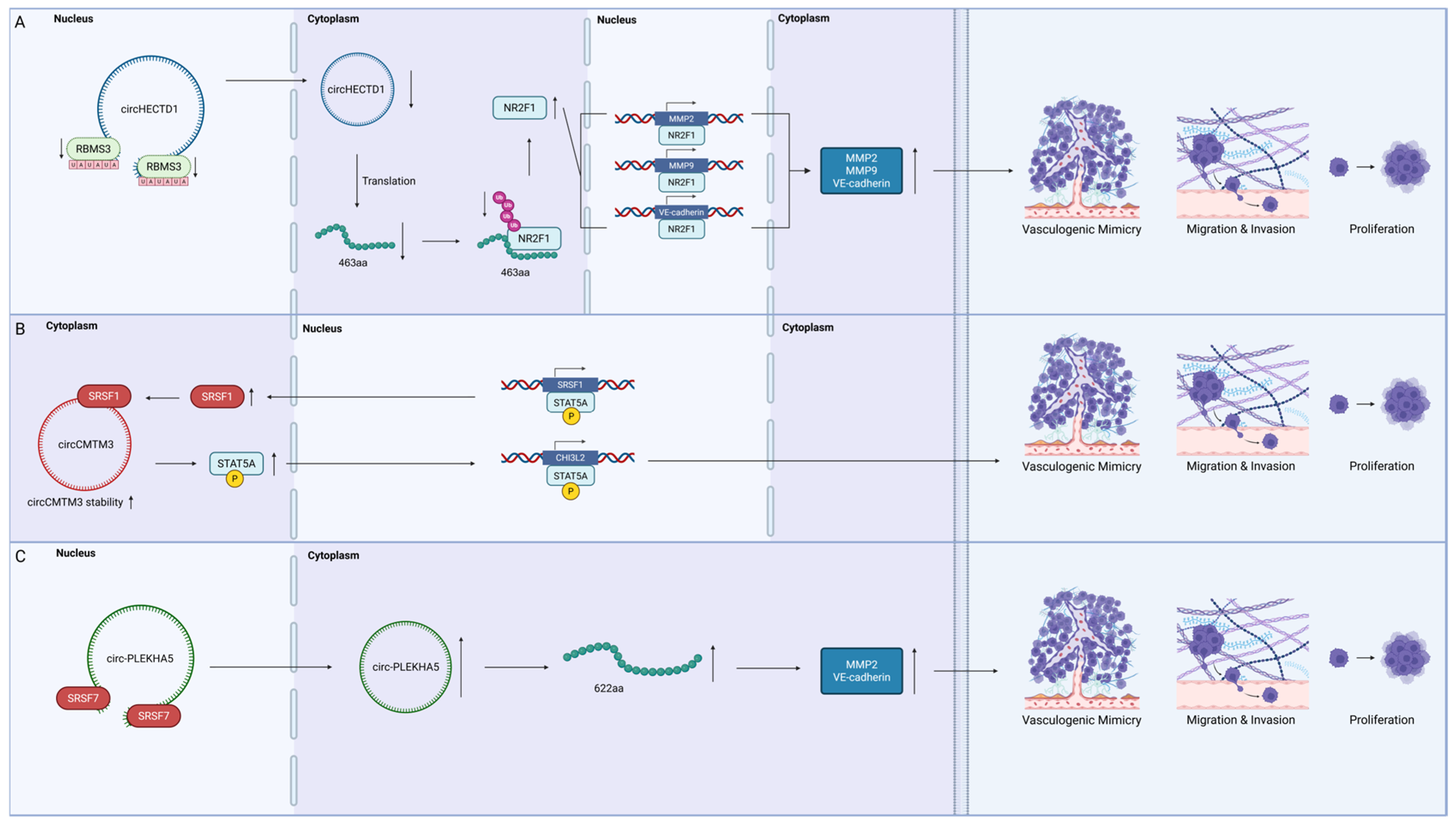
4.2.1. RNA-Binding Motif Single-Stranded Interacting Protein 3 (RBMS3)
4.2.2. The Serine/Arginine-Rich Splicing Factor (SRSF) Family
4.3. Long Non-Coding RNA-Binding Proteins
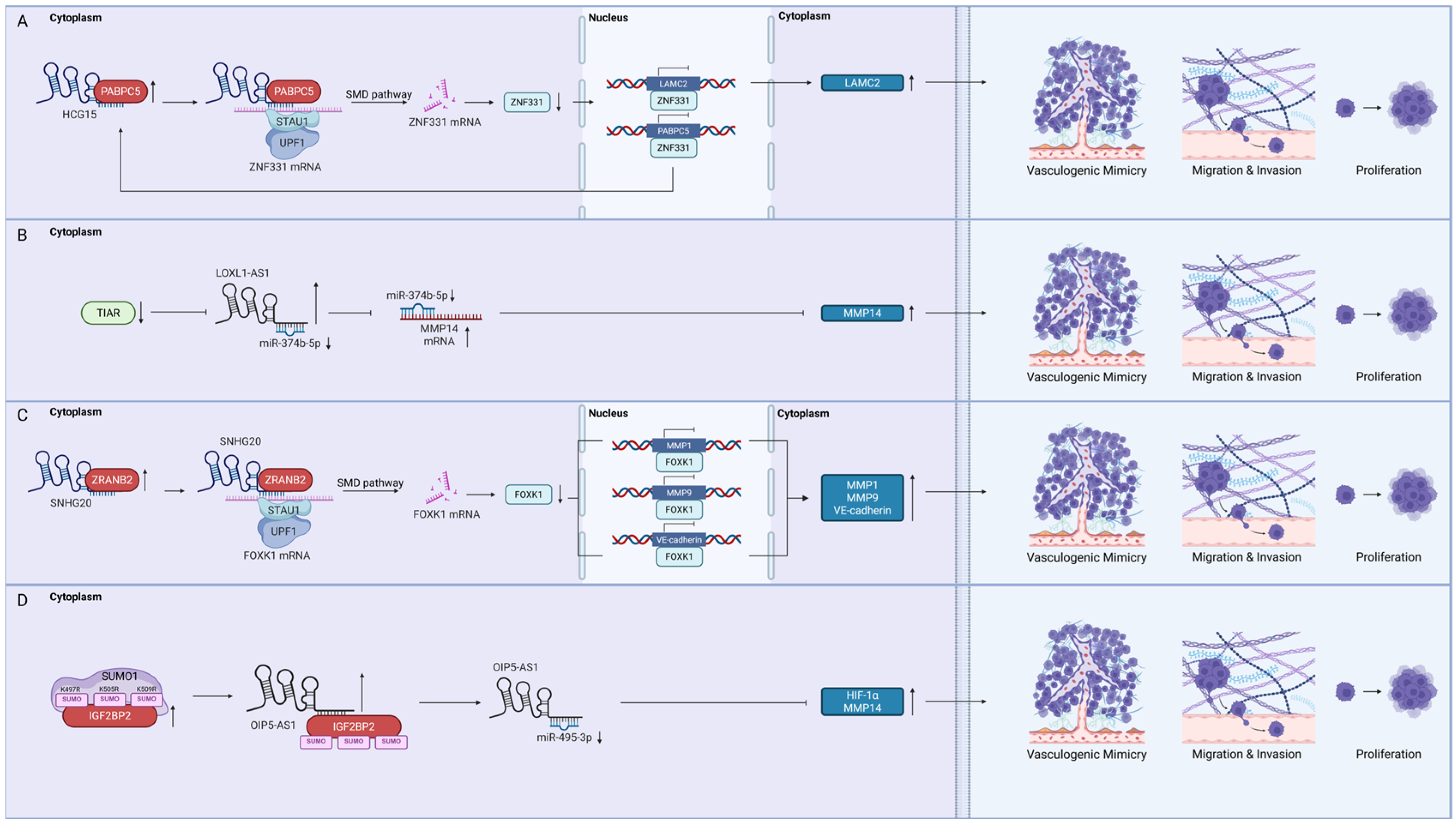
4.3.1. Poly(A) Binding Protein Cytoplasmic 5 (PABPC5)
4.3.2. T-Cell Intracellular Antigen 1-Related Protein (TIAR)
4.3.3. Zinc Finger Ran-Binding Domain-Containing Protein 2 (ZRANB2)
4.3.4. Insulin-like Growth Factor 2 mRNA-Binding Protein 2 (IGF2BP2)
4.4. Methyltransferase-like 3 (METTL3)
5. Exploring the Possibilities of RNA-Binding Proteins as Therapeutic Targets in Vasculogenic Mimicry Formation of Glioblastoma
5.1. Juglone: A Novel Inhibitor of Glioblastoma Vasculogenic Mimicry Targeting HuR
5.2. Possible Strategies—Small Molecule Inhibitors, Decoy Oligonucleotides, and Post-Translational Modification Targeting
5.3. Translational Potential of Vasculogenic Mimicry Targeting
6. Perspectives
6.1. Vasculogenic Mimicry Marker Identification and Functional Profiling
6.2. In Situ Mass Profiling of RBP-RNA Interactions
6.3. Accounting for Genomic Variations Across Glioblastoma Subtypes
6.4. Blood–Brain Barrier Penetration and Target-Based Drug Discovery
7. Concluding Remarks
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-HG | 2-hydroxyglutarate |
| 3′ UTR | 3′ untranslated region |
| AAT | Anti-angiogenic therapy |
| ARE | Adenine- and uridine-rich elements |
| ARTR-seq | Assay of reverse transcription-based RBP binding sites sequencing |
| BBB | Blood–brain barrier |
| BCL2 | B-cell lymphoma 2 protein |
| BTG1 | BTG anti-proliferation factor 1 |
| BUD13 | BUD13 homolog |
| c-Myc | Cellular myelocytomatosis |
| CD34 | Cluster of differentiation 34 |
| CD144 | Cluster of differentiation 144 |
| CDH2 | Cadherin 2 |
| CDH5 | Cadherin 5 |
| CDK12 | Cyclin-dependent kinase 12 |
| CEBPD | CCAAT/enhancer-binding protein delta |
| CHI3L2 | Chitinase 3-like 2 |
| circRNA | Circular RNA |
| COX-2 | Cyclooxygenase-2 |
| DART-seq | Deamination adjacent to RNA modification targets sequencing |
| DHTS | 15,16-dihydrotanshinone-I |
| DKK1 | Dickkopf WNT signalling pathway inhibitor 1 |
| ECM | Extracellular matrix |
| EGFR | Epidermal growth factor receptor |
| eIF | Eukaryotic initiation factor |
| EMT | Epithelial–mesenchymal transition |
| EP1 | Prostaglandin E2 receptor 1 |
| EphA2 | Ephrin type-A receptor 2 |
| ERK | Extracellular signal-regulated kinase |
| FDA | Food and Drug Administration |
| FN1 | Fibronectin 1 |
| FOXC2 | Forkhead box C2 |
| FOSL1 | FOS-like 1 |
| FOXD1 | Forkhead box D1 |
| FOXK1 | Forkhead box K1 |
| FOXO | Forkhead Box O |
| GBM | Glioblastoma |
| GSK3 | Glycogen synthase kinase 3 |
| HCG15 | HLA complex group 15 |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| HIF-2α | Hypoxia-inducible factor 2-alpha |
| HK2 | Hexokinase 2 |
| HNRNP | Heterogeneous nuclear ribonucleoprotein |
| HNRNP A2/B1 | heterogeneous nuclear ribonucleoprotein A2/B1 |
| HNRNP D | heterogeneous nuclear ribonucleoprotein D |
| HOTAIRM1 | HOXA transcript antisense RNA myeloid-specific 1 |
| Hsp90 | Heat shock protein 90 |
| HuR | Human antigen R |
| IGF2BP2 | Insulin-like growth factor 2 mRNA-binding protein 2 |
| IGFBP2 | Insulin-like growth factor-binding protein 2 |
| IGFBP7 | Insulin-like growth factor-binding protein 7 |
| IL-10 | Interleukin-10 |
| L1CAM | L1 cell adhesion molecule |
| LAMC2 | Laminin subunit gamma 2 |
| LINC00339 | Long intergenic non-protein coding RNA 339 |
| lncRNA | Long non-coding RNA |
| LIN28 | Lin-28 homolog A |
| LOXL1-AS1 | Lysyl oxidase-like 1 antisense RNA 1 |
| LRIG1 | Leucine rich repeats and immunoglobulin-like domains 1 |
| m6A | N6-methyladenosine |
| MAPK | Mitogen-activated protein kinase |
| MBNL1 | Muscleblind-like splicing regulator 1 |
| METTL3 | Methyltransferase-like 3 |
| miCLIP | m6A individual-nucleotide resolution UV crosslinking and immunoprecipitation |
| MMP | Matrix metalloproteinase |
| MMP2 | Matrix metalloproteinase 2 |
| MMP9 | Matrix metalloproteinase 9 |
| MMP14 | Matrix metalloproteinase 14 |
| mTOR | Mechanistic target of rapamycin |
| N-cadherin | Neural cadherin |
| NF2 | Neurofibromin 2 |
| NFATC3 | Nuclear factor of activated T cells 3 |
| NF-κB | Nuclear factor kappa B |
| NR2F1 | Nuclear receptor subfamily 2 group F member 1 |
| OIP5-AS1 | Opa interacting protein 5 antisense RNA 1 |
| OS | Overall survival |
| PABPC1 | Poly(A)-binding protein cytoplasmic 1 |
| PABPC5 | Poly(A)-binding protein cytoplasmic 5 |
| PAS | Periodic Acid Schiff |
| PFS | Progression-free survival |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphatidylinositol 3-kinase |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PIP3 | Phosphatidylinositol 3,4,5-trisphosphate |
| PKC | Protein kinase C |
| PLK4 | Polo-like kinase 4 |
| PRRX1 | Paired-related homeobox 1 |
| PUF | Pumilio and FBF |
| PUM2 | Pumilio RNA-binding family member 2 |
| RALY | RNA-binding protein Raly |
| RBMS3 | RNA-binding motif single-stranded interacting protein 3 |
| RBP | RNA-binding protein |
| RhoA | Ras homolog family member A |
| ROS | Reactive oxygen species |
| RRM | RNA recognition motif |
| RTK | Receptor tyrosine kinase |
| scRibo-seq | Single-cell ribosome sequencing |
| SMD | Staufen-mediated mRNA decay |
| SNHG20 | Small nucleolar RNA host gene 20 |
| SP2 | Sp2 transcription factor |
| SRSF | Serine/arginine-rich splicing factor |
| SRSF1 | Serine/arginine-rich splicing factor 1 |
| SRSF3 | Serine/arginine-rich splicing factor 3 |
| SRSF7 | Serine/arginine-rich splicing factor 7 |
| SRSF9 | Serine/arginine-rich splicing factor 9 |
| STAT5A | Signal transducer and activator of transcription 5A |
| STK11 | Serine/threonine kinase 11 |
| SUMO1 | Small ubiquitin-like modifier 1 |
| SUMO2/3 | Small ubiquitin-like modifier 2/3 |
| TEAD1 | Transcriptional enhanced associate domain family member 1 |
| TF | Transcriptional factor |
| TGF-β | Transforming growth factor β |
| TIAR | T-cell intracellular antigen 1-related protein |
| TRAP1 | Tumour necrosis factor receptor-associated protein 1 |
| TWIST-1 | Twist-related protein 1 |
| UBA2 | Ubiquitin-like modifier activating enzyme 2 |
| VE-cadherin | Vascular endothelial cadherin |
| VEGF | Vascular endothelial growth factor |
| VEGF-A | Vascular endothelial growth factor-A |
| VEGFR-1 | Vascular endothelial growth factor receptor 1 |
| VEGFR-2 | Vascular endothelial growth factor receptor 2 |
| VM | Vasculogenic mimicry |
| YAP | Yes-associated protein |
| YAP1 | Yes-associated protein 1 |
| ZHX2 | Zinc fingers and homeoboxes 2 |
| ZNF331 | Zinc finger protein 331 |
| ZRANB2 | Zinc finger Ran-binding domain-containing protein 2 |
References
- Ohgaki, H.; Kleihues, P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005, 109, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Delgado-López, P.D.; Corrales-García, E.M. Survival in glioblastoma: A review on the impact of treatment modalities. Clin. Transl. Oncol. 2016, 18, 1062–1071. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Narayana, A.; Gruber, D.; Kunnakkat, S.; Golfinos, J.G.; Parker, E.; Raza, S.; Zagzag, D.; Eagan, P.; Gruber, M.L. A clinical trial of bevacizumab, temozolomide, and radiation for newly diagnosed glioblastoma. J. Neurosurg. 2012, 116, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef] [PubMed]
- de Groot, J.F.; Lamborn, K.R.; Chang, S.M.; Gilbert, M.R.; Cloughesy, T.F.; Aldape, K.; Yao, J.; Jackson, E.F.; Lieberman, F.; Robins, H.I.; et al. Phase II Study of Aflibercept in Recurrent Malignant Glioma: A North American Brain Tumor Consortium Study. J. Clin. Oncol. 2011, 29, 2689–2695. [Google Scholar] [CrossRef]
- Lai, A.; Tran, A.; Nghiemphu, P.L.; Pope, W.B.; Solis, O.E.; Selch, M.; Filka, E.; Yong, W.H.; Mischel, P.S.; Liau, L.M.; et al. Phase II Study of Bevacizumab Plus Temozolomide During and After Radiation Therapy for Patients With Newly Diagnosed Glioblastoma Multiforme. J. Clin. Oncol. 2011, 29, 142–148. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.; Won, M.; Blumenthal, D.T.; Vogelbaum, M.A.; Aldape, K.D.; Colman, H.; Chakravarti, A.; Jeraj, R.; Armstrong, T.S.; et al. RTOG 0825: Phase III double-blind placebo-controlled trial evaluating bevacizumab (Bev) in patients (Pts) with newly diagnosed glioblastoma (GBM). J. Clin. Oncol. 2013, 31, 1. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- El Hallani, S.; Boisselier, B.; Peglion, F.; Rousseau, A.; Colin, C.; Idbaih, A.; Marie, Y.; Mokhtari, K.; Thomas, J.-L.; Eichmann, A.; et al. A new alternative mechanism in glioblastoma vascularization: Tubular vasculogenic mimicry. Brain 2010, 133, 973–982. [Google Scholar] [CrossRef]
- McDonald, D.M.; Munn, L.; Jain, R.K. Vasculogenic Mimicry: How Convincing, How Novel, and How Significant? Am. J. Pathol. 2000, 156, 383–388. [Google Scholar] [CrossRef]
- Yue, W.-Y.; Chen, Z.-P. Does Vasculogenic Mimicry Exist in Astrocytoma? J. Histochem. Cytochem. 2005, 53, 997–1002. [Google Scholar] [CrossRef]
- Dang, S.-M.; Yang, D.; Wang, Z.-Y.; Ding, X.-M.; Li, X.-L.; Li, D.-Y.; Li, D.-X. Vasculogenic mimicry: A pivotal mechanism con-tributing to drug resistance in antiangiogenic therapy. Oncol. Transl. Med. 2024, 10, 119–125. [Google Scholar] [CrossRef]
- Hentze, M.W.; Castello, A.; Schwarzl, T.; Preiss, T. A brave new world of RNA-binding proteins. Nat. Rev. Mol. Cell Biol. 2018, 19, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Guha, A.; Waris, S.; Nabors, L.B.; Filippova, N.; Gorospe, M.; Kwan, T.; King, P.H. The versatile role of HuR in Glioblastoma and its potential as a therapeutic target for a multi-pronged attack. Adv. Drug Deliv. Rev. 2022, 181, 114082. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.; Huang, T.; Qi, J.; An, S.; Burket, N.; Cooper, S.; Nazarian, J.; Saratsis, A.M. LIN28B and Let-7 in Diffuse Midline Glioma: A Review. Cancers 2023, 15, 3241. [Google Scholar] [CrossRef]
- Dong, Z.; Cui, H. The Emerging Roles of RNA Modifications in Glioblastoma. Cancers 2020, 12, 736. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Zhang, C.; Guan, X.; Li, X.; Jia, W.; Chen, A. Old players and new insights: Unraveling the role of RNA-binding proteins in brain tumors. Theranostics 2025, 15, 5238–5257. [Google Scholar] [CrossRef]
- Maj, E.; Papiernik, D.; Wietrzyk, J. Antiangiogenic cancer treatment: The great discovery and greater complexity (Review). Int. J. Oncol. 2016, 49, 1773–1784. [Google Scholar] [CrossRef]
- Jain, R.K. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat. Med. 2001, 7, 987–989. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Sorensen, A.G.; di Tomaso, E.; Zhang, W.-T.; Duda, D.G.; Cohen, K.S.; Kozak, K.R.; Cahill, D.P.; Chen, P.-J.; Zhu, M.; et al. AZD2171, a Pan-VEGF Receptor Tyrosine Kinase Inhibitor, Normalizes Tumor Vasculature and Alleviates Edema in Glioblastoma Patients. Cancer Cell 2007, 11, 83–95. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Won, M.; Chakravarti, A.; Hadjipanayis, C.G.; Shi, W.; Ashby, L.S.; Stieber, V.W.; Robins, H.I.; Gray, H.J.; Voloschin, A.; et al. NRG/RTOG 0837: Randomized, phase II, double-blind, placebo-controlled trial of chemoradiation with or without cediranib in newly diagnosed glioblastoma. Neuro-Oncol. Adv. 2023, 5, vdad116. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalizing Tumor Microenvironment to Treat Cancer: Bench to Bedside to Biomarkers. J. Clin. Oncol. 2013, 31, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.T.; Gerstner, E.R.; Emblem, K.E.; Duda, D.G.; Kalpathy-Cramer, J.; Snuderl, M.; Ancukiewicz, M.; Polaskova, P.; Pinho, M.C.; Jennings, D.; et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc. Natl. Acad. Sci. USA 2013, 110, 19059–19064. [Google Scholar] [CrossRef] [PubMed]
- Obad, N.; Espedal, H.; Jirik, R.; Sakariassen, P.O.; Rygh, C.B.; Lund-Johansen, M.; Taxt, T.; Niclou, S.P.; Bjerkvig, R.; Keunen, O. Lack of functional normalisation of tumour vessels following anti-angiogenic therapy in glioblastoma. J. Cereb. Blood Flow Metab. 2017, 38, 1741–1753. [Google Scholar] [CrossRef]
- Mei, X.; Chen, Y.; Zhang, Q.; Chen, F.; Xi, S.; Long, Y.; Zhang, J.; Cai, H.; Ke, C.; Wang, J.; et al. Association between glioblastoma cell-derived vessels and poor prognosis of the patients. Cancer Commun. 2020, 40, 211–221. [Google Scholar] [CrossRef]
- Maddison, K.; Faulkner, S.; Graves, M.C.; Fay, M.; Bowden, N.A.; Tooney, P.A. Vasculogenic Mimicry Occurs at Low Levels in Primary and Recurrent Glioblastoma. Cancers 2023, 15, 3922. [Google Scholar] [CrossRef]
- Chen, Y.; Jing, Z.; Luo, C.; Zhuang, M.; Xia, J.; Chen, Z.; Wang, Y. Vasculogenic mimicry–potential target for glioblastoma therapy: An in vitro and in vivo study. Med Oncol. 2010, 29, 324–331. [Google Scholar] [CrossRef]
- Huang, M.; Ke, Y.; Sun, X.; Yu, L.; Yang, Z.; Zhang, Y.; DU, M.; Wang, J.; Liu, X.; Huang, S. Mammalian target of rapamycin signaling is involved in the vasculogenic mimicry of glioma via hypoxia-inducible factor-1α. Oncol. Rep. 2014, 32, 1973–1980. [Google Scholar] [CrossRef]
- Chiba, R.; Akiya, M.; Hashimura, M.; Oguri, Y.; Inukai, M.; Hara, A.; Saegusa, M.; Lee, S.-G. ALK signaling cascade confers multiple advantages to glioblastoma cells through neovascularization and cell proliferation. PLoS ONE 2017, 12, e0183516. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Zhao, W.; Ma, Y.; Yang, X. Demonstration of vasculogenic mimicry in astrocytomas and effects of Endostar on U251 cells. Pathol. Res. Pract. 2011, 207, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-M.; Zhang, Q.-P.; Mu, Y.-G.; Zhang, X.-H.; Sai, K.; Pang, J.C.-S.; Ng, H.-K.; Chen, Z.-P. Clinical significance of vasculogenic mimicry in human gliomas. J. Neuro-Oncol. 2011, 105, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, J.; Li, S.; Li, L.; Huang, M.; Zhang, Y.; Liu, Y.; Yang, Y.; Ding, R.; Ke, Y. Histone deacetylase 3 expression correlates with vasculogenic mimicry through the phosphoinositide3-kinase/ERK–MMP–laminin5γ2 signaling pathway. Cancer Sci. 2015, 106, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Ke, Y.-Q.; Lu, G.-H.; Song, Z.-H.; Yu, L.; Xiao, S.; Sun, X.-L.; Jiang, X.-D.; Yang, Z.-L.; Hu, C.-C. Vasculogenic mimicry is a prognostic factor for postoperative survival in patients with glioblastoma. J. Neuro-Oncol. 2013, 112, 339–345. [Google Scholar] [CrossRef]
- Xue, W.; Du, X.; Wu, H.; Liu, H.; Xie, T.; Tong, H.; Chen, X.; Guo, Y.; Zhang, W. Aberrant glioblastoma neovascularization patterns and their correlation with DCE-MRI-derived parameters following temozolomide and bevacizumab treatment. Sci. Rep. 2017, 7, 13894. [Google Scholar] [CrossRef]
- Angara, K.; Rashid, M.H.; Shankar, A.; Ara, R.; Iskander, A.; Borin, T.F.; Jain, M.; Achyut, B.R.; Arbab, A.S. Vascular mimicry in glioblastoma following anti-angiogenic and anti-20-HETE therapies. Histol. Histopathol. 2017, 32, 917–928. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Chen, Y.; Lin, W.; Zhang, Y.; Cai, G.; Sun, X.; Zheng, K.; He, J.; Ai, T.; et al. Prrx1 promotes resistance to temozolomide by upregulating ABCC1 and inducing vasculogenic mimicry in glioma. Am. J. Cancer Res. 2022, 12, 3892–3912. [Google Scholar]
- Shaifer, C.A.; Huang, J.; Lin, P.C. Glioblastoma cells incorporate into tumor vasculature and contribute to vascular radioresistance. Int. J. Cancer 2010, 127, 2063–2075. [Google Scholar] [CrossRef]
- Ran, Y.; Han, S.; Gao, D.; Chen, X.; Liu, C. Interference of FZD2 suppresses proliferation, vasculogenic mimicry and stemness in glioma cells via blocking the Notch/NF-κB signaling pathway. Exp. Ther. Med. 2024, 28, 373. [Google Scholar] [CrossRef]
- Rong, X.; Huang, B.; Qiu, S.; Li, X.; He, L.; Peng, Y. Tumor-associated macrophages induce vasculogenic mimicry of glioblastoma multiforme through cyclooxygenase-2 activation. Oncotarget 2016, 7, 83976–83986. [Google Scholar] [CrossRef]
- Wu, H.-B.; Yang, S.; Weng, H.-Y.; Chen, Q.; Zhao, X.-L.; Fu, W.-J.; Niu, Q.; Ping, Y.-F.; Wang, J.M.; Zhang, X.; et al. Autophagy-induced KDR/VEGFR-2 activation promotes the formation of vasculogenic mimicry by glioma stem cells. Autophagy 2017, 13, 1528–1542. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Zhao, P.; Zhao, H.; Gao, W.; Wang, L. Celastrol Suppresses Glioma Vasculogenic Mimicry Formation and Angiogenesis by Blocking the PI3K/Akt/mTOR Signaling Pathway. Front. Pharmacol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, Y.; Tao, X.; Zhang, W.; Wang, X.; Wang, X.; Ruan, Z.; Chen, Z. INPP4B inhibits glioma cell proliferation and immune escape via inhibition of the PI3K/AKT signaling pathway. Front. Oncol. 2022, 12, 983537. [Google Scholar] [CrossRef] [PubMed]
- Colardo, M.; Segatto, M.; Di Bartolomeo, S. Targeting RTK-PI3K-mTOR Axis in Gliomas: An Update. Int. J. Mol. Sci. 2021, 22, 4899. [Google Scholar] [CrossRef]
- Tanase, C.P.; Cruceru, M.L.; Enciu, A.-M.; Popa, A.C.; Albulescu, R.; Neagu, M.; Constantinescu, S.N. Signal transduction molecule patterns indicating potential glioblastoma therapy approaches. OncoTargets Ther. 2013, 6, 1737–1749. [Google Scholar] [CrossRef]
- Yao, X.; Ping, Y.; Liu, Y.; Chen, K.; Yoshimura, T.; Liu, M.; Gong, W.; Chen, C.; Niu, Q.; Guo, D.; et al. Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2) Plays a Key Role in Vasculogenic Mimicry Formation, Neovascularization and Tumor Initiation by Glioma Stem-like Cells. PLoS ONE 2013, 8, e57188. [Google Scholar] [CrossRef]
- Wang, B.; Yu, R.-Z.; Zhang, X.-Y.; Ren, Y.; Zhen, Y.-W.; Han, L. Polo-like kinase 4 accelerates glioma malignant progression and vasculogenic mimicry by phosphorylating EphA2. Cancer Lett. 2024, 611, 217397. [Google Scholar] [CrossRef]
- Fu, H.; Wu, S.; Shen, H.; Luo, K.; Huang, Z.; Lu, N.; Li, Y.; Lan, Q.; Xian, Y. Dihydroartemisinin inhibits EphA2/PI3K/Akt pathway-mediated malignant behaviors and vasculogenic mimicry in glioma stem cells. Heliyon 2025, 11, e42095. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Q.; Wei, C.; Qu, J. LRIG1 inhibits hypoxia-induced vasculogenic mimicry formation via suppression of the EGFR/PI3K/AKT pathway and epithelial-to-mesenchymal transition in human glioma SHG-44 cells. Cell Stress Chaperon 2015, 20, 631–641. [Google Scholar] [CrossRef]
- Xie, R.; Yang, H.; Xiao, Q.; Mao, F.; Zhang, S.; Ye, F.; Wan, F.; Wang, B.; Lei, T.; Guo, D. Downregulation of LRIG1 expression by RNA interference promotes the aggressive properties of glioma cells via EGFR/Akt/c-Myc activation. Oncol. Rep. 2012, 29, 177–184. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, C.; Liu, P.; Ouyang, F.; Luo, J.; Lu, C.; Tang, B.; Yang, X. L1CAM promotes vasculogenic mimicry formation by miR-143-3p-induced expression of hexokinase 2 in glioma. Mol. Oncol. 2023, 17, 664–685. [Google Scholar] [CrossRef]
- Mao, X.-G.; Xue, X.-Y.; Wang, L.; Zhang, X.; Yan, M.; Tu, Y.-Y.; Lin, W.; Jiang, X.-F.; Ren, H.-G.; Zhang, W.; et al. CDH5 is specifically activated in glioblastoma stemlike cells and contributes to vasculogenic mimicry induced by hypoxia. Neuro-Oncol. 2013, 15, 865–879. [Google Scholar] [CrossRef]
- Delgado-Bellido, D.; Zamudio-Martínez, E.; Fernández-Cortés, M.; Herrera-Campos, A.B.; Olmedo-Pelayo, J.; Perez, C.J.; Expósito, J.; de Álava, E.; Amaral, A.T.; Valle, F.O.; et al. VE-Cadherin modulates β-catenin/TCF-4 to enhance Vasculogenic Mimicry. Cell Death Dis. 2023, 14, 135. [Google Scholar] [CrossRef]
- Mao, J.-M.; Liu, J.; Guo, G.; Mao, X.-G.; Li, C.-X. Glioblastoma vasculogenic mimicry: Signaling pathways progression and potential anti-angiogenesis targets. Biomark. Res. 2015, 3, 8. [Google Scholar] [CrossRef]
- Golán-Cancela, I.; Caja, L. The TGF-β Family in Glioblastoma. Int. J. Mol. Sci. 2024, 25, 1067. [Google Scholar] [CrossRef]
- Pen, A.; Moreno, M.J.; Durocher, Y.; Deb-Rinker, P.; Stanimirovic, D.B. Glioblastoma-secreted factors induce IGFBP7 and angiogenesis by modulating Smad-2-dependent TGF-β signaling. Oncogene 2008, 27, 6834–6844. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Szabo, E.; Burghardt, I.; Frei, K.; Tabatabai, G.; Weller, M. Modulation of cerebral endothelial cell function by TGF-β in glioblastoma: VEGF-dependent angiogenesis versus endothelial mesenchymal transition. Oncotarget 2015, 6, 22480–22495. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, X.; Zhang, Y.; Mo, Y.; Sun, X.; Shu, L.; Ke, Y. Paradoxical role of β8 integrin on angiogenesis and vasculogenic mimicry in glioblastoma. Cell Death Dis. 2022, 13, 536. [Google Scholar] [CrossRef] [PubMed]
- Ling, G.; Wang, S.; Song, Z.; Sun, X.; Liu, Y.; Jiang, X.; Cai, Y.; Du, M.; Ke, Y. Transforming growth factor-β is required for vasculogenic mimicry formation in glioma cell line U251MG. Cancer Biol. Ther. 2011, 12, 978–988. [Google Scholar] [CrossRef]
- Roy, M.; Elimam, R.; Zgheib, A.; Annabi, B. A Role for the Hippo/YAP1 Pathway in the Regulation of In Vitro Vasculogenic Mimicry in Glioblastoma Cells. J. Cell. Mol. Med. 2024, 28, e70304. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.-E.; Veilleux, C.; Paquin, A.; Gagnon, A.; Annabi, B. Transcriptional regulation of CYR61 and CTGF by LM98: A synthetic YAP-TEAD inhibitor that targets in-vitro vasculogenic mimicry in glioblastoma cells. Anti-Cancer Drugs 2024, 35, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Hamill, K.J.; Kligys, K.; Hopkinson, S.B.; Jones, J.C.R. Laminin deposition in the extracellular matrix: A complex picture emerges. J. Cell Sci. 2009, 122, 4409–4417. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Sierra, E.; Bermúdez, M.; Villegas-Mercado, C.E.; Silva-Cázares, M.B.; López-Camarillo, C. LncRNAs Regulate Vasculogenic Mimicry in Human Cancers. Cells 2025, 14, 616. [Google Scholar] [CrossRef]
- Shao, Y.; Lu, B. The emerging roles of circular RNAs in vessel co-option and vasculogenic mimicry: Clinical insights for anti-angiogenic therapy in cancers. Cancer Metastasis Rev. 2021, 41, 173–191. [Google Scholar] [CrossRef]
- García-Hernández, A.P.; Sánchez-Sánchez, G.; Carlos-Reyes, A.; López-Camarillo, C. Functional roles of microRNAs in vasculogenic mimicry and resistance to therapy in human cancers: An update. Expert Rev. Clin. Immunol. 2024, 20, 913–926. [Google Scholar] [CrossRef]
- Guo, J.; Cai, H.; Liu, X.; Zheng, J.; Liu, Y.; Gong, W.; Chen, J.; Xi, Z.; Xue, Y. Long Non-coding RNA LINC00339 Stimulates Glioma Vasculogenic Mimicry Formation by Regulating the miR-539-5p/TWIST1/MMPs Axis. Mol. Ther. Nucleic Acids 2018, 10, 170–186. [Google Scholar] [CrossRef]
- Luo, C.; Chen, G.; Li, R.; Peng, S.; Zhang, P.; Wang, F.; Yu, S.; Zhu, Y.; Zhang, J. Juglone suppresses vasculogenic mimicry in glioma through inhibition of HuR-mediated VEGF-A expression. Biochem. Pharmacol. 2024, 227, 116458. [Google Scholar] [CrossRef]
- Wang, D.; Ruan, X.; Liu, X.; Xue, Y.; Shao, L.; Yang, C.; Zhu, L.; Yang, Y.; Li, Z.; Yu, B.; et al. SUMOylation of PUM2 promotes the vasculogenic mimicry of glioma cells via regulating CEBPD. Clin. Transl. Med. 2020, 10, e168. [Google Scholar] [CrossRef]
- Wang, H.; Shi, Y.; Zhou, X.; Zhang, L.; Yang, A.; Zhou, D.; Ma, T. HNRNPA2B1 stabilizes NFATC3 levels to potentiate its combined actions with FOSL1 to mediate vasculogenic mimicry in GBM cells. Cell Biol. Toxicol. 2024, 40, 44. [Google Scholar] [CrossRef]
- Yu, S.; Ruan, X.; Liu, X.; Zhang, F.; Wang, D.; Liu, Y.; Yang, C.; Shao, L.; Liu, Q.; Zhu, L.; et al. HNRNPD interacts with ZHX2 regulating the vasculogenic mimicry formation of glioma cells via linc00707/miR-651-3p/SP2 axis. Cell Death Dis. 2021, 12, 153. [Google Scholar] [CrossRef]
- Cao, S.; Wang, D.; Wang, P.; Liu, Y.; Dong, W.; Ruan, X.; Liu, L.; Xue, Y.; E, T.; Lin, H.; et al. SUMOylation of RALY promotes vasculogenic mimicry in glioma cells via the FOXD1/DKK1 pathway. Cell Biol. Toxicol. 2023, 39, 3323–3340. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Liu, Y.; Wang, P.; Liu, L.; Ma, T.; Xue, Y.; Dong, W.; Zhao, Y.; E, T.; Lin, H.; et al. RBMS3-induced circHECTD1 encoded a novel protein to suppress the vasculogenic mimicry formation in glioblastoma multiforme. Cell Death Dis. 2023, 14, 745. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Zuo, Z.; Cui, D.; Xu, Y.; Li, L.; Jiang, Y. Dual role of exosomal circCMTM3 derived from GSCs in impeding degradation and promoting phosphorylation of STAT5A to facilitate vasculogenic mimicry formation in glioblastoma. Theranostics 2024, 14, 5698–5724. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Liu, X.; Teng, H.; Ruan, X.; Wang, D.; Yang, C.; Shao, L.; Liu, Y.; Cai, H.; Li, Z.; et al. Biosynthetic circ-PLEKHA5 stabilized by SRSF7 promotes the vasculogenic mimicry of glioblastoma cells by encoding a novel protein 622aa. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Jing, F.; Ruan, X.; Liu, X.; Yang, C.; Wang, D.; Zheng, J.; Xue, Y.; Shen, S.; Shao, L.; Yang, Y.; et al. The PABPC5/HCG15/ZNF331 Feedback Loop Regulates Vasculogenic Mimicry of Glioma via STAU1-Mediated mRNA Decay. Mol. Ther. Oncolytics 2020, 17, 216–231. [Google Scholar] [CrossRef]
- Yi, B.; Li, H.; Cai, H.; Lou, X.; Yu, M.; Li, Z. LOXL1-AS1 communicating with TIAR modulates vasculogenic mimicry in glioma via regulation of the miR-374b-5p/MMP14 axis. J. Cell. Mol. Med. 2021, 26, 475–490. [Google Scholar] [CrossRef]
- Li, X.; Xue, Y.; Liu, X.; Zheng, J.; Shen, S.; Yang, C.; Chen, J.; Li, Z.; Liu, L.; Ma, J.; et al. ZRANB2/SNHG20/FOXK1 Axis regulates Vasculogenic mimicry formation in glioma. J. Exp. Clin. Cancer Res. 2019, 38, 68. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Yi, B.; Cai, H.; Wang, Y.; Lou, X.; Xi, Z.; Li, Z. SUMOylation of IGF2BP2 promotes vasculogenic mimicry of glioma via regulating OIP5-AS1/miR-495-3p axis. Int. J. Biol. Sci. 2021, 17, 2912–2930. [Google Scholar] [CrossRef]
- Tao, M.; Li, X.; He, L.; Rong, X.; Wang, H.; Pan, J.; Lu, Z.; Zhang, X.; Peng, Y. Decreased RNA m6A methylation enhances the process of the epithelial mesenchymal transition and vasculogenic mimicry in glioblastoma. Am. J. Cancer Res. 2022, 12, 893–906. [Google Scholar]
- Wu, Z.; Wei, N. METTL3-mediated HOTAIRM1 promotes vasculogenic mimicry icontributionsn glioma via regulating IGFBP2 expression. J. Transl. Med. 2023, 21, 855. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ruan, X.; Liu, X.; Dong, W.; Wang, D.; Yang, C.; Liu, L.; Wang, P.; Zhang, M.; Xue, Y. The mechanism of BUD13 m6A methylation mediated MBNL1-phosphorylation by CDK12 regulating the vasculogenic mimicry in glioblastoma cells. Cell Death Dis. 2022, 13, 1017. [Google Scholar] [CrossRef] [PubMed]
- White, E.K.; Moore-Jarrett, T.; Ruley, H.E. PUM2, a novel murine puf protein, and its consensus RNA-binding site. RNA 2001, 7, 1855–1866. [Google Scholar]
- Wang, Y.; Sun, W.; Yang, J.; Yang, L.; Li, C.; Liu, H.; Liu, X.; Jiao, B. PUM2 Promotes Glioblastoma Cell Proliferation and Migration via Repressing BTG1 Expression. Cell Struct. Funct. 2019, 44, 29–39. [Google Scholar] [CrossRef]
- Wu, B.; Su, S.; Patil, D.P.; Liu, H.; Gan, J.; Jaffrey, S.R.; Ma, J. Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat. Commun. 2018, 9, 420. [Google Scholar] [CrossRef]
- Górnicki, T.; Lambrinow, J.; Mrozowska, M.; Podhorska-Okołów, M.; Dzięgiel, P.; Grzegrzółka, J. Role of RBMS3 Novel Potential Regulator of the EMT Phenomenon in Physiological and Pathological Processes. Int. J. Mol. Sci. 2022, 23, 10875. [Google Scholar] [CrossRef]
- Zhu, L.; Xi, P.-W.; Li, X.-X.; Sun, X.; Zhou, W.-B.; Xia, T.-S.; Shi, L.; Hu, Y.; Ding, Q.; Wei, J.-F. The RNA binding protein RBMS3 inhibits the metastasis of breast cancer by regulating Twist1 expression. J. Exp. Clin. Cancer Res. 2019, 38, 105. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, R.; Li, X.; Yu, L.; Hua, D.; Sun, C.; Shi, C.; Luo, W.; Rao, C.; Jiang, Z.; et al. Splicing factor SRSF1 promotes gliomagenesis via oncogenic splice-switching of MYO1B. J. Clin. Investig. 2019, 129, 676–693. [Google Scholar] [CrossRef]
- Ye, L.-J.; Xu, K.-M.; Bai, G.; Yuan, J.; Ran, F.-M. SRSF1 induces glioma progression and has a potential diagnostic application in grading primary glioma. Oncol. Lett. 2023, 26, 348. [Google Scholar] [CrossRef]
- Song, X.; Wan, X.; Huang, T.; Zeng, C.; Sastry, N.; Wu, B.; James, C.D.; Horbinski, C.; Nakano, I.; Zhang, W.; et al. SRSF3-Regulated RNA Alternative Splicing Promotes Glioblastoma Tumorigenicity by Affecting Multiple Cellular Processes. Cancer Res. 2019, 79, 5288–5301. [Google Scholar] [CrossRef]
- Luo, C.; He, J.; Yang, Y.; Wu, K.; Fu, X.; Cheng, J.; Ming, Y.; Liu, W.; Peng, Y. SRSF9 promotes cell proliferation and migration of glioblastoma through enhancing CDK1 expression. J. Cancer Res. Clin. Oncol. 2024, 150, 292. [Google Scholar] [CrossRef]
- Cléry, A.; Krepl, M.; Nguyen, C.K.X.; Moursy, A.; Jorjani, H.; Katsantoni, M.; Okoniewski, M.; Mittal, N.; Zavolan, M.; Sponer, J.; et al. Structure of SRSF1 RRM1 bound to RNA reveals an unexpected bimodal mode of interaction and explains its involvement in SMN1 exon7 splicing. Nat. Commun. 2021, 12, 428. [Google Scholar] [CrossRef] [PubMed]
- Farouk, I.A.; Low, Z.Y.; Yip, A.J.W.; Lal, S.K. Pabpc1: A novel emerging target for cancer prognostics and anti-cancer. In Molecular Biomarkers for Cancer Diagnosis and Therapy; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Su, R.; Ma, J.; Zheng, J.; Liu, X.; Liu, Y.; Ruan, X.; Shen, S.; Yang, C.; Wang, D.; Cai, H.; et al. PABPC1-induced stabilization of BDNF-AS inhibits malignant progression of glioblastoma cells through STAU1-mediated decay. Cell Death Dis. 2020, 11, 81. [Google Scholar] [CrossRef]
- Mazan-Mamczarz, K.; Lal, A.; Martindale, J.L.; Kawai, T.; Gorospe, M. Translational Repression by RNA-Binding Protein TIAR. Mol. Cell. Biol. 2006, 26, 2716–2727. [Google Scholar] [CrossRef] [PubMed]
- Loughlin, F.E.; Mansfield, R.E.; Vaz, P.M.; McGrath, A.P.; Setiyaputra, S.; Gamsjaeger, R.; Chen, E.S.; Morris, B.J.; Guss, J.M.; Mackay, J.P. The zinc fingers of the SR-like protein ZRANB2 are single-stranded RNA-binding domains that recognize 5′ splice site-like sequences. Proc. Natl. Acad. Sci. USA 2009, 106, 5581–5586. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Yang, Y.T.; Xu, X.D.; Chen, J.S.; Chen, T.L.; Chen, H.J.; Zhu, Y.B.; Lin, J.Y.; Li, Y.; et al. IGFBP2 promotes vasculogenic mimicry formation via regulating CD144 and MMP2 expression in glioma. Oncogene 2018, 38, 1815–1831. [Google Scholar] [CrossRef]
- Jungfleisch, J.; Gebauer, F. RNA-binding proteins as therapeutic targets in cancer. RNA Biol. 2025, 22, 1–8. [Google Scholar] [CrossRef]
- Schmeing, S.; Hart, P.t. Challenges in Therapeutically Targeting the RNA-Recognition Motif. Wiley Interdiscip. Rev. RNA 2024, 15, e1877. [Google Scholar] [CrossRef]
- Tang, Y.T.; Li, Y.; Chu, P.; Ma, X.D.; Tang, Z.Y.; Sun, Z.L. Molecular biological mechanism of action in cancer therapies: Juglone and its derivatives, the future of development. Biomed. Pharmacother. 2022, 148, 112785. [Google Scholar] [CrossRef]
- Guo, F.; Ling, G.; Qiu, J.; Li, J.; Gan, Y.; Yu, Y.; Tang, J.; Mo, L.; Piao, H. Juglone induces ferroptosis in glioblastoma cells by inhibiting the Nrf2-GPX4 axis through the phosphorylation of p38MAPK. Chin. Med. 2024, 19, 52. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Xu, Y.; Zhang, J.; Zhu, W.; Zhang, Y.; Chen, L.; Hua, W.; Mao, Y. Juglone induces apoptosis of tumor stem-like cells through ROS-p38 pathway in glioblastoma. BMC Neurol. 2017, 17, 70. [Google Scholar] [CrossRef]
- Paulsen, M.T.; Ljungman, M. The natural toxin juglone causes degradation of p53 and induces rapid H2AX phosphorylation and cell death in human fibroblasts. Toxicol. Appl. Pharmacol. 2005, 209, 1–9. [Google Scholar] [CrossRef]
- Filippova, N.; Yang, X.; Ananthan, S.; Sorochinsky, A.; Hackney, J.R.; Gentry, Z.; Bae, S.; King, P.; Nabors, L.B. Hu antigen R (HuR) multimerization contributes to glioma disease progression. J. Biol. Chem. 2017, 292, 16999–17010. [Google Scholar] [CrossRef]
- Chellappan, R.; Guha, A.; Si, Y.; Kwan, T.; Nabors, L.B.; Filippova, N.; Yang, X.; Myneni, A.S.; Meesala, S.; Harms, A.S.; et al. SRI-42127, a novel small molecule inhibitor of the RNA regulator HuR, potently attenuates glial activation in a model of lipopolysaccharide-induced neuroinflammation. Glia 2021, 70, 155–172. [Google Scholar] [CrossRef]
- Wang, J.; Hjelmeland, A.B.; Nabors, L.B.; King, P.H. Anti-cancer effects of the HuR inhibitor, MS-444, in malignant glioma cells. Cancer Biol. Ther. 2019, 20, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Filippova, N.; Yang, X.; Ananthan, S.; Calano, J.; Pathak, V.; Bratton, L.; Vekariya, R.H.; Zhang, S.; Ofori, E.; Hayward, E.N.; et al. Targeting the HuR Oncogenic Role with a New Class of Cytoplasmic Dimerization Inhibitors. Cancer Res. 2021, 81, 2220–2233. [Google Scholar] [CrossRef] [PubMed]
- Denichenko, P.; Mogilevsky, M.; Cléry, A.; Welte, T.; Biran, J.; Shimshon, O.; Barnabas, G.D.; Danan-Gotthold, M.; Kumar, S.; Yavin, E.; et al. Specific inhibition of splicing factor activity by decoy RNA oligonucleotides. Nat. Commun. 2019, 10, 1590. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Fan, Y.; Wang, Q.; Li, B.; Qiu, W.; Qi, Y.; Pan, Z.; Zhang, S.; Zhao, S.; Yang, K.; et al. Glioblastoma upregulates SUMOylation of hnRNP A2/B1 to eliminate the tumor suppressor miR-204-3p, accelerating angiogenesis under hypoxia. Cell Death Dis. 2023, 14, 147. [Google Scholar] [CrossRef]
- Shen, L.; Chen, Y.-L.; Huang, C.-C.; Shyu, Y.-C.; Seftor, R.E.B.; Seftor, E.A.; Hendrix, M.J.C.; Chien, D.-S.; Chu, Y.-W. CVM-1118 (foslinanib), a 2-phenyl-4-quinolone derivative, promotes apoptosis and inhibits vasculogenic mimicry via targeting TRAP1. Pathol. Oncol. Res. 2023, 29, 1611038. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Su, W.-C.; Lakhani, N.; Gutheil, J.; Melink, T.J.; Chu, Y.-W.; Chen, Y.-L.; Chien, D.-S. Phase I dose escalation study of CVM-1118, a novel anti-vascular mimicry agent, in patients with advanced cancers. J. Clin. Oncol. 2017, 35, 2580. [Google Scholar] [CrossRef]
- Su, W.-C.; Chen, M.H.; Bai, L.-Y.; Chen, J.-S.; Shih, Y.-H.; Wu, I.-C.; Gutheil, J.; Melink, T.J.; Chu, Y.-W.; Chen, Y.-L.; et al. CVM-1118: A potent oral anti-vasculogenic mimicry (VM) agent in patients with advanced neuroendocrine tumors (NETs)—A phase IIa study. J. Clin. Oncol. 2023, 41, e16235. [Google Scholar] [CrossRef]
- Maddison, K.; Bowden, N.A.; Graves, M.C.; Tooney, P.A. Characteristics of vasculogenic mimicry and tumour to endothelial transdifferentiation in human glioblastoma: A systematic review. BMC Cancer 2023, 23, 185. [Google Scholar] [CrossRef]
- Valdivia, A.; Mingo, G.; Aldana, V.; Pinto, M.P.; Ramirez, M.; Retamal, C.; Gonzalez, A.; Nualart, F.; Corvalan, A.H.; Owen, G.I. Fact or Fiction, It Is Time for a Verdict on Vasculogenic Mimicry? Front. Oncol. 2019, 9, 680. [Google Scholar] [CrossRef]
- Janesick, A.; Shelansky, R.; Gottscho, A.D.; Wagner, F.; Williams, S.R.; Rouault, M.; Beliakoff, G.; Morrison, C.A.; Oliveira, M.F.; Sicherman, J.T.; et al. High resolution mapping of the tumor microenvironment using integrated single-cell, spatial and in situ analysis. Nat. Commun. 2023, 14, 8353. [Google Scholar] [CrossRef]
- He, S.; Bhatt, R.; Brown, C.; Brown, E.A.; Buhr, D.L.; Chantranuvatana, K.; Danaher, P.; Dunaway, D.; Garrison, R.G.; Geiss, G.; et al. High-plex imaging of RNA and proteins at subcellular resolution in fixed tissue by spatial molecular imaging. Nat. Biotechnol. 2022, 40, 1794–1806. [Google Scholar] [CrossRef] [PubMed]
- Sahadevan, S.; Pérez-Berlanga, M.; Polymenidou, M. Identification of RNA–RBP Interactions in Subcellular Compartmen. In The Integrated Stress Response: Methods and Protocols; Methods in Molecular Biology 2428; Humana Press: New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Grozhik, A.V.; Linder, B.; Olarerin-George, A.O.; Jaffrey, S.R. Mapping m6a at individual-nucleotide resolution using crosslinking and immunoprecipitation (miclip). In RNA Methylation: Methods and Protocols; Methods in Molecular Biology 1562; Humana Press: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- VanInsberghe, M.; Berg, J.v.D.; Andersson-Rolf, A.; Clevers, H.; van Oudenaarden, A. Single-cell Ribo-seq reveals cell cycle-dependent translational pausing. Nature 2021, 597, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.D. DART-seq: An antibody-free method for global m6A detection. Nat. Methods 2019, 16, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, Y.-M.; Zou, Z.; Ye, C.; Dou, X.; Wu, J.; Liu, C.; Liu, S.; Yan, H.; Wang, P.; et al. Profiling of RNA-binding protein binding sites by in situ reverse transcription-based sequencing. Nat. Methods 2024, 21, 247–258. [Google Scholar] [CrossRef]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Jill, P.; Alexe, G.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef]
- Lobbous, M.; Bernstock, J.D.; Coffee, E.; Friedman, G.K.; Metrock, L.K.; Chagoya, G.; Elsayed, G.; Nakano, I.; Hackney, J.R.; Korf, B.R.; et al. An Update on Neurofibromatosis Type 1-Associated Gliomas. Cancers 2020, 12, 114. [Google Scholar] [CrossRef]
- Xu, H.; Cao, Y.; Ruan, J.; Wang, F.; He, Y.; Yang, L.; Yu, T.; Du, F.; Zhang, N.; Cao, X. The effects of BMP2 and the mechanisms involved in the invasion and angiogenesis of IDH1 mutant glioma cells. J. Neuro-Oncol. 2024, 170, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, W.; Yuan, J.; Qiang, B.; Han, W.; Peng, X. Integrated Analysis of RNA-Binding Proteins in Glioma. Cancers 2020, 12, 892. [Google Scholar] [CrossRef] [PubMed]
- Choate, K.A.; Pratt, E.P.S.; Jennings, M.J.; Winn, R.J.; Mann, P.B. IDH Mutations in Glioma: Molecular, Cellular, Diagnostic, and Clinical Implications. Biology 2024, 13, 885. [Google Scholar] [CrossRef]
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Moving beyond Rules: The Development of a Central Nervous System Multiparameter Optimization (CNS MPO) Approach To Enable Alignment of Druglike Properties. ACS Chem. Neurosci. 2010, 1, 435–449. [Google Scholar] [CrossRef]
- Urbina, F.; Zorn, K.M.; Brunner, D.; Ekins, S. Comparing the Pfizer Central Nervous System Multiparameter Optimization Calculator and a BBB Machine Learning Model. ACS Chem. Neurosci. 2021, 12, 2247–2253. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, B.; Li, J.; Guo, Z.; Zhang, C.; Chen, X.; Ma, L.; Wang, Z.; Yang, H.; Li, Y.; et al. Bioengineered protein nanocarrier facilitating siRNA escape from lysosomes for targeted RNAi therapy in glioblastoma. Sci. Adv. 2025, 11, eadr9266. [Google Scholar] [CrossRef]
- Naveed, S.; Husnain, M.; Samad, A.; Ikram, A.; Afreen, H.; Gilanie, G.; Alsubaie, N. Drug Efficacy Recommendation System of Glioblastoma (GBM) Using Deep Learning. IEEE Access 2024, 13, 10398–10411. [Google Scholar] [CrossRef]
- Anwer, M.S.; Abdel-Rasol, M.A.; El-Sayed, W.M. Emerging therapeutic strategies in glioblastsoma: Drug repurposing, mechanisms of resistance, precision medicine, and technological innovations. Clin. Exp. Med. 2025, 25, 117. [Google Scholar] [CrossRef]
- Yan, J.; Kang, D.D.; Turnbull, G.; Dong, Y. Delivery of CRISPR-Cas9 system for screening and editing RNA binding proteins in cancer. Adv. Drug Deliv. Rev. 2021, 180, 114042. [Google Scholar] [CrossRef]
- Capozzi, A.; Riitano, G.; Recalchi, S.; Manganelli, V.; Costi, R.; Saccoliti, F.; Pulcinelli, F.; Garofalo, T.; Misasi, R.; Longo, A.; et al. Effect of heparanase inhibitor on tissue factor overexpression in platelets and endothelial cells induced by anti-β2-GPI antibodies. J. Thromb. Haemost. 2021, 19, 2302–2313. [Google Scholar] [CrossRef]
- Manganelli, V.; Misasi, R.; Riitano, G.; Capozzi, A.; Mattei, V.; Caglar, T.R.; Ialongo, D.; Madia, V.N.; Messore, A.; Costi, R.; et al. Role of a Novel Heparanase Inhibitor on the Balance between Apoptosis and Autophagy in U87 Human Glioblastoma Cells. Cells 2023, 12, 1891. [Google Scholar] [CrossRef]

| RBP | Expression Level in GBM | Effect of the RBP on GBM VM | Effect of the RBP on Target Transcripts Involved in GBM VM | Cell Lines | Experimental Models Verifying VM Formation | Citation |
|---|---|---|---|---|---|---|
| HuR | Increased | Promoted | ↑ VEGFA mRNA | U251 | 3D sphere sprouting assay, tube formation assay | [68] |
| PUM2 | Decreased | Suppressed | ↓ CEBPD mRNA | U251, U373 | Tube formation assay, nude mouse xenograft | [69] |
| HNRNP A2/B1 | Increased | Promoted | ↑ NFATC3 mRNA | U251, U373 | Tube formation assay, nude mouse xenograft | [70] |
| HNRNP D | Increased | Promoted | ↑ ZHX2 mRNA | U87, U251 | Tube formation assay, nude mouse xenograft | [71] |
| RALY | Increased | Promoted | ↑ FOXD1 mRNA | U251, U373 | Tube formation assay, nude mouse xenograft | [72] |
| RBMS3 | Decreased | Suppressed | ↓ circHECTD1 | U87, U251 | Tube formation assay, nude mouse xenograft | [73] |
| SRSF1 | Increased | Promoted | ↑ circCMTM3 | Patient-derived | Tube formation assay, nude mouse xenograft | [74] |
| SRSF7 | Increased | Promoted | ↑ circPLEKHA5 | U251, U373 | Tube formation assay, nude mouse xenograft | [75] |
| PABPC5 | Increased | Promoted | ↑ HCG15 | U87, U251 | Tube formation assay, nude mouse xenograft | [76] |
| TIAR | Decreased | Suppressed | ↓ LOXL1-AS1 | U87, U251 | Tube formation assay, nude mouse xenograft | [77] |
| ZRANB2 | Increased | Promoted | ↑ SNHG20 | U87, U251 | Tube formation assay, nude mouse xenograft | [78] |
| IGF2BP2 | Increased | Promoted | ↑ OIP5-AS1 | U87, U251 | Tube formation assay, nude mouse xenograft | [79] |
| METTL3 | Uncertain | Suppressed | -- | U87 | Tube formation assay, nude mouse xenograft | [80] |
| Promoted | ↑ HOTAIRM1 | U87, U251 | Tube formation assay, nude mouse xenograft | [81] | ||
| Promotion | ↑ BUD13 mRNA | U251, U373 | Tube formation assay, nude mouse xenograft | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoi, P.K.; Liu, X.; Wong, M.D.; Lin, L.-T. The Roles of RNA-Binding Proteins in Vasculogenic Mimicry Regulation in Glioblastoma. Int. J. Mol. Sci. 2025, 26, 7976. https://doi.org/10.3390/ijms26167976
Tsoi PK, Liu X, Wong MD, Lin L-T. The Roles of RNA-Binding Proteins in Vasculogenic Mimicry Regulation in Glioblastoma. International Journal of Molecular Sciences. 2025; 26(16):7976. https://doi.org/10.3390/ijms26167976
Chicago/Turabian StyleTsoi, Pok Kong, Xian Liu, Man Ding Wong, and Liang-Ting Lin. 2025. "The Roles of RNA-Binding Proteins in Vasculogenic Mimicry Regulation in Glioblastoma" International Journal of Molecular Sciences 26, no. 16: 7976. https://doi.org/10.3390/ijms26167976
APA StyleTsoi, P. K., Liu, X., Wong, M. D., & Lin, L.-T. (2025). The Roles of RNA-Binding Proteins in Vasculogenic Mimicry Regulation in Glioblastoma. International Journal of Molecular Sciences, 26(16), 7976. https://doi.org/10.3390/ijms26167976







