Cholesterol-Conjugated Polyion Complex Nanoparticles for Combination Delivery of Hydrophobic Paclitaxel and Hydrophilic miR-34a for Colon Cancer Therapy
Abstract
1. Introduction
2. Results and Discussion
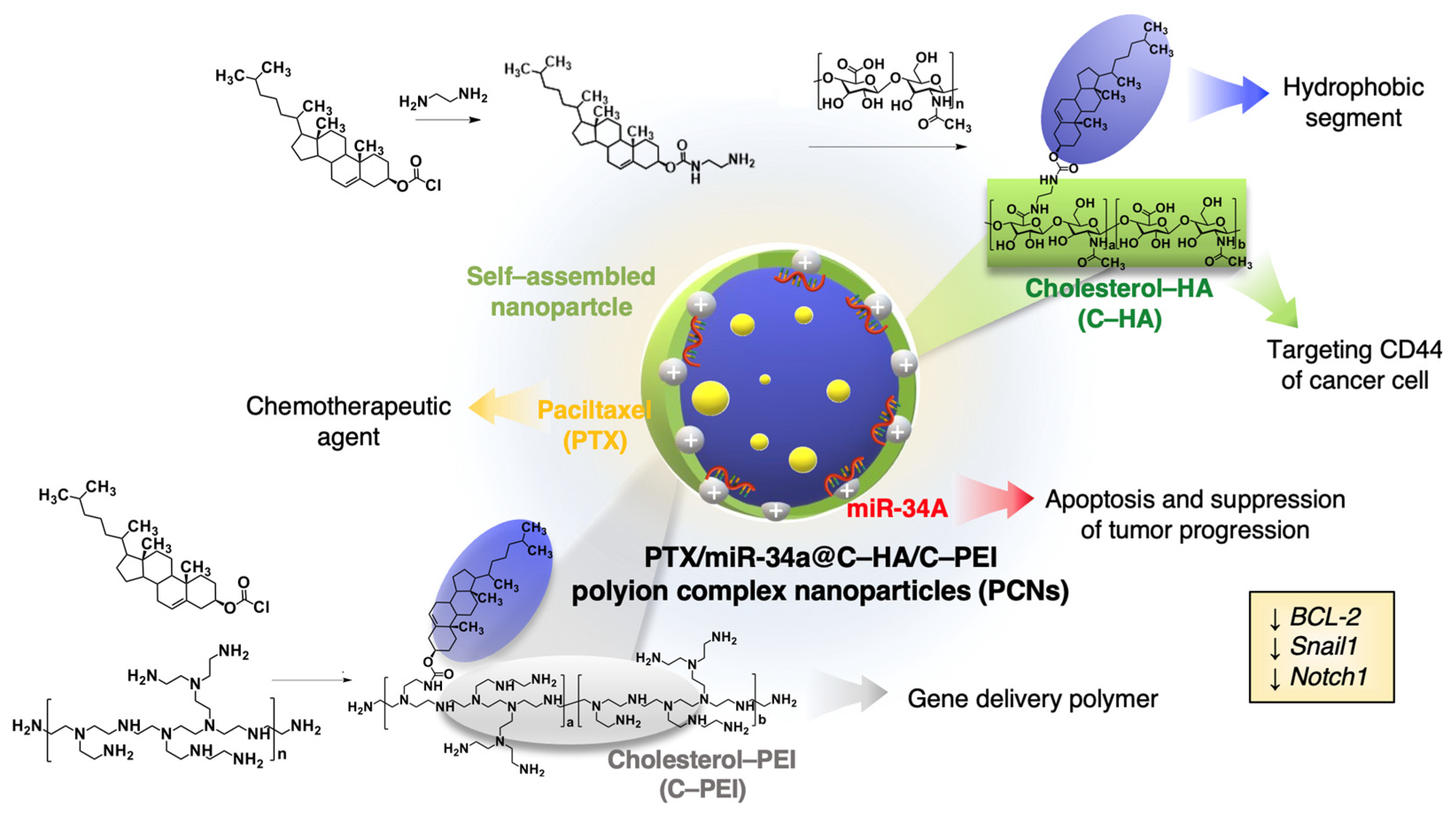
2.1. Preparation of Polyion Complex Nanocarriers
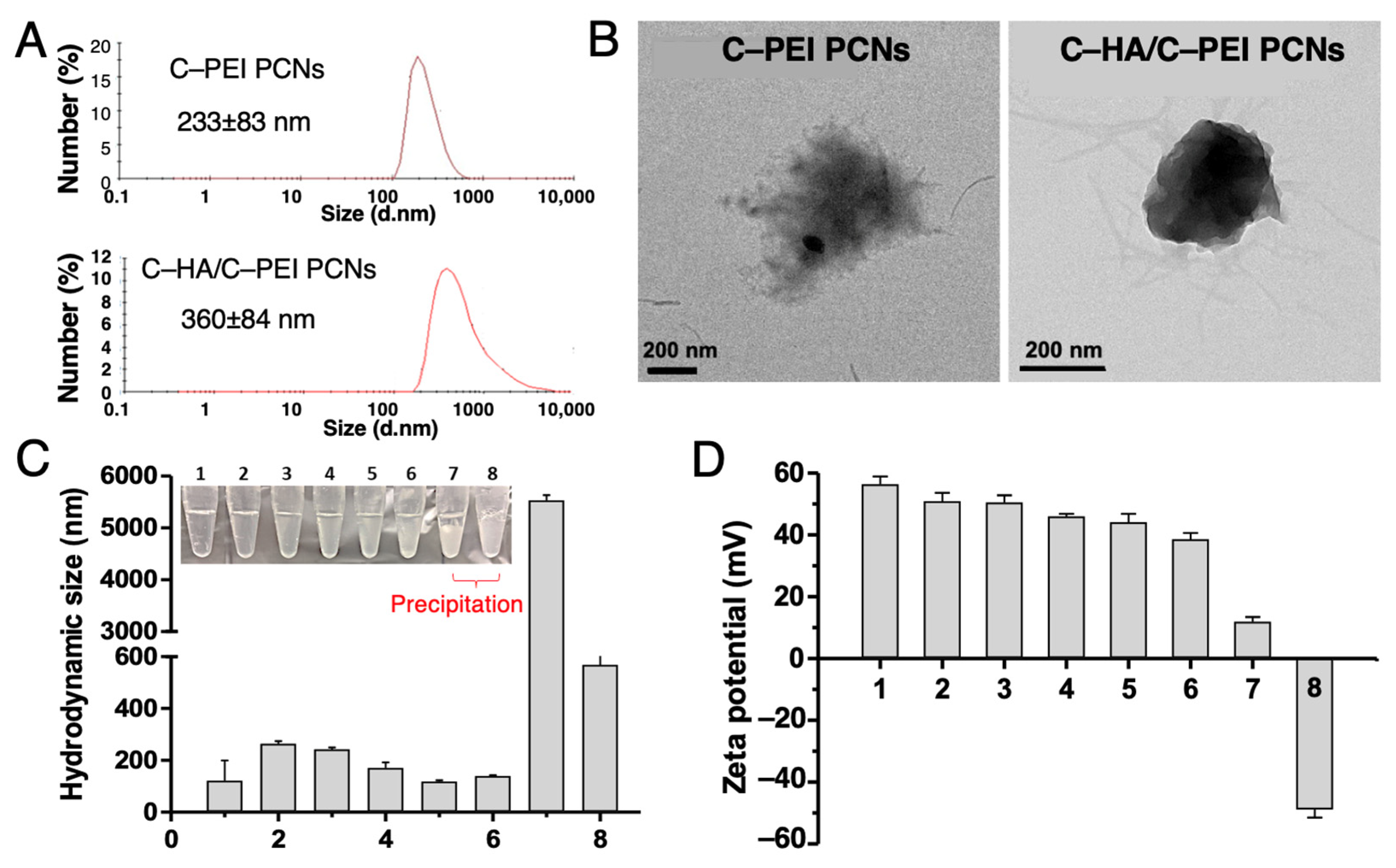
2.2. Characterization of Nanocarriers and Encapsulation Function for PTX and miR-34A
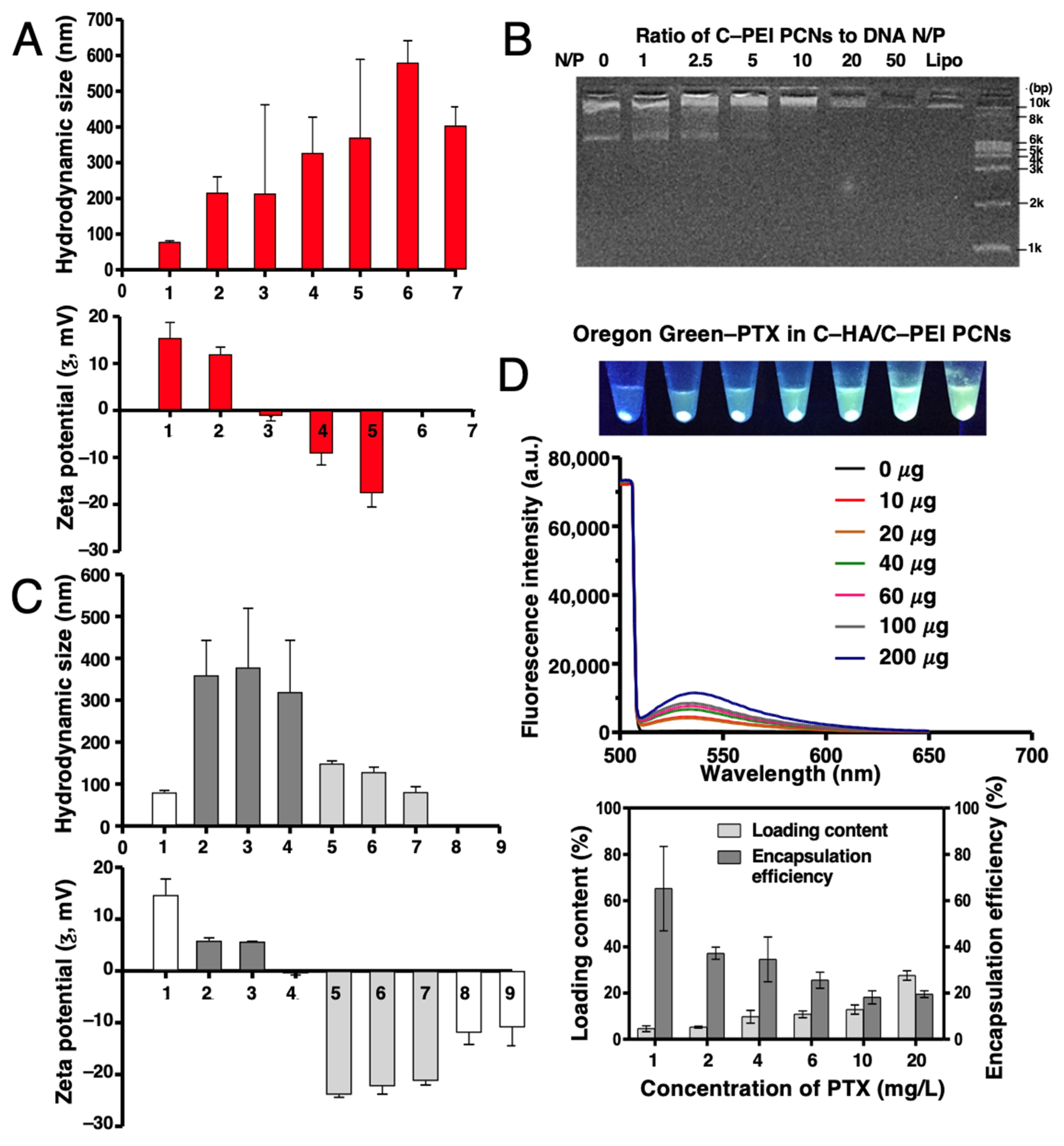
2.3. Optimization and Characterization of DNA and Paclitaxel Encapsulation in C–HA/C–PEI Polyion Complex Nanoparticles
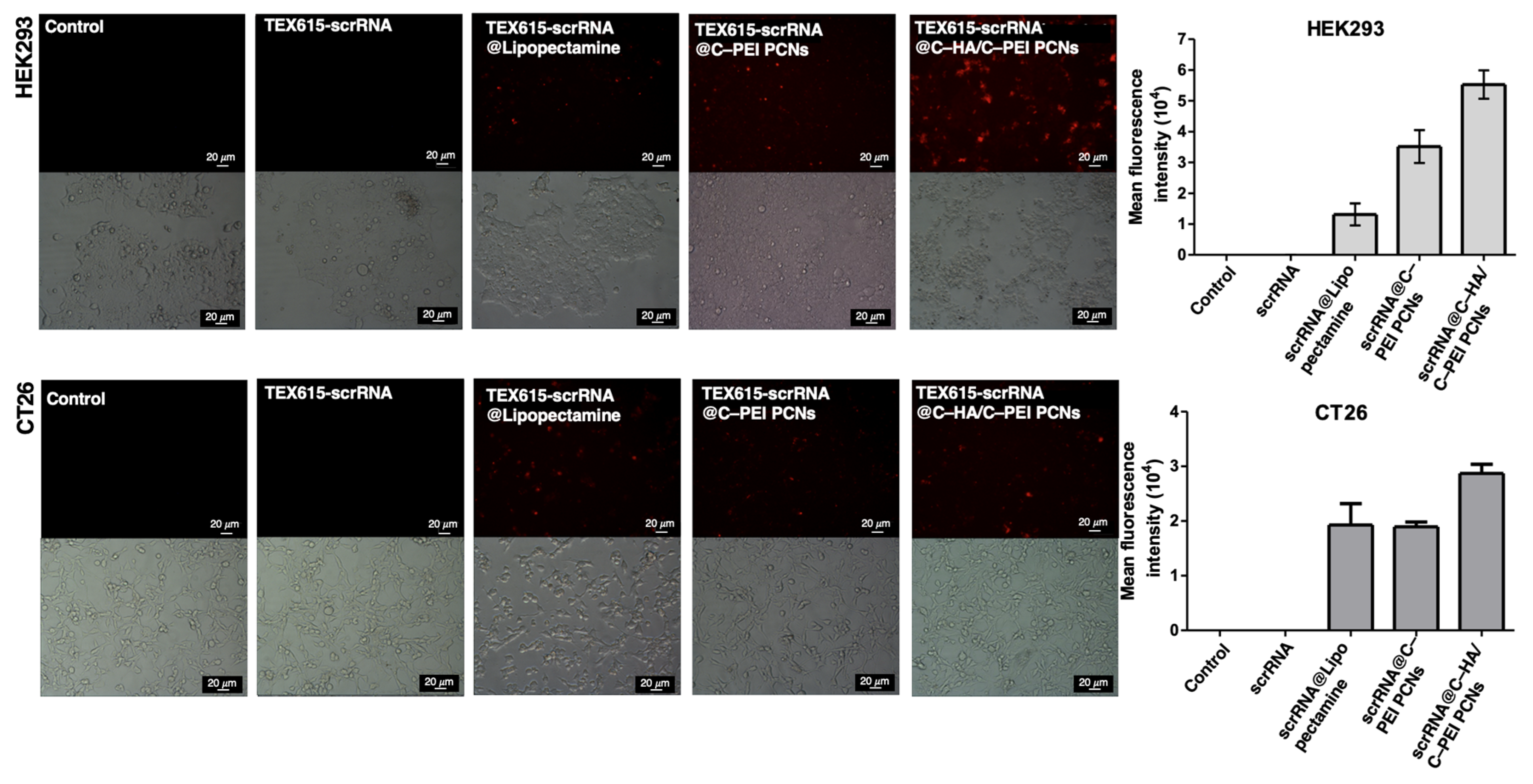
2.4. In Vitro Cellular Uptake of Polyion Complex Nanoparticles
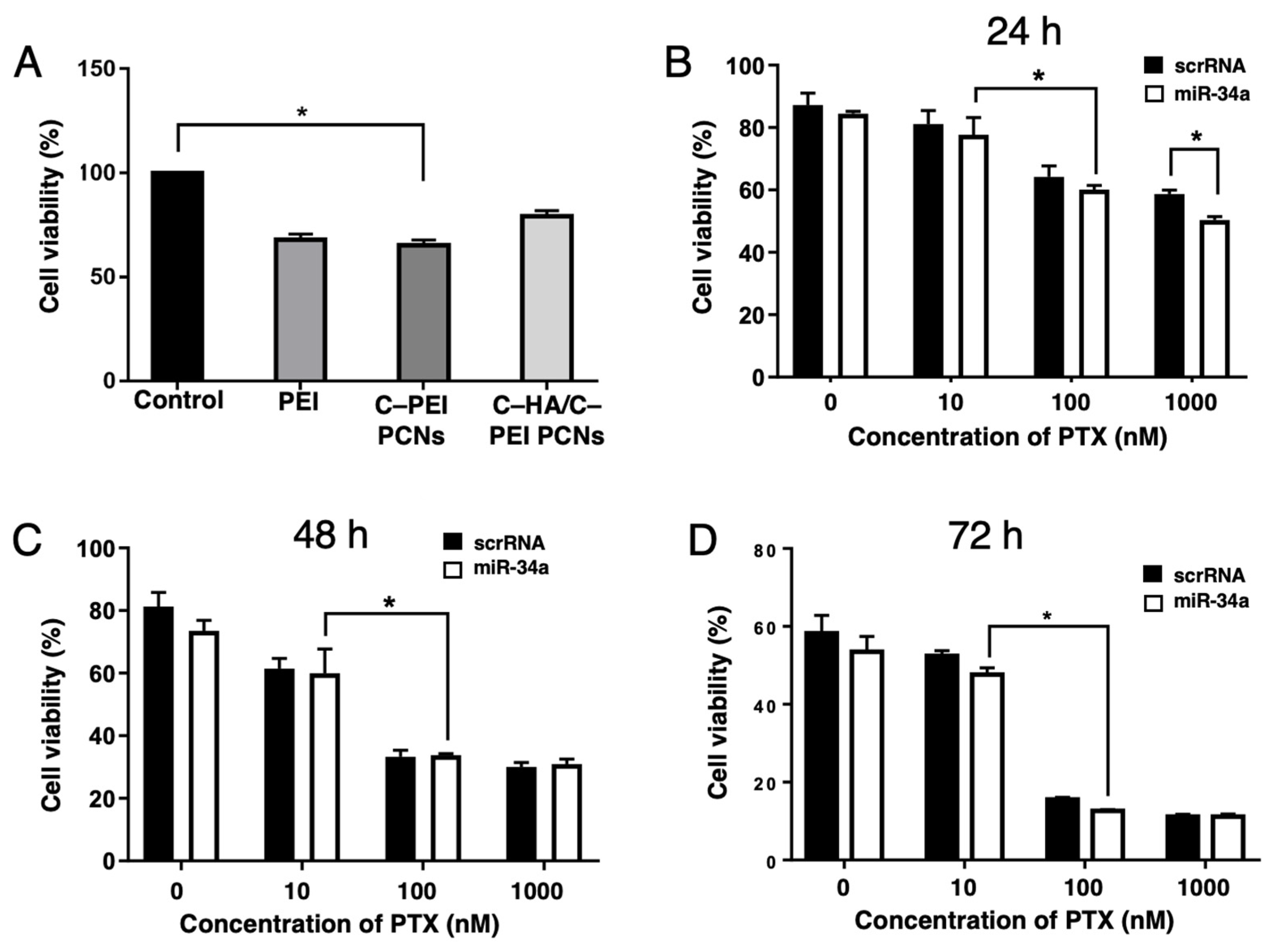
2.5. Evaluation of In Vitro Cytotoxicity and Gene Transfection Efficiency of Polyion Complex Nanoparticles
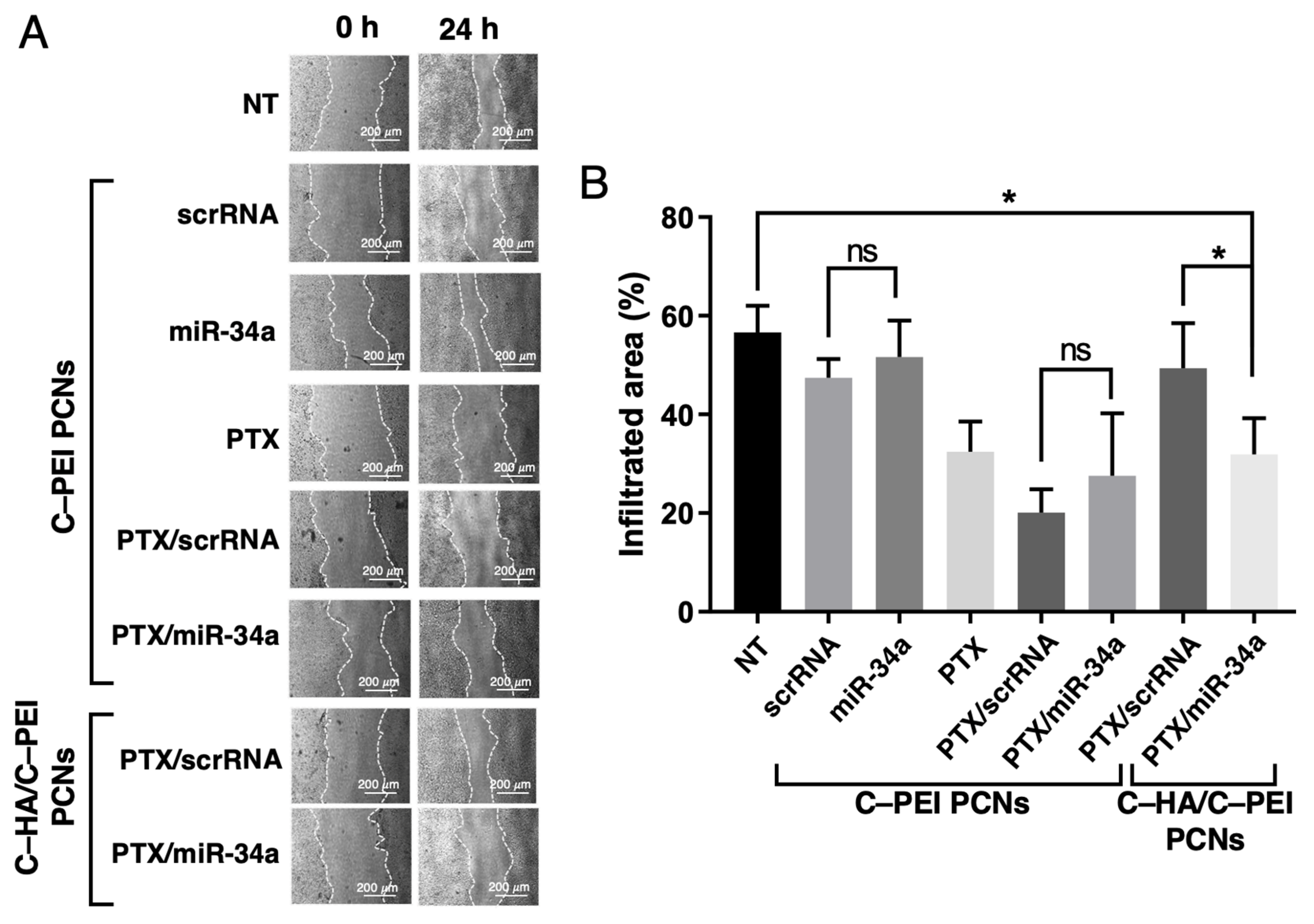
2.6. Cell Migration Assay of HCT116 Cells Treated with PTX and miR-34a Co-Delivery System
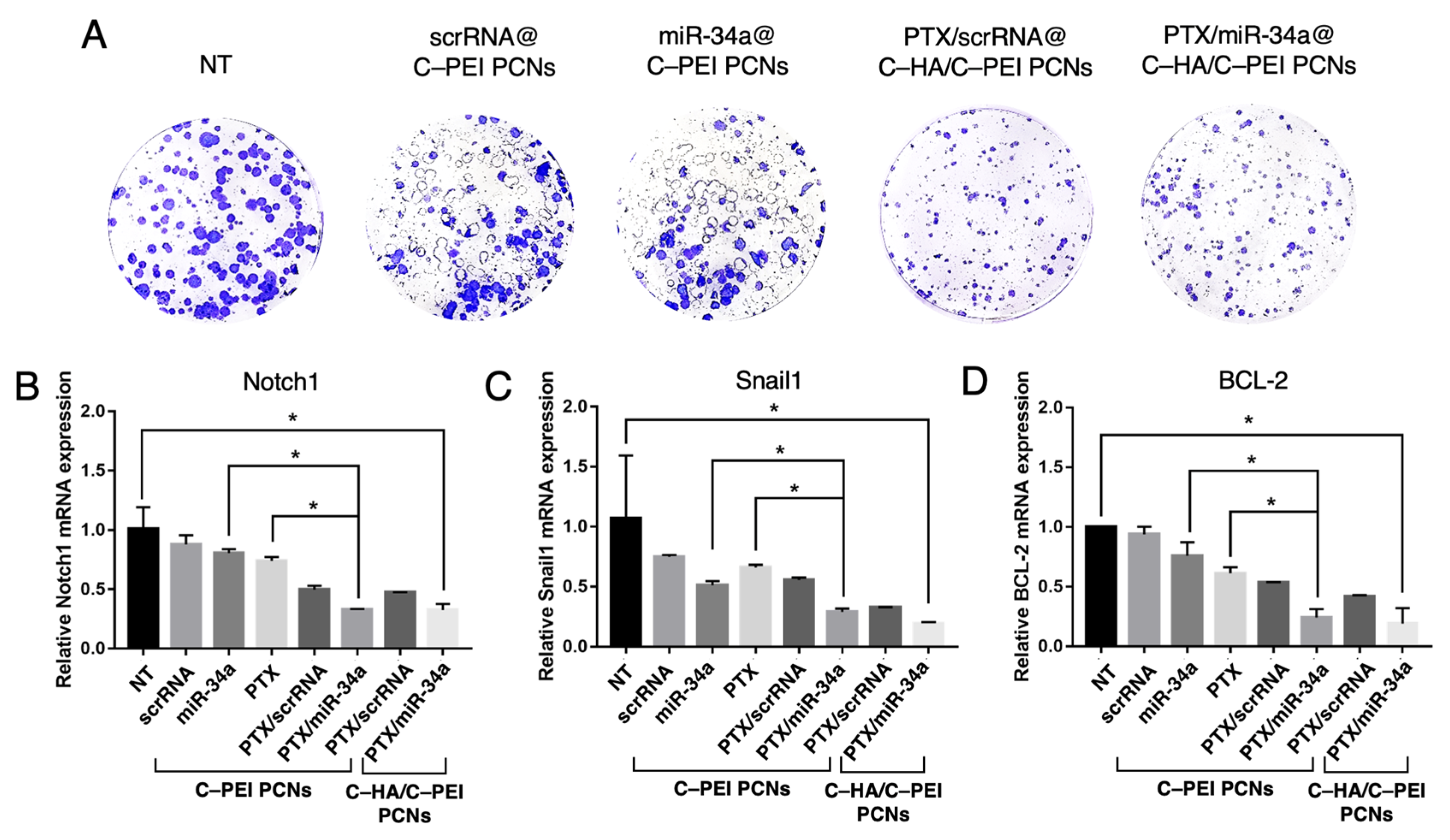
2.7. Clonogenic Assay and Gene Expression Analysis
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Amine-Modified Cholesterol
3.3. Synthesis of Cholesterol-Conjugated Hyaluronic Acid (C–HA)
3.4. Synthesis of Cholesterol-Conjugated Polyethyleneimine (C–PEI)
3.5. Loading of Paclitaxel (PTX) and Nucleic Acids into C–PEI and C–HA Polyion Complex Nanoparticles (C–HA/C–PEI PCNs)
3.6. Characterization of C–HA/C–PEI PCNs
3.7. Cell Culture
3.8. Cell Viability Test
3.9. Cellular Uptake
3.10. Wound Healing Assay
3.11. Colony-Forming Assay
3.12. Reverse Transcription Polymerase Chain Reaction (RT-PCR) for Snail1, Notch1, and BCL-2
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C–HA | Cholesterol-conjugated hyaluronic acid |
| C–PEI | Cholesterol-conjugated polyethylenimine |
| C–HA/C–PEI PCNs | Cholesterol-conjugated polyethylenimine and cholesterol-conjugated hyaluronic acid polyion complex nanoparticles |
| CRC | Colorectal cancer |
| HA | Hyaluronic acid |
| PEI | Polyethylenimine |
| PCNs | Polyion complex nanoparticles |
| PTX | Paclitaxel |
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef] [PubMed]
- Argiles, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Kerr, J.; Anderson, C.; Lippman, S.M. Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. Lancet Oncol. 2017, 18, E457–E471. [Google Scholar] [CrossRef] [PubMed]
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Claney, T.E.; Zhang, X.Y.; Cai, D.N.; Neuberg, D.; Huang, K.; Guevara, F.; Nelson, T.; et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017, 358, 1443–1448. [Google Scholar] [CrossRef]
- Pleguezuelos-Manzano, C.; Puschhof, J.; Huber, A.R.; van Hoeck, A.; Wood, H.M.; Nomburg, J.; Gurjao, C.; Manders, F.; Dalmasso, G.; Stege, P.B.; et al. Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nature 2020, 580, 269–273. [Google Scholar] [CrossRef]
- Asare, E.A.; Evans, D.B.; Erickson, B.A.; Aburajab, M.; Tolat, P.; Tsai, S. Neoadjuvant treatment sequencing adds value to the care of patients with operable pancreatic cancer. J. Surg. Oncol. 2016, 114, 291–295. [Google Scholar] [CrossRef]
- Lee, J.C.; Ahn, S.; Paik, K.H.; Kim, H.W.; Kang, J.; Kim, J.; Hwang, J.H. Clinical impact of neoadjuvant treatment in resectable pancreatic cancer: A systematic review and meta-analysis protocol. BMJ Open 2016, 6, e010491. [Google Scholar] [CrossRef]
- Sati, P.; Sharma, E.; Dhyani, P.; Attri, D.C.; Rana, R.; Kiyekbayeva, L.; Büsselberg, D.; Samuel, S.M.; Sharifi-Rad, J. Paclitaxel and its semi-synthetic derivatives: Comprehensive insights into chemical structure, mechanisms of action, and anticancer properties. Eur. J. Med. Res. 2024, 29, 90. [Google Scholar] [CrossRef]
- Wall, M.E.; Wani, M.C. Camptothecin and taxol: From discovery to clinic. J. Ethnopharmacol. 1996, 51, 239–253. [Google Scholar] [CrossRef]
- Cavallaro, G.; Licciardi, M.; Caliceti, P.; Salmaso, S.; Giammona, G. Synthesis, physico-chemical and biological characterization of a paclitaxel macromolecular prodrug. Eur. J. Pharm. Biopharm. 2004, 58, 151–159. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, X.Y.; Mei, H.; Wang, Y.; Liao, Z.W.; Chen, J.; Zhang, Q.Z.; Hu, Y.; Pang, Z.Q.; Jiang, X.G. LDLR-mediated peptide-22-conjugated nanoparticles for dual-targeting therapy of brain glioma. Biomaterials 2013, 34, 9171–9182. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Patra, J.K.; Singh, Y.D.; Panda, M.K.; Das, G.; Adetunji, C.O.; Michael, O.S.; Sytar, O.; Polito, L. Paclitaxel: Application in modern oncology and nanomedicine-based cancer therapy. Oxid. Med. Cell. Longev. 2021, 2021, 3687700. [Google Scholar] [CrossRef]
- Dorton, B.J.; Elias, K.M.; Growdon, W.; Horowitz, N.S. Biomarkers in Gynecologic Cancers Red cell distribution width (RDW) as a novel marker for predicting recurrence of high-grade cervical dysplasia or carcinoma in active smokers. Gynecol. Oncol. 2015, 136, 400. [Google Scholar] [CrossRef]
- Kong, Y.W.; Ferland-McCollough, D.; Jackson, T.J.; Bushell, M. microRNAs in cancer management. Lancet Oncol. 2012, 13, E249–E258. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M. miRNAs versus oncogenes: The power of social networking. Mol. Syst. Biol. 2012, 8, 569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Mo, R.; Yin, B.D.; Zhou, L.B.; Liu, Y.C.; Fan, J. Tumor suppressor microRNA-34a inhibits cell proliferation by targeting Notch1 in renal cell carcinoma. Oncol. Lett. 2014, 7, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Ren, Z.J.; Tang, J.H. MicroRNA-34a: A potential therapeutic target in human cancer. Cell Death Dis. 2014, 5, e1327. [Google Scholar] [CrossRef]
- Bader, A.G.; Brown, D.; Winkler, M. The Promise of MicroRNA Replacement Therapy. Cancer Res. 2010, 70, 7027–7030. [Google Scholar] [CrossRef]
- Joshua, B.; Kaplan, M.J.; Doweck, I.; Pai, R.; Weissman, I.L.; Prince, M.E.; Ailles, L.E. Frequency of cells expressing CD44, a Head and Neck cancer stem cell marker: Correlation with tumor aggressiveness. Head Neck 2012, 34, 42–49. [Google Scholar] [CrossRef]
- Liu, C.; Kelnar, K.; Liu, B.G.; Chen, X.; Calhoun-Davis, T.; Li, H.W.; Patrawala, L.; Yan, H.; Jeter, C.; Honorio, S.; et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 2011, 17, 211–215. [Google Scholar] [CrossRef]
- Bu, P.; Chen, K.-Y.; Chen, J.H.; Wang, L.; Walters, J.; Shin, Y.J.; Goerger, J.P.; Sun, J.; Witherspoon, M.; Rakhilin, N. A microRNA miR-34a-regulated bimodal switch targets Notch in colon cancer stem cells. Cell Stem Cell 2013, 12, 602–615. [Google Scholar] [CrossRef]
- Shi, S.; Han, L.; Deng, L.; Zhang, Y.; Shen, H.; Gong, T.; Zhang, Z.; Sun, X. Dual drugs (microRNA-34a and paclitaxel)-loaded functional solid lipid nanoparticles for synergistic cancer cell suppression. J. Control. Release 2014, 194, 228–237. [Google Scholar] [CrossRef]
- Choosakoonkriang, S.; Lobo, B.A.; Koe, G.S.; Koe, J.G.; Middaugh, C.R. Biophysical characterization of PEI/DNA complexes. J. Pharm. Sci. 2003, 92, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Y.; Nie, Y.; Xie, L.; Song, H.M.; Gu, Z.W. p53 mediated apoptosis by reduction sensitive shielding ternary complexes based on disulfide linked PEI ternary complexes. Biomaterials 2014, 35, 1657–1666. [Google Scholar] [CrossRef]
- Lee, T.; Lim, E.K.; Lee, J.; Kang, B.; Choi, J.; Park, H.S.; Suh, J.S.; Huh, Y.M.; Haam, S. Efficient CD44-targeted magnetic resonance imaging (MRI) of breast cancer cells using hyaluronic acid (HA)-modified MnFe2O4 nanocrystals. Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Miao, W.; Shim, G.; Kang, C.M.; Lee, S.; Choe, Y.S.; Choi, H.G.; Oh, Y.K. Cholesteryl hyaluronic acid-coated, reduced graphene oxide nanosheets for anti-cancer drug delivery. Biomaterials 2013, 34, 9638–9647. [Google Scholar] [CrossRef]
- Kulig, W.; Jurkiewicz, P.; Olżyńska, A.; Tynkkynen, J.; Javanainen, M.; Manna, M.; Rog, T.; Hof, M.; Vattulainen, I.; Jungwirth, P. Experimental determination and computational interpretation of biophysical properties of lipid bilayers enriched by cholesteryl hemisuccinate. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 422–432. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, B. Polyethyleneimine-based drug delivery systems for cancer theranostics. J. Funct. Biomater. 2022, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Geng, J.; An, P.; Xu, Y.; Huang, J.; Lu, W.; Liu, S.; Yu, J. Cathepsin B-sensitive cholesteryl hemisuccinate–gemcitabine prodrug nanoparticles: Enhanced cellular uptake and intracellular drug controlled release. RSC Adv. 2015, 5, 6985–6992. [Google Scholar] [CrossRef]
- Cho, S.; Dang, C.; Wang, X.; Ragan, R.; Kwon, Y. Mixing-sequence-dependent nucleic acid complexation and gene transfer efficiency by polyethylenimine. Biomater. Sci. 2015, 3, 1124–1133. [Google Scholar] [CrossRef]
- Yang, J.; Huang, L. Time-dependent maturation of cationic liposome–DNA complex for serum resistance. Gene Ther. 1998, 5, 380–387. [Google Scholar] [CrossRef]
- Shi, S.J.; Han, L.; Gong, T.; Zhang, Z.R.; Sun, X. Systemic Delivery of microRNA-34a for Cancer Stem Cell Therapy. Angew. Chem. Int. Ed. 2013, 52, 3901–3905. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Guessous, F.; Zhang, Y.; DiPierro, C.; Kefas, B.; Johnson, E.; Marcinkiewicz, L.; Jiang, J.M.; Yang, Y.Z.; Schmittgen, T.D.; et al. MicroRNA-34a Inhibits Glioblastoma Growth by Targeting Multiple Oncogenes. Cancer Res. 2009, 69, 7569–7576. [Google Scholar] [CrossRef]
- Pang, R.T.K.; Leung, C.O.N.; Ye, T.M.; Liu, W.M.; Chiu, P.C.N.; Lam, K.K.W.; Lee, K.F.; Yeung, W.S.B. MicroRNA-34a suppresses invasion through downregulation of Notch1 and Jagged1 in cervical carcinoma and choriocarcinoma cells. Carcinogenesis 2010, 31, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- de Antonellis, P.; Medaglia, C.; Cusanelli, E.; Andolfo, I.; Liguori, L.; De Vita, G.; Carotenuto, M.; Bello, A.; Formiggini, F.; Galeone, A.; et al. MiR-34a Targeting of Notch Ligand Delta-Like 1 Impairs CD15(+)/CD133(+) Tumor-Propagating Cells and Supports Neural Differentiation in Medulloblastoma. PLoS ONE 2011, 6, e24584. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Xue, C.; Zeng, Y.; Yuan, X.; Chu, Q.; Jiang, S.; Wang, J.; Zhang, Y.; Zhu, D.; Li, L. Notch signaling pathway in cancer: From mechanistic insights to targeted therapies. Signal Transduct. Target. Ther. 2024, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Sun, X.; Sun, J.; Zhang, J.; Wang, Y. Epithelial-mesenchymal transition in colorectal cancer metastasis and progression: Molecular mechanisms and therapeutic strategies. Cell Death Discov. 2025, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Roy, S.; Zhu, D.; Parak, W.J.; Feliu, N. Lysosomal proton buffering of poly (ethylenimine) measured in situ by fluorescent pH-sensor microcapsules. ACS Nano 2020, 14, 8012–8023. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jobdeedamrong, A.; Yoo, H.J.; Jung, H.; Pechyen, C.; Natphopsuk, S.; Thongnuek, P.; Jeong, S.; Lee, J.; Yang, S.-G. Cholesterol-Conjugated Polyion Complex Nanoparticles for Combination Delivery of Hydrophobic Paclitaxel and Hydrophilic miR-34a for Colon Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 7965. https://doi.org/10.3390/ijms26167965
Jobdeedamrong A, Yoo HJ, Jung H, Pechyen C, Natphopsuk S, Thongnuek P, Jeong S, Lee J, Yang S-G. Cholesterol-Conjugated Polyion Complex Nanoparticles for Combination Delivery of Hydrophobic Paclitaxel and Hydrophilic miR-34a for Colon Cancer Therapy. International Journal of Molecular Sciences. 2025; 26(16):7965. https://doi.org/10.3390/ijms26167965
Chicago/Turabian StyleJobdeedamrong, Arjaree, Hye Jin Yoo, Hosun Jung, Chiravoot Pechyen, Sitakan Natphopsuk, Peerapat Thongnuek, Seok Jeong, Junghan Lee, and Su-Geun Yang. 2025. "Cholesterol-Conjugated Polyion Complex Nanoparticles for Combination Delivery of Hydrophobic Paclitaxel and Hydrophilic miR-34a for Colon Cancer Therapy" International Journal of Molecular Sciences 26, no. 16: 7965. https://doi.org/10.3390/ijms26167965
APA StyleJobdeedamrong, A., Yoo, H. J., Jung, H., Pechyen, C., Natphopsuk, S., Thongnuek, P., Jeong, S., Lee, J., & Yang, S.-G. (2025). Cholesterol-Conjugated Polyion Complex Nanoparticles for Combination Delivery of Hydrophobic Paclitaxel and Hydrophilic miR-34a for Colon Cancer Therapy. International Journal of Molecular Sciences, 26(16), 7965. https://doi.org/10.3390/ijms26167965





