The Multifaceted Role of Mitochondria in Angiogenesis
Abstract
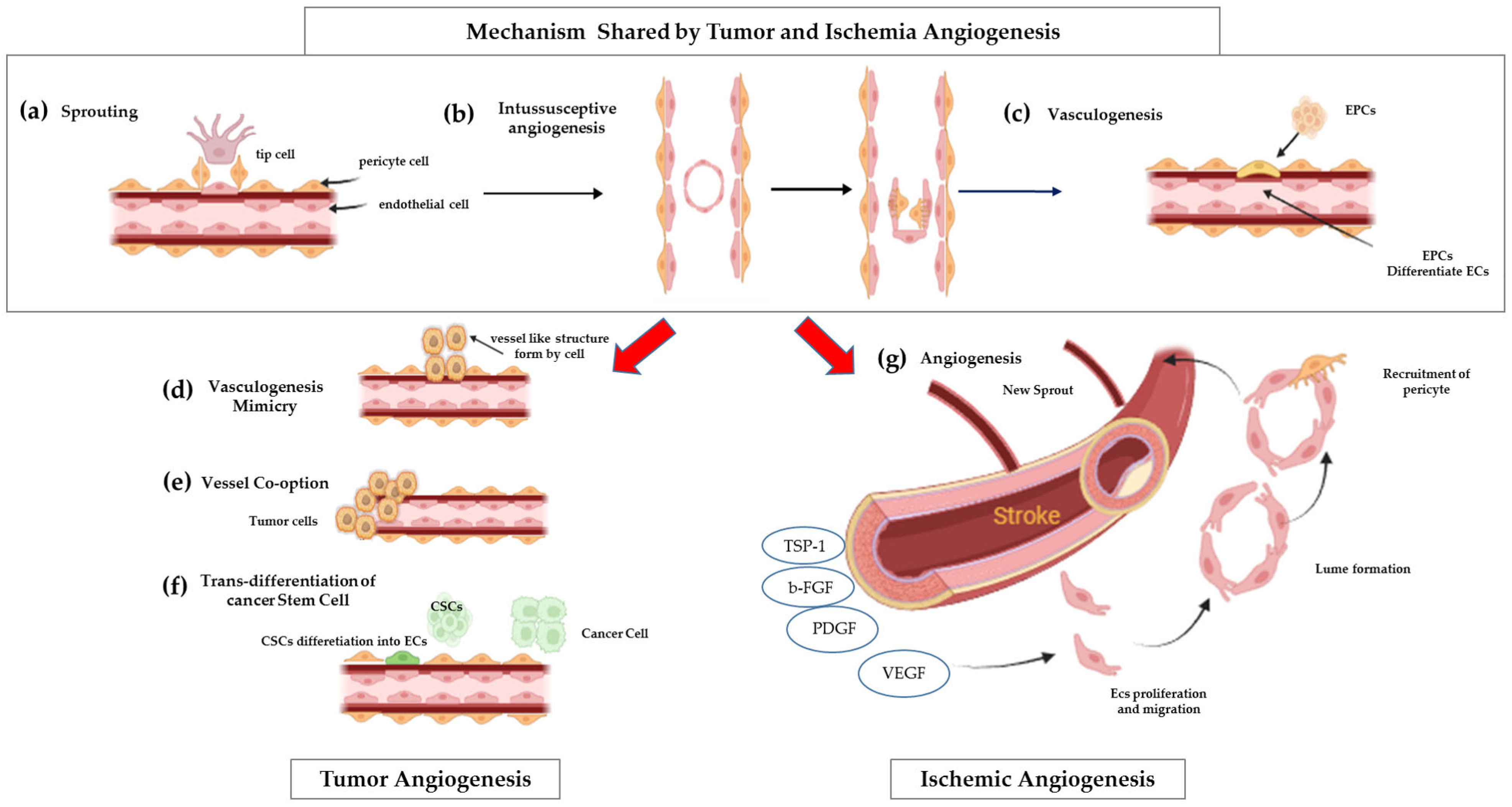
1. Introduction
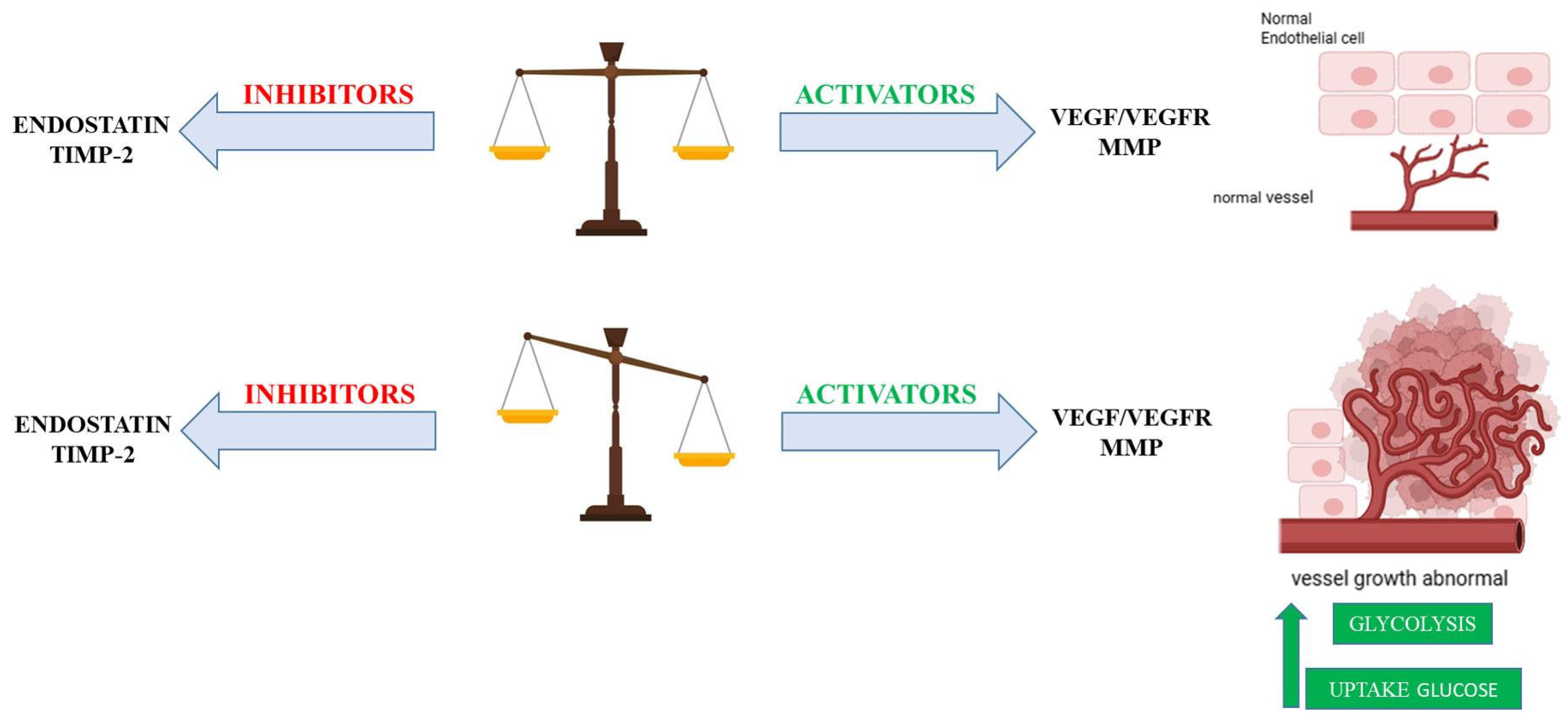
2. Metabolic Interplay in Angiogenesis: Balancing Glycolysis and Oxidative Phosphorylation
- Rapid ATP Production. Glycolysis, as an anaerobic process, can generate ATP at a faster rate compared with oxidative phosphorylation. This rapid energy production is crucial for the bursts of energy required for swift cell migration and proliferation, particularly at the leading edge of newly forming vessels [7].
- Biomass Synthesis. Glycolytic intermediates are shunted into anabolic pathways (e.g., pentose phosphate pathway, serine synthesis pathway). These pathways produce essential building blocks such as nucleotides, lipids, and amino acids, which are vital for rapid cell division and the extensive new vessel formation that characterizes angiogenesis [8].
- Adaptation to Hypoxia. This metabolic preference allows endothelial cells to adapt to hypoxic environments, which are frequently encountered during angiogenesis (e.g., in rapidly growing tissues or ischemic areas). By limiting their own oxygen consumption through reliance on glycolysis, endothelial cells may indirectly facilitate a more efficient transfer of available oxygen to the surrounding metabolically active tissues [7].
3. Mitochondrial Functions in Endothelial Cells: More than Just Powerhouses
3.1. Mitochondria as Biosynthetic Hubs
3.2. Mitochondria Respiration and ROS Production
| Protein Name | Location Within Mitochondria | Primary Function | Role in Angiogenesis | Ref. |
|---|---|---|---|---|
| POLRMT (RNA Polymerase Mitochondrial) | Mitochondrial matrix | Regulates mitochondrial transcription and oxidative phosphorylation | Anti-angiogenic: Silencing/knockout Impedes proliferation, migration, and tube formation. Linked to pathological angiogenesis in diabetic retinopathy. | [29,30] |
| TIMM44 (Translocase of Inner Mitochondrial Membrane 44) | Inner mitochondrial membrane | Essential for mitochondrial integrity and function | Anti-angiogenic: Silencing/blocking Inhibits proliferation, migration, and tube formation in vitro and in vivo. A potential therapeutic target for abnormal Angiogenesis. | [31] |
| SIRT3 (Sirtuin 3) | Outer mitochondrial membrane | Regulates ROS formation and glycolysis | Anti-proliferative: Knockdown decreases ATP production and inhibits mTOR activity, affecting cell proliferation. | [24] |
| VDAC1 (Voltage-Dependent Anion Channel 1) | Outer mitochondrial membrane | Regulates metabolite exchange (ATP/ADP) | Anti-proliferative: Knockdown decreases ATP production and inhibits mTOR activity, affecting cell proliferation. | [32,33] |
| Drp1 (Dynamin- Related Protein 1) | Outer mitochondrial membrane | Mediates mitochondrial fission | Anti-migratory/proliferative: Knockdown impairs cell migration and proliferation. Inhibition can protect against ischemia–reperfusion injury. | [34] |
| p66Shc (66 kDa proto-oncogene Src homologous-collagen homolog adaptor protein) | Inner mitochondrial membrane | Involved in ROS dependent signaling | Pro-angiogenic: Critical role in ROS-dependent VEGF signaling and angiogenesis. Regulates oxidative stress and apoptosis pathways. | [22] |
| UQCRB (Ubiquinol-Cytochrome c Reductase Binding Protein) | Inner mitochondrial membrane (Complex III subunit) | Regulates electron transport and ROS Production | Anti-angiogenic: Inhibition reduces VEGF-mediated cell proliferation and angiogenesis. | [23] |
| ALDH2 (Aldehyde Dehydrogenase 2) | Mitochondrial matrix | Antioxidant by detoxifying aldehydes | Pro-angiogenic: Overexpression promotes endothelial cell migration, proliferation, and angiogenesis. | [35] |
| CypD (Cyclophilin D) | Mitochondrial matrix | Regulates calcium levels, energy metabolism, and apoptosis | Pro-angiogenic: Deficiency can increase VEGF-induced proliferation and angiogenesis. | [33,36] |
4. Mitochondrial Signaling and Angiogenesis: Orchestrating New Vessel Growth
4.1. Mitochondria as Central Oxygen Sensors in Endothelial Cells
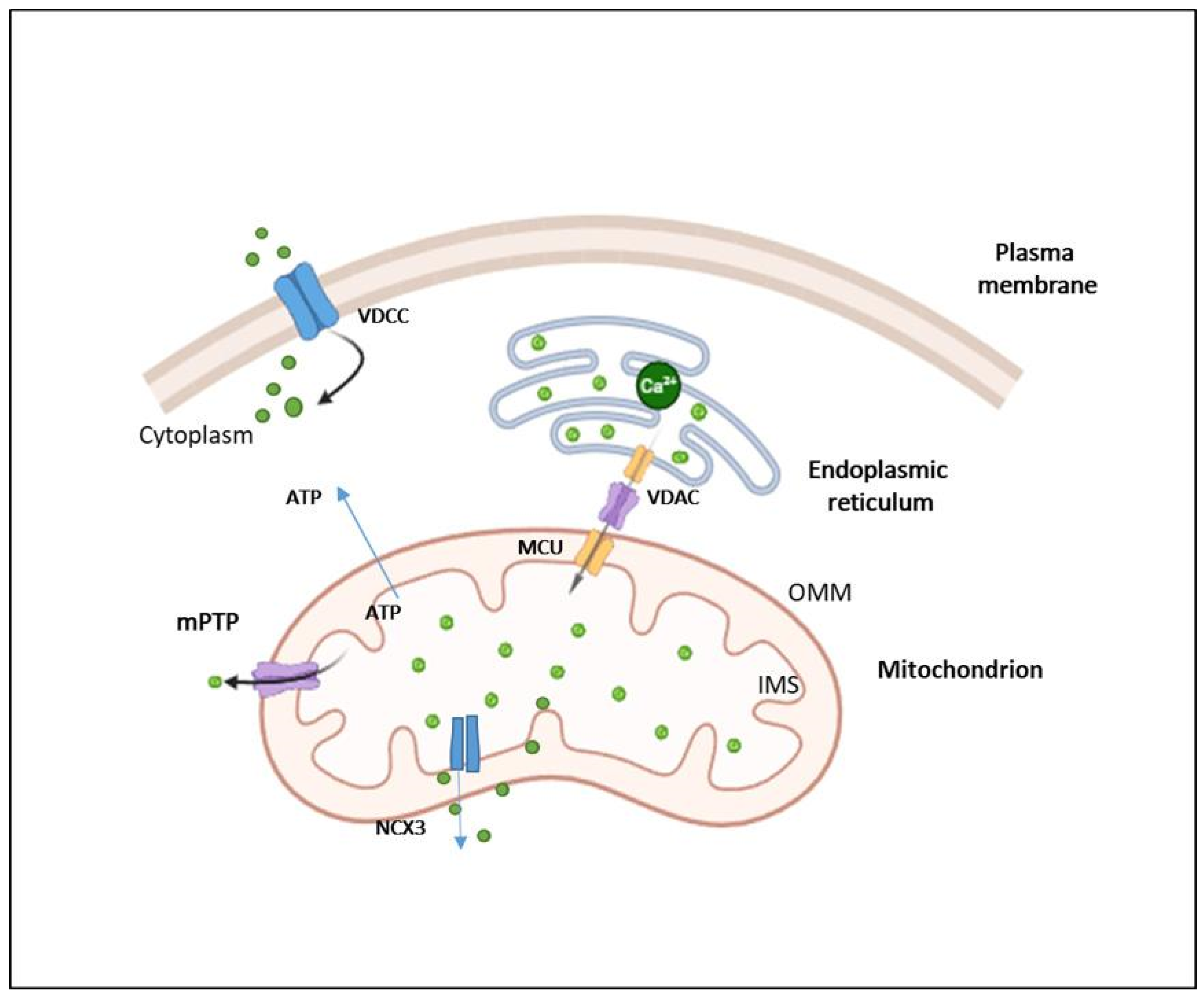
4.2. Mitochondria as Responsible of Intracellular Calcium Homeostasis
4.3. Mitochondrial Dynamics in Angiogenesis
4.4. Mitochondrial Quality Control
4.5. Extracellular Vesicles (EVs): Mitochondrial Modulators in Vascular Health
5. The Impact of Mitochondrial DNA Mutation on Angiogenesis: When Power Fails
5.1. Mitochondrial DNA Mutations: A Context-Dependent Modulator of Angiogenesis in Ischemia vs. Cancer
5.2. Cell-Type Specificity: Endothelial vs. Tumor Cell Responses
5.3. Heteroplasmy Level: High vs. Low Mutation Burden
5.4. Compensatory Mechanisms: Metabolic Reprogramming and mtDNA Transfer
5.5. Integration of POLRMT Regulation: A Central Transcriptional Control Node
6. Therapeutic Potential of Targeting Mitochondria for Angiogenesis Modulation
6.1. Mitochondria-Targeted Antioxidants
6.2. Angiogenesis Inhibitors
7. Conclusions and Future Directions
- How does mitochondrial heterogeneity across different vascular beds affect angiogenic responses?
- To what extent do mtDNA mutations exert context-dependent effects on angiogenesis?
- Can we selectively modulate mitochondrial dynamics (fusion, fission, mitophagy) to enhance therapeutic outcomes?
- How do mitochondria communicate with the nucleus and other organelles to coordinate angiogenesis?
- What delivery strategies can ensure safe and tissue-specific mitochondrial targeting in clinical applications?
- What role does mitochondrial signaling play in the immune regulation of angiogenesis?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| ASNS | Asparagine synthetase |
| ATP | Adenosine triphosphate |
| b-FGF | Basic fibroblast growth factor |
| BINP3 | Bcl-2 interacting protein 3 |
| Ca2+ | Calcium |
| CAD | Coronary artery disease |
| cGAS-STING | cyclic GMP-AMP synthase-stimulator of interferon genes |
| CPT1A | Carnitine palmitoyl transferase 1 A |
| CXCL12 | C-X-C Motif Chemokine Ligand 12 |
| CypD | Cyclophilin D |
| DAMP | Damage-associated molecular pattern |
| Drp1 | Dynamin-related protein 1 |
| EC | Endothelial cell |
| EMRE | Essential MUC Regulator |
| EPCs | Endothelial progenitor cells |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| ETC | Mitochondrial electron transport chain |
| EVs | Extracellular vesicles |
| FADH2 | Flavin adenine dinucleotide |
| FAO | Fatty acid oxidation |
| Fe2+ | Ferrous iron |
| FECH | Ferrochelatase |
| Fis1 | Fission 1 |
| FUNDC1 | FUN14 domain containing 1 |
| GLS1 | Glutaminase 1 |
| HIF-1α | Hypoxia-Inducible Factor-1 alpha |
| hCMEC/D3 | Human cerebral microvascular endothelial cell line |
| HUVECs | Human umbilical vein endothelial cells |
| IMM | Inner mitochondrial membrane |
| IMS | Intermembrane space |
| IP3R | Inositol 1,4,5-trisphosphate receptor |
| MAMs | Mitochondria-associated membranes |
| MCU | Mitochondrial calcium uniporter complex |
| MCUb | Mitochondrial Calcium Uniporter Dominant Negative Subunit Beta |
| MCUC | Mitochondrial calcium uniporter complex |
| MCUR1 | Mitochondrial calcium uniporter regulator 1 |
| Mfn1/2 | Mitofusins 1 and 2 |
| Mff | Mitochondrial fission factor |
| mitoK_ATP | Mitochondrial ATP-sensitive potassium channel |
| MitoQ | Mitochinone mesilato |
| mtDNA | Mitochondrial DNA |
| MMPs | Matrix metalloproteinases |
| ΔΨm | Mitochondrial membrane potential |
| MPC | Mitochondrial pyruvate carrier |
| mPTP | Mitochondrial permeability transition pore |
| mtROS | Mitochondrial ROS |
| mTOR | Mammalian target of rapamycin |
| mTORC1/2 | Mechanistic Target of Rapamycin Complex 1/2 |
| NAC | N-acetylcysteine |
| NADH | Nicotinamide adenine dinucleotide |
| NCLX | Mitochondrial Na+/Li+/Ca2+ exchanger |
| NCX3 | Mitochondrial Na+/Ca2+ exchanger |
| ND6 | Mitochondrially encoded NADH dehydrogenase 6 |
| NFU1 | Iron-Sulfur Cluster Scaffold |
| NOX4 | NADPH oxidase 4 |
| NPs | Nanoparticles |
| NSCLC | Non-Small Cell Lung Cancer |
| OCR | Oxygen consumption rate |
| OMM | Outer mitochondrial membrane |
| Opa1 | Optic atrophy 1 |
| oxLDL | Oxidized low-density lipoprotein |
| OXPHOS | Oxidative phosphorylation |
| PAH | Pulmonary arterial hypertension |
| PDGF | Platelet-derived growth factor |
| PFKFB3 | 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 |
| PHD | Prolyl hydroxylase domain |
| PTEN | Phosphatase and tensin homolog |
| PINK1 | PTEN-induced kinase 1 |
| POLRMT | Mitochondrial RNA polymerase |
| ROS | Reactive oxygen species |
| SIRT3 | Sirtuin 3 |
| SkQ1 | Visomitin |
| TCA | Tricarboxylic Acid |
| TIMM44 | Translocase of the inner mitochondrial membrane 44 |
| TIMP2 | Tissue inhibitor of metalloproteinases-2 |
| TLR9 | Toll-like receptor 9 |
| TPP | Triphenylphosphonium |
| TSP1 | Thrombospondin-1 |
| TSPO | Translocator protein |
| UQCRB | Ubiquinol-cytochrome c reductase binding protein |
| VDAC1 | Voltage-dependent anion channel 1 |
| VEGF | Vascular Endothelial Growth Factor |
| VEGFR2 | Vascular Endothelial Growth Factor Receptor 2 |
| VDCC | Voltage-dependent calcium channels |
References
- Guo, D.; Wang, Q.; Li, C.; Wang, Y.; Chen, X. VEGF stimulated the angiogenesis by promoting the mitochondrial functions. Oncotarget 2017, 8, 77020–77027. [Google Scholar] [CrossRef]
- Ionescu, C.; Oprea, B.; Ciobanu, G.; Georgescu, M.; Bica, R.; Mateescu, G.O.; Huseynova, F.; Barragan-Montero, V. The Angiogenic Balance and Its Implications in Cancer and Cardiovascular Diseases: An Overview. Medicina 2022, 58, 903. [Google Scholar] [CrossRef]
- Luo, Z.; Yao, J.; Wang, Z.; Xu, J. Mitochondria in endothelial cells angiogenesis and function: Current understanding and future perspectives. J. Transl. Med. 2023, 21, 441. [Google Scholar] [CrossRef]
- Marcu, R.; Zheng, Y.; Hawkins, B.J. Mitochondria and Angiogenesis. Adv. Exp. Med. Biol. 2017, 982, 371–406. [Google Scholar]
- Reichard, A.; Asosingh, K. The role of mitochondria in angiogenesis. Mol. Biol. Rep. 2019, 46, 1393–1400. [Google Scholar] [CrossRef]
- Pang, B.; Dong, G.; Pang, T.; Sun, X.; Liu, X.; Nie, Y.; Chang, X. Advances in pathogenesis and treatment of vascular endothelial injury-related diseases mediated by mitochondrial abnormality. Front. Pharmacol. 2024, 15, 1422686. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Ren, L.; Hamblin, M.H.; Fan, Y. Endothelial Cell Glucose Metabolism and Angiogenesis. Biomedicines 2021, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Aden, D.; Sureka, N.; Zaheer, S.; Chaurasia, J.K.; Zaheer, S. Metabolic Reprogramming in Cancer: Implications for Immunosuppressive Microenvironment. Immunology 2025, 174, 30–72. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wu, Q.; Horbinski, C.M.; Flavahan, W.A.; Yang, K.; Zhou, W.; Dombrowski, S.M.; Huang, Z.; Fang, X.; Shi, Y.; et al. Mitochondrial control by DRP1 in brain tumor initiating cells. Nat. Neurosci. 2015, 18, 501. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Y.; Wang, L.; Wang, Y.S.; Dou, G.R. PFKFB3: A Potential Key to Ocular Angiogenesis. Front. Cell Dev. Biol. 2021, 9, 628317. [Google Scholar] [CrossRef]
- Xiong, Q.W.; Jiang, K.; Shen, X.W.; Ma, Z.R.; Yan, X.M.; Xia, H.; Cao, X. The requirement of the mitochondrial protein NDUFS8 for angiogenesis. Cell Death Dis. 2024, 15, 253. [Google Scholar] [CrossRef] [PubMed]
- Kulovic-Sissawo, A.; Tocantins, C.; Diniz, M.S.; Weiss, E.; Steiner, A.; Tokic, S.; Madreiter-Sokolowski, C.T.; Pereira, S.P.; Hiden, U. Mitochondrial Dysfunction in Endothelial Progenitor Cells: Unraveling Insights from Vascular Endothelial Cells. Biology 2024, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Margaglione, M.; D’Apolito, M.; Santocroce, R.; Maffione, A.B. Hereditary angioedema: Looking for bradykinin production and triggers of vascular permeability. Clin. Exp. Allergy 2019, 49, 1395–1402. [Google Scholar] [CrossRef]
- Kluge, M.A.; Fetterman, J.L.; Vita, J.A. Mitochondria and endothelial function. Circ. Res. 2013, 112, 1171. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, K.; Liu, F.; Zhang, J.; Chen, X.; Chen, T.; Chen, Q.; Yao, Y.; Hu, W.; Wang, L.; et al. Developmental Angiogenesis Requires the Mitochondrial Phenylalanyl-tRNA Synthetase. Front. Cardiovasc. Med. 2021, 8, 724846. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar] [CrossRef]
- Kalucka, J.; Bierhansl, L.; Conchinha, N.V.; Missiaen, R.; Elia, I.; Bruning, U.; Scheinok, S.; Treps, L.; Cantelmo, A.R.; Dubois, C.; et al. Quiescent Endothelial Cells Upregulate Fatty Acid beta-Oxidation for Vasculoprotection via Redox Homeostasis. Cell Metab. 2018, 28, 881–894 e13. [Google Scholar] [CrossRef]
- Fitzgerald, G.; Soro-Arnaiz, I.; De Bock, K. The Warburg Effect in Endothelial Cells and its Potential as an Anti-angiogenic Target in Cancer. Front. Cell Dev. Biol. 2018, 6, 100. [Google Scholar] [CrossRef]
- Chen, F.; Haigh, S.; Barman, S.; Fulton, D.J. From form to function: The role of Nox4 in the cardiovascular system. Front. Physiol. 2012, 3, 412. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, S.J.; Tatsunami, R.; Yamamura, H.; Fukai, T.; Ushio-Fukai, M. ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis. Am. J. Physiol. Cell Physiol. 2017, 312, C749–C764. [Google Scholar] [CrossRef]
- Pecchillo Cimmino, T.; Ammendola, R.; Cattaneo, F.; Esposito, G. NOX Dependent ROS Generation and Cell Metabolism. Int. J. Mol. Sci. 2023, 24, 2086. [Google Scholar] [CrossRef]
- Caja, S.; Enriquez, J.A. Mitochondria in endothelial cells: Sensors and integrators of environmental cues. Redox Biol. 2017, 12, 821–827. [Google Scholar] [CrossRef]
- Chandel, N.S. Mitochondrial complex III: An essential component of universal oxygen sensing machinery? Respir. Physiol. Neurobiol. 2010, 174, 175. [Google Scholar] [CrossRef]
- He, X.; Zeng, H.; Chen, J.X. Emerging role of SIRT3 in endothelial metabolism, angiogenesis, and cardiovascular disease. J. Cell Physiol. 2018, 234, 2252–2265. [Google Scholar] [CrossRef] [PubMed]
- D’Apolito, M.; Colia, A.L.; Lasalvia, M.; Capozzi, V.; Falcone, M.P.; Pettoello-Mantovani, M.; Brownlee, M.; Maffione, A.B.; Giardino, I. Urea-induced ROS accelerate senescence in endothelial progenitor cells. Atherosclerosis 2017, 263, 127–136. [Google Scholar] [CrossRef] [PubMed]
- D’Apolito, M.; Du, X.; Pisanelli, D.; Pettoello-Mantovani, M.; Campanozzi, A.; Giacco, F.; Maffione, A.B.; Colia, A.L.; Brownlee, M.; Giardino, I. Urea-induced ROS cause endothelial dysfunction in chronic renal failure. Atherosclerosis 2015, 239, 393–400. [Google Scholar] [CrossRef]
- Giardino, I.; D’Apolito, M.; Brownlee, M.; Maffione, A.B.; Colia, A.L.; Sacco, M.; Ferrara, P.; Pettoello-Mantovani, M. Vascular toxicity of urea, a new “old player” in the pathogenesis of chronic renal failure induced cardiovascular diseases. Turk. Pediatri Ars. 2017, 52, 187–193. [Google Scholar] [CrossRef]
- d’Apolito, M.; Colia, A.L.; Manca, E.; Pettoello-Mantovani, M.; Sacco, M.; Maffione, A.B.; Brownlee, M.; Giardino, I. Urea Memory: Transient Cell Exposure to Urea Causes Persistent Mitochondrial ROS Production and Endothelial Dysfunction. Toxins 2018, 10, 410. [Google Scholar] [CrossRef]
- Miller, D.J.; Cascio, M.A.; Rosca, M.G. Diabetic Retinopathy: The Role of Mitochondria in the Neural Retina and Microvascular Disease. Antioxidants 2020, 9, 905. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Zhang, S.; Wang, Y.; Pei, X.; Chen, Y.; Zhang, J.; Zhang, Y.; Du, Y.; Hao, S.; et al. Mitochondrial dysfunction in the regulation of aging and aging-related diseases. Cell Commun. Signal. 2025, 23, 290. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.R.; Li, H.P.; Cai, S.Z.; Du, S.Y.; Chen, X.; Yao, J.; Cao, X.; Zhen, Y.F.; Wang, Q. The mitochondrial protein TIMM44 is required for angiogenesis in vitro and in vivo. Cell Death Dis. 2023, 14, 307. [Google Scholar] [CrossRef]
- Filippini, A.; D’Amore, A.; D’Alessio, A. Calcium Mobilization in Endothelial Cell Functions. Int. J. Mol. Sci. 2019, 20, 4525. [Google Scholar] [CrossRef] [PubMed]
- de Ridder, I.; Kerkhofs, M.; Lemos, F.O.; Loncke, J.; Bultynck, G.; Parys, J.B. The ER-mitochondria interface, where Ca2+ and cell death meet. Cell Calcium 2023, 112, 102743. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, Y.L.; Chien, C.L. Mitochondrial aldehyde dehydrogenase restores the migratory capacity inhibited by high glucose-induced hyperosmolality. Sci. Rep. 2025, 15, 17741. [Google Scholar] [CrossRef]
- Amanakis, G.; Murphy, E. Cyclophilin D: An Integrator of Mitochondrial Function. Front. Physiol. 2020, 11, 595. [Google Scholar] [CrossRef]
- Shah, F.H.; Nam, Y.S.; Bang, J.Y.; Hwang, I.S.; Kim, D.H.; Ki, M.; Lee, H.W. Targeting vascular endothelial growth receptor-2 (VEGFR-2): Structural biology, functional insights, and therapeutic resistance. Arch. Pharm. Res. 2025, 48, 404–425. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Yan, Z.; Zhu, Z. Mitochondria-Associated Endoplasmic Reticulum Membranes in Cardiovascular Diseases. Front. Cell Dev. Biol. 2020, 8, 604240. [Google Scholar] [CrossRef] [PubMed]
- Vervloessem, T.; Yule, D.I.; Bultynck, G.; Parys, J.B. The type 2 inositol 1, 4, 5-trisphosphate receptor, emerging functions for an intriguing Ca2+-release channel. Biochim. Biophys. Acta 2015, 1853, 1992–2005. [Google Scholar] [CrossRef]
- Wang, C.; Dai, X.; Wu, S.; Xu, W.; Song, P.; Huang, K.; Zou, M.H. FUNDC1-dependent mitochondria-associated endoplasmic reticulum membranes are involved in angiogenesis and neoangiogenesis. Nat. Commun. 2021, 12, 2616, Erratum in: Nat. Commun. 2024, 15, 4572. [Google Scholar] [CrossRef]
- Tang, X.; Luo, Y.X.; Chen, H.Z.; Liu, D.P. Mitochondria, endothelial cell function, and vascular diseases. Front. Physiol. 2014, 5, 175. [Google Scholar] [CrossRef]
- Wang, L.T.; He, P.C.; Li, A.Q.; Cao, K.X.; Yan, J.W.; Guo, S.; Jiang, L.; Yao, L.; Dai, X.Y.; Feng, D.; et al. Caffeine promotes angiogenesis through modulating endothelial mitochondrial dynamics. Acta Pharmacol. Sin. 2021, 42, 2033–2045. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, J.; Yu, M.; Xie, Y.; Huang, Y.; Wolff, D.W.; Abel, P.W.; Tu, Y. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene 2013, 32, 4814. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Ren, K.D.; Luan, Y.; Chen, X.; Yang, Y. Mitochondrial Dynamics: Pathogenesis and Therapeutic Targets of Vascular Diseases. Front. Cardiovasc. Med. 2021, 8, 770574. [Google Scholar] [CrossRef]
- Marsboom, G.; Toth, P.T.; Ryan, J.J.; Hong, Z.; Wu, X.; Fang, Y.H.; Thenappan, T.; Piao, L.; Zhang, H.J.; Pogoriler, J.; et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ. Res. 2012, 110, 1484. [Google Scholar] [CrossRef] [PubMed]
- Nagarkoti, S.; Kim, Y.M.; Das, A.; Ash, D.; A. Vitriol, E.; Read, T.A.; Sudhahar, V.; Hossain, M.S.; Yadav, S.; McMenamin, M.; et al. Endothelial Drp1 Couples VEGF-induced Redox Signaling with Glycolysis Through Cysteine Oxidation to Drive Angiogenesis. bioRxiv 2024. [Google Scholar] [CrossRef]
- Herkenne, S.; Ek, O.; Zamberlan, M.; Pellattiero, A.; Chergova, M.; Chivite, I.; Novotna, E.; Rigoni, G.; Fonseca, T.B.; Samardzic, D.; et al. Developmental and Tumor Angiogenesis Requires the Mitochondria-Shaping Protein Opa1. Cell Metab. 2020, 31, 987–1003 e8. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Long, H.; Hou, L.; Feng, B.; Ma, Z.; Wu, Y.; Zeng, Y.; Cai, J.; Zhang, D.W.; Zhao, G. The mitophagy pathway and its implications in human diseases. Signal Transduct. Target. Ther. 2023, 8, 304. [Google Scholar] [CrossRef]
- Wang, X.L.; Feng, S.T.; Wang, Z.Z.; Chen, N.H.; Zhang, Y. Role of mitophagy in mitochondrial quality control: Mechanisms and potential implications for neurodegenerative diseases. Pharmacol. Res. 2021, 165, 105433. [Google Scholar] [CrossRef]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef]
- Moeinabadi-Bidgoli, K.; Rezaee, M.; Hossein-Khannazer, N.; Babajani, A.; Aghdaei, H.A.; Arki, M.K.; Afaghi, S.; Niknejad, H.; Vosough, M. Exosomes for angiogenesis induction in ischemic disorders. J. Cell Mol. Med. 2023, 27, 763–787. [Google Scholar] [CrossRef]
- Chance, T.C.; Herzig, M.C.; Christy, B.A.; Delavan, C.; Rathbone, C.R.; Cap, A.P.; Bynum, J.A. Human mesenchymal stromal cell source and culture conditions influence extracellular vesicle angiogenic and metabolic effects on human endothelial cells in vitro. J. Trauma. Acute Care Surg. 2020, 89 (Suppl. 2), S100–S108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, X.; Shen, K.; Luo, L.; Zhao, M.; Xu, C.; Jia, Y.; Xiao, D.; Li, Y.; Gao, X.; et al. Exosomes Derived from Adipose Mesenchymal Stem Cells Promote Diabetic Chronic Wound Healing through SIRT3/SOD2. Cells 2022, 11, 2568. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, J.; Li, J.; Ma, C.; Chen, S.; Lei, W.; Yang, Y.; Liu, S.; Bihl, J.; Chen, C. Loading MiR-210 in Endothelial Progenitor Cells Derived Exosomes Boosts Their Beneficial Effects on Hypoxia/Reoxygeneation-Injured Human Endothelial Cells via Protecting Mitochondrial Function. Cell Physiol. Biochem. 2018, 46, 664–675. [Google Scholar] [CrossRef]
- Hayakawa, K.; Chan, S.J.; Mandeville, E.T.; Park, J.H.; Bruzzese, M.; Montaner, J.; Arai, K.; Rosell, A.; Lo, E.H. Protective Effects of Endothelial Progenitor Cell-Derived Extracellular Mitochondria in Brain Endothelium. Stem Cells 2018, 36, 1404–1410. [Google Scholar] [CrossRef]
- D’Souza, A.; Burch, A.; Dave, K.M.; Sreeram, A.; Reynolds, M.J.; Dobbins, D.X.; Kamte, Y.S.; Zhao, W.; Sabatelle, C.; Joy, G.M.; et al. Microvesicles transfer mitochondria and increase mitochondrial function in brain endothelial cells. J. Control. Release 2021, 338, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhang, Y.; Li, Q.; Ye, Z.; Liu, Y.; Fu, C.; Cang, X.; Wang, M.; Guan, M.X. A coronary artery disease-associated tRNAThr mutation altered mitochondrial function, apoptosis and angiogenesis. Nucleic Acids Res. 2019, 47, 2056–2074. [Google Scholar] [CrossRef]
- Grossini, E.; Venkatesan, S.; Ola Pour, M.M. Mitochondrial Dysfunction in Endothelial Cells: A Key Driver of Organ Disorders and Aging. Antioxidants 2025, 14, 372. [Google Scholar] [CrossRef]
- Quan, Y.; Xin, Y.; Tian, G.; Zhou, J.; Liu, X. Mitochondrial ROS-Modulated mtDNA: A Potential Target for Cardiac Aging. Oxid. Med. Cell Longev. 2020, 2020, 9423593. [Google Scholar] [CrossRef]
- d’Apolito, M.; Pisanelli, D.; Faletra, F.; Giardino, I.; Gigante, M.; Pettoello-Mantovani, M.; Goulet, O.; Gasparini, P.; Campanozzi, A. Genetic analysis of Italian patients with congenital tufting enteropathy. World J. Pediatr. 2016, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Zemskova, M.; Eccles, C.A.; Varghese, M.V.; Niihori, M.; Barker, N.K.; Luo, M.; Mandarino, L.J.; Langlais, P.R.; Rafikova, O.; et al. Single Mutation in the NFU1 Gene Metabolically Reprograms Pulmonary Artery Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 734–754. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, P.; Cheng, H.; Xu, F.; Ye, Y. The impact of glycolysis on ischemic stroke: From molecular mechanisms to clinical applications. Front. Neurol. 2025, 16, 1514394. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.; Liu, E.M.; Shergold, A.L.; Tolla, E.; Tait-Mulder, J.; Huerta-Uribe, A.; Shokry, E.; Young, A.L.; Lilla, S.; Kim, M.; et al. Mitochondrial DNA mutations drive aerobic glycolysis to enhance checkpoint blockade response in melanoma. Nat. Cancer 2024, 5, 659–672. [Google Scholar] [CrossRef]
- Boso, D.; Piga, I.; Trento, C.; Minuzzo, S.; Angi, E.; Iommarini, L.; Lazzarini, E.; Caporali, L.; Fiorini, C.; D’Angelo, L.; et al. Pathogenic mitochondrial DNA variants are associated with response to anti-VEGF therapy in ovarian cancer PDX models. J. Exp. Clin. Cancer Res. 2024, 43, 325. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.M.; Whitehall, J.C.; Greaves, L.C. Mitochondrial DNA mutations in ageing and cancer. Mol. Oncol. 2022, 16, 3276–3294. [Google Scholar] [CrossRef]
- Beerkens, A.P.M.; Heskamp, S.; Reinema, F.V.; Adema, G.J.; Span, P.N.; Bussink, J. Mitochondria Targeting of Oxidative Phosphorylation Inhibitors to Alleviate Hypoxia and Enhance Anticancer Treatment Efficacy. Clin. Cancer Res. 2025, 31, 1186–1193. [Google Scholar] [CrossRef]
- Perez-Amado, C.J.; Bazan-Cordoba, A.; Hidalgo-Miranda, A.; Jimenez-Morales, S. Mitochondrial Heteroplasmy Shifting as a Potential Biomarker of Cancer Progression. Int. J. Mol. Sci. 2021, 22, 7369. [Google Scholar] [CrossRef]
- Wu, Q.; Tsai, H.I.; Zhu, H.; Wang, D. The Entanglement between Mitochondrial DNA and Tumor Metastasis. Cancers 2022, 14, 1862. [Google Scholar] [CrossRef]
- Girolimetti, G.G.F.; Eusebi, L.H.U.; Ricci, C.; Marzetti, E.; Picca, A.; Bucci, C. Circulating Cell-Free Mitochondrial DNA and Inflammation in Older Adults with Pancreatic Cancer: Results from an Exploratory Study. Appl. Sci. 2025, 15, 4410. [Google Scholar] [CrossRef]
- Inatomi, T.; Matsuda, S.; Ishiuchi, T.; Do, Y.; Nakayama, M.; Abe, S.; Kasho, K.; Wanrooij, S.; Nakada, K.; Ichiyanagi, K.; et al. TFB2M and POLRMT are essential for mammalian mitochondrial DNA replication. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119167. [Google Scholar] [CrossRef]
- Huan, M.J.; Fu, P.P.; Chen, X.; Wang, Z.X.; Ma, Z.R.; Cai, S.Z.; Jiang, Q.; Wang, Q. Identification of the central role of RNA polymerase mitochondrial for angiogenesis. Cell Commun. Signal. 2024, 22, 343. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, B.; Huang, Y.; Zhang, Y.; Jiang, Y.; Ma, L.; Shen, Y.Q. Mitochondrial DNA-targeted therapy: A novel approach to combat cancer. Cell Insight 2023, 2, 100113. [Google Scholar] [CrossRef]
- Lyu, Y.; Wang, T.; Huang, S.; Zhang, Z. Mitochondrial Damage-Associated Molecular Patterns and Metabolism in the Regulation of Innate Immunity. J. Innate Immun. 2023, 15, 665–679. [Google Scholar] [CrossRef]
- Ghazizadeh, Y.; Sharifi-Ardani, S.E.; Tajik, N.; Mirzaei, R.; Pourahmad, J. Exploring the Potential of Mitochondria-Targeted Drug Delivery for Enhanced Breast Cancer Therapy. Int. J. Breast Cancer 2025, 2025, 3013009. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.A.; Fink, B.D.; Gibbs, B.E.; Chheda, P.R.; Wu, M.; Sivitz, W.I.; Kerns, R.J. A Novel Triphenylphosphonium Carrier to Target Mitochondria without Uncoupling Oxidative Phosphorylation. J. Med. Chem. 2021, 64, 662–676. [Google Scholar] [CrossRef]
- Buchke, S.; Sharma, M.; Bora, A.; Relekar, M.; Bhanu, P.; Kumar, J. Mitochondria-Targeted, Nanoparticle-Based Drug-Delivery Systems: Therapeutics for Mitochondrial Disorders. Life 2022, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Xiao, Y.; Zhao, C.; Deng, H.; Liu, H.; Ke, S.; Zhou, H.; Chen, G.; Wang, H. Ultrasmall PtIr Bimetallic Nanozyme Treats Myocardial Infarction via Ischemic/Inflammatory Cardiac Microenvironment Remodeling. ACS Nano 2025, 19, 13723–13739. [Google Scholar] [CrossRef]
- Herrera, E.A.; Cifuentes-Zuniga, F.; Figueroa, E.; Villanueva, C.; Hernandez, C.; Alegria, R.; Arroyo-Jousse, V.; Penaloza, E.; Farias, M.; Uauy, R.; et al. N-Acetylcysteine, a glutathione precursor, reverts vascular dysfunction and endothelial epigenetic programming in intrauterine growth restricted guinea pigs. J. Physiol. 2017, 595, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- Schwalfenberg, G.K. N-Acetylcysteine: A Review of Clinical Usefulness (an Old Drug with New Tricks). J. Nutr. Metab. 2021, 2021, 9949453. [Google Scholar] [CrossRef]
- Murray, K.O.; Ludwig, K.R.; Darvish, S.; Coppock, M.E.; Seals, D.R.; Rossman, M.J. Chronic mitochondria antioxidant treatment in older adults alters the circulating milieu to improve endothelial cell function and mitochondrial oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H187–H194. [Google Scholar] [CrossRef]
- Pang, M.; Wang, S.; Shi, T.; Chen, J. Overview of MitoQ on prevention and management of cardiometabolic diseases: A scoping review. Front. Cardiovasc. Med. 2025, 12, 1506460. [Google Scholar] [CrossRef]
- Zadeh, H.J.; Roholamini, Z.; Aminizadeh, S.; Deh-Ahmadi, M.A. Endurance training and MitoQ supplementation improve spatial memory, VEGF expression, and neurogenic factors in hippocampal tissue of rats. J. Clin. Transl. Res. 2022, 9, 1–7. [Google Scholar] [PubMed]
- Tang, D.; Liu, X.; Chen, J. Mitoquinone intravitreal injection ameliorates retinal ischemia-reperfusion injury in rats involving SIRT1/Notch1/NADPH axis. Drug. Dev. Res. 2022, 83, 800–810. [Google Scholar]
- Chang, J.; Jung, H.J.; Park, H.J.; Cho, S.W.; Lee, S.K.; Kwon, H.J. Cell-permeable mitochondrial ubiquinol-cytochrome c reductase binding protein induces angiogenesis in vitro and in vivo. Cancer Lett. 2015, 366, 52–60. [Google Scholar] [CrossRef]
- Jung, H.J.; Shim, J.S.; Lee, J.; Song, Y.M.; Park, K.C.; Choi, S.H.; Kim, N.D.; Yoon, J.H.; Mungai, P.T.; Schumacker, P.T.; et al. Terpestacin inhibits tumor angiogenesis by targeting UQCRB of mitochondrial complex III and suppressing hypoxia-induced reactive oxygen species production and cellular oxygen sensing. J. Biol. Chem. 2010, 285, 11584. [Google Scholar] [CrossRef]
- Jung, H.J.; Kim, Y.; Chang, J.; Kang, S.W.; Kim, J.H.; Kwon, H.J. Mitochondrial UQCRB regulates VEGFR2 signaling in endothelial cells. J. Mol. Med. 2013, 91, 1117. [Google Scholar] [CrossRef]
- Gwak, E.J.; Kim, D.; Hwang, H.Y.; Kwon, H.J. Mitochondrial ROS Produced in Human Colon Carcinoma Associated with Cell Survival via Autophagy. Cancers 2022, 14, 1883. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Chang, J.; Lee, H.S.; Kwon, H.J. Mitochondrial UQCRB as a new molecular prognostic biomarker of human colorectal cancer. Exp. Mol. Med. 2017, 49, e391. [Google Scholar] [CrossRef]
- Monti, E.; Mancini, A.; Marras, E.; Gariboldi, M.B. Targeting Mitochondrial ROS Production to Reverse the Epithelial-Mesenchymal Transition in Breast Cancer Cells. Curr. Issues Mol. Biol. 2022, 44, 5277–5293. [Google Scholar] [CrossRef]
- Sotgia, F.; Fiorillo, M.; Lisanti, M.P. Mitochondrial markers predict recurrence, metastasis and tamoxifen-resistance in breast cancer patients: Early detection of treatment failure with companion diagnostics. Oncotarget 2017, 8, 68730–68745. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Tsai, C.C.; Lin, H.Y.; Chen, H.L.; Ou, Y.C.; Chiang, P.H.; Suen, J.L.; Tsai, E.M. Gene Expression Profile Analysis of the Molecular Mechanism of HOXD10 Regulation of Epithelial Ovarian Cancer Cells. J. Cancer 2024, 15, 1213–1224. [Google Scholar] [CrossRef]
- Brzheskiy, V.V.; Efimova, E.L.; Vorontsova, T.N.; Alekseev, V.N.; Gusarevich, O.G.; Shaidurova, K.N.; Ryabtseva, A.A.; Andryukhina, O.M.; Kamenskikh, T.G.; Sumarokova, E.S.; et al. Results of a Multicenter, Randomized, Double-Masked, Placebo-Controlled Clinical Study of the Efficacy and Safety of Visomitin Eye Drops in Patients with Dry Eye Syndrome. Adv. Ther. 2015, 32, 1263. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.; Perekhvatova, N.; Skulachev, M.; Stein, L.; Ousler, G. SkQ1 Ophthalmic Solution for Dry Eye Treatment: Results of a Phase 2 Safety and Efficacy Clinical Study in the Environment and During Challenge in the Controlled Adverse Environment Model. Adv. Ther. 2016, 33, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Vasan, K.; Chandel, N.S. Molecular and cellular mechanisms underlying the failure of mitochondrial metabolism drugs in cancer clinical trials. J. Clin. Investig. 2024, 134, e176736. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, L.; Deng, H.; Huang, C.; Wang, N.; Yue, L.; Zhang, P.; Zhou, Y.; Zhou, W.; Gao, Y. Targeting OPA1-Mediated Mitochondrial Fusion Contributed to Celastrol’s Anti-Tumor Angiogenesis Effect. Pharmaceutics 2022, 15, 48. [Google Scholar] [CrossRef]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef]


| Drp1/Fis1 (fission) | Generates mitochondrial fragments, which increase mtROS, inducing HIF-1α/VEGF activation and promoting sprouting angiogenesis. In tumor cells, hyperactive Drp1 enhances its own angiogenesis and remodels the microenvironment. | [34,41,44] |
| Opa1/Mfn1/2 (fusion) | Promotes network elongation, which decreases mtROS, dampening angiogenic signals. | [34,41,45,47] |
| Mitochondrial trafficking | Relocates mitochondria to EC leading edge, supporting migration via ATP/ROS supply. | [44] |
| Mitophagy & quality control | PINK1/FUNDC1/BINP3 pathways regulate vessel density and sprouting through organelle turnover. | [48] |
| mtDNA Mutational Effect | Angiogenic Outcome | Ref | |
|---|---|---|---|
| Pulmonary vascular disease/PAH | NFU1 mutation induces Fe-S cluster dysfunction causing ROS imbalance | Impaired angiogenesis reduces capillary density; rescued by metabolic cofactors | [61] |
| Cardiovascular disease (e.g., CAD variant) | tRNAThr mutation induces mitochondrial instability | Impaired EC survival and angiogenesis | [57] |
| Tumor context, high-heteroplasmy | Disrupted respiratory gene variants cause glycolytic switch | Enhanced sensitivity to anti-angiogenic therapy; possibly reduced angiogenesis | [66,67] |
| Tumor context, low-heteroplasmy or rescue | mtDNA transfer or specific pathogenic mutations | Increased VEGF/MMP/CXCL12 expression, angiogenesis, and metastasis | [65,67] |
| Context | Target/Agent | Benefit | Limitations/Risks | Ref. |
|---|---|---|---|---|
| Ischemic Disease | MitoQ |
|
| [80,81] |
| Cancer | UQCRB inhibitors |
|
| [84,89] |
| Complex III inhibitors |
|
| [8] |
| Agent | Target | Mechanism | Strengths | Limitations/Challenges | Ref |
|---|---|---|---|---|---|
| Terpestacin | Inhibits HIF-1α/VEGF activation by reduction of hypoxia-driven mtROS production | Targeted mtROS inhibition without disrupting respiration | Needs validation in chronic diseases; EC-specific effects unknown | [84,85] | |
| Stigmatellin/ Matairesinol | Complex III Qo/Qi | Broad inhibition of electron flow that reduces mtROS production | mtROS suppression confirmed | High mitochondrial toxicity; limited in vivo efficacy data | [3] |
| Celastrol | Mito structure + AKT/mTOR | Mitochondrial disruption; AKT/mTOR inhibition | Multi-pathway anti-angiogenic and antitumor activity | Non-specific effects; limited mitochondrial-specific data | [95] |
| Rapamycin | mTORC1 | Impairs mitochondria via mitophagy suppression | Has clinical approval; effective in endothelial models | Broad metabolic impact; compensatory | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannito, S.; Giardino, I.; d’Apolito, M.; Pettoello-Mantovani, M.; Scaltrito, F.; Mangieri, D.; Piscazzi, A. The Multifaceted Role of Mitochondria in Angiogenesis. Int. J. Mol. Sci. 2025, 26, 7960. https://doi.org/10.3390/ijms26167960
Cannito S, Giardino I, d’Apolito M, Pettoello-Mantovani M, Scaltrito F, Mangieri D, Piscazzi A. The Multifaceted Role of Mitochondria in Angiogenesis. International Journal of Molecular Sciences. 2025; 26(16):7960. https://doi.org/10.3390/ijms26167960
Chicago/Turabian StyleCannito, Sara, Ida Giardino, Maria d’Apolito, Massimo Pettoello-Mantovani, Francesca Scaltrito, Domenica Mangieri, and Annamaria Piscazzi. 2025. "The Multifaceted Role of Mitochondria in Angiogenesis" International Journal of Molecular Sciences 26, no. 16: 7960. https://doi.org/10.3390/ijms26167960
APA StyleCannito, S., Giardino, I., d’Apolito, M., Pettoello-Mantovani, M., Scaltrito, F., Mangieri, D., & Piscazzi, A. (2025). The Multifaceted Role of Mitochondria in Angiogenesis. International Journal of Molecular Sciences, 26(16), 7960. https://doi.org/10.3390/ijms26167960







