Inhibition of Metalloproteinases-2, -9, and -14 Suppresses Papillary Thyroid Carcinoma Cell Migration and Invasion
Abstract
1. Introduction
2. Results
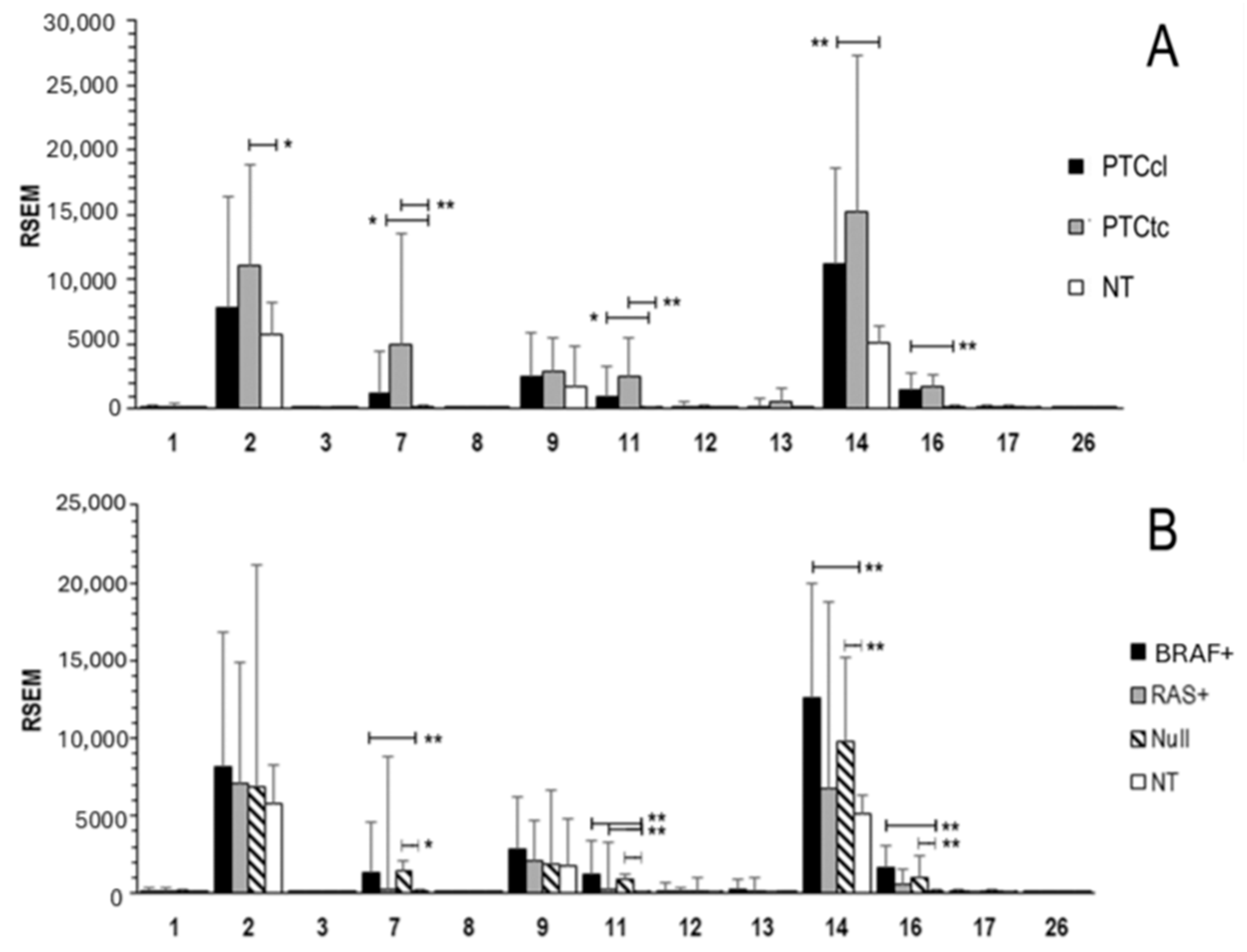
2.1. MMPs Expression in Normal Thyroid Tissue and in PTC
2.2. Association of MMPs Expression and Clinical Features
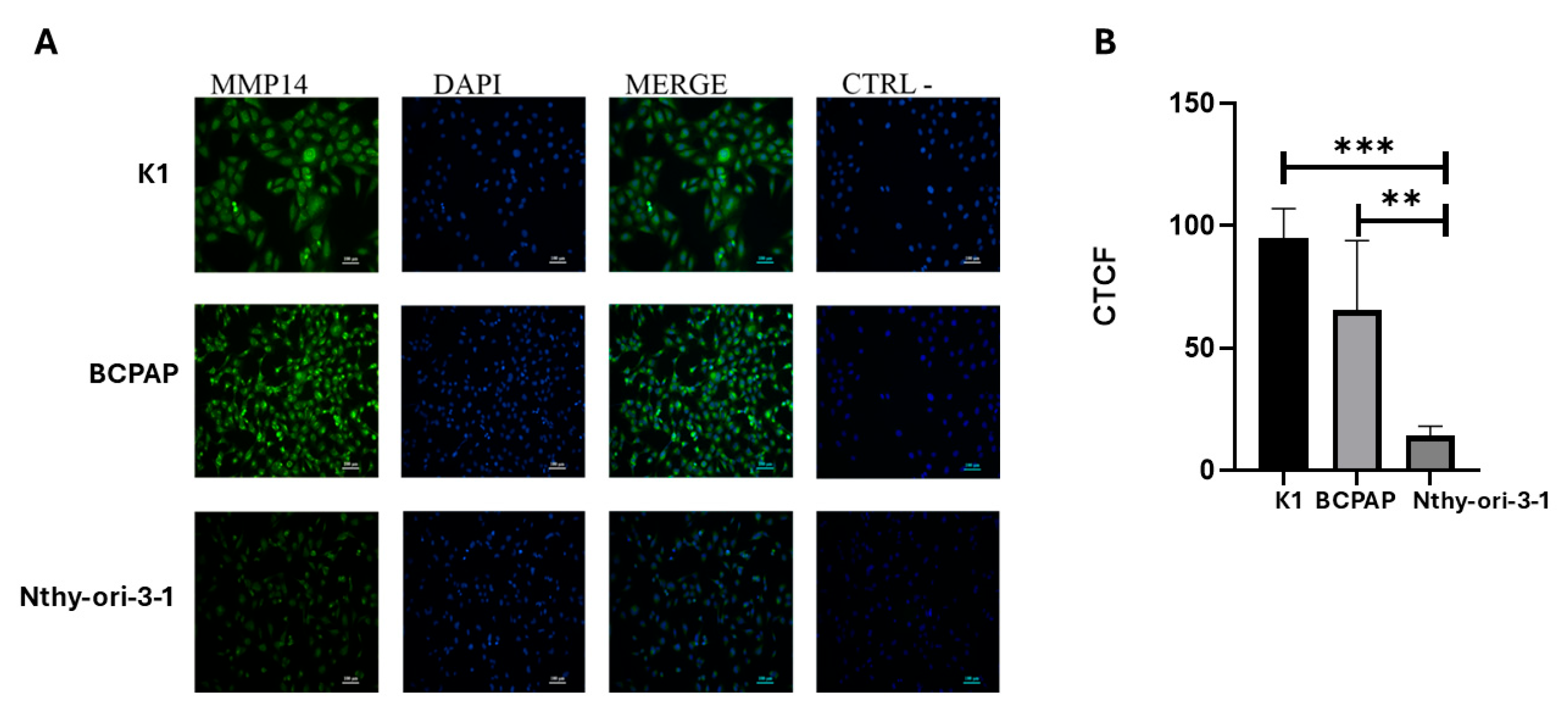
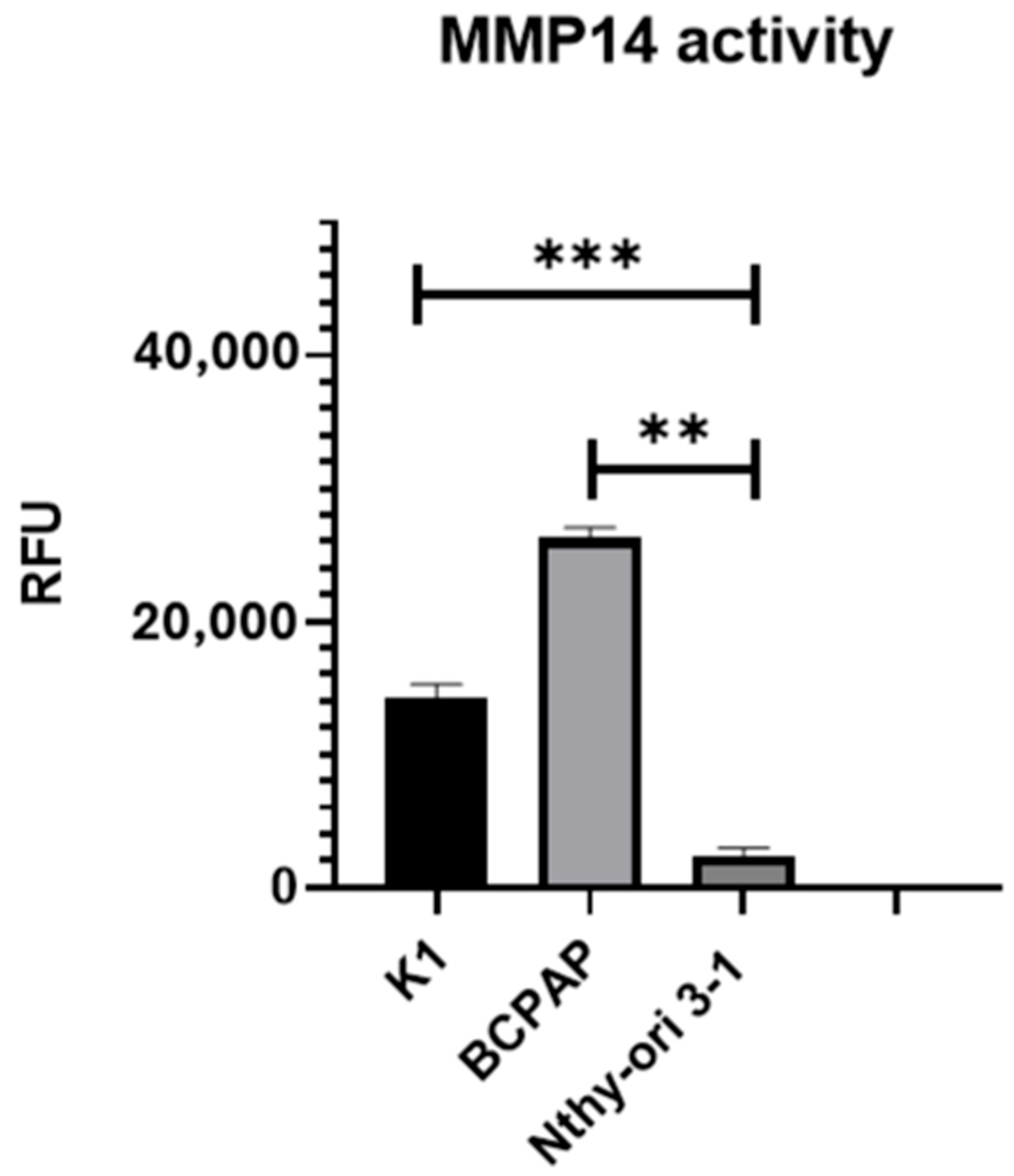
2.3. MMP-14 Expression and Activity in PTC Cell Lines
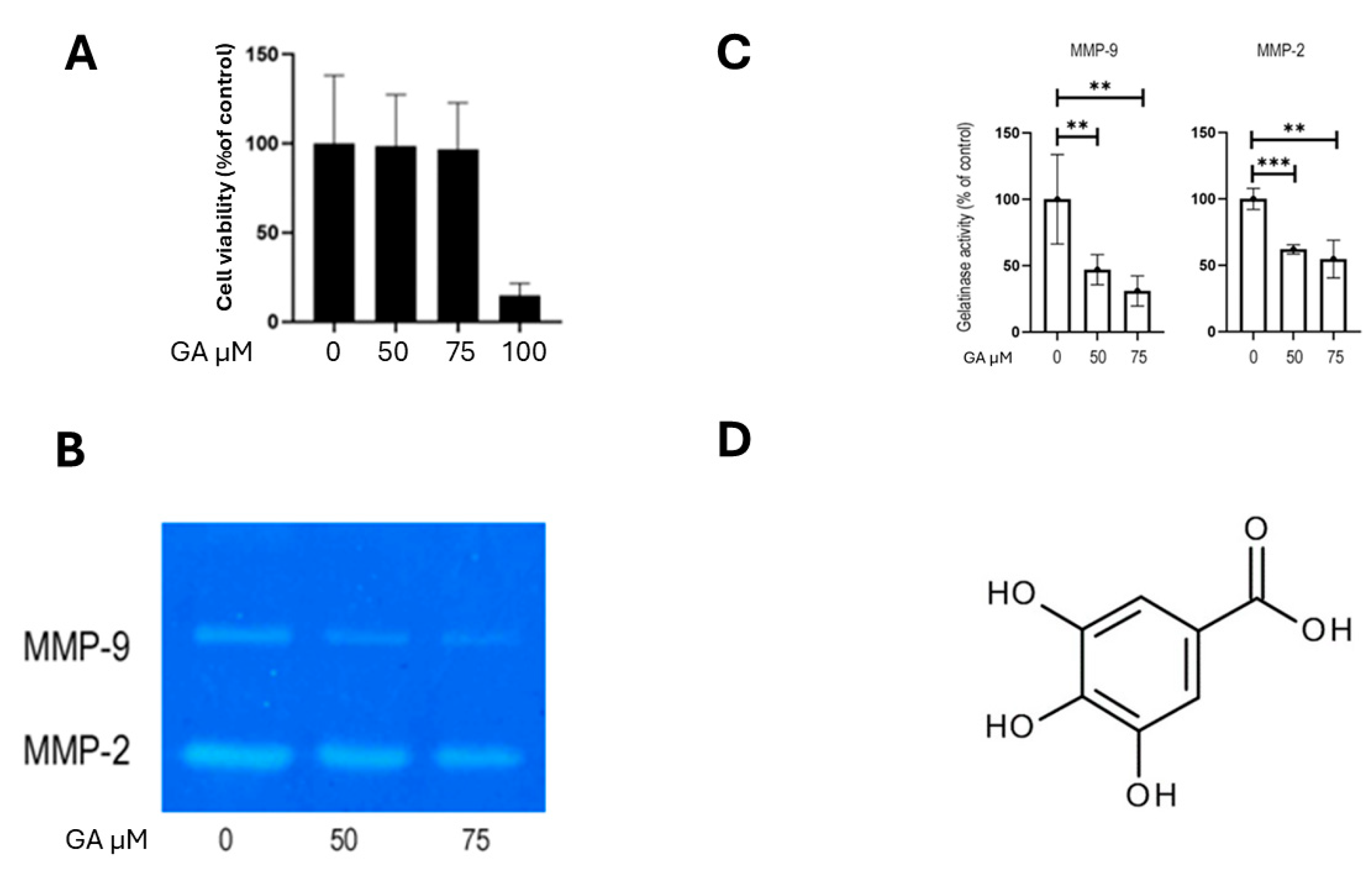
2.4. MMP-2 and MMP-9 Activity in PTC Cell Lines
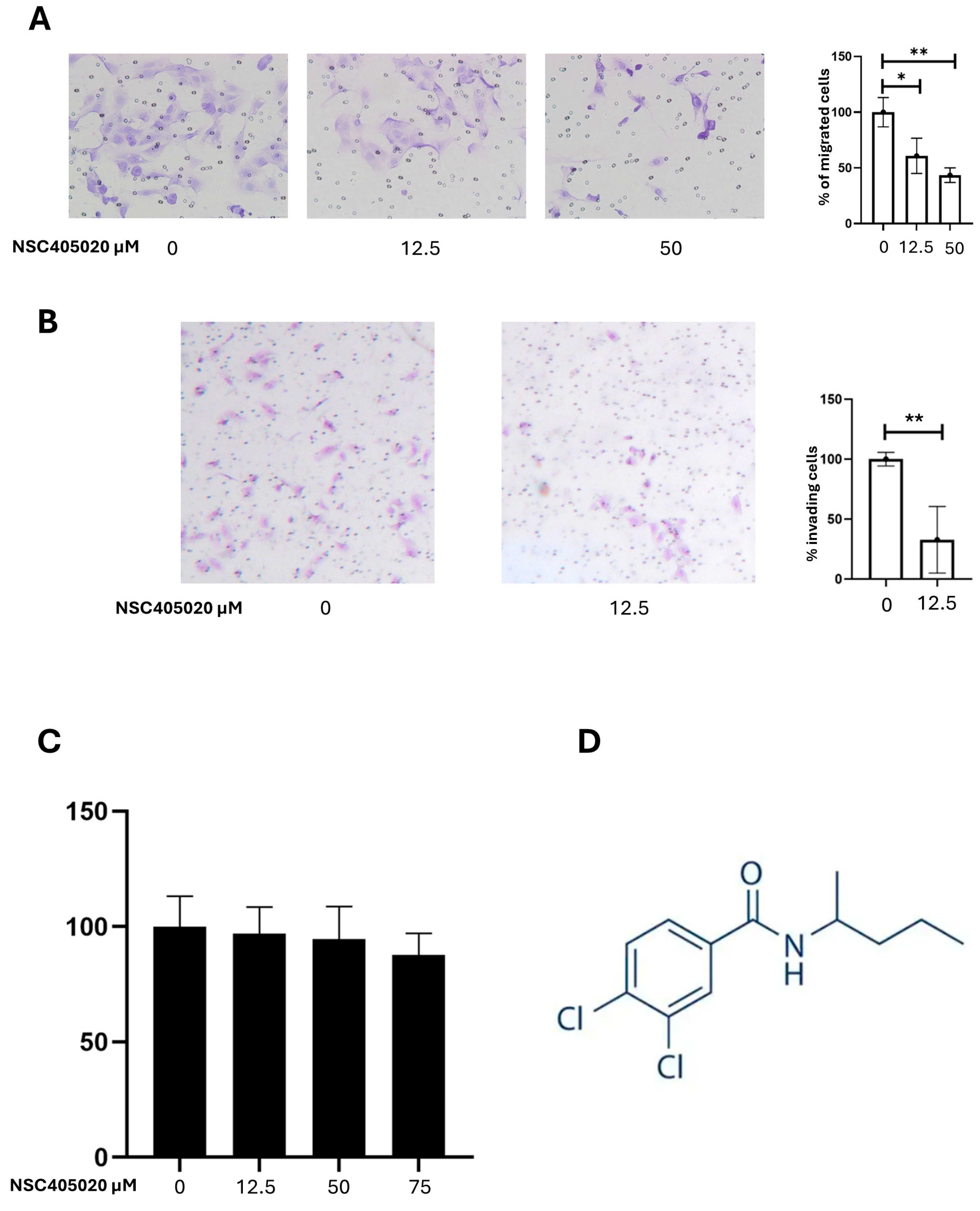
2.5. Inhibition of MMP-14 Activity Reduces K1 Cell Migration and Invasion
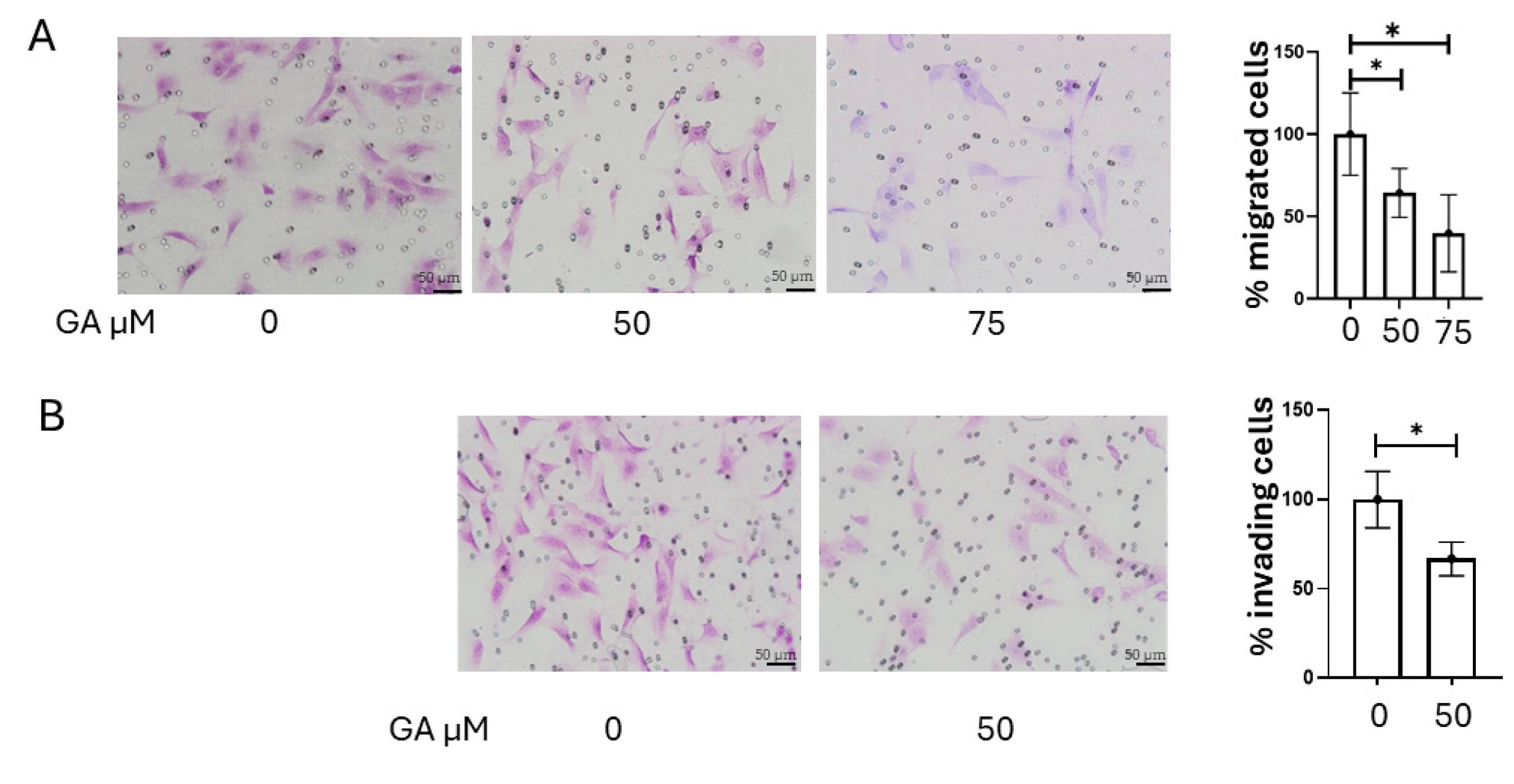
2.6. Inhibition of MMP-2 and MMP-9 Activity Reduces K1 Cell Invasion and Migration
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. RNA-Seq Analysis
4.3. Cell Lines and Culture Mediums
4.4. Fluorimetric MMP-14 Activity Assay
4.5. Immunofluorescence
4.6. Gelatin Gel Zymography
4.7. Cell Migration and Matrigel Invasion Assays
4.8. Cell-Viability (MTT) Assay
4.9. Crystal Violet-Based Cell-Viability Assay
4.10. Statical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| APMA | 4-aminophenylmercuric acetate |
| BCA | bicinchoninic acid |
| BSA | bovine serum albumin |
| CO2 | carbon dioxide |
| CTCF | Corrected Total Cell Fluorescence |
| DMEM/F12 | Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 |
| DMSO | dimethyl sulfoxide |
| ECM | extracellular matrix |
| FBS | Fetal Bovine Serum |
| FN | fibronectin |
| MMP | matrix metalloproteinase |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NT | normal thyroid |
| PBS | phosphate-buffered saline |
| PEX | hemopexin domain |
| PTC | papillary thyroid carcinoma |
| PTCcl | classical papillary thyroid carcinoma |
| PTCtc | tall cell-variant papillary thyroid carcinoma |
| RFU | relative fluorescence units |
| RPMI-1640 | Roswell Park Memorial Institute 1640 medium |
| RSEM | RNA-Seq by Expectation Maximization |
| SD | standard deviation |
| TCGA | The Cancer Genome Atlas |
| TIMP | tissue inhibitor of metalloproteinases |
References
- Lloyd, R.V.; Erickson, L.A.; Casey, M.B.; Lam, K.Y.; Lohse, C.M.; Asa, S.L.; Chan, J.K.C.; DeLellis, R.A.; Harach, H.R.; Kakudo, K.; et al. Observer Variation in the Diagnosis of Follicular Variant of Papillary Thyroid Carcinoma. Am. J. Surg. Pathol. 2004, 28, 1336–1340. [Google Scholar] [CrossRef]
- Limaiem, F.; Rehman, A.; Mazzoni, T. Papillary Thyroid Carcinoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sun, J.-H.; Li, Y.-R.; Chang, K.-H.; Liou, M.-J.; Lin, S.-F.; Tsai, S.-S.; Yu, M.-C.; Hsueh, C.; Chen, S.-T. Evaluation of Recurrence Risk in Patients with Papillary Thyroid Cancer through Tumor-Node-Metastasis Staging: A Single-Center Observational Study in Taiwan. Biomed. J. 2022, 45, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, F.; Dedhia, P.H.; Ringel, M.D. Thyroid Cancer, Recent Advances in Diagnosis and Therapy. Int. J. Cancer 2021, 149, 984–992. [Google Scholar] [CrossRef]
- Curran, S.; Murray, G.I. Matrix Metalloproteinases in Tumour Invasion and Metastasis. J. Pathol. 1999, 189, 300–308. [Google Scholar] [CrossRef]
- Li, Z.; Wei, J.; Chen, B.; Wang, Y.; Yang, S.; Wu, K.; Meng, X. The Role of MMP-9 and MMP-9 Inhibition in Different Types of Thyroid Carcinoma. Molecules 2023, 28, 3705. [Google Scholar] [CrossRef]
- Scannevin, R.H.; Alexander, R.; Haarlander, T.M.; Burke, S.L.; Singer, M.; Huo, C.; Zhang, Y.-M.; Maguire, D.; Spurlino, J.; Deckman, I.; et al. Discovery of a Highly Selective Chemical Inhibitor of Matrix Metalloproteinase-9 (MMP-9) That Allosterically Inhibits Zymogen Activation. J. Biol. Chem. 2017, 292, 17963–17974. [Google Scholar] [CrossRef]
- Hadler-Olsen, E.; Fadnes, B.; Sylte, I.; Uhlin-Hansen, L.; Winberg, J. Regulation of Matrix Metalloproteinase Activity in Health and Disease. FEBS J. 2011, 278, 28–45. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New Functions for the Matrix Metalloproteinases in Cancer Progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Fanjul-Fernández, M.; Folgueras, A.R.; Cabrera, S.; López-Otín, C. Matrix Metalloproteinases: Evolution, Gene Regulation and Functional Analysis in Mouse Models. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 3–19. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and Function of Matrix Metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Adley, B.P.; Gleason, K.J.; Yang, X.J.; Stack, M.S. Expression of Membrane Type 1 Matrix Metalloproteinase (MMP-14) in Epithelial Ovarian Cancer: High Level Expression in Clear Cell Carcinoma. Gynecol. Oncol. 2009, 112, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-C.; Huang, A.-C.; Wu, P.-P.; Lin, H.-Y.; Chueh, F.-S.; Yang, J.-S.; Lu, C.-C.; Chiang, J.-H.; Meng, M.; Chung, J.-G. Gallic Acid Suppresses the Migration and Invasion of PC-3 Human Prostate Cancer Cells via Inhibition of Matrix Metalloproteinase-2 and -9 Signaling Pathways. Oncol. Rep. 2011, 26, 177–184. [Google Scholar] [CrossRef]
- Maeta, H.; Ohgi, S.; Terada, T. Protein Expression of Matrix Metalloproteinases 2 and 9 and Tissue Inhibitors of Metalloproteinase 1 and 2 in Papillary Thyroid Carcinomas. Virchows Arch. 2001, 438, 121–128. [Google Scholar] [CrossRef]
- Marečko, I.; Cvejić, D.; Šelemetjev, S.; Paskaš, S.; Tatić, S.; Paunović, I.; Savin, S. Enhanced Activation of Matrix Metalloproteinase-9 Correlates with the Degree of Papillary Thyroid Carcinoma Infiltration. Croat. Med. J. 2014, 55, 128–137. [Google Scholar] [CrossRef]
- Zarkesh, M.; Zadeh-Vakili, A.; Akbarzadeh, M.; Fanaei, S.A.; Hedayati, M.; Azizi, F. The Role of Matrix Metalloproteinase-9 as a Prognostic Biomarker in Papillary Thyroid Cancer. BMC Cancer 2018, 18, 1199. [Google Scholar] [CrossRef]
- Ivković, I.; Limani, Z.; Jakovčević, A.; Huić, D.; Prgomet, D. Role of Matrix Metalloproteinases and Their Inhibitors in Locally Invasive Papillary Thyroid Cancer. Biomedicines 2022, 10, 3178. [Google Scholar] [CrossRef] [PubMed]
- Šelemetjev, S.; Đorić, I.; Paunović, I.; Tatić, S.; Cvejić, D. Coexpressed High Levels of VEGF-C and Active MMP-9 Are Associated with Lymphatic Spreading and Local Invasiveness of Papillary Thyroid Carcinoma. Am. J. Clin. Pathol. 2016, 146, 594–602. [Google Scholar] [CrossRef]
- Xu, D.; Su, C.; Guo, L.; Yan, H.; Wang, S.; Yuan, C.; Chen, G.; Pang, L.; Zhang, N. Predictive Significance of Serum MMP-9 in Papillary Thyroid Carcinoma. Open Life Sci. 2019, 14, 275–287. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Matrix Metalloproteinases and the Development of Cancer. Chem. Biol. 1996, 3, 895–904. [Google Scholar] [CrossRef]
- Overall, C.M.; Kleifeld, O. Tumour Microenvironment—Opinion: Validating Matrix Metalloproteinases as Drug Targets and Anti-Targets for Cancer Therapy. Nat. Rev. Cancer 2006, 6, 227–239. [Google Scholar] [CrossRef]
- Fidler, I.J. The Organ Microenvironment and Cancer Metastasis. Differentiation 2002, 70, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Takagi, J.; Coller, B.S.; Wang, J.-H.; Springer, T.A. Structural Basis for Allostery in Integrins and Binding to Fibrinogen-Mimetic Therapeutics. Nature 2004, 432, 59–67. [Google Scholar] [CrossRef]
- Marotta, V.; Rocco, D.; Crocco, A.; Deiana, M.G.; Martinelli, R.; Di Gennaro, F.; Valeriani, M.; Valvano, L.; Caleo, A.; Pezzullo, L.; et al. Survival Predictors of Radioiodine-Refractory Differentiated Thyroid Cancer Treated with Lenvatinib in Real Life. J. Clin. Endocrinol. Metab. 2024, 109, 2541–2552. [Google Scholar] [CrossRef]
- Prete, A.; Borges De Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef]
- Vitale, M.; Illario, M.; Di Matola, T.; Casamassima, A.; Fenzi, G.; Rossi, G. Integrin Binding to Immobilized Collagen and Fibronectin Stimulates the Proliferation of Human Thyroid Cells in Culture. Endocrinology 1997, 138, 1642–1648. [Google Scholar] [CrossRef]
- Vitale, M. Fibronectin Is Required to Prevent Thyroid Cell Apoptosis through an Integrin-Mediated Adhesion Mechanism. J. Clin. Endocrinol. Metab. 1998, 83, 3673–3680. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mautone, L.; Ferravante, C.; Tortora, A.; Tarallo, R.; Giurato, G.; Weisz, A.; Vitale, M. Higher Integrin Alpha 3 Beta1 Expression in Papillary Thyroid Cancer Is Associated with Worst Outcome. Cancers 2021, 13, 2937. [Google Scholar] [CrossRef] [PubMed]
- Rocco, D.; Tortora, A.; Marotta, V.; Machado, A.M.; Selistre-de-Araujo, H.S.; Vitale, M. Integrin-Fibronectin Interaction Is a Pivotal Biological and Clinical Determinant in Papillary Thyroid Carcinoma. Endocr. Relat. Cancer 2025, 32, e250101. [Google Scholar] [CrossRef]
- Remacle, A.G.; Golubkov, V.S.; Shiryaev, S.A.; Dahl, R.; Stebbins, J.L.; Chernov, A.V.; Cheltsov, A.V.; Pellecchia, M.; Strongin, A.Y. Novel MT1-MMP Small-Molecule Inhibitors Based on Insights into Hemopexin Domain Function in Tumor Growth. Cancer Res. 2012, 72, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Woessner, J.F. Matrix Metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef]
- Chakraborti, S.; Mandal, M.; Das, S.; Mandal, A.; Chakraborti, T. Regulation of Matrix Metalloproteinases: An Overview. Mol. Cell Biochem. 2003, 253, 269–285. [Google Scholar] [CrossRef]
- Sato, H.; Takino, T.; Okada, Y.; Cao, J.; Shinagawa, A.; Yamamoto, E.; Seiki, M. A Matrix Metalloproteinase Expressed on the Surface of Invasive Tumour Cells. Nature 1994, 370, 61–65. [Google Scholar] [CrossRef]
- Strongin, A.Y.; Collier, I.; Bannikov, G.; Marmer, B.L.; Grant, G.A.; Goldberg, G.I. Mechanism of Cell Surface Activation of 72-kDa Type IV Collagenase. Isolation of the Activated Form of the Membrane Metalloprotease. J. Biol. Chem. 1995, 270, 5331–5338. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Tomasetto, C.; Lutz, Y.; Bellocq, J.P.; Rio, M.C.; Basset, P. Expression of Matrix Metalloproteinases during Rat Skin Wound Healing: Evidence That Membrane Type-1 Matrix Metalloproteinase Is a Stromal Activator of pro-Gelatinase A. J. Cell Biol. 1997, 137, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, J.; Feng, J.; Klocker, H.; Lee, C.; Zhang, J. Type IV Collagenase (Matrix Metalloproteinase-2 and -9) in Prostate Cancer. Prostate Cancer Prostatic Dis. 2004, 7, 327–332. [Google Scholar] [CrossRef]
- Bjørnland, K.; Flatmark, K.; Pettersen, S.; Aaasen, A.O.; Fodstad, O.; Maelandsmo, G.M. Matrix Metalloproteinases Participate in Osteosarcoma Invasion. J. Surg. Res. 2005, 127, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Guo, Y.; Liu, L.; Ye, R.; Liang, W.; Li, H.; Xiao, H.; Li, Y. INAVA Promotes Aggressiveness of Papillary Thyroid Cancer by Upregulating MMP9 Expression. Cell Biosci. 2018, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-T.; Li, Y.; Jiang, Q.-L.; Jiang, R.; Zeng, Y.; Jiang, J. Human Adipose-Derived Stem Cells Promote Migration of Papillary Thyroid Cancer Cell via Leptin Pathway. Ann. Med. 2024, 56, 2419990. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.; Barralet, J.; Harvey, E.J.; Merle, G. Detecting the PEX Like Domain of Matrix Metalloproteinase-14 (MMP-14) with Therapeutic Conjugated CNTs. Biosensors 2022, 12, 884. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Lai, K.-C.; Ma, Y.-S.; Weng, S.-W.; Lin, J.-P.; Chung, J.-G. Gallic Acid Inhibits Migration and Invasion of SCC-4 Human Oral Cancer Cells through Actions of NF-κB, Ras and Matrix Metalloproteinase-2 and -9. Oncol. Rep. 2014, 32, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.-H.S.; Yen, J.-H.; Wu, H.-T.; Huang, S.-T. Gallic Acid Inhibited Matrix Invasion and AP-1/ETS-1-Mediated MMP-1 Transcription in Human Nasopharyngeal Carcinoma Cells. Int. J. Mol. Sci. 2017, 18, 1354. [Google Scholar] [CrossRef]
- Libertini, S.; Iacuzzo, I.; Ferraro, A.; Vitale, M.; Bifulco, M.; Fusco, A.; Portella, G. Lovastatin Enhances the Replication of the Oncolytic Adenovirus Dl1520 and Its Antineoplastic Activity against Anaplastic Thyroid Carcinoma Cells. Endocrinology 2007, 148, 5186–5194. [Google Scholar] [CrossRef]
- Giuffrida, D.; Prestifilippo, A.; Scarfia, A.; Martino, D.; Marchisotta, S. New Treatment in Advanced Thyroid Cancer. J. Oncol. 2012, 2012, 391629. [Google Scholar] [CrossRef]
- Cherifi, F.; Awada, A. Molecular Oncology of Iodine Refractory Thyroid Cancer Current Therapies and Perspective. Crit. Rev. Oncol./Hematol. 2025, 209, 104679. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]

| Nodal Status | ||||||
|---|---|---|---|---|---|---|
| Gene | N0 | N1 | p | |||
| MMP14 | 9735 | 13,210 | 0.0001 | |||
| MMP16 | 1172 | 1657 | 0.0012 | |||
| Disease stage | ||||||
| I | II | III | IV | p | ||
| MMP14 | 10,454 | 10,313 | 12,475 | 13,769 | 0.0419 | |
| Extra thyroidal extension | ||||||
| None | Minimal | Moderate/advanced | p | |||
| MMP14 | 10,439 | 13,385 | 14,231 | 0.0045 | ||
| Risk group a | ||||||
| Low | Intermediate | High | p | |||
| MMP14 | 9196 | 11,875 | 15,930 | 0.0002 | ||
| MMP16 | 979 | 1615 | 1797 | 0.0003 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocco, D.; Marotta, V.; Palumbo, D.; Vitale, M. Inhibition of Metalloproteinases-2, -9, and -14 Suppresses Papillary Thyroid Carcinoma Cell Migration and Invasion. Int. J. Mol. Sci. 2025, 26, 7956. https://doi.org/10.3390/ijms26167956
Rocco D, Marotta V, Palumbo D, Vitale M. Inhibition of Metalloproteinases-2, -9, and -14 Suppresses Papillary Thyroid Carcinoma Cell Migration and Invasion. International Journal of Molecular Sciences. 2025; 26(16):7956. https://doi.org/10.3390/ijms26167956
Chicago/Turabian StyleRocco, Domenico, Vincenzo Marotta, Domenico Palumbo, and Mario Vitale. 2025. "Inhibition of Metalloproteinases-2, -9, and -14 Suppresses Papillary Thyroid Carcinoma Cell Migration and Invasion" International Journal of Molecular Sciences 26, no. 16: 7956. https://doi.org/10.3390/ijms26167956
APA StyleRocco, D., Marotta, V., Palumbo, D., & Vitale, M. (2025). Inhibition of Metalloproteinases-2, -9, and -14 Suppresses Papillary Thyroid Carcinoma Cell Migration and Invasion. International Journal of Molecular Sciences, 26(16), 7956. https://doi.org/10.3390/ijms26167956






