Maternal Extra Virgin Olive Oil Supplementation Enhances Offspring Immune Function: A Preclinical Study
Abstract
1. Introduction
2. Results
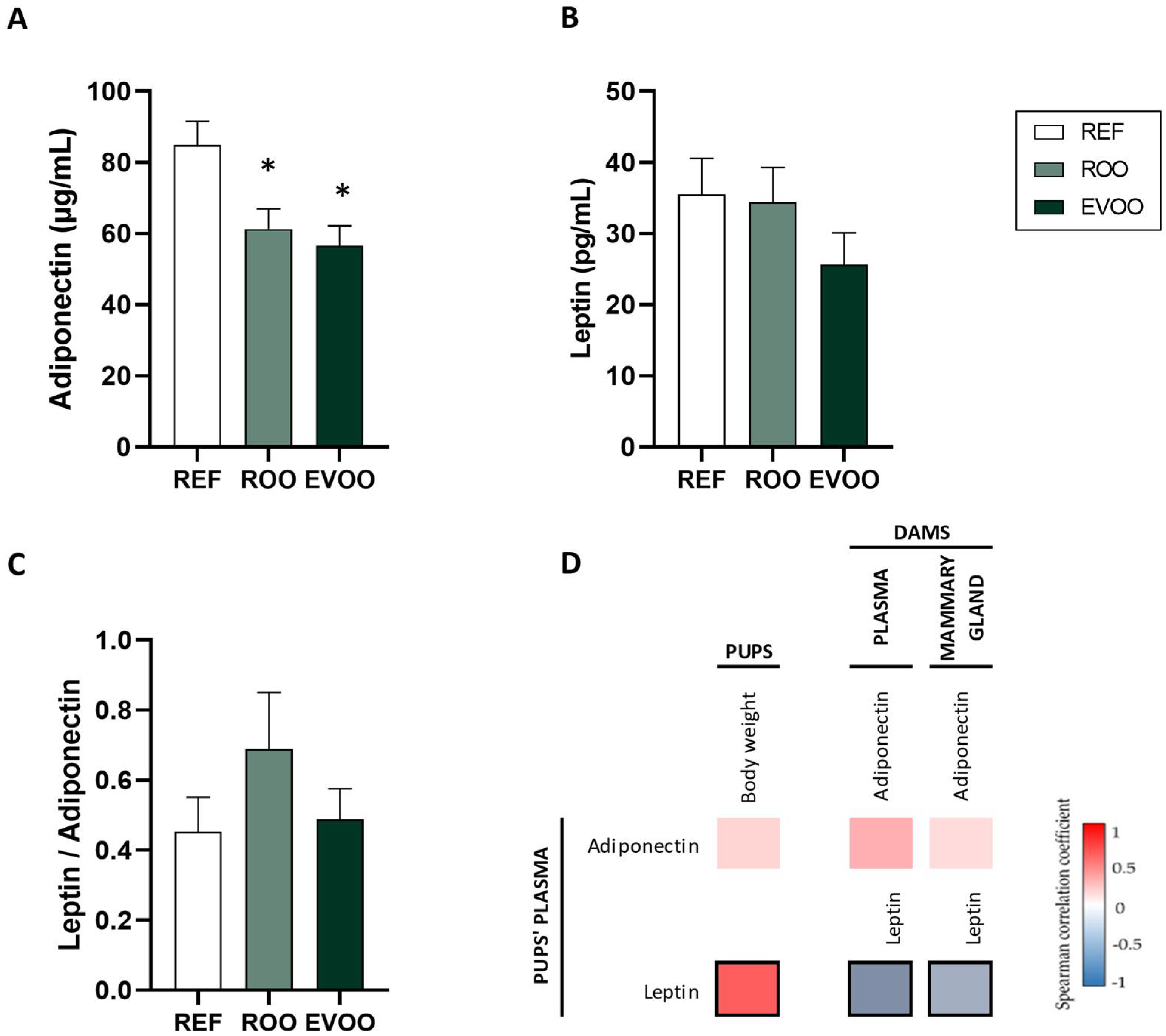
2.1. Body Weight and Morphometric Variables
2.2. Immunoglobulin Profile in Plasma
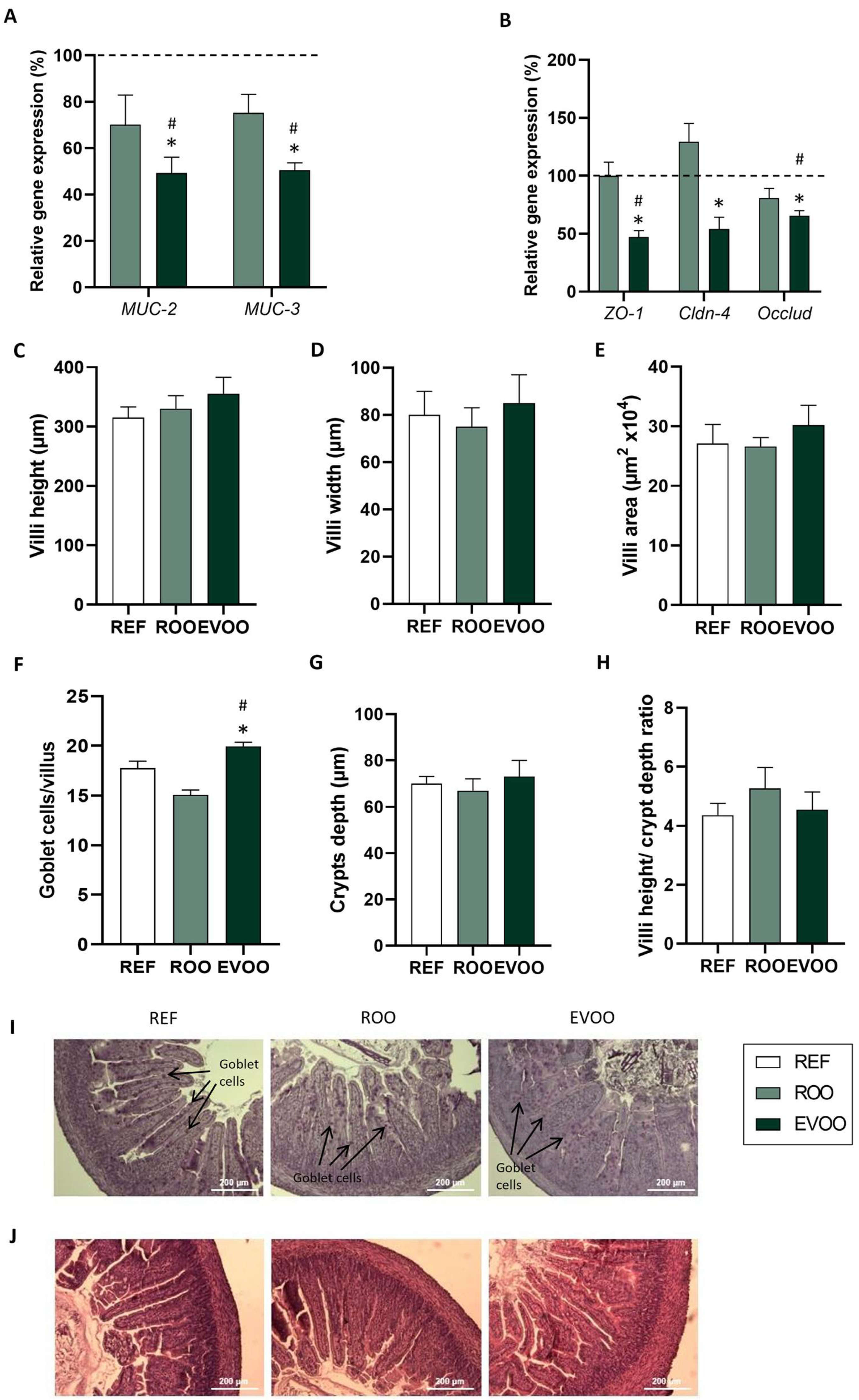
2.3. Intestinal Effects of EVOO
2.4. In Vitro Effect of EVOO Metabolites on Caco-2 Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Sample Collection and Processing
4.4. Immunoglobulins Quantification
4.5. Galectins, Adiponectin, and Leptin Quantification
4.6. Histology
4.7. Gene Expression Study
4.8. In Vitro Experimentation
4.8.1. Cell Culture
4.8.2. Lactate Dehydrogenase Activity
4.8.3. Measurement of Trans-Epithelial Electrical Resistance (TEER)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BW | Body weight |
| EVOO | Extra virgin olive oil |
| HCM | Mean corpuscular hemoglobin |
| HCT | Hematocrit |
| HFA | Hydroxyphenylacetic acid |
| HGB | Hemoglobulin |
| HPP | Hippuric acid |
| HV | Homovanillic acid |
| Ig | Immunoglobulin |
| IS | Immune system |
| LDH | Lactate dehydrogenase |
| REF | Reference |
| ROO | Refined olive oil |
| TLR | Toll-like receptors |
| TEER | Trans-Epithelial Electrical Resistance |
| VCM | Mean corpuscular volume |
References
- Schelonka, R.L.; Infante, A.J. Neonatal Immunology. Semin. Perinatol. 1998, 22, 2–14. [Google Scholar] [CrossRef]
- Agnovia, A.M. The Role of Innate and Acquired Immunity in Susceptibility to Infections in Neonates. IDOSR J. Biol. Chem. Pharm. 2025, 10, 19–23. [Google Scholar] [CrossRef]
- Cacho, N.; Lawrence, R. Innate Immunity and Breast Milk. Front. Immunol. 2017, 8, 584. [Google Scholar] [CrossRef]
- Grases-Pintó, B.; Torres-Castro, P.; Marín-Morote, L.; Abril-Gil, M.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J.; Franch, À. Leptin and EGF Supplementation Enhance the Immune System Maturation in Preterm Suckling Rats. Nutrients 2019, 11, 2380. [Google Scholar] [CrossRef] [PubMed]
- Dzidic, M.; Boix-Amorós, A.; Selma-Royo, M.; Mira, A.; Collado, M.C. Gut Microbiota and Mucosal Immunity in the Neonate. Med. Sci. 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Falize, C.; Savage, M.; Jeanes, Y.M.; Dyall, S.C. Evaluating the Relationship between the Nutrient Intake of Lactating Women and Their Breast Milk Nutritional Profile: A Systematic Review and Narrative Synthesis. Br. J. Nutr. 2024, 131, 1196–1224. [Google Scholar] [CrossRef]
- Favara, G.; Maugeri, A.; Barchitta, M.; Lanza, E.; Lio, M.S.; Agodi, A. Maternal Lifestyle Factors Affecting Breast Milk Composition and Infant Health: A Systematic Review. Nutrients 2024, 17, 62. [Google Scholar] [CrossRef]
- Hyötyläinen, T.; Ghaffarzadegan, T.; Karthikeyan, B.; Triplett, E.; Orešič, M.; Ludvigsson, J. Impact of Environmental Exposures on Human Breast Milk Lipidome in Future Immune-Mediated Diseases. Environ. Sci. Technol. 2024, 58, 2214–2223. [Google Scholar] [CrossRef] [PubMed]
- Hascoët, J.M.; Chauvin, M.; Pierret, C.; Skweres, S.; Van Egroo, L.D.; Rougé, C.; Franck, P. Impact of Maternal Nutrition and Perinatal Factors on Breast Milk Composition after Premature Delivery. Nutrients 2019, 11, 366. [Google Scholar] [CrossRef]
- Martínez García, R.M.; Jiménez Ortega, A.I.; Peral Suárez, Á.; Bermejo López, L.M.; Rodríguez-Rodríguez, E. Importance of nutrition during pregnancy. Impact on the composition of breast milk. Nutr. Hosp. 2021, 37, 38–42. [Google Scholar] [CrossRef]
- Hoppu, U.; Isolauri, E.; Laakso, P.; Matomäki, J.; Laitinen, K. Probiotics and Dietary Counselling Targeting Maternal Dietary Fat Intake Modifies Breast Milk Fatty Acids and Cytokines. Eur. J. Nutr. 2012, 51, 211–219. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra Virgin Olive Oil: More than a Healthy Fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Castellón, J.; López-Yerena, A.; Rinaldi de Alvarenga, J.F.; Romero del Castillo-Alba, J.; Vallverdú-Queralt, A.; Escribano-Ferrer, E.; Lamuela-Raventós, R.M. Health-Promoting Properties of Oleocanthal and Oleacein: Two Secoiridoids from Extra-Virgin Olive Oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 2532–2548. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, Antioxidant and Anti-Inflammatory Phenolic Activities in Extra Virgin Olive Oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Zhan-Dai, S.; Grases-Pintó, B.; Lamuela-Raventós, R.M.; Castell, M.; Pérez-Cano, F.J.; Vallverdú-Queralt, A.; Rodríguez-Lagunas, M.J. Exploring the Impact of Extra Virgin Olive Oil on Maternal Immune System and Breast Milk Composition in Rats. Nutrients 2024, 16, 1785. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, R.P.; Quesada-Granados, J.J.; del Pilar Ramírez-Anaya, J.; Peña-Díaz, J.; Blanca-Herrera, R.; Samaniego-Sánchez, C. Bioactive Compounds in Spanish Extra Virgin Olive Oils: Migration and Stability According to the Culinary Technique Used. Food Res. Int. 2023, 172, 113191. [Google Scholar] [CrossRef] [PubMed]
- Nikou, T.; Liaki, V.; Stathopoulos, P.; Sklirou, A.D.; Tsakiri, E.N.; Jakschitz, T.; Bonn, G.; Trougakos, I.P.; Halabalaki, M.; Skaltsounis, L.A. Comparison Survey of EVOO Polyphenols and Exploration of Healthy Aging-Promoting Properties of Oleocanthal and Oleacein. Food Chem. Toxicol. 2019, 125, 403–412. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic Molecules in Virgin Olive Oils: A Survey of Their Sensory Properties, Health Effects, Antioxidant Activity and Analytical Methods. An Overview of the Last Decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Deiana, M.; Serra, G.; Corona, G. Modulation of Intestinal Epithelium Homeostasis by Extra Virgin Olive Oil Phenolic Compounds. Food Funct. 2018, 9, 4085–4099. [Google Scholar] [CrossRef]
- Cuffaro, D.; Bertini, S.; Macchia, M.; Digiacomo, M. Enhanced Nutraceutical Properties of Extra Virgin Olive Oil Extract by Olive Leaf Enrichment. Nutrients 2023, 15, 1073. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Rodríguez, L.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Illescas-Montesa, R.; Costela-Ruiz, V.; García-Martínez, O. Potential Effects of Phenolic Compounds That Can Be Found in Olive Oil on Wound Healing. Foods 2021, 10, 1642. [Google Scholar] [CrossRef]
- Francisco, V.; Ruiz-Fernández, C.; Lahera, V.; Lago, F.; Pino, J.; Skaltsounis, L.; González-Gay, M.A.; Mobasheri, A.; Gómez, R.; Scotece, M.; et al. Natural Molecules for Healthy Lifestyles: Oleocanthal from Extra Virgin Olive Oil. J. Agric. Food Chem. 2019, 67, 3845–3853. [Google Scholar] [CrossRef]
- Nocella, C.; Cammisotto, V.; Fianchini, L.; D’Amico, A.; Novo, M.; Castellani, V.; Stefanini, L.; Violi, F.; Carnevale, R. Extra Virgin Olive Oil and Cardiovascular Diseases: Benefits for Human Health. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 4–13. [Google Scholar] [CrossRef]
- Milena, E.; Maurizio, M. Exploring the Cardiovascular Benefits of Extra Virgin Olive Oil: Insights into Mechanisms and Therapeutic Potential. Biomolecules 2025, 15, 284. [Google Scholar] [CrossRef]
- García, R.G.; Claros, C.F.G. Heart-Healthy Effect of Extra Virgin Olive Oil: A Bibliographic Review. Enfermería Cuid. 2023, 6, 71–84. [Google Scholar] [CrossRef]
- Marrone, G.; Urciuoli, S.; Di Lauro, M.; Ruzzolini, J.; Ieri, F.; Vignolini, P.; Di Daniele, F.; Guerriero, C.; Nediani, C.; Di Daniele, N.; et al. Extra Virgin Olive Oil and Cardiovascular Protection in Chronic Kidney Disease. Nutrients 2022, 14, 4265. [Google Scholar] [CrossRef] [PubMed]
- López-Yerena, A.; Grases-Pintó, B.; Zhan-Dai, S.; Pérez-Cano, F.J.; Lamuela-Raventos, R.M.; Rodríguez-Lagunas, M.J.; Vallverdú-Queralt, A. Nutrition during Pregnancy and Lactation: New Evidence for the Vertical Transmission of Extra Virgin Olive Oil Phenolic Compounds in Rats. Food Chem. 2022, 391, 133211. [Google Scholar] [CrossRef]
- Ogawa, C.; Bankoti, R.; Nguyen, T.; Hassanzadeh-Kiabi, N.; Nadeau, S.; Porritt, R.; Couse, M.; Fan, X.; Dhall, D.; Eberl, G.; et al. Blimp-1 Functions as a Molecular Switch to Prevent Inflammatory Activity in Foxp3+RORγt+ Regulatory T Cells. Cell Rep. 2018, 25, 19–28. [Google Scholar] [CrossRef]
- Giragossian, C.; Clark, T.; Piché-Nicholas, N.; Bowman, C. Neonatal Fc Receptor and Its Role in the Absorption, Distribution, Metabolism and Excretion of Immunoglobulin G-Based Biotherapeutics. Curr. Drug Metab. 2013, 14, 764–790. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.N.; Koohdani, F.; Shidfar, F.; Eslaminejad, M.B.; Izadi, P.; Eshraghian, M.; Shafieineek, L.; Tohidinik, H. Effects of Maternal Isocaloric Diet Containing Different Amounts of Soy Oil and Extra Virgin Olive Oil on Weight, Serum Glucose, and Lipid Profile of Female Mice Offspring. Iran. J. Med. Sci. 2017, 42, 161–169. [Google Scholar] [PubMed]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.-Y.; Shi, Y.; et al. Hydroxytyrosol Prevents Diet-Induced Metabolic Syndrome and Attenuates Mitochondrial Abnormalities in Obese Mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Jemai, H.; Fki, I.; Bouaziz, M.; Bouallagui, Z.; El Feki, A.; Isoda, H.; Sayadi, S. Lipid-Lowering and Antioxidant Effects of Hydroxytyrosol and Its Triacetylated Derivative Recovered from Olive Tree Leaves in Cholesterol-Fed Rats. J. Agric. Food Chem. 2008, 56, 2630–2636. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef]
- Abou-Samra, M.; Selvais, C.; Dubuisson, N.; Brichard, S. Adiponectin and Its Mimics on Skeletal Muscle: Insulin Sensitizers, Fat Burners, Exercise Mimickers, Muscling Pills … or Everything Together? Int. J. Mol. Sci. 2020, 21, 2620. [Google Scholar] [CrossRef] [PubMed]
- Laws, J.; Litten, J.C.; Laws, A.; Lean, I.J.; Dodds, P.F.; Clarke, L. Effect of Type and Timing of Oil Supplements to Sows during Pregnancy on the Growth Performance and Endocrine Profile of Low and Normal Birth Weight Offspring. Br. J. Nutr. 2008, 101, 240–249. [Google Scholar] [CrossRef]
- Oh, Y.T.; Oh, H.H.; Nguyen, A.-K.; Choi, C.S.; Youn, J.H. Circulating Free Fatty Acids Inhibit Food Intake in an Oleate-Specific Manner in Rats. Physiol. Behav. 2016, 167, 194–201. [Google Scholar] [CrossRef]
- Priego, T.; Sánchez, J.; García, A.P.; Palou, A.; Picó, C. Maternal Dietary Fat Affects Milk Fatty Acid Profile and Impacts on Weight Gain and Thermogenic Capacity of Suckling Rats. Lipids 2013, 48, 481–495. [Google Scholar] [CrossRef]
- Harmer, N.J.; Chahwan, R. Isotype Switching: Mouse IgG3 Constant Region Drives Increased Affinity for Polysaccharide Antigens. Virulence 2016, 7, 623–626. [Google Scholar] [CrossRef]
- Der Balian, G.P.; Slack, J.; Clevinger, B.L.; Bazin, H.; Davie, J.M. Subclass Restriction of Murine Antibodies. III. Antigens That Stimulate IgG3 in Mice Stimulate IgG2c in Rats. J. Exp. Med. 1980, 152, 209–218. [Google Scholar] [CrossRef]
- Sáez-Fuertes, L.; Kapravelou, G.; Grases-Pintó, B.; Bernabeu, M.; Knipping, K.; Garssen, J.; Bourdet-Sicard, R.; Castell, M.; Collado, M.C.; Pérez-Cano, F.J.; et al. Maternal Synbiotic Supplementation with B. Breve M-16V and ScGOS/LcFOS Shape Offspring Immune Development and Gut Microbiota at the End of Suckling. Nutrients 2024, 16, 1890. [Google Scholar] [CrossRef]
- Caballero-Flores, G.; Sakamoto, K.; Zeng, M.; Wang, Y.; Hakim, J.; Matus-Acuña, V.; Inohara, N.; Núñez, G. Maternal Immunization Confers Protection to the Offspring against an Attaching and Effacing Pathogen through Delivery of IgG in Breast Milk. Cell Host Microbe 2019, 25, 313–323. [Google Scholar] [CrossRef]
- Zhu, J.; Naughton, S.; Bowman, N.; LeRioth, T.; Luo, X.; Leeth, C. Maternal Antibody Repertoire Restriction Modulates the Development of Lupus-like Disease in BXSB Offspring. Int. Immunol. 2022, 35, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Capano, G.; Bloch, K.J.; Schiffrin, E.J.; Dascoli, J.A.; Israel, E.J.; Harmatz, P.R. Influence of the Polyamine, Spermidine, on Intestinal Maturation and Dietary Antigen Uptake in the Neonatal Rat. J. Pediatr. Gastroenterol. Nutr. 1994, 19, 34–42. [Google Scholar] [CrossRef]
- Szikora, B.; Marx, A.; Jani, P.K.; Pipek, O.; Müller, V.; Csabai, I.; Kacskovics, I. FcRn Overexpression Expands Diversity of the Humoral Immune Response in BFcRn Transgenic Mice. Front. Immunol. 2020, 11, 1887. [Google Scholar] [CrossRef]
- Cervenak, J.; Bender, B.; Schneider, Z.; Magna, M.; Carstea, B.V.; Liliom, K.; Erdei, A.; Bosze, Z.; Kacskovics, I. Neonatal FcR Overexpression Boosts Humoral Immune Response in Transgenic Mice. J. Immunol. 2011, 186, 959–968. [Google Scholar] [CrossRef]
- Birchenough, G.; Johansson, M.; Gustafsson, J.; Bergström, J.; Hansson, G. New Developments in Goblet Cell Mucus Secretion and Function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Hansson, G. Immunological Aspects of Intestinal Mucus and Mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef]
- Yang, S.; Yu, M. Role of Goblet Cells in Intestinal Barrier and Mucosal Immunity. J. Inflamm. Res. 2021, 14, 3171–3183. [Google Scholar] [CrossRef] [PubMed]
- Nyström, E.; Martinez-Abad, B.; Arike, L.; Birchenough, G.; Nonnecke, E.; Castillo, P.; Svensson, F.; Bevins, C.; Hansson, G.; Johansson, M. An Intercrypt Subpopulation of Goblet Cells Is Essential for Colonic Mucus Barrier Function. Science 2021, 372, eabb1590. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. The Role of MUC2 Mucin in Intestinal Homeostasis and the Impact of Dietary Components on MUC2 Expression. Int. J. Biol. Macromol. 2020, 164, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Pierre, J.; Heneghan, A.; Feliciano, R.; Shanmuganayagam, D.; Roenneburg, D.; Krueger, C.; Reed, J.; Kudsk, K. Cranberry Proanthocyanidins Improve the Gut Mucous Layer Morphology and Function in Mice Receiving Elemental Enteral Nutrition. JPEN J. Parenter. Enter. Nutr. 2013, 37, 401–409. [Google Scholar] [CrossRef]
- Hill, A.; Diehl, G. Identifying the Patterns of Pattern Recognition Receptors. Immunity 2018, 49, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.; Kuolee, R.; Chen, W. Role of Toll-like Receptors in Health and Diseases of Gastrointestinal Tract. World J. Gastroenterol. 2006, 12, 2149–2160. [Google Scholar] [CrossRef] [PubMed]
- Price, A.; Shamardani, K.; Lugo, K.; Deguine, J.; Roberts, A.; Lee, B.; Barton, G. A Map of Toll-like Receptor Expression in the Intestinal Epithelium Reveals Distinct Spatial, Cell Type-Specific, and Temporal Patterns. Immunity 2018, 49, 560. [Google Scholar] [CrossRef]
- Millman, J.; Okamoto, S.; Teruya, T.; Uema, T.; Ikematsu, S.; Shimabukuro, M.; Masuzaki, H. Extra-Virgin Olive Oil and the Gut-Brain Axis: Influence on Gut Microbiota, Mucosal Immunity, and Cardiometabolic and Cognitive Health. Nutr. Rev. 2021, 79, 1362–1374. [Google Scholar] [CrossRef]
- Millman, J.; Okamoto, S.; Kimura, A.; Uema, T.; Higa, M.; Yonamine, M.; Namba, T.; Ogata, E.; Yamazaki, S.; Shimabukuro, M.; et al. Metabolically and Immunologically Beneficial Impact of Extra Virgin Olive and Flaxseed Oils on Composition of Gut Microbiota in Mice. Eur. J. Nutr. 2019, 59, 2411–2425. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Shi, A.-M.; Wang, Q.; Zhou, J. High Oleic Acid Peanut Oil and Extra Virgin Olive Oil Supplementation Attenuate Metabolic Syndrome in Rats by Modulating the Gut Microbiota. Nutrients 2019, 11, 3005. [Google Scholar] [CrossRef]
- Correia, M.; Gomes, A.; Moreira, I.; El Maghariki, J.; Mendes, K.; Correia, M.; Barros, R.; Barbosa, J.; Rosa, N.; Gomes, A. Unraveling the Extra Virgin Olive Oil Effect on Inflammation and on Gut and Saliva Microbiota. Biomolecules 2025, 15, 338. [Google Scholar] [CrossRef]
- Lieder, B.; Hans, J.; Hentschel, F.; Geissler, K.; Ley, J. Biological Evaluation of Natural and Synthesized Homovanillic Acid Esters as Inhibitors of Intestinal Fatty Acid Uptake in Differentiated Caco-2 Cells. Molecules 2019, 24, 3559. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, S.; Liao, Y.; Wu, X.; Zhang, C.; Wang, X.; Yang, Z. Hippuric Acid Alleviates Dextran Sulfate Sodium-Induced Colitis via Suppressing Inflammatory Activity and Modulating Gut Microbiota. Biochem. Biophys. Res. Commun. 2024, 710, 149879. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C.J. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Van’t Land, B.; Stahl, B.; Garssen, J.; Rodríguez-Lagunas, M.J.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Supplementation With 2′-FL and ScGOS/LcFOS Ameliorates Rotavirus-Induced Diarrhea in Suckling Rats. Front. Cell Infect. Microbiol. 2018, 8, 372. [Google Scholar] [CrossRef]
- Sáez-Fuertes, L.; Kapravelou, G.; Grases-Pintó, B.; Massot-Cladera, M.; Bernabeu, M.; Knipping, K.; Garssen, J.; Bourdet-Sicard, R.; Castell, M.; Rodríguez-Lagunas, M.J.; et al. Impact of Maternal Bifidobacterium Breve M-16V and ScGOS/LcFOS Supplementation during Pregnancy and Lactation on the Maternal Immune System and Milk Composition. Front. Immunol. 2024, 15, 1418594. [Google Scholar] [CrossRef]
- Ceballos-Sánchez, D.; Sáez-Fuertes, L.; Casanova-Crespo, S.; Rodríguez-Lagunas, M.J.; Castell, M.; Pérez-Cano, F.J.; Massot-Cladera, M. Influence of Dietary Fiber and Polyphenols During Pre-Gestation, Gestation, or Lactation on Intestinal Gene Expression. Nutrients 2025, 17, 341. [Google Scholar] [CrossRef]
- Massot-Cladera, M.; Rigo-Adrover, M.D.M.; Herrero, L.; Franch, À.; Castell, M.; Vulevic, J.; Pérez-Cano, F.J.; Lagunas, M.J.R. A Galactooligosaccharide Product Decreases the Rotavirus Infection in Suckling Rats. Cells 2022, 11, 1669. [Google Scholar] [CrossRef]
- Rodríguez-Lagunas, M.; Martín-Venegas, R.; Moreno, J.J.; Ferrer, R. PGE2 Promotes Ca2+-Mediated Epithelial Barrier Disruption through EP1 and EP4 Receptors in Caco-2 Cell Monolayers. Am. J. Physiol. Cell Physiol. 2010, 299, C324–C334. [Google Scholar] [CrossRef]
- Moral-Anter, D.; Campo Sabariz, J.; Ferrer, R.; Martin-Venegas, R. Cyperus esculentus L. Tubers (Tiger Nuts) Protect Epithelial Barrier Function in Caco-2 Cells Infected by Salmonella Enteritidis and Promote Lactobacillus Plantarum Growth. Nutrients 2020, 13, 71. [Google Scholar] [CrossRef]



| REF | ROO | EVOO | |
|---|---|---|---|
| Body weight (g) | 32.69 ± 1.50 | 28.77 ± 0.61 * | 27.10 ± 0.71 * |
| Naso-anal length (cm) | 10.51 ± 0.17 | 10.29 ± 0.07 | 9.93 ± 0.12 * |
| BMI (g/cm2) | 0.29 ± 0.01 | 0.27 ± 0.01 * | 0.27 ± 0.01 * |
| Lee index | 303.10 ± 2.01 | 297.46 ± 1.46 | 302.40 ± 1.60 |
| Stomach (%) | 0.82 ± 0.02 | 0.88 ± 0.02 * | 0.88 ± 0.01 * |
| Liver (%) | 3.43 ± 0.09 | 3.66 ± 0.1 | 3.30 ± 0.05 # |
| Spleen (%) | 0.29 ± 0.01 | 0.28 ± 0.01 | 0.28 ± 0.01 |
| Thymus (%) | 0.45 ± 0.02 | 0.41 ± 0.01 | 0.46 ± 0.02 |
| Right kidney (%) | 0.71 ± 0.01 | 0.67 ± 0.01 | 0.68 ± 0.01 |
| Heart (%) | 0.72 ± 0.01 | 0.97 ± 0.26 | 0.70 ± 0.01 |
| Caecum (%) | 1.34 ± 0.07 | 1.47 ± 0.1 | 1.16 ± 0.08 |
| Small intestine weight (%) | 3.52 ± 0.08 | 3.86 ± 0.11 * | 3.55 ± 0.05 # |
| Small intestine length (%) | 117.38 ± 5.53 | 129.63 ± 3.14 * | 127.90 ± 3.40 * |
| REF | ROO | EVOO | |
|---|---|---|---|
| Leukocytes (×109/L) | 2.38 ± 0.65 | 1.78 ± 0.37 | 1.33 ± 0.18 |
| Lymphocytes (×109/L) | 1.78 ± 0.52 | 1.13 ± 0.23 | 0.91 ± 0.12 |
| Monocytes (×109/L) | 0.08 ± 0.03 | 0.07 ± 0.05 | 0.02 ± 0.01 |
| Granulocytes (×109/L) | 0.52 ± 0.11 | 0.58 ± 0.10 | 0.40 ± 0.05 |
| Lymphocytes (%) | 75.35 ± 1.29 | 66.65 ± 2.15 | 71.63 ± 1.92 |
| Monocytes (%) | 5.88 ± 0.52 | 6.45 ± 1.08 | 6.29 ± 0.44 |
| Granulocytes (%) | 18.77 ± 1.24 | 26.91 ± 1.68 | 22.08 ± 1.65 |
| Platelets (×109/L) | 217.67 ± 84.09 | 217.33 ± 79.17 | 183.56 ± 86.86 |
| Erythrocytes (×109/L) | 3.73 ± 0.42 | 4.75 ± 0.19 * | 3.94 ± 0.26 # |
| HGB (g/L) | 70.17 ± 8.41 | 84.01 ± 2.38 | 65.22 ± 5.12 # |
| HCT (%) | 20.98 ± 2.33 | 26.02 ± 0.91 * | 21.63 ± 1.48 # |
| VCM (fL) | 56.53 ± 0.48 | 55.03 ± 0.72 | 55.10 ± 0.56 |
| HCM (pg) | 18.68 ± 0.65 | 17.70 ± 0.35 | 17.38 ± 86.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan-Dai, S.; Grases-Pintó, B.; García-Vara, A.; Ferrer, R.; Martín-Venegas, R.; Lamuela-Raventós, R.M.; Castell, M.; Pérez-Cano, F.J.; Vallverdú-Queralt, A.; Rodríguez-Lagunas, M.J. Maternal Extra Virgin Olive Oil Supplementation Enhances Offspring Immune Function: A Preclinical Study. Int. J. Mol. Sci. 2025, 26, 7946. https://doi.org/10.3390/ijms26167946
Zhan-Dai S, Grases-Pintó B, García-Vara A, Ferrer R, Martín-Venegas R, Lamuela-Raventós RM, Castell M, Pérez-Cano FJ, Vallverdú-Queralt A, Rodríguez-Lagunas MJ. Maternal Extra Virgin Olive Oil Supplementation Enhances Offspring Immune Function: A Preclinical Study. International Journal of Molecular Sciences. 2025; 26(16):7946. https://doi.org/10.3390/ijms26167946
Chicago/Turabian StyleZhan-Dai, Sonia, Blanca Grases-Pintó, Adriana García-Vara, Ruth Ferrer, Raquel Martín-Venegas, Rosa M. Lamuela-Raventós, Margarida Castell, Francisco J. Pérez-Cano, Anna Vallverdú-Queralt, and Maria J. Rodríguez-Lagunas. 2025. "Maternal Extra Virgin Olive Oil Supplementation Enhances Offspring Immune Function: A Preclinical Study" International Journal of Molecular Sciences 26, no. 16: 7946. https://doi.org/10.3390/ijms26167946
APA StyleZhan-Dai, S., Grases-Pintó, B., García-Vara, A., Ferrer, R., Martín-Venegas, R., Lamuela-Raventós, R. M., Castell, M., Pérez-Cano, F. J., Vallverdú-Queralt, A., & Rodríguez-Lagunas, M. J. (2025). Maternal Extra Virgin Olive Oil Supplementation Enhances Offspring Immune Function: A Preclinical Study. International Journal of Molecular Sciences, 26(16), 7946. https://doi.org/10.3390/ijms26167946











