Molecular Characterization of Hemopexin in the Siberian Sturgeon (Acipenser baerii): Evolutionary Insights and Differential Expression Under Immune and Thermal Stresses
Abstract
1. Introduction
2. Results
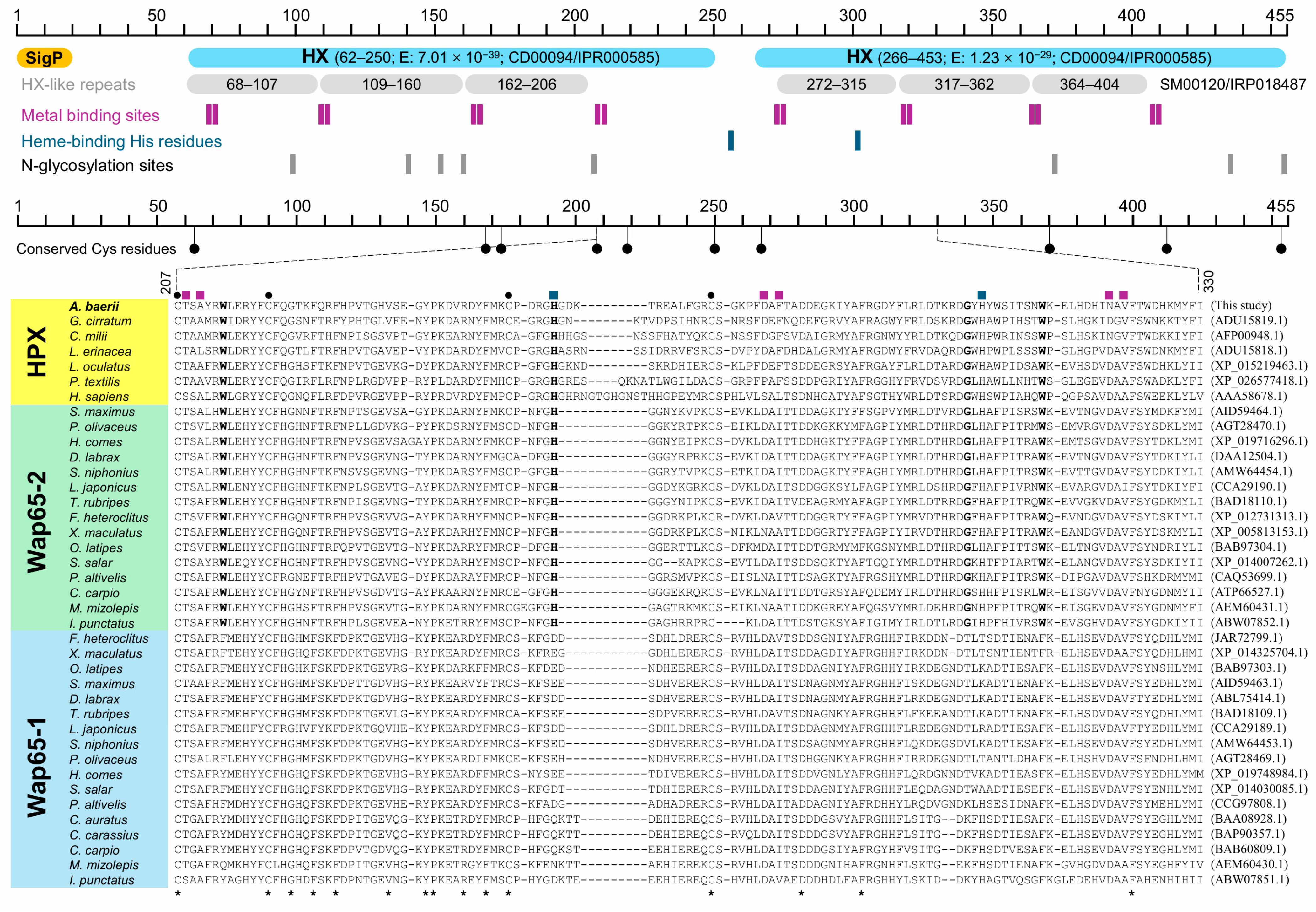
2.1. Characterization of Acipenser baerii HPX cDNA and Deduced Amino Acid Sequences
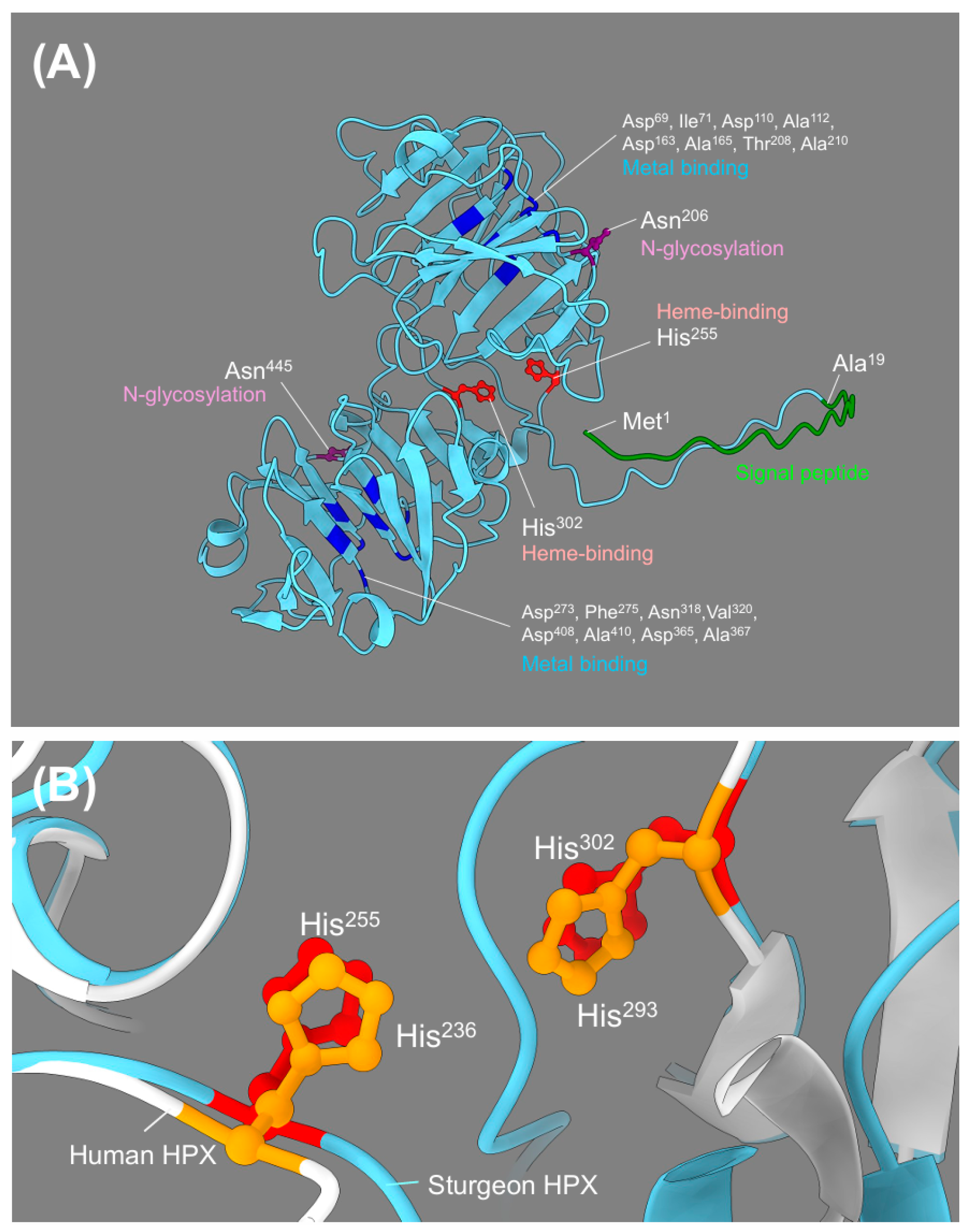
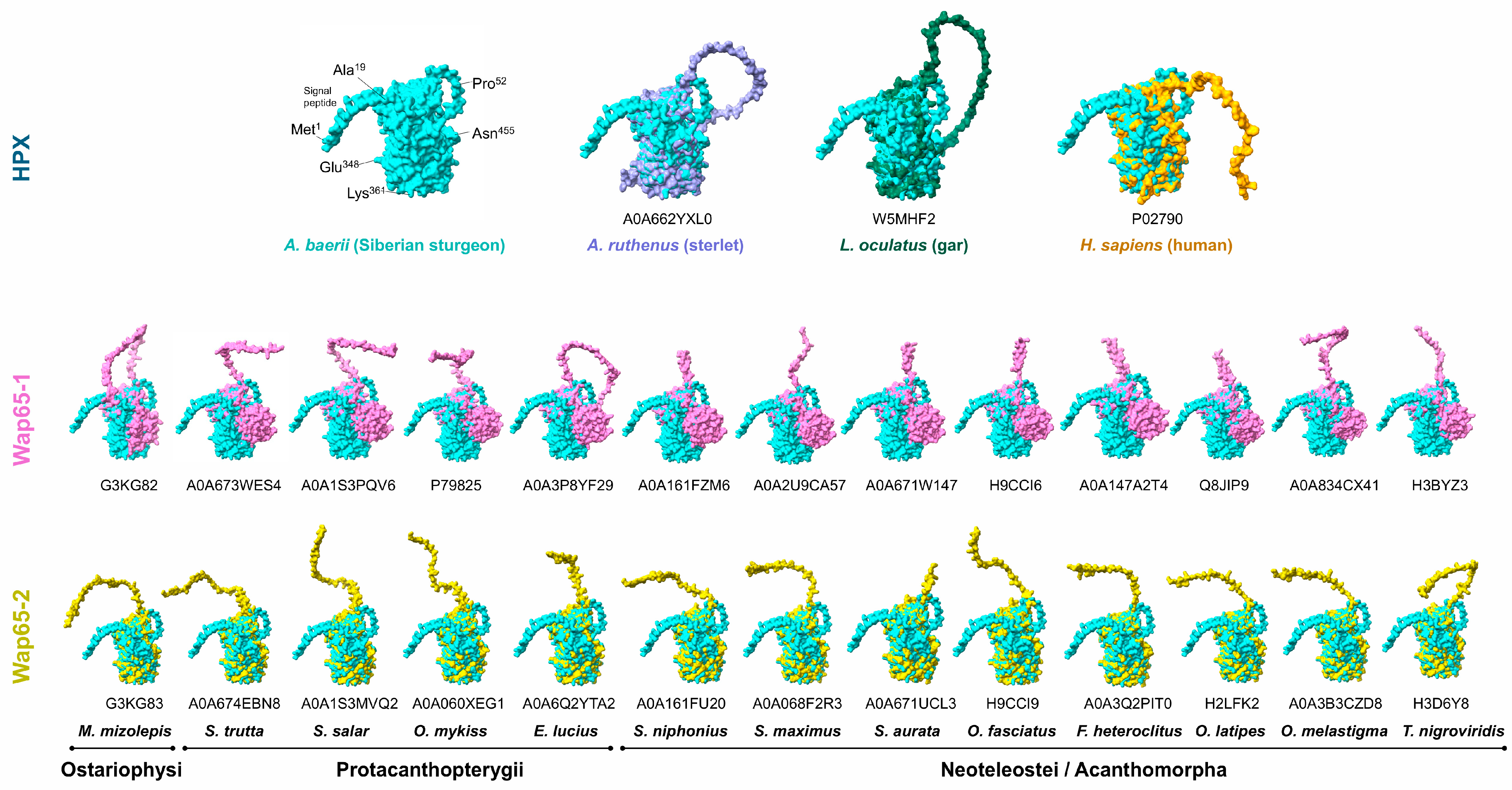
2.2. Predicted 3D Structure of A. baerii HPX and Superimposed Visualization of Conserved Motifs with Human HPX
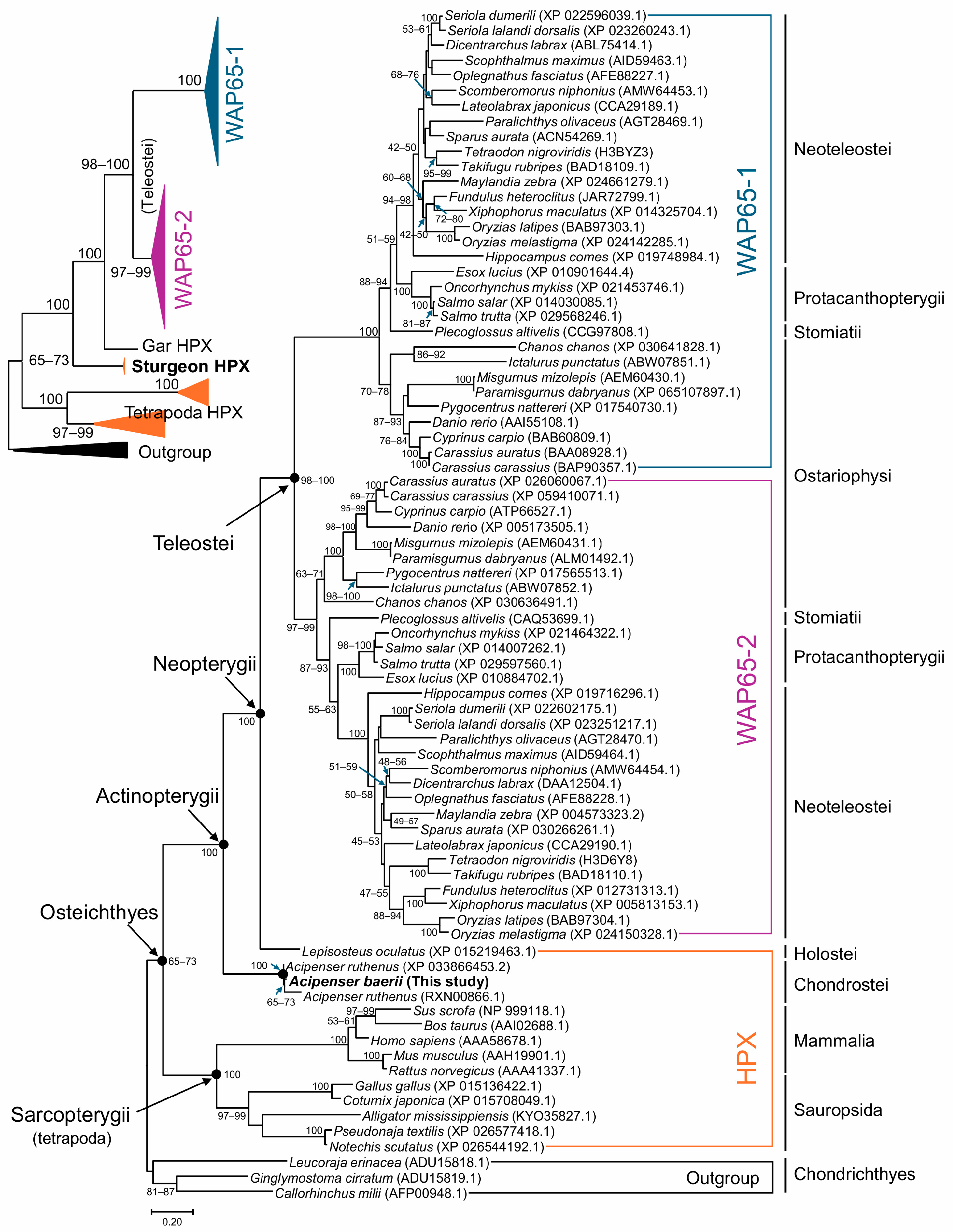
2.3. Molecular Phylogenetic Analysis of Vertebrate HPX and Wap65 Orthologs
2.4. Structural Comparison of A. baerii HPX with Other HPX and Wap65 Orthologs
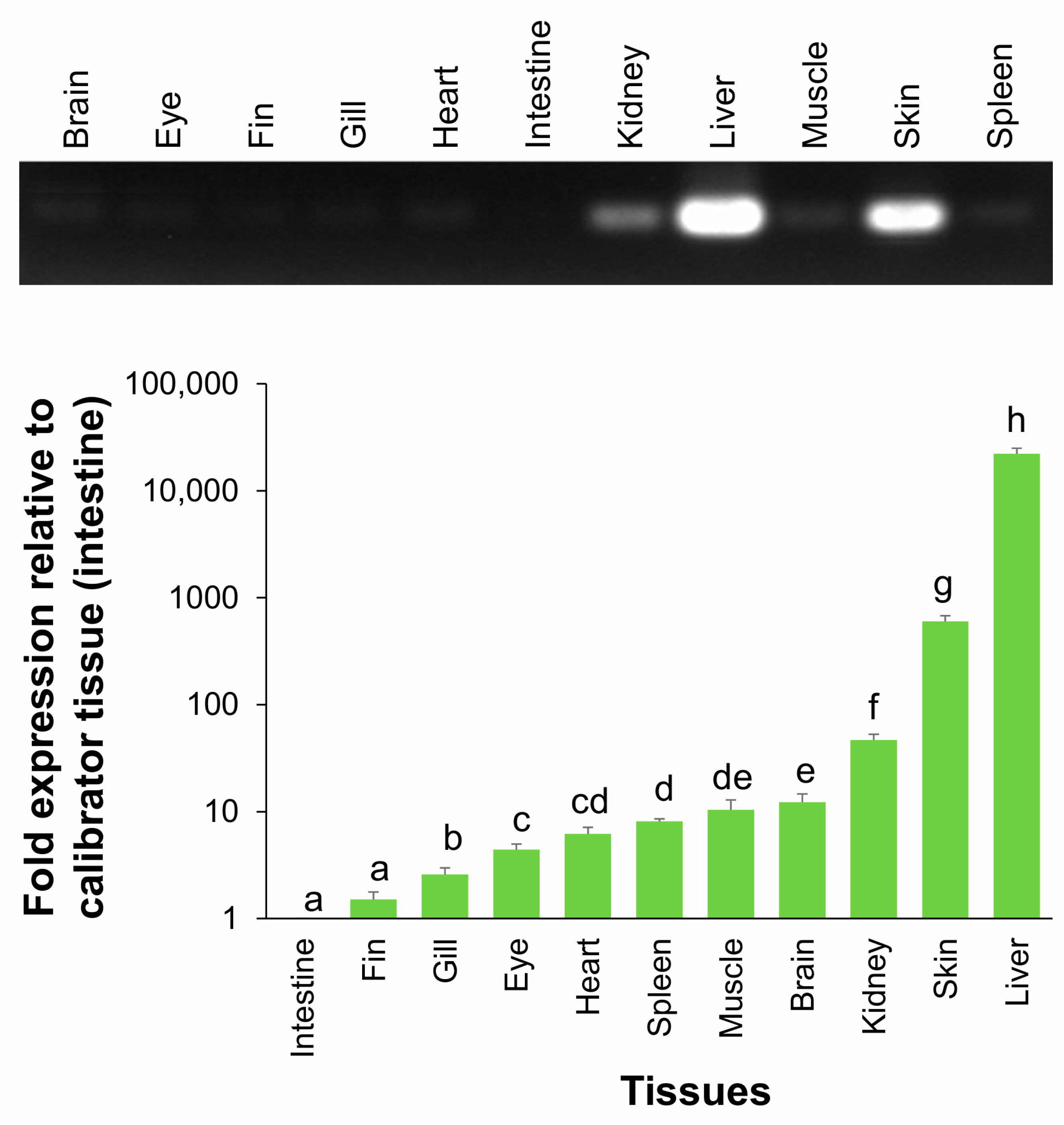
2.5. Tissue Distribution of HPX mRNA Expression in Fingerling A. baerii
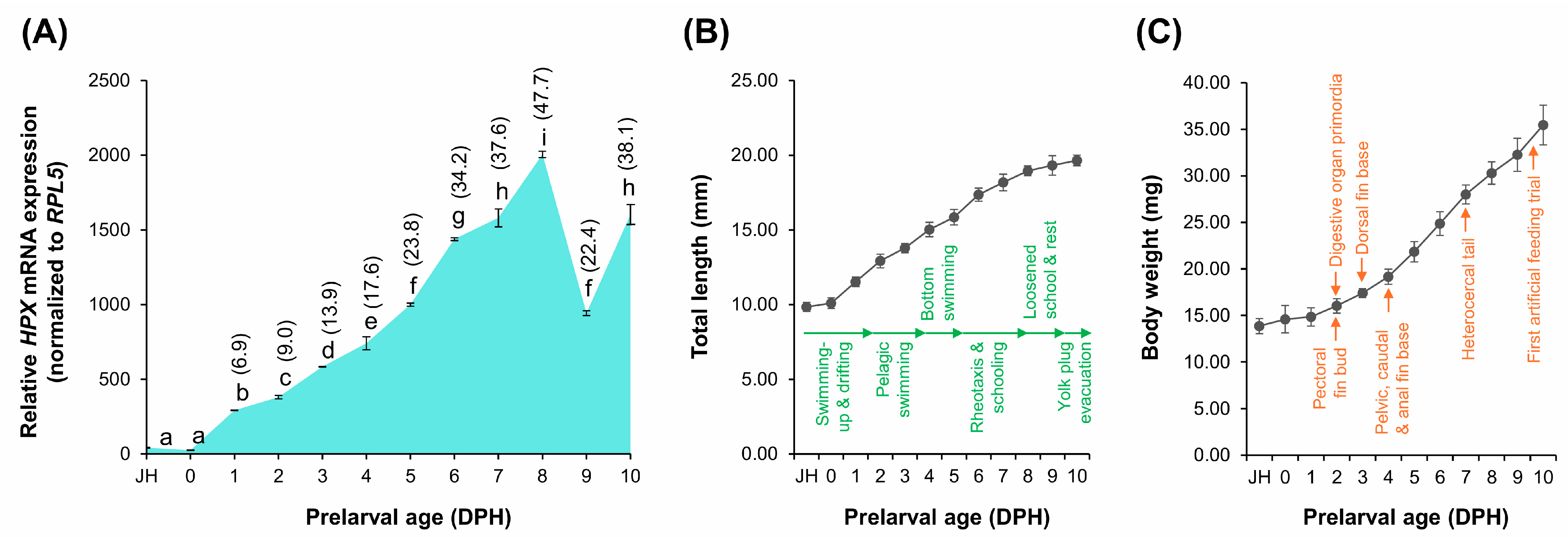
2.6. Ontogenetic Expression of HPX During Early Development in A. baerii
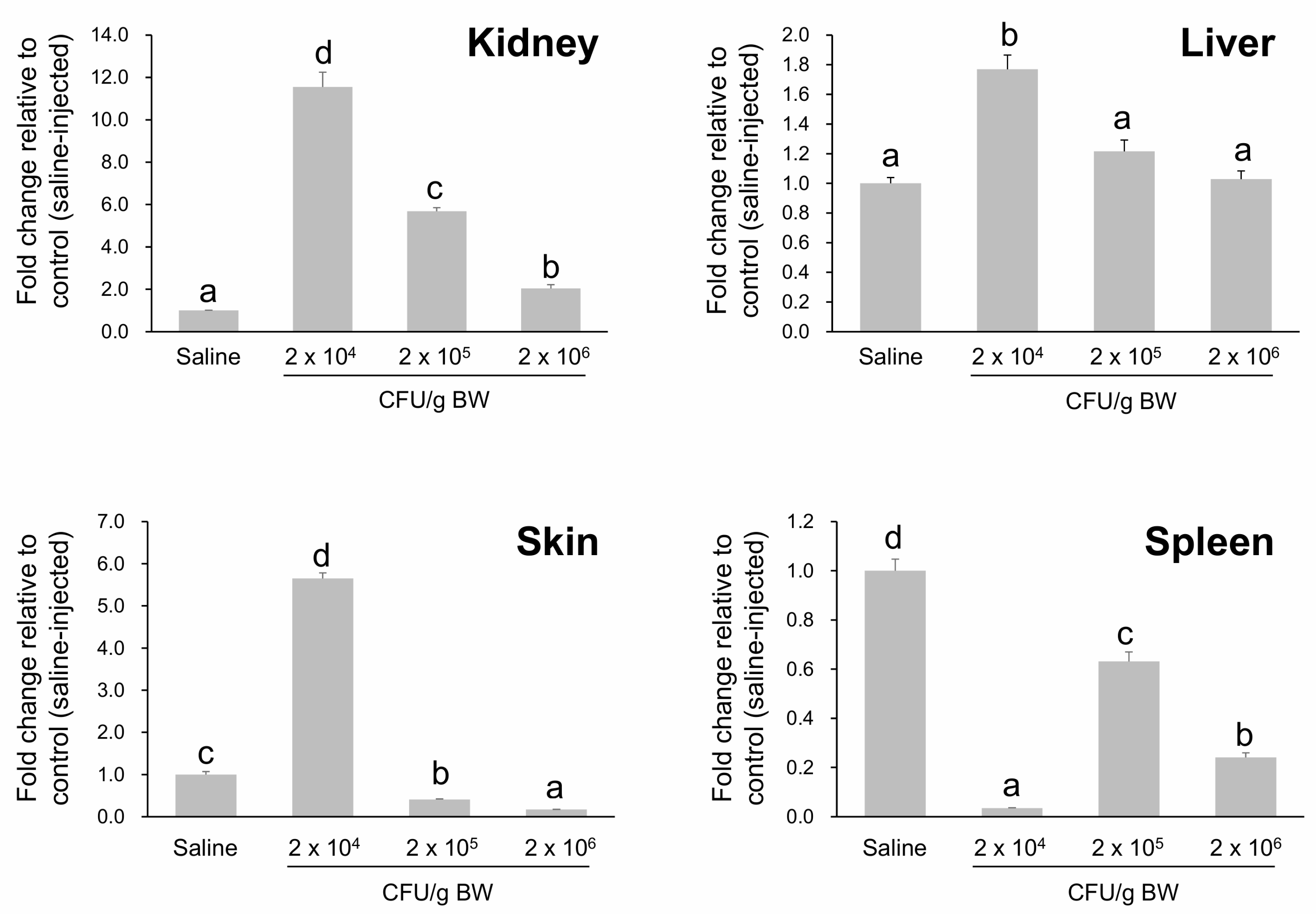
2.7. Dose-Dependent Modulation of HPX Expression Following A. hydrophila Challenge in A. baerii Fingerlings
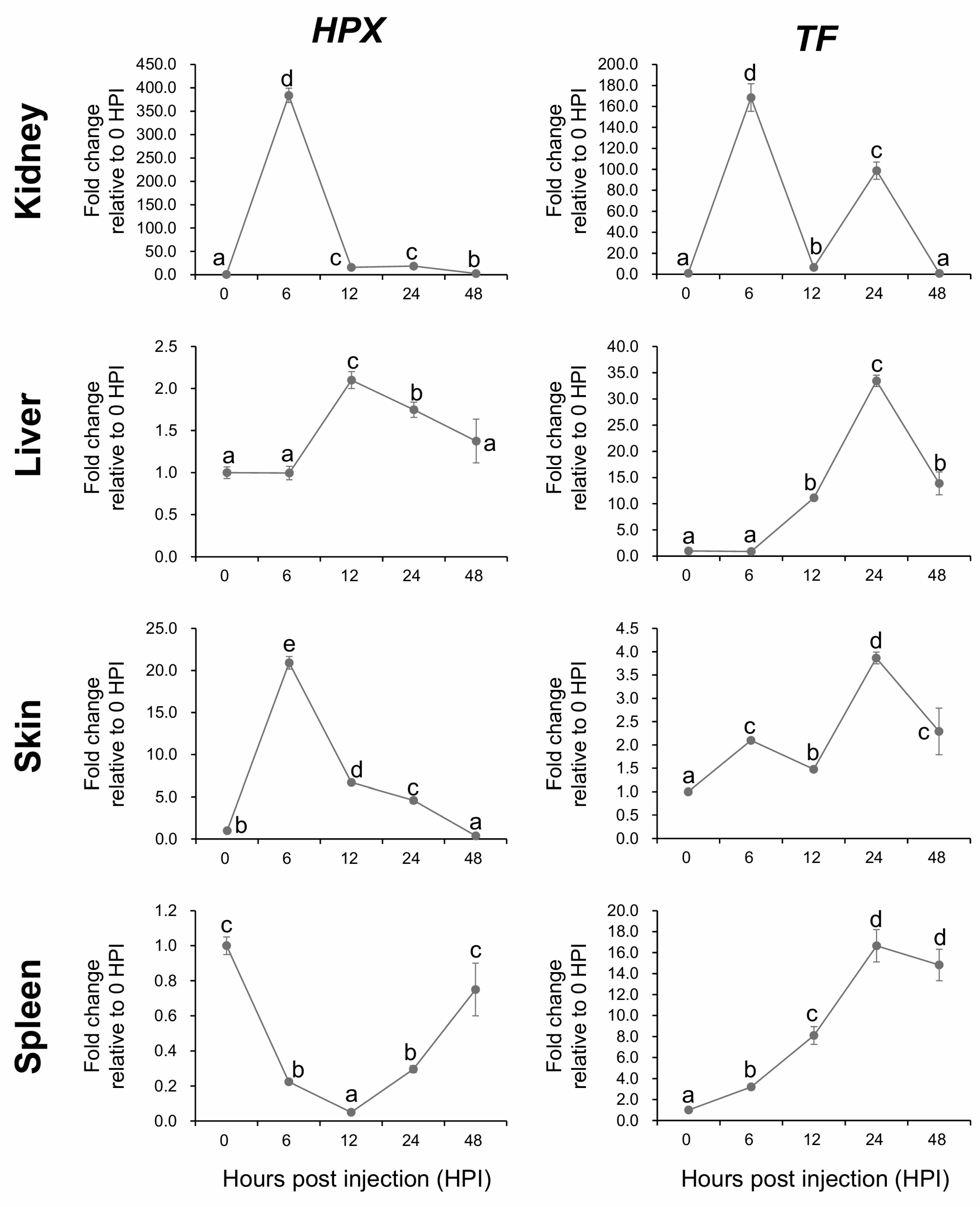
2.8. Temporal Expression Dynamics of HPX and TF Following A. hydrophila Challenge
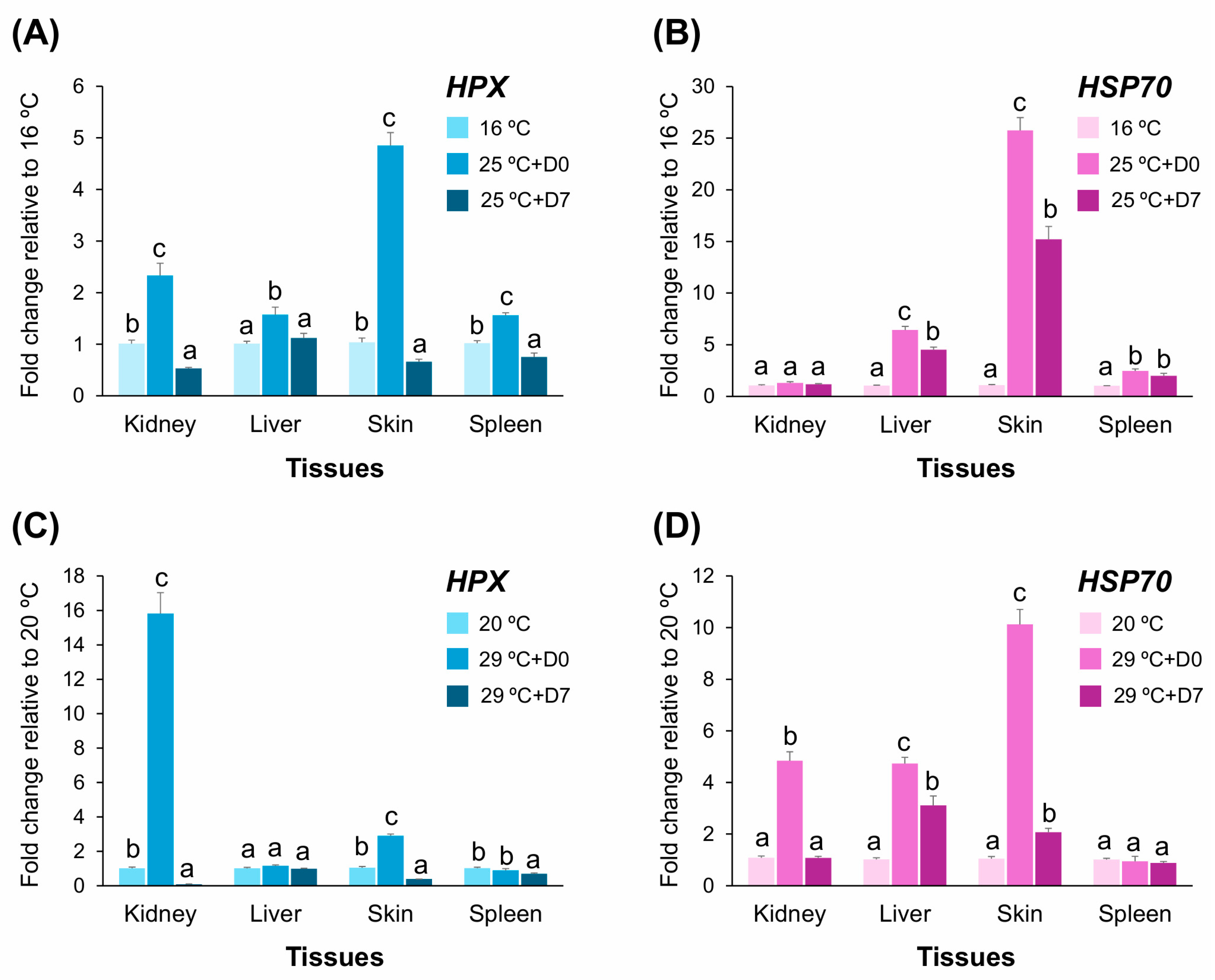
2.9. HPX Expression Under Mild and Severe Thermal Stress in A. baerii Fingerlings
3. Discussion
3.1. Structural Features and Evolutionary Context of A. baerii HPX
3.2. Basal Expression Patterns of HPX in Tissues and Early Ontogenic Development
3.3. Multivalent Stress Responsiveness: HPX Under Infection and Heat Challenge
4. Materials and Methods
4.1. Molecular Cloning of Acipenser baerii Hemopexin (HPX) cDNA
4.2. Bioinformatic Sequence Analysis
4.2.1. Sequence Characterization
4.2.2. Multiple Sequence Alignments and Molecular Phylogeny
4.2.3. Three-Dimensional Structural Modeling and Superimposed Analysis
4.3. Tissue and Prelarval Sampling for Basal Expression Analysis
4.4. Bacterial and Thermal Challenges for Differential Expression Analyses
4.4.1. Bacterial Challenge
4.4.2. Thermal Treatment
4.5. Nucleic Acid Preparation and RT-qPCR
4.5.1. RNA Extraction and cDNA Synthesis
4.5.2. Reference Gene Selection and Quantitative PCR Conditions
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPX | Hemopexin |
| Wap65 | Warm-temperature acclimation-associated 65 kDa protein |
| TF | Transferrin |
| HSP70 | Heat shock protein 70 |
| RPL5 | Ribosomal protein L5 |
| RPL7 | Ribosomal protein L7 |
| RPL7A | Ribosomal protein L7A |
| DPH | Days post-hatching |
| HPI | Hours post-injection |
| CFU | Colony-forming unit |
| CT | Threshold cycle |
References
- Tolosano, E.; Fagoonee, S.; Morello, N.; Vinchi, F.; Fiorito, V. Heme scavenging and the other facets of hemopexin. Antioxid. Redox Signal. 2010, 12, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Matalon, S. The long road to Ithaca: A physiologist’s journey. Am. J. Physiol. Cell Physiol. 2025, 328, C1526–C1534. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, R.; Wang, C.; Deng, J.; Luo, S. Double-edged functions of hemopexin in hematological related diseases: From basic mechanisms to clinical application. Front. Immunol. 2023, 14, 1274333. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Watabe, S.; Suzuki, Y.; Aida, K.; Nakajima, H. The 65-kDa cytosolic protein associated with warm temperature acclimation in goldfish, Carassius auratus. J. Comp. Physiol. B 1993, 163, 349–354. [Google Scholar] [CrossRef]
- Watabe, S.; Kikuchi, K.; Aida, K. Cold-and Warm-temperature Acclimation Induces Specific Cytosolic Proteins in Goldfish and Carp. Nippon Suisan Gakkaishi 1993, 59, 151–156. [Google Scholar] [CrossRef]
- Kikuchi, K.; Watabe, S.; Aida, K. The Wap65 gene expression of goldfish (Carassius auratus) in association with warm water temperature as well as bacterial lipopolysaccharide (LPS). Fish Physiol. Biochem. 1997, 17, 423–432. [Google Scholar] [CrossRef]
- Dooley, H.; Buckingham, E.B.; Criscitiello, M.F.; Flajnik, M.F. Emergence of the acute-phase protein hemopexin in jawed vertebrates. Mol. Immunol. 2010, 48, 147–152. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kim, B.S.; Kim, D.S.; Nam, Y.K. Modulation of warm-temperature-acclimation-associated 65-kDa protein genes (Wap65-1 and Wap65-2) in mud loach (Misgurnus mizolepis, Cypriniformes) liver in response to different stimulatory treatments. Fish Shellfish Immunol. 2012, 32, 662–669. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, B.S.; Noh, C.H.; Nam, Y.K. Genomic organization and functional diversification of two warm-temperature-acclimation-associated 65-kDa protein genes in rockbream (Oplegnathus fasciatus; Perciformes). Fish Shellfish Immunol. 2014, 37, 11–21. [Google Scholar] [CrossRef]
- Machado, J.P.; Vasconcelos, V.; Antunes, A. Adaptive functional divergence of the warm temperature acclimation related protein (WAP65) in fishes and the ortholog Hemopexin (HPX) in Mammals. J. Hered. 2014, 105, 237–252. [Google Scholar] [CrossRef]
- Du, K.; Stöck, M.; Kneitz, S.; Klopp, C.; Woltering, J.M.; Adolfi, M.C.; Feron, R.; Prokopov, D.; Makunin, A.; Kichigin, I.; et al. The sterlet sturgeon genome sequence and the mechanisms of segmental rediploidization. Nat. Ecol. Evol. 2020, 4, 841–852. [Google Scholar] [CrossRef]
- Shedko, S.V. Molecular dating of phylogeny of sturgeons (Acipenseridae) based on total evidence analysis. Russ. J. Genet. 2022, 58, 718–729. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, E.J.; Nam, Y.K. Chondrostean sturgeon hepcidin: An evolutionary link between teleost and tetrapod hepcidins. Fish Shellfish Immunol. 2019, 88, 117–125. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, E.J.; Nam, Y.K. Subfunctionalization and evolution of liver-expressed antimicrobial peptide 2 (LEAP2) isoform genes in Siberian sturgeon (Acipenser baerii), a primitive chondrostean fish species. Fish Shellfish Immunol. 2019, 93, 161–173. [Google Scholar] [CrossRef]
- Höhne, C.; Prokopov, D.; Kuhl, H.; Du, K.; Klopp, C.; Wuertz, S.; Trifonov, V.; Stöck, M. The immune system of sturgeons and paddlefish (Acipenseriformes): A review with new data from a chromosome-scale sturgeon genome. Rev. Aquac. 2021, 13, 1709–1729. [Google Scholar] [CrossRef]
- Aversa-Marnai, M.; Castellano, M.; Quartiani, I.; Conijesky, D.; Perretta, A.; Villarino, A.; Silva-Álvarez, V.; Ferreira, A.M. Different response of Acipenser gueldenstaedtii CRP/SAP and SAA to bacterial challenge and chronic thermal stress sheds light on the innate immune system of sturgeons. Fish Shellfish Immunol. 2022, 121, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Aversa-Marnai, M.; Villarino, A.; Silva-Álvarez, V. Innate immune and chronic heat stress responses in sturgeons: Advances and insights from studies on Russian sturgeons. Fish Shellfish Immunol. Rep. 2023, 5, 100121. [Google Scholar] [CrossRef] [PubMed]
- Afanasyev, S.G. Ecological and biological characteristics of the Siberian sturgeon (Acipenser baeri Brandt, 1869) in Lake Baikal. Nat. Inn. Asia 2025, 1, 6–19. (In Russian) [Google Scholar]
- Ruban, G.I. On the structure of the Siberian sturgeon species Acipenser baerii Brandt. J. Ichthyol. (Vopr. Ikhtiol.) 1998, 38, 307–327. [Google Scholar]
- Ruban, G.I. Siberian sturgeon Acipenser baerii Brandt: Species Structure and Ecology, 1st ed.; GEOS: Moscow, Russia, 2008; pp. 1–235. [Google Scholar]
- Ruban, G.I. Adaptive ecological and morphological features of the Siberian sturgeon (Acipenser baerii Brandt). Biol. Inland Waters 2019, 2, 71–78. [Google Scholar] [CrossRef]
- Ruban, G.I. Geographical distribution, ecological and biological characteristics of the Siberian sturgeon species. In The Siberian Sturgeon (Acipenser baerii, Brandt, 1869) Volume 1—Biology; Williot, P., Nonnotte, G., Vizziano-Cantonnet, D., Chebanov, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 3–28. [Google Scholar] [CrossRef]
- Park, C.; Lee, S.Y.; Kim, D.S.; Nam, Y.K. Embryonic development of Siberian Sturgeon Acipenser baerii under hatchery conditions: An image guide with embryological descriptions. Fish. Aquat. Sci. 2013, 16, 15–23. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, C.; Nam, Y.K. Behavioral characteristics of a chondrostean sturgeon species Acipenser baerii prelarvae in response to different environmental light intensities in a diel photoperiodic cycle. J. Anim. Reprod. Biotechnol. 2020, 35, 245–257. [Google Scholar] [CrossRef]
- Mugue, N.; Terekhanova, N.; Afanasyev, S.; Krasnov, A. Transcriptome sequencing of hybrid bester sturgeon: Responses to poly (I:C) in the context of comparative immunogenomics. Fish Shellfish Immunol. 2019, 93, 888–894. [Google Scholar] [CrossRef]
- Castellano, M.; Silva-Álvarez, V.; Aversa-Marnai, M.; Lamas-Bervejillo, M.; Quartiani, I.; Perretta, A.; Villarino, A.; Ferreira, A.M. Serum amyloid A is a positive acute phase protein in Russian sturgeon challenged with Aeromonas hydrophila. Sci. Rep. 2020, 17, 22162. [Google Scholar] [CrossRef]
- Kim, Y.O.; Park, E.M.; Moon, J.Y.; Nam, B.H.; Kim, D.G.; Kong, H.J.; Kim, W.J.; Lee, Y.J.; Lee, S.J. Genetic organization of two types of Flounder warm-temperature acclimation-associated 65 kDa protein and their gene expression profile. Biosci. Biotechnol. Biochem. 2013, 77, 2065–2072. [Google Scholar] [CrossRef]
- Detzel, M.S.; Schmalohr, B.F.; Steinbock, F.; Hopp, M.; Ramoji, A.; George, A.A.P.; Neugebauer, U.; Imhof, D. Revisiting the interaction of heme with hemopexin. Biol. Chem. 2021, 402, 675–691. [Google Scholar] [CrossRef]
- Hopp, M.; George, A.A.P.; Ramoji, A.; Pepanian, A.; Detzel, M.S. A model peptide reveals insights into the interaction of human Hemopexin with Heme. Int. J. Pept. Res. Ther. 2022, 28, 129. [Google Scholar] [CrossRef]
- Near, T.J.; Eytan, R.I.; Dornburg, A.; Kuhn, K.L.; Moore, J.A.; Davis, M.P.; Wainwright, P.C.; Friedman, M.; Smith, W.L. Resolution of ray-finned fish phylogeny and timing of diversification. Proc. Natl. Acad. Sci. USA 2012, 109, 13698–13703. [Google Scholar] [CrossRef]
- Hughes, L.C.; Ortí, G.; Huang, Y.; Sun, Y.; Baldwin, C.C.; Thompson, A.W.; Arcila, D.; Betancur-R, R.; Li, C.; Becker, L.; et al. Comprehensive phylogeny of ray-finned fishes (Actinopterygii) based on transcriptomic and genomic data. Proc. Natl. Acad. Sci. USA 2018, 115, 6249–6254. [Google Scholar] [CrossRef]
- Harvey, V.L.; Keating, J.N.; Buckley, M. Phylogenetic analyses of ray finned fishes (Actinopterygii) using collagen type I protein sequences. R. Soc. Open Sci. 2021, 8, 201955. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.; Fuerst, P.A. Evidence for a slowed rate of molecular evolution in the order Acipenseriformes. Mol. Biol. Evol. 2002, 19, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Delanghe, J.R.; Langlois, M.R. Hemopexin: A review of biological aspects and the role in laboratory medicine. Clin. Chim. Acta 2001, 312, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Tolosano, E.; Altruda, F. Hemopexin: Structure, function, and regulation. DNA Cell Biol. 2002, 21, 297–306. [Google Scholar] [CrossRef]
- Sha, Z.; Xu, P.; Takano, T.; Liu, H.; Terhune, J.; Liu, Z. The warm temperature acclimation protein Wap65 as an immune response gene: Its duplicates are differentially regulated by temperature and bacterial infections. Mol. Immunol. 2008, 45, 1458–1469. [Google Scholar] [CrossRef]
- Hirayama, M.; Kobiyama, A.; Kinoshita, S.; Watabe, S. The occurrence of two types of hemopexin-like protein in medaka and differences in their affinity to heme. J. Exp. Biol. 2004, 207, 1387–1398. [Google Scholar] [CrossRef]
- Gisbert, E.; Nam, Y.K. Early Ontogeny in the Siberian Sturgeon. In The Siberian Sturgeon (Acipenser baerii, Brandt, 1869) Volume 1—Biology; Williot, P., Nonnotte, G., Vizziano-Cantonnet, D., Chebanov, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 131–158. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, E.J.; Nam, Y.K. Superoxide dismutase multigene family from a primitive chondrostean sturgeon, Acipenser baerii: Molecular characterization, evolution, and antioxidant defense during development and pathogen infection. Antioxidants 2021, 10, 232. [Google Scholar] [CrossRef]
- Kim, E.J.; Nam, Y.K. Anesthetic protocol for microinjection-related handling of Siberian sturgeon (Acipenser baerii; Acipenseriformes) prolarvae. PLoS ONE 2018, 13, e0209928. [Google Scholar] [CrossRef] [PubMed]
- Nakaniwa, M.; Hirayama, M.; Shimizu, A.; Sasaki, T.; Asakawa, S.; Shimizu, N.; Watabe, S. Genomic sequences encoding two types of medaka hemopexin-like protein Wap65, and their gene expression profiles in embryos. J. Exp. Biol. 2005, 208, 1915–1925. [Google Scholar] [CrossRef]
- Sarropoulou, E.; Fernandes, J.M.O.; Mitter, K.; Magoulas, A.; Mulero, V.; Sepulcre, M.P.; Figueras, A.; Novoa, B.; Kotoulas, G. Evolution of a multifunctional gene: The warm temperature acclimation protein Wap65 in the European seabass Dicentrarchus labrax. Mol. Phylogenet. Evol. 2010, 55, 640–649. [Google Scholar] [CrossRef]
- Kumar, N.; Thorat, S.T.; Gunaware, M.A.; Kumar, P.; Reddy, K.S. Unraveling gene regulation mechanisms in fish: Insights into multistress responses and mitigation through iron nanoparticles. Front. Immunol. 2024, 15, 1410150. [Google Scholar] [CrossRef] [PubMed]
- Alotiby, A. Immunology of stress: A Review article. J. Clin. Med. 2024, 13, 6394. [Google Scholar] [CrossRef]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef]
- Salinas, I.; Ding, Y.; Fernández-Montero, Á.; Sunyer, J.O. Mucosal Immunity in Fish. In Principles of Fish Immunology: From Cells and Molecules to Host Protection; Buchmann, K., Secombes, C.J., Eds.; Springer: Cham, Switzerland, 2022; pp. 387–444. [Google Scholar]
- Secombes, C.J.; Wang, T. The innate and adaptive immune system of fish. In Infectious Disease in Aquaculture: Prevention and Control; Austin, B., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 3–68. [Google Scholar]
- Dalmo, R.A.; Bøgwald, J. Innate Immunity. In Principles of Fish Immunology: From Cells and Molecules to Host Protection; Buchmann, K., Secombes, C.J., Eds.; Springer: Cham, Switzerland, 2022; pp. 31–104. [Google Scholar]
- Wang, W.; Deng, D.; Riu, N.D.; Moniello, G.; Hung, S.S.O. Heat shock protein 70 (HSP70) responses in tissues of White Sturgeon and Green Sturgeon exposed to different stressors. N. Am. J. Aquac. 2013, 75, 164–169. [Google Scholar] [CrossRef]
- Yang, S.; Xu, W.; Tan, C.; Li, M.; Li, D.; Zhang, C.; Feng, L.; Chen, Q.; Jiang, J.; Li, Y.; et al. Heat stress weakens the skin barrier function in sturgeon by decreasing mucus secretion and disrupting the mucosal microbiota. Front. Microbiol. 2022, 13, 860079. [Google Scholar] [CrossRef] [PubMed]
- Bugg, W.S.; Yoon, G.R.; Schoen, A.N.; Weinrauch, A.M.; Jeffries, K.M.; Anderson, W.G. Elevated temperatures dampen the innate immune capacity of developing lake sturgeon (Acipenser fulvescens). J. Exp. Biol. 2023, 226, jeb245335. [Google Scholar] [CrossRef] [PubMed]
- Penny, F.M.; Bugg, W.S.; Kieffer, J.D.; Jeffries, K.M.; Pavey, S.A. Atlantic sturgeon and shortnose sturgeon exhibit highly divergent transcriptomic responses to acute heat stress. Comp. Biochem. Physiol. D 2023, 45, 101058. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real-Time Quantitative PCR and the 2 −ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.J.; Kim, K.H.; Nam, Y.K. Molecular Characterization of Hemopexin in the Siberian Sturgeon (Acipenser baerii): Evolutionary Insights and Differential Expression Under Immune and Thermal Stresses. Int. J. Mol. Sci. 2025, 26, 7934. https://doi.org/10.3390/ijms26167934
Kim EJ, Kim KH, Nam YK. Molecular Characterization of Hemopexin in the Siberian Sturgeon (Acipenser baerii): Evolutionary Insights and Differential Expression Under Immune and Thermal Stresses. International Journal of Molecular Sciences. 2025; 26(16):7934. https://doi.org/10.3390/ijms26167934
Chicago/Turabian StyleKim, Eun Jeong, Ki Hong Kim, and Yoon Kwon Nam. 2025. "Molecular Characterization of Hemopexin in the Siberian Sturgeon (Acipenser baerii): Evolutionary Insights and Differential Expression Under Immune and Thermal Stresses" International Journal of Molecular Sciences 26, no. 16: 7934. https://doi.org/10.3390/ijms26167934
APA StyleKim, E. J., Kim, K. H., & Nam, Y. K. (2025). Molecular Characterization of Hemopexin in the Siberian Sturgeon (Acipenser baerii): Evolutionary Insights and Differential Expression Under Immune and Thermal Stresses. International Journal of Molecular Sciences, 26(16), 7934. https://doi.org/10.3390/ijms26167934





