Applications of Tailored Mesoporous Silicate Nanomaterials in Regenerative Medicine and Theranostics
Abstract
1. Introduction
2. Application of Mesoporous Silicate in Regenerative Medicine
2.1. Application of Mesoporous Silicate in Hard-Tissue Regeneration
2.2. Application of Mesoporous Silicate in Soft-Tissue Regeneration and Wound Healing
3. Application of Mesoporous Silicates in Theranostics
3.1. Application in Drug Delivery and Stimuli-Responsive Therapy
3.2. Application in Photodynamic and Photothermal Therapies, Bioimaging and Theranostics
3.3. Application in Proteins, Genes and Antigens Delivery
4. Perspective and Challenges
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSCs: | adipose-derived mesenchymal stem cells |
| AgNP-MSG: | silver nanoparticle-incorporated mesoporous silica granules |
| ALI: | acute lung injury |
| APCs: | antigen-presenting cells |
| APTES: | 3-aminopropyltriethoxysilane |
| BBB: | blood–brain barrier |
| BMSCs: | bone marrow mesenchymal stromal cells |
| B-MSs: | Bacillus-shaped mesoporous silica nanoparticles |
| CAB: | cabotegravir |
| cAMP: | cyclic adenosine monophosphate |
| CBP: | carboplatin |
| CDM-PEG: | cis-aconitic anhydride-polyethylene glycol |
| CDP: | Cistanche deserticola polysaccharide |
| Chic: | chitosan-catechol |
| CMC: | carboxymethylcellulose |
| CNI: | cavernous nerve injury |
| CNS: | reduced central nervous system |
| COL1α1: | collagen type I alpha 1 |
| PBA: | 4-carboxyphenylboronic acid |
| CPPs: | cell-penetrating peptides |
| CRISPR: | clustered regularly interspaced short palindromic repeats |
| dANA: | diphtheria anatoxins |
| DC: | dendritic cell |
| DES: | deep eutectic system |
| DFNS: | dendritic fibrous nano-silica |
| DUSP26: | dual-specificity phosphatase 26 |
| DSNs: | dendritic silica nanoparticles |
| dtANA: | diphtheria-tetanus anatoxins |
| EAE: | autoimmune encephalomyelitis |
| ED: | erectile dysfunction |
| EI-ADSCs: | endothelial-induced adipose-derived mesenchymal stem cells |
| EGCG: | epigallocatechin-3-gallate |
| EpCAM: | epithelial cell adhesion molecule |
| EPR: | enhanced permeability and retention |
| ERK: | extracellular signal-regulated kinase |
| FDA: | U.S. Food and Drug Administration |
| GFP: | green fluorescent protein |
| GI: | gastrointestinal |
| HA: | hyaluronic acid |
| HCC: | hepatocellular carcinoma |
| hDPSCs: | human dental pulp stem cells |
| HEK-293: | human embryonic kidney cells |
| HIV-1: | human immunodeficiency virus type 1 |
| HMSNs: | hollow mesoporous silica nanoparticles |
| HUVECs: | human umbilical vein endothelial cells |
| ICB: | immune checkpoint blockade |
| IL-1β: | interleukin-1 beta |
| L/D-Ala: | L/D-alanine |
| MBGs: | mesoporous bioactive glasses |
| MBGNs: | mesoporous bioactive glass nanoparticles |
| MCNSs: | mesoporous silica nanoscrews |
| MERS-CoV: | Middle East respiratory syndrome coronavirus |
| MPLA: | agonist monophosphoryl lipid A |
| MPTP: | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MS: | multiple sclerosis |
| MSCs: | mesenchymal stromal cells |
| MSNs: | mesoporous silicate nanoparticles or mesoporous silica nanoparticles |
| MTBS: | microtensile bond strength |
| NADH: | nicotinamide adenine dinucleotide |
| NFC: | nanofibrillated cellulose |
| NIR: | near-infrared |
| NSCs: OMVs: | neural stem cells outer membrane vesicles |
| ORMOSILs: | organically modified silicates |
| OVA: | ovalbumin |
| PCR: | polymerase chain reaction |
| PD: | Parkinson’s disease |
| PD-L1: | programmed death-ligand 1 |
| PEDV: | porcine epidemic diarrhea virus |
| PEI: | polyethyleneimine |
| PEG: | polyethylene glycol |
| PEGS: | poly(glycerol sebacate) |
| PMOs: | periodic mesoporous organosilica nanoparticles |
| R837: | imiquimod |
| RGD: | arginine-glycine-aspartic acid |
| rmTBI: | repeated mild traumatic brain injury |
| ROS: | reactive oxygen species |
| RPV: | rilpivirine |
| Ru(tpy)DPPZ: | [Ru(terpyridine)(dipyridophenazine)(H2O)]2+ |
| RVG: | rabies virus glycopeptide |
| SARS-CoV-2: | severe acute respiratory syndrome coronavirus 2 |
| SCI: | spinal cord injury |
| SCPs: | silica-coated calcium phosphate nanoparticles |
| SDT: | sonodynamic therapy |
| SEM: | scanning electron microscopy |
| tANA: | tetanus anatoxins |
| TEM: | transmission electron microscopy |
| TEOS: | tetraethyl orthosilicate |
| TfR: | transferrin receptor |
| TLR9: | toll-like receptor 9 |
| TMOS: | tetramethyl orthosilicate |
| TNF-α: | tumor necrosis factor-alpha |
| UCNPs: | upconversion nanoparticles |
| VMSNs: | virus-like mesoporous silica nanoparticles |
References
- Lazar, T. Biomineralization: Progress in biology, molecular biology and application., 2nd revised ed. Edited by E. Baeuerlein. Eng. Life Sci. 2005, 5, 286–287. [Google Scholar]
- Wegner, G. Biomineralization: Progress in biology, molecular biology and application, 2nd revised ed. Edited by E. Baeuerlein. ChemBioChem 2005, 6, 762–763. [Google Scholar] [CrossRef]
- Weiner, S. Biomineralization: Progress in biology, molecular biology and application, 2nd revised ed. Edited by E. Baeuerlein. Angew. Chem. Int. Ed. 2005, 44, 4833–4834. [Google Scholar] [CrossRef]
- McCutchin, C.A.; Edgar, K.J.; Chen, C.-L.; Dove, P.M. Silica-biomacromolecule interactions: Toward a mechanistic understanding of silicification. Biomacromolecules 2025, 26, 43–84. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, C.; Zhang, L.; Chen, X.; Fan, W. Chemical synthesis and multihybridization of small-sized hollow mesoporous organosilica nanoparticles toward advanced theranostics. Acc. Chem. Res. 2024, 57, 3465–3477. [Google Scholar] [CrossRef]
- Das, P.; Albertazzi, L.; Durand, J.-O. Silica-based nanoparticles: From understanding to biomedical applications. ACS Mater. Lett. 2025, 7, 1297–1312. [Google Scholar] [CrossRef]
- Wang, W.; Wang, P.; Tang, X.; Elzatahry, A.A.; Wang, S.; Al-Dahyan, D.; Zhao, M.; Yao, C.; Hung, C.-T.; Zhu, X.; et al. Facile synthesis of uniform virus-like mesoporous silica nanoparticles for enhanced cellular internalization. ACS Cent. Sci. 2017, 3, 839–846. [Google Scholar] [CrossRef]
- Gupta, A.; Choudhury, A.M.; Meena, J.; Bauri, S.; Maiti, P. Ordered mesoporous silica delivering siRNA as cancer nanotherapeutics: A comprehensive review. ACS Biomater. Sci. Eng. 2024, 10, 2636–2658. [Google Scholar] [CrossRef]
- Su, H.; Song, Y.; Yang, S.; Zhang, Z.; Shen, Y.; Yu, L.; Chen, S.; Gao, L.; Chen, C.; Hou, D.; et al. Plasmonic alloys enhanced metabolic fingerprints for the diagnosis of COPD and exacerbations. ACS Cent. Sci. 2024, 10, 331–343. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Chen, X.; Fang, W.; Yu, K.; Gu, W.; Wei, Y.; Zheng, H.; Piao, J.; Li, F. Strategies to regulate the degradation and clearance of mesoporous silica nanoparticles: A review. Int. J. Nanomedicine 2024, 19, 5859–5878. [Google Scholar] [CrossRef]
- Nair, A.; Chandrashekhar, H.R.; Day, C.M.; Garg, S.; Nayak, Y.; Shenoy, P.A.; Nayak, U.Y. Polymeric functionalization of mesoporous silica nanoparticles: Biomedical insights. Int. J. Pharm. 2024, 660, 124314. [Google Scholar] [CrossRef] [PubMed]
- Payamifar, S.; Khalili, Y.; Foroozandeh, A.; Abdouss, M.; Hasanzadeh, M. Magnetic mesoporous silica nanoparticles as advanced polymeric scaffolds for efficient cancer chemotherapy: Recent progress and challenges. RSC Adv. 2025, 15, 16050–16074. [Google Scholar] [CrossRef]
- Bhattacharjee, S. Craft of co-encapsulation in nanomedicine: A struggle to achieve synergy through reciprocity. ACS Pharmacol. Transl. Sci. 2022, 5, 278–298. [Google Scholar] [CrossRef]
- Kanungo, A.; Tripathy, N.S.; Sahoo, L.; Acharya, S.; Dilnawaz, F. Theranostic siRNA loaded mesoporous silica nanoplatforms: A game changer in gene therapy for cancer treatment. OpenNano 2024, 15, 100195. [Google Scholar] [CrossRef]
- Mahmoudi Gharehbaba, A.; Soltanmohammadi, F.; Vandghanooni, S.; Eskandani, M.; Adibkia, K. A comprehensive review on overcoming the multifaceted challenge of cancer multidrug resistance: The emerging role of mesoporous silica nanoparticles. Biomed. Pharmacother. 2025, 186, 118045. [Google Scholar] [CrossRef]
- Huq, T.B.; Anil Kumar Jeeja, P.; Dam, S.K.; Das, S.S.; Vivero-Escoto, J.L. Recent applications of mesoporous silica nanoparticles in gene therapy. Adv. Healthcare Mater. 2025, 2404781. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Qiu, X.; Zuo, F.; Wang, B. Mesoporous silica nanoparticles as a drug delivery mechanism. Open Life Sci. 2024, 19, 20220867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Dong, H.-X.; Chou, Y.-Y. Porous silicon as functionalized material for biomedical application. Appl. Mech. Mater. 2014, 618, 431–436. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Zhang, H.; Zhang, Q.; Bo, D.; Zhong, H.; Jiao, L.; Yuan, H.; Lu, G. Tumor cell membrane biomimetic mesoporous silicon materials in combination with PD-L1 knockout achieved through the CRISPR/Cas9 system for targeted and immunotherapeutic purposes. Bioconjugate Chem. 2025, 36, 971–979. [Google Scholar] [CrossRef]
- Belton, D.J.; Deschaume, O.; Perry, C.C. An overview of the fundamentals of the chemistry of silica with relevance to biosilicification and technological advances. FEBS J. 2012, 279, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Annenkov, V.V.; Danilovtseva, E.N.; Pal’shin, V.A.; Verkhozina, O.N.; Zelinskiy, S.N.; Krishnan, U.M. Silicic acid condensation under the influence of water-soluble polymers: From biology to new materials. RSC Adv. 2017, 7, 20995–21027. [Google Scholar] [CrossRef]
- Kovtareva, S.; Kusepova, L.; Tazhkenova, G.; Mashan, T.; Bazarbaeva, K.; Kopishev, E. Surface modification of mesoporous silica nanoparticles for application in targeted delivery systems of antitumour drugs. Polymers 2024, 16, 1105. [Google Scholar] [CrossRef]
- Janeta, M.; John, L.; Ejfler, J.; Szafert, S. Novel organic-inorganic hybrids based on T8 and T10 silsesquioxanes: Synthesis, cage-rearrangement and properties. RSC Adv. 2015, 5, 72340–72351. [Google Scholar] [CrossRef]
- Janeta, M.; John, L.; Ejfler, J.; Szafert, S. High-yield synthesis of amido-functionalized polyoctahedral oligomeric silsesquioxanes by using acyl chlorides. Chem.—Eur. J. 2014, 20, 15966–15974. [Google Scholar] [CrossRef]
- John, L.; Janeta, M.; Szafert, S. Synthesis of cubic spherosilicates for self-assembled organic-inorganic biohybrids based on functionalized methacrylates. New J. Chem. 2018, 42, 39–47. [Google Scholar] [CrossRef]
- Fu, J.; Wang, S.; He, C.; Lu, Z.; Huang, J.; Chen, Z. Facilitated fabrication of high strength silica aerogels using cellulose nanofibrils as scaffold. Carbohydr. Polym. 2016, 147, 89–96. [Google Scholar] [CrossRef]
- Curley, R.; Holmes, J.D.; Flynn, E.J. Can sustainable, monodisperse, spherical silica be produced from biomolecules? A review. Appl. Nanosci. 2021, 11, 1777–1804. [Google Scholar] [CrossRef]
- Cui, Y.; Hong, S.; Jiang, W.; Li, X.; Zhou, X.; He, X.; Liu, J.; Lin, K.; Mao, L. Engineering mesoporous bioactive glasses for emerging stimuli-responsive drug delivery and theranostic applications. Bioact. Mater. 2024, 34, 436–462. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Zhao, Q.; Ao, Y. Advances in immunomodulatory mesoporous silica nanoparticles for inflammatory and cancer therapies. Biomolecules 2024, 14, 1057. [Google Scholar] [CrossRef] [PubMed]
- Gu, L. Tailored silica nanomaterials for immunotherapy. ACS Cent. Sci. 2018, 4, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, Y.; Wu, J.; Hou, H. Heterogeneous nanosized metal (metallic compound)@metal-organic framework composites: Recent advances in the preparation and applications. Adv. Funct. Mater. 2023, 33, 2302573. [Google Scholar] [CrossRef]
- Haffner, S.M.; Parra-Ortiz, E.; Browning, K.L.; Joergensen, E.; Skoda, M.W.A.; Montis, C.; Li, X.; Berti, D.; Zhao, D.; Malmsten, M. Membrane interactions of virus-like mesoporous silica nanoparticles. ACS Nano 2021, 15, 6787–6800. [Google Scholar] [CrossRef]
- Picchetti, P.; Volpi, S.; Rossetti, M.; Dore, M.D.; Trinh, T.; Biedermann, F.; Neri, M.; Bertucci, A.; Porchetta, A.; Corradini, R.; et al. Responsive nucleic acid-based organosilica nanoparticles. J. Am. Chem. Soc. 2023, 145, 22896–22902. [Google Scholar] [CrossRef]
- Picchetti, P.; Volpi, S.; Sancho-Albero, M.; Rossetti, M.; Dore, M.D.; Trinh, T.; Biedermann, F.; Neri, M.; Bertucci, A.; Porchetta, A.; et al. Supramolecular nucleic acid-based organosilica nanoparticles responsive to physical and biological inputs. J. Am. Chem. Soc. 2023, 145, 22903–22912. [Google Scholar] [CrossRef]
- Tripathi, A.D.; Labh, Y.; Katiyar, S.; Singh, A.K.; Chaturvedi, V.K.; Mishra, A. Folate-mediated targeting and controlled release: PLGA-encapsulated mesoporous silica nanoparticles delivering capecitabine to pancreatic tumor. ACS Appl. Bio Mater. 2024, 7, 7838–7851. [Google Scholar] [CrossRef]
- Cha, B.G.; Jeong, J.H.; Kim, J. Extra-large pore mesoporous silica nanoparticles enabling co-delivery of high amounts of protein antigen and toll-like receptor 9 agonist for enhanced cancer vaccine efficacy. ACS Cent. Sci. 2018, 4, 484–492. [Google Scholar] [CrossRef]
- Tng, D.J.H.; Low, J.G.H. Current status of silica-based nanoparticles as therapeutics and its potential as therapies against viruses. Antiviral Res. 2023, 210, 105488. [Google Scholar] [CrossRef]
- Ni, D.; Jiang, D.; Ehlerding, E.B.; Huang, P.; Cai, W. Radiolabeling silica-based nanoparticles via coordination chemistry: Basic principles, strategies, and applications. Acc. Chem. Res. 2018, 51, 778–788. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, S.; Ma, J.; Fan, L.; Yin, C.; Lin, G.; Li, Q. Double loaded self-decomposable SiO2 nanoparticles for sustained drug release. Nanoscale 2015, 7, 16389–16398. [Google Scholar] [CrossRef] [PubMed]
- Watermann, A.; Brieger, J. Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.; Dunnill, P. A brief definition of regenerative medicine. Regen. Med. 2008, 3, 1–5. [Google Scholar] [CrossRef]
- Mahla, R.S. Stem cells applications in regenerative medicine and disease therapeutics. Int. J. Cell Biol. 2016, 6940283. [Google Scholar] [CrossRef]
- Napolitano, F.; Giudice, V.; D’Esposito, V.; Prevete, N.; Scala, P.; de Paulis, A.; Selleri, C.; Formisano, P.; Rossi, F.W.; Montuori, N. Cell-free regenerative medicine: Identifying the best source of mesenchymal stem cells for skin therapy in Systemic Sclerosis. Front. Cell Dev. Biol. 2025, 13, 1518412. [Google Scholar] [CrossRef]
- Preynat-Seauve, O.; Krause, K.-H. Stem cell sources for regenerative medicine: The immunological point of view. Semin. Immunopathol. 2011, 33, 519–524. [Google Scholar] [CrossRef]
- Riazi, A.M.; Kwon, S.Y.; Stanford, W.L. Stem cell source for regenerative medicine. Methods Mol. Biol. 2009, 482, 55–90. [Google Scholar]
- Hipp, J.; Atala, A. Sources of stem cells for regenerative medicine. Stem Cell Rev. 2008, 4, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kuci, S.; Kuci, Z.; Latifi-Pupovci, H.; Niethammer, D.; Handgretinger, R.; Schumm, M.; Bruchelt, G.; Bader, P.; Klingebiel, T. Adult stem cells as an alternative source of multipotential (pluripotential) cells in regenerative medicine. Curr. Stem Cell Res. Ther. 2009, 4, 107–117. [Google Scholar] [CrossRef]
- Rammal, H.; Harmouch, C.; Lataillade, J.-J.; Laurent-Maquin, D.; Labrude, P.; Menu, P.; Kerdjoudj, H. Stem cells: A promising source for vascular regenerative medicine. Stem Cells Dev. 2014, 23, 2931–2949. [Google Scholar] [CrossRef]
- Vapniarsky, N.; Arzi, B.; Hu, J.C.; Nolta, J.A.; Athanasiou, K.A. Concise review: Human dermis as an autologous source of stem cells for tissue engineering and regenerative medicine. Stem Cells Transl. Med. 2015, 4, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.; Dienelt, A.; Blankenstein, A.; Duda, G.N.; Geissler, S. Qualifying stem cell sources: How to overcome potential pitfalls in regenerative medicine? J. Tissue Eng. Regener. Med. 2016, 10, 3–10. [Google Scholar] [CrossRef]
- Haring, G.; Zupan, J. Tissues from post-mortem donors as alternative sources of stem cells for regenerative medicine. Adv. Exp. Med. Biol. 2020, 1288, 33–46. [Google Scholar]
- Naeem, A.; Gupta, N.; Naeem, U.; Elrayess, M.A.; Albanese, C. Amniotic stem cells as a source of regenerative medicine to treat female infertility. Hum. Cell 2023, 36, 15–25. [Google Scholar] [CrossRef]
- Pablos, J.L.; Lozano, D.; Manzano, M.; Vallet-Regi, M. Regenerative medicine: Hydrogels and mesoporous silica nanoparticles. Mater. Today Bio. 2024, 29, 101342. [Google Scholar] [CrossRef]
- Jia, S.; Liu, Y.; Ma, Z.; Liu, C.; Chai, J.; Li, Z.; Song, W.; Hu, K. A novel vertical aligned mesoporous silica coated nanohydroxyapatite particle as efficient dexamethasone carrier for potential application in osteogenesis. Biomed. Mater. 2021, 16, 035030. [Google Scholar] [CrossRef]
- Kaya, S.; Cresswell, M.; Boccaccini, A.R. Mesoporous silica-based bioactive glasses for antibiotic-free antibacterial applications. Mater. Sci. Eng. C 2018, 83, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yu, P.; Chen, H.; Wang, J.; Lu, B.; Cai, X.; Gu, C.; Liang, G.; Hao, D.; Ma, Q.; et al. Icariin controlled release on a silk fibroin/mesoporous bioactive glass nanoparticles scaffold for promoting stem cell osteogenic differentiation. RSC Adv. 2020, 10, 12105–12112. [Google Scholar] [CrossRef]
- Hay, J.N.; Raval, H.M. Synthesis of organic-inorganic hybrids via the nonhydrolytic sol-gel process. Chem. Mater. 2001, 13, 3396–3403. [Google Scholar] [CrossRef]
- John, L. Selected developments and medical applications of organic-inorganic hybrid biomaterials based on functionalized spherosilicates. Mater. Sci. Eng. C 2018, 88, 172–181. [Google Scholar] [CrossRef] [PubMed]
- John, L.; Ejfler, J. A brief review on selected applications of hybrid materials based on functionalized cage-like silsesquioxanes. Polymers 2023, 15, 1452. [Google Scholar] [CrossRef]
- Seaborn, C.D.; Nielsen, F.H. Silicon deprivation decreases collagen formation in wounds and bone, and ornithine transaminase enzyme activity in liver. Biol. Trace Elem. Res. 2002, 89, 251–261. [Google Scholar] [CrossRef]
- Perez, R.; Sanchez-Salcedo, S.; Lozano, D.; Heras, C.; Esbrit, P.; Vallet-Regi, M.; Salinas, A.J. Osteogenic effect of ZnO-mesoporous glasses loaded with osteostatin. Nanomaterials 2018, 8, 592. [Google Scholar] [CrossRef]
- Salinas, A.J.; Vallet-Regi, M. Glasses in bone regeneration: A multiscale issue. J. Non-Cryst. Solids 2016, 432, 9–14. [Google Scholar] [CrossRef]
- John, L.; Janeta, M.; Rajczakowska, M.; Ejfler, J.; Lydzba, D.; Szafert, S. Synthesis and microstructural properties of the scaffold based on a 3-(trimethoxysilyl)propyl methacrylate-POSS hybrid towards potential tissue engineering applications. RSC Adv. 2016, 6, 66037–66047. [Google Scholar] [CrossRef]
- Salle, L.; Laloze, J.; Usseglio, J.; Salle, H. Extensive scalp defect with exposed bone after a head trauma. Intern. Emerg. Med. 2021, 16, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Kitaguchi, K.; Kashii, M.; Ebina, K.; Kaito, T.; Okada, R.; Makino, T.; Noguchi, T.; Ishimoto, T.; Nakano, T.; Yoshikawa, H. Effects of single or combination therapy of teriparatide and anti-RANKL monoclonal antibody on bone defect regeneration in mice. Bone 2018, 106, 1–10. [Google Scholar] [CrossRef]
- Keppler, A.M.; Saller, M.M.; Alberton, P.; Westphal, I.; Heidenau, F.; Schönitzer, V.; Böcker, W.; Kammerlander, C.; Schieker, M.; Aszodi, A.; et al. Bone defect reconstruction with a novel biomaterial containing calcium phosphate and aluminum oxide reinforcement. J. Orthop. Surg. Res. 2020, 15, 287. [Google Scholar] [CrossRef]
- Etani, Y.; Ebina, K.; Hirao, M.; Kitaguchi, K.; Kashii, M.; Ishimoto, T.; Nakano, T.; Okamura, G.; Miyama, A.; Takami, K.; et al. Combined effect of teriparatide and an anti-RANKL monoclonal antibody on bone defect regeneration in mice with glucocorticoid-induced osteoporosis. Bone 2020, 139, 115525. [Google Scholar] [CrossRef]
- Kitaguchi, K.; Kashii, M.; Ebina, K.; Kaito, T.; Okada, R.; Makino, T.; Etani, Y.; Ishimoto, T.; Nakano, T.; Yoshikawa, H. The combined effects of teriparatide and anti-RANKL monoclonal antibody on bone defect regeneration in ovariectomized mice. Bone 2020, 130, 115077. [Google Scholar] [CrossRef]
- Ding, Y.; Cui, Y.; Yang, X.; Wang, X.; Tian, G.; Peng, J.; Wu, B.; Tang, L.; Cui, C.-P.; Zhang, L. Anti-RANKL monoclonal antibody and bortezomib prevent mechanical unloading-induced bone loss. J. Bone Miner. Metab. 2021, 39, 974–983. [Google Scholar] [CrossRef]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef]
- Byun, S.-Y.; Han, A.R.; Kim, K.-M.; Kwon, J.-S. Antibacterial properties of mesoporous silica coated with cerium oxide nanoparticles in dental resin composite. Sci. Rep. 2024, 14, 18014. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Mohammed, H.; Al-Hadeethi, Y.; Bakry, A.S.; Umar, A.; Hussein, M.A.; Abbassy, M.A.; Vaidya, K.G.; Al Berakdar, G.; Mkawi, E.M.; et al. Silica-based bioactive glasses and their applications in hard tissue regeneration: A review. Pharmaceuticals 2021, 14, 75. [Google Scholar] [CrossRef]
- Rahmati, M.; Mozafari, M. Selective contribution of bioactive glasses to Molecular and Cellular Pathways. ACS Biomater. Sci. Eng. 2020, 6, 4–20. [Google Scholar] [CrossRef]
- Arcos, D.; Gomez-Cerezo, N.; Saiz-Pardo, M.; de Pablo, D.; Ortega, L.; Enciso, S.; Fernandez-Tome, B.; Diaz-Guemes, I.; Sanchez-Margallo, F.M.; Casarrubios, L.; et al. Injectable mesoporous bioactive nanoparticles regenerate bone tissue under osteoporosis conditions. Acta Biomater. 2022, 151, 501–511. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regi, M. Sol-gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Mihaila, S.M.; Swami, A.; Patel, A.; Sant, S.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells. Adv. Mater. 2023, 35, 2300774. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Mihaila, S.M.; Swami, A.; Patel, A.; Sant, S.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells. Adv. Mater. 2013, 25, 3329–3336. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Liu, X.; Huang, Q.; Zhang, R.; Feng, Q. Incorporation of silica nanoparticles to PLGA electrospun fibers for osteogenic differentiation of human osteoblast-like cells. Regener. Biomater. 2018, 5, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Li, D.; Mou, X.; Li, J.; Guo, W.; Wang, S.; Yu, X.; Ma, B.; Zhang, S.; Tang, W.; et al. Effects of graphene quantum dots on the self-renewal and differentiation of mesenchymal stem cells. Adv. Healthcare Mater. 2016, 5, 702–710. [Google Scholar] [CrossRef]
- Garcia, A.; Cicuendez, M.; Izquierdo-Barba, I.; Arcos, D.; Vallet-Regi, M. Essential role of calcium phosphate heterogeneities in 2D-hexagonal and 3D-cubic SiO2-CaO-P2O5 mesoporous bioactive glasses. Chem. Mater. 2009, 21, 5474–5484. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, I.-R.; Kim, Y.; Kim, D.-H.; Park, S.-B.; Park, B.-S.; Bae, M.-K.; Kim, Y.-I. The effect of mesoporous bioactive glass nanoparticles/graphene oxide composites on the differentiation and mineralization of human dental pulp stem cells. Nanomaterials 2020, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, Z.; Zhang, M.; Ju, Z.; Niu, Z.; Ma, Y.; Xu, Z.; Zhang, T.; Shi, F. Mesoporous silica nanoparticles: A synthesis guide and research progress in the biomedical field. Mater. Today Chem. 2024, 42, 102426. [Google Scholar] [CrossRef]
- Kuang, Z.; Dai, G.; Wan, R.; Zhang, D.; Zhao, C.; Chen, C.; Li, J.; Gu, H.; Huang, W. Osteogenic and antibacterial dual functions of a novel levofloxacin loaded mesoporous silica microspheres/nano-hydroxyapatite/polyurethane composite scaffold. Genes Dis. 2021, 8, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Belancon, M.P.; Sandrini, M.; Zanuto, V.S.; Muniz, R.F. Glassy materials for silicon-based solar panels: Present and future. J. Non-Cryst. Solids 2023, 619, 122548. [Google Scholar] [CrossRef]
- Du, J.; Xie, P.; Lin, S.; Wu, Y.; Zeng, D.; Li, Y.; Jiang, X. Time-phase sequential utilization of adipose-derived mesenchymal stem cells on mesoporous bioactive glass for restoration of critical size bone defects. ACS Appl. Mater. Interfaces 2018, 10, 28340–28350. [Google Scholar] [CrossRef]
- Kermani, F.; Beidokhti, S.M.; Baino, F.; Gholamzadeh-Virany, Z.; Mozafari, M.; Kargozar, S. Strontium- and cobalt-doped multicomponent mesoporous bioactive glasses (MBGs) for potential use in bone tissue engineering applications. Materials 2020, 13, 1348. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Gargiulo, N.; Jindal, H.M.; Hasikin, K.; Naveen, S.V.; Sekaran, S.D.; Kamarul, T. Elastomeric biocomposite of silver-containing mesoporous bioactive glass and poly(1,8-octanediol citrate): Physiochemistry and in vitro antibacterial capacity in tissue engineering applications. Mater. Sci. Eng. C 2019, 98, 1022–1033. [Google Scholar] [CrossRef]
- Han, W.; Qiu, S.; Chen, J.; Zhong, X.; Hao, L.; Chen, H.; Zhou, X.; Zhou, H. One-pot synthesis of mesoporous silica-supported nano-metal oxide composites with enhanced antibacterial properties. Mater. Chem. Phys. 2022, 290, 126618. [Google Scholar] [CrossRef]
- Qiu, S.; Gao, F.; Liang, Z.; Zhong, X.; Hao, L.; Chen, H.; Zhou, X.; Zhou, H. Rosin modified aminated mesoporous silica adsorbed tea tree oil sustained-release system for improve synergistic antibacterial and long-term antibacterial effects. Nanotechnology 2021, 32, 275707. [Google Scholar] [CrossRef]
- Su, G.; Qiu, S.; Lin, J.; Zhong, X.; Zhou, H.; Zhou, X. Mesoporous silica doped with different water-soluble ligands to enhance the antibacterial performance of nano zinc oxides by coordination effect. Colloids Surf. A 2022, 640, 128414. [Google Scholar] [CrossRef]
- Zhong, X.; Gao, F.; Lin, H.; Su, G.; Zhou, H.; Zhou, X. One-pot self-assembly strategy to prepare mesoporous silica-based nanocomposites with enhanced and long-term antibacterial performance. Colloids Surf. A 2022, 650, 129654. [Google Scholar] [CrossRef]
- Li, D.; Xie, J.; Qiu, Y.; Zhang, S.; Chen, J. Multifunctional mesoporous silica nanoparticles reinforced silk fibroin composite with antibacterial and osteogenic effects for infectious bone rehabilitation. Int. J. Nanomed. 2022, 17, 5661–5678. [Google Scholar] [CrossRef]
- Xie, H.; He, Z.; Liu, Y.; Zhao, C.; Guo, B.; Zhu, C.; Xu, J. Efficient antibacterial agent delivery by mesoporous silica aerogel. ACS Omega 2022, 7, 7638–7647. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Lin, W.; Sun, S.; Zhang, M.; Liang, G.; Xie, L.; Song, W.; Zhou, Y. Mesoporous Silica Polylysine Nanocomposite Antibacterial Agent, Its Preparation Method and Application in Medical Device. Jiangsu University of Technology China CN118402522, 30 July 2024. [Google Scholar]
- Lin, D.; Cai, B.; Wang, L.; Cai, L.; Wang, Z.; Xie, J.; Lv, Q.-x.; Yuan, Y.; Liu, C.; Shen, S.G.F. A viscoelastic PEGylated poly(glycerol sebacate)-based bilayer scaffold for cartilage regeneration in full-thickness osteochondral defect. Biomaterials 2020, 253, 120095, Erratum in: Biomaterials 2021, 269, 120618. [Google Scholar] [CrossRef] [PubMed]
- Krakor, E.; Saniternik, S.; Gessner, I.; Frohnhoven, R.; Wilhelm, M.; Drexelius, M.; Tosun, N.; Neundorf, I.; Mathur, S. Hollow mesoporous silica capsules loaded with copper, silver, and zinc oxide nanoclusters for sustained antibacterial efficacy. J. Am. Ceram. Soc. 2022, 105, 1685–1696. [Google Scholar] [CrossRef]
- Li, B.; Liao, Y.; Su, X.; Chen, S.; Wang, X.; Shen, B.; Song, H.; Yue, P. Powering mesoporous silica nanoparticles into bioactive nanoplatforms for antibacterial therapies: Strategies and challenges. J. Nanobiotechnol. 2023, 21, 325. [Google Scholar] [CrossRef]
- Li, D.; Qiu, Y.; Zhang, S.; Zhang, M.; Chen, Z.; Chen, J. A multifunctional antibacterial and osteogenic nanomedicine: QAS-modified core-shell mesoporous silica containing ag nanoparticles. BioMed. Res. Int. 2020, 2020, 4567049. [Google Scholar] [CrossRef]
- Li, Y.; Tiwari, A.K.; Ng, J.S.; Seah, G.L.; Lim, H.K.; Suteewong, T.; Tay, C.Y.; Lam, Y.M.; Tan, K.W. One-pot synthesis of aminated bimodal mesoporous silica nanoparticles as silver-embedded antibacterial nanocarriers and CO2 capture sorbents. ACS Appl. Mater. Interfaces 2022, 14, 52279–52288. [Google Scholar] [CrossRef]
- Vickram, A.S.; Infant, S.S.; Manikandan, S.; Sowndharya, B.B.; Gulothungan, G.; Chopra, H. 3D bio-printed scaffolds and smart implants: Evaluating functional performance in animal surgery models. Ann. Med. Surg. 2025, 87, 3618–3634. [Google Scholar] [CrossRef] [PubMed]
- Dawood, R.M.; Mahdee, A.F. Fabrication and characterization of 3D-printed polymeric-based scaffold coated with bioceramic and naringin for a potential use in dental pulp regeneration (in vitro study). Int. Endod. J. 2025, 58, 627–642. [Google Scholar] [CrossRef]
- Behrooznia, Z.; Nourmohammadi, J.; Mohammadi, Z.; Shabani, F.; Mashhadi, R. Biological evaluation of 3D-Printed chitosan-based scaffolds for tissue engineering. Carbohydr. Res. 2025, 551, 109416. [Google Scholar] [CrossRef]
- Shen YWu, Y. Review on synthesis of carbon aerogels for CO2 capture. Fuel 2025, 387, 134370. [Google Scholar] [CrossRef]
- Zhao, P.; Zhu, Y.; Kim, M.; Zhao, G.; Wang, Y.; Collins, C.P.; Mei, O.; Zhang, Y.; Duan, C.; Zhong, J.; et al. Effective bone tissue fabrication using 3D-printed citrate-based nanocomposite scaffolds laden with BMP9-stimulated human urine stem cells. ACS Appl. Mater. Interfaces 2025, 17, 197–210. [Google Scholar] [CrossRef]
- Hong, X.; Du, Z.; Li, L.; Jiang, K.; Chen, D.; Shen, G. Biomimetic honeycomb-like Ti3C2Tx MXene/bacterial cellulose aerogel-based flexible pressure sensor for the human-computer interface. ACS Sens. 2025, 10, 417–426. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Bai, H.; Wang, C.; Li, Z.; Wang, Z.; Liu, Q.; Wang, Z.; Wang, X.; Zhu, X.; et al. 3D printed cell-free bilayer porous scaffold based on alginate with biomimetic microenvironment for osteochondral defect repair. Biomater. Adv. 2025, 167, 214092. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.; Pinho, A.R.; Gomes, M.C.; Demidov, Y.; Krakor, E.; Grume, D.; Herb, M.; Le, K.; Mano, J.; Mathur, S.; et al. Fabrication of antibacterial, osteo-inductor 3D printed aerogel-based scaffolds by incorporation of drug laden hollow mesoporous silica microparticles into the self-assembled silk fibroin biopolymer. Macromol. Biosci. 2022, 22, 2100442. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Yang, Y.; Yuan, K.; Zhou, F.; Liu, H.; Zhou, Q.; Yang, S.; Tang, T. A 3D-bioprinted scaffold with doxycycline-controlled BMP2-expressing cells for inducing bone regeneration and inhibiting bacterial infection. Bioact. Mater. 2021, 6, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, K.; Thevissen, K.; Mesquita, M.F.; Coropciuc, R.-G.; Agbaje, J.; Thevissen, P.; da Silva, W.J.; Vleugels, J.; De Cremer, K.; Gerits, E.; et al. Implant functionalization with mesoporous silica: A promising antibacterial strategy, but does such an implant osseointegrate? Clin. Exp. Dent. Res. 2021, 7, 502–511. [Google Scholar] [CrossRef]
- Jung, J.-H.; Kim, D.-H.; Yoo, K.-H.; Yoon, S.-Y.; Kim, Y.; Bae, M.-K.; Chung, J.; Ko, C.-C.; Kwon, Y.H.; Kim, Y.-I. Dentin sealing and antibacterial effects of silver-doped bioactive glass/mesoporous silica nanocomposite: An in vitro study. Clin. Oral Investig. 2019, 23, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Lin, C.; Wang, Y.; Ma, J.; Wang, X.; Yao, X.; Tang, B. Preparation of Zn doped mesoporous silica nanoparticles (Zn-MSNs) for the improvement of mechanical and antibacterial properties of dental resin composites. Dent. Mater. 2020, 36, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Shirmohammadi, A.; Balazadeh, Y.; Negahdari, R.; Dizaj, S.M.; Memar, M.Y. In vitro antibacterial effects of curcumin-loaded mesoporous silica nanoparticles inside the dental implant fixture. Open Dent. J. 2023, 17, e187421062307120. [Google Scholar] [CrossRef]
- Liao, Y.; Li, B.; Chen, H.; Ma, Y.; Wang, F.; Huang, L.; Shen, B.; Song, H.; Yue, P. Stimuli-responsive mesoporous silica nanoplatforms for smart antibacterial therapies: From single to combination strategies. J. Control. Release 2025, 378, 60–91. [Google Scholar] [CrossRef]
- Zhang, J.F.; Wu, R.; Fan, Y.; Liao, S.; Wang, Y.; Wen, Z.T.; Xu, X. Antibacterial dental composites with chlorhexidine and mesoporous silica. J. Dent. Res. 2014, 93, 1283. [Google Scholar] [CrossRef] [PubMed]
- Son, M.J.; Lee, S.-W. Antibacterial toxicity of mesoporous silica nanoparticles with functional decoration of specific organic moieties. Colloids Surf. A 2021, 630, 127612. [Google Scholar] [CrossRef]
- Son, S.-A.; Kim, D.-H.; Yoo, K.-H.; Yoon, S.-Y.; Kim, Y.-I. Mesoporous bioactive glass combined with graphene oxide quantum dot as a new material for a new treatment option for dentin hypersensitivity. Nanomaterials 2020, 10, 621. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kang, M.-S.; Mahapatra, C.; Kim, H.-W. Effect of aminated mesoporous bioactive glass nanoparticles on the differentiation of dental pulp stem cells. PLoS ONE 2016, 11, e0150727/1. [Google Scholar] [CrossRef]
- Youness, R.A.; Tag El-deen, D.M.; Taha, M.A. A review on calcium silicate ceramics: Properties, limitations, and solutions for their use in biomedical applications. Silicon 2023, 15, 2493–2505. [Google Scholar] [CrossRef]
- Xiong, S.; Jia, Y.; Liang, X. Polysaccharides coated on mesoporous silica nanoparticles inhibit the inflammatory response for the treatment of sepsis. Carbohydr. Res. 2025, 555, 109580. [Google Scholar] [CrossRef]
- Mu, Y.; Li, M.; Zhao, X.; Gong, C.; Luo, Z.; Li, B.; Zhang, W.; Ge, X.; Chen, S.; Zhou, J. TiO2 nanotube implants modified with silk fibroin and mesoporous silica nanocomposite coatings enable efficient drug release to promote osteogenesis. ACS Appl. Mater. Interfaces 2025, 17, 30600–30612. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Cabanas, M.V.; Pena, J.; Sanchez-Salcedo, S. Design of 3D scaffolds for hard tissue engineering: From apatites to silicon mesoporous materials. Pharmaceutics 2021, 13, 1981. [Google Scholar] [CrossRef]
- Du, S.; Du, L.; Liu, H.; Liu, Y.; Qin, B.; He, L.; Chang, X.; Song, W.; Li, Z. Amino-functionalized mesoporous silica film as a spatiotemporally matched degradable nanotopography to enhance early bioactivity and osteogenesis on titania nanotube surfaces. ACS Appl. Mater. Interfaces 2025, 17, 20957–20967. [Google Scholar] [CrossRef]
- Liao, T.; Liu, C.; Wu, X.; Liu, J.; Yu, W.; Xu, Z.; Kuang, Y.; Li, C. Degradable mesoporous silica nanoparticle/peptide-based “trojan horse”-like drug delivery system for deep intratumoral penetration and cancer therapy. ACS Appl. Nano Mater. 2024, 7, 9518–9531. [Google Scholar] [CrossRef]
- Moeller, K.; Bein, T. Degradable drug carriers: Vanishing mesoporous silica nanoparticles. Chem. Mater. 2019, 31, 4364–4378. [Google Scholar] [CrossRef]
- Moya, S.E.; Hernandez, R.R.; Angelome, P.C. Degradation of mesoporous silica materials in biological milieu: The gateway for therapeutic applications. Adv. NanoBiomed. Res. 2024, 4, 2400005. [Google Scholar] [CrossRef]
- Godakhindi, V.; Tarannum, M.; Dam, S.K.; Vivero-Escoto, J.L. Mesoporous silica nanoparticles as an ideal platform for cancer immunotherapy: Recent advances and future directions. Adv. Healthcare Mater. 2024, 13, 2400323. [Google Scholar] [CrossRef]
- Godakhindi, V.; Yazdimamaghani, M.; Dam, S.K.; Ferdous, F.; Wang, A.Z.; Tarannum, M.; Serody, J.; Vivero-Escoto, J.L. Optimized fabrication of dendritic mesoporous silica nanoparticles as efficient delivery system for cancer immunotherapy. Small 2024, 20, 2402802. [Google Scholar] [CrossRef] [PubMed]
- Funk, N.L.; Tavaniello, F.C.; Dos Santos, J.; Benvenutti, E.V.; Buchner, S.; Paese, K.; Deon, M.; Beck, R.C.R. Naproxen-loaded mesoporous silica nanoparticles: How can the process of incorporation affect drug placement, drug release and the in vitro naproxen irritant potential. Microporous Mesoporous Mater. 2025, 384, 113458. [Google Scholar] [CrossRef]
- Baumann, F.; Paul, T.; Ossmann, S.; Enke, D.; Aigner, A. Mesoporous silica-based membranes in transdermal drug delivery: The role of drug loss in the skin. Pharmaceutics 2024, 16, 995. [Google Scholar] [CrossRef] [PubMed]
- Stappenbeck, T.S.; Miyoshi, H. The role of stromal stem cells in tissue regeneration and wound repair. Science 2009, 324, 1666–1669. [Google Scholar] [CrossRef] [PubMed]
- Pena, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Li, H.; Fu, X. Mechanisms of action of mesenchymal stem cells in cutaneous wound repair and regeneration. Cell Tissue Res. 2012, 348, 371–377. [Google Scholar] [CrossRef]
- Akita, S. Wound repair and regeneration: Mechanisms, signaling. Int. J. Mol. Sci. 2019, 20, 6328. [Google Scholar] [CrossRef]
- Sheikholeslam, M.; Wright, M.E.E.; Cheng, N.; Oh, H.H.; Wang, Y.; Datu, A.K.; Santerre, J.P.; Amini-Nik, S.; Jeschke, M.G. Electrospun polyurethane-gelatin composite: A new tissue-engineered scaffold for application in skin regeneration and repair of complex wounds. ACS Biomater. Sci. Eng. 2020, 6, 505–516. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, J. Editorial: Advances in wound repair and regeneration: Novel materials, targets and applications. Front. Chem. 2024, 12, 1487091. [Google Scholar] [CrossRef] [PubMed]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional hydrogels for treatment of chronic wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef]
- Xue, L.; Deng, T.; Guo, R.; Peng, L.; Guo, J.; Tang, F.; Lin, J.; Jiang, S.; Lu, H.; Liu, X.; et al. A composite hydrogel containing mesoporous silica nanoparticles loaded with Artemisia argyi extract for improving chronic wound healing. Front. Bioeng. Biotechnol. 2022, 10, 825339. [Google Scholar] [CrossRef] [PubMed]
- Ugalde-Arbizu, M.; Aguilera-Correa, J.J.; San Sebastian, E.; Paez, P.L.; Nogales, E.; Esteban, J.; Gomez-Ruiz, S. Antibacterial properties of mesoporous silica nanoparticles modified with fluoroquinolones and copper or silver species. Pharmaceuticals 2023, 16, 961. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, H.; Chen, Q. Near-infrared light-mediated antibacterial photodynamic therapy based on erythrosine-functionalized mesoporous silica-coated upconversion nanoplatform. ACS Omega 2024, 9, 34799–34807. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Chen, W.; Lu, X.; Liu, Z.; Rao, Q.; Xiao, W.; Zhan, X.; Yang, S.; Gao, F.; Zhang, Q. Mesoporous silica with dual stimuli-microenvironment responsiveness via the pectin-gated strategy for controlled release of rosmarinic Acid. ACS Appl. Bio Mater. 2025, 8, 1583–1593. [Google Scholar] [CrossRef]
- Zayed, M.; Jeong, B.-H. Mesenchymal stem cell secretome attenuates PrP106-126-induced neurotoxicity by suppressing neuroinflammation and apoptosis and enhances cell migration. Cells 2025, 14, 851. [Google Scholar] [CrossRef] [PubMed]
- Riza, Y.M.; Alzahrani, F.A. Rewiring the spine-cutting-edge stem cell therapies for spinal cord repair. Int. J. Mol. Sci. 2025, 26, 5048. [Google Scholar] [CrossRef]
- Wang, X.; Dong, J.; Kang, J.; Chen, X.; Hong, X.; Chen, J.; Du, W.; Cai, H.; Liu, D. Self-adaptive release of stem cell-derived exosomes from a multifunctional hydrogel for accelerating MRSA-infected diabetic wound repair. J. Am. Chem. Soc. 2025, 147, 16362–16378. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, R.; Wu, X.; Zhou, Y.; Wang, Z.; Zhang, H.; Zhu, H.; Sun, L.; Shuai, Z. Bioinspired exosome-SiO2 nanohybrid therapeutic for rheumatoid arthritis treatment. Theranostics 2025, 15, 6553–6571. [Google Scholar] [CrossRef] [PubMed]
- Serafin, A.; Collins, M.N. Are emerging electroconductive biomaterials for spinal cord injury repair the future? Neural Regen. Res. 2025, 21, 1140–1141. [Google Scholar] [CrossRef]
- Li, C.; Luo, Y.; Li, S. Mechanistic insights of neuronal death and neuroprotective therapeutic approaches in stroke. Neural Regen. Res. 2026, 21, 869–886. [Google Scholar] [CrossRef]
- Chen, X.; Wei, M.; Li, G. Molecular mechanisms after optic nerve injury: Neurorepair strategies from a transcriptomic perspective. Neural Regen. Res. 2026, 21, 989–999. [Google Scholar] [CrossRef]
- Zhang, K.; Wen, R.; Ma, W.; He, X.; Yang, Z.; Liu, D.; Li, X. Neuronal dual-specificity phosphatase 26 inhibition via reactive-oxygen-species responsive mesoporous-silica-loaded hydrogel for spinal cord injury repair. ACS Nano 2025, 19, 4942–4958. [Google Scholar] [CrossRef]
- Liu, D.; Niu, R.; Wang, S.; Shao, L.; Yang, X.; Liu, X.; Ma, X.; Zhu, Z.; Zhang, J.; Shi, B.; et al. Nitric oxide-releasing mesoporous hollow cerium oxide nanozyme-based hydrogel synergizes with neural stem cell for spinal cord injury repair. ACS Nano 2025, 19, 2591–2614. [Google Scholar] [CrossRef]
- Shah, D.; Das, P.; Acharya, S.; Agarwal, B.; Christensen, D.J.; Robertson, S.M.; Bhandari, V. Small immunomodulatory molecules as potential therapeutics in experimental murine models of acute lung injury (ALI)/acute respiratory distress syndrome (ARDS). Int. J. Mol. Sci. 2021, 22, 2573. [Google Scholar] [CrossRef]
- Kellner, M.; Noonepalle, S.; Lu, Q.; Srivastava, A.; Zemskov, E.; Black, S.M. ROS signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Adv. Exp. Med. Biol. 2017, 967, 105–137. [Google Scholar]
- Han, Y.; Liu, T.; Wei, R.; Dai, C.; Huang, X.; Hu, D. Inflammation-responsive biomimetic nanoparticles with epigallocatechin-3-gallate for acute lung injury therapy via autophagy enhancement. iScience 2025, 28, 112318. [Google Scholar] [CrossRef]
- Shu, T.; Ren, D.; Wang, R. The role of vacuum erection device and penile traction therapy in the patients after radical prostatectomy: A narrative review. Int. J. Impot. Res. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Suçeken, F.Y.; Coşer, Ş.; Kurtuluş, D.; Akgül, M.; Kül, K.; Sevim, M.; Ekşi, M.; Küçük, E.V.; Aras, B. Transcutaneous electrical nerve stimulation (TENS) therapy in rehabilitating erectile dysfunction after bilateral nerve sparing robotic assisted radical prostatectomy. World J. Urol. 2025, 43, 337. [Google Scholar] [CrossRef] [PubMed]
- Unal, S.; Kutluhan, M.A.; Soydas, T.; Uzundal, H.; Okulu, E.; Ozayar, A.; Kayigil, O. Evaluation of electrophysiological changes after radical prostatectomy and their relationship with erectile function recovery. Int. J. Impot. Res. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Liang, X.; Wang, Z.; Wang, S.; Ruan, F.; Zhang, Y.; Shao, D.; Liu, X.; Chen, F.; Shi, X. Magnetic mesoporous silica nanoparticles loaded with peptides for the targeted repair of cavernous nerve injury underlying erectile dysfunction. Biomaterials 2025, 314, 122811. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Dai, X.; Li, D.; McCoul, D.; Gillispie, G.J.; Zhang, Y.; Yu, B.; He, C. One-pot synthesis of silver nanoparticle incorporated mesoporous silica granules for hemorrhage control and antibacterial treatment. ACS Biomater. Sci. Eng. 2018, 4, 3588–3599. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, J.; Wang, Y.; Chen, C.; Gu, H.; Chai, Y.; Wang, Y. Silver nanoparticles-decorated and mesoporous silica coated single-walled carbon nanotubes with an enhanced antibacterial activity for killing drug-resistant bacteria. Nano Res. 2020, 13, 389–400. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, F.; Liu, J.; Smatt, J.-H.; Gepperth, D.; Lastusaari, M.; Xu, C.; Hupa, L. Biocomposites of copper-containing mesoporous bioactive glass and nanofibrillated cellulose: Biocompatibility and angiogenic promotion in chronic wound healing application. Acta Biomater. 2016, 46, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, S.; Goyal, S.; Thirumal, D.; Sharma, P.; Kaur, G.; Mittal, N. Mesoporous silica nanoparticles: A versatile carrier platform in lung cancer management. Nanomedicine 2024, 19, 1331–1346. [Google Scholar] [CrossRef]
- Iriarte-Mesa, C.; Kleitz, F. Tailored mesoporous silica nanoparticles for overcoming gastrointestinal barriers: A perspective on advanced strategies for oral delivery. New J. Chem. 2025, 49, 10018–10034. [Google Scholar] [CrossRef]
- Grini, M.I.; Benbayer, C.; Saidi-Besbes, S.; Elaissari, A. Advances in mesoporous silica nanoparticles as carriers for drug delivery and other biomedical applications. Microporous Mesoporous Mater. 2025, 391, 113603. [Google Scholar] [CrossRef]
- Yousefiasl, S.; Ghovvati, M.; Alibakhshi, A.; Azizi, M.; Samadi, P.; Kumar, A.; Shojaeian, A.; Sharifi, E.; Zare, E.N.; Dey, A.D.; et al. Smart mesoporous silica nanoparticles in cancer: Diagnosis, treatment, immunogenicity, and clinical translation. Small 2025, 21, 2408898. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, X.; Duan, Y.; Shi, J. Hollow mesoporous silica nanoparticles for drug formulation and delivery: Opportunities for cancer therapy. Colloids Surf. B 2025, 249, 114534. [Google Scholar] [CrossRef] [PubMed]
- Anas Al Tahan, M.; Marwah, M.; El-Zein, H.; Al Tahan, S.; Sanchez-Aranguren, L. Exploring mesoporous silica microparticles in pharmaceutical sciences: Drug delivery and therapeutic insights. Int. J. Pharm. 2025, 678, 125656. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Mei, H.; Cui, H.; Song, M.; Qiao, Y.; Chen, J.; Lei, Y. Permeation dynamics of organic moiety-tuned organosilica nanoparticles across porcine corneal barriers: Experimental and mass transfer analysis for glaucoma drug delivery. Biomed. Phys. Eng. Express 2025, 11, 045015. [Google Scholar] [CrossRef]
- Mohanty, D.L.; Divya, N.; Zafar, A.; Warsi, M.H.; Parida, G.R.; Padhi, P.; Khalid, M.; Yasir, M.; Mujtaba, A.M. Development of etoricoxib-loaded mesoporous silica nanoparticles laden gel as vehicle for transdermal delivery: Optimization, ex vivo permeation, histopathology, and in vivo anti-inflammatory study. Drug Dev. Ind. Pharm. 2025, 51, 506–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, W.; He, Z.; Yin, W.; Chen, X.; Zhang, J.; Li, H. Multichiral mesoporous silica screws with chiral differential mucus penetration and mucosal adhesion for oral drug delivery. ACS Nano 2024, 18, 16166–16183. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Wang, Y.; Ren, Y.; Tian, Y.; Hou, L. Thrombin-responsive and sequential targeted nanoplatform for synergistic thrombolysis therapy. ACS Appl. Mater. Interfaces 2025, 17, 16696–16707. [Google Scholar] [CrossRef]
- Attia, M.S.; Hasan, A.A.; Ghazy, F.-E.S.; Gomaa, E. Mesoporous silica nanoparticles-embedded hydrogel: A potential approach for transdermal delivery of carvedilol to pediatric population. Int. J. Pharm. X 2025, 676, 125605. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Y.; Wei, Q.; Zhang, A.; Wang, S.; Wang, D.; Dong, Z.; Ma, X.; Yan, R.; Wang, Y. 4T1 cell membrane biomimetic nanovehicle for enhanced breast cancer treatment. ACS Med. Chem. Lett. 2025, 16, 51–58. [Google Scholar] [CrossRef]
- Arvejeh, P.M.; Chermahini, F.A.; Soltani, A.; Lorigooini, Z.; Rafieian-Kopaei, M.; Mobini, G.R.; Khosravian, P. Improved therapeutic efficacy: Liposome-coated mesoporous silica nanoparticles delivering thymoquinone to MCF-7 cells. Curr. Drug Deliv. 2024, 22, 956–967. [Google Scholar] [CrossRef]
- Zhang, J.; Shang, J.; Tang, X.; Xu, X. TfR aptamer-functionalized MSNs for enhancing targeted cellular uptake and therapy of cancer cells. ACS Omega 2023, 8, 48975–48983. [Google Scholar] [CrossRef]
- Sah, R.K.; Kumar, B.S. Development of PLGA-SPC3 functionalized gefitinib mesoporous silica nano-scaffolds for breast cancer targeting: Biodistribution and cytotoxicity analysis. Pharm. Dev. Technol. 2025, 30, 160–176. [Google Scholar] [CrossRef]
- Grayton, Q.E.; Phan, T.T.; Kussatz, C.C.; Schoenfisch, M.H. Hyaluronic acid-coated silica nanoparticles for targeted delivery of nitric oxide to cancer cells. ACS Appl. Bio. Mater. 2024, 7, 3796–3809. [Google Scholar] [CrossRef]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A multifunctional mediator of cancer progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef]
- Lin, C.-K.; Yang, Y.-S.; Liu, T.-P.; Lin, J.-C.; Bupphathong, S.; Tamanoi, F.; Chen, Y.-P. Tailored mesoporous silica nanoparticles and the chick chorioallantoic membrane: A promising strategy and model for efficient Blood–Brain Barrier crossing. ACS Appl. Mater. Interfaces 2025, 17, 29437–29454. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-A.; Wu, C.-H.; Wu, S.-H.; Huang, C.-Y.; Mou, C.-Y.; Wei, K.-C.; Yen, Y.; Chien, I.T.; Runa, S.; Chen, Y.-P.; et al. Receptor ligand-free mesoporous silica nanoparticles: A streamlined strategy for targeted drug delivery across the blood-brain barrier. ACS Nano 2024, 18, 12716–12736. [Google Scholar] [CrossRef] [PubMed]
- Algethami, H.; Lim, W.K.; Chitayat, D.; Tein, I.; Fasano, A.; Gorodetsky, C. Levodopa-responsive dystonia secondary to CTNNB1 neurodevelopmental disorder. Mov. Disord. Clin. Pract. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Barbosa, R.; Bastos, P.; Pita Lobo, P.; Rodrigues, C.C.; Valadas, A.; Correia Guedes, L.; Mano, B.; Alberto, S.; Paixão, V.; Rosa, M.M.; et al. Differential effects of levodopa and stimulation on post-surgery freezing of gait in subthalamic nucleus deep brain stimulation Parkinson’s Disease patients: A clinical and kinematic analysis. Mov. Disord. Clin. Pract. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Guo, X.; Qiao, X.; Li, X.; Zhou, W.; Liu, C.; Yu, F.; Chen, Q.; Pan, M.; Niu, X.; Wang, X.; et al. Lactoferrin-modified organic-inorganic hybrid mesoporous silica for co-delivery of levodopa and curcumin in the synergistic treatment of Parkinson′s disease. Phytomedicine 2025, 140, 156547. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Xu, L.; Ding, R.; Wang, M.; Yang, X.; Li, X.; Zhou, B.; Gou, K.; Han, Y.; Liu, T.; et al. Nanoparticles exhibiting virus-mimic surface topology for enhanced oral delivery. Nat. Commun. 2023, 14, 7694. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.; Xia, H.; Ren, Y.; Zhang, H.; Huang, S.; Li, M.; Wang, Y.; Li, H.; Liu, H. Biomimetic virus-like mesoporous silica nanoparticles improved cellular internalization for co-delivery of antigen and agonist to enhance tumor immunotherapy. Drug Deliv. 2023, 30, 2183814. [Google Scholar] [CrossRef]
- Phan, N.M.; Nguyen, T.L.; Choi, Y.; Mo, X.W.; Trinh, T.A.; Yi, G.-R.; Kim, J. High cellular internalization of virus-like mesoporous silica nanoparticles enhances adaptive antigen-specific immune responses against cancer. ACS Appl. Mater. Interfaces 2024, 16, 45917–45928. [Google Scholar] [CrossRef]
- Duncan, G.A. Integrative approaches to enhance adeno-associated viral gene delivery. J. Control. Release 2022, 341, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A.; Ragaisyte, I.; Morton, W.; Angioletti-Uberti, S.; Proust, A.; Dantuono, R.; Luk, C.H.; Gutierrez, M.G.; Cerrone, M.; Wilkinson, K.A.; et al. Virus-shaped mesoporous silica nanostars to improve the transport of drugs across the blood-brain barrier. ACS Appl. Mater. Interfaces 2024, 16, 37623–37640. [Google Scholar] [CrossRef]
- Yang, X.; Xie, Y.; Liao, X.; Zheng, T. Virus-bionic mesoporous silica nanoplatform for malignant tumor inhibition via effective cellular uptake and precise drug delivery. ChemMedChem 2023, 18, e202300439. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Chen, J.; Rui, S.; Li, S.; Ding, Y.; Feng, S.; Liu, Z.; Liu, Q.; Wang, S.; Zhao, Q. Variable pore size of mesoporous silica in improving physical stability and oral bioavailability of insoluble drugs. Int. J. Pharm. X 2025, 674, 125394. [Google Scholar] [CrossRef]
- Shakeel, F.; Alshehri, S.; Ibrahim, M.A.; Altamimi, M.; Haq, N.; Elzayat, E.M.; Shazly, G.A. Solubilization and thermodynamic properties of simvastatin in various micellar solutions of different non-ionic surfactants: Computational modeling and solubilization capacity. PLoS ONE 2021, 16, e0249485. [Google Scholar] [CrossRef]
- Bessa, I.A.A.; Cruz, J.V.R.; da Silva, A.F.M.; Damato, D.L.; Ligiero, C.B.P.; Gomes-da-Silva, N.C.; Archanjo, B.S.; Pinto, L.F.R.; Santos-Oliveira, R.; Rossi, A.M.; et al. Hydroxyapatite nanocrystals integrated into mesoporous silica for sustained delivery of doxorubicin. ACS Appl. Nano Mater. 2024, 7, 18450–18466. [Google Scholar] [CrossRef]
- He, L.; Javid Anbardan, Z.; Habibovic, P.; van Rijt, S. Doxorubicin- and selenium-incorporated mesoporous silica nanoparticles as a combination therapy for osteosarcoma. ACS Appl. Nano Mater. 2024, 7, 25400–25411. [Google Scholar] [CrossRef]
- Ouyang, R.; Xue, W.; Cao, P.; Sun, Z.; Wang, H.; Zhang, X.; Zhou, S.; Miao, Y. Superhydrophilic bi-methylimidazole for preferred pH-responsive, targeted drug delivery to tumor. Colloids Surf. B 2025, 253, 114701. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Wang, X.; Bai, S.; Bi, Y.; Liu, F.; Sun, J.; Fu, W.; Xu, D. Dual pH- and temperature-responsive performance and cytotoxicity of N-isopropylacrylamide and acrylic acid functionalized bimodal mesoporous silicas with core-shell structure and fluorescent feature for Hela cell. Pharmaceutics 2025, 17, 206. [Google Scholar] [CrossRef] [PubMed]
- Tarin, M.; Babaei, M.; Eshghi, H.; Matin, M.M.; Saljooghi, A.S. Targeted delivery of elesclomol using a magnetic mesoporous platform improves prostate cancer treatment both in vitro and in vivo. Talanta 2024, 270, 125539. [Google Scholar] [CrossRef]
- Ortiz, A.C.; Jackson, N.; Buelvas, N.; Jerez, A.; Lopez-Munoz, R.A.; Morales, J.; Arriagada, F. Release kinetics approach of stimuli-responsive mesoporous silica nanocarriers: pH-sensitive linker versus pH-sensitive framework. J. Drug Deliv. Sci. Technol. 2024, 91, 105212. [Google Scholar] [CrossRef]
- Shirazian, S.; Alzhrani, R.M.; Zare, M.H. Design and synthesis of drug hydrogels containing carboxymethylcellulose with honeycomb structure and pH-sensitivity as drug delivery systems for adriamycin, metformin, and naproxen. Int. J. Biol. Macromol. 2024, 271, 132568. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Huang, S.; Li, M.; Ouyang, X.-K.; Ling, J. Efficient delivery of fucoxanthin using alginate oligosaccharide-coated mesoporous silica. Mater. Today Commun. 2024, 38, 108564. [Google Scholar] [CrossRef]
- Elkady, O.A.; Zaafan, M.A.; George, M.; Elsayed, N.A.; Mettias, V.G.; Edward, V.S.; Ghataty, D.S. Metformin-loaded bioinspired mesoporous silica nanoparticles for targeted melanoma therapy: Nanotopographical design with in vitro and in vivo evaluation. Int. J. Pharm. X 2025, 674, 125499. [Google Scholar] [CrossRef]
- Mayol, B.; Pradana-Lopez, S.; Garcia, A.; de la Torre, C.; Diez, P.; Villalonga, A.; Anillo, C.; Vilela, D.; Sanchez, A.; Martinez-Ruiz, P.; et al. Self-propelled enzyme-controlled IR-mesoporous silica Janus nanomotor for smart delivery. J. Colloid Interface Sci. 2024, 671, 294–302. [Google Scholar] [CrossRef]
- He, Y.; Wang, C.; Wu, Q.; Zhang, G. Magnetic targeting and pH-microwave dual responsive Janus mesoporous silica nanoparticles for drug encapsulation and delivery. Nanotechnology 2024, 35, 315701. [Google Scholar] [CrossRef]
- Zam, W.; Ahmed, I.; Yousef, H. The warburg effect on cancer cells survival: The role of sugar starvation in cancer therapy. Curr. Rev. Clin. Exp. Pharmacol. 2021, 16, 30–38. [Google Scholar] [CrossRef]
- Xie, W.; Deng, W.-W.; Zan, M.; Rao, L.; Yu, G.-T.; Zhu, D.-M.; Wu, W.-T.; Chen, B.; Ji, L.-W.; Chen, L.; et al. Cancer cell membrane camouflaged nanoparticles to realize starvation therapy together with sheckpoint blockades for enhancing cancer therapy. ACS Nano 2019, 13, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Liu, Y.; Liu, Z.; Tan, J.; Xu, W.; Huang, G.; He, Z. Glutathione-responsive organosilica hybrid nanosystems for targeted dual-starvation therapy in luminal breast cancer. Mol. Pharm. 2024, 21, 745–759. [Google Scholar] [CrossRef]
- Sabbah, A.; Delouya, G.; Laskine, M.; Taussky, D. Metabolic plasticity in prostate cancer: The Warburg effect and its clinical relevance. Am. J. Clin. Oncol. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Shiomura, M.; Watanabe, T.; Yasuda, S.; Fukuda, I.; Yamada, T.; Shichiri, M. Refractory hypoglycemia secondary to the Warburg effect in diffuse large B-cell lymphoma. JCEM Case Rep. 2025, 3, luaf038. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, T.; Lee, J.; Goldberg, S.; King, S.A.; Tang, E.; Hu, Y.; Chen, L.; Hoover, A.; Zhu, L.; et al. Enabling tumor-specific drug delivery by targeting the Warburg effect of cancer. Cell Rep. Med. 2025, 6, 101920. [Google Scholar] [CrossRef]
- Tran, J.; Estevez, J.J.; Howard, N.J.; Kumar, S. Barriers and enablers influencing the implementation of artificial intelligence for diabetic retinopathy screening in clinical practice: A scoping review. Clin. Exp. Ophthalmol. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Dai, X.; Hui, X.; Xi, M. Critical factors driving diabetic retinopathy pathogenesis and a promising interventional strategy. Biomed. Pharmacother. 2025, 189, 118106. [Google Scholar] [CrossRef]
- Sarkar, S.; Osman, N.; Thrimawithana, T.; Wann, S.B.; Kalita, J.; Manna, P. Alleviation of diabetic retinopathy by glucose-triggered delivery of vitamin D via dextran-gated functionalized mesoporous silica nanoparticles. ACS Appl. Bio Mater. 2024, 7, 1260–1270. [Google Scholar] [CrossRef]
- Murugesan, A.; Ravi, S.; Ekambaram, M.; Balakrishnan, S.; Indrapriyadharshini, K. Tumor hypoxia and cancer stem cell markers expression in oral squamous cell carcinoma- An Immunohistochemical analysis. Indian. J. Pathol. Microbiol. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Shang, T.; Jia, Z.; Li, J.; Cao, H.; Xu, H.; Cong, L.; Ma, D.; Wang, X.; Liu, J. Unraveling the triad of hypoxia, cancer cell stemness, and drug resistance. J. Hematol. Oncol. 2025, 18, 32. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Qian, Y.; Wu, Y.; Li, T.; Zheng, Y.; Luo, C.; Wu, X.; Chen, T.; Ou, L. Hypoxia-induced VGF promotes cell migration and invasion in prostate cancer via the PI3K/Akt Axis. Front. Biosci. 2025, 30, 25522. [Google Scholar] [CrossRef]
- Barros, M.; Saez, J.A.; Arroyo, P.; Vicente Ros-Lis, J.; Dolores Garrido, M.; Martinez-Manez, R.; Carmen Terencio, M.; Carmen Montesinos, M.; Gavina, P. Nitroreductase-responsive gated mesoporous silica nanocarriers for hypoxia-targeted drug delivery. Int. J. Pharm. 2025, 672, 125326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Song, H.; Dai, Q.; Liu, C. Tumor microenvironment-responsive polymer-based RNA delivery systems for cancer treatment. Small Methods 2025, 9, 2400278. [Google Scholar] [CrossRef] [PubMed]
- Egorova, V.S.; Kolesova, E.P.; Lopus, M.; Yan, N.; Parodi, A.; Zamyatnin, A.A., Jr. Smart delivery systems responsive to Cathepsin B activity for cancer treatment. Pharmaceutics 2023, 15, 1848. [Google Scholar] [CrossRef]
- Jia, C.; Guo, Y.; Wu, F.-G. Chemodynamic therapy via Fenton and Fenton-like nanomaterials: Strategies and Recent Advances. Small 2022, 18, 2103868. [Google Scholar] [CrossRef]
- Liu, Z.; He, Y.; Ling, J.; Yi, G.; Ouyang, X.-K.; Wang, N. MnO2 modified hollow mesoporous silica nanoparticles for enhanced chemodynamic therapy. J. Drug Deliv. Sci. Technol. 2024, 95, 105604. [Google Scholar] [CrossRef]
- Cao, K.; Zhou, Y.; Shen, Y.; Wang, Y.; Huang, H.; Zhu, H. Combined photothermal therapy and cancer immunotherapy by immunogenic hollow mesoporous silicon-shelled gold nanorods. J. Pharm. Sci. 2024, 113, 2232–2244. [Google Scholar] [CrossRef]
- Zhang, Z.; Sui, D.; Xu, M.; Yang, L.; Lai, C.; Ji, Y.; Wang, K. A Multifunctional mesoporous silica-coated UCNP/Au nanoplatform for near-infrared light-triggered photothermal conversion/photoluminescence and drug delivery. Ind. Eng. Chem. Res. 2024, 63, 20191–20198. [Google Scholar] [CrossRef]
- de Lara Andrade, J.; Goncalves de Oliveira, A.; Augusto Moreira, C.; Scanferla, C.E.; Lima, S.M.; da Cunha Andrade, L.H.; Stival Bittencourt, P.R.; Martins Fernandes de Oliveira, D. Multifunctional nanoplatforms based on alumina-coated mesoporous silica with potential for cancer theranostics applications. Microporous Mesoporous Mater. 2025, 382, 113373. [Google Scholar] [CrossRef]
- Nsubuga, A.; Fayad, N.; Pini, F.; Natile, M.M.; Hildebrandt, N. Small upconversion-ruthenium nanohybrids for cancer theranostics. Nanoscale 2025, 17, 3809–3821. [Google Scholar] [CrossRef]
- Ma, Q.; Xiao, J.; Qiu, Z.; Huang, H.; Yan, D.; You, Y.; Wang, L.; Guo, X. Indocyanine green-loaded mesoporous silica nanocomposite for breast cancer imaging. ACS Appl. Nano Mater. 2024, 7, 11377–11385. [Google Scholar] [CrossRef]
- Liang, J.; Ling, J.; Zhang, X.; Ouyang, X.-K.; Omer, A.M.; Yang, G. pH/glutathione dual-responsive copper sulfide-coated organic mesoporous silica for synergistic chemo-photothermal therapy. J. Colloid Interface Sci. 2024, 657, 1–14. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, J.; Zhang, C.; Zhai, H.; Yang, P.; Chen, M. Mesoporous silica coated spicules for photodynamic therapy of metastatic melanoma. J. Nanobiotechnol. 2024, 22, 179. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, B.; Zhu, J.; Cui, X.; Yang, Y.; Zhang, W.; Wang, C. Transdermal sequential delivery of functionalized nano-deep eutectic system for enhanced treatment of melanoma. Int. J. Pharm. X 2025, 674, 125466. [Google Scholar] [CrossRef]
- Abrishami, A.; Bahrami, A.R.; Nekooei, S.; Sh Saljooghi, A.; Matin, M.M. Hybridized quantum dot, silica, and gold nanoparticles for targeted chemo-radiotherapy in colorectal cancer theranostics. Commun. Biol. 2024, 7, 393. [Google Scholar] [CrossRef]
- Chu, Y.; Wang, L.; Ke, Y.; Feng, X.; Rao, W.; Ren, W.; Xin, K.; Wang, Y.; Yu, L.; Liu, B.; et al. A multifunctional mesoporous silica drug delivery nanosystem that ameliorates tumor hypoxia and increases radiotherapy efficacy. NPG Asia Mater. 2024, 16, 40. [Google Scholar] [CrossRef]
- Zhang, N.; Zeng, W.; Xu, Y.; Li, R.; Wang, M.; Liu, Y.; Qu, S.; Ferrara, K.W.; Dai, Z. Pyroptosis induction with nanosonosensitizer-augmented sonodynamic therapy combined with PD-L1 blockade boosts efficacy against liver cancer. Adv. Healthcare Mater. 2024, 13, 2302606. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, T.; Liu, K.; Bao, K.; Liu, X.; Cui, S.; Chen, D.; Li, M.J.; Bao, S.; Hu, C.; et al. Ultrasonic diagnosis and treatment of tumors using multifunctional hollow mesoporous silicon nanoparticles. ACS Appl. Nano Mater. 2024, 7, 15446–15458. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, Y.; Yang, H.; Zheng, G.; Ren, L.; Zhang, Z.; Lin, L.; He, Y.; Wang, Q.; Zou, J. Bacteria-based drug-loaded mesoporous silica nanoparticles for synergistic FUAS of breast cancer. Cancer Nanotechnol. 2025, 16, 23. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Wang, J.; Zhang, L.; Liu, C.; Guo, D.; Yu, Y.; Ye, R.; Wang, Y.; Xu, B.; et al. Nucleolin-targeted silicon-based nanoparticles for enhanced chemo-sonodynamic therapy of diffuse large B-cell lymphoma. Int. J. Pharm. X 2025, 671, 125294. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Jian, J.-Y.; Lin, H.-M. Functionalization of rice husk-derived mesoporous silica nanoparticles for targeted and imaging in cancer drug delivery. J. Sci. Food Agric. 2024, 104, 2120–2129. [Google Scholar] [CrossRef]
- Daksh, S.; Bose, P.; Kumar, N.; Ojha, H.; Deep, S.; Datta, A. Mesoporous silica nanoparticles for targeted Aβ42 imaging and therapy in traumatic brain injury: Enhanced blood-brain barrier penetration and sustained drug delivery. ACS Appl. Nano Mater. 2025, 8, 11322–11337. [Google Scholar] [CrossRef]
- Na, H.; Venedicto, M.; Chang, C.-Y.; Carrier, J.; Lai, C.-Y. Infrared-activated bactericide: Rhenium disulfide (ReS2)-functionalized mesoporous silica nanoparticles. ACS Appl. Bio Mater. 2023, 6, 1577–1585. [Google Scholar] [CrossRef]
- Cao, C.; Ge, W.; Yin, J.; Yang, D.; Wang, W.; Song, X.; Hu, Y.; Yin, J.; Dong, X. Mesoporous silica supported silver-bismuth nanoparticles as photothermal agents for skin infection synergistic antibacterial therapy. Small 2020, 16, 2000436. [Google Scholar] [CrossRef]
- Wu, Y.; Li, H.; Wang, Y.; Zhang, B.; Bi, C.; Li, X. Evaluation of the therapeutic effect of repeated photodynamic therapy-assisted SRP treatments on class II furcation. Photodiagnosis Photodyn. Ther. 2025, 53, 104629. [Google Scholar] [CrossRef]
- Li, C.; Yang, Q.; Nie, C.; Ni, Q.; Li, M.; Xing, C.; Wang, B. Target-activated selective photodynamic antibacterial therapy: In situ enhancing 1O2 yield of conjugated polyelectrolytes by E. coli surface. J. Colloid Interface Sci. 2025, 699 Pt 1, 138162. [Google Scholar] [CrossRef]
- Huang, X.; Zhan, Y.; Xiao, Z.; He, S.; Hu, L.; Zhu, H.; Guo, H.; Sun, H.; Liu, M. Photodynamic antibacterial research on hypericin-loaded PEGylated mesoporous silica delivery system. J. Biomater. Sci., Polym. Ed. 2024, 35, 1795–1818. [Google Scholar] [CrossRef]
- Malech, H.L.; Garabedian, E.K.; Hsieh, M.M. Evolution of gene therapy, historical perspective. Hematol. Oncol. Clin. N. Am. 2022, 36, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.M.; Bankiewicz, K.S.; Christine, C.W.; Van Laar, A.D.; Gross, R.E.; Lonser, R.; Factor, S.A.; Kostyk, S.K.; Kells, A.P.; Ravina, B.; et al. Data-driven evolution of neurosurgical gene therapy delivery in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1210–1218. [Google Scholar] [CrossRef]
- Moscoso, C.G.; Steer, C.J. The evolution of gene therapy in the treatment of metabolic liver diseases. Genes 2020, 11, 915. [Google Scholar] [CrossRef]
- Lam, P.; Khan, G.; Stripecke, R.; Hui, K.M.; Kasahara, N.; Peng, K.W.; Guinn, B.A. The innovative evolution of cancer gene and cellular therapies. Cancer Gene Ther. 2013, 20, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Siwakoti, P.; Shaw, S.; Bose, S.; Kokil, G.; Kumeria, T. Porous silicon and silica carriers for delivery of peptide therapeutics. Drug Deliv. Transl. Res. 2024, 14, 3549–3567. [Google Scholar] [CrossRef] [PubMed]
- Kotadiya, D.D.; Patel, P.; Patel, H.D. Cell-penetrating peptides: A powerful tool for targeted drug delivery. Curr. Drug Deliv. 2024, 21, 368–388. [Google Scholar] [CrossRef]
- Xie, X.; Yue, T.; Gu, W.; Cheng, W.; He, L.; Ren, W.; Li, F.; Piao, J.-G. Recent advances in mesoporous silica nanoparticles delivering siRNA for cancer treatment. Pharmaceutics 2023, 15, 2483. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Chatterjee, D.; Datta, A. Mesoporous silica nanoparticles in smart drug delivery and siRNA precision therapy for cancer: Nanomedicine′s new frontier. J. Drug Deliv. Sci. Technol. 2025, 110, 107068. [Google Scholar] [CrossRef]
- Syed, O.; Jancic, P.; Knezevic, N.N. A Review of recent pharmacological advances in the management of diabetes-associated peripheral neuropathy. Pharmaceuticals 2023, 16, 801. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, Y.; Teng, W.; Li, F.; Li, L.; Li, M.; Tan, L.; Wu, Z. pH-responsive mesoporous silica nanorod for high load and oral delivery of insulin. J. Drug Deliv. Sci. Technol. 2024, 91, 105256. [Google Scholar] [CrossRef]
- Juere, E.; Caillard, R.; Marko, D.; Del Favero, G.; Kleitz, F. Smart protein-based formulation of dendritic mesoporous silica nanoparticles: Toward oral delivery of insulin. Chem.—Eur. J. 2020, 26, 5195–5199. [Google Scholar] [CrossRef]
- Hou, L.; Zheng, Y.; Wang, Y.; Hu, Y.; Shi, J.; Liu, Q.; Zhang, H.; Zhang, Z. Self-regulated carboxyphenylboronic acid-modified mesoporous silica nanoparticles with “touch switch” releasing property for insulin delivery. ACS Appl. Mater. Interfaces 2018, 10, 21927–21938. [Google Scholar] [CrossRef]
- Zhao, Y.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.Y. Mesoporous silica nanoparticle-based double drug delivery system for glucose-responsive controlled release of insulin and cyclic AMP. J. Am. Chem. Soc. 2009, 131, 8398–8400. [Google Scholar] [CrossRef]
- Mandic, L.; Sadzak, A.; Strasser, V.; Baranovic, G.; Jurasin, D.D.; Sikiric, M.D.; Segota, S. Enhanced protection of biological membranes during lipid peroxidation: Study of the interactions between flavonoid loaded mesoporous silica nanoparticles and model cell membranes. Int. J. Mol. Sci. 2019, 20, 2709. [Google Scholar] [CrossRef]
- Tada, D.B.; Suraniti, E.; Rossi, L.M.; Leite, C.A.P.; Oliveira, C.S.; Tumolo, T.C.; Calemczuk, R.; Livache, T.; Baptista, M.S. Effect of lipid coating on the interaction between silica nanoparticles and membranes. J. Biomed. Nanotechnol. 2014, 10, 519–528. [Google Scholar] [CrossRef]
- Sriwastava, S.; Elkhooly, M.; Amatya, S.; Shrestha, K.; Kagzi, Y.; Bhatia, D.; Gupta, R.; Jaiswal, S.; Lisak, R.P. Recent advances in the treatment of primary and secondary progressive Multiple Sclerosis. J. Neuroimmunol. 2024, 390, 578315. [Google Scholar] [CrossRef]
- Zolfaghari Baghbadorani, P.; Rayati Damavandi, A.; Moradi, S.; Ahmadi, M.; Bemani, P.; Aria, H.; Mottedayyen, H.; Rayati Damavandi, A.; Eskandari, N.; Fathi, F. Current advances in stem cell therapy in the treatment of multiple sclerosis. Rev. Neurosci. 2023, 34, 613–633. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Choi, Y.; Im, J.; Shin, H.; Phan, N.M.; Kim, M.K.; Choi, S.W.; Kim, J. Immunosuppressive biomaterial-based therapeutic vaccine to treat multiple sclerosis via re-establishing immune tolerance. Nat. Commun. 2022, 13, 7449. [Google Scholar] [CrossRef]
- Zang, H.; Fofana, J.; Xu, F.; Nodder, S.B.; Gummuluru, S.; Reinhard, B.M. Characterizing lipid-coated mesoporous silica nanoparticles as CD169-binding delivery system for rilpivirine and cabotegravir. Adv. NanoBiomed. Res. 2022, 2, 2100157. [Google Scholar] [CrossRef]
- Ren, M.; Zhou, Y.; Tu, T.; Jiang, D.; Pang, M.; Li, Y.; Luo, Y.; Yao, X.; Yang, Z.; Wang, Y. RVG peptide-functionalized favipiravir nanoparticle delivery system facilitates antiviral therapy of neurotropic virus infection in a mouse model. Int. J. Mol. Sci. 2023, 24, 5851. [Google Scholar] [CrossRef]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.M.; Schubert, M.S.; Friedmann-Morvinski, D.; Cohen, Z.R.; Behlke, M.A.; et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020, 6, eabc9450. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Xie, L.; Hassanin, A.A.; Zuo, E.; Lu, Y. The potential of CRISPR/Cas9 gene editing as a treatment strategy for inherited diseases. Front. Cell Dev. Biol. 2021, 9, 699597. [Google Scholar] [CrossRef]
- LaBauve, A.E.; Saada, E.A.; Jones, I.K.A.; Mosesso, R.; Noureddine, A.; Techel, J.; Gomez, A.; Collette, N.; Sherman, M.B.; Serda, R.E.; et al. Lipid-coated mesoporous silica nanoparticles for anti-viral applications via delivery of CRISPR-Cas9 ribonucleoproteins. Sci. Rep. 2023, 13, 6873. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.A.; El-Tanani, M.; Tambuwala, M.M. Principles of CRISPR-Cas9 technology: Advancements in genome editing and emerging trends in drug delivery. J. Drug Deliv. Sci. Technol. 2024, 92, 105338. [Google Scholar] [CrossRef]
- Wang, A.Z.; Brink, H.J.; Bouma, R.G.; Affandi, A.J.; Nijen Twilhaar, M.K.; Heijnen, D.A.M.; van Elk, J.; Maaskant, J.J.; Konijn, V.A.L.; Stolwijk, J.G.C.; et al. Development of a versatile cancer vaccine format targeting antigen-presenting cells using proximity-based sortase a-mediated ligation of T-cell epitopes. Bioconjugate Chem. 2024, 35, 1805–1814. [Google Scholar] [CrossRef]
- Lu, T.; Xu, R.; Wang, C.-H.; Zhao, J.-Y.; Peng, B.; Wang, J.; Zhang, L.-Y. Identification of tumor antigens and immune subtypes of esophageal squamous cell carcinoma for mRNA vaccine development. Front. Genet. 2022, 13, 853113. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Toapanta, F.R.; McArthur, M.A.; Qadri, F.; Svennerholm, A.-M.; Devriendt, B.; Phalipon, A.; Cohen, D.; Sztein, M.B. Role of antigen specific T and B cells in systemic and mucosal immune responses in ETEC and Shigella infections, and their potential to serve as correlates of protection in vaccine development. Vaccine 2019, 37, 4787–4793. [Google Scholar] [CrossRef]
- Magana, G.; Harvey, C.; Taggart, C.C.; Rodgers, A.M. Bacterial outer membrane vesicles: Role in pathogenesis and host-cell interactions. Antibiotics 2024, 13, 32. [Google Scholar]
- Lieberman, L.A. Outer membrane vesicles: A bacterial-derived vaccination system. Front. Microbiol. 2022, 13, 1029146. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chen, C.-W.; Chen, M.-Y.; Xu, L.; Tian, X.; Cheung, S.-H.; Wu, Y.-L.; Siriwon, N.; Wu, S.-H.; Mou, K.Y. The bacterial outer membrane vesicle-cloaked immunostimulatory nanoplatform reinvigorates T cell function and reprograms tumor immunity. ACS Nano 2025, 19, 19866–19889. [Google Scholar] [CrossRef]
- Da, X.; Cao, B.; Mo, J.; Xiang, Y.; Hu, H.; Qiu, C.; Zhang, C.; Lv, B.; Zhang, H.; He, C.; et al. Inhibition of growth of hepatocellular carcinoma by co-delivery of anti-PD-1 antibody and sorafenib using biomimetic nano-platelets. BMC Cancer 2024, 24, 273. [Google Scholar] [CrossRef]
- Yang, J.; Liu, B.; Wang, Q.; Yan, H.; Li, G.; Wang, X.; Shang, Z.; Ou, T.; Chen, W. Carboxylated mesoporous silica nanoparticle-nucleic acid chimera conjugate-assisted delivery of siRNA and doxorubicin effectively treat drug-resistant bladder cancer. Biomed. Pharmacother. 2024, 178, 117185. [Google Scholar] [CrossRef] [PubMed]
- Sheykhhasan, M.; Ahmadieh-Yazdi, A.; Heidari, R.; Chamanara, M.; Akbari, M.; Poondla, N.; Yang, P.; Malih, S.; Manoochehri, H.; Tanzadehpanah, H.; et al. Revolutionizing cancer treatment: The power of dendritic cell-based vaccines in immunotherapy. Biomed. Pharmacother. 2025, 184, 117858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Q.; Yang, H.; Shi, X.; Su, Y.; Xia, J.; Li, Y.; Liu, X. Lipid coating of mesoporous silica nanoparticles leads to efficient antigen delivery to lymph nodes for cancer vaccination. ACS Appl. Bio Mater. 2025, 8, 4294–4302. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Chen, Y.; Tao, S.; Du, S.; Liang, C.; Teng, Z.; Gao, Y. Exploring hollow mesoporous silica nanoparticles as a nanocarrier in the delivery of foot-and-mouth disease virus-like particle vaccines. ACS Appl. Bio Mater. 2024, 7, 1064–1072. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Y.; Shi, Z.; Zink, J.I.; Yu, Q. Virus-like magnetic mesoporous silica particles as a universal vaccination platform against pathogenic infections. ACS Nano 2023, 17, 6899–6911. [Google Scholar] [CrossRef]
- Wan, H.; Yang, Y.; Tu, Z.; Tang, M.; Jing, B.; Feng, Y.; Xie, J.; Gao, H.; Song, X.; Zhao, X. Enhanced mucosal immune response through nanoparticle delivery system based on chitosan-catechol and a recombinant antigen targeted towards M cells. Int. J. Biol. Macromol. 2025, 306, 141345. [Google Scholar] [CrossRef]
- Guo, W.; Wang, Z.; Xue, R.; Zhang, X.; Zhao, Q.; Gao, Y.; Wang, S.; Mao, Y. The minimalist epigenetic nano-adjuvant gMSN reprograms dendritic cells to enhance the mucosal immune response of oral vaccines. Biomaterials 2026, 324, 123459. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, T.; Mao, N.; Cai, G.; Gu, P.; Song, Z.; Lu, X.; Yang, Y.; Wang, D. Cistanche deserticola polysaccharide-functionalized dendritic fibrous nano-silica as oral vaccine adjuvant delivery enhancing both the mucosal and systemic immunity. Int. J. Biol. Macromol. 2024, 262, 129982. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Ye, X.; Chen, W.; Liu, L.; Hu, F.; Qi, Y.; Yu, W.; Lin, Z.; Yan, J.; Guo, Z.; et al. A mild bioinspiration route to bacillus-shaped silica with enhanced immune responses. ACS Sustain. Chem. Eng. 2023, 11, 1324–1332. [Google Scholar] [CrossRef]
- Zhang, C.; Li, F.; Yu, X.; Tian, H.; Li, Y.; Liu, X.; Liu, W.; Yu, B.; Qiao, Z.-A.; Yu, X. Periodic mesoporous organosilica-loaded mincle agonists enhance the immunogenicity of COVID-19 subunit vaccines by dual activation of B cells and dendritic cells. Acta Biomater. 2025, 193, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Cheng, B.; Ru, J.; Li, X.; Fang, G.; Xie, Y.; Shi, G.; Hou, J.; Zhao, L.; Gan, L.; et al. Nano-carrier DMSN for effective multi-antigen vaccination against SARS-CoV-2. J. Nanobiotechnol. 2024, 22, 11. [Google Scholar] [CrossRef]
- Ajumobi, O.; Davis, M.; George, C.M.; Rosman, L.; Von Dobschuetz, S.; Watson, C.; Nuzzo, J.B. Improving risk analysis of the environmental drivers of the spillover, emergence/re-emergence and spread of Crimean-Congo haemorrhagic fever virus, Marburg virus and Middle East respiratory syndrome coronavirus in the East Africa Region. BMJ Glob. Health 2025, 10, e019162. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A. Developments in treatment for middle east respiratory syndrome coronavirus (MERS-CoV). Expert Rev. Respir. Med. 2024, 18, 295–307. [Google Scholar] [CrossRef]
- Almansour, I.; Jermy, B.R. Nucleic acid vaccine candidates encapsulated with mesoporous silica nanoparticles against MERS-CoV. Hum. Vaccines Immunother. 2024, 20, 2346390. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, F.; Ru, J.; Liu, L.; Wang, Z.; Wang, N.; Shu, X.; Wei, Z.; Guo, H. Evaluation of the effect of inactivated transmissible gastroenteritis virus vaccine with nano silicon on the phenotype and function of porcine dendritic cells. Viruses 2021, 13, 2158. [Google Scholar] [CrossRef]
- Joseph-Munné, J.; Maya-Hoyos, M.; Saubi, N.; Perez, S.; Lopez, M.A.M.; Baron, E.; Soto, C.Y. Recombinant mycobacterium bovis BCG-Based HIV vaccine: Failures and promising approaches for a successful vaccine strategy. Vaccines 2025, 13, 606. [Google Scholar] [CrossRef]
- Boomgarden, A.C.; Upadhyay, C. Progress and challenges in HIV-1 vaccine research: A Comprehensive overview. Vaccines 2025, 13, 148. [Google Scholar] [CrossRef]
- Li, S.; Wang, B.; Jiang, S.; Pan, Y.; Shi, Y.; Kong, W.; Shan, Y. Surface-functionalized silica-coated calcium phosphate nanoparticles efficiently deliver DNA-based HIV-1 trimeric envelope vaccines against HIV-1. ACS Appl. Mater. Interfaces 2021, 13, 53630–53645. [Google Scholar] [CrossRef]
- Trezena, A.G.; Oseliero Filho, P.L.; Cides da Silva, L.C.; Oliveira, C.L.P.; Lopes, J.L.d.S.; Antonio, N.d.S.; Dettmann, V.F.B.; Akamatsu, M.A.; Martins, T.d.S.; Ribeiro, O.G.; et al. Adjuvant effect of mesoporous silica SBA-15 on anti-diphtheria and anti-tetanus humoral immune response. Biologicals 2022, 80, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Guo, J.; Noureddine, A.; Wang, A.; Wuttke, S.; Brinker, C.J.; Zhu, W. Sol-gel-based advanced porous silica materials for biomedical applications. Adv. Funct. Mater. 2020, 30, 1909539. [Google Scholar] [CrossRef]
- Croissant, J.G.; Butler, K.S.; Zink, J.I.; Brinker, C.J. Synthetic amorphous silica nanoparticles: Toxicity, biomedical and environmental implications. Nat. Rev. Mater. 2020, 5, 886–909. [Google Scholar] [CrossRef]
- Lerida-Viso, A.; Estepa-Fernandez, A.; Garcia-Fernandez, A.; Marti-Centelles, V.; Martinez-Manez, R. Biosafety of mesoporous silica nanoparticles; towards clinical translation. Adv. Drug Deliv. Rev. 2023, 201, 115049. [Google Scholar] [CrossRef]
- Brinker, C.J.; Lu, Y.; Sellinger, A.; Fan, H. Evaporation-induced self-assembly. Nanostructures made easy. Adv. Mater. 1999, 11, 579–585. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, H.; Stump, A.; Ward, T.L.; Rieker, T.; Brinker, C.J. Aerosol-assisted self-assembly of mesostructured spherical nanoparticles. Nature 1999, 398, 223–226. [Google Scholar] [CrossRef]
- Esa, M.; Kaewpaiboon, S.; Teerapol Srichana, T. Fabrication, biodistribution, and toxicological evaluation of mesoporous silica nanoparticles based on preclinical studies intended for cancer therapy: A review. J. Appl. Pharm. Sci. 2025, 15, 001–026. [Google Scholar] [CrossRef]
- Janjua, T.I.; Cao, Y.; Yu, C.; Popat, A. Clinical translation of silica nanoparticles. Nat. Rev. Mater. 2021, 6, 1072–1074. [Google Scholar] [CrossRef]
- Benezra, M.; Penate-Medina, O.; Zanzonico, P.B.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Iyer, S.; et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 2011, 121, 2768–2780. [Google Scholar] [CrossRef]
- Bukara, K.; Schueller, L.; Rosier, J.; Martens, M.A.; Daems, T.; Verheyden, L.; Eelen, S.; Van Speybroeck, M.; Libanati, C.; Martens, J.A.; et al. Ordered mesoporous silica to enhance the bioavailability of poorly water-soluble drugs: Proof of concept in man. Eur. J. Pharm. Biopharm. 2016, 108, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Eskandar, N.G.; Rao, S.; Prestidge, C.A. First in man bioavailability and tolerability studies of a silica-lipid hybrid (Lipoceramic) formulation: A Phase I study with ibuprofen. Drug Deliv. Transl. Res. 2014, 4, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Caruana, P.; De la Fuente, N.; Espanol, P.; Gamez, M.; Balart, J.; Llurba, E.; Rovira, R.; Ruiz, R.; Martin-Lorente, C.; et al. Nano-based approved pharmaceuticals for cancer treatment: Present and future challenges. Biomolecules 2022, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Tao, J.; Zhang, Y.; Xi, L.; Han, R.; Xu, M.; Lee, S.M.-Y.; Ge, W.; Gan, Y.; Zheng, Y. Shape and shear stress impact on the toxicity of mesoporous silica nanoparticles: In vitro and in vivo evidence. Mol. Pharmaceutics 2023, 20, 3187–3201. [Google Scholar] [CrossRef]
- Hu, Y.; Bai, S.; Wu, X.; Tan, S.; He, Y. Biodegradability of mesoporous silica nanoparticles. Ceram. Int. 2021, 47, 31031–31041. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Zhong, W.; Xiao, H.; Liu, X.; Yu, T.; Zhou, C. Tuning biodegradability and biocompatibility of mesoporous silica nanoparticles by doping strontium. Ceram. Int. 2020, 46, 11762–11769. [Google Scholar] [CrossRef]
- Ratirotjanakul, W.; Suteewong, T.; Polpanich, D.; Tangboriboonrat, P. Amino acid as a biodegradation accelerator of mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2019, 282, 243–251. [Google Scholar] [CrossRef]
- Sere, S.; De Roo, B.; Vervaele, M.; Van Gool, S.; Jacobs, S.; Seo, J.W.; Locquet, J.-P. Altering the biodegradation of mesoporous silica nanoparticles by means of experimental parameters and surface functionalization. J. Nanomater. 2018, 2018, 7390618. [Google Scholar] [CrossRef]
- Kempen, P.J.; Greasley, S.; Parker, K.A.; Campbell, J.L.; Chang, H.-Y.; Jones, J.R.; Sinclair, R.; Gambhir, S.S.; Jokerst, J.V. Theranostic mesoporous silica nanoparticles biodegrade after pro-survival drug delivery and ultrasound/magnetic resonance imaging of stem cells. Theranostics 2015, 5, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Shi, J. Defect engineering of mesoporous silica nanoparticles for biomedical applications. Acc. Mater. Res. 2021, 2, 581–593. [Google Scholar] [CrossRef]
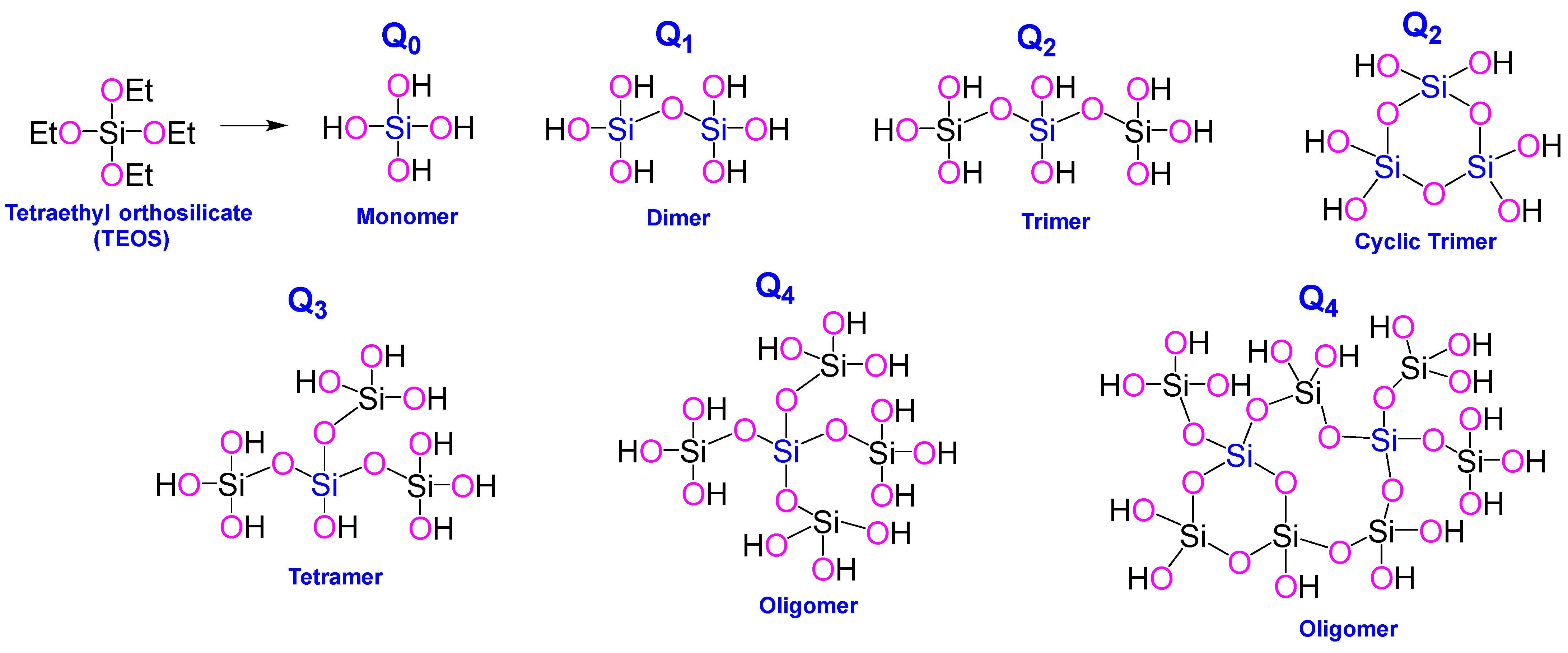
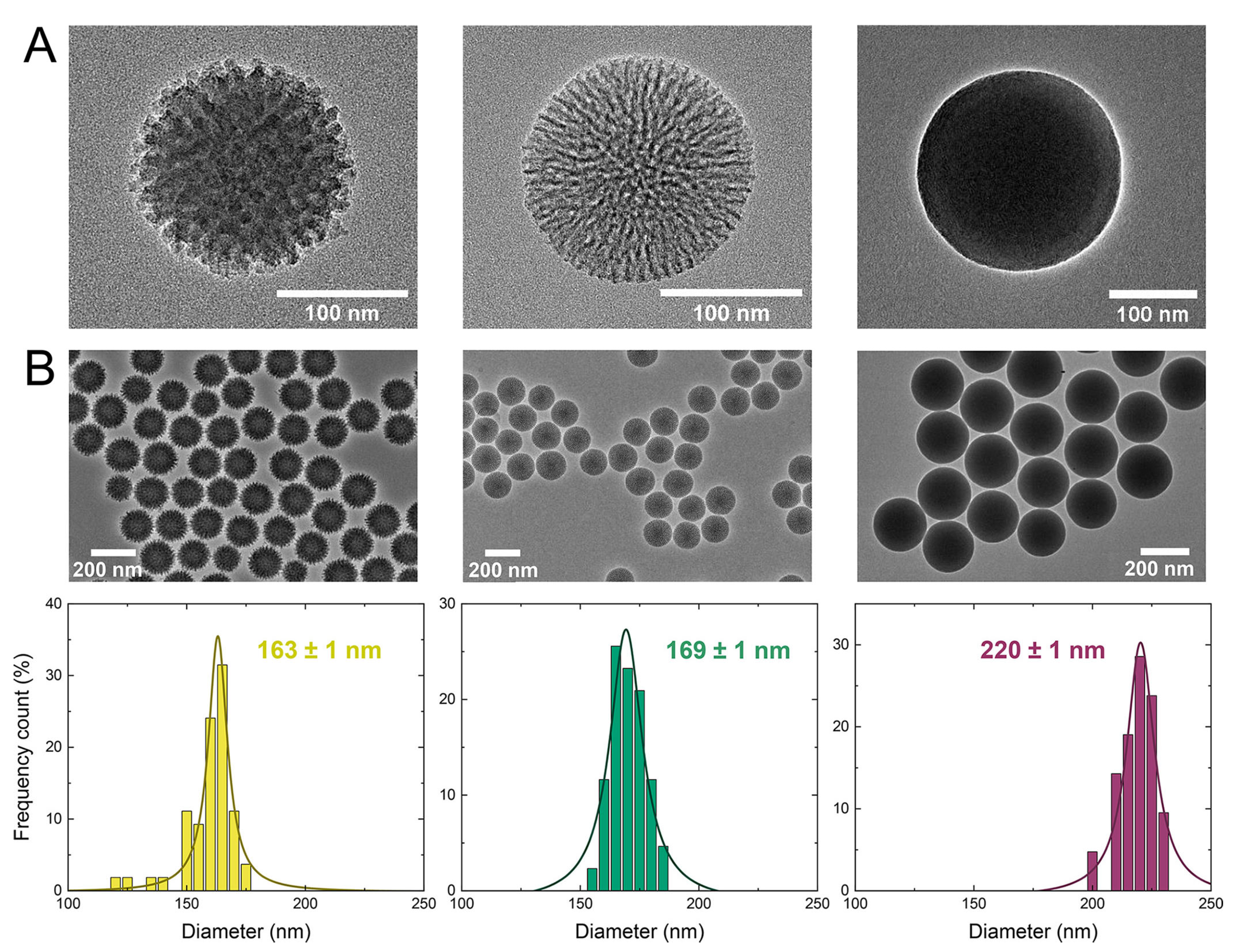

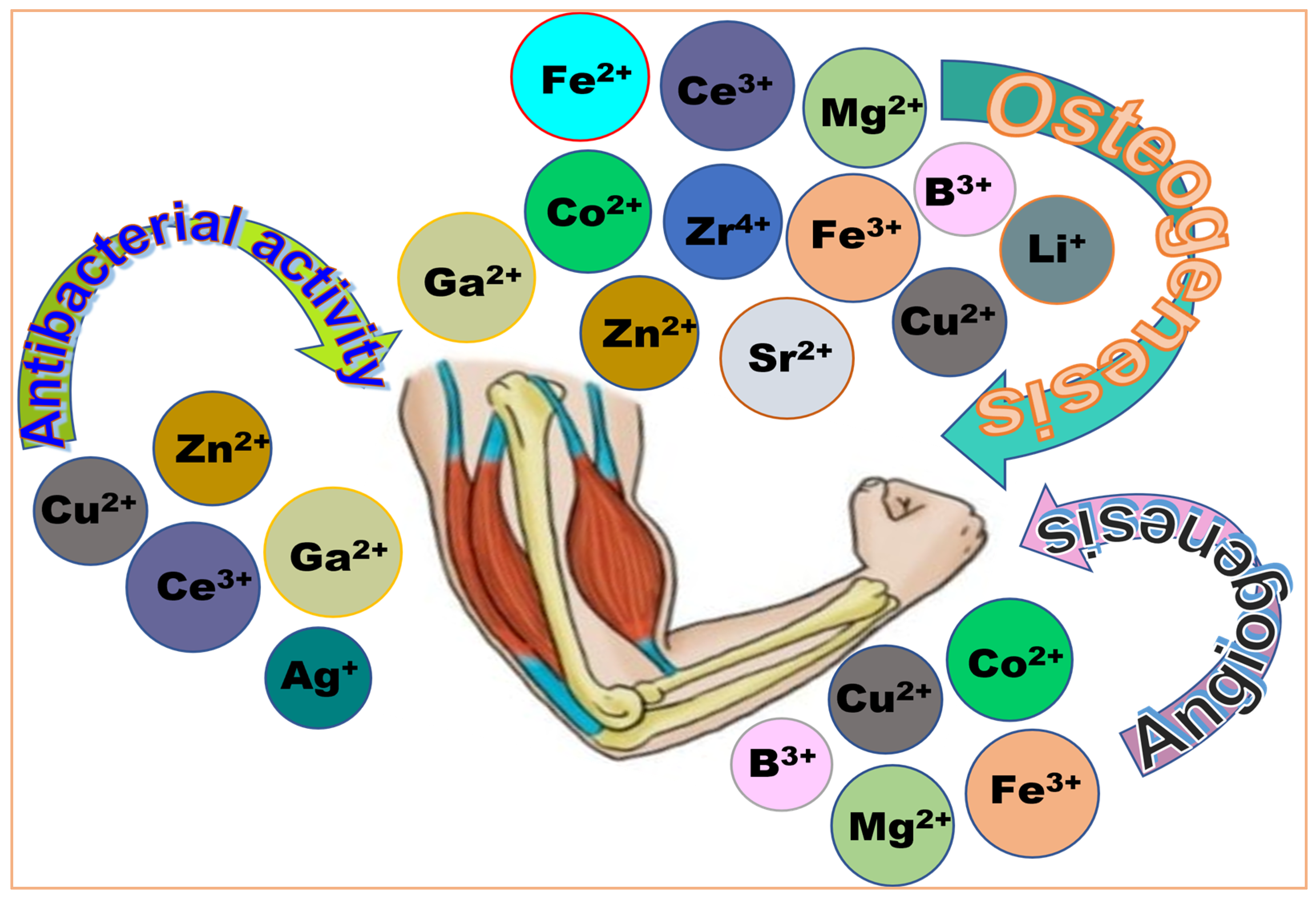
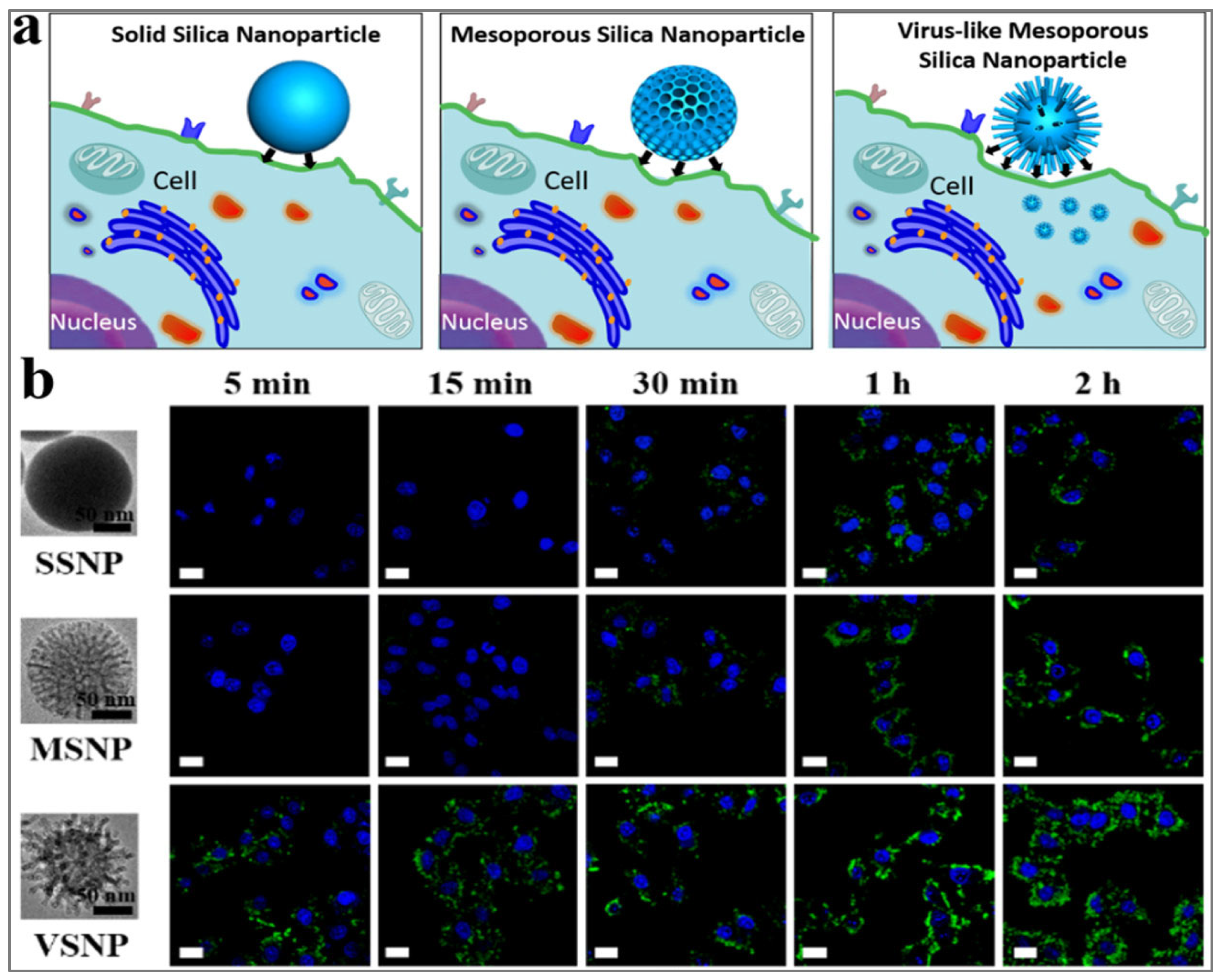
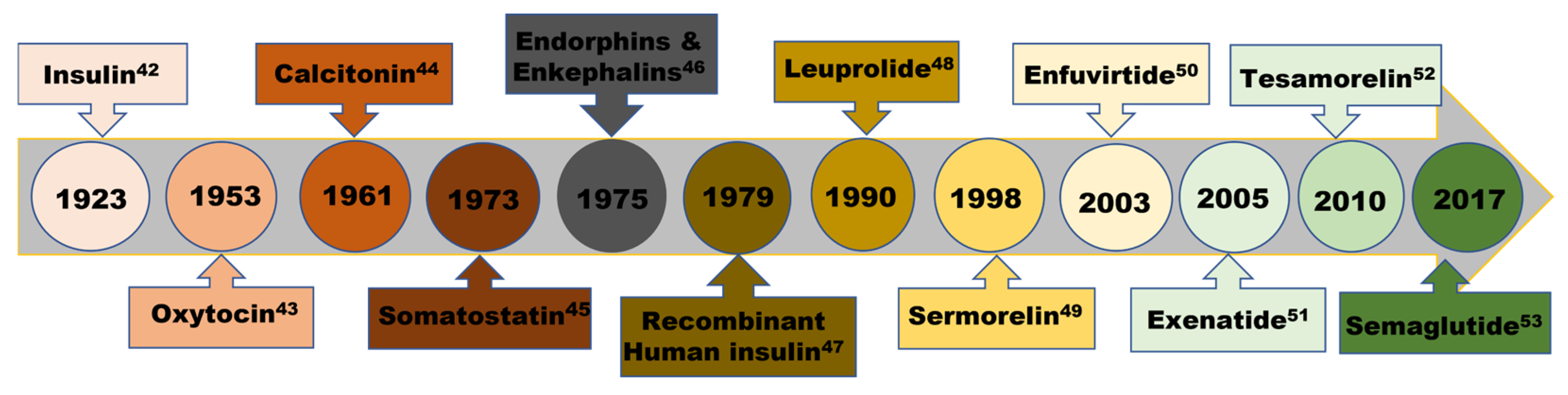
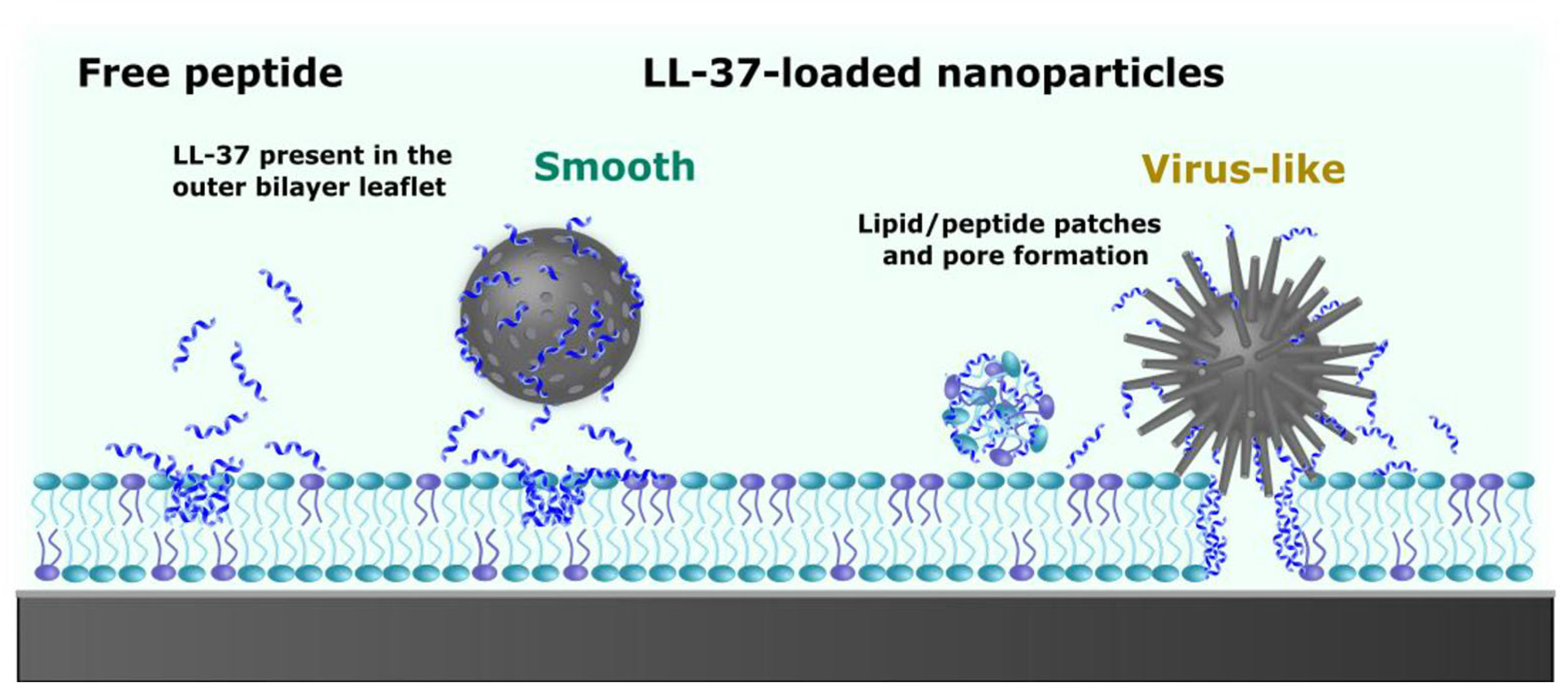
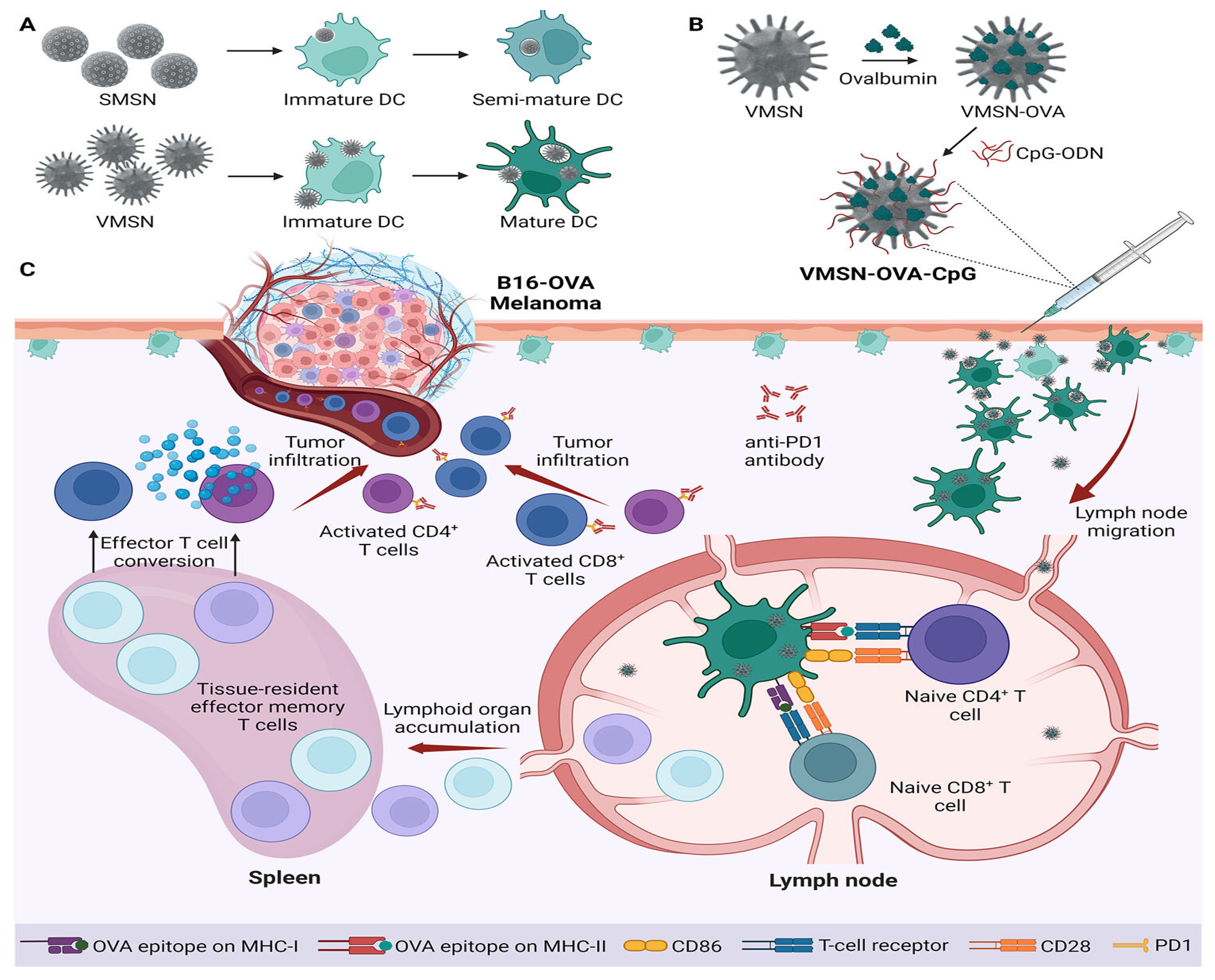
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotie, J. Applications of Tailored Mesoporous Silicate Nanomaterials in Regenerative Medicine and Theranostics. Int. J. Mol. Sci. 2025, 26, 7918. https://doi.org/10.3390/ijms26167918
Fotie J. Applications of Tailored Mesoporous Silicate Nanomaterials in Regenerative Medicine and Theranostics. International Journal of Molecular Sciences. 2025; 26(16):7918. https://doi.org/10.3390/ijms26167918
Chicago/Turabian StyleFotie, Jean. 2025. "Applications of Tailored Mesoporous Silicate Nanomaterials in Regenerative Medicine and Theranostics" International Journal of Molecular Sciences 26, no. 16: 7918. https://doi.org/10.3390/ijms26167918
APA StyleFotie, J. (2025). Applications of Tailored Mesoporous Silicate Nanomaterials in Regenerative Medicine and Theranostics. International Journal of Molecular Sciences, 26(16), 7918. https://doi.org/10.3390/ijms26167918






