Biomarkers and Clinical Evaluation in the Detection of Frailty
Abstract
1. Introduction
2. Epidemiology of Frailty
Healthcare Outcomes
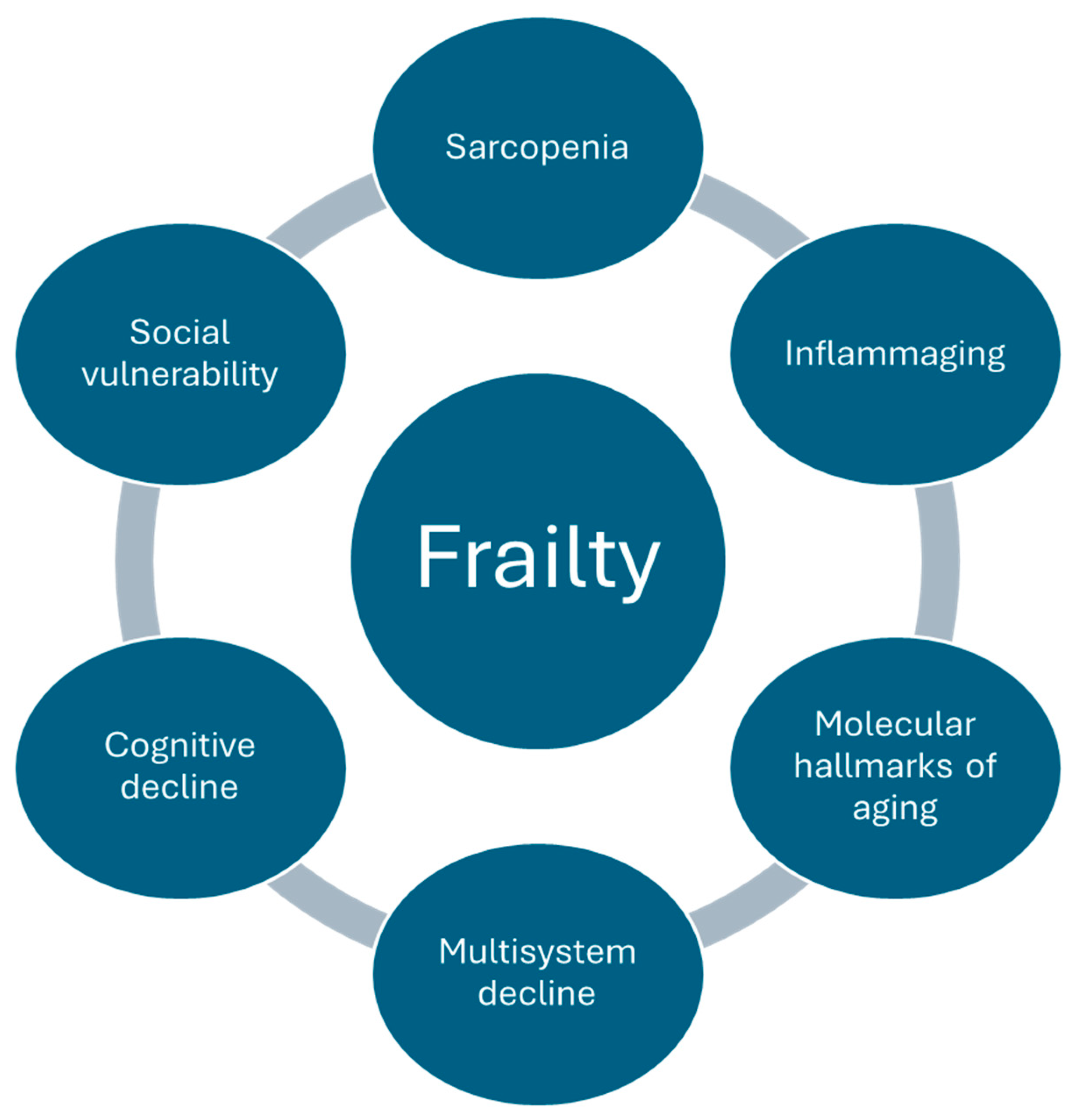
3. Pathophysiology of Frailty
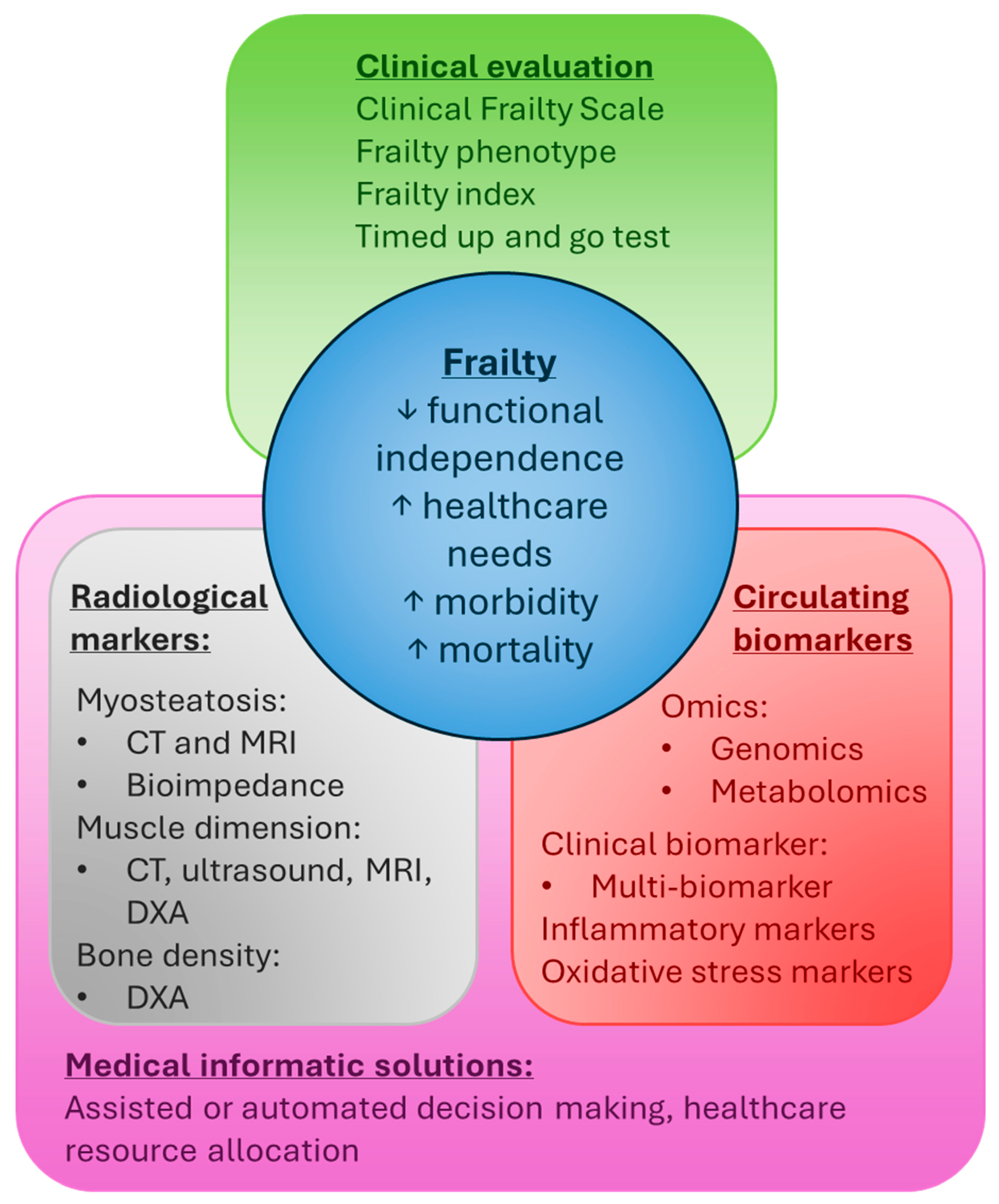
4. Clinical Measures of Frailty
5. Circulating Frailty Biomarkers
5.1. Clinical Biomarkers
5.2. Metabolic Profile and Metabolomics
5.3. Genetic and Epigenetic Markers
5.4. Inflammatory Markers
5.5. Markers of Oxidative Stress
5.6. Multi-Biomarker Approach and Machine Learning
| Category | Findings/Associations |
|---|---|
| Clinical markers |
|
| Metabolic profile (metabolomics) |
|
| Genetic and Epigenetic Markers | |
| Oxidative stress | |
| Inflammatory markers |
6. Radiological Markers of Frailty
7. The Future of Early Frailty Intervention
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADLs | Activities of daily living |
| CRP | C reactive protein |
| CVD | Cardiovascular diseases |
| CT | Computed Tomography |
| DAMPS | Damage-associated molecular patterns |
| DXA | Dual X ray Absorptiometry |
| FP | Frailty Phenotype |
| GAMA | Gamma-aminobutyric acid |
| GDF-15 | Growth/differentiation factor 15 |
| HGS | Hand grip strength |
| IFN- γ | Interferon- γ |
| IL | Interleukin |
| Lp-PLA2 | Lipoprotein phospholipase A2 |
| MetS | Metabolic Syndrome |
| MRI | Magnetic Resonance Imaging |
| MDA | Malondialdehyde |
| miRs | microRNAs |
| mitomiRs | mitochondrial microRNAs |
| NAAG | N-acetyl-aspartyl-glutamate |
| NSQIP | National Surgical Quality Improvement Program |
| SNP | Single nucleotide polymorphisms |
| TGF | Transforming Growth Factor β |
| TUG | Timed up and go test |
| TNF-α | Tumor necrosis factor-α |
| VASQIP | Veterans Affairs Surgical Quality Improvement Program |
References
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef]
- Walsh, B.; Fogg, C.; Harris, S.; Roderick, P.; de Lusignan, S.; England, T.; Clegg, A.; Brailsford, S.; Fraser, S.D.S. Frailty transitions and prevalence in an ageing population: Longitudinal analysis of primary care data from an open cohort of adults aged 50 and over in England, 2006–2017. Age Ageing 2023, 52, afad058. [Google Scholar] [CrossRef]
- The Older Population: 2020. Available online: https://www2.census.gov/library/publications/decennial/2020/census-briefs/c2020br-07.pdf (accessed on 29 June 2025).
- Hall, M.J.; DeFrances, C.J.; Williams, S.N.; Golosinskiy, A.; Schwartzman, A. National Hospital Discharge Survey: 2007 Summary; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2010; pp. 1–20. [Google Scholar]
- Bicket, M.C.; Chua, K.P.; Lagisetty, P.; Li, Y.; Waljee, J.F.; Brummett, C.M.; Nguyen, T.D. Prevalence of Surgery Among Individuals in the United States. Ann. Surg. Open 2024, 5, e421. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef]
- Sieber, F.; McIsaac, D.I.; Deiner, S.; Azefor, T.; Berger, M.; Hughes, C.; Leung, J.M.; Maldon, J.; McSwain, J.R.; Neuman, M.D.; et al. 2025 American Society of Anesthesiologists Practice Advisory for Perioperative Care of Older Adults Scheduled for Inpatient Surgery. Anesthesiology 2025, 142, 22–51. [Google Scholar] [CrossRef]
- George, E.L.; Hall, D.E.; Youk, A.; Chen, R.; Kashikar, A.; Trickey, A.W.; Varley, P.R.; Shireman, P.K.; Shinall, M.C., Jr.; Massarweh, N.N.; et al. Association Between Patient Frailty and Postoperative Mortality Across Multiple Noncardiac Surgical Specialties. JAMA Surg. 2021, 156, e205152. [Google Scholar] [CrossRef]
- Panayi, A.C.; Orkaby, A.R.; Sakthivel, D.; Endo, Y.; Varon, D.; Roh, D.; Orgill, D.P.; Neppl, R.L.; Javedan, H.; Bhasin, S.; et al. Impact of frailty on outcomes in surgical patients: A systematic review and meta-analysis. Am. J. Surg. 2019, 218, 393–400. [Google Scholar] [CrossRef]
- Kahlon, S.; Pederson, J.; Majumdar, S.R.; Belga, S.; Lau, D.; Fradette, M.; Boyko, D.; Bakal, J.A.; Johnston, C.; Padwal, R.S.; et al. Association between frailty and 30-day outcomes after discharge from hospital. Can. Med. Assoc. J. 2015, 187, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Iliffe, S.; Jivraj, S.; Walters, K. Association between frailty and quality of life among community-dwelling older people: A systematic review and meta-analysis. J. Epidemiol. Community Health 2016, 70, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G. Frailty as a predictor of disabilities among community-dwelling older people: A systematic review and meta-analysis. Disabil. Rehabil. 2017, 39, 1897–1908. [Google Scholar] [CrossRef]
- Joynt Maddox, K.E.; Elkind, M.S.V.; Aparicio, H.J.; Commodore-Mensah, Y.; de Ferranti, S.D.; Dowd, W.N.; Hernandez, A.F.; Khavjou, O.; Michos, E.D.; Palaniappan, L.; et al. Forecasting the Burden of Cardiovascular Disease and Stroke in the United States Through 2050-Prevalence of Risk Factors and Disease: A Presidential Advisory from the American Heart Association. Circulation 2024, 150, e65–e88. [Google Scholar] [CrossRef]
- Shi, Q.; Huang, J.; Wan, J.; Zhong, Z.; Sun, Y.; Zhou, Y.; Li, J.; Tan, X.; Yu, B.; Lu, Y.; et al. Physical Frailty, Genetic Predisposition, and Incident Heart Failure. JACC Asia 2024, 4, 547–556. [Google Scholar] [CrossRef]
- Damluji, A.A.; Chung, S.E.; Xue, Q.L.; Hasan, R.K.; Moscucci, M.; Forman, D.E.; Bandeen-Roche, K.; Batchelor, W.; Walston, J.D.; Resar, J.R.; et al. Frailty and cardiovascular outcomes in the National Health and Aging Trends Study. Eur. Heart J. 2021, 42, 3856–3865. [Google Scholar] [CrossRef]
- McGinn, R.; Agung, Y.; Grudzinski, A.L.; Talarico, R.; Hallet, J.; McIsaac, D.I. Attributable Perioperative Cost of Frailty after Major, Elective Noncardiac Surgery: A Population-based Cohort Study. Anesthesiology 2023, 139, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Crooms, R.C.; Gelfman, L.P. Palliative Care and End-of-Life Considerations for the Frail Patient. Anesth. Analg. 2020, 130, 1504–1515. [Google Scholar] [CrossRef]
- Rockwood, K.; Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Cohen, A.A.; Xue, Q.L.; Walston, J.; Bandeen-Roche, K.; Varadhan, R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat. Aging 2021, 1, 36–46. [Google Scholar] [CrossRef]
- Xue, Q.L.; Tian, J.; Walston, J.D.; Chaves, P.H.M.; Newman, A.B.; Bandeen-Roche, K. Discrepancy in Frailty Identification: Move Beyond Predictive Validity. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 387–393. [Google Scholar] [CrossRef]
- van Sleen, Y.; Shetty, S.A.; van der Heiden, M.; Venema, M.C.A.; Gutiérrez-Melo, N.; Toonen, E.J.M.; van Beek, J.; Buisman, A.; van Baarle, D.; Sauce, D. Frailty is related to serum inflammageing markers: Results from the VITAL study. Immun. Ageing 2023, 20, 68. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.M.; Vijg, J.; Van Steeg, H.; Dollé, M.E.; et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef]
- Idda, M.L.; McClusky, W.G.; Lodde, V.; Munk, R.; Abdelmohsen, K.; Rossi, M.; Gorospe, M. Survey of senescent cell markers with age in human tissues. Aging 2020, 12, 4052–4066. [Google Scholar] [CrossRef]
- Fielding, R.A.; Atkinson, E.J.; Aversa, Z.; White, T.A.; Heeren, A.A.; Achenbach, S.J.; Mielke, M.M.; Cummings, S.R.; Pahor, M.; Leeuwenburgh, C.; et al. Associations between biomarkers of cellular senescence and physical function in humans: Observations from the lifestyle interventions for elders (LIFE) study. GeroScience 2022, 44, 2757–2770. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Júnior, H.J.; Marini, F.; Landi, F.; Marzetti, E. Circulating Inflammatory, Mitochondrial Dysfunction, and Senescence-Related Markers in Older Adults with Physical Frailty and Sarcopenia: A BIOSPHERE Exploratory Study. Int. J. Mol. Sci. 2022, 23, 14006. [Google Scholar] [CrossRef]
- Bombelli, S.; Grasselli, C.; Mazzola, P.; Veronesi, V.; Morabito, I.; Zucchini, N.; Scollo, C.M.; Blanco, S.I.; De Marco, S.; Torsello, B.; et al. Impairment of Renal and Hematopoietic Stem/Progenitor Cell Compartments in Frailty Syndrome: Link with Oxidative Stress, Plasma Cytokine Profiles, and Nuclear DNA Damage. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae188. [Google Scholar] [CrossRef]
- Varadhan, R.; Chaves, P.H.; Lipsitz, L.A.; Stein, P.K.; Tian, J.; Windham, B.G.; Berger, R.D.; Fried, L.P. Frailty and impaired cardiac autonomic control: New insights from principal components aggregation of traditional heart rate variability indices. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Parvaneh, S.; Howe, C.L.; Toosizadeh, N.; Honarvar, B.; Slepian, M.J.; Fain, M.; Mohler, J.; Najafi, B. Regulation of Cardiac Autonomic Nervous System Control across Frailty Statuses: A Systematic Review. Gerontology 2015, 62, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Gielen, E.; Dupont, J.; Dejaeger, M.; Laurent, M.R. Sarcopenia, osteoporosis and frailty. Metabolism 2023, 145, 155638. [Google Scholar] [CrossRef]
- Aleixo, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Battaglini, C.L.; Williams, G.R. Bioelectrical Impedance Analysis for the Assessment of Sarcopenia in Patients with Cancer: A Systematic Review. Oncologist 2020, 25, 170–182. [Google Scholar] [CrossRef]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Chow, S.K.; Hung, V.W.; Wong, C.H.; Wong, R.M.; Tsang, C.S.; Kwok, T.; Cheung, W.H. Diagnosis of sarcopenia by evaluating skeletal muscle mass by adjusted bioimpedance analysis validated with dual-energy X-ray absorptiometry. J. Cachexia Sarcopenia Muscle 2021, 12, 2163–2173. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X. Comparison between bioelectrical impedance analyses and dual-energy X-ray absorptiometry for accuracy in assessing appendicular skeletal muscle mass and diagnosing sarcopenia in hospitalized Chinese older adults. Medicine 2023, 102, e35250. [Google Scholar] [CrossRef]
- Sousa-Santos, A.R.; Barros, D.; Montanha, T.L.; Carvalho, J.; Amaral, T.F. Which is the best alternative to estimate muscle mass for sarcopenia diagnosis when DXA is unavailable? Arch. Gerontol. Geriatr. 2021, 97, 104517. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, P.; Wightman, H.; Politis, M.; Kirkpatrick, S.; Jones, C.; Andrew, M.K.; Vetrano, D.L.; Dent, E.; Hoogendijk, E.O. The relationship between frailty and social vulnerability: A systematic review. Lancet Healthy Longev. 2024, 5, e214–e226. [Google Scholar] [CrossRef]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Massa, M.S.; Potter, C.M.; Clarke, R.; Bennett, D.A. Systematic review of the utility of the frailty index and frailty phenotype to predict all-cause mortality in older people. Syst. Rev. 2022, 11, 187. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.Y. Predictive Validity of Two Physical Frailty Phenotype Specifications Developed for Investigation of Frailty Pathways in Older People. Gerontology 2017, 63, 401–410. [Google Scholar] [CrossRef]
- Buta, B.J.; Walston, J.D.; Godino, J.G.; Park, M.; Kalyani, R.R.; Xue, Q.L.; Bandeen-Roche, K.; Varadhan, R. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res. Rev. 2016, 26, 53–61. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. Can. Med. Assoc. J. 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Amon, J.N.; Ridley, E.J. Clinimetrics: Clinical Frailty Scale. J. Physiother. 2022, 68, 147. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Theou, O. Using the Clinical Frailty Scale in Allocating Scarce Health Care Resources. Can. Geriatr. J. 2020, 23, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Savva, G.M.; Donoghue, O.A.; Horgan, F.; O’Regan, C.; Cronin, H.; Kenny, R.A. Using timed up-and-go to identify frail members of the older population. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 441–446. [Google Scholar] [CrossRef]
- Jung, H.W.; Kim, S.; Jang, I.Y.; Shin, D.W.; Lee, J.E.; Won, C.W. Screening Value of Timed Up and Go Test for Frailty and Low Physical Performance in Korean Older Population: The Korean Frailty and Aging Cohort Study (KFACS). Ann. Geriatr. Med. Res. 2020, 24, 259–266. [Google Scholar] [CrossRef]
- Sison, S.D.M.; Shi, S.M.; Kim, K.M.; Steinberg, N.; Jeong, S.; McCarthy, E.P.; Kim, D.H. A crosswalk of commonly used frailty scales. J. Am. Geriatr. Soc. 2023, 71, 3189–3198. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Ewing, S.K.; Taylor, B.C.; Fink, H.A.; Cawthon, P.M.; Stone, K.L.; Hillier, T.A.; Cauley, J.A.; Hochberg, M.C.; Rodondi, N.; et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch. Intern. Med. 2008, 168, 382–389. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef]
- Saliba, D.; Elliott, M.; Rubenstein, L.Z.; Solomon, D.H.; Young, R.T.; Kamberg, C.J.; Roth, C.; MacLean, C.H.; Shekelle, P.G.; Sloss, E.M.; et al. The Vulnerable Elders Survey: A tool for identifying vulnerable older people in the community. J. Am. Geriatr. Soc. 2001, 49, 1691–1699. [Google Scholar] [CrossRef]
- Rolfson, D.B.; Majumdar, S.R.; Tsuyuki, R.T.; Tahir, A.; Rockwood, K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006, 35, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Fritzenschaft, L.; Boehm, F.; Rothenbacher, D.; Denkinger, M.; Dallmeier, D. Association of blood biomarkers with frailty-A mapping review. Ageing Res. Rev. 2025, 109, 102761. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Yang, C.; Hua, R.; Xie, W.; Zhang, L. Association of Cystatin C Kidney Function Measures with Long-term Deficit-Accumulation Frailty Trajectories and Physical Function Decline. JAMA Netw. Open 2022, 5, e2234208. [Google Scholar] [CrossRef]
- Potok, O.A.; Ix, J.H.; Shlipak, M.G.; Katz, R.; Hawfield, A.T.; Rocco, M.V.; Ambrosius, W.T.; Cho, M.E.; Pajewski, N.M.; Rastogi, A.; et al. The Difference Between Cystatin C- and Creatinine-Based Estimated GFR and Associations with Frailty and Adverse Outcomes: A Cohort Analysis of the Systolic Blood Pressure Intervention Trial (SPRINT). Am. J. Kidney Dis. 2020, 76, 765–774. [Google Scholar] [CrossRef]
- Potok, O.A.; Ix, J.H.; Shlipak, M.G.; Bansal, N.; Katz, R.; Kritchevsky, S.B.; Rifkin, D.E. Cystatin C- and Creatinine-Based Glomerular Filtration Rate Estimation Differences and Muscle Quantity and Functional Status in Older Adults: The Health, Aging, and Body Composition Study. Kidney Med. 2022, 4, 100416. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.H.; Rifkin, D.E.; Ginsberg, C.; Cawthon, P.M.; Kado, D.M.; Bauer, S.R.; Ensrud, K.E.; Hoffman, A.R.; Potok, O.A. Difference between kidney function by cystatin C versus creatinine and association with muscle mass and frailty. J. Am. Geriatr. Soc. 2024, 72, 3163–3170. [Google Scholar] [CrossRef]
- Yao, S.; Guo, J.; Shi, G.; Zhu, Y.; Wang, Y.; Chu, X.; Jiang, X.; Jin, L.; Wang, Z.; Wang, X. Association of BNP with Frailty in Elderly Population: Rugao Longevity and Ageing Study. J. Nutr. Health Aging 2019, 23, 73–78. [Google Scholar] [CrossRef]
- Camino-Willhuber, G.; Tani, S.; Schonnagel, L.; Caffard, T.; Haffer, H.; Chiapparelli, E.; Sarin, M.; Shue, J.; Soffin, E.M.; Zelenty, W.D.; et al. Association of Frailty and Preoperative Hypoalbuminemia with the Risk of Complications, Readmission, and Mortality After Spine Surgery. World Neurosurg. 2023, 174, e152–e158. [Google Scholar] [CrossRef]
- Zeng, P.; Li, M.; Cao, J.; Zeng, L.; Jiang, C.; Lin, F. Association of metabolic syndrome severity with frailty progression among Chinese middle and old-aged adults: A longitudinal study. Cardiovasc. Diabetol. 2024, 23, 302. [Google Scholar] [CrossRef] [PubMed]
- Rattray, N.J.W.; Trivedi, D.K.; Xu, Y.; Chandola, T.; Johnson, C.H.; Marshall, A.D.; Mekli, K.; Rattray, Z.; Tampubolon, G.; Vanhoutte, B.; et al. Metabolic dysregulation in vitamin E and carnitine shuttle energy mechanisms associate with human frailty. Nat. Commun. 2019, 10, 5027. [Google Scholar] [CrossRef]
- Westbrook, R.; Zhang, C.; Yang, H.; Tian, J.; Guo, S.; Xue, Q.L.; Walston, J.; Le, A.; Abadir, P.M. Metabolomics-Based Identification of Metabolic Dysfunction in Frailty. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 2367–2372. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Rodriguez-Manas, L.; Tosato, M.; Coelho-Junior, H.J.; Biancolillo, A.; Laosa, O.; Gervasoni, J.; Primiano, A.; Santucci, L.; et al. Amino Acid Profiles in Older Adults with Frailty: Secondary Analysis from MetaboFrail and BIOSPHERE Studies. Metabolites 2023, 13, 542. [Google Scholar] [CrossRef]
- Ni Lochlainn, M.; Cox, N.J.; Wilson, T.; Hayhoe, R.P.G.; Ramsay, S.E.; Granic, A.; Isanejad, M.; Roberts, H.C.; Wilson, D.; Welch, C.; et al. Nutrition and Frailty: Opportunities for Prevention and Treatment. Nutrients 2021, 13, 2349. [Google Scholar] [CrossRef]
- Ye, Y.; Noche, R.B.; Szejko, N.; Both, C.P.; Acosta, J.N.; Leasure, A.C.; Brown, S.C.; Sheth, K.N.; Gill, T.M.; Zhao, H.; et al. A genome-wide association study of frailty identifies significant genetic correlation with neuropsychiatric, cardiovascular, and inflammation pathways. GeroScience 2023, 45, 2511–2523. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Ipson, B.R.; Fletcher, M.B.; Espinoza, S.E.; Fisher, A.L. Identifying Exosome-Derived MicroRNAs as Candidate Biomarkers of Frailty. J. Frailty Aging 2018, 7, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Machida, T.; Tomofuji, T.; Ekuni, D.; Maruyama, T.; Yoneda, T.; Kawabata, Y.; Mizuno, H.; Miyai, H.; Kunitomo, M.; Morita, M. MicroRNAs in Salivary Exosome as Potential Biomarkers of Aging. Int. J. Mol. Sci. 2015, 16, 21294–21309. [Google Scholar] [CrossRef]
- Wang, B.; Yin, Z.; Lin, Y.; Deng, X.; Liu, F.; Tao, H.; Dong, R.; Lin, X.; Bi, Y. Correlation between microRNA-320 and postoperative delirium in patients undergoing tibial fracture internal fixation surgery. BMC Anesthesiol. 2022, 22, 75. [Google Scholar] [CrossRef]
- Kern, A.E.; Pereyra, D.; Santol, J.; Ammann, M.; Kim, S.; Huber, F.X.; Weninger, J.; Brunner, S.; Laferl, V.; Herrmann, Y.; et al. MicroRNA based Prediction of Posthepatectomy Liver Failure and Mortality Outperforms Established Markers of Preoperative Risk Assessment. Ann. Surg. Oncol. 2025, 32, 6283–6294. [Google Scholar] [CrossRef] [PubMed]
- Rusanova, I.; Fernández-Martínez, J.; Fernández-Ortiz, M.; Aranda-Martínez, P.; Escames, G.; García-García, F.J.; Mañas, L.; Acuña-Castroviejo, D. Involvement of plasma miRNAs, muscle miRNAs and mitochondrial miRNAs in the pathophysiology of frailty. Exp. Gerontol. 2019, 124, 110637. [Google Scholar] [CrossRef]
- Rusanova, I.; Diaz-Casado, M.E.; Fernández-Ortiz, M.; Aranda-Martínez, P.; Guerra-Librero, A.; García-García, F.J.; Escames, G.; Mañas, L.; Acuña-Castroviejo, D. Analysis of Plasma MicroRNAs as Predictors and Biomarkers of Aging and Frailty in Humans. Oxidative Med. Cell. Longev. 2018, 2018, 7671850. [Google Scholar] [CrossRef]
- Olivieri, F.; Spazzafumo, L.; Santini, G.; Lazzarini, R.; Albertini, M.C.; Rippo, M.R.; Galeazzi, R.; Abbatecola, A.M.; Marcheselli, F.; Monti, D.; et al. Age-related differences in the expression of circulating microRNAs: miR-21 as a new circulating marker of inflammaging. Mech. Ageing Dev. 2012, 133, 675–685. [Google Scholar] [CrossRef]
- Mak, J.K.L.; Skovgaard, A.C.; Nygaard, M.; Kananen, L.; Reynolds, C.A.; Wang, Y.; Kuja-Halkola, R.; Karlsson, I.K.; Pedersen, N.L.; Hägg, S.; et al. Epigenome-wide analysis of frailty: Results from two European twin cohorts. Aging Cell 2024, 23, e14135. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.L.; Jylhävä, J.; Pedersen, N.L.; Magnusson, P.K.; Lu, Y.; Wang, Y.; Hägg, S.; Melzer, D.; Williams, D.M.; Pilling, L.C. A genome-wide association study of the frailty index highlights brain pathways in ageing. Aging Cell 2021, 20, e13459. [Google Scholar] [CrossRef]
- Vatic, M.; von Haehling, S.; Ebner, N. Inflammatory biomarkers of frailty. Exp. Gerontol. 2020, 133, 110858. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, M.; Chen, D.; Jiang, X.; Xiong, Z. Inflammatory biomarkers in older adults with frailty: A systematic review and meta-analysis of cross-sectional studies. Aging Clin. Exp. Res. 2022, 34, 971–987. [Google Scholar] [CrossRef]
- Kamper, R.S.; Alcazar, J.; Andersen, L.L.; Haddock, B.; Jorgensen, N.R.; Hovind, P.; Suetta, C. Associations between inflammatory markers, body composition, and physical function: The Copenhagen Sarcopenia Study. J. Cachexia Sarcopenia Muscle 2021, 12, 1641–1652. [Google Scholar] [CrossRef]
- Mekli, K.; Nazroo, J.Y.; Marshall, A.D.; Kumari, M.; Pendleton, N. Proinflammatory genotype is associated with the frailty phenotype in the English Longitudinal Study of Ageing. Aging Clin. Exp. Res. 2016, 28, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Collerton, J.; Martin-Ruiz, C.; Davies, K.; Hilkens, C.M.; Isaacs, J.; Kolenda, C.; Parker, C.; Dunn, M.; Catt, M.; Jagger, C.; et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: Cross-sectional findings from the Newcastle 85+ Study. Mech. Ageing Dev. 2012, 133, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Langmann, G.A.; Perera, S.; Ferchak, M.A.; Nace, D.A.; Resnick, N.M.; Greenspan, S.L. Inflammatory Markers and Frailty in Long-Term Care Residents. J. Am. Geriatr. Soc. 2017, 65, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Puts, M.T.; Visser, M.; Twisk, J.W.; Deeg, D.J.; Lips, P. Endocrine and inflammatory markers as predictors of frailty. Clin. Endocrinol. 2005, 63, 403–411. [Google Scholar] [CrossRef]
- Luo, Y.F.; Cheng, Z.J.; Wang, Y.F.; Jiang, X.Y.; Lei, S.F.; Deng, F.Y.; Ren, W.Y.; Wu, L.F. Unraveling the relationship between high-sensitivity C-reactive protein and frailty: Evidence from longitudinal cohort study and genetic analysis. BMC Geriatr. 2024, 24, 222. [Google Scholar] [CrossRef]
- Walker, K.A.; Walston, J.; Gottesman, R.F.; Kucharska-Newton, A.; Palta, P.; Windham, B.G. Midlife Systemic Inflammation Is Associated with Frailty in Later Life: The ARIC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 343–349. [Google Scholar] [CrossRef]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jørgensen, S.B.; Steinberg, G.R. GDF15: Emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 2021, 17, 592–607. [Google Scholar] [CrossRef]
- Conte, M.; Martucci, M.; Mosconi, G.; Chiariello, A.; Cappuccilli, M.; Totti, V.; Santoro, A.; Franceschi, C.; Salvioli, S. GDF15 Plasma Level Is Inversely Associated with Level of Physical Activity and Correlates with Markers of Inflammation and Muscle Weakness. Front. Immunol. 2020, 11, 915. [Google Scholar] [CrossRef] [PubMed]
- Kamper, R.S.; Nygaard, H.; Praeger-Jahnsen, L.; Ekmann, A.; Ditlev, S.B.; Schultz, M.; Hansen, S.K.; Hansen, P.; Pressel, E.; Suetta, C. GDF-15 is associated with sarcopenia and frailty in acutely admitted older medical patients. J. Cachexia Sarcopenia Muscle 2024, 15, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Walston, J.D.; Won, C.W. Associations Between Elevated Growth Differentiation Factor-15 and Sarcopenia Among Community-dwelling Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 770–780. [Google Scholar] [CrossRef]
- Álvarez-Satta, M.; Berna-Erro, A.; Carrasco-Garcia, E.; Alberro, A.; Saenz-Antoñanzas, A.; Vergara, I.; Otaegui, D.; Matheu, A. Relevance of oxidative stress and inflammation in frailty based on human studies and mouse models. Aging 2020, 12, 9982–9999. [Google Scholar] [CrossRef]
- Liu, C.K.; Lyass, A.; Larson, M.G.; Massaro, J.M.; Wang, N.; D’Agostino, R.B., Sr.; Benjamin, E.J.; Murabito, J.M. Biomarkers of oxidative stress are associated with frailty: The Framingham Offspring Study. Age 2016, 38, 1. [Google Scholar] [CrossRef]
- Inglés, M.; Gambini, J.; Carnicero, J.A.; García-García, F.J.; Rodríguez-Mañas, L.; Olaso-González, G.; Dromant, M.; Borrás, C.; Viña, J. Oxidative stress is related to frailty, not to age or sex, in a geriatric population: Lipid and protein oxidation as biomarkers of frailty. J. Am. Geriatr. Soc. 2014, 62, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Cumming, R.G.; Naganathan, V.; Blyth, F.; Ribeiro, R.V.; Le Couteur, D.G.; Handelsman, D.J.; Waite, L.M.; Simpson, S.J.; Hirani, V. Prospective Associations Between Dietary Antioxidant Intake and Frailty in Older Australian Men: The Concord Health and Ageing in Men Project. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 348–356. [Google Scholar] [CrossRef]
- Ble, A.; Cherubini, A.; Volpato, S.; Bartali, B.; Walston, J.D.; Windham, B.G.; Bandinelli, S.; Lauretani, F.; Guralnik, J.M.; Ferrucci, L. Lower plasma vitamin E levels are associated with the frailty syndrome: The InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 278–283. [Google Scholar] [CrossRef]
- Saum, K.U.; Dieffenbach, A.K.; Jansen, E.H.; Schöttker, B.; Holleczek, B.; Hauer, K.; Brenner, H. Association between Oxidative Stress and Frailty in an Elderly German Population: Results from the ESTHER Cohort Study. Gerontology 2015, 61, 407–415. [Google Scholar] [CrossRef]
- Sajeev, S.; Champion, S.; Maeder, A.; Gordon, S. Machine learning models for identifying pre-frailty in community dwelling older adults. BMC Geriatr. 2022, 22, 794. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.H.; Chattopadhyay, A.; Phan, N.N.; Chuang, E.Y.; Lee, O.K. Utilizing multimodal approach to identify candidate pathways and biomarkers and predicting frailty syndrome in individuals from UK Biobank. GeroScience 2024, 46, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Sevini, F.; Conte, G.; Tognocchi, M.; Ciurca, E.; Trofarello, L.; Chiariello, A.; Capri, M.; Franceschi, C.; Monti, D.; et al. The combination of GDF15, FGF21, sRAGE and NfL plasma levels can identify frailty in community-dwelling people across old age. Mech. Ageing Dev. 2025, 226, 112077. [Google Scholar] [CrossRef]
- Chou, Y.Y.; Wang, M.S.; Lin, C.F.; Lee, Y.S.; Lee, P.H.; Huang, S.M.; Wu, C.L.; Lin, S.Y. The application of machine learning for identifying frailty in older patients during hospital admission. BMC Med. Inform. Decis. Mak. 2024, 24, 270. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Guglielmi, G.; Ponti, F.; Agostini, M.; Amadori, M.; Battista, G.; Bazzocchi, A. The role of DXA in sarcopenia. Aging Clin. Exp. Res. 2016, 28, 1047–1060. [Google Scholar] [CrossRef]
- Derstine, B.A.; Holcombe, S.A.; Ross, B.E.; Wang, N.C.; Su, G.L.; Wang, S.C. Optimal body size adjustment of L3 CT skeletal muscle area for sarcopenia assessment. Sci. Rep. 2021, 11, 279. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J. Appl. Physiol. 2001, 90, 2157–2165. [Google Scholar] [CrossRef]
- Beavers, K.M.; Beavers, D.P.; Houston, D.K.; Harris, T.B.; Hue, T.F.; Koster, A.; Newman, A.B.; Simonsick, E.M.; Studenski, S.A.; Nicklas, B.J.; et al. Associations between body composition and gait-speed decline: Results from the Health, Aging, and Body Composition study. Am. J. Clin. Nutr. 2013, 97, 552–560. [Google Scholar] [CrossRef]
- Thai, S.T.; Lund, J.L.; Poole, C.; Buse, J.B.; Stürmer, T.; Harmon, C.A.; Al-Obaidi, M.; Williams, G.R. Skeletal muscle density performance for screening frailty in older adults with cancer and the impact of diabetes: The CARE Registry. J. Geriatr. Oncol. 2024, 15, 101815. [Google Scholar] [CrossRef]
- Wang, L.; Yin, L.; Zhao, Y.; Su, Y.; Sun, W.; Chen, S.; Liu, Y.; Yang, M.; Yu, A.; Guglielmi, G.; et al. Muscle Density, but Not Size, Correlates Well with Muscle Strength and Physical Performance. J. Am. Med. Dir. Assoc. 2021, 22, 751–759.E2. [Google Scholar] [CrossRef]
- Santanasto, A.J.; Zmuda, J.M.; Cvejkus, R.K.; Gordon, C.L.; Nair, S.; Carr, J.J.; Terry, J.G.; Wheeler, V.W.; Miljkovic, I. Thigh and Calf Myosteatosis are Strongly Associated with Muscle and Physical Function in African Caribbean Men. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Lenchik, L.; Lenoir, K.M.; Tan, J.; Boutin, R.D.; Callahan, K.E.; Kritchevsky, S.B.; Wells, B.J. Opportunistic Measurement of Skeletal Muscle Size and Muscle Attenuation on Computed Tomography Predicts 1-Year Mortality in Medicare Patients. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Reinders, I.; Murphy, R.A.; Brouwer, I.A.; Visser, M.; Launer, L.; Siggeirsdottir, K.; Eiriksdottir, G.; Gudnason, V.; Jonsson, P.V.; Lang, T.F.; et al. Muscle Quality and Myosteatosis: Novel Associations with Mortality Risk: The Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study. Am. J. Epidemiol. 2016, 183, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Nachit, M.; Horsmans, Y.; Summers, R.M.; Leclercq, I.A.; Pickhardt, P.J. AI-based CT Body Composition Identifies Myosteatosis as Key Mortality Predictor in Asymptomatic Adults. Radiology 2023, 307, e222008. [Google Scholar] [CrossRef]
- Cheung, A.; Haas, B.; Ringer, T.J.; McFarlan, A.; Wong, C.L. Canadian Study of Health and Aging Clinical Frailty Scale: Does It Predict Adverse Outcomes among Geriatric Trauma Patients? J. Am. Coll. Surg. 2017, 225, 658–665e3. [Google Scholar] [CrossRef]
- Di Cola, S.; D’Amico, G.; Caraceni, P.; Schepis, F.; Loredana, S.; Lampertico, P.; Toniutto, P.; Martini, S.; Maimone, S.; Colecchia, A.; et al. Myosteatosis is closely associated with sarcopenia and significantly worse outcomes in patients with cirrhosis. J. Hepatol. 2024, 81, 641–650. [Google Scholar] [CrossRef]
- Ebadi, M.; Tsien, C.; Bhanji, R.A.; Dunichand-Hoedl, A.R.; Rider, E.; Motamedrad, M.; Mazurak, V.C.; Baracos, V.; Montano-Loza, A.J. Skeletal Muscle Pathological Fat Infiltration (Myosteatosis) Is Associated with Higher Mortality in Patients with Cirrhosis. Cells 2022, 11, 1345. [Google Scholar] [CrossRef]
- Erlandson, K.M.; Umbleja, T.; Lu, M.T.; Taron, J.; Ribaudo, H.J.; Overton, E.T.; Presti, R.M.; Haas, D.W.; Sax, P.E.; Yin, M.T.; et al. Associations of Muscle Density and Area with Coronary Artery Plaque and Physical Function. J. Acquir. Immune Defic. Syndr. 2023, 94, 174–184. [Google Scholar] [CrossRef]
- Horwich, T.B.; Fonarow, G.C.; Clark, A.L. Obesity and the Obesity Paradox in Heart Failure. Prog. Cardiovasc. Dis. 2018, 61, 151–156. [Google Scholar] [CrossRef]
- Gravina, G.; Ferrari, F.; Nebbiai, G. The obesity paradox and diabetes. Eat. Weight. Disord.-Stud. Anorex. Bulim. Obes. 2021, 26, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zeng, L.; Wang, F.; Chen, K. Obesity Paradox in Lung Diseases: What Explains It? Obes. Facts 2023, 16, 411–426. [Google Scholar] [CrossRef]
- Gao, Q.; Mei, F.; Shang, Y.; Hu, K.; Chen, F.; Zhao, L.; Ma, B. Global prevalence of sarcopenic obesity in older adults: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 4633–4641. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Hu, K.; Gao, J.; Shang, Y.; Mei, F.; Zhao, L.; Chen, F.; Ma, B. Prevalence and prognostic value of sarcopenic obesity in patients with cancer: A systematic review and meta-analysis. Nutrition 2022, 101, 111704. [Google Scholar] [CrossRef]
- Batsis, J.A.; Mackenzie, T.A.; Barre, L.K.; Lopez-Jimenez, F.; Bartels, S.J. Sarcopenia, sarcopenic obesity and mortality in older adults: Results from the National Health and Nutrition Examination Survey III. Eur. J. Clin. Nutr. 2014, 68, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; Chin, S.H.; Whittaker, A.C.; Jones, D.; Kaur, O.; Bosch, J.A.; Borrows, R. The Associations of Muscle Strength, Muscle Mass, and Adiposity with Clinical Outcomes and Quality of Life in Prevalent Kidney Transplant Recipients. J. Ren. Nutr. 2019, 29, 536–547. [Google Scholar] [CrossRef]
- Chauvot de Beauchene, R.; Souweine, B.; Bonnet, B.; Evrard, B.; Boirie, Y.; Cassagnes, L.; Dupuis, C. Sarcopenia, myosteatosis and inflammation are independent prognostic factors of SARS-CoV-2 pneumonia patients admitted to the ICU. Sci. Rep. 2025, 15, 4373. [Google Scholar] [CrossRef]
- Bonatti, M.; Lombardo, F.; Valletta, R.; Comai, A.; Petralia, B.; Avesani, G.; Franchini, E.; Rossi, A.; De Santis, N.; Guerriero, M.; et al. Myosteatosis as an independent predictor of short-term mortality in successfully reperfused acute ischemic stroke. Neuroradiol. J. 2023, 36, 17–22. [Google Scholar] [CrossRef]
- Park, B.; Vandal, A.; Welsh, F.; Eglinton, T.; Koea, J.; Taneja, A.; Barazanchi, A.; Hill, A.G.; MacCormick, A.D. Sarcopenia, myosteatosis, and frailty parameters to predict adverse outcomes in patients undergoing emergency laparotomy: Prospective observational multicentre cohort study. BJS Open 2025, 9, zraf016. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, I.A.; Le, S.T.; Peng, P.D.; Kipnis, P.; Liu, V.X.; Caan, B.; Chow, V.; Beg, M.F.; Popuri, K.; Cespedes Feliciano, E.M. Automated CT Analysis of Body Composition as a Frailty Biomarker in Abdominal Surgery. JAMA Surg. 2024, 159, 766–774. [Google Scholar] [CrossRef]
- Tokuda, T.; Yamamoto, M.; Kagase, A.; Koyama, Y.; Otsuka, T.; Tada, N.; Naganuma, T.; Araki, M.; Yamanaka, F.; Shirai, S.; et al. Importance of combined assessment of skeletal muscle mass and density by computed tomography in predicting clinical outcomes after transcatheter aortic valve replacement. Int. J. Cardiovasc. Imaging 2020, 36, 929–938. [Google Scholar] [CrossRef]
- Soud, M.; Alahdab, F.; Ho, G.; Kuku, K.O.; Cejudo-Tejeda, M.; Hideo-Kajita, A.; de Araujo Gonçalves, P.; Teles, R.C.; Waksman, R.; Garcia-Garcia, H.M. Usefulness of skeletal muscle area detected by computed tomography to predict mortality in patients undergoing transcatheter aortic valve replacement: A meta-analysis study. Int. J. Cardiovasc. Imaging 2019, 35, 1141–1147. [Google Scholar] [CrossRef]
- Huangfu, G.; Kumar, A.A.; Yong, G.; Shetty, S.; He, A.; Raju, V.; Dwivedi, G.; Ihdayhid, A.R. CT-derived frailty score outperforms clinical frailty scale for mortality prediction following TAVR. J. Cardiovasc. Comput. Tomogr. 2024, 18, 314–315. [Google Scholar] [CrossRef]
- Bradley, N.A.; Walter, A.; Roxburgh, C.S.D.; McMillan, D.C.; Guthrie, G.J.K. The Relationship between Clinical Frailty Score, CT-Derived Body Composition, Systemic Inflammation, and Survival in Patients with Chronic Limb-Threatening Ischemia. Ann. Vasc. Surg. 2024, 104, 18–26. [Google Scholar] [CrossRef]
- Kärkkäinen, J.M.; Tenorio, E.R.; Oksala, N.; Macedo, T.A.; Sen, I.; Mendes, B.C.; DeMartino, R.R.; Jacobs, M.J.; Mees, B.; Oderich, G.S. Pre-operative Psoas Muscle Size Combined with Radiodensity Predicts Mid-Term Survival and Quality of Life After Fenestrated-Branched Endovascular Aortic Repair. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kärkkäinen, J.M.; Oderich, G.S.; Tenorio, E.R.; Pather, K.; Oksala, N.; Macedo, T.A.; Vrtiska, T.; Mees, B.; Jacobs, M.J. Psoas muscle area and attenuation are highly predictive of complications and mortality after complex endovascular aortic repair. J. Vasc. Surg. 2021, 73, 1178–1188.E1. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.; Ouamri, Y.; Canouï-Poitrine, F.; Mulé, S.; Champy, C.M.; Ingels, A.; Audard, V.; Luciani, A.; Grimbert, P.; Matignon, M.; et al. Myosteatosis as an independent risk factor for mortality after kidney allograft transplantation: A retrospective cohort study. J. Cachexia Sarcopenia Muscle 2022, 13, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Caan, B.J.; Cespedes Feliciano, E.M.; Meyerhardt, J.A.; Peng, P.D.; Baracos, V.E.; Lee, V.S.; Ely, S.; Gologorsky, R.C.; Weltzien, E.; et al. Association of Low Muscle Mass and Low Muscle Radiodensity with Morbidity and Mortality for Colon Cancer Surgery. JAMA Surg. 2020, 155, 942–949. [Google Scholar] [CrossRef]
- Margadant, C.C.; Bruns, E.R.; Sloothaak, D.A.; van Duijvendijk, P.; van Raamt, A.F.; van der Zaag, H.J.; Buskens, C.J.; van Munster, B.C.; van der Zaag, E.S. Lower muscle density is associated with major postoperative complications in older patients after surgery for colorectal cancer. Eur. J. Surg. Oncol. 2016, 42, 1654–1659. [Google Scholar] [CrossRef]
- Kroenke, C.H.; Prado, C.M.; Meyerhardt, J.A.; Weltzien, E.K.; Xiao, J.; Cespedes Feliciano, E.M.; Caan, B.J. Muscle radiodensity and mortality in patients with colorectal cancer. Cancer 2018, 124, 3008–3015. [Google Scholar] [CrossRef]
- Murnane, L.C.; Forsyth, A.K.; Koukounaras, J.; Pilgrim, C.H.; Shaw, K.; Brown, W.A.; Mourtzakis, M.; Tierney, A.C.; Burton, P.R. Myosteatosis predicts higher complications and reduced overall survival following radical oesophageal and gastric cancer surgery. Eur. J. Surg. Oncol. 2021, 47, 2295–2303. [Google Scholar] [CrossRef]
- Haehl, E.; Alvino, L.; Rühle, A.; Zou, J.; Fabian, A.; Grosu, A.L.; Nicolay, N.H. Sarcopenia as a Prognostic Marker in Elderly Head and Neck Squamous Cell Carcinoma Patients Undergoing (Chemo-)Radiation. Cancers 2022, 14, 5536. [Google Scholar] [CrossRef]
- Canales, C.; Mazor, E.; Coy, H.; Grogan, T.R.; Duval, V.; Raman, S.; Cannesson, M.; Singh, S.P. Preoperative Point-of-Care Ultrasound to Identify Frailty and Predict Postoperative Outcomes: A Diagnostic Accuracy Study. Anesthesiology 2022, 136, 268–278. [Google Scholar] [CrossRef]
- Jiang, R.; Noble, S.; Sui, J.; Yoo, K.; Rosenblatt, M.; Horien, C.; Qi, S.; Liang, Q.; Sun, H.; Calhoun, V.D.; et al. Associations of physical frailty with health outcomes and brain structure in 483 033 middle-aged and older adults: A population-based study from the UK Biobank. Lancet Digit. Health 2023, 5, e350–e359. [Google Scholar] [CrossRef] [PubMed]
- Nishita, Y.; Nakamura, A.; Kato, T.; Otsuka, R.; Iwata, K.; Tange, C.; Ando, F.; Ito, K.; Shimokata, H.; Arai, H. Links Between Physical Frailty and Regional Gray Matter Volumes in Older Adults: A Voxel-Based Morphometry Study. J. Am. Med. Dir. Assoc. 2019, 20, 1587–1592.e7. [Google Scholar] [CrossRef]
- Apóstolo, J.; Cooke, R.; Bobrowicz-Campos, E.; Santana, S.; Marcucci, M.; Cano, A.; Vollenbroek-Hutten, M.; Germini, F.; D’Avanzo, B.; Gwyther, H.; et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: A systematic review. JBI Evid. Synth. 2018, 16, 140–232. [Google Scholar] [CrossRef]
- Travers, J.; Romero-Ortuno, R.; Langan, J.; MacNamara, F.; McCormack, D.; McDermott, C.; McEntire, J.; McKiernan, J.; Lacey, S.; Doran, P.; et al. Building resilience and reversing frailty: A randomised controlled trial of a primary care intervention for older adults. Age Ageing 2023, 52, afad012. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Ryu, H.K.; Lyu, S.J.; Yi, H.J.; Lee, B.H. Effects of Preoperative Telerehabilitation on Muscle Strength, Range of Motion, and Functional Outcomes in Candidates for Total Knee Arthroplasty: A Single-Blind Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 6071. [Google Scholar] [CrossRef]
- Lai, Y.; Huang, J.; Yang, M.; Su, J.; Liu, J.; Che, G. Seven-day intensive preoperative rehabilitation for elderly patients with lung cancer: A randomized controlled trial. J. Surg. Res. 2017, 209, 30–36. [Google Scholar] [CrossRef]
- Carli, F.; Bousquet-Dion, G.; Awasthi, R.; Elsherbini, N.; Liberman, S.; Boutros, M.; Stein, B.; Charlebois, P.; Ghitulescu, G.; Morin, N.; et al. Effect of Multimodal Prehabilitation vs Postoperative Rehabilitation on 30-Day Postoperative Complications for Frail Patients Undergoing Resection of Colorectal Cancer: A Randomized Clinical Trial. JAMA Surg. 2020, 155, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, C.J.L.; Minnella, E.M.; Coca-Martinez, M.; Ten Cate, D.W.G.; Regis, M.; Awasthi, R.; Martinez-Palli, G.; Lopez-Baamonde, M.; Sebio-Garcia, R.; Feo, C.V.; et al. Effect of Multimodal Prehabilitation on Reducing Postoperative Complications and Enhancing Functional Capacity Following Colorectal Cancer Surgery: The PREHAB Randomized Clinical Trial. JAMA Surg. 2023, 158, 572–581. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devitt, C.; Patel, D.; Mahboubi Ardakani, R.; Poovathoor, S.; Jin, Z.; Moller, D. Biomarkers and Clinical Evaluation in the Detection of Frailty. Int. J. Mol. Sci. 2025, 26, 7888. https://doi.org/10.3390/ijms26167888
Devitt C, Patel D, Mahboubi Ardakani R, Poovathoor S, Jin Z, Moller D. Biomarkers and Clinical Evaluation in the Detection of Frailty. International Journal of Molecular Sciences. 2025; 26(16):7888. https://doi.org/10.3390/ijms26167888
Chicago/Turabian StyleDevitt, Catherine, Devon Patel, Rustin Mahboubi Ardakani, Shaji Poovathoor, Zhaosheng Jin, and Daryn Moller. 2025. "Biomarkers and Clinical Evaluation in the Detection of Frailty" International Journal of Molecular Sciences 26, no. 16: 7888. https://doi.org/10.3390/ijms26167888
APA StyleDevitt, C., Patel, D., Mahboubi Ardakani, R., Poovathoor, S., Jin, Z., & Moller, D. (2025). Biomarkers and Clinical Evaluation in the Detection of Frailty. International Journal of Molecular Sciences, 26(16), 7888. https://doi.org/10.3390/ijms26167888






