Fibroblast–Myofibroblast Transition in Osteoarthritis Progression: Current Insights
Abstract
1. Introduction
1.1. Osteoarthritis
1.2. Fibroblast-like Synoviocytes: Definition, Features, and Heterogeneity
1.3. OA-Associated FLS Changes and Synovial Fibrosis
2. Molecular Pathways of Pathological FLS Activation
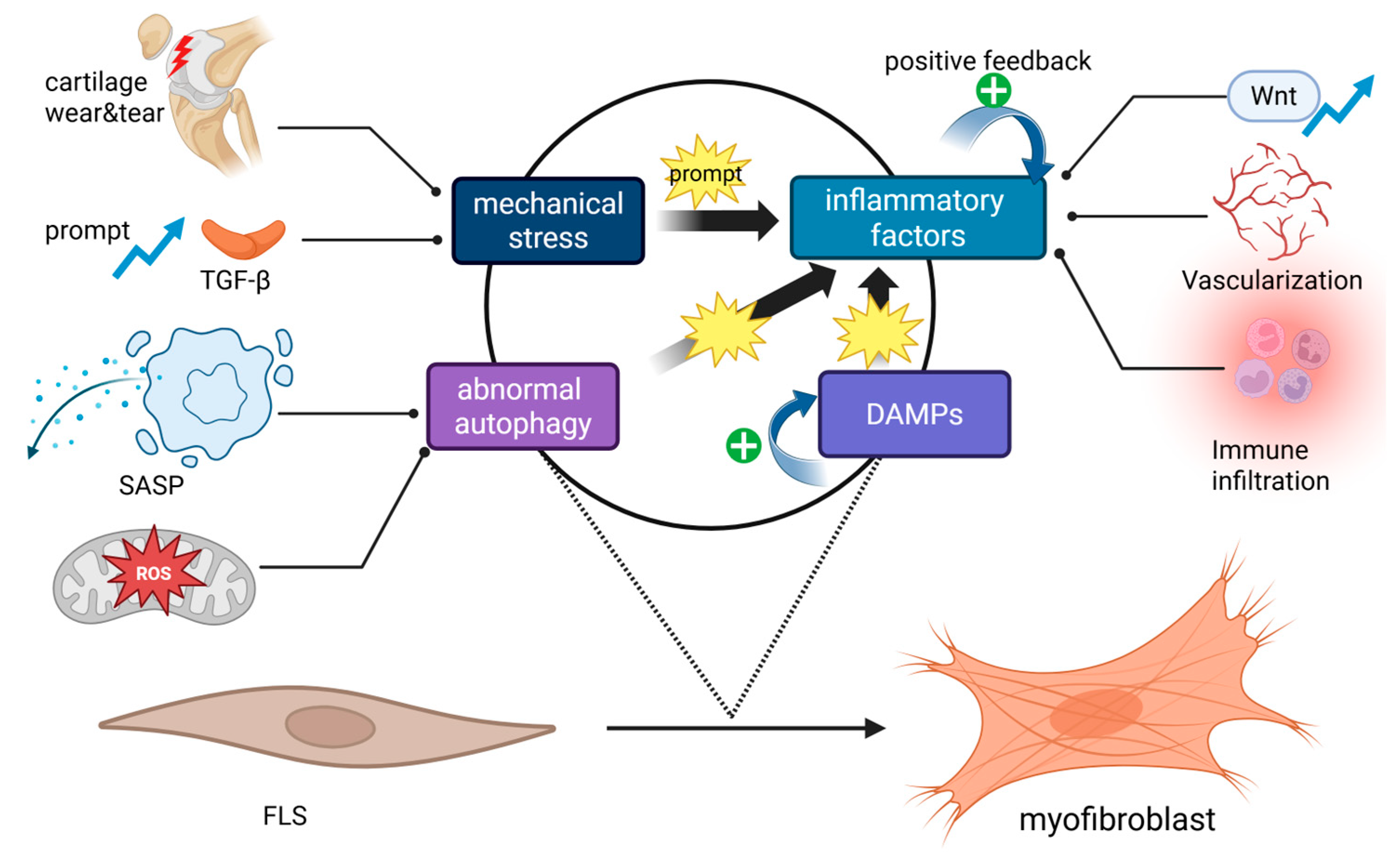
2.1. Inducing Factors
2.1.1. Mechanical Stress
2.1.2. Inflammatory Factors
2.1.3. Damage-Associated Molecular Patterns (DAMPs)
2.1.4. Abnormal Cell Autophagy
2.2. Intracellular Signaling Pathways
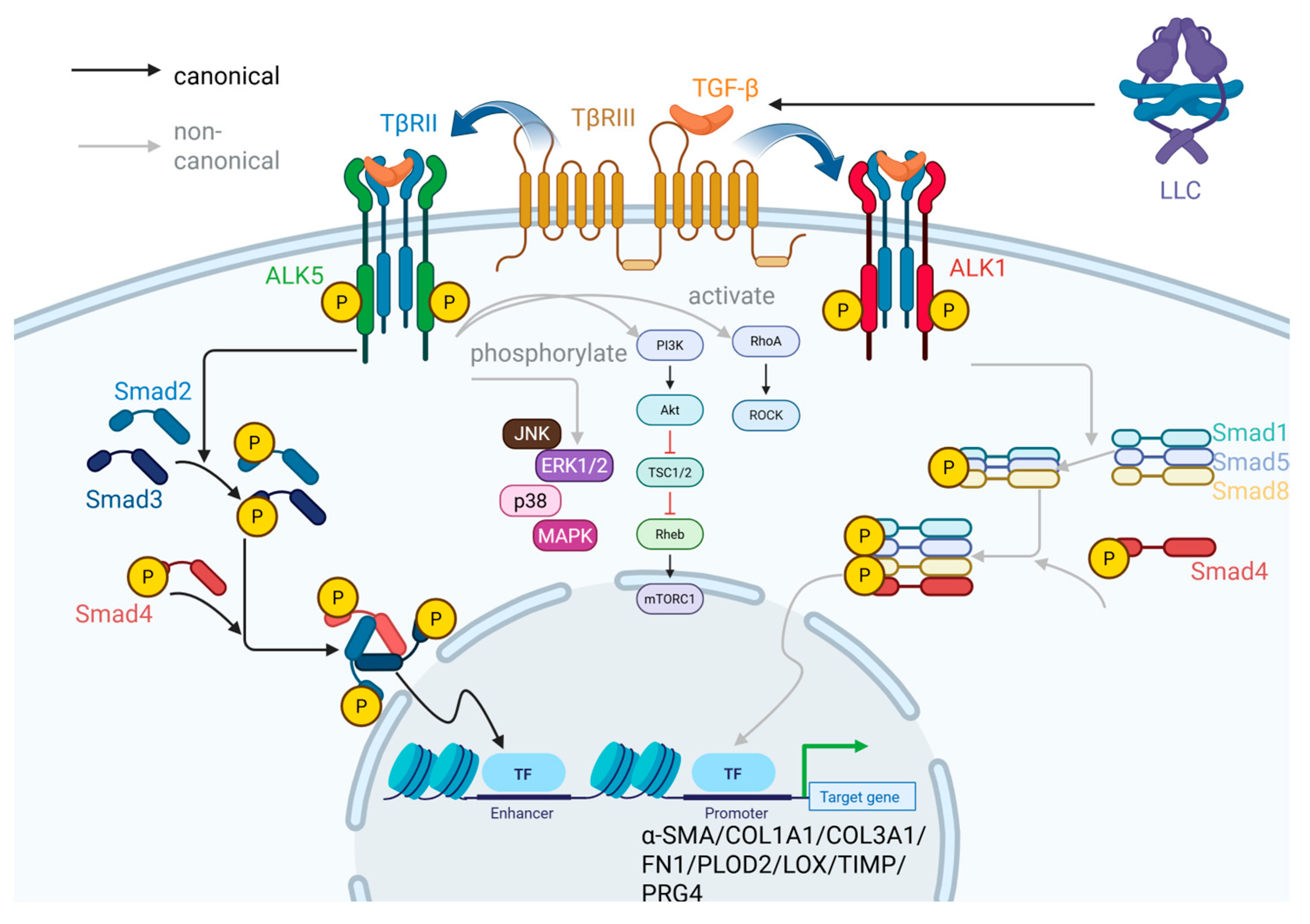
2.2.1. TGF-β/Smad Signaling Pathway
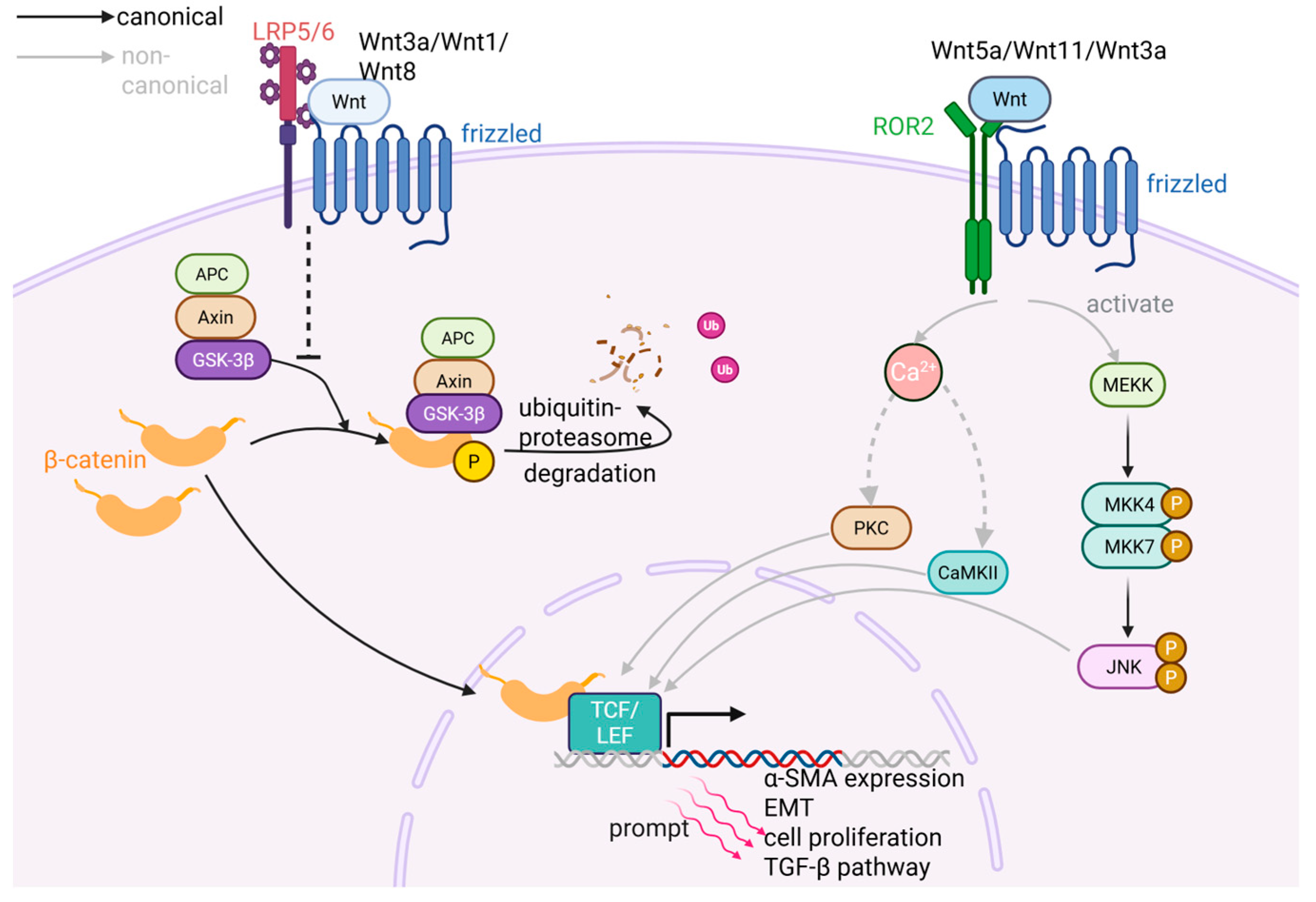
2.2.2. Wnt/β-Catenin Pathway
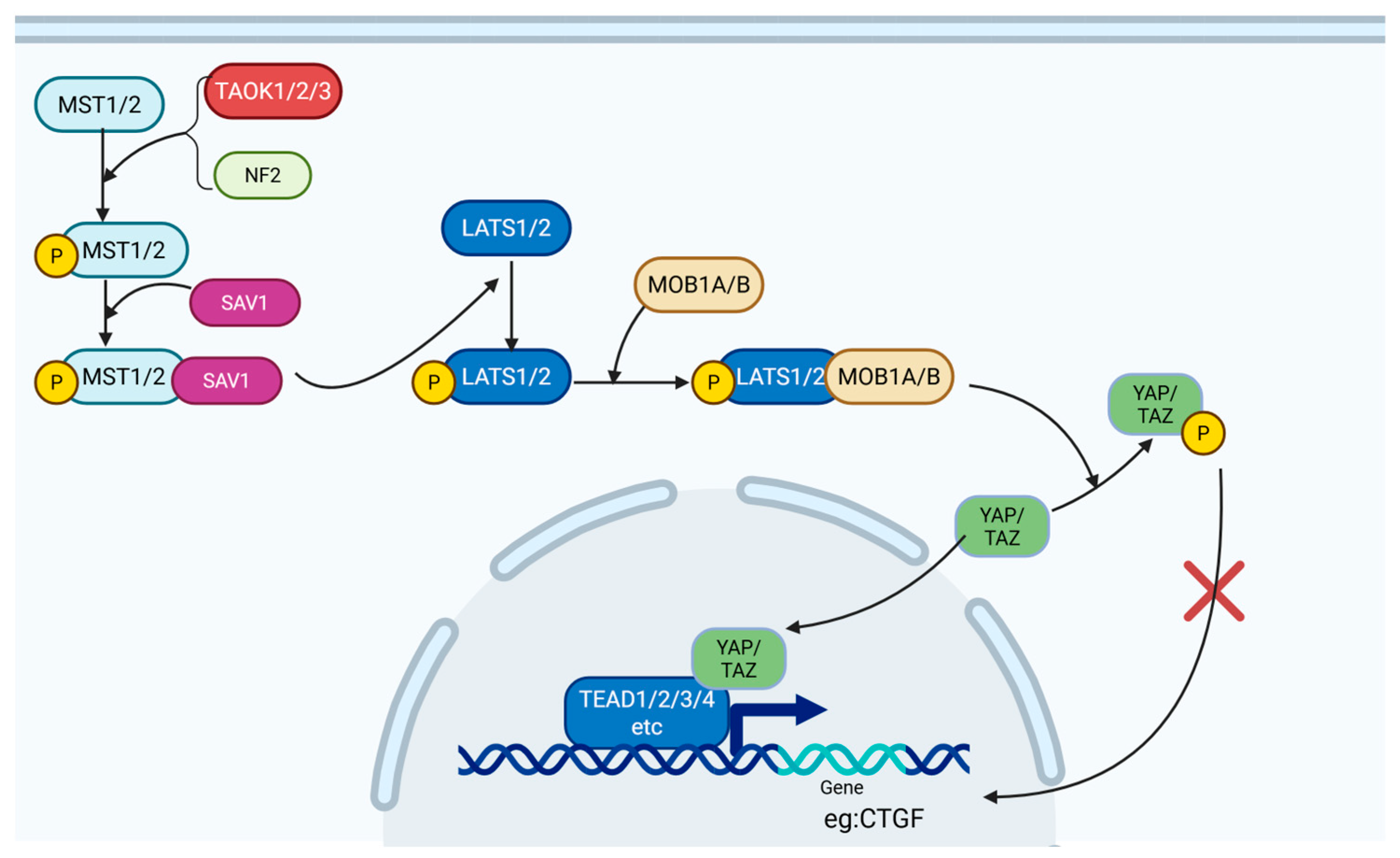
2.2.3. Hippo-YAP/TAZ Pathway
2.2.4. The Roles of Metabolic Reprogramming
3. Impact of FLS Phenotypic Changes in OA
4. Corresponding Clinical Strategy
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| FLSs | Fibroblast-like synoviocytes |
| FN | Fibronectin |
| PRG4 | Proteoglycan 4 |
| HBEGF | Heparin-binding epidermal growth factor-like growth factor |
| CLIC5 | Chloride intracellular channel 5 |
| BMP | Bone morphogenetic protein |
| RANKL | Receptor activator of NF-κB ligand |
| CCL | CC motif chemokine ligand |
| ECM | Extracellular matrix |
| DKK | Dickkopf |
| MMPs | Matrix metalloproteinases |
| α-SMA | A-smooth muscle actin |
| TGF-β | Transforming growth factor beta |
| DMM | Destabilization of the medial meniscus |
| YAP | Yes-associated protein |
| TAZ | Transcriptional Coactivator with Pdz-Binding Motif Proteins |
| COL1A1 | Collagen type I, alpha 1 chain |
| mRNA | Messenger RNA |
| ADAMTS4 | A disintegrin and metalloproteinase with thrombospondin motifs 4 protein |
| ADAMTS5 | A disintegrin and metalloproteinase with thrombospondin motifs 5 protein |
| IL-1β | Interleukin-1beta |
| TNF-α | Tumor necrosis factor-alpha |
| IL | Interleukin- |
| CCL2 | C-C motif chemokine ligand 2 |
| IL-1RI | Interleukin 1 receptor, Type I |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular signal-regulated kinase |
| NF-κB | Nuclear factor kappa-B |
| JAK | Janus kinase |
| STAT | Signal transducer and activator of transcription |
| PI3K | Phosphatidylinositol 3-kinase |
| AP-1 | Activator protein-1 |
| DAMPs | Damage-associated molecular patterns |
| PRRs | Pattern recognition receptors |
| TLRs | Toll-like receptors |
| NLRs | Nucleotide oligomerization domain (NOD)-like receptors |
| RIG-I | Retinoic acid-inducible gene-I |
| NLRP3 | NOD-like receptor thermal protein domain-associated protein 3 |
| ASC | Apoptosis-Associated Speck-Like Protein Containing A CARD (Caspase-Activation and Recruitment Domain) |
| COMP | Cartilage oligomeric matrix protein |
| SASP | Senescence-associated secretory phenotype |
| TIMPs | Tissue inhibitors of metalloproteinases |
| ROS | Reactive oxygen species |
| mtROS | Mitochondrial ROS |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| LAP | Latency-associated peptide |
| SLC | Small latent complex |
| LTBPs | Latent TGF-Β binding proteins |
| LLC | Large latent complex |
| TβR | TGF-Β receptor |
| ALK | Activin receptor-like kinase |
| RhoA-ROCK | Rhoa (Ras Homolog Gene Family Member A)-Associated Coiled-Coil Forming Protein Kinase |
| AKT | Protein Kinase B |
| JNK | C-Jun N-terminal kinase |
| ACTA2 | Actin Alpha 2, Smooth Muscle |
| PLOD2 | Procollagen-Lysine,2-Oxoglutarate 5-Dioxygenase 2 |
| P4HB | Prolyl 4-Hydroxylase Subunit Beta |
| LEPRE1 | Leucine Proline-Enriched Proteoglycan (Leprecan) 1 |
| LOX | Lysyl oxidase |
| LH2 | Lysyl Hydroxylase 2 |
| PRG | Plasticity-Related Gene |
References
- Courties, A.; Kouki, I.; Soliman, N.; Mathieu, S.; Sellam, J. Osteoarthritis year in review 2024: Epidemiology and therapy. Osteoarthr. Cartil. 2024, 32, 1397–1404. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, R.; Huang, Z.; Ding, L.; Zhang, L.; Li, M.; Li, X.; Wang, P.; Mao, J. Synovial Fibrosis Involvement in Osteoarthritis. Front. Med. 2021, 8, 684389. [Google Scholar] [CrossRef]
- Han, D.; Fang, Y.; Tan, X.; Jiang, H.; Gong, X.; Wang, X.; Hong, W.; Tu, J.; Wei, W. The emerging role of fibroblast-like synoviocytes-mediated synovitis in osteoarthritis: An update. J. Cell Mol. Med. 2020, 24, 9518–9532. [Google Scholar] [CrossRef]
- Barland, P.; Novikoff, A.B.; Hamerman, D. Electron microscopy of the human synovial membrane. J. Cell Biol. 1962, 14, 207–220. [Google Scholar] [CrossRef]
- Schuster, R.; Rockel, J.S.; Kapoor, M.; Hinz, B. The inflammatory speech of fibroblasts. Immunol. Rev. 2021, 302, 126–146. [Google Scholar] [CrossRef]
- Pap, T.; Dankbar, B.; Wehmeyer, C.; Korb-Pap, A.; Sherwood, J. Synovial fibroblasts and articular tissue remodelling: Role and mechanisms. Semin. Cell Dev. Biol. 2020, 101, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ye, Z.; Wang, J.; Chen, Q.; Huang, D.; Liu, H. USP13 mediates PTEN to ameliorate osteoarthritis by restraining oxidative stress, apoptosis and inflammation via AKT-dependent manner. Biomed. Pharmacother. 2021, 133, 111089. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Potin, I.; Largo, R.; Roman-Blas, J.A.; Herrero-Beaumont, G.; Walsh, D.A. Characterization of multinucleated giant cells in synovium and subchondral bone in knee osteoarthritis and rheumatoid arthritis. BMC Musculoskelet. Disord. 2015, 16, 226. [Google Scholar] [CrossRef]
- Walker, C.J.; Crocini, C.; Ramirez, D.; Killaars, A.R.; Grim, J.C.; Aguado, B.A.; Clark, K.; Allen, M.A.; Dowell, R.D.; Leinwand, L.A.; et al. Nuclear mechanosensing drives chromatin remodelling in persistently activated fibroblasts. Nat. Biomed. Eng. 2021, 5, 1485–1499. [Google Scholar] [CrossRef]
- Stephenson, W.; Donlin, L.T.; Butler, A.; Rozo, C.; Bracken, B.; Rashidfarrokhi, A.; Goodman, S.M.; Ivashkiv, L.B.; Bykerk, V.P.; Orange, D.E.; et al. Single-cell RNA-seq of rheumatoid arthritis synovial tissue using low-cost microfluidic instrumentation. Nat. Commun. 2018, 9, 791. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, F.; Slowikowski, K.; Wei, K.; Marshall, J.L.; Rao, D.A.; Chang, S.K.; Nguyen, H.N.; Noss, E.H.; Turner, J.D.; Earp, B.E.; et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 2018, 9, 789. [Google Scholar] [CrossRef]
- Croft, A.P.; Campos, J.; Jansen, K.; Turner, J.D.; Marshall, J.; Attar, M.; Savary, L.; Wehmeyer, C.; Naylor, A.J.; Kemble, S.; et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 2019, 570, 246–251. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, K.; Slowikowski, K.; Fonseka, C.Y.; Rao, D.A.; Kelly, S.; Goodman, S.M.; Tabechian, D.; Hughes, L.B.; Salomon-Escoto, K.; et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 2019, 20, 928–942. [Google Scholar] [CrossRef]
- Damerau, A.; Rosenow, E.; Alkhoury, D.; Buttgereit, F.; Gaber, T. Fibrotic pathways and fibroblast-like synoviocyte phenotypes in osteoarthritis. Front. Immunol. 2024, 15, 1385006. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Liang, Y.; Peng, P.; Ma, X.; Zhang, X. DKK3 regulates cell proliferation, apoptosis and collagen synthesis in keloid fibroblasts via TGF-β1/Smad signaling pathway. Bioimed. Pharmacother. 2017, 91, 174–180. [Google Scholar] [CrossRef]
- Chen, X.; Gong, L.; Li, C.; Wang, S.; Wang, Z.; Chu, M.; Zhou, Y. Single-cell and bulk tissue sequencing unravels the heterogeneity of synovial microenvironment in arthrofibrosis. iScience 2023, 26, 107379. [Google Scholar] [CrossRef]
- Younesi, F.S.; Miller, A.E.; Barker, T.H.; Rossi, F.M.V.; Hinz, B. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.; Younesi, F.; Ezzo, M.; Hinz, B. The Role of Myofibroblasts in Physiological and Pathological Tissue Repair. Cold Spring Harb. Perspect. Biol. 2023, 15, a041231. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Feng, G.; Yang, Y.; Zhao, X.; Ma, L.; Guo, H.; Chen, X.; Wang, H.; Chen, Z.; Jin, Q. Nintedanib ameliorates osteoarthritis in mice by inhibiting synovial inflammation and fibrosis caused by M1 polarization of synovial macrophages via the MAPK/PI3K-AKT pathway. FASEB J 2023, 37, e23177. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Kong, N.; Liu, X.; Tian, R.; Jiao, M.; Li, Y.; Guan, H.; Wang, K.; Yang, P. Pirfenidone attenuates synovial fibrosis and postpones the progression of osteoarthritis by anti-fibrotic and anti-inflammatory properties in vivo and in vitro. J. Transl. Med. 2021, 19, 157. [Google Scholar] [CrossRef]
- Jamal, J.; Roebuck, M.M.; Lee, S.Y.; Frostick, S.P.; Abbas, A.A.; Merican, A.M.; Teo, S.H.; Wood, A.; Tan, S.L.; Bou-Gharios, G.; et al. Modulation of the mechanical responses of synovial fibroblasts by osteoarthritis-associated inflammatory stressors. Int. J. Biochem. Cell Biol. 2020, 126, 105800. [Google Scholar] [CrossRef]
- Bolia, I.K.; Mertz, K.; Faye, E.; Sheppard, J.; Telang, S.; Bogdanov, J.; Hasan, L.K.; Haratian, A.; Evseenko, D.; Weber, A.E.; et al. Cross-Communication Between Knee Osteoarthritis and Fibrosis: Molecular Pathways and Key Molecules. Open Access J. Sports Med. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Remst, D.F.; Blaney Davidson, E.N.; van der Kraan, P.M. Unravelling osteoarthritis-related synovial fibrosis: A step closer to solving joint stiffness. Rheumatology 2015, 54, 1954–1963. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Baran, P.; Hansen, S.; Waetzig, G.H.; Akbarzadeh, M.; Lamertz, L.; Huber, H.J.; Ahmadian, M.R.; Moll, J.M.; Scheller, J. The balance of interleukin (IL)-6, IL-6·soluble IL-6 receptor (sIL-6R), and IL-6·sIL-6R·sgp130 complexes allows simultaneous classic and trans-signaling. J. Biol. Chem. 2018, 293, 6762–6775. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, Y.; Li, Q. Therapeutic mechanisms of ibuprofen, prednisone and betamethasone in osteoarthritis. Mol. Med. Rep. 2017, 15, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Shiau, A.L.; Li, Y.T.; Lin, C.C.; Jou, I.M.; Liu, M.F.; Wu, C.L.; Wang, C.R. Transcription factor snail regulates tumor necrosis factor α-mediated synovial fibroblast activation in the rheumatoid joint. Arthritis Rheumatol. 2015, 67, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Osta, B.; Roux, J.P.; Lavocat, F.; Pierre, M.; Ndongo-Thiam, N.; Boivin, G.; Miossec, P. Differential Effects of IL-17A and TNF-α on Osteoblastic Differentiation of Isolated Synoviocytes and on Bone Explants from Arthritis Patients. Front. Immunol. 2015, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Liu, P.; Yang, D.; Wang, F.; Yuan, L.; Lin, Z.; Jiang, J. Interleukin-18-induced inflammatory responses in synoviocytes and chondrocytes from osteoarthritic patients. Int. J. Mol. Med. 2012, 30, 805–810. [Google Scholar] [CrossRef]
- Bale, S.; Verma, P.; Varga, J.; Bhattacharyya, S. Extracellular Matrix-Derived Damage-Associated Molecular Patterns (DAMP): Implications in Systemic Sclerosis and Fibrosis. J. Investig. Dermatol. 2023, 143, 1877–1885. [Google Scholar] [CrossRef]
- Roškar, S.; Hafner-Bratkovič, I. The Role of Inflammasomes in Osteoarthritis and Secondary Joint Degeneration Diseases. Life 2022, 12, 731. [Google Scholar] [CrossRef]
- Lu, A.; Magupalli, V.G.; Ruan, J.; Yin, Q.; Atianand, M.K.; Vos, M.R.; Schröder, G.F.; Fitzgerald, K.A.; Wu, H.; Egelman, E.H. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 2014, 156, 1193–1206. [Google Scholar] [CrossRef]
- Kayagaki, N.; Kornfeld, O.S.; Lee, B.L.; Stowe, I.B.; O’Rourke, K.; Li, Q.; Sandoval, W.; Yan, D.; Kang, J.; Xu, M.; et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 2021, 591, 131–136. [Google Scholar] [CrossRef]
- Ding, D.F.; Xue, Y.; Wu, X.C.; Zhu, Z.H.; Ding, J.Y.; Song, Y.J.; Xu, X.L.; Xu, J.G. Recent Advances in Reactive Oxygen Species (ROS)-Responsive Polyfunctional Nanosystems 3.0 for the Treatment of Osteoarthritis. J. Inflamm. Res. 2022, 15, 5009–5026. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Rivera, S.; Monclus, E.A.; Synenki, L.; Zirk, A.; Eisenbart, J.; Feghali-Bostwick, C.; Mutlu, G.M.; Budinger, G.R.; Chandel, N.S. Mitochondrial reactive oxygen species regulate transforming growth factor-β signaling. J. Biol. Chem. 2013, 288, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.; Dieudé, M.; Yang, B.; Hamelin, K.; Underwood, K.; Hébert, M.J. Autophagy fosters myofibroblast differentiation through MTORC2 activation and downstream upregulation of CTGF. Autophagy 2014, 10, 2193–2207. [Google Scholar] [CrossRef]
- Del Principe, D.; Lista, P.; Malorni, W.; Giammarioli, A.M. Fibroblast autophagy in fibrotic disorders. J. Pathol. 2013, 229, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef]
- Brown, P.D.; Wakefield, L.M.; Levinson, A.D.; Sporn, M.B. Physicochemical activation of recombinant latent transforming growth factor-beta’s 1, 2, and 3. Growth Factors 1990, 3, 35–43. [Google Scholar] [CrossRef]
- Shi, M.; Zhu, J.; Wang, R.; Chen, X.; Mi, L.; Walz, T.; Springer, T.A. Latent TGF-β structure and activation. Nature 2011, 474, 343–349. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β signaling in health, disease, and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Liu, W.J.; Wu, L.Y.; Wang, G.; Zhang, C.L.; Liu, J. The Research Progress in Transforming Growth Factor-β2. Cells 2023, 12, 2739. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Remst, D.F.; Blom, A.B.; Vitters, E.L.; Bank, R.A.; van den Berg, W.B.; Blaney Davidson, E.N.; van der Kraan, P.M. Gene expression analysis of murine and human osteoarthritis synovium reveals elevation of transforming growth factor β-responsive genes in osteoarthritis-related fibrosis. Arthritis Rheumatol. 2014, 66, 647–656. [Google Scholar] [CrossRef]
- Drabsch, Y.; ten Dijke, P. TGF-β signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev. 2012, 31, 553–568. [Google Scholar] [CrossRef]
- Giarratana, A.O.; Prendergast, C.M.; Salvatore, M.M.; Capaccione, K.M. TGF-β signaling: Critical nexus of fibrogenesis and cancer. J. Transl. Med. 2024, 22, 594. [Google Scholar] [CrossRef]
- Distler, J.H.W.; Györfi, A.H.; Ramanujam, M.; Whitfield, M.L.; Königshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730. [Google Scholar] [CrossRef]
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of β-catenin. EMBO J 2012, 31, 2714–2736. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Alok, A.; Lei, Z.; Jagannathan, N.S.; Kaur, S.; Harmston, N.; Rozen, S.G.; Tucker-Kellogg, L.; Virshup, D.M. Wnt proteins synergize to activate β-catenin signaling. J. Cell Sci. 2017, 130, 1532–1544. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Cherifi, C.; Monteagudo, S.; Lories, R.J. Promising targets for therapy of osteoarthritis: A review on the Wnt and TGF-β signalling pathways. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720x211006959. [Google Scholar] [CrossRef]
- Lietman, C.; Wu, B.; Lechner, S.; Shinar, A.; Sehgal, M.; Rossomacha, E.; Datta, P.; Sharma, A.; Gandhi, R.; Kapoor, M.; et al. Inhibition of Wnt/β-catenin signaling ameliorates osteoarthritis in a murine model of experimental osteoarthritis. JCI Insight 2018, 3, e96308. [Google Scholar] [CrossRef]
- Liao, B.; Guan, M.; Tan, Q.; Wang, G.; Zhang, R.; Huang, J.; Liu, M.; Chen, H.; Li, K.; Bai, D.; et al. Low-intensity pulsed ultrasound inhibits fibroblast-like synoviocyte proliferation and reduces synovial fibrosis by regulating Wnt/β-catenin signaling. J. Orthop. Transl. 2021, 30, 41–50. [Google Scholar] [CrossRef]
- Tsai, H.W.; Cheng, S.W.; Chen, C.C.; Chen, I.W.; Ho, C.L. A combined bioinformatics and experimental approach identifies RMI2 as a Wnt/beta-catenin signaling target gene related to hepatocellular carcinoma. BMC Cancer 2023, 23, 1025. [Google Scholar] [CrossRef]
- Song, P.; Gao, Z.; Bao, Y.; Chen, L.; Huang, Y.; Liu, Y.; Dong, Q.; Wei, X. Wnt/β-catenin signaling pathway in carcinogenesis and cancer therapy. J. Hematol. Oncol. 2024, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Trinh-Minh, T.; Chen, C.W.; Tran Manh, C.; Li, Y.N.; Zhu, H.; Zhou, X.; Chakraborty, D.; Zhang, Y.; Rauber, S.; Dees, C.; et al. Noncanonical WNT5A controls the activation of latent TGF-beta to drive fibroblast activation and tissue fibrosis. J. Clin. Investig. 2024, 134, e159884. [Google Scholar] [CrossRef]
- Tian, Y.; Xia, J.; Yang, G.; Li, C.; Qi, Y.; Dai, K.; Wu, C.; Guo, Y.; Yao, W.; Hao, C. A2aR inhibits fibrosis and the EMT process in silicosis by regulating Wnt/beta-catenin pathway. Ecotoxicol. Environ. Saf. 2023, 249, 114410. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Moroishi, T.; Guan, K.L. Mechanisms of Hippo pathway regulation. Genes. Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef]
- Han, J.; Zhang, J.; Zhang, X.; Luo, W.; Liu, L.; Zhu, Y.; Liu, Q.; Zhang, X.A. Emerging role and function of Hippo-YAP/TAZ signaling pathway in musculoskeletal disorders. Stem Cell Res. Ther. 2024, 15, 386. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yu, H.; Liu, J.; Yuan, L.; Li, F. Role of Hippo-YAP/TAZ signaling pathway in organ fibrosis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024, 49, 1509–1516. [Google Scholar] [CrossRef]
- Su, S.; Jiang, W.; Wang, X.; Du, S.; Qi, J.; Jia, Q.; Song, H. Resolvin D1 inhibits the proliferation of osteoarthritis fibroblast-like synoviocytes through the Hippo-YAP signaling pathway. BMC Musculoskelet. Disord. 2022, 23, 149. [Google Scholar] [CrossRef]
- Kubota, S.; Takigawa, M. Cellular and molecular actions of CCN2/CTGF and its role under physiological and pathological conditions. Clin. Sci. 2015, 128, 181–196. [Google Scholar] [CrossRef]
- Blaney Davidson, E.N.; Vitters, E.L.; Mooren, F.M.; Oliver, N.; Berg, W.B.; van der Kraan, P.M. Connective tissue growth factor/CCN2 overexpression in mouse synovial lining results in transient fibrosis and cartilage damage. Arthritis Rheum. 2006, 54, 1653–1661. [Google Scholar] [CrossRef]
- Rebolledo, D.L.; Acuña, M.J.; Brandan, E. Role of Matricellular CCN Proteins in Skeletal Muscle: Focus on CCN2/CTGF and Its Regulation by Vasoactive Peptides. Int. J. Mol. Sci. 2021, 22, 5234. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, L.; Liu, H.; Liu, Y.; Xing, J.; Song, J.; Luo, E. Hippo/YAP1 promotes osteoporotic mice bone defect repair via the activating of Wnt signaling pathway. Cell Signal 2024, 116, 111037. [Google Scholar] [CrossRef]
- Varelas, X.; Miller, B.W.; Sopko, R.; Song, S.; Gregorieff, A.; Fellouse, F.A.; Sakuma, R.; Pawson, T.; Hunziker, W.; McNeill, H.; et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev. Cell 2010, 18, 579–591. [Google Scholar] [CrossRef]
- Azzolin, L.; Panciera, T.; Soligo, S.; Enzo, E.; Bicciato, S.; Dupont, S.; Bresolin, S.; Frasson, C.; Basso, G.; Guzzardo, V.; et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 2014, 158, 157–170. [Google Scholar] [CrossRef]
- Qin, Z.; Xia, W.; Fisher, G.J.; Voorhees, J.J.; Quan, T. YAP/TAZ regulates TGF-β/Smad3 signaling by induction of Smad7 via AP-1 in human skin dermal fibroblasts. Cell Commun. Signal 2018, 16, 18. [Google Scholar] [CrossRef]
- Ge, J.; Cui, H.; Xie, N.; Banerjee, S.; Guo, S.; Dubey, S.; Barnes, S.; Liu, G. Glutaminolysis Promotes Collagen Translation and Stability via α-Ketoglutarate-mediated mTOR Activation and Proline Hydroxylation. Am. J. Respir. Cell Mol. Biol. 2018, 58, 378–390. [Google Scholar] [CrossRef]
- Rim, Y.A.; Ju, J.H. The Role of Fibrosis in Osteoarthritis Progression. Life 2020, 11, 3. [Google Scholar] [CrossRef]
- Maglaviceanu, A.; Wu, B.; Kapoor, M. Fibroblast-like synoviocytes: Role in synovial fibrosis associated with osteoarthritis. Wound Repair. Regen. 2021, 29, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. Viscoelasticity, Like Forces, Plays a Role in Mechanotransduction. Front. Cell Dev. Biol. 2022, 10, 789841. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, S.N.; Ditchfield, C.; Flynn, S.; Agrawal, J.; Davis, E.T.; Dajas-Bailador, F.; Chapman, V.; Jones, S.W. Immunomodulation and fibroblast dynamics driving nociceptive joint pain within inflammatory synovium: Unravelling mechanisms for therapeutic advancements in osteoarthritis. Osteoarthr. Cartil. 2024, 32, 1358–1370. [Google Scholar] [CrossRef]
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 35–44. [Google Scholar] [CrossRef]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef]
- Kuang, S.; Sheng, W.; Meng, J.; Liu, W.; Xiao, Y.; Tang, H.; Fu, X.; Kuang, M.; He, Q.; Gao, S. Pyroptosis-related crosstalk in osteoarthritis: Macrophages, fibroblast-like synoviocytes and chondrocytes. J. Orthop. Transl. 2024, 47, 223–234. [Google Scholar] [CrossRef]
- Piersma, B.; Bank, R.A. Collagen cross-linking mediated by lysyl hydroxylase 2: An enzymatic battlefield to combat fibrosis. Essays Biochem. 2019, 63, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Liao, T.; Yang, N.; Wei, Y.; Xing, R.; Wu, P.; Li, X.; Mao, J.; Wang, P. Chrysin ameliorates synovitis and fibrosis of osteoarthritic fibroblast-like synoviocytes in rats through PERK/TXNIP/NLRP3 signaling. Front. Pharmacol. 2023, 14, 1170243. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Shi, L.; He, C.; Liu, D.; Wei, Y.; Ma, Z.; Wang, P.; Mao, J.; Wu, P. Suppression of NUPR1 in fibroblast-like synoviocytes reduces synovial fibrosis via the Smad3 pathway. J. Transl. Med. 2024, 22, 715. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Wu, S.; Li, X.; Yuan, L.X. Danggui niantong decoction attenuates synovial fibrosis through regulating PI3k/AKT signaling pathway. J. Ethnopharmacol. 2025, 342, 119381. [Google Scholar] [CrossRef]
- Piñeiro-Ramil, M.; Flórez-Fernández, N.; Ramil-Gómez, O.; Torres, M.D.; Dominguez, H.; Blanco, F.J.; Meijide-Faílde, R.; Vaamonde-García, C. Antifibrotic effect of brown algae-derived fucoidans on osteoarthritic fibroblast-like synoviocytes. Carbohydr. Polym. 2022, 282, 119134. [Google Scholar] [CrossRef] [PubMed]
- Vaamonde-Garcia, C.; Malaise, O.; Charlier, E.; Deroyer, C.; Neuville, S.; Gillet, P.; Kurth, W.; Meijide-Failde, R.; Malaise, M.G.; de Seny, D. 15-Deoxy-Δ-12, 14-prostaglandin J2 acts cooperatively with prednisolone to reduce TGF-β-induced pro-fibrotic pathways in human osteoarthritis fibroblasts. Biochem. Pharmacol. 2019, 165, 66–78. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, D.; Zhou, F.; Yu, J.; Wu, X.; Yu, D.; Zou, Y.; Hong, Y.; Yuan, C.; Chen, Y.; et al. Targeting downstream subcellular YAP activity as a function of matrix stiffness with Verteporfin-encapsulated chitosan microsphere attenuates osteoarthritis. Biomaterials 2020, 232, 119724. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Efficacy and safety of adalimumab by intra-articular injection for moderate to severe knee osteoarthritis: An open-label randomized controlled trial. J. Int. Med. Res. 2018, 46, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.D.; Malone, J.B.; Kean, T.J. Advances and Challenges in the Pursuit of Disease-Modifying Osteoarthritis Drugs: A Review of 2010-2024 Clinical Trials. Biomedicines 2025, 13, 355. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Ong, K.L.; Lau, E.; Bozic, K.J. Impact of the economic downturn on total joint replacement demand in the United States: Updated projections to 2021. J. Bone Joint Surg. Am. 2014, 96, 624–630. [Google Scholar] [CrossRef]
- Yazici, Y.; McAlindon, T.E.; Gibofsky, A.; Lane, N.E.; Lattermann, C.; Skrepnik, N.; Swearingen, C.J.; Simsek, I.; Ghandehari, H.; DiFrancesco, A.; et al. A Phase 2b randomized trial of lorecivivint, a novel intra-articular CLK2/DYRK1A inhibitor and Wnt pathway modulator for knee osteoarthritis. Osteoarthr. Cartil. 2021, 29, 654–666. [Google Scholar] [CrossRef]
- Yazici, Y.; Tambiah, J.R.S.; Swearingen, C.J.; Britt, J.; Kennedy, S.; Fineman, M.S.; Simsek, I.; Solomon, E.; McAlindon, T.E. A Phase 3, 56-week, randomised, double-blind, placebo-controlled study (OA-11) utilising patient-reported and radiographic outcomes evaluating the efficacy and safety of a lorecivivint injection in patients with moderate to severe knee osteoarthritis. Clin. Exp. Rheumatol. 2025, 43, 854–860. [Google Scholar] [CrossRef]
- Yazici, Y.; Tambiah, J.R.S.; Swearingen, C.J.; Lopez, V.A.; Kennedy, S.; Fineman, M.S.; Simsek, I.; Solomon, E.; McAlindon, T.E. A Phase 3, 28-week, multicentre, randomised, double-blind, placebo-controlled trial (OA-10) to evaluate the efficacy and safety of a single injection of lorecivivint in the target knee joint of moderately to severely symptomatic osteoarthritis patients. Clin. Exp. Rheumatol. 2025, 43, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Tambiah, J.; Kennedy, S.; Swearingen, C.; McAlindon, T.; Yazici, Y. Impact of structural severity on outcomes in knee osteoarthritis: An analysis of data from phase 2 and phase 3 lorecivivint clinical trials. Rheumatology 2025, 64, 2583–2590. [Google Scholar] [CrossRef]

| Molecule/Drug Name | Target | Status | Preclinical/Clinical Trial Result | NCT |
|---|---|---|---|---|
| Chrysin | PERK/TXNIP/NLRP3 signaling | Preclinical trial | Effective [86] | \ |
| Trifluoperazine (TFP) | NUPR1, Smad3 pathway | Preclinical trial | Effective [87] | \ |
| Danggui Niantong | PI3k/AKT signaling, non-canonical TGF-β pathway | Preclinical trial | Effective [88] | \ |
| Fucoidans | Nitric oxide production; TGF-β/Smad pathway | Preclinical trial | Effective [89] | \ |
| Prednisolone | TGF-β/Smad pathway; ALK5/Smad2 signaling | Preclinical trial | Effective [90] | \ |
| Adalimumab | TNF-α | Clinical trial, Phase 2 | Completed | NCT00296894 |
| Lorecivivint | CLK2 kinase, Wnt pathway | Clinical trial, Phase 3 | Completed | NCT05603754 |
| Verteporfin | YAP, Hippo pathway | Preclinical trial | Effective [91] | \ |
| Resolvin D1 | YAP, Hippo pathway | Preclinical trial | Effective [68] | \ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, R.; Lin, Q.; Yang, Z.; Li, H.; Li, J.J.; Xing, D. Fibroblast–Myofibroblast Transition in Osteoarthritis Progression: Current Insights. Int. J. Mol. Sci. 2025, 26, 7881. https://doi.org/10.3390/ijms26167881
Peng R, Lin Q, Yang Z, Li H, Li JJ, Xing D. Fibroblast–Myofibroblast Transition in Osteoarthritis Progression: Current Insights. International Journal of Molecular Sciences. 2025; 26(16):7881. https://doi.org/10.3390/ijms26167881
Chicago/Turabian StylePeng, Ruixin, Qiyuan Lin, Zhen Yang, Hui Li, Jiao Jiao Li, and Dan Xing. 2025. "Fibroblast–Myofibroblast Transition in Osteoarthritis Progression: Current Insights" International Journal of Molecular Sciences 26, no. 16: 7881. https://doi.org/10.3390/ijms26167881
APA StylePeng, R., Lin, Q., Yang, Z., Li, H., Li, J. J., & Xing, D. (2025). Fibroblast–Myofibroblast Transition in Osteoarthritis Progression: Current Insights. International Journal of Molecular Sciences, 26(16), 7881. https://doi.org/10.3390/ijms26167881







