Complete Loss of Cramp Promotes Experimental Osteoarthritis with Enhanced Chondrocyte Apoptosis in Mice
Abstract
1. Introduction
2. Results
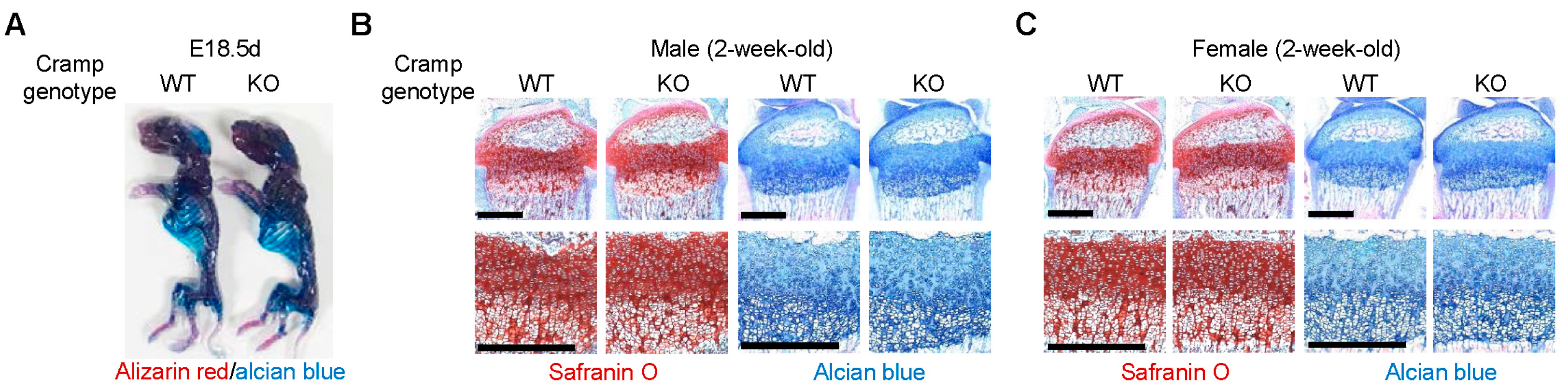
2.1. Cramp Knockout (KO) Mice Exhibit Normal Skeletal Development
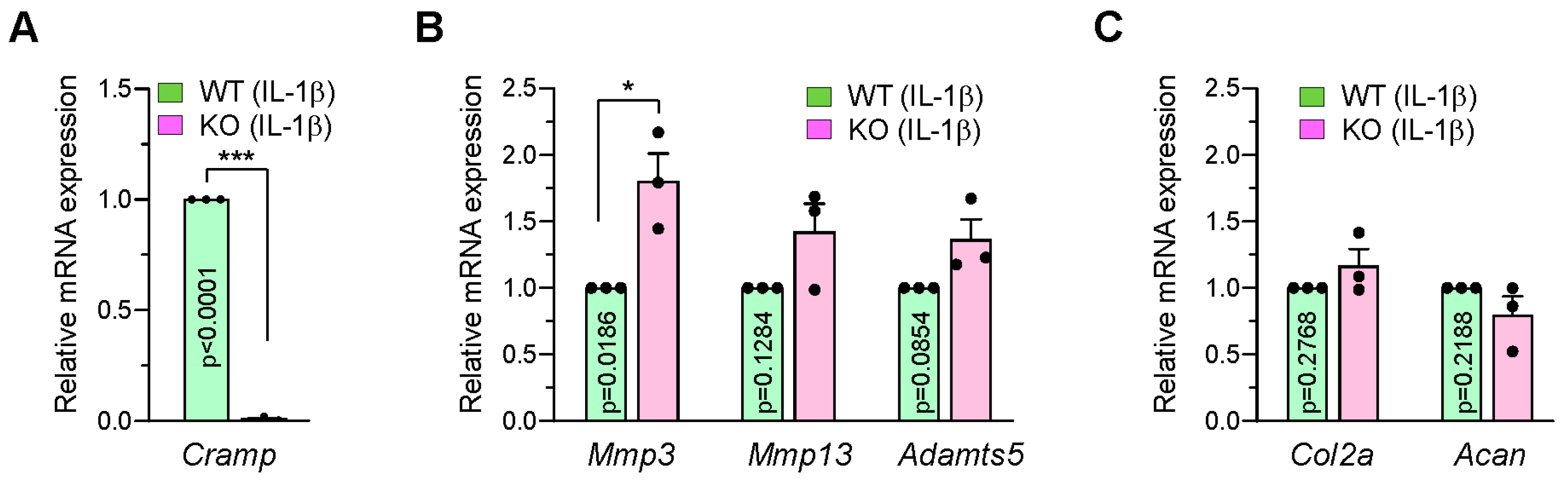
2.2. Cramp-Deficient Chondrocytes Exhibit Partial Upregulation of Matrix-Degrading Enzymes Following IL-1β Stimulation
2.3. Loss of Cramp Accelerates Monosodium Iodoacetate (MIA)-Induced Cartilage Lesions
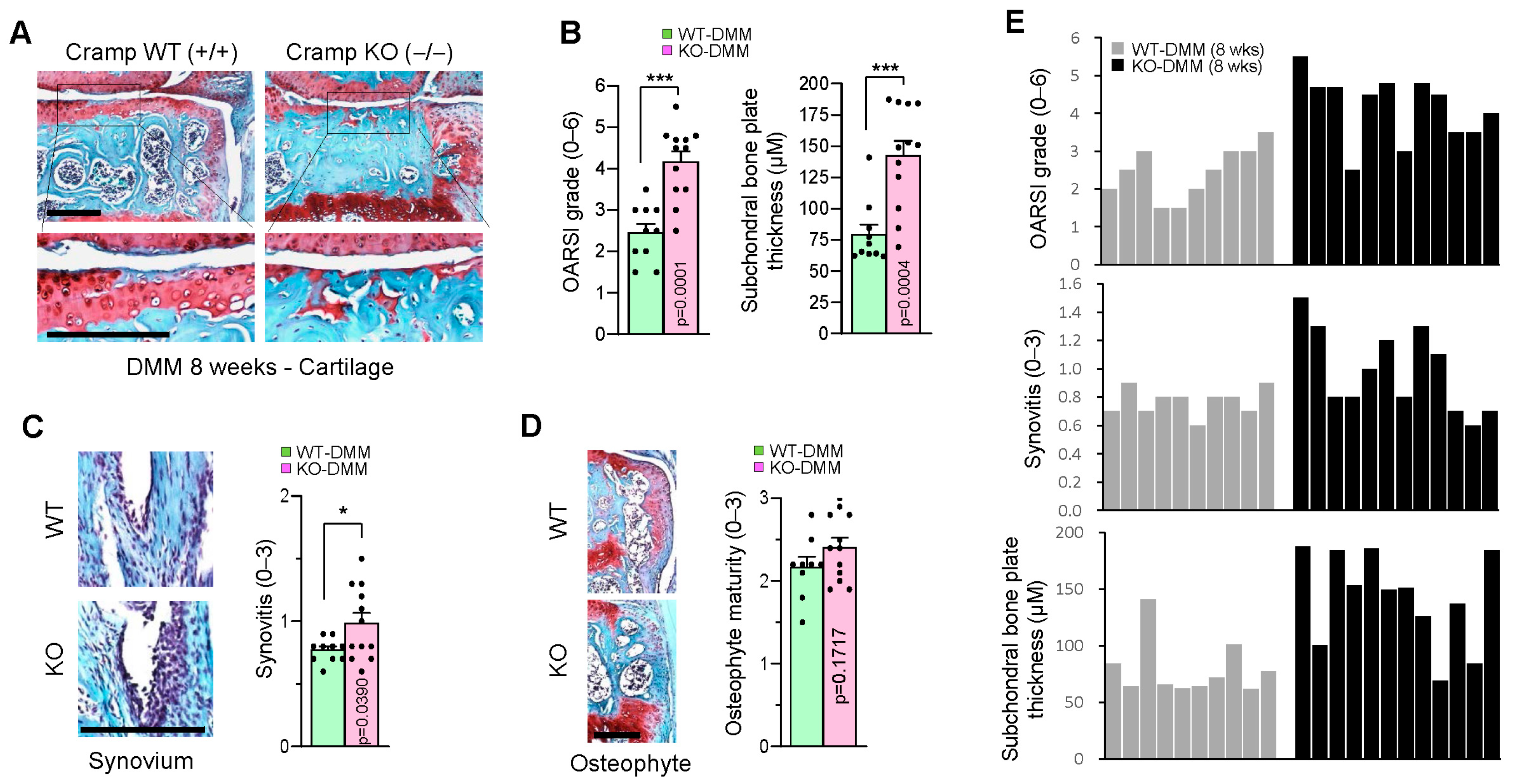
2.4. Genetic Ablation of Cramp Exacerbates the Destabilization of the Medial Meniscus (DMM)-Induced Experimental OA
2.5. Cramp Deficiency Increases Chondrocyte Apoptosis in DMM-Induced Experimental OA
3. Discussion
4. Materials and Methods
4.1. Chondrocyte Culture and RNA Analysis
4.2. Mice and Experimental OA
4.3. Skeletal Staining, Histological Analysis and In Situ Apoptosis Detection
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheumatol. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Heinegard, D.; Saxne, T. The role of the cartilage matrix in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Nakasa, T.; Hikata, T.; Asahara, H. Molecular network of cartilage homeostasis and osteoarthritis. Med. Res. Rev. 2008, 28, 464–481. [Google Scholar] [CrossRef]
- Little, C.B.; Barai, A.; Burkhardt, D.; Smith, S.M.; Fosang, A.J.; Werb, Z.; Shah, M.; Thompson, E.W. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheumatol. 2009, 60, 3723–3733. [Google Scholar] [CrossRef]
- Glasson, S.S.; Askew, R.; Sheppard, B.; Carito, B.; Blanchet, T.; Ma, H.L.; Flannery, C.R.; Peluso, D.; Kanki, K.; Yang, Z.; et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 2005, 434, 644–648. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, H.A. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef] [PubMed]
- Zamli, Z.; Sharif, M. Chondrocyte apoptosis: A cause or consequence of osteoarthritis? Int. J. Rheum. Dis. 2011, 14, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Alford, M.A.; Baquir, B.; Santana, F.L.; Haney, E.F.; Hancock, R.E.W. Cathelicidin Host Defense Peptides and Inflammatory Signaling: Striking a Balance. Front. Microbiol. 2020, 11, 1902. [Google Scholar] [CrossRef]
- Pircher, J.; Czermak, T.; Ehrlich, A.; Eberle, C.; Gaitzsch, E.; Margraf, A.; Grommes, J.; Saha, P.; Titova, A.; Ishikawa-Ankerhold, H.; et al. Cathelicidins prime platelets to mediate arterial thrombosis and tissue inflammation. Nat. Commun. 2018, 9, 1523. [Google Scholar] [CrossRef]
- Doring, Y.; Drechsler, M.; Wantha, S.; Kemmerich, K.; Lievens, D.; Vijayan, S.; Gallo, R.L.; Weber, C.; Soehnlein, O. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ. Res. 2012, 110, 1052–1056. [Google Scholar] [CrossRef]
- Zhang, L.J.; Guerrero-Juarez, C.F.; Hata, T.; Bapat, S.P.; Ramos, R.; Plikus, M.V.; Gallo, R.L. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 2015, 347, 67–71. [Google Scholar] [CrossRef]
- Edfeldt, K.; Agerberth, B.; Rottenberg, M.E.; Gudmundsson, G.H.; Wang, X.B.; Mandal, K.; Xu, Q.; Yan, Z.Q. Involvement of the antimicrobial peptide LL-37 in human atherosclerosis. Arter. Thromb. Vasc. Biol. 2006, 26, 1551–1557. [Google Scholar] [CrossRef]
- Chen, K.; Yoshimura, T.; Yao, X.; Gong, W.; Huang, J.; Dzutsev, A.K.; McCulloch, J.; O’HUigin, C.; Bian, X.W.; Trinchieri, G.; et al. Distinct contributions of cathelin-related antimicrobial peptide (CRAMP) derived from epithelial cells and macrophages to colon mucosal homeostasis. J. Pathol. 2020, 253, 339–350. [Google Scholar] [CrossRef]
- Pan, L.L.; Liang, W.; Ren, Z.; Li, C.; Chen, Y.; Niu, W.; Fang, X.; Liu, Y.; Zhang, M.; Diana, J.; et al. Cathelicidin-related antimicrobial peptide protects against ischaemia reperfusion-induced acute kidney injury in mice. Br. J. Pharmacol. 2020, 177, 2726–2742. [Google Scholar] [CrossRef]
- Wertenbruch, S.; Drescher, H.; Grossarth, V.; Kroy, D.; Giebeler, A.; Erschfeld, S.; Heinrichs, D.; Soehnlein, O.; Trautwein, C.; Brandenburg, L.O.; et al. The Anti-Microbial Peptide LL-37/CRAMP Is Elevated in Patients with Liver Diseases and Acts as a Protective Factor during Mouse Liver Injury. Digestion 2015, 91, 307–317. [Google Scholar] [CrossRef]
- Choi, M.C.; Jo, J.; Lee, M.; Park, J.; Yao, T.P.; Park, Y. Cathelicidin-related antimicrobial peptide mediates skeletal muscle degeneration caused by injury and Duchenne muscular dystrophy in mice. J. Cachexia Sarcopenia Muscle 2022, 13, 3091–3105. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.; Ariga, S.K.; Barbeiro, H.V.; Volpini, R.A.; Barbeiro, D.F.; Seguro, A.C.; Pinheiro da Silva, F. Cathelicidin protects mice from Rhabdomyolysis-induced Acute Kidney Injury. Int. J. Med. Sci. 2021, 18, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Jo, J.; Lee, M.; Park, J.; Park, Y. Intra-Articular Administration of Cramp into Mouse Knee Joint Exacerbates Experimental Osteoarthritis Progression. Int. J. Mol. Sci. 2021, 22, 3429. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Marker, C.L.; Pomonis, J.D. The monosodium iodoacetate model of osteoarthritis pain in the rat. Methods Mol. Biol. 2012, 851, 239–248. [Google Scholar] [CrossRef]
- Pinheiro da Silva, F.; Machado, M.C. The dual role of cathelicidins in systemic inflammation. Immunol. Lett. 2017, 182, 57–60. [Google Scholar] [CrossRef]
- Scheenstra, M.R.; van Harten, R.M.; Veldhuizen, E.J.A.; Haagsman, H.P.; Coorens, M. Cathelicidins Modulate TLR-Activation and Inflammation. Front. Immunol. 2020, 11, 1137. [Google Scholar] [CrossRef]
- Kress, E.; Merres, J.; Albrecht, L.J.; Hammerschmidt, S.; Pufe, T.; Tauber, S.C.; Brandenburg, L.O. CRAMP deficiency leads to a pro-inflammatory phenotype and impaired phagocytosis after exposure to bacterial meningitis pathogens. Cell Commun. Signal 2017, 15, 32. [Google Scholar] [CrossRef]
- Merres, J.; Hoss, J.; Albrecht, L.J.; Kress, E.; Soehnlein, O.; Jansen, S.; Pufe, T.; Tauber, S.C.; Brandenburg, L.O. Role of the cathelicidin-related antimicrobial peptide in inflammation and mortality in a mouse model of bacterial meningitis. J. Innate. Immun. 2014, 6, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.C.; Enee, E.; Manasse, K.; Rebhi, F.; Penc, A.; Romeo-Guitart, D.; Bui Thi, C.; Titeux, M.; Oury, F.; Fillatreau, S.; et al. Cathelicidin antimicrobial peptide expression in neutrophils and neurons antagonistically modulates neuroinflammation. J. Clin. Investig. 2024, 135, e184502. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-kappaB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef]
- Murahashi, Y.; Yano, F.; Kobayashi, H.; Makii, Y.; Iba, K.; Yamashita, T.; Tanaka, S.; Saito, T. Intra-articular administration of IkappaBalpha kinase inhibitor suppresses mouse knee osteoarthritis via downregulation of the NF-kappaB/HIF-2alpha axis. Sci. Rep. 2018, 8, 16475. [Google Scholar] [CrossRef]
- Kobayashi, H.; Chang, S.H.; Mori, D.; Itoh, S.; Hirata, M.; Hosaka, Y.; Taniguchi, Y.; Okada, K.; Mori, Y.; Yano, F.; et al. Biphasic regulation of chondrocytes by Rela through induction of anti-apoptotic and catabolic target genes. Nat. Commun. 2016, 7, 13336. [Google Scholar] [CrossRef]
- Choi, M.C.; MaruYama, T.; Chun, C.H.; Park, Y. Alleviation of Murine Osteoarthritis by Cartilage-Specific Deletion of IkappaBzeta. Arthritis Rheumatol. 2018, 70, 1440–1449. [Google Scholar] [CrossRef]
- Won, Y.; Shin, Y.; Chun, C.H.; Cho, Y.; Ha, C.W.; Kim, J.H.; Chun, J.S. Pleiotropic roles of metallothioneins as regulators of chondrocyte apoptosis and catabolic and anabolic pathways during osteoarthritis pathogenesis. Ann. Rheum. Dis. 2016, 75, 2045–2052. [Google Scholar] [CrossRef]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Choi, W.H. Mithramycin A Alleviates Osteoarthritic Cartilage Destruction by Inhibiting HIF-2alpha Expression. Int. J. Mol. Sci. 2018, 19, 1411. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S17–S23. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.-C.; Jo, J.; Park, J. Complete Loss of Cramp Promotes Experimental Osteoarthritis with Enhanced Chondrocyte Apoptosis in Mice. Int. J. Mol. Sci. 2025, 26, 7874. https://doi.org/10.3390/ijms26167874
Choi M-C, Jo J, Park J. Complete Loss of Cramp Promotes Experimental Osteoarthritis with Enhanced Chondrocyte Apoptosis in Mice. International Journal of Molecular Sciences. 2025; 26(16):7874. https://doi.org/10.3390/ijms26167874
Chicago/Turabian StyleChoi, Moon-Chang, Jiwon Jo, and Junghee Park. 2025. "Complete Loss of Cramp Promotes Experimental Osteoarthritis with Enhanced Chondrocyte Apoptosis in Mice" International Journal of Molecular Sciences 26, no. 16: 7874. https://doi.org/10.3390/ijms26167874
APA StyleChoi, M.-C., Jo, J., & Park, J. (2025). Complete Loss of Cramp Promotes Experimental Osteoarthritis with Enhanced Chondrocyte Apoptosis in Mice. International Journal of Molecular Sciences, 26(16), 7874. https://doi.org/10.3390/ijms26167874





