Loss of Myh11 K1256 Dysregulates the Extracellular Matrix and Focal Adhesion by Inhibiting Zyxin-Activated Transcription
Abstract
1. Introduction
2. Results
2.1. Proteomics
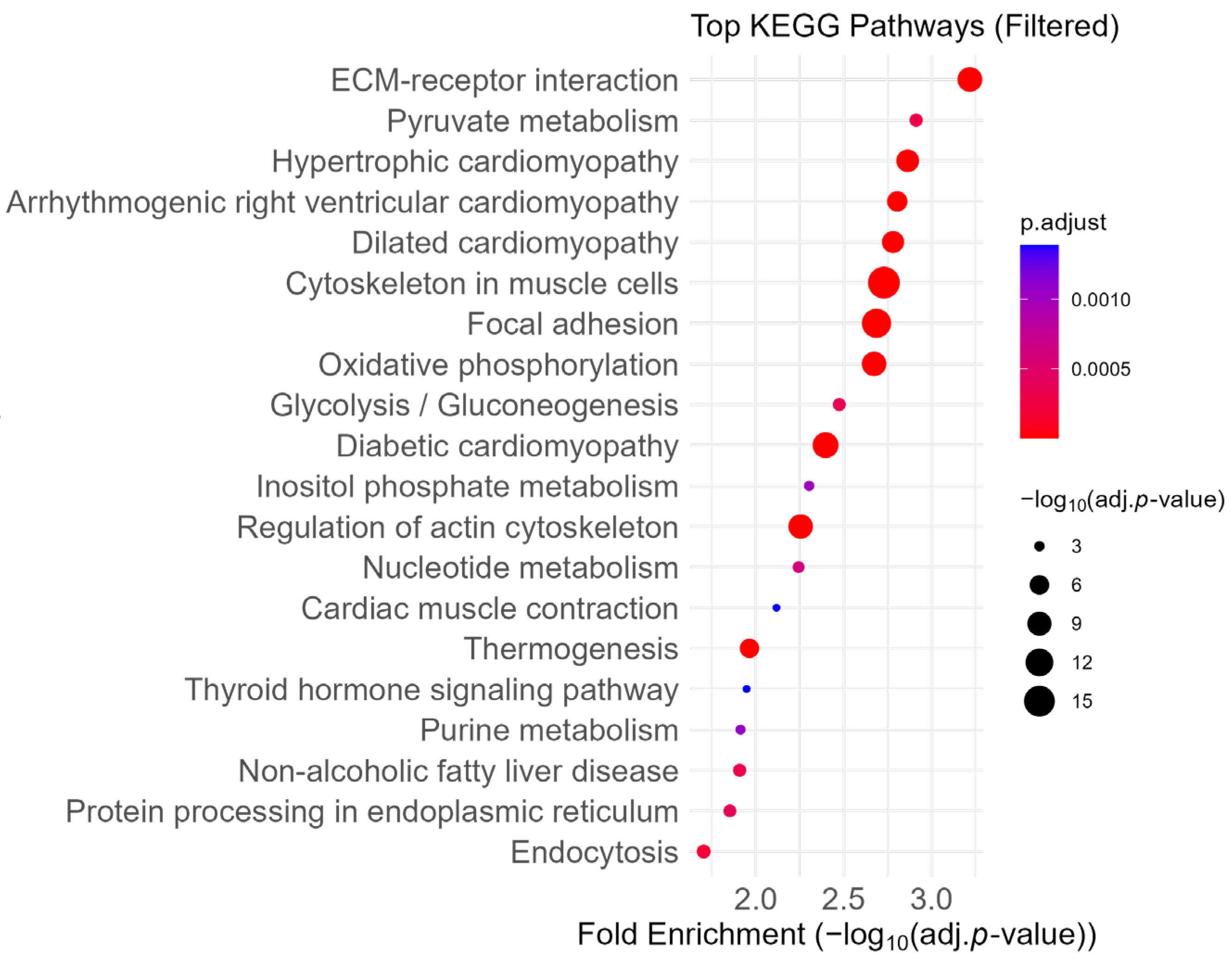
2.2. Enrichment of ECM and Focal Adhesion Pathways
2.3. Attenuation of ECM- and Coagulation-Related Pathways
2.4. Enrichment of Upregulated Proteins in Aerobic Respiration- and Ribosome-Related Pathways Revealed by Fold-Change-Specific Enrichment Analysis
2.5. Enriched Pathways Common to Proteomic and Transcriptomic Data
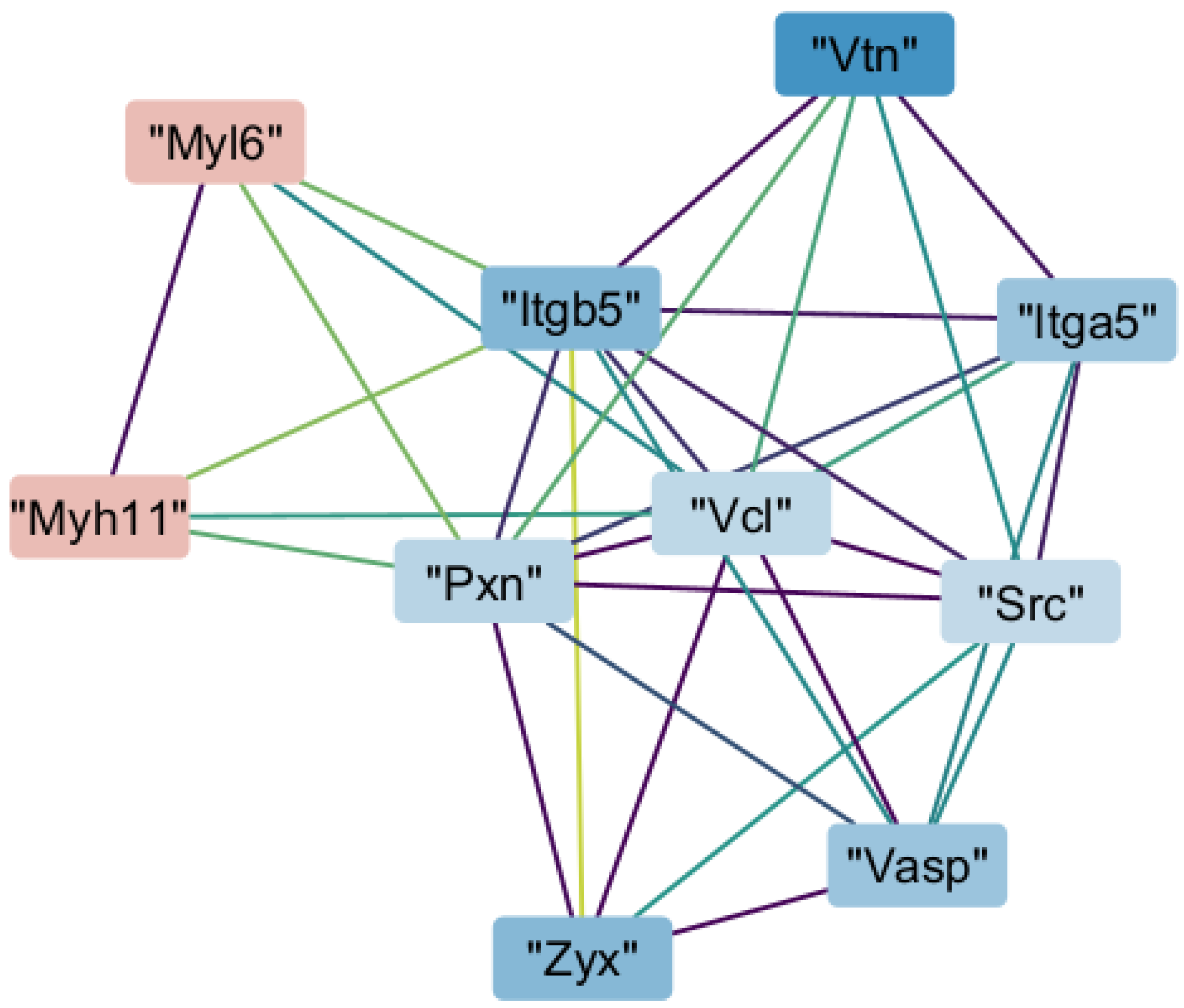
2.6. Interaction of Myh11 with Components of Focal Adhesion
2.7. Transcription Promoted by Zyxin
2.8. Downregulation of Proteins Whose Malfuntion Causes Loeys–Dietz and Ehlers–Danlos Syndromes
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Protein Extraction, Digestion, and Peptide Purification
4.3. NanoLC-MS/MS Analysis
4.4. Protein Quantification and Statistical Analysis
4.5. Fold-Change-Specific Enrichment Analysis
4.6. KEGG Pathway Enrichment Analysis
4.7. Protein–Protein Interaction (PPI) Network and MCODE Clustering
4.8. Identification of Genes Regulated by Zyxin
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hagan, P.G.; Nienaber, C.A.; Isselbacher, E.M.; Bruckman, D.; Karavite, D.J.; Russman, P.L.; Evangelista, A.; Fattori, R.; Suzuki, T.; Oh, J.K.; et al. The International Registry of Acute Aortic Dissection (IRAD)New Insights Into an Old Disease. JAMA 2000, 283, 897–903. [Google Scholar] [CrossRef]
- Negishi, K.; Aizawa, K.; Shindo, T.; Suzuki, T.; Sakurai, T.; Saito, Y.; Miyakawa, T.; Tanokura, M.; Kataoka, Y.; Maeda, M.; et al. An Myh11 single lysine deletion causes aortic dissection by reducing aortic structural integrity and contractility. Sci. Rep. 2022, 12, 8844. [Google Scholar] [CrossRef]
- Coady, M.A.; Davies, R.R.; Roberts, M.; Goldstein, L.J.; Rogalski, M.J.; Rizzo, J.A.; Hammond, G.L.; Kopf, G.S.; Elefteriades, J.A. Familial Patterns of Thoracic Aortic Aneurysms. Arch. Surg. 1999, 134, 361–367. [Google Scholar] [CrossRef]
- Biddinger, A.; Rocklin, M.; Coselli, J.; Milewicz, D.M. Familial thoracic aortic dilatations and dissections: A case control study. J. Vasc. Surg. 1997, 25, 506–511. [Google Scholar] [CrossRef]
- Albornoz, G.; Coady, M.A.; Roberts, M.; Davies, R.R.; Tranquilli, M.; Rizzo, J.A.; Elefteriades, J.A. Familial Thoracic Aortic Aneurysms and Dissections—Incidence, Modes of Inheritance, and Phenotypic Patterns. Ann. Thorac. Surg. 2006, 82, 1400–1405. [Google Scholar] [CrossRef]
- Zhu, L.; Vranckx, R.; Van Kien, P.K.; Lalande, A.; Boisset, N.; Mathieu, F.; Wegman, M.; Glancy, L.; Gasc, J.-M.; Brunotte, F.; et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat. Genet. 2006, 38, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Harakalova, M.; van der Smagt, J.; de Kovel, C.G.F.; van’t Slot, R.; Poot, M.; Nijman, I.J.; Medic, J.; Joziasse, I.; Deckers, J.; Roos-Hesselink, J.W.; et al. Incomplete segregation of MYH11 variants with thoracic aortic aneurysms and dissections and patent ductus arteriosus. Eur. J. Hum. Genet. 2013, 21, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Morita, H.; Takeda, N.; Miya, F.; Hyodo, H.; Fujita, D.; Tajima, T.; Tsunoda, T.; Nagai, R.; Kubo, M.; et al. A deletion mutation in myosin heavy chain 11 causing familial thoracic aortic dissection in two Japanese pedigrees. Int. J. Cardiol. 2015, 195, 290–292. [Google Scholar] [CrossRef]
- Tomida, S.; Ishima, T.; Sawaki, D.; Imai, Y.; Nagai, R.; Aizawa, K. Multi-Omics of Familial Thoracic Aortic Aneurysm and Dissection: Calcium Transport Impairment Predisposes Aortas to Dissection. Int. J. Mol. Sci. 2023, 24, 15213. [Google Scholar] [CrossRef]
- Rai, P.; Robinson, L.; Davies, H.A.; Akhtar, R.; Field, M.; Madine, J. Is There Enough Evidence to Support the Role of Glycosaminoglycans and Proteoglycans in Thoracic Aortic Aneurysm and Dissection?—A Systematic Review. Int. J. Mol. Sci. 2022, 23, 9200. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Shen, Y.H.; Russell, L.; Coselli, J.S.; LeMaire, S.A. Molecular mechanisms of thoracic aortic dissection. J. Surg. Res. 2013, 184, 907–924. [Google Scholar] [CrossRef] [PubMed]
- Banceu, C.M.; Gurzu, S.; Satala, C.-B.; Ghiga, D.; Neamtu, M.H.; Voth, V.; Liebrich, M.; Suciu, H. Histopathological Gap in Aortic Diseases: A Prospective Analysis. Int. J. Mol. Sci. 2023, 24, 15470. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.K.; Davis, G.E. Extracellular Matrix Remodeling in Vascular Disease: Defining Its Regulators and Pathological Influence. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1599–1616. [Google Scholar] [CrossRef]
- Cho, M.J.; Lee, M.-R.; Park, J.-G. Aortic aneurysms: Current pathogenesis and therapeutic targets. Exp. Mol. Med. 2023, 55, 2519–2530. [Google Scholar] [CrossRef]
- Bishop, J.E.; Lindahl, G. Regulation of cardiovascular collagen synthesis by mechanical load. Cardiovasc. Res. 1999, 42, 27–44. [Google Scholar] [CrossRef]
- Babici, D.; Kudej, R.K.; McNulty, T.; Zhang, J.; Oydanich, M.; Berkman, T.; Nishimura, K.; Bishop, S.P.; Vatner, D.E.; Vatner, S.F. Mechanisms of increased vascular stiffness down the aortic tree in aging, premenopausal female monkeys. Am. J. Physiol.-Heart Circ. Physiol. 2020, 319, H222–H234. [Google Scholar] [CrossRef]
- Zieman, S.J.; Melenovsky, V.; Kass, D.A. Mechanisms, Pathophysiology, and Therapy of Arterial Stiffness. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 932–943. [Google Scholar] [CrossRef]
- Wiebe, D.S.; Omelyanchuk, N.A.; Mukhin, A.M.; Grosse, I.; Lashin, S.A.; Zemlyanskaya, E.V.; Mironova, V.V. Fold-Change-Specific Enrichment Analysis (FSEA): Quantification of Transcriptional Response Magnitude for Functional Gene Groups. Genes 2020, 11, 434. [Google Scholar] [CrossRef]
- Signorile, A.; De Rasmo, D. Mitochondrial Complex I, a Possible Sensible Site of cAMP Pathway in Aging. Antioxidants 2023, 12, 221. [Google Scholar] [CrossRef]
- Bedi, M.; Ray, M.; Ghosh, A. Active mitochondrial respiration in cancer: A target for the drug. Mol. Cell. Biochem. 2022, 477, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Hock, D.H.; Robinson, D.R.L.; Stroud, D.A. Blackout in the powerhouse: Clinical phenotypes associated with defects in the assembly of OXPHOS complexes and the mitoribosome. Biochem. J. 2020, 477, 4085–4132. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, D.; Zhong, Q.; Zou, X.; Liu, Z.; Long, H.; Wei, J.; Li, X.; Dai, F. The role of zyxin in signal transduction and its relationship with diseases. Front. Mol. Biosci. 2024, 11, 1371549. [Google Scholar] [CrossRef]
- Ghosh, S.; Kollar, B.; Nahar, T.; Suresh Babu, S.; Wojtowicz, A.; Sticht, C.; Gretz, N.; Wagner, A.H.; Korff, T.; Hecker, M. Loss of the Mechanotransducer Zyxin Promotes a Synthetic Phenotype of Vascular Smooth Muscle Cells. J. Am. Heart Assoc. 2015, 4, e001712. [Google Scholar] [CrossRef]
- Kaufman, C.S.; Butler, M.G. Mutation in TNXB gene causes moderate to severe Ehlers-Danlos syndrome. World J. Med. Genet. 2016, 6, 17–21. [Google Scholar] [CrossRef]
- Zweers, M.C.; Bristow, J.; Steijlen, P.M.; Dean, W.B.; Hamel, B.C.; Otero, M.; Kucharekova, M.; Boezeman, J.B.; Schalkwijk, J. Haploinsufficiency of TNXB Is Associated with Hypermobility Type of Ehlers-Danlos Syndrome. Am. J. Hum. Genet. 2003, 73, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.L.; Chen, J.; Neptune, E.R.; Judge, D.P.; Podowski, M.; Holm, T.; Meyers, J.; Leitch, C.C.; Katsanis, N.; Sharifi, N.; et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005, 37, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Schepers, D.; Tortora, G.; Morisaki, H.; MacCarrick, G.; Lindsay, M.; Liang, D.; Mehta, S.G.; Hague, J.; Verhagen, J.; van de Laar, I.; et al. A mutation update on the LDS-associated genes TGFB2/3 and SMAD2/3. Hum. Mutat. 2018, 39, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.-Q.; Kwartler, C.S.; Byanova, K.L.; Pham, J.; Gong, L.; Prakash, S.K.; Huang, J.; Kamm, K.E.; Stull, J.T.; Sweeney, H.L.; et al. Rare, Nonsynonymous Variant in the Smooth Muscle-Specific Isoform of Myosin Heavy Chain, MYH11, R247C, Alters Force Generation in the Aorta and Phenotype of Smooth Muscle Cells. Circ. Res. 2012, 110, 1411–1422. [Google Scholar] [CrossRef]
- Suresh Babu, S.; Wojtowicz, A.; Freichel, M.; Birnbaumer, L.; Hecker, M.; Cattaruzza, M. Mechanism of Stretch-Induced Activation of the Mechanotransducer Zyxin in Vascular Cells. Sci. Signal. 2012, 5, ra91. [Google Scholar] [CrossRef]
- Derbyshire, E.R.; Marletta, M.A. Structure and Regulation of Soluble Guanylate Cyclase. Annu. Rev. Biochem. 2012, 81, 533–559. [Google Scholar] [CrossRef]
- Renard, M.; Callewaert, B.; Baetens, M.; Campens, L.; MacDermot, K.; Fryns, J.-P.; Bonduelle, M.; Dietz, H.C.; Gaspar, I.M.; Cavaco, D.; et al. Novel MYH11 and ACTA2 mutations reveal a role for enhanced TGFβ; signaling in FTAAD. Int. J. Cardiol. 2013, 165, 314–321. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, W.; Liu, S.; Song, B.; Xie, L.; Liu, R. Does False Lumen Thrombosis Lead to Better Outcomes in Patients with Aortic Dissection: A Meta-Analysis and Systematic Review. Heart Surg. Forum 2023, 26, E628–E638. [Google Scholar] [CrossRef] [PubMed]
- Kozai, Y.; Watanabe, S.; Yonezawa, M.; Itani, Y.; Inoue, T.; Takasu, J.; Masuda, Y. Long-Term Prognosis of Acute Aortic Dissection With Medical Treatment: A Survey of 263 Unoperated Patients. Jpn. Circ. J. 2001, 65, 359–363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pataky, M.W.; Nair, K.S. Too much of a good thing: Excess exercise can harm mitochondria. Cell Metab. 2021, 33, 847–848. [Google Scholar] [CrossRef] [PubMed]
- Alkazemi, D.; Rahman, A.; Habra, B. Alterations in glutathione redox homeostasis among adolescents with obesity and anemia. Sci. Rep. 2021, 11, 3034. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P.; et al. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021, 7, 193. [Google Scholar] [CrossRef]
- Xu, S.; Han, X.; Wang, X.; Yu, Y.; Qu, C.; Liu, X.; Yang, B. The role of oxidative stress in aortic dissection: A potential therapeutic target. Front. Cardiovasc. Med. 2024, 11, 1410477. [Google Scholar] [CrossRef]
- Shi, C.; Zhou, X.; Yang, W.; Wu, J.; Bai, M.; Zhang, Y.; Zhao, W.; Yang, H.; Nagai, A.; Yin, M.; et al. Proteomic Analysis of Plasma-Derived Extracellular Vesicles From Mice With Echinococcus granulosus at Different Infection Stages and Their Immunomodulatory Functions. Front. Cell. Infect. Microbiol. 2022, 12, 805010. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal Research: Reporting in vivo Experiments—The ARRIVE Guidelines. J. Cereb. Blood Flow Metab. 2011, 31, 991–993. [Google Scholar] [CrossRef]
- Konno, R.; Ishikawa, M.; Nakajima, D.; Endo, Y.; Ohara, O.; Kawashima, Y. Universal Pretreatment Development for Low-input Proteomics Using Lauryl Maltose Neopentyl Glycol. Mol. Cell. Proteom. 2024, 23, 100745. [Google Scholar] [CrossRef] [PubMed]

| GO Term | Fold Change |
|---|---|
| Extracellular region | −0.4–−4.56 |
| Extracellular space | −0.4–−4.56 |
| Extracellular matrix | −0.4–−4.56 |
| Extracellular region part | −0.4–−4.56 |
| Collagen-containing extracellular matrix | −0.4–−4.56 |
| Basement membrane | −0.304–−4.56 |
| Cell projection | −0.304–−0.4 |
| Plasma membrane-bound cell projection | −0.304–−0.4 |
| Nucleosome | −0.178–−0.234 |
| Nuclear nucleosome | −0.178–−0.234 |
| GO Term | Fold-Change |
|---|---|
| Extracellular matrix structural constituent conferring tensile strength | −0.623–−4.56 |
| Serine-type endopeptidase activity | −0.4–−4.56 |
| Enzyme inhibitor activity | −0.4–−4.56 |
| Endopeptidase inhibitor activity | −0.4–−4.56 |
| Extracellular matrix structural constituent | −0.4–−4.56 |
| Glycosaminoglycan binding | −0.4–−4.56 |
| Heparin binding | −0.4–−4.56 |
| Serine-type peptidase activity | −0.4–−4.56 |
| Serine hydrolase activity | −0.4–−4.56 |
| Peptidase inhibitor activity | −0.4–−4.56 |
| GO Term | Fold Change |
|---|---|
| Blood coagulation | −0.623–−4.56 |
| Hemostasis | −0.623–−4.56 |
| Regulation of blood coagulation | −0.623–−4.56 |
| Negative regulation of blood coagulation | −0.623–−4.56 |
| Coagulation | −0.623–−4.56 |
| Regulation of coagulation | −0.623–−4.56 |
| Negative regulation of coagulation | −0.623–−4.56 |
| Regulation of body fluid levels | −0.623–−4.56 |
| Negative regulation of wound healing | −0.623–−4.56 |
| Regulation of hemostasis | −0.623–−4.56 |
| Negative regulation of hemostasis | −0.623–−4.56 |
| Negative regulation of response to wounding | −0.623–−4.56 |
| GO Term | Fold-Change |
|---|---|
| Organellar ribosome | 0.358–4.43 |
| Organellar large ribosomal subunit | 0.358–4.43 |
| Mitochondrial respiratory chain | 0.358–4.43 |
| Mitochondrial matrix | 0.358–4.43 |
| Mitochondrial ribosome | 0.358–4.43 |
| Mitochondrial large ribosomal subunit | 0.358–4.43 |
| Mitochondrial part | 0.358–4.43 |
| Mitochondrial membrane part | 0.358–4.43 |
| Respiratory chain | 0.358–4.43 |
| Inner mitochondrial membrane protein complex | 0.358–4.43 |
| Respiratory chain complex | 0.358–4.43 |
| Oxidoreductase complex | 0.358–4.43 |
| GO ** Term | Fold-Change |
|---|---|
| Cell projection | −0.304–−4.56 |
| Inorganic molecular entity transmembrane transporter activity | −0.304–−0.623 |
| Plasma membrane-bound cell projection | −0.304–−0.4 |
| Response to wounding | −0.304–−0.4 |
| Organelle organization | −0.094–−0.4 |
| Intracellular | −0.094–−0.4 |
| Nucleus | −0.094–−0.4 |
| Cytoplasm | −0.094–−0.4 |
| Organelle | −0.094–−0.4 |
| Membrane-bound organelle | −0.094–−0.4 |
| Intracellular organelle | −0.094–−0.4 |
| Intracellular membrane-bound organelle | −0.094–−0.4 |
| Intracellular part | −0.094–−0.4 |
| Nuclear part | −0.094–−0.4 |
| Cytoplasmic part | −0.094–−0.4 |
| Intracellular organelle part | −0.094–−0.4 |
| Catalytic complex | −0.094–−0.304 |
| Organic cyclic compound binding | −0.094–−0.304 |
| Heterocyclic compound binding | −0.094–−0.178 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomida, S.; Okuhata, H.; Ishima, T.; Nagai, R.; Aizawa, K. Loss of Myh11 K1256 Dysregulates the Extracellular Matrix and Focal Adhesion by Inhibiting Zyxin-Activated Transcription. Int. J. Mol. Sci. 2025, 26, 7853. https://doi.org/10.3390/ijms26167853
Tomida S, Okuhata H, Ishima T, Nagai R, Aizawa K. Loss of Myh11 K1256 Dysregulates the Extracellular Matrix and Focal Adhesion by Inhibiting Zyxin-Activated Transcription. International Journal of Molecular Sciences. 2025; 26(16):7853. https://doi.org/10.3390/ijms26167853
Chicago/Turabian StyleTomida, Shota, Hironori Okuhata, Tamaki Ishima, Ryozo Nagai, and Kenichi Aizawa. 2025. "Loss of Myh11 K1256 Dysregulates the Extracellular Matrix and Focal Adhesion by Inhibiting Zyxin-Activated Transcription" International Journal of Molecular Sciences 26, no. 16: 7853. https://doi.org/10.3390/ijms26167853
APA StyleTomida, S., Okuhata, H., Ishima, T., Nagai, R., & Aizawa, K. (2025). Loss of Myh11 K1256 Dysregulates the Extracellular Matrix and Focal Adhesion by Inhibiting Zyxin-Activated Transcription. International Journal of Molecular Sciences, 26(16), 7853. https://doi.org/10.3390/ijms26167853





