Neurochemical Aspects of the Role of Thirst in Body Fluid Homeostasis and Their Significance in Health and Disease: A Literature Review
Abstract
1. Introduction
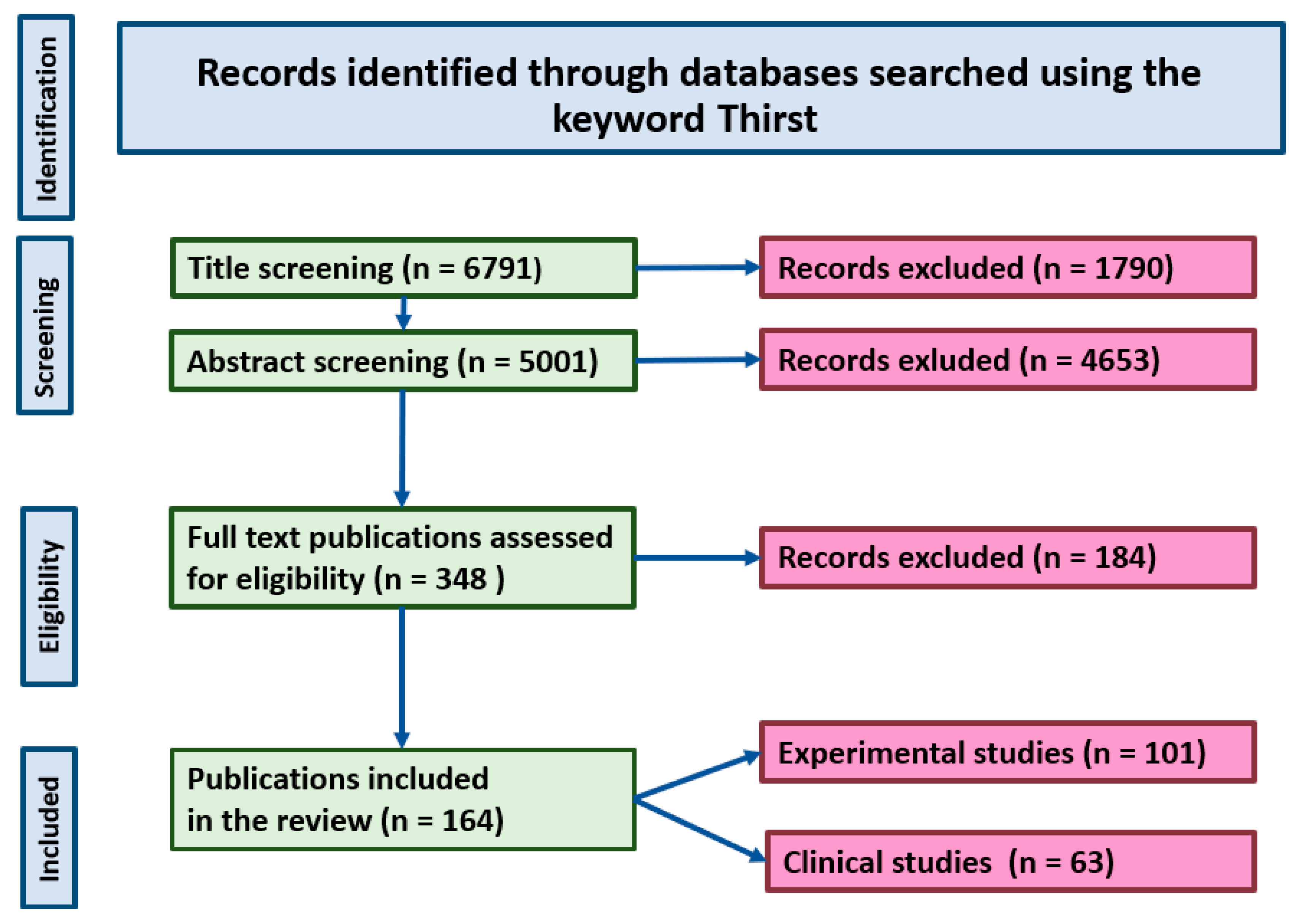
2. Methodology
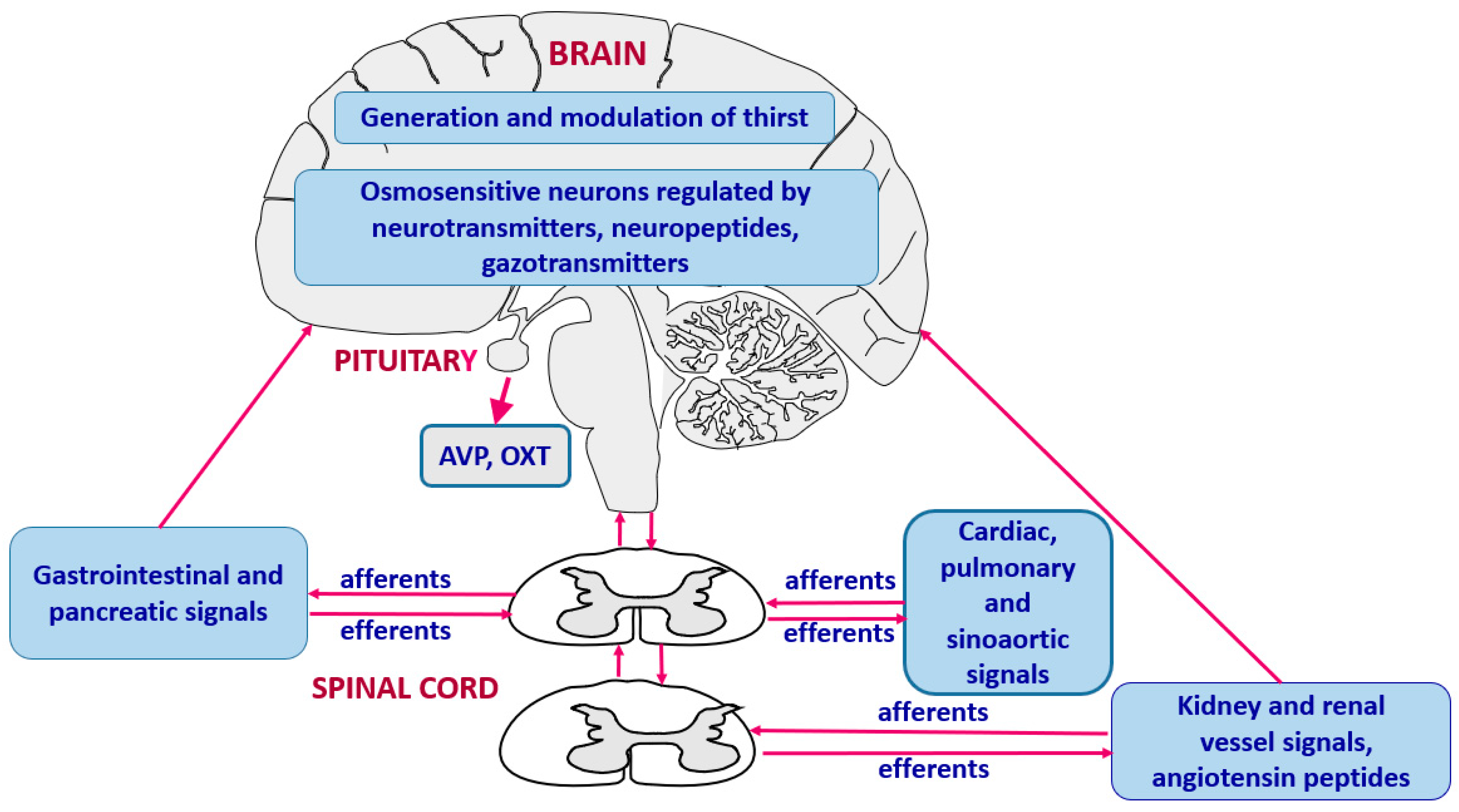
3. Generation of Thirst and Integration of Thirst Signals
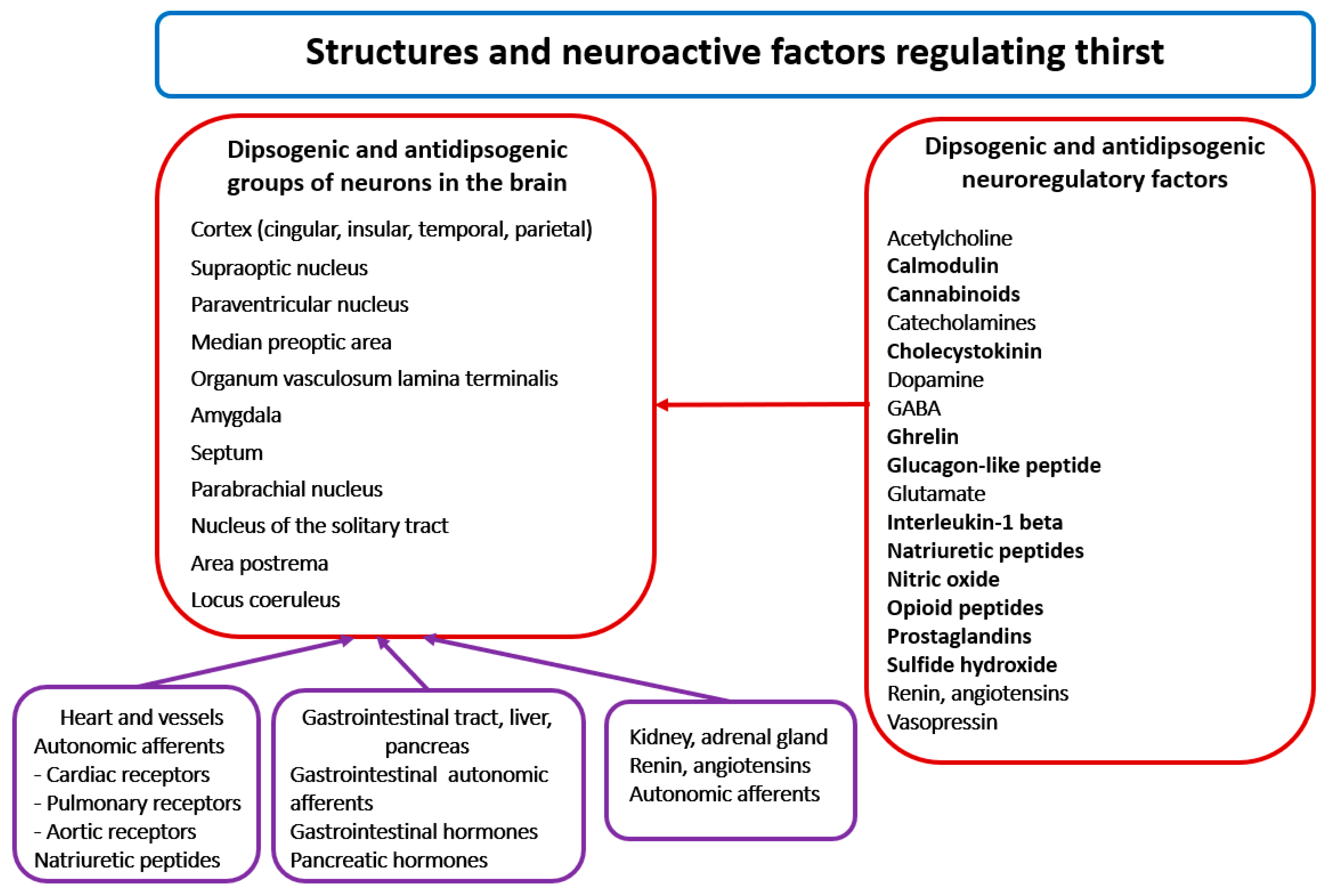
3.1. Neuroanatomy and Neurochemistry of Thirst
3.2. Primary Role of Osmosensitive Neurons in Regulation of Thirst and Sodium Appetite
3.3. Regulation of Thirst by Angiotensin II and Vasopressin
3.4. Signals from the Alimentary Tract
3.5. Gaseous Transmitters, Cytokines and Prostaglandins
3.6. Cardiovascular Regulatory Signals
4. Other Factors Influencing Thirst
4.1. Heat, Fever and Heat Acclimation
4.2. Aging
5. Regulation of Thirst in Pathological Conditions
5.1. Heart Failure
5.2. Diabetes
5.3. Psychogenic Thirst Abnormalities
6. Limitations and Future Directions
7. Summary
8. Conclusions
- 1.
- Thirst is activated during elevation of the osmolality of body fluid, which causes dehydration of osmosensitive neurons located in multiple regions of the brain and in some peripheral organs.
- 2.
- Proper neurogenic and endocrine regulation of thirst is based on multiple micro- and macro-feedback interdependences that allows maintenance of body fluid osmolality with a precision of 1–2%.
- 3.
- Stimulation and inhibition of thirst can be modulated by signals generated in the brain and in the peripheral organs during changes of blood pressure, blood volume, body temperature and digestive processes, especially in situations disturbing homeostatic conditions.
- 4.
- Abnormal regulation of thirst that may be associated with inappropriate salt appetite is observed during hyperthermia, during aging, in heart failure, in diabetes insipidus and diabetes mellitus and in some psychogenic disorders.
- 5.
- The molecular background of the abnormal regulation of thirst under pathological conditions is not yet well recognized. Complexity of these processes may require application of a sophisticated mathematical approach, which may be necessary to define multiple interactions of cellular processes during homeostatic disorders that challenge thirst regulation.
- 6.
- Currently available studies do not allow for correlation of the behavioral symptoms of thirst with molecular processes occurring in cells in the same experimental models. Progress in this field is necessary for elaboration of more effective dipsogenic and antidipsogenic treatments.
- 7.
- Regulation of thirst in human beings is not fully recognized. Future studies should more deeply investigate thirst in patients suffering from the neuropsychiatric, cardiovascular, visceral, metabolic and renal diseases, especially when these diseases occur jointly.
- 8.
- Future studies should elaborate more efficient biomolecular determinants of body hydration, which would correspond to the intensity of thirst and allow evaluation of thirst in human patients who are not able to communicate their water needs.
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Anderson, C.R.; Houpt, T.R. Hypertonic and hypovolemic stimulation of thirst in pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1990, 258 Pt 2, R149–R154. [Google Scholar] [CrossRef]
- Fitzsimons, J.T. The physiological basis of thirst. Kidney Int. 1976, 10, 3–11. [Google Scholar] [CrossRef][Green Version]
- Pool, A.H.; Wang, T.; Stafford, D.A.; Chance, R.K.; Lee, S.; Ngai, J.; Oka, Y. The cellular basis of distinct thirst modalities. Nature 2020, 588, 112–117. [Google Scholar] [CrossRef]
- Suwa, Y.; Kunimatsu, J.; Kamata, A.; Matsumoto, M.; Yamada, H.A. Method for Evaluating Hunger and Thirst in Monkeys by Measuring Blood Ghrelin and Osmolality Levels. eNeuro 2024, 11, ENEURO.0481-23.2024. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimons, J.T. Angiotensin, thirst, and sodium appetite. Physiol. Rev. 1998, 78, 583–686. [Google Scholar] [CrossRef] [PubMed]
- Key, B.; Brown, D.J. Making sense of feelings. Neurosci. Conscious. 2024, 2024, niae034. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Giersch, G.E.W.; Dunn, L.; Fiol, A.; Muñoz, C.X.; Lee, E.C. Inputs to Thirst and Drinking during Water Restriction and Rehydration. Nutrients 2020, 12, 2554. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Rodrigues, J.; de Castro, M.; Elias, L.L.; Valença, M.M.; McCann, S.M. Neuroendocrine control of body fluid metabolism. Physiol. Rev. 2004, 84, 169–208. [Google Scholar] [CrossRef]
- Fitzsimons, J.T. Bengt Andersson’s pioneering demonstration of the hypothalamic “drinking area” and the subsequent osmoreceptor/sodium receptor controversy. Acta Physiol. Scand. Suppl. 1989, 583, 15–25. [Google Scholar] [PubMed]
- Gardner, J.W. Death by water intoxication. Mil. Med. 2002, 167, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Hawken, E.R.; Crookall, J.M.; Reddick, D.; Millson, R.C.; Milev, R.; Delva, N. Mortality over a 20-year period in patients with primary polydipsia associated with schizophrenia: A retrospective study. Schizophr. Res. 2009, 107, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Andersson, B.; McCann, S.M. A further study of polydipsia evoked by hypothalamic stimulation in the goat. Acta Physiol. Scand. 1955, 33, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Franzé, G.M.; Andrade, C.A.; Gasparini, S.; De Luca, L.A., Jr.; De Paula, P.M.; Colombari, D.S.; Colombari, E.; Menani, J.V. Importance of the central nucleus of the amygdala on sodium intake caused by deactivation of lateral parabrachial nucleus. Brain Res. 2015, 1625, 238–245. [Google Scholar] [CrossRef]
- Becker, C.A.; Flaisch, T.; Renner, B.; Schupp, H.T. From Thirst to Satiety: The Anterior Mid-Cingulate Cortex and Right Posterior Insula Indicate Dynamic Changes in Incentive Value. Front. Hum. Neurosci. 2017, 11, 234. [Google Scholar] [CrossRef]
- Callera, J.C.; Oliveira, L.B.; Barbosa, S.P.; Colombari, D.S.; De Luca, L.A., Jr.; Menani, J.V. GABA(A) receptor activation in the lateral parabrachial nucleus induces water and hypertonic NaCl intake. Neuroscience 2005, 134, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska-Sadowska, E.; Sobocińska, J.; Sadowski, B.; Kosowski, S. Osmotic thirst suppression elicited by electrical stimulation of the basal forebrain in the dog. Arch. Int. Physiol. Biochim. 1978, 86, 169–173. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E.; Cudnoch-Jedrzejewska, A.; Ufnal, M.; Zera, T. Brain and cardiovascular diseases: Common neurogenic background of cardiovascular, metabolic and inflammatory diseases. J. Physiol. Pharmacol. 2010, 61, 509–521. [Google Scholar] [PubMed]
- Fitts, D.A.; Freece, J.A.; Van Bebber, J.E.; Zierath, D.K.; Bassett, J.E. Effects of forebrain circumventricular organ ablation on drinking or salt appetite after sodium depletion or hypernatremia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1325–R1334. [Google Scholar] [CrossRef]
- Stricker, E.M.; Craver, C.F.; Curtis, K.S.; Peacock-Kinzig, K.A.; Sved, A.F.; Smith, J.C. Osmoregulation in water-deprived rats drinking hypertonic saline: Effect of area postrema lesions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R831–R842. [Google Scholar] [CrossRef]
- Matsuda, T.; Hiyama, T.Y.; Kobayashi, K.; Kobayashi, K.; Noda, M. Distinct CCK-positive SFO neurons are involved in persistent or transient suppression of water intake. Nat. Commun. 2020, 11, 5692. [Google Scholar] [CrossRef]
- Fitzsimons, J.T.; Szczepańska-Sadowska, E. Drinking and antidiuresis elicited by isoprenaline in the dog. J. Physiol. 1974, 239, 251–267. [Google Scholar] [CrossRef]
- Leibowitz, S.F. Hypothalamic alpha-and beta-adrenergic systems regulate both thirst and hunger in the rat. Proc. Natl. Acad. Sci. USA 1971, 68, 332–334. [Google Scholar] [CrossRef]
- Hollis, J.H.; McKinley, M.J.; D’Souza, M.; Kampe, J.; Oldfield, B.J. The trajectory of sensory pathways from the lamina terminalis to the insular and cingulate cortex: A neuroanatomical framework for the generation of thirst. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1390–R1401. [Google Scholar] [CrossRef]
- Zhao, Z.; Soria-Gómez, E.; Varilh, M.; Covelo, A.; Julio-Kalajzić, F.; Cannich, A.; Castiglione, A.; Vanhoutte, L.; Duveau, A.; Zizzari, P.; et al. A Novel Cortical Mechanism for Top-Down Control of Water Intake. Curr. Biol. 2020, 30, 4789–4798.e4. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.K. The periventricular anteroventral third ventricle (AV3V): Its relationship with the subfornical organ and neural systems involved in maintaining body fluid homeostasis. Brain Res. Bull. 1985, 15, 595–601. [Google Scholar] [CrossRef]
- Johnson, A.K.; Thunhorst, R.L. The neuroendocrinology of thirst and salt appetite: Visceral sensory signals and mechanisms of central integration. Front. Neuroendocrinol. 1997, 18, 292–353. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.M.; Bazzino, P.; Hurh, S.J.; Konanur, V.R.; Roitman, J.D.; Roitman, M.F. Thirst recruits phasic dopamine signaling through subfornical organ neurons. Proc. Natl. Acad. Sci. USA 2020, 117, 30744–30754. [Google Scholar] [CrossRef]
- Denton, D.A.; McKinley, M.J.; Weisinger, R.S. Hypothalamic integration of body fluid regulation. Proc. Natl. Acad. Sci. USA 1996, 93, 7397–7404. [Google Scholar] [CrossRef]
- Denton, D.; Shade, R.; Zamarippa, F.; Egan, G.; Blair-West, J.; McKinley, M.; Lancaster, J.; Fox, P. Neuroimaging of genesis and satiation of thirst and an interoceptor-driven theory of origins of primary consciousness. Proc. Natl. Acad. Sci. USA 1999, 96, 5304–5309. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.J.; Bowala, T.K.; Gavrilescu, M.; Phillips, P.A.; McKinley, M.J.; McAllen, R.M.; Denton, D.A.; Egan, G.F. Cortical activation and lamina terminalis functional connectivity during thirst and drinking in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R623–R631. [Google Scholar] [CrossRef]
- Saker, P.; Farrell, M.J.; Egan, G.F.; McKinley, M.J.; Denton, D.A. Influence of anterior midcingulate cortex on drinking behavior during thirst and following satiation. Proc. Natl. Acad. Sci. USA 2018, 115, 786–791. [Google Scholar] [CrossRef]
- Bichet, D.G. Vasopressin and the Regulation of Thirst. Ann Nutr. Metab. 2018, 72 (Suppl. 2), 3–7. [Google Scholar] [CrossRef]
- Wang, J.; Lv, F.; Yin, W.; Gao, Z.; Liu, H.; Wang, Z.; Sun, J. The organum vasculosum of the lamina terminalis and subfornical organ: Regulation of thirst. Front. Neurosci. 2023, 17, 1223836. [Google Scholar] [CrossRef]
- Kinsman, B.J.; Simmonds, S.S.; Browning, K.N.; Wenner, M.M.; Farquhar, W.B.; Stocker, S.D. Integration of Hypernatremia and Angiotensin II by the Organum Vasculosum of the Lamina Terminalis Regulates Thirst. J. Neurosci. 2020, 40, 2069–2079. [Google Scholar] [CrossRef]
- Hiyama, T.Y. Brain sodium sensing for regulation of thirst, salt appetite, and blood pressure. Physiol. Rep. 2024, 12, e15970. [Google Scholar] [CrossRef]
- Watanabe, E.; Fujikawa, A.; Matsunaga, H.; Yasoshima, Y.; Sako, N.; Yamamoto, T.; Saegusa, C.; Noda, M. Nav2/NaG channel is involved in control of salt-intake behavior in the CNS. J. Neurosci. 2000, 20, 7743–7751. [Google Scholar] [CrossRef] [PubMed]
- Marciante, A.B.; Wang, L.A.; Farmer, G.E.; Cunningham, J.T. Selectively Inhibiting the Median Preoptic Nucleus Attenuates Angiotensin II and Hyperosmotic-Induced Drinking Behavior and Vasopressin Release in Adult Male Rats. Fitts eNeuro 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Ciura, S.; Liedtke, W.; Bourque, C.W. Hypertonicity sensing in organum vasculosum lamina terminalis neurons: A mechanical process involving TRPV1 but not TRPV4. J. Neurosci. 2011, 31, 14669–14676. [Google Scholar] [CrossRef]
- Sladek, C.D.; Johnson, A.K. Integration of thermal and osmotic regulation of water homeostasis: The role of TRPV channels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R669–R678. [Google Scholar] [CrossRef] [PubMed]
- Zaelzer, C.; Hua, P.; Prager-Khoutorsky, M.; Ciura, S.; Voisin, D.L.; Liedtke, W.; Bourque, C.W. ΔN-TRPV1: A Molecular Co-detector of Body Temperature and Osmotic Stress. Cell Rep. 2015, 13, 23–30. [Google Scholar] [CrossRef]
- Tsushima, H.; Mori, M. Antidipsogenic effects of a TRPV4 agonist, 4alpha-phorbol 12,13-didecanoate, injected into the cerebroventricle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1736–R1741. [Google Scholar] [CrossRef]
- Zimmerman, C.A.; Lin, Y.C.; Leib, D.E.; Guo, L.; Huey, E.L.; Daly, G.E.; Chen, Y.; Knight, Z.A. Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature 2016, 537, 680–684. [Google Scholar] [CrossRef]
- Northeast, R.C.; Chrobok, L.; Hughes, A.T.L.; Petit, C.; Piggins, H.D. Keeping time in the lamina terminalis: Novel oscillator properties of forebrain sensory circumventricular organs. FASEB J. 2020, 34, 974–987. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.J.; Egan, G.F.; Zamarripa, F.; Shade, R.; Blair-West, J.; Fox, P.; Denton, D.A. Unique, common, and interacting cortical correlates of thirst and pain. Proc. Natl. Acad. Sci. USA 2006, 103, 2416–2421. [Google Scholar] [CrossRef]
- De Castro, E.; Silva, E.; Fregoneze, J.B.; Johnson, A.K. Corticotropin-releasing hormone in the lateral parabrachial nucleus inhibits sodium appetite in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1136–R1141. [Google Scholar] [CrossRef]
- Noda, M.; Matsuda, T. Central regulation of body fluid homeostasis. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2022, 98, 283–324. [Google Scholar] [CrossRef]
- Hurley, S.W.; Johnson, A.K. Dissociation of thirst and sodium appetite in the furo/cap model of extracellular dehydration and a role for N-methyl-D-aspartate receptors in the sensitization of sodium appetite. Behav. Neurosci. 2013, 127, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Jalowiec, J.E.; Stricker, E.M. Sodium appetite in rats after apparent recovery from acute sodium deficiency. J. Comp. Physiol. Psychol. 1970, 73, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Takamata, A.; Mack, G.W.; Gillen, C.M.; Nadel, E.R. Sodium appetite, thirst, and body fluid regulation in humans during rehydration without sodium replacement. Am J Physiol. 1994, 266 Pt 2, R1493–R1502. [Google Scholar] [CrossRef]
- Epstein, A.N.; Fitzsimons, J.T.; Rolls, B.J. Drinking induced by injection of angiotensin into the brain of the rat. J. Physiol. 1970, 210, 457–474. [Google Scholar] [CrossRef]
- Fitzsimons, J.T. The effect on drinking of peptide precursors and of shorter chain peptide fragments of angiotensin II injected into the rat’s diencephalon. J. Physiol. 1971, 214, 295–303. [Google Scholar] [CrossRef]
- Beresford, M.J.; Fitzsimons, J.T. Intracerebroventricular angiotensin II-induced thirst and sodium appetite in rat are blocked by the AT1 receptor antagonist, Losartan (DuP 753), but not by the AT2 antagonist, CGP 42112B. Exp. Physiol. 1992, 77, 761–764. [Google Scholar] [CrossRef]
- Fitzsimons, J.T.; Kucharczyk, J. Drinking and haemodynamic changes induced in the dog by intracranial injection of components of the renin-angiotensin system. J. Physiol. 1978, 276, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Cooney, A.S.; Fitzsimons, J.T. The effect of the putative AT2 agonist, p-aminophenylalanine6 angiotensin II, on thirst and sodium appetite in rats. Exp. Physiol. 1993, 78, 767–774. [Google Scholar] [CrossRef]
- Mecawi, A.S.; Araujo, I.G.; Rocha, F.F.; Coimbra, T.M.; Antunes-Rodrigues, J.; Reis, L.C. Ontogenetic role of angiontensin-converting enzyme in rats: Thirst and sodium appetite evaluation. Physiol. Behav. 2010, 99, 118–124. [Google Scholar] [CrossRef]
- Fitzsimons, J.T.; Moore-Gillon, M.J. Drinking and antidiuresis in response to reductions in venous return in the dog: Neural and endocrine mechanisms. J. Physiol. 1980, 308, 403–416. [Google Scholar] [CrossRef]
- Fitzsimons, J.T.; Moore-Gillon, M.J. Renin-dependence of drinking induced by partial aortic obstruction in the dog. J. Physiol. 1981, 320, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Weisinger, R.S.; Blair-West, J.R.; Denton, D.A.; Tarjan, E. Central administration of atrial natriuretic peptide suppresses sodium and water intake of sheep. Brain Res. 1992, 579, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Szczepanska-Sadowska, E.; Kozlowski, S.; Sobocińska, J. Blood antidiuretic hormone level and osmotic reactivity of thirst mechanism in dogs. Am. J. Physiol. 1974, 227, 766–770. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E.; Sobocinska, J.; Kozłowski, S. Thirst impairment elicited by intraventricular administration of vasopressin antagonists. Peptides 1987, 8, 1003–1009. [Google Scholar] [CrossRef]
- Greenberg, A.; Verbalis, J.G. Vasopressin receptor antagonists. Kidney Int. 2006, 69, 2124–2130. [Google Scholar] [CrossRef]
- Smith, D.; Moore, K.; Tormey, W.; Baylis, P.H.; Thompson, C.J. Downward resetting of the osmotic threshold for thirst in patients with SIADH. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E1019–E1023. [Google Scholar] [CrossRef]
- Verbalis, J.G.; Greenberg, A.; Burst, V.; Haymann, J.P.; Johannsson, G.; Peri, A.; Poch, E.; Chiodo, J.A., 3rd; Dave, J. Diagnosing and Treating the Syndrome of Inappropriate Antidiuretic Hormone Secretion. Am. J. Med. 2016, 129, e9–e537. [Google Scholar] [CrossRef] [PubMed]
- Bichet, D.G. Regulation of Thirst and Vasopressin Release. Annu. Rev. Physiol. 2019, 81, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Nakamura-Utsunomiya, A. Autoimmunity related to anti-Nax and anti-ZSCAN1 autoantibodies in adipsic hypernatremia. Endocr. J. 2024, 71, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Augustine, V.; Ebisu, H.; Zhao, Y.; Lee, S.; Ho, B.; Mizuno, G.O.; Tian, L.; Oka, Y. Temporally and Spatially Distinct Thirst Satiation Signals. Neuron 2019, 103, 242–249.e4. [Google Scholar] [CrossRef]
- Zocchi, D.; Wennemuth, G.; Oka, Y. The cellular mechanism for water detection in the mammalian taste system. Nat. Neurosci. 2017, 20, 927–933. [Google Scholar] [CrossRef]
- Geerling, J.C.; Loewy, A.D. Central regulation of sodium appetite. Exp. Physiol. 2008, 93, 177–209. [Google Scholar] [CrossRef]
- Hoffmann, M.L.; DenBleyker, M.; Smith, J.C.; Stricker, E.M. Inhibition of thirst when dehydrated rats drink water or saline. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1199–R1207. [Google Scholar] [CrossRef]
- Peyrot des Gachons, C.; Avrillier, J.; Gleason, M.; Algarra, L.; Zhang, S.; Mura, E.; Nagai, H.; Breslin, P.A. Oral Cooling and Carbonation Increase the Perception of Drinking and Thirst Quenching in Thirsty Adults. PLoS ONE 2016, 11, e0162261. [Google Scholar] [CrossRef]
- Allen, W.E.; Chen, M.Z.; Pichamoorthy, N.; Tien, R.H.; Pachitariu, M.; Luo, L.; Deisseroth, K. Thirst regulates motivated behavior through modulation of brain wide neural population dynamics. Science 2019, 364, 253. [Google Scholar] [CrossRef]
- Menani, J.V.; Johnson, A.K. Cholecystokinin actions in the parabrachial nucleus: Effects on thirst and salt appetite. Am. J. Physiol. 1998, 275 Pt 2, R1431–R1437. [Google Scholar] [CrossRef]
- Matsuda, T.; Kobayashi, K.; Kobayashi, K.; Noda, M. Two parabrachial Cck neurons involved in the feedback control of thirst or salt appetite. Cell Rep. 2024, 43, 113619. [Google Scholar] [CrossRef] [PubMed]
- McKay, N.J.; Galante, D.L.; Daniels, D. Endogenous glucagon-like peptide-1 reduces drinking behavior and is differentially engaged by water and food intakes in rats. J. Neurosci. 2014, 34, 16417–16423. [Google Scholar] [CrossRef] [PubMed]
- Augustine, V.; Gokce, S.K.; Lee, S.; Wang, B.; Davidson, T.J.; Reimann, F.; Gribble, F.; Deisseroth, K.; Lois, C.; Oka, Y. Hierarchical neural architecture underlying thirst regulation. Nature 2018, 555, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Volcko, K.L.; Brakey, D.J.; McNamara, T.E.; Meyer, M.J.; McKay, N.J.; Santollo, J.; Daniels, D. Control of water intake by a pathway from the nucleus of the solitary tract to the paraventricular hypothalamic nucleus. Appetite 2022, 172, 105943. [Google Scholar] [CrossRef]
- Nakhleh, A.; Shehadeh, N.; Mansour, B. GLP-1 receptor agonists may enhance the effects of desmopressin in individuals with AVP deficiency: A case series and proposed mechanism. Pituitary 2024, 27, 731–736. [Google Scholar] [CrossRef]
- Samson, W.K.; White, M.M.; Price, C.; Ferguson, A.V. Obestatin acts in brain to inhibit thirst. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R637–R643. [Google Scholar] [CrossRef]
- Mietlicki, E.G.; Daniels, D. Ghrelin reduces hypertonic saline intake in a variety of natriorexigenic conditions. Exp. Physiol. 2011, 96, 1072–1083. [Google Scholar] [CrossRef]
- Crabtree, D.R.; Chambers, E.S.; Hardwick, R.M.; Blannin, A.K. The effects of high-intensity exercise on neural responses to images of food. Am. J. Clin. Nutr. 2014, 99, 258–267. [Google Scholar] [CrossRef]
- Vestergaard, E.T.; Møller, N.; Andersen, R.F.; Rittig, S.; Jørgensen, J.O.L. Acute intravenous acyl ghrelin infusion induces thirst but does not affect sodium excretion: Two randomized, double-blind, placebo-controlled crossover studies in hypopituitary patients. Eur. J. Endocrinol. 2019, 181, 23–30. [Google Scholar] [CrossRef]
- Liu, H.; Terrell, M.; Summy-Long, J.Y.; Kadekaro, M. Drinking and blood pressure responses to central injection of L-NAME in conscious rats. Physiol. Behav. 1996, 59, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Calapai, G.; Squadrito, F.; Altavilla, D.; Zingarelli, B.; Campo, G.M.; Cilia, M.; Caputi, A.P. Evidence that nitric oxide modulates drinking behaviour. Neuropharmacology 1992, 31, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.D.; Rowland, N.E. Effects of L-arginine on angiotensin II-related water and salt intakes. Physiol. Behav. 1998, 63, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Coletti, R.; Almeida-Pereira, G.; Elias, L.L.; Antunes-Rodrigues, J. Effects of hydrogen sulfide (H2S) on water intake and vasopressin and oxytocin secretion induced by fluid deprivation. Horm. Behav. 2015, 67, 12–20. [Google Scholar] [CrossRef]
- Luz, P.A.; Andrade, L.; Miranda, N.; Pereira, V.; Fregoneze, J.; De Castro e Silva, E. Inhibition of water intake by the central administration of IL-1beta in rats: Role of the central opioid system. Neuropeptides 2006, 40, 85–94. [Google Scholar] [CrossRef]
- Osaka, T.; Kannan, H.; Kawano, S.; Ueta, Y.; Yamashita, H. Intraperitoneal administration of recombinant human interleukin-1 beta inhibits osmotic thirst in the rat. Physiol. Behav. 1992, 51, 1267–1270. [Google Scholar] [CrossRef]
- Andersson, B.; Leksell, L.G.; Rundgren, M. Physiological and pharmacological aspects of the control of hunger and thirst. Annu. Rev. Nutr. 1982, 2, 73–89. [Google Scholar] [CrossRef]
- Fitzsimons, J.T. Drinking by rats depleted of body fluid without increase in osmotic pressure. J. Physiol. 1961, 159, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimons, J.T.; Elfont, R.M. Angiotensin does contribute to drinking induced by caval ligation in rat. Am. J. Physiol. 1982, 243, R558–R562. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E.; Ruka, M.; Sobocinska, J.; Kozlowski, S. Suppression of thirst in dogs with arteriovenous fistula. Arch. Int. Physiol. Biochim. 1981, 89, 269–273. [Google Scholar] [CrossRef]
- Moore-Gillon, M.J.; Fitzsimons, J.T. Pulmonary vein-atrial junction stretch receptors and the inhibition of drinking. Am. J. Physiol. 1982, 242, R452–R457. [Google Scholar] [CrossRef]
- Stocker, S.D.; Stricker, E.M.; Sved, A.F. Arterial baroreceptors mediate the inhibitory effect of acute increases in arterial blood pressure on thirst. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R1718–R1729. [Google Scholar] [CrossRef]
- Coble, J.P.; Grobe, J.L.; Johnson, A.K.; Sigmund, C.D. Mechanisms of brain renin angiotensin system-induced drinking and blood pressure: Importance of the subfornical organ. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R238–R249. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E. Plasma ADH increase and thirst suppression elicited by preoptic heating in the dog. Am. J. Physiol. 1974, 226, 155–161. [Google Scholar] [CrossRef]
- Szczepańska-Sadowska, E. Osmotic thirst suppression during 2,4-dinitrophenol (DNP) hyperthermia in the dog. Pflugers Arch. 1975, 355, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska-Sadowska, E.; Sobocińska, J.; Kozłowski, S. Thirst and renal excretion of water and electrolytes during pyrogen fever in dogs. Arch. Int. Physiol. Biochim. 1979, 87, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Focà, A.; Matera, G.; Mastroeni, P.; Caputi, A.P. Evidence that prostaglandins within preoptic area (POA) may mediate the antidipsogenic effect of Escherichia coli endotoxin in the rat. Circ. Shock 1985, 17, 137–145. [Google Scholar]
- Nava, F.; Calapai, G.; Facciolà, G.; Cuzzocrea, S.; Giuliani, G.; De Sarro, A.; Caputi, A.P. Melatonin effects on inhibition of thirst and fever induced by lipopolysaccharide in rat. Eur. J. Pharmacol. 1997, 331, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Nava, F.; Carta, G. Repeated lipopolysaccharide administration produces tolerance to anorexia and fever but not to inhibition of thirst in rat. Int. J. Immunopharmacol. 2000, 22, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, V.; Agrewala, J.N.; Kulkarni, S.K. Role of centrally administered melatonin and inhibitors of COX and NOS in LPS-induced hyperthermia and adipsia. Prostaglandins Leukot. Essent. Fatty Acids 1999, 60, 249–253. [Google Scholar] [CrossRef]
- Barney, C.C.; Schanhals, E.M.; Grobe, J.L.; Andresen, B.T.; Traver, M. Heat acclimation and thirst in rats. Physiol. Rep. 2015, 3, e12642. [Google Scholar] [CrossRef]
- Rosinger, A.Y.; Bethancourt, H.J.; Swanson, Z.S.; Lopez, K.; Kenney, W.L.; Huanca, T.; Conde, E.; Nzunza, R.; Ndiema, E.; Braun, D.R.; et al. Cross-cultural variation in thirst perception in hot-humid and hot-arid environments: Evidence from two small-scale populations. Am. J. Hum. Biol. 2022, 34, e23715. [Google Scholar] [CrossRef]
- Taim, B.C.; Suppiah, H.T.; Wee, J.; Lee, M.; Lee, J.K.W.; Chia, M. Palatable Flavoured Fluids without Carbohydrates and Electrolytes Do Not Enhance Voluntary Fluid Consumption in Male Collegiate Basketball Players in the Heat. Nutrients 2021, 13, 4197. [Google Scholar] [CrossRef]
- Mack, G.W.; Weseman, C.A.; Langhans, G.W.; Scherzer, H.; Gillen, C.M.; Nadel, E.R. Body fluid balance in dehydrated healthy older men: Thirst and renal osmoregulation. J. Appl. Physiol. 1994, 76, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P.A.; Bretherton, M.; Johnston, C.I.; Gray, L. Reduced osmotic thirst in healthy elderly men. Am. J. Physiol. 1991, 261 Pt 2, R166–R171. [Google Scholar] [CrossRef]
- Stachenfeld, N.S.; DiPietro, L.; Nadel, E.R.; Mack, G.W. Mechanism of attenuated thirst in aging: Role of central volume receptors. Am. J. Physiol. 1997, 272 Pt 2, R148–R157. [Google Scholar] [CrossRef]
- Begg, D.P.; Sinclair, A.J.; Weisinger, R.S. Impaired Fluid Intake, but Not Sodium Appetite, in Aged Rats Is Mediated by the Cyclooxygenase-Prostaglandin E2 Pathway. Front. Aging Neurosci. 2020, 12, 19. [Google Scholar] [CrossRef]
- Whyte, D.G.; Thunhorst, R.L.; Johnson, A.K. Reduced thirst in old, thermally dehydrated rats. Physiol. Behav. 2004, 81, 569–576. [Google Scholar] [CrossRef] [PubMed]
- McKinley, M.J.; Denton, D.A.; Thomas, C.J.; Woods, R.L.; Mathai, M.L. Differential effects of aging on fluid intake in response to hypovolemia, hypertonicity, and hormonal stimuli in Munich Wistar rats. Proc. Natl. Acad. Sci. USA 2006, 103, 3450–3455. [Google Scholar] [CrossRef]
- Silver, A.J.; Morley, J.E.; Ishimaru-Tseng, T.V.; Morley, P.M. Angiotensin II and fluid ingestion in old rats. Neurobiol. Aging 1993, 14, 519–522. [Google Scholar] [CrossRef]
- Thunhorst, R.L.; Beltz, T.G.; Johnson, A.K. Hypotension- and osmotically induced thirst in old Brown Norway rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R149–R157. [Google Scholar] [CrossRef]
- Thunhorst, R.L.; Johnson, A.K. Thirst and salt appetite responses in young and old Brown Norway rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R317–R327. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Friedrichsen, M.; Lythell, C.; Waldréus, N.; Jaarsma, T.; Ångström, H.; Milovanovic, M.; Karlsson, M.; Milberg, A.; Thulesius, H.; Hedman, C.; et al. Ethical challenges around thirst in end-of-life care -experiences of palliative care physicians. BMC Med. Ethics 2023, 24, 61. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, D.J.; Rolls, B.J.; Wood, R.J. The relationship between elevated water intake and oedema associated with congestive cardiac failure in the dog. J. Physiol. 1975, 244, 303–312. [Google Scholar] [CrossRef] [PubMed]
- De Smet, H.R.; Menadue, M.F.; Oliver, J.R.; Phillips, P.A. Increased thirst and vasopressin secretion after myocardial infarction in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R1203–R1211. [Google Scholar] [CrossRef][Green Version]
- Bolger, A.P.; Sharma, R.; Li, W.; Leenarts, M.; Kalra, P.R.; Kemp, M.; Coats, A.J.; Anker, S.D.; Gatzoulis, M.A. Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation. 2002, 106, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; He, R.L.; Yang, L.M.; Qin, D.N.; Guggilam, A.; Elks, C.; Yan, N.; Guo, Z.; Francis, J. Brain tumour necrosis factor-alpha modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovasc. Res. 2009, 83, 737–746. [Google Scholar] [CrossRef]
- Rouleau, J.L.; Packer, M.; Moyé, L.; de Champlain, J.; Bichet, D.; Klein, M.; Rouleau, J.R.; Sussex, B.; Arnold, J.M.; Sestier, F.; et al. Prognostic value of neurohumoral activation in patients with an acute myocardial infarction: Effect of captopril. J. Am. Coll. Cardiol. 1994, 24, 583–591. [Google Scholar] [CrossRef][Green Version]
- Eng, S.H.; Jaarsma, T.; Lupón, J.; González, B.; Ehrlin, J.; Díaz, V.; Bayes-Genis, A.; Waldréus, N. Thirst and factors associated with frequent thirst in patients with heart failure in Spain. Heart Lung 2021, 50, 86–91. [Google Scholar] [CrossRef]
- Gong, J.; Waldréus, N.; Hu, S.; Luo, Z.; Xu, M.; Zhu, L. Thirst and factors associated with thirst in hospitalized patients with heart failure in China. Heart Lung 2022, 53, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.M.; Higgins, M.; Smith, A.; Culler, S.D.; Dunbar, S.B. Isolating the benefits of fluid restriction in patients with heart failure: A pilot study. Eur. J. Cardiovasc. Nurs. 2015, 14, 495–505. [Google Scholar] [CrossRef]
- Waldréus, N.; Hahn, R.G.; Jaarsma, T. Thirst in heart failure: A systematic literature review. Eur. J. Heart Fail. 2013, 15, 141–149. [Google Scholar] [CrossRef]
- Younes, K.; Massouh, A.; Deek, H.; Nasreddine, L.; Waldréus, N.; Noureddine, S. Prevalence and Predictors of Thirst in Patients With Heart Failure. J. Cardiovasc. Nurs. 2025, 40, 431–440. [Google Scholar] [CrossRef]
- van der Wal, M.H.L.; Jaarsma, T.; Jenneboer, L.C.; Linssen, G.C.M. Thirst in stable heart failure patients; time to reconsider fluid restriction and prescribed diuretics. ESC Heart Fail. 2022, 9, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Garrahy, A.; Moran, C.; Thompson, C.J. Diagnosis and management of central diabetes insipidus in adults. Clin. Endocrinol. 2019, 90, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Nigro, N.; Grossmann, M.; Chiang, C.; Inder, W.J. Polyuria-polydipsia syndrome: A diagnostic challenge. Intern. Med. J. 2018, 48, 244–253. [Google Scholar] [CrossRef]
- Atila, C.; Loughrey, P.B.; Garrahy, A.; Winzeler, B.; Refardt, J.; Gildroy, P.; Hamza, M.; Pal, A.; Verbalis, J.G.; Thompson, C.J.; et al. Central diabetes insipidus from a patient’s perspective: Management, psychological co-morbidities, and renaming of the condition: Results from an international web-based survey. Lancet Diabetes Endocrinol. 2022, 10, 700–709. [Google Scholar] [CrossRef]
- Bankir, L.; Guerrot, D.; Bichet, D.G. Vaptans or voluntary increased hydration to protect the kidney: How do they compare? Nephrol. Dial. Transplant. 2023, 38, 562–574. [Google Scholar] [CrossRef]
- Cherchir, F.; Oueslati, I.; Salhi, S.; Ben Hamida, A.; Yazidi, M.; Chihaoui, M. Persistent hypernatremia secondary to adipsic central diabetes insipidus in a patient with herpes-induced meningoencephalitis and COVID-19 infection: A case report. J. Int. Med. Res. 2024, 52, 3000605241235747. [Google Scholar] [CrossRef] [PubMed]
- Seki, Y.; Sugawara, S.; Suzuki, S.; Minakuchi, Y.; Kusuki, K.; Mizuno, Y. Hyponatremia due to preserved non-osmotic arginine vasopressin secretion in adipsic diabetes insipidus: A case report with review of literature. Endocr. J. 2024, 71, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Wakabayashi, T.; Nagatani, T.; Fujii, M.; Hirakawa, A.; Murase, T.; Yambe, Y.; Yamada, T.; Yamakawa, F.; Yamamori, I.; et al. Adipsia increases risk of death in patients with central diabetes insipidus. Endocr. J. 2014, 61, 143–148. [Google Scholar] [CrossRef]
- Latcha, S.; Lubetzky, M.; Weinstein, A.M. Severe hyperosmolarity and hypernatremia in an adipsic young woman. Clin. Nephrol. 2011, 76, 407–411. [Google Scholar] [CrossRef]
- Yang, T.; Wu, W.; Liu, X.; Xiang, B.; Sun, Q.; Zhang, S.; Zhuang, Y.; Yin, Z.; Zhang, Q.; Cao, Y.; et al. Clinical Characteristics of Adipsic Diabetes Insipidus. Endocr. Pract. 2024, 30, 141–145. [Google Scholar] [CrossRef]
- Hayashi, T.; Murata, M.; Saito, T.; Ikoma, A.; Tamemoto, H.; Kawakami, M.; Ishikawa, S.E. Pathogenesis of chronic hypernatremia with dehydrated and non-dehydrated states in hypothalamic space-occupying lesions. Endocr. J. 2008, 55, 651–655. [Google Scholar] [CrossRef]
- Leroy, C.; Karrouz, W.; Douillard, C.; Do Cao, C.; Cortet, C.; Wémeau, J.L.; Vantyghem, M.C. Diabetes insipidus. Ann. Endocrinol. 2013, 74, 496–507. [Google Scholar] [CrossRef]
- Tran, V.; Flores, J.; Sheldon, M.; Pena, C.; Nugent, K. Fluid and Electrolyte Disorders in Traumatic Brain Injury: Clinical Implications and Management Strategies. J. Clin. Med. 2025, 14, 756. [Google Scholar] [CrossRef]
- Genuth, S.M.; Palmer, J.P.; Nathan, D.M.; Cowie, C.C.; Casagrande, S.S.; Menke, A.; Cissell, M.A.; Eberhardt, M.S.; Meigs, J.B.; Gregg, E.W.; et al. (Eds.) Classification and Diagnosis of Diabetes. In Diabetes in America, 3rd ed.; National Institute of Diabetes and Digestive and Kidney Diseases (US): Bethesda, MD, USA, 2018; Chapter 1. [Google Scholar] [PubMed]
- Chang, D.C.; Stinson, E.J.; Piaggi, P.; Krakoff, J.; Gluck, M.E. Disinhibition augments thirst perception from two dehydrating stimuli in men. Appetite 2023, 182, 106429. [Google Scholar] [CrossRef]
- Kamoi, K.; Ishibashi, M.; Yamaji, T. Thirst and plasma levels of vasopressin, angiotensin II and atrial natriuretic peptide in patients with non-insulin-dependent diabetes mellitus. Diabetes Res. Clin. Pract. 1991, 11, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.J.; Davis, S.N.; Butler, P.C.; Charlton, J.A.; Baylis, P.H. Osmoregulation of thirst and vasopressin secretion in insulin-dependent diabetes mellitus. Clin. Sci. 1988, 74, 599–606. [Google Scholar] [CrossRef]
- Kavelaars, J.; Tamsma, J.T.; Meinders, A.E. Hypernatremia in a non insulin dependent (type 2) diabetic patient with central diabetes insipidus. Neth. J. Med. 2001, 58, 150–154. [Google Scholar] [CrossRef]
- Wagner, B.; Ing, T.S.; Roumelioti, M.E.; Sam, R.; Argyropoulos, C.P.; Lew, S.Q.; Unruh, M.L.; Dorin, R.I.; Degnan, J.H.; Tzamaloukas, A.H. Hypernatremia in Hyperglycemia: Clinical Features and Relationship to Fractional Changes in Body Water and Monovalent Cations during Its Development. J. Clin. Med. 2024, 13, 1957. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.A.; Campbell, R.G.; Lilavivat, U.; Welle, S.L.; Robertson, G.L. Increased thirst and plasma arginine vasopressin levels during 2-deoxy-D-glucose-induced glucoprivation in humans. J. Clin. Investig. 1981, 67, 1083–1093. [Google Scholar] [CrossRef]
- Oikonomakos, I.T.; Steenblock, C.; Bornstein, S.R. Artificial intelligence in diabetes mellitus and endocrine diseases—What can we expect? Nat. Rev. Endocrinol. 2023, 19, 375–376. [Google Scholar] [CrossRef]
- Ravaut, M.; Harish, V.; Sadeghi, H.; Leung, K.K.; Volkovs, M.; Kornas, K.; Watson, T.; Poutanen, T.; Rosella, L.C. Development and Validation of a Machine Learning Model Using Administrative Health Data to Predict Onset of Type 2 Diabetes. JAMA Netw. Open 2021, 4, e2111315. [Google Scholar] [CrossRef]
- Siegel, A.J. Hyponatremia in psychiatric patients: Update on evaluation and management. Harv. Rev. Psychiatry 2008, 16, 13–24. [Google Scholar] [CrossRef]
- Goldman, M.B.; Gnerlich, J.; Hussain, N. Neuroendocrine responses to a cold pressor stimulus in polydipsic hyponatremic and in matched schizophrenic patients. Neuropsychopharmacology 2007, 32, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Hurwit, A.A.; Parker, J.M.; Uhlyar, S. Treatment of Psychogenic Polydipsia and Hyponatremia: A Case Report. Cureus 2023, 15, e47719. [Google Scholar] [CrossRef] [PubMed]
- Kawai, N.; Baba, A.; Suzuki, T.; Shiraishi, H. Roles of arginine vasopressin and atrial natriuretic peptide in polydipsia-hyponatremia of schizophrenic patients. Psychiatry Res. 2001, 101, 39–45. [Google Scholar] [CrossRef]
- Nickles, M.R.; Singh, G. Hyponatremia Secondary to Psychogenic Polydipsia and Schizophrenia: A Case Report. Cureus 2024, 16, e64600. [Google Scholar] [CrossRef]
- Parag, S.; Espiridion, E.D. Hyponatremia Presenting with Recurrent Mania. Cureus 2018, 10, e3645. [Google Scholar] [CrossRef]
- Torres, I.J.; Keedy, S.; Marlow-O’Connor, M.; Beenken, B.; Goldman, M.B. Neuropsychological impairment in patients with schizophrenia and evidence of hyponatremia and polydipsia. Neuropsychology 2009, 23, 307–314. [Google Scholar] [CrossRef]
- Farley, P.C.; Lau, K.Y.; Suba, S. Severe hypernatremia in a patient with psychiatric illness. Arch. Intern. Med. 1986, 146, 1214–1215. [Google Scholar] [CrossRef] [PubMed]
- Liebrand, M.; Rebsamen, M.; Nakamura-Utsunomiya, A.; von den Driesch, L.; Köck, P.; Caccia, J.; Hamann, C.; Wiest, R.; Kaess, M.; Walther, S.; et al. Case report: Psychosis and catatonia in an adolescent patient with adipsic hypernatremia and autoantibodies against the subfornical organ. Front. Psychiatry 2023, 14, 1206226. [Google Scholar] [CrossRef]
- Rodriguez, A.; Fogelfeld, L.; Robertson, G. Hypernatremic Hydrophobic Transient Adipsia Without Organic or Severe Psychiatric Disorder. J. Clin. Endocrinol. Metab. 2019, 104, 5427–5430. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Alkhidir, A.; Lagos, Y.V.; Le, P.T.V.; Nguyen, L.H. Challenges in Diagnosing and Managing Severe Hypernatremia in Patients With Major Depressive Disorder: A Case Report. Cureus 2024, 16, e64281. [Google Scholar] [CrossRef] [PubMed]
- Donka, R.M.; Hsu, T.; Roitman, M.F.; Roitman, J.D. Chronic water restriction reduces sensitivity to brain stimulation reward in male and female rats. Physiol. Behav. 2023, 263, 114110. [Google Scholar] [CrossRef]
- Pearlstein, H.; Dayno, A.; Zook, J.; Crowley, J.; Alter, C.; Gutmark-Little, I.; McCormack, S.E. Management of Arginine Vasopressin Deficiency (Central Diabetes Insipidus) in Neonates and Infants. Horm. Res. Paediatr. 2025, 1–20. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, Q.; Xia, C.; Liu, H. Thirst symptoms in patients with heart failure: An integrative review. J. Adv. Nurs. 2025, 81, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, C.; Wehner, F. The epithelial Na(+) channel (ENaC) is related to the hypertonicity-induced Na(+) conductance in rat hepatocytes. FEBS Lett. 2001, 494, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, P.; Elfers, K.; Maurer, S.; Klingenspor, M.; Schemann, M.; Mazzuoli-Weber, G. Submucosal enteric neurons of the cavine distal colon are sensitive to hypoosmolar stimuli. J. Physiol. 2020, 598, 5317–5332. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepanska-Sadowska, E. Neurochemical Aspects of the Role of Thirst in Body Fluid Homeostasis and Their Significance in Health and Disease: A Literature Review. Int. J. Mol. Sci. 2025, 26, 7850. https://doi.org/10.3390/ijms26167850
Szczepanska-Sadowska E. Neurochemical Aspects of the Role of Thirst in Body Fluid Homeostasis and Their Significance in Health and Disease: A Literature Review. International Journal of Molecular Sciences. 2025; 26(16):7850. https://doi.org/10.3390/ijms26167850
Chicago/Turabian StyleSzczepanska-Sadowska, Ewa. 2025. "Neurochemical Aspects of the Role of Thirst in Body Fluid Homeostasis and Their Significance in Health and Disease: A Literature Review" International Journal of Molecular Sciences 26, no. 16: 7850. https://doi.org/10.3390/ijms26167850
APA StyleSzczepanska-Sadowska, E. (2025). Neurochemical Aspects of the Role of Thirst in Body Fluid Homeostasis and Their Significance in Health and Disease: A Literature Review. International Journal of Molecular Sciences, 26(16), 7850. https://doi.org/10.3390/ijms26167850





