The Association of Adropin with Asymptomatic Coronary Calcification in Patients in Early Stages of Chronic Kidney Disease
Abstract
1. Introduction
2. Results
2.1. Baseline Clinical Characteristics
2.2. Spearman’s Correlations Between the Levels of Circulating Biomarkers and Other Parameters in CKD G1–2 Patients with Asymptomatic Coronary Artery Calcification
2.3. The Levels of Adropin Depending on the Weighted Sum of Coronary Artery Lesions with a Density
2.4. Receiver Operating Characteristic Curve Analysis for Adropin
2.5. Predictors of Asymptomatic Coronary Calcification: Univariate and Multivariate Logistic Regression Analyses
2.6. Comparison of the Predictive Models
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Determination of Early Stages of CKD
4.3. Native Coronary Multi-Detector Computed Tomography Angiography
4.4. Determination of Coronary Artery Calcification
4.5. Echocardiography Examination
4.6. Clinical Data
4.7. Blood Sampling and Biomarker Assessment
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under curve |
| BMI | body mass index |
| BP | blood pressure |
| CAD | coronary artery disease |
| CKD | chronic kidney disease |
| CV | cardiovascular |
| DPP-4 | dipeptidyl peptidase-4 |
| eGFR | estimated glomerular filtration rate |
| FGF-23 | fibroblast growth factor 23 |
| GLP-1 | glucagon-like peptide-1 |
| GLS | global longitudinal strain |
| HDL-C | high-density lipoprotein cholesterol |
| HFpEF | heart failure with preserved ejection fraction |
| hs-CRP | high-sensitivity C-reactive protein |
| HU | Hounsfield units |
| IL | interleukin |
| LAVI | left atrial volume index |
| LDL-C | low-density lipoprotein cholesterol |
| LVEDV | left ventricular end-diastolic volume |
| LVEF | left ventricular ejection fraction |
| LVESV | left ventricular end-systolic volume |
| LVH | left ventricular hypertrophy |
| LVMMI | left ventricle myocardial mass index |
| MRA | mineralocorticoid receptor antagonists |
| NT-proBNP | N-terminal natriuretic pro-peptide |
| PI3K | phosphatidylinositol 3-kinase |
| ROC | Receiver Operating Curve |
| SGLT2 | sodium–glucose cotransporter-2 |
| SUA | serum uric acid |
| T2DM | type 2 diabetes mellitus |
| TNF-alpha | tumor necrosis factor-alpha |
| UACR | urinary albumin/creatinine ratio |
| WHR | waist-to-hip ratio |
References
- Brück, K.; Stel, V.S.; Gambaro, G.; Hallan, S.; Völzke, H.; Ärnlöv, J.; Kastarinen, M.; Guessous, I.; Vinhas, J.; Stengel, B.; et al. CKD Prevalence Varies across the European General Population. J. Am. Soc. Nephrol. 2016, 27, 2135–2147. [Google Scholar] [CrossRef]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef]
- Minutolo, R.; Gabbai, F.B.; Chiodini, P.; Provenzano, M.; Borrelli, S.; Garofalo, C.; Bellizzi, V.; Russo, D.; Conte, G.; De Nicola, L.; et al. Sex Differences in the Progression of CKD Among Older Patients: Pooled Analysis of 4 Cohort Studies. Am. J. Kidney Dis. 2020, 75, 30–38. [Google Scholar] [CrossRef]
- Nair, N.; Kalra, R.; Chandra Bhatt, G.; Narang, A.; Kumar, G.; Raina, R. The Effect and Prevalence of Comorbidities in Adolescents With CKD and Obesity. Adv. Chronic Kidney Dis. 2022, 29, 251–262. [Google Scholar] [CrossRef]
- Adler, J.; Taneva, E.; Ansorge, T.; Mertens, P.R. CKD prevalence based on real-world data: Continuous age-dependent lower reference limits of eGFR with CKD-EPI, FAS and EKFC algorithms. Int. Urol. Nephrol. 2022, 54, 2929–2937. [Google Scholar] [CrossRef]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Ding, N.; Lv, Y.; Su, H.; Wang, Z.; Kong, X.; Zhen, J.; Lv, Z.; Wang, R. Vascular calcification in CKD: New insights into its mechanisms. J. Cell. Physiol. 2023, 238, 1160–1182. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Adamczak, M.; de Oliveira, R.B.; Massy, Z.A.; Sarafidis, P.; Agarwal, R.; Mark, P.B.; Kotanko, P.; Ferro, C.J.; et al. Cardiovascular complications in chronic kidney disease: A review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association. Cardiovasc. Res. 2023, 119, 2017–2032. [Google Scholar] [CrossRef]
- Charytan, D.M.; Skali, H.; Shah, N.R.; Veeranna, V.; Cheezum, M.K.; Taqueti, V.R.; Kato, T.; Bibbo, C.R.; Hainer, J.; Dorbala, S.; et al. Coronary flow reserve is predictive of the risk of cardiovascular death regardless of chronic kidney disease stage. Kidney Int. 2018, 93, 501–509. [Google Scholar] [CrossRef]
- Murthy, V.L.; Naya, M.; Foster, C.R.; Hainer, J.; Gaber, M.; Dorbala, S.; Charytan, D.M.; Blankstein, R.; Di Carli, M.F. Coronary vascular dysfunction and prognosis in patients with chronic kidney disease. JACC Cardiovasc. Imaging 2012, 5, 1025–1034. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Neves, P.O.; Andrade, J.; Monção, H. Coronary artery calcium score: Current status. Radiol. Bras. 2017, 50, 182–189. [Google Scholar] [CrossRef]
- Nasir, K.; Clouse, M. Role of nonenhanced multidetector CT coronary artery calcium testing in asymptomatic and symptomatic individuals. Radiology 2012, 264, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Pluquet, M.; Kamel, S.; Choukroun, G.; Liabeuf, S.; Laville, S.M. Serum Calcification Propensity Represents a Good Biomarker of Vascular Calcification: A Systematic Review. Toxins 2022, 14, 637. [Google Scholar] [CrossRef]
- Liabeuf, S.; Okazaki, H.; Desjardins, L.; Fliser, D.; Goldsmith, D.; Covic, A.; Wiecek, A.; Ortiz, A.; Martinez-Castelao, A.; Lindholm, B.; et al. Vascular calcification in chronic kidney disease: Are biomarkers useful for probing the pathobiology and the health risks of this process in the clinical scenario? Nephrol. Dial. Transpl. 2014, 29, 1275–1284. [Google Scholar] [CrossRef]
- Bozic, M.; Méndez-Barbero, N.; Gutiérrez-Muñoz, C.; Betriu, A.; Egido, J.; Fernández, E.; Martín-Ventura, J.L.; Valdivielso, J.M.; Blanco-Colio, L.M.; investigators from the NEFRONA study. Combination of biomarkers of vascular calcification and sTWEAK to predict cardiovascular events in chronic kidney disease. Atherosclerosis 2018, 270, 13–20. [Google Scholar] [CrossRef]
- Kaur, R.; Krishan, P.; Kumari, P.; Singh, T.; Singh, V.; Singh, R.; Ahmad, S.F. Clinical Significance of Adropin and Afamin in Evaluating Renal Function and Cardiovascular Health in the Presence of CKD-MBD Biomarkers in Chronic Kidney Disease. Diagnostics 2023, 13, 3158. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.; Berezina, T.A.; Hoppe, U.C.; Lichtenauer, M.; Berezin, A.A. An overview of circulating and urinary biomarkers capable of predicting the transition of acute kidney injury to chronic kidney disease. Expert. Rev. Mol. Diagn. 2024, 24, 627–647. [Google Scholar] [CrossRef]
- Chen, I.W.; Lin, C.W.; Lin, C.N.; Chen, S.T. Serum adropin levels as a potential biomarker for predicting diabetic kidney disease progression. Front. Endocrinol. 2025, 16, 1511730. [Google Scholar] [CrossRef]
- Ali, I.I.; D’Souza, C.; Singh, J.; Adeghate, E. Adropin’s Role in Energy Homeostasis and Metabolic Disorders. Int. J. Mol. Sci. 2022, 23, 8318. [Google Scholar] [CrossRef]
- Rooban, S.; Arul Senghor, K.A.; Vinodhini, V.M.; Kumar, J.S. Adropin: A crucial regulator of cardiovascular health and metabolic balance. Metabol. Open 2024, 23, 100299. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liu, H.; Qiu, X.; Zhang, J.; Huang, J.; Chen, H.; Qiu, S.; Lin, R.; Li, S.; Tu, M. The association between serum adropin and carotid atherosclerosis in patients with type 2 diabetes mellitus: A cross-sectional study. Diabetol. Metab. Syndr. 2022, 14, 27. [Google Scholar] [CrossRef]
- Zhao, L.P.; You, T.; Chan, S.P.; Chen, J.C.; Xu, W.T. Adropin is associated with hyperhomocysteine and coronary atherosclerosis. Exp. Ther. Med. 2016, 11, 1065–1070. [Google Scholar] [CrossRef]
- Wu, L.; Fang, J.; Chen, L.; Zhao, Z.; Luo, Y.; Lin, C.; Fan, L. Low serum adropin is associated with coronary atherosclerosis in type 2 diabetic and non-diabetic patients. Clin. Chem. Lab. Med. 2014, 52, 751–758. [Google Scholar] [CrossRef]
- El Moneem Elfedawy, M.A.; El Sadek Elsebai, S.A.; Tawfik, H.M.; Youness, E.R.; Zaki, M. Adropin a candidate diagnostic biomarker for cardiovascular disease in patients with chronic kidney disease. J. Genet. Eng. Biotechnol. 2024, 22, 100438. [Google Scholar] [CrossRef]
- Morena-Carrere, M.; Jaussent, I.; Chenine, L.; Dupuy, A.M.; Bargnoux, A.S.; Leray-Moragues, H.; Klouche, K.; Vernhet, H.; Canaud, B.; Cristol, J.P. Severe Coronary Artery Calcifications in Chronic Kidney Disease Patients, Coupled with Inflammation and Bone Mineral Disease Derangement, Promote Major Adverse Cardiovascular Events through Vascular Remodeling. Kidney Blood Press. Res. 2025, 50, 33–45. [Google Scholar] [CrossRef]
- Wang, X.R.; Yuan, L.; Shi, R.; Li, H.; Wang, D.G.; Wu, Y.G. Predictors of coronary artery calcification and its association with cardiovascular events in patients with chronic kidney disease. Ren. Fail. 2021, 43, 1172–1179. [Google Scholar] [CrossRef]
- Jin, H.; Ji, J.J.; Zhu, Y.; Wang, X.D.; Li, Y.P.; Shi, Q.Y.; Chen, Y.F. Brain-Derived Neurotrophic Factor, a New Predictor of Coronary Artery Calcification. Clin. Appl. Thromb. Hemost. 2021, 27, 1076029621989813. [Google Scholar] [CrossRef]
- Berlot, A.A.; Fu, X.; Shea, M.K.; Tracy, R.; Budoff, M.; Kim, R.S.; Naveed, M.; Booth, S.L.; Kizer, J.R.; Bortnick, A.E. Matrix Gla protein and the long-term incidence and progression of coronary artery and aortic calcification in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2024, 392, 117505. [Google Scholar] [CrossRef] [PubMed]
- Golüke, N.M.S.; Schoffelmeer, M.A.; De Jonghe, A.; Emmelot-Vonk, M.H.; De Jong, P.A.; Koek, H.L. Serum biomarkers for arterial calcification in humans: A systematic review. Bone Rep. 2022, 17, 101599. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.D.; Budoff, M.J.; Ferdinand, K.; Graham, I.M.; Michos, E.D.; Reddy, T.; Shapiro, M.D.; Toth, P.P. Atherosclerotic cardiovascular disease risk assessment: An American Society for Preventive Cardiology clinical practice statement. Am. J. Prev. Cardiol. 2022, 10, 100335. [Google Scholar] [CrossRef]
- Berezina, T.A.; Obradovic, Z.; Boxhammer, E.; Berezin, A.A.; Lichtenauer, M.; Berezin, A.E. Adropin Predicts Chronic Kidney Disease in Type 2 Diabetes Mellitus Patients with Chronic Heart Failure. J. Clin. Med. 2023, 12, 2231. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.A.; Havel, P.J. Adropin: A cardio-metabolic hormone in the periphery, a neurohormone in the brain? Peptides 2025, 187, 171391. [Google Scholar] [CrossRef]
- Lovren, F.; Pan, Y.; Quan, A.; Singh, K.K.; Shukla, P.C.; Gupta, M.; Al-Omran, M.; Teoh, H.; Verma, S. Adropin is a novel regulator of endothelial function. Circulation 2010, 122 (Suppl. 11), S185–S192. [Google Scholar] [CrossRef] [PubMed]
- Bozic, J.; Kumric, M.; Ticinovic Kurir, T.; Males, I.; Borovac, J.A.; Martinovic, D.; Vilovic, M. Role of Adropin in Cardiometabolic Disorders: From Pathophysiological Mechanisms to Therapeutic Target. Biomedicines 2021, 9, 1407. [Google Scholar] [CrossRef]
- Boric-Skaro, D.; Mizdrak, M.; Luketin, M.; Martinovic, D.; Tokic, D.; Vilovic, M.; Supe-Domic, D.; Kurir, T.T.; Bozic, J. Serum Adropin Levels in Patients on Hemodialysis. Life 2021, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, A.F.; Erkuş, E.; Duysak, L. Measurement of serum adropin levels in chronic renal failure patients receiving routine hemodialysis treatment. Medicine 2025, 104, e41860. [Google Scholar] [CrossRef]
- Soltani, S.; Beigrezaei, S.; Malekahmadi, M.; Clark, C.C.T.; Abdollahi, S. Circulating levels of adropin and diabetes: A systematic review and meta-analysis of observational studies. BMC Endocr Disord. 2023, 23, 73. [Google Scholar] [CrossRef]
- Es-Haghi, A.; Al-Abyadh, T.; Mehrad-Majd, H. The Clinical Value of Serum Adropin Level in Early Detection of Diabetic Nephropathy. Kidney Blood Press Res. 2021, 46, 734–740. [Google Scholar] [CrossRef]
- Berezina, T.A.; Fushtey, I.M.; Berezin, A.A.; Pavlov, S.V.; Berezin, A.E. Predictors of Kidney Function Outcomes and Their Relation to SGLT2 Inhibitor Dapagliflozin in Patients with Type 2 Diabetes Mellitus Who Had Chronic Heart Failure. Adv. Ther. 2024, 41, 292–314. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Abbara, S.; Blanke, P.; Maroules, C.D.; Cheezum, M.; Choi, A.D.; Han, B.K.; Marwan, M.; Naoum, C.; Norgaard, B.L.; Rubinshtein, R.; et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2016, 10, 435–449. [Google Scholar] [CrossRef]
- Budoff, M.J.; Achenbach, S.; Blumenthal, R.S.; Carr, J.J.; Goldin, J.G.; Greenland, P.; Guerci, A.D.; Lima, J.A.; Rader, D.J.; Rubin, G.D.; et al. Assessment of coronary artery disease by cardiac computed tomography: A scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 2006, 114, 1761–1791. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESCGuidelines for the management of elevated blood pressure hypertension. Eur. Heart J. 2024, 45, 3912–4018, Erratum in Eur. Heart J. 2025, 46, 1300. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. 1), S20–S42. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188, Erratum in Eur. Heart J. 2020, 41, 4255. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4901. [Google Scholar] [CrossRef]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089, Erratum in Stroke 2019, 50, e239. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]

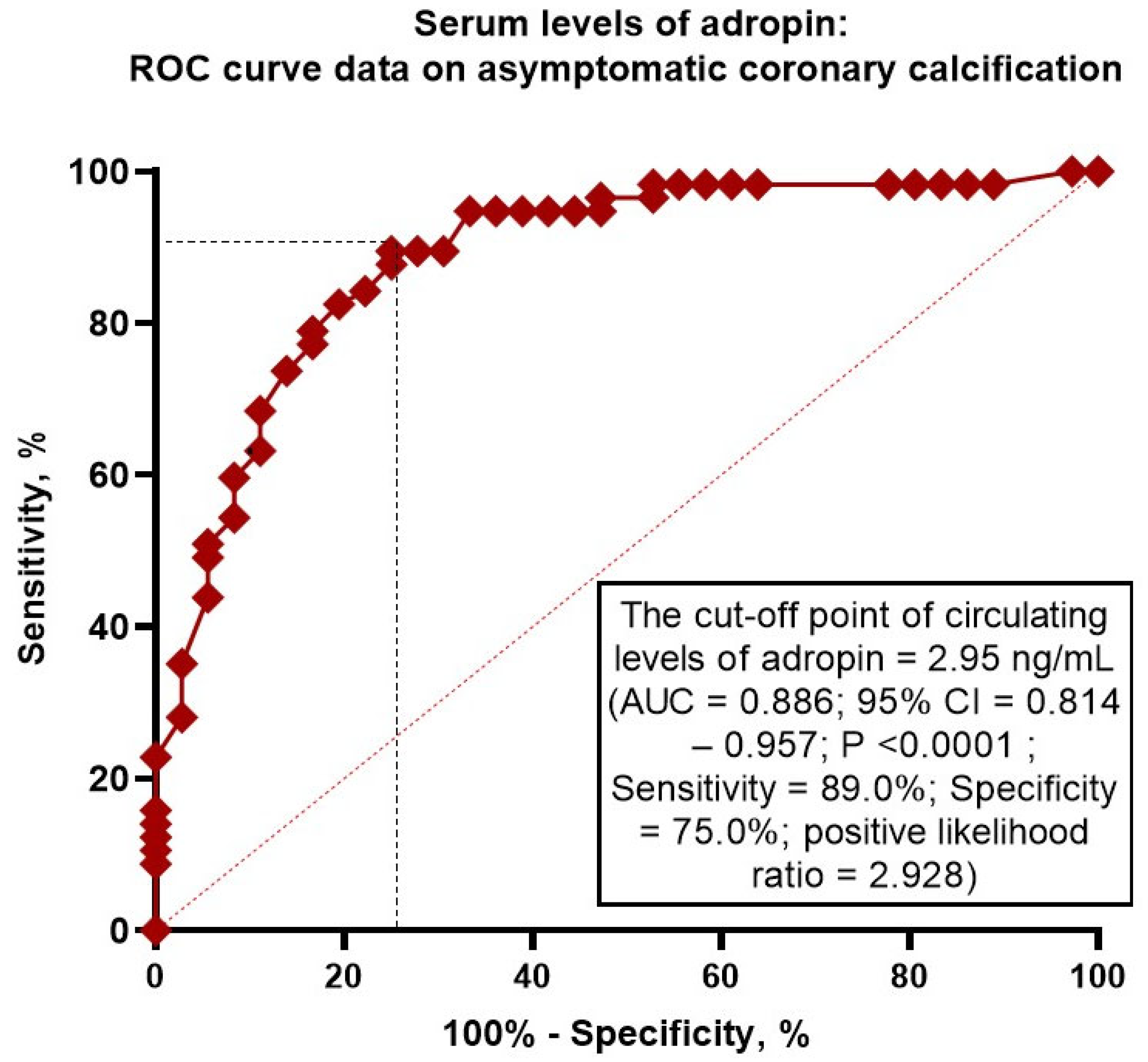
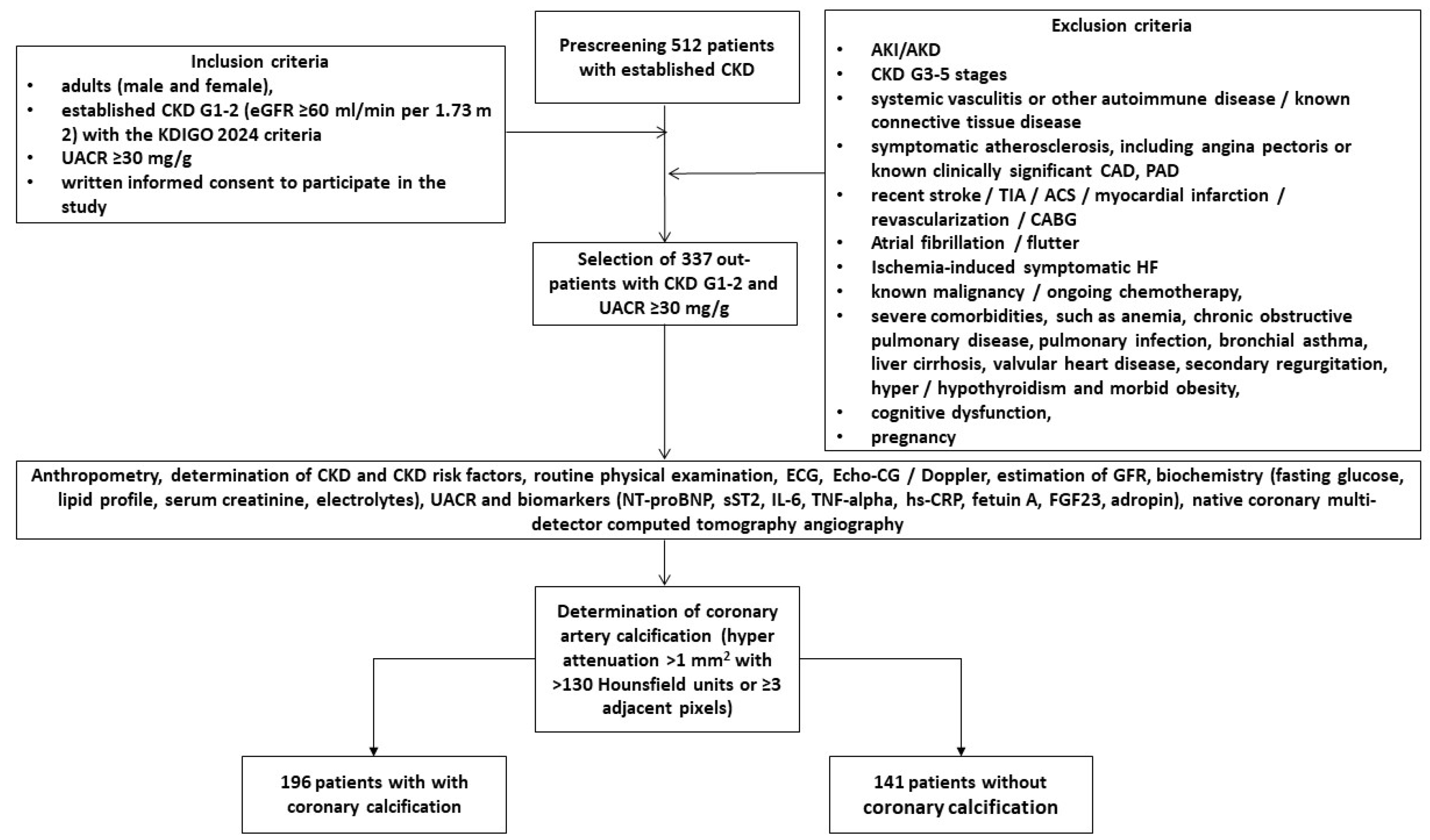
| Variables | Entire Group Patients with Early (G1–2) CKD (n = 337) | Patients with Coronary Calcification (n = 196) | Patients Without Coronary Calcification (n = 141) | p Value |
|---|---|---|---|---|
| Age (years) | 65 (54–77) | 68 (55–79) | 63 (52–74) | 0.044 |
| Male (n (%)) | 216 (64.1) | 125 (63.8) | 91 (64.5) | 0.822 |
| BMI (kg/m2) | 28.4 ± 6.7 | 29.6 ± 5.9 | 27.1 ± 4.6 | 0.710 |
| Waist circumference (cm) | 98 ± 5 | 98 ± 4 | 97 ± 6 | 0.810 |
| WHR (units) | 0.90 ± 0.2 | 0.91 ± 0.1 | 0.88 ± 0.1 | 0.750 |
| Smoking (n (%)) | 115 (34.1) | 71 (36.2) | 44 (31.2) | 0.870 |
| Dyslipidemia (n (%)) | 283 (84.0) | 172 (87.8) | 111 (78.7) | 0.061 |
| Hypertension (n (%)) | 269 (79.8) | 170 (86.7) | 99 (70.2) | 0.046 |
| Abdominal obesity (n (%)) | 92 (27.3) | 55 (28.1) | 37 (26.2) | 0.687 |
| T2DM (n (%)) | 128 (38.0) | 82 (41.8) | 46 (32.6) | 0.044 |
| LVH (n (%)) | 273 (81.0) | 158 (80.6) | 115 (81.6) | 0.812 |
| HFpEF (n (%)) | 138 (40.9) | 79 (40.3) | 59 (41.8) | 0.790 |
| Systolic BP (mm Hg) | 142 ± 10 | 143 ± 9 | 138 ± 7 | 0.660 |
| Diastolic BP (mm Hg) | 84 ± 8 | 86 ± 6 | 83 ± 5 | 0.830 |
| LVEDV (mL) | 149 (140–161) | 150 (140–163) | 149 (138–160) | 0.810 |
| LVESV (mL) | 68 (61–77) | 70 (62–79) | 67 (60–78) | 0.322 |
| LVEF (%) | 55 (51–59) | 53 (50–57) | 55 (51–59) | 0.384 |
| LVMMI (g/m2) | 142 ± 19 | 142 ± 16 | 140 ± 15 | 0.622 |
| LAVI (mL/m2) | 34 (31–38) | 35 (30–39) | 33 (30–37) | 0.646 |
| E/e′ (units) | 13 ± 6 | 13 ± 4 | 12 ± 5 | 0.716 |
| GLS (%) | −14.5 (−11.6; −17.0) | −14.7 (−11.2; −17.2) | −14.3 (−12.1; −16.7) | 0.884 |
| eGFR (mL/min/1.73 m2) | 78 ± 15 | 75 ± 13 | 80 ± 14 | 0.776 |
| UACR (mg/g) | 49 (33–217) | 52 (37–226) | 46 (32–211) | 0.644 |
| Fasting glucose (mmol/L) | 4.81 ± 1.24 | 5.22 ± 1.25 | 4.67 ± 1.30 | 0.292 |
| Creatinine (µmol/L) | 166 ± 39.1 | 173 ± 27 | 159 ± 24 | 0.655 |
| SUA (mcmol/L) | 365 ± 126 | 370 ± 115 | 356 ± 119 | 0.362 |
| Phosphorus (mmol/L) | 1.15 ± 0.28 | 1.15 ± 0.22 | 1.13 ± 0.20 | 0.773 |
| Calcium (mmol/L) | 2.24 (2.06–2.53) | 2.24 (2.10–2.62) | 2.22 (2.02–2.50) | 0.633 |
| Total cholesterol (mmol/L) | 5.70 ± 1.50 | 5.72 ± 1.42 | 5.66 ± 1.38 | 0.551 |
| HDL-C (mmol/L) | 0.99 ± 0.17 | 0.97 ± 0.15 | 0.99 ± 0.17 | 0.446 |
| LDL-C (mmol/L) | 3.82± 0.21 | 3.88 ± 0.20 | 3.79± 0.19 | 0.515 |
| Triglycerides (mmol/L) | 2.21 ± 0.17 | 2.27 ± 0.16 | 2.20 ± 0.15 | 0.524 |
| Lp(a), ng/mL | 8.54 (6.32–11.85) | 9.77 (6.52–12.40) | 8.09 (5.78–11.52) | 0.115 |
| sST2 (ng/mL) | 9.8 (1.25–16.2) | 10.6 (0.77–17.1) | 8.5 (1.25–14.6) | 0.228 |
| hs-CRP (mg/L) | 5.15 (2.23–7.16) | 5.21 (2.30–7.30) | 5.03 (2.02–6.43) | 0.048 |
| TNF-alpha (pg/mL) | 2.61 (1.60–3.70) | 2.84 (1.92–4.15) | 2.32 (1.40–3.53) | 0.046 |
| IL-6 (pg/mL) | 1.67 (0.54–3.92) | 1.74 (0.62–4.15) | 1.58 (0.51–3.77) | 0.128 |
| NT-proBNP (pmol/mL) | 138 (55–219) | 142 (53–233) | 135 (47–215) | 0.563 |
| Adropin (ng/mL) | 3.50 (1.90–5.40) | 2.85 (1.85–4.07) | 3.94 (2.92–5.67) | 0.012 |
| Fetuin-A (μg/mL) | 54.2 (31.2–72.4) | 55.9 (33.6–75.1) | 53.8 (30.2–72.5) | 0.592 |
| FGF-23 (pg/mL) | 93.8 ± 15.2 | 105.5 ± 13.6 | 88.2 ± 17.8 | 0.055 |
| ACEIs (n (%)) | 217 (64.4) | 116 (59.2) | 101 (71.6) | 0.046 |
| Angiotensin-II receptor blockers (n (%)) | 48 (14.2) | 37 (18.9) | 11 (7.80) | 0.026 |
| Beta-blockers (n (%)) | 276 (81.9) | 157 (80.1) | 119 (84.4) | 0.659 |
| Ivabradine (n (%)) | 27 (8.0) | 17(8.7) | 10 (7.1) | 0.769 |
| Calcium channel blockers (n (%)) | 75 (22.3) | 37 (18.9) | 38 (27.0) | 0.040 |
| Loop or thiazide-like diuretics (n (%)) | 161 (47.8) | 95 (48.5) | 66 (46.8) | 0.725 |
| MRA (n (%)) | 95 (28.2) | 57 (29.1) | 38 (27.0) | 0.488 |
| Antiplatelet agents (n (%)) | 87 (25.8) | 51 (26.0) | 36 (25.5) | 0.873 |
| Metformin (n (%)) | 92 (27.3) | 58 (30.0) | 34 (24.1) | 0.554 |
| DPP4 inhibitors (n (%)) | 18 (5.3) | 9 (4.6) | 9 (6.4) | 0.120 |
| GLP-1 receptor agonists (n (%)) | 11 (3.2) | 5 (2.6) | 6 (4.2) | 0.066 |
| SGLT2 inhibitors (n (%)) | 65 (19.3) | 39 (19.9) | 26 (18.4) | 0.854 |
| Statins (n (%)) | 283 (84.0) | 172 (87.8) | 111 (78.7) | 0.061 |
| Variables | Adropin | hs-CRP | TNF-Alpha | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Age (years) | −0.21 | 0.024 | 0.16 | 0.142 | 0.12 | 0.180 |
| BMI (kg/m2) | −0.23 | 0.001 | 0.19 | 0.043 | 0.18 | 0.050 |
| Systolic BP (mm Hg) | −0.25 | 0.001 | 0.08 | 0.431 | 0.12 | 0.220 |
| Diastolic BP (mm Hg) | −0.24 | 0.001 | 0.10 | 0.422 | 0.12 | 0.210 |
| LVEF (%) | 0.26 | 0.001 | −0.14 | 0.313 | −0.19 | 0.050 |
| LVMMI (g/m2) | −0.31 | 0.001 | −0.21 | 0.026 | −0.13 | 0.110 |
| LAVI (mL/m2) | −0.26 | 0.016 | 0.18 | 0.172 | 0.14 | 0.450 |
| GLS (%) | 0.32 | 0.001 | −0.17 | 0.406 | −0.13 | 0.601 |
| Agatston density range | 0.42 | 0.001 | −0.20 | 0.070 | −0.21 | 0.051 |
| eGFR (mL/min/1.73 m2) | 0.11 | 0.262 | −0.09 | 0.622 | −0.11 | 0.472 |
| UACR (mg/g) | −0.21 | 0.012 | 0.13 | 0.473 | 0.19 | 0.121 |
| Fasting glucose (mmol/L) | −0.19 | 0.050 | 0.07 | 0.542 | 0.08 | 0.493 |
| Total cholesterol (mmol/L) | −0.25 | 0.032 | −0.08 | 0.571 | −0.10 | 0.552 |
| LDL-C (mmol/L) | −0.22 | 0.040 | 0.11 | 0.293 | 0.13 | 0.431 |
| Variables | Dependent Variable: Asymptomatic Coronary Calcification | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Logistic Regression | Multivariate Logistic Regression | |||||||
| OR | 95% CI | p-Value | C-Index | OR | 95% CI | p-Value | C-Index | |
| Low adropin vs. elevated adropin | 1.26 | 1.08–1.52 | 0.001 | 0.66 | 1.27 | 1.13–1.40 | 0.001 | 0.01 |
| UACR ≥ 49 mg/g vs. UACR < 49 mg/g | 1.02 | 0.97–1.08 | 0.438 | 0.09 | - | |||
| hs-CRP ≥ 5.15 mg/L vs. hs-CRP < 5.15 mg/L | 1.06 | 1.01–1.18 | 0.052 | 0.12 | 1.03 | 1.00–1.10 | 0.182 | 0.14 |
| TNF-α ≥ 2.61 pg/mL vs. TNF-α < 2.61 pg/mL | 1.09 | 1.02–1.23 | 0.048 | 0.19 | 1.05 | 1.00–1.18 | 0.068 | 0.13 |
| Hypertension vs. non-hypertension | 1.09 | 1.03–1.22 | 0.044 | 0.32 | 1.09 | 1.07–1.23 | 0.042 | 0.36 |
| T2DM vs. non-T2DM | 1.07 | 1.02–1.15 | 0.042 | 0.31 | 1.05 | 1.01–1.10 | 0.044 | 0.31 |
| LVH vs. non-LVH | 1.08 | 0.96–1.25 | 0.672 | 0.11 | - | |||
| HFpEF vs. non-HFpEF | 1.11 | 1.02–1.24 | 0.046 | 0.37 | 1.14 | 1.00–1.28 | 0.422 | 0.13 |
| Administration of CCB | 0.89 | 0.71–0.99 | 0.042 | 0.39 | 0.90 | 0.70–1.02 | 0.068 | 0.22 |
| Administration of SGLT2i | 0.90 | 0.82–0.98 | 0.040 | 0.42 | 0.91 | 0.78–1.00 | 0.062 | 0.28 |
| Predictive Models | Dependent Variable: Asymptomatic Coronary Calcification | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC | NRI | IDI | |||||||
| M | 95% CI | p Value | M | 95% CI | p Value | M | 95% CI | p Value | |
| Model 1 | 0.886 | 0.814–0.957 | - | Reference | Reference | ||||
| Model 2 | 0.724 | 0.699–0.751 | 0.001 | 0.09 | 0.05–0.15 | 0.688 | 0.11 | 0.08–0.16 | 0.426 |
| Model 3 (T2DM) | 0.706 | 0.625–0.784 | 0.001 | 0.07 | 0.03–0.09 | 0.772 | 0.10 | 0.06–0.17 | 0.455 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berezina, T.A.; Berezin, O.O.; Novikov, E.V.; Berezin, A.E. The Association of Adropin with Asymptomatic Coronary Calcification in Patients in Early Stages of Chronic Kidney Disease. Int. J. Mol. Sci. 2025, 26, 7816. https://doi.org/10.3390/ijms26167816
Berezina TA, Berezin OO, Novikov EV, Berezin AE. The Association of Adropin with Asymptomatic Coronary Calcification in Patients in Early Stages of Chronic Kidney Disease. International Journal of Molecular Sciences. 2025; 26(16):7816. https://doi.org/10.3390/ijms26167816
Chicago/Turabian StyleBerezina, Tetiana A., Oleksandr O. Berezin, Evgen V. Novikov, and Alexander E. Berezin. 2025. "The Association of Adropin with Asymptomatic Coronary Calcification in Patients in Early Stages of Chronic Kidney Disease" International Journal of Molecular Sciences 26, no. 16: 7816. https://doi.org/10.3390/ijms26167816
APA StyleBerezina, T. A., Berezin, O. O., Novikov, E. V., & Berezin, A. E. (2025). The Association of Adropin with Asymptomatic Coronary Calcification in Patients in Early Stages of Chronic Kidney Disease. International Journal of Molecular Sciences, 26(16), 7816. https://doi.org/10.3390/ijms26167816






