Development of an Antibacterial Poly(Lactic Acid)/Poly(ε-Caprolactone)/Tributyl Citrate Film Loaded with Staphylococcus aureus Bacteriophages Using a Sodium Alginate Coating
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of the PLA/PCL Film Composition
2.2. Determination of TBC Composition in the PLA/PCL Film
2.3. Film Characterization
2.3.1. Optical Properties of Films
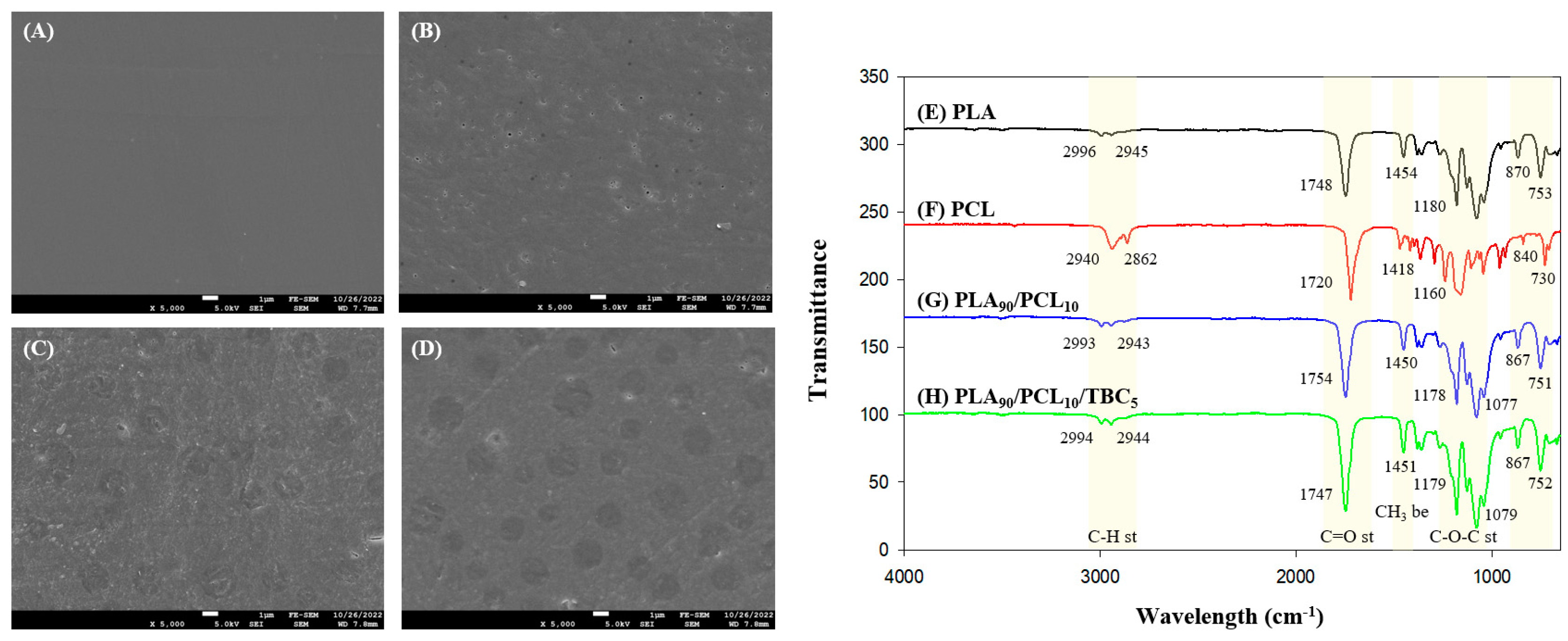
2.3.2. Morphologies of Films
2.3.3. FTIR Analysis
2.4. Determination of the SA Coating Solution Composition
2.5. Phage Characterization
2.5.1. Morphological Analysis
2.5.2. Host Range and Efficiency of Plating Analysis
2.5.3. Bacteriolytic Efficacy Against S. aureus
2.5.4. Temperature and pH Stability Assessment
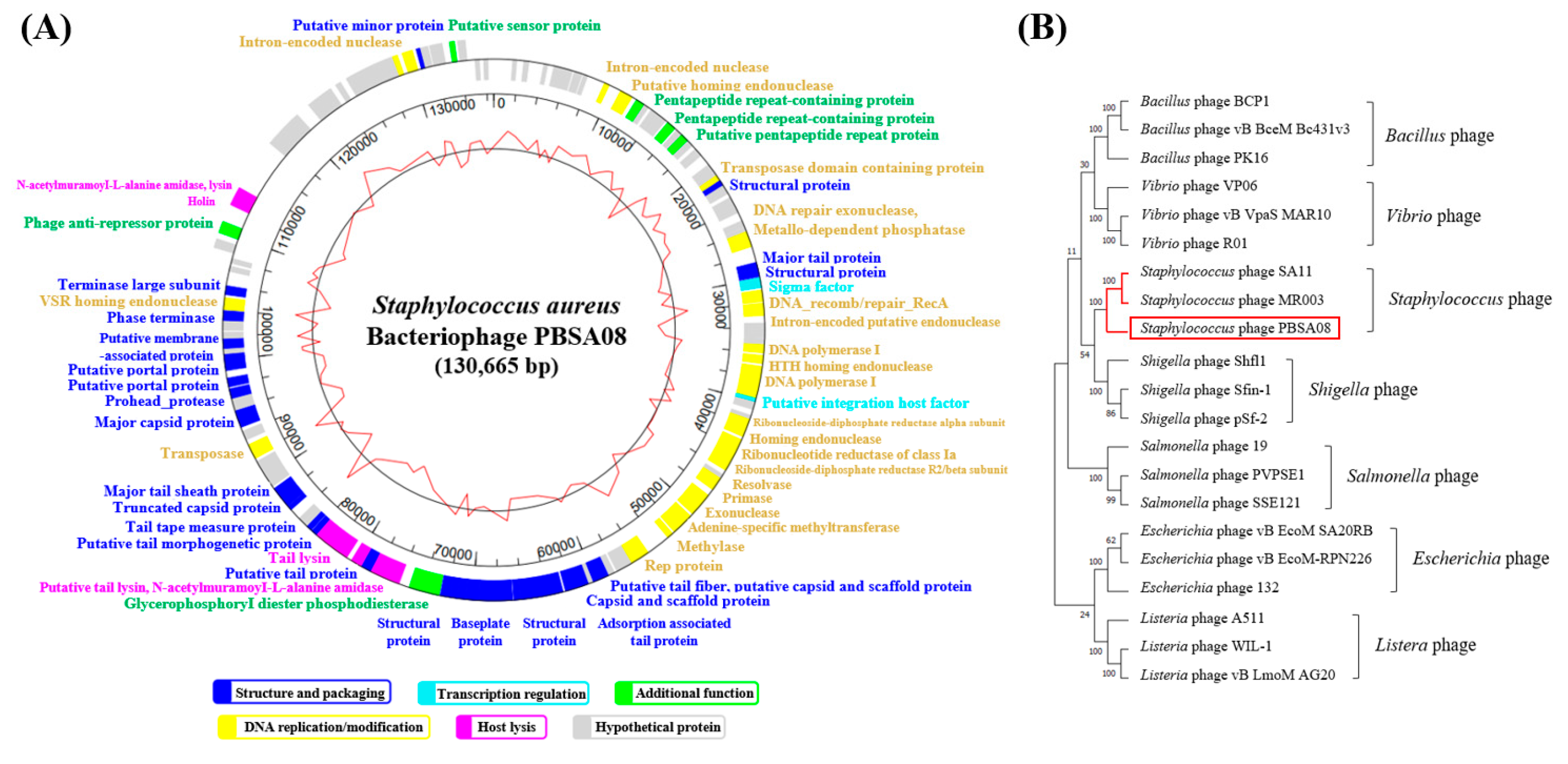
2.5.5. Phage Genome Analysis
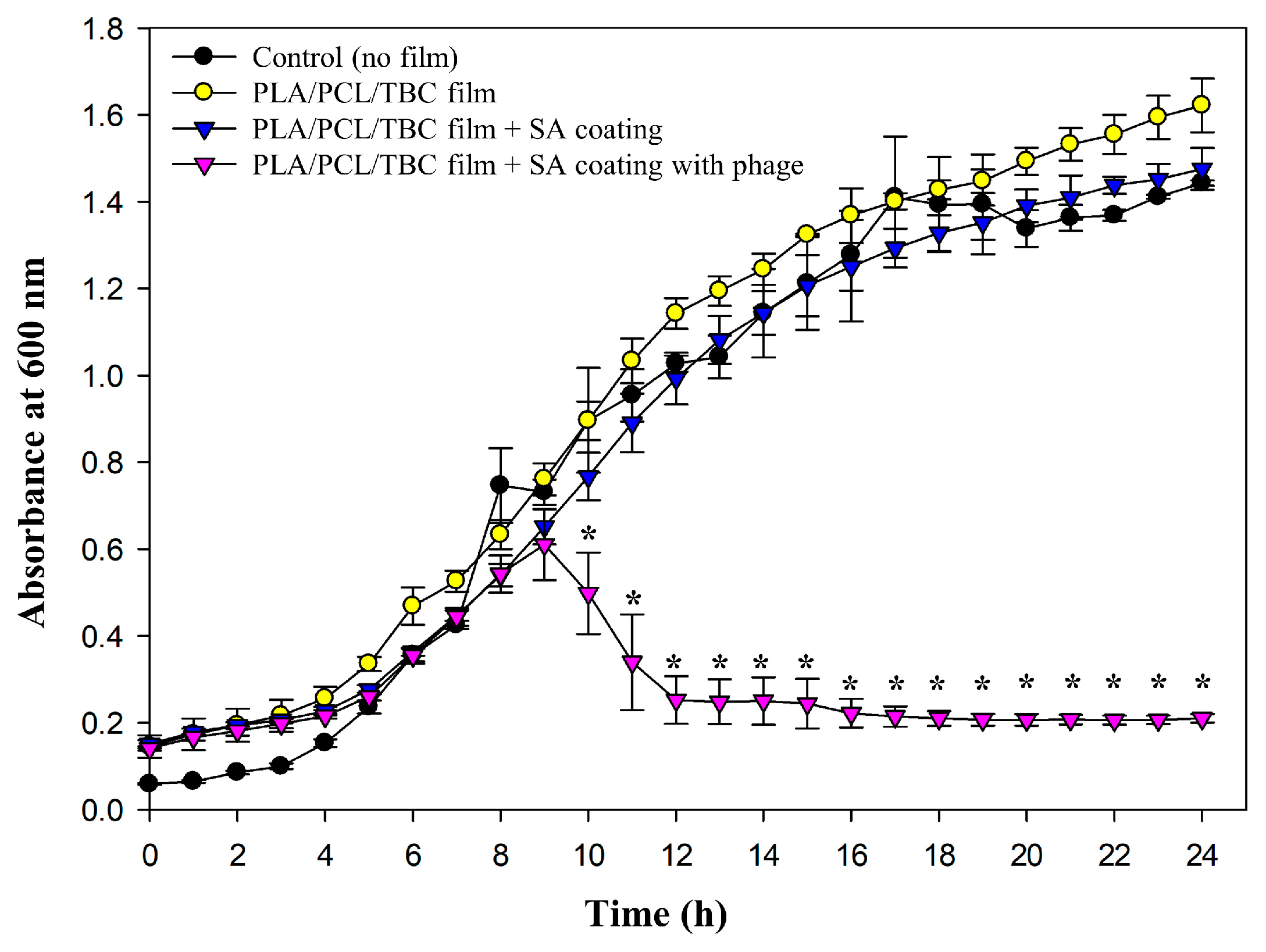
2.6. Antibacterial Efficacy of SA-Coated PLA/PCL/TBC Films Containing S. aureus Phage PBSA08
3. Materials and Methods
3.1. Materials
3.2. Preparation of PLA/PCL/TBC Composite Films
3.3. Sodium Alginate Coating Preparation and Treatment
3.4. Characterization of the Films
3.4.1. Film Thickness and Mechanical Properties
3.4.2. Water Vapor Permeability
3.4.3. Optical Properties
3.4.4. Scanning Electron Microscopy Analysis
3.4.5. Fourier-Transform Infrared (FTIR) Spectroscopy
3.5. Characterization of the SA Coating Solution
3.5.1. Viscosity Analysis
3.5.2. Atomic Force Microscopy
3.6. Bacteriophage (Phage) Preparation
3.6.1. Bacterial Strains and Culture Conditions
3.6.2. Phage Propagation and Stock Production
3.7. Characterization of the Phage PBSA08
3.7.1. Transmission Electron Microscopy (TEM) Analysis
3.7.2. Bacteriolytic Activity
3.7.3. Temperature and pH Stability
3.7.4. Host Range Determination
3.8. Phage DNA Extraction and Genome Sequence Analysis
3.8.1. Genomic DNA Extraction
3.8.2. Genome Sequencing and Analysis
3.9. Antibacterial Efficacy of the Phage-Coated Film
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Peltzer, M.A. Characterization of PLA-limonene blends for food packaging applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.-Y.; Kim, J.; Moon, K.-D. Effects of edible coatings with various natural browning inhibitors on the qualitative characteristics of banana (Musa acuminata Cavendish Subgroup) during storage. Korean J. Food Preserv. 2021, 28, 13–22. [Google Scholar] [CrossRef]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-based polymers with potential for biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Zhang, K.; Ran, X.; Wang, X.; Han, C.; Han, L.; Wen, X.; Zhuang, Y.; Dong, L. Improvement in toughness and crystallization of poly(L -lactic acid) by melt blending with poly(epichlorohydrin-co-ethylene oxide). Polym. Eng. Sci. 2011, 51, 2370–2380. [Google Scholar] [CrossRef]
- Zhang, C.; Zhai, T.; Turng, L.-S.; Dan, Y. Morphological, mechanical, and crystallization behavior of polylactide/polycaprolactone blends compatibilized by L-lactide/caprolactone copolymer. Ind. Eng. Chem. Res. 2015, 54, 9505–9511. [Google Scholar] [CrossRef]
- Patrício, T.; Bártolo, P. Thermal stability of PCL/PLA blends produced by physical blending process. Procedia Eng. 2013, 59, 292–297. [Google Scholar] [CrossRef]
- Semba, T.; Kitagawa, K.; Ishiaku, U.S.; Hamada, H. The effect of crosslinking on the mechanical properties of polylactic acid/polycaprolactone blends. J. Appl. Polym. Sci. 2006, 101, 1816–1825. [Google Scholar] [CrossRef]
- Shin, B.Y.; Han, D.H. Viscoelastic properties of PLA/PCL blends compatibilized with different methods. Korea-Aust. Rheol. J. 2017, 29, 295–302. [Google Scholar] [CrossRef]
- Wang, L.; Ma, W.; Gross, R.A.; McCarthy, S.P. Reactive compatibilization of biodegradable blends of poly(lactic acid) and poly(ε-caprolactone). Polym. Degrad. Stab. 1998, 59, 161–168. [Google Scholar] [CrossRef]
- Jeong, H.; Rho, J.; Shin, J.Y.; Lee, D.Y.; Hwang, T.; Kim, K.J. Mechanical properties and cytotoxicity of PLA/PCL films. Biomed. Eng. Lett. 2018, 8, 267–272. [Google Scholar] [CrossRef]
- Maiza, M.; Benaniba, M.T.; Massardier-Nageotte, V. Plasticizing effects of citrate esters on properties of poly(lactic acid). J. Polym. Eng. 2016, 36, 371–380. [Google Scholar] [CrossRef]
- Abbasifar, R.; Griffiths, M.W.; Sabour, P.M.; Ackermann, H.W.; Vandersteegen, K.; Lavigne, R.; Noben, J.P.; Villa, A.A.; Abbasifar, A.; Nash, J.H.; et al. Supersize me: Cronobacter sakazakii phage GAP32. Virology 2014, 460–461, 138–146. [Google Scholar] [CrossRef]
- Kazi, M.; Annapure, U.S. Bacteriophage biocontrol of foodborne pathogens. J. Food Sci. Technol. 2016, 53, 1355–1362. [Google Scholar] [CrossRef]
- Domingo-Calap, P.; Delgado-Martínez, J. Bacteriophages: Protagonists of a post-antibiotic era. Antibiotics 2018, 7, 66. [Google Scholar] [CrossRef]
- Meireles Gouvêa Boggione, D.; Santos, I.J.B.; Menezes de Souza, S.; Santos Mendonça, R.C. Preparation of polyvinyl alcohol hydrogel containing bacteriophage and its evaluation for potential use in the healing of skin wounds. J. Drug Deliv. Sci. Technol. 2021, 63, 102484. [Google Scholar] [CrossRef]
- Imm, S.; Chang, Y. Evaluation of the biocontrol potential of a collagen peptide/trehalose-based Cronobacter sakazakii phage powder in rehydrated powdered infant formula. Food Res. Int. 2023, 173, 113257. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chang, Y. Anti-Salmonella polyvinyl alcohol coating containing a virulent phage PBSE191 and its application on chicken eggshell. Food Res. Int. 2022, 162, 111971. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xu, F.; Wang, H.; Liu, H.; Yan, F.; Ma, C. Manufacturing technologies of polymer composites—A Review. Polymers 2023, 15, 712. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Conte, A.; Buonocore, G.G.; Incoronato, A.L.; Massaro, A.; Panza, O. Active packaging by extrusion processing of recyclable and biodegradable polymers. J. Food Eng. 2009, 93, 1–6. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Development of biodegradable films loaded with phages with antilisterial properties. Food Control 2015, 55, 66–72. [Google Scholar]
- Pereira, C.; Silva, J.; Teixeira, P. The role of active packaging in the defense against food-borne pathogens with particular attention to bacteriophages. Trends Food Sci. Technol. 2016, 58, 110–121. [Google Scholar]
- Zhang, H.; Xue, J.; Dong, K.; Lu, W.; Gao, S.; Wang, J. Application of bacteriophages in biopolymer-based functional food packaging films. Trends Food Sci. Technol. 2023, 134, 300–311. [Google Scholar]
- Sadeghi, A.; Razavi, S.M.A.; Shahrampour, D. Fabrication and characterization of biodegradable active films with modified morphology based on polycaprolactone-polylactic acid-green tea extract. Int. J. Biol. Macromol. 2022, 205, 341–356. [Google Scholar] [CrossRef]
- Ferri, J.M.; Fenollar, O.; Jorda-Vilaplana, A.; García-Sanoguera, D.; Balart, R. Effect of miscibility on mechanical and thermal properties of poly(lactic acid)/polycaprolactone blends. Polym. Int. 2016, 65, 453–463. [Google Scholar] [CrossRef]
- Qin, Y.; Li, W.; Liu, D.; Yuan, M.; Li, L. Development of active packaging film made from poly (lactic acid) incorporated essential oil. Prog. Org. Coat. 2017, 103, 76–82. [Google Scholar] [CrossRef]
- Wang, X.; Pan, H.; Jia, S.; Wang, Z.; Tian, H.; Han, L.; Zhang, H. In-situ reaction compatibilization modification of poly(butylene succinate-co-terephthalate)/polylactide acid blend films by multifunctional epoxy compound. Int. J. Biol. Macromol. 2022, 213, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Chinsirikul, W.; Rojsatean, J.; Hararak, B.; Kerddonfag, N.; Aontee, A.; Jaieau, K.; Kumsang, P.; Sripethdee, C. Flexible and tough poly(lactic acid) films for packaging applications: Property and processability improvement by effective reactive blending. Packag. Technol. Sci. 2015, 28, 741–759. [Google Scholar] [CrossRef]
- Yang, W.; Qi, G.; Ding, H.; Xu, P.; Dong, W.; Zhu, X.; Zheng, T.; Ma, P. Biodegradable poly (lactic acid)-poly (ε-caprolactone)-nanolignin composite films with excellent flexibility and UV barrier performance. Compos. Commun. 2020, 22, 100497. [Google Scholar] [CrossRef]
- Fortelny, I.; Ujcic, A.; Fambri, L.; Slouf, M. Phase structure, compatibility, and toughness of PLA/PCL blends: A review. Front. Mater. 2019, 6, 206. [Google Scholar] [CrossRef]
- Wang, L.-F.; Rhim, J.W.; Hong, S.I. Preparation of poly(lactide)/poly(butylene adipate-co-terephthalate) blend films using a solvent casting method and their food packaging application. LWT—Food Sci. Technol. 2016, 68, 454–461. [Google Scholar] [CrossRef]
- Paul, U.C.; Fragouli, D.; Bayer, I.S.; Zych, A.; Athanassiou, A. Effect of green plasticizer on the performance of microcrystalline cellulose/polylactic acid biocomposites. ACS Appl. Polym. Mater. 2021, 3, 3071–3081. [Google Scholar] [CrossRef]
- Kumar, M.; Mohanty, S.; Nayak, S.K.; Parvaiz, M.R. Effect of glycidyl methacrylate (GMA) on the thermal, mechanical and morphological property of biodegradable PLA/PBAT blend and its nanocomposites. Bioresour. Technol. 2010, 101, 8406–8415. [Google Scholar] [CrossRef]
- Phetwarotai, W.; Zawong, M.; Phusunti, N.; Aht-Ong, D. Toughening and thermal characteristics of plasticized polylactide and poly(butylene adipate-co-terephthalate) blend films: Influence of compatibilization. Int. J. Biol. Macromol. 2021, 183, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Ahi, Z.B.; Renkler, N.Z.; Seker, M.G.; Tuzlakoglu, K. Biodegradable polymer films with a natural antibacterial extract as novel periodontal barrier membranes. Int. J. Biomater. 2019, 2019, 7932470. [Google Scholar] [CrossRef] [PubMed]
- Åkerlund, E.; Diez-Escudero, A.; Grzeszczak, A.; Persson, C. The effect of PCL addition on 3D-printable PLA/HA composite filaments for the treatment of bone defects. Polymers 2022, 14, 3305. [Google Scholar] [CrossRef]
- Sundar, N.; Keerthana, P.; Kumar, S.A.; Kumar, G.A.; Ghosh, S. Dual purpose, bio-based polylactic acid (PLA)-polycaprolactone (PCL) blends for coated abrasive and packaging industrial coating applications. J. Polym. Res. 2020, 27, 386. [Google Scholar] [CrossRef]
- Zhong, T.; Wolcott, M.P.; Liu, H.; Wang, J. Developing chitin nanocrystals for flexible packaging coatings. Carbohydr. Polym. 2019, 226, 115276. [Google Scholar] [CrossRef]
- Belalia, F.; Djelali, N.-E. Rheological properties of sodium alginate solutions. Rev. Roum. Chim. 2014, 59, 135–145. [Google Scholar]
- Ma, J.; Lin, Y.; Chen, X.; Zhao, B.; Zhang, J. Flow behavior, thixotropy and dynamical viscoelasticity of sodium alginate aqueous solutions. Food Hydrocoll. 2014, 38, 119–128. [Google Scholar] [CrossRef]
- Duffy, J. Getting the complete picture: How to best measure a viscosity flow curve. Am. Lab. 2016, 48, 26–29. [Google Scholar]
- Ackermann, H.W. Phage classification and characterization. In Bacteriophages: Methods and Protocols; Clokie, M.R.J., Kropinski, A.M., Eds.; Humana Press: Totowa, NJ, USA, 2009; Volume 1, pp. 127–140. [Google Scholar]
- Hamza, A.; Perveen, S.; Abbas, Z.; Rehman, S.U. The lytic SA phage demonstrate bactericidal activity against mastitis causing Staphylococcus aureus. Open Life Sci. 2016, 11, 39–45. [Google Scholar] [CrossRef]
- Chang, Y.; Shin, H.; Lee, J.H.; Park, C.J.; Paik, S.Y.; Ryu, S. Isolation and genome characterization of the virulent Staphylococcus aureus bacteriophage SA97. Viruses 2015, 7, 5225–5242. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.; Marques, A.; Milho, C.; Costa, M.J.; Pastrana, L.M.; Cerqueira, M.A.; Sillankorva, S.M. Bacteriophage ϕIBB-PF7A loaded on sodium alginate-based films to prevent microbial meat spoilage. Int. J. Food Microbiol. 2019, 291, 121–127. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Guo, J.; Yu, Y.; Li, J.; Guo, Y.; Liu, C. Comparing two functions for optical density and cell numbers in bacterial exponential growth phase. J. Pure Appl. Microbiol. 2015, 9, 299–305. [Google Scholar]
- ISO 3219-1:2021; Rheology—Part 1: Vocabulary and Symbols for Rotational and Oscillatory Rheometry. International Organization for Standardization: Geneva, Switzerland, 2021.




| Bacterial Strain | Lysis Sensitivity a | Source b |
|---|---|---|
| Staphylococcus aureus strains | ||
| ATCC 29213 | + + + | ATCC |
| ATCC 6538 | + + + | ATCC |
| ATCC 12600 | + + + | ATCC |
| ATCC 13301 | + + | ATCC |
| ATCC 23235 | + + + | ATCC |
| ATCC 25923 | + + | ATCC |
| ATCC 27664 | + + + | ATCC |
| ATCC 33586 | + + + | ATCC |
| ATCC 33593 | + + | ATCC |
| CCARM 3089 | + + + | CCARM |
| CCARM 3090 | + | CCARM |
| CCARM 3793 | + | CCARM |
| KCCM 12103 | + | KCCM |
| KCTC 1916 | + + + | KCTC |
| Gram-negative bacteria | ||
| Escherichia coli O157:H7 ATCC 43890 | - | ATCC |
| Klebsiella pneumoniae KCTC 2242 | - | KCTC |
| Vibrio parahaemolyticus KCTC 2471 | - | KCTC |
| Vibrio cholerae NCCP 13589 | - | NCCP |
| Shigella flexneri KCTC 2517 | - | KCTC |
| Pseudomonas aeruginosa ATCC 27853 | - | ATCC |
| Yersinia enterocolitica ATCC 55075 | - | ATCC |
| Salmonella enterica Enteritidis ATCC 13076 | - | ATCC |
| Salmonella enterica Typhimurium KCTC 1425 | - | KCTC |
| Pectobacterium carotovorum KACC 21701 | - | KACC |
| Cronobacter sakazakii ATCC 29544 | - | ATCC |
| Gram-positive bacteria | ||
| Listeria monocytogenes ATCC 15313 | - | ATCC |
| Bacillus cereus ATCC 14579 | - | ATCC |
| Enterococcus faecalis ATCC 19433 | - | ATCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imm, S.; Bai, J.; Chang, Y. Development of an Antibacterial Poly(Lactic Acid)/Poly(ε-Caprolactone)/Tributyl Citrate Film Loaded with Staphylococcus aureus Bacteriophages Using a Sodium Alginate Coating. Int. J. Mol. Sci. 2025, 26, 7793. https://doi.org/10.3390/ijms26167793
Imm S, Bai J, Chang Y. Development of an Antibacterial Poly(Lactic Acid)/Poly(ε-Caprolactone)/Tributyl Citrate Film Loaded with Staphylococcus aureus Bacteriophages Using a Sodium Alginate Coating. International Journal of Molecular Sciences. 2025; 26(16):7793. https://doi.org/10.3390/ijms26167793
Chicago/Turabian StyleImm, Seulgi, Jaewoo Bai, and Yoonjee Chang. 2025. "Development of an Antibacterial Poly(Lactic Acid)/Poly(ε-Caprolactone)/Tributyl Citrate Film Loaded with Staphylococcus aureus Bacteriophages Using a Sodium Alginate Coating" International Journal of Molecular Sciences 26, no. 16: 7793. https://doi.org/10.3390/ijms26167793
APA StyleImm, S., Bai, J., & Chang, Y. (2025). Development of an Antibacterial Poly(Lactic Acid)/Poly(ε-Caprolactone)/Tributyl Citrate Film Loaded with Staphylococcus aureus Bacteriophages Using a Sodium Alginate Coating. International Journal of Molecular Sciences, 26(16), 7793. https://doi.org/10.3390/ijms26167793






