IgG4-Mediated Sclerosing Riedel Thyroiditis: A Multidisciplinary Case Study and Literature Review
Abstract
1. Introduction
2. Case Report
2.1. Clinical Assessment
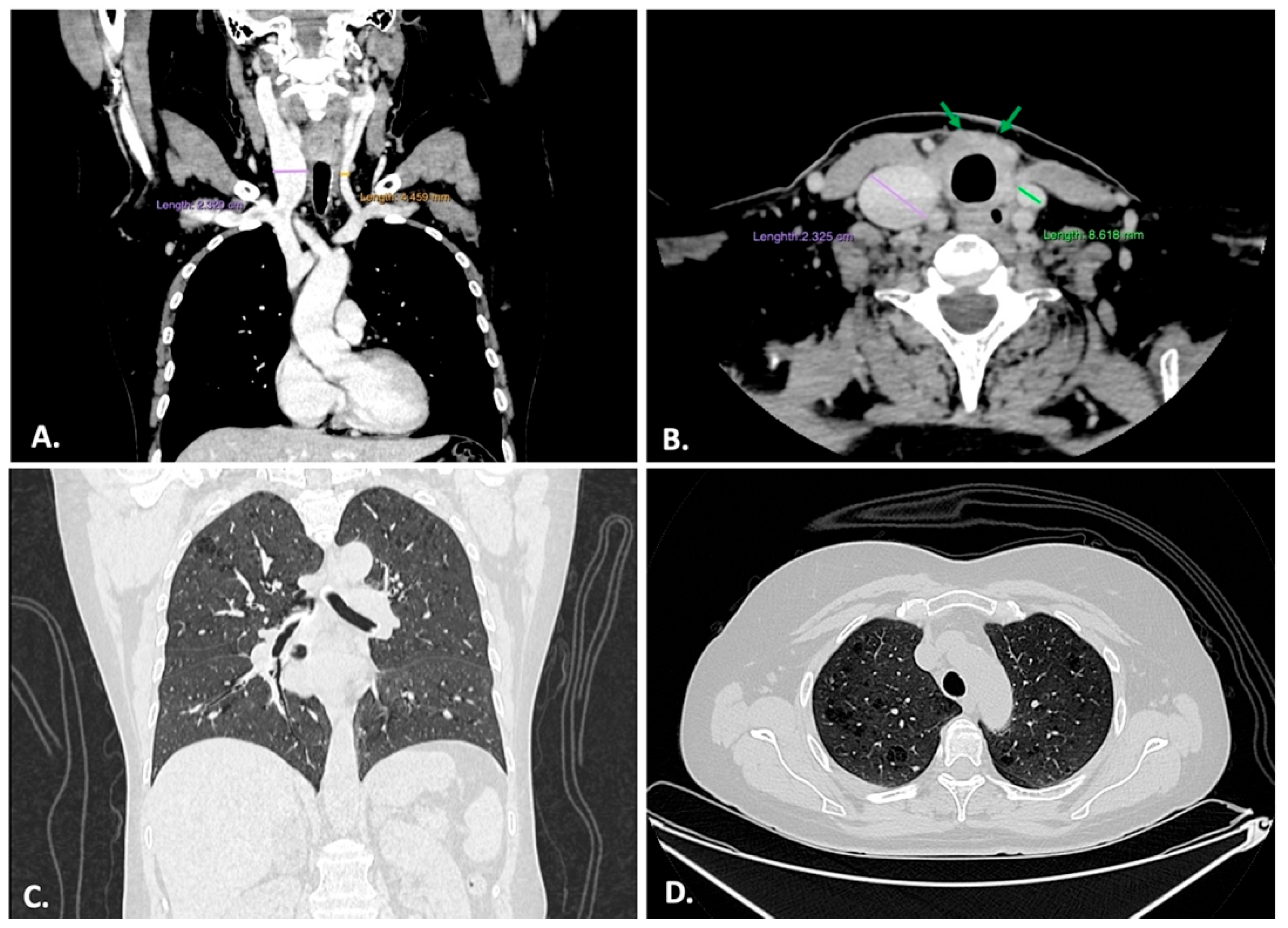
2.2. Imagistic Examination
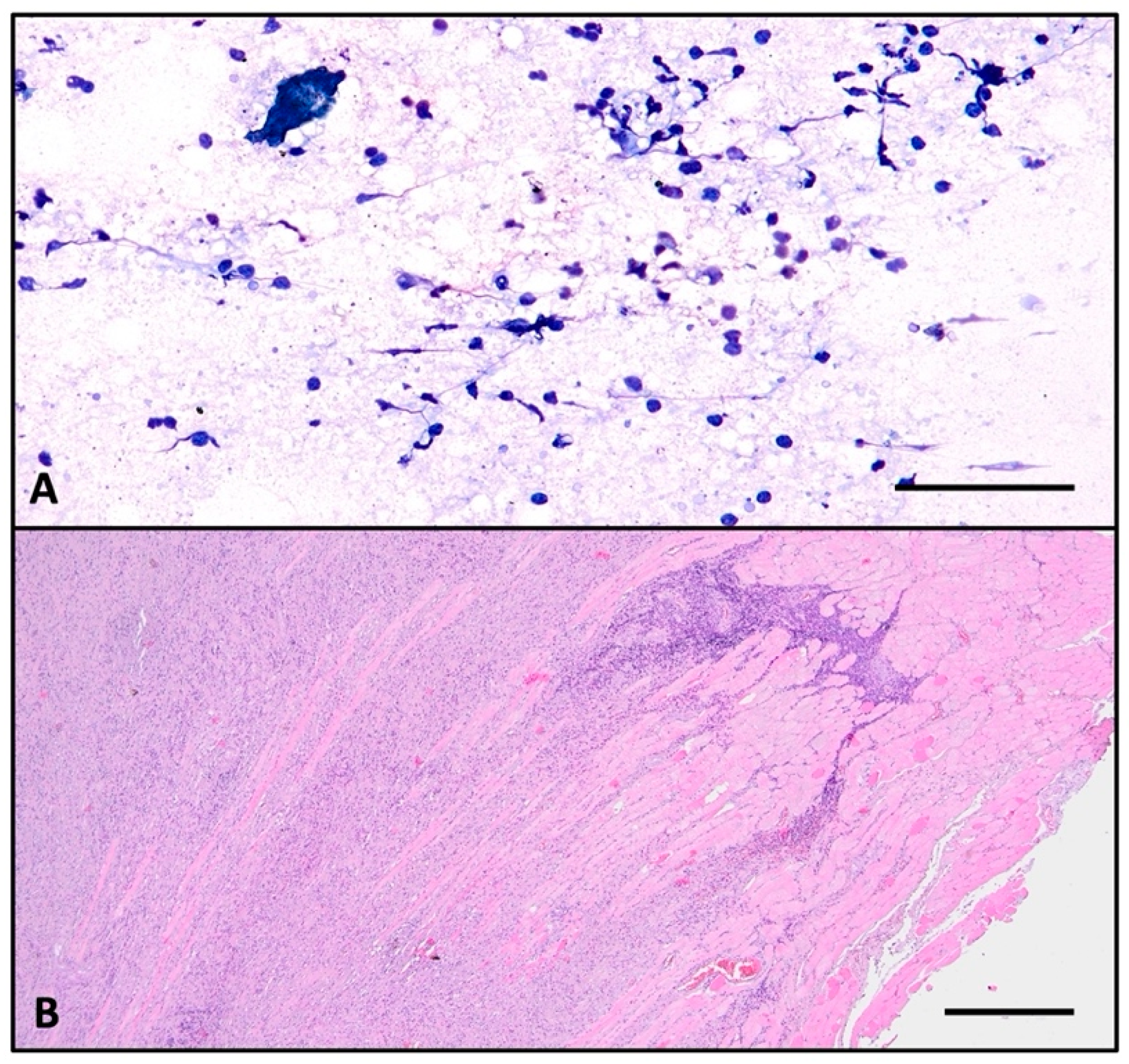
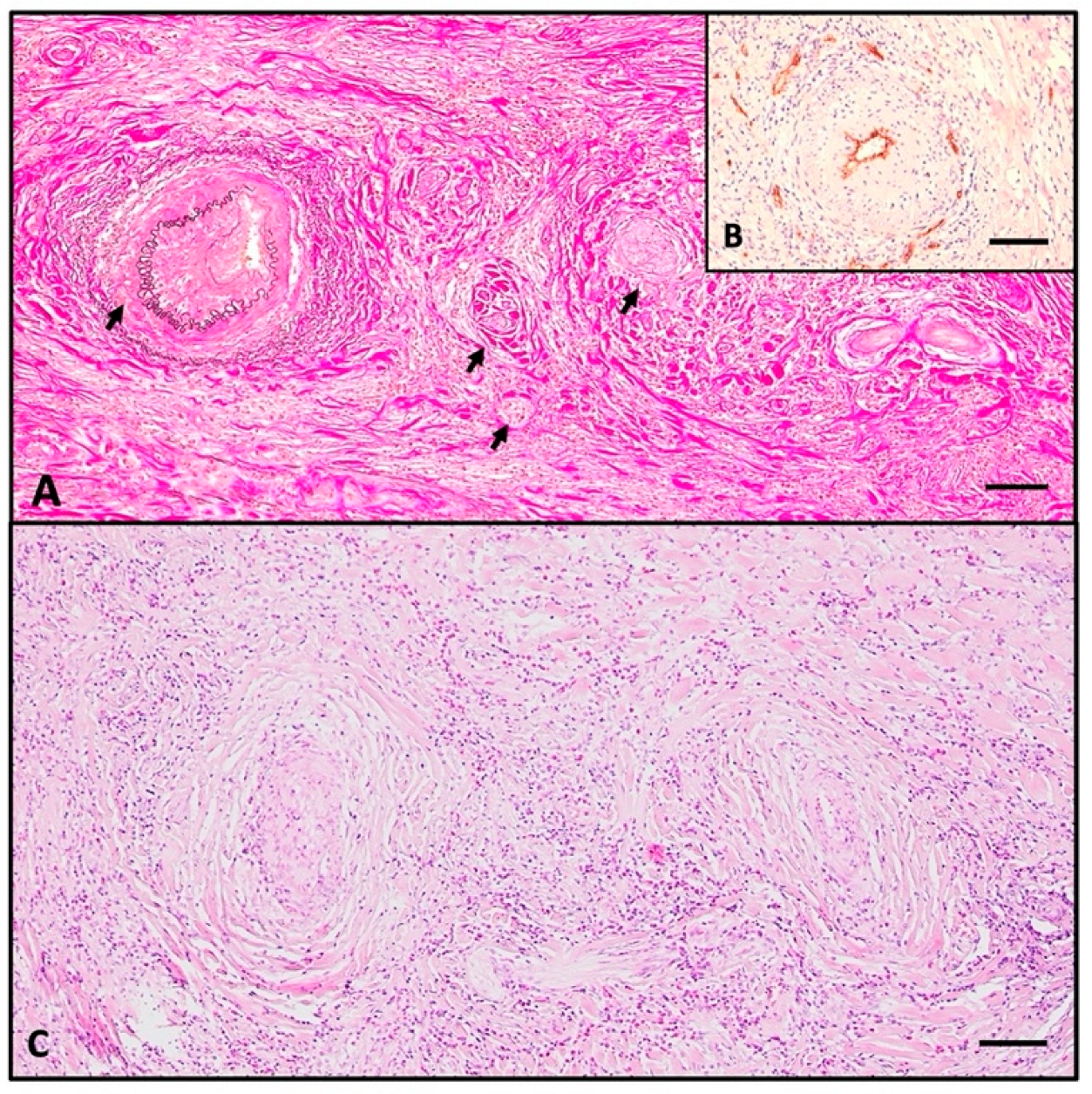
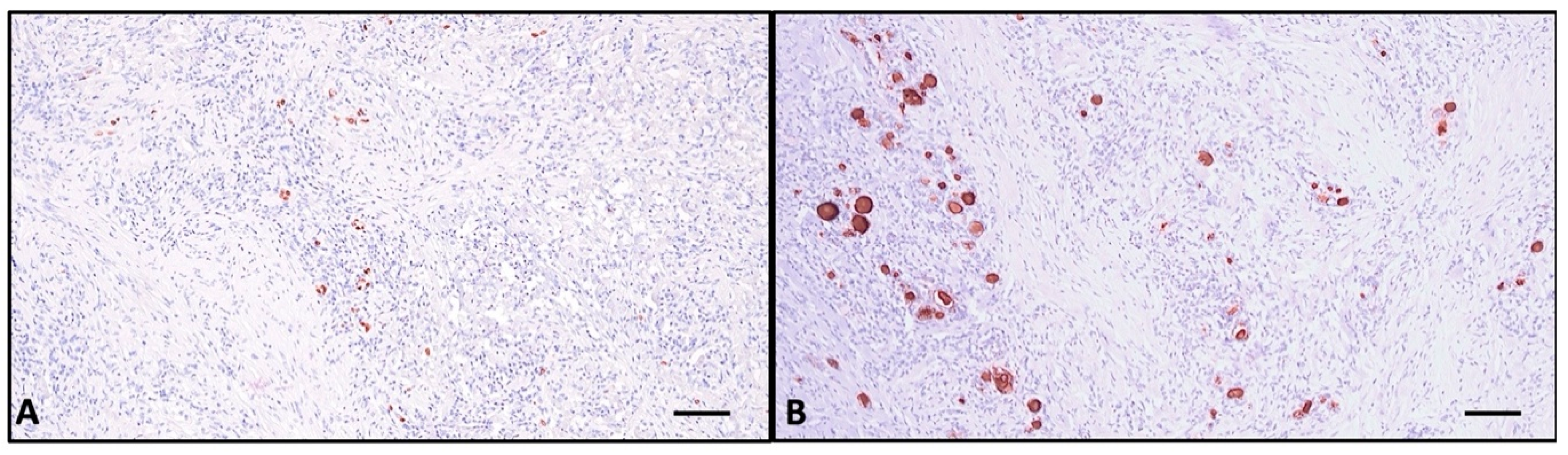
2.3. Histopathologic Investigation
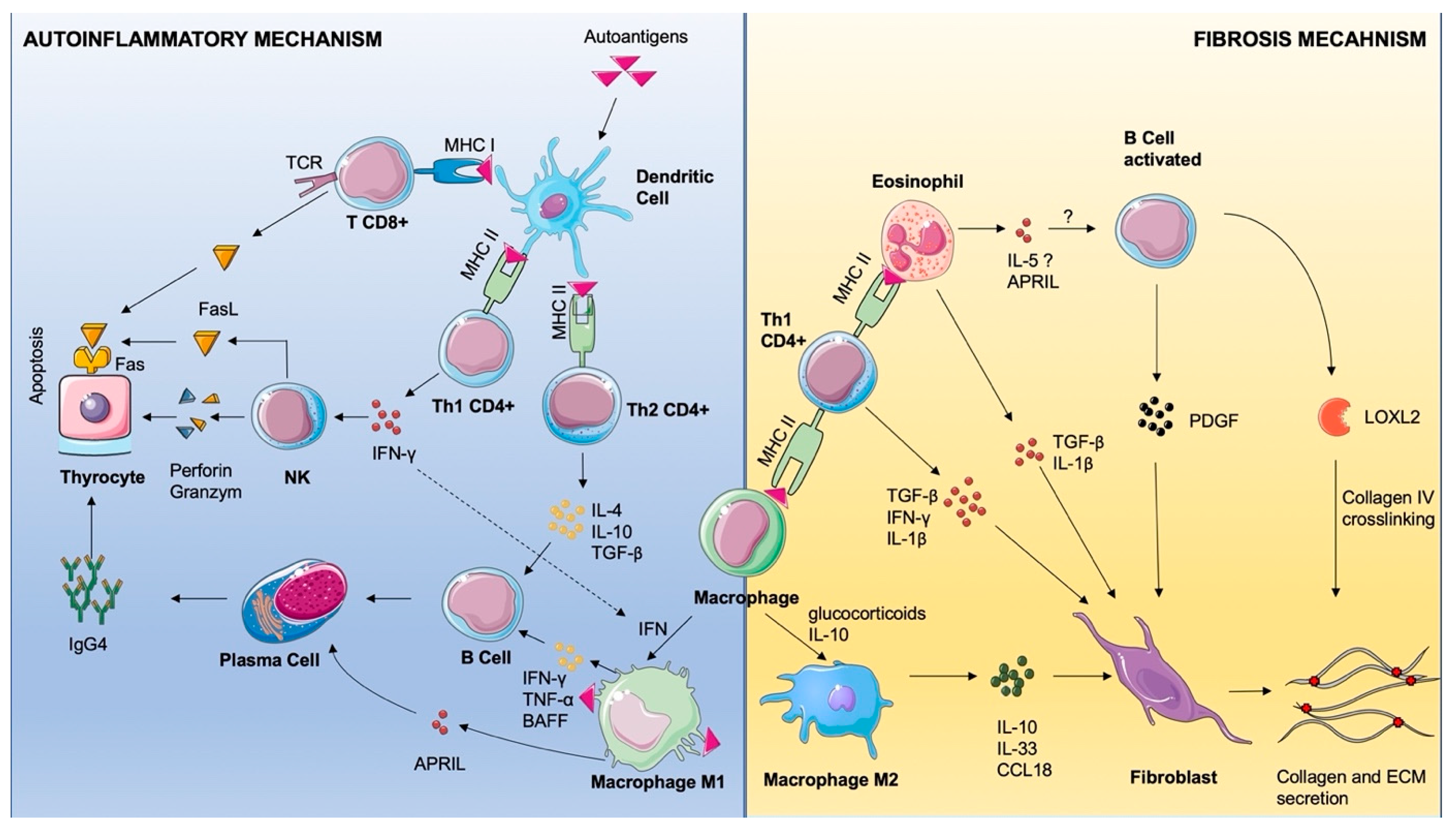
3. Discussion and Literature Review
| First Author | Year | n | Thyroid Function at Presentation | Inflammation | Antithyroid Antibodies | Serum IgG | IgG/IgG4 Tissue > 40% | Main Manifestations | Secondary Fibrosis Involvement | Tobacco Use | Associated Thyroid Condition |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pandev [3] | 2023 | 1 |
| 1 ESR ↑, CRP ↑ (1) | aTP ↑, aTG ↑ | NA | NA |
| NA | NA | Hashimoto’s thyroiditis |
| Falhammar [5] | 2018 | 6 |
| ESR ↑, CRP ↑ (1) | NA | IgG4 ↑ (1) unknown (2) | yes (1) |
| yes (1) | NA | NA |
| Won [8] | 2006 | 1 | NA | NA | NA | NA | NA | NA | NA | NA | goiter |
| Shafi [10] | 2020 | 1 | hypothyroidism | NA | aTP ↑ aTG ↑ | normal | NA |
| no | yes | goiter |
| Sadacharan [13] | 2022 | 6 |
| NA | aTP ↑ aTG ↑ | NA | NA |
| no | NA | NA |
| Fatourechi [14] | 2011 | 21 |
| NA | NA | NA | NA |
| yes (8) | yes (16) | Grave’s disease (3) |
| Lee [15] | 2013 | 1 | hyperthyroidism | ESR N CRP N | aTSH-R ↑ | NA | NA |
| yes | NA | Grave’s disease |
| Schwagerle [16] | 1988 | 1 | NA | NA | NA | NA | NA |
| no | NA | NA |
| Boakye [17] | 2025 | 1 | hypothyroidism | ESR N CRP N | aTP ↑, aTG ↑ | normal | NA | enlarging neck mass | no | yes | hypothyroidism |
| Ezanolue [18] | 2021 | 1 | normal | NA | NA | NA | NA |
| no | no | euthyroid goiter |
| McIver [19] | 2010 | 1 | normal | NA | aTSH-R ↑ | NA | NA |
| no | NA | Grave’s disease |
| Salhi [20] | 2023 | 1 |
| CRP ↑ | NA | NA | NA |
| NA | no | goiter |
| Navarro-Sánchez [22] | 2020 | 1 |
| ESR ↑ | aTP ↑, aTG ↑ | IgG1 N IgG2 ↑ IgG3 N IgG4 ↑ | 40% |
| no | no | hypothyroidism |
| Góralska [23] | 2021 | 1 |
| ESR ↑ CRP ↑ | aTP ↑, aTG ↑ | IgG4 ↑ | NA |
| no | NA | nodular goiter |
| Canpolat [24] | 2020 | 8 |
| ESR ↑ (4) CRP ↑ (7) | aTP ↑, aTG ↑ (3) aTSH-R ↑ | IgG N (8) IgG4 N (8) | yes (2) |
| yes (3) | yes (3) | NA |
| Nielsen [25] | 2003 | 1 |
| ESR ↑ CRP ↑ | aTP ↑ aTSH-R N | NA | NA |
| yes | NA | NA |
| Wang [4] | 2012 | 1 |
| ESR ↑ CRP ↑ | aTP ↑, aTG ↑ aTSH-R N | NA | NA |
| NA | NA | Hashimoto’s thyroiditis |
| Pusztaszeri [26] | 2012 | 1 |
| NA | aTP ↑, aTG ↑ | IgG ↑ IgG4 N | no |
| no | no | goiter |
| Jin [27] | 2022 | 2 |
| NA | aTP ↑ (1) aTG ↑ (2) | IgG4 ↑ (1) | yes (1) |
| NA | NA | NA |
| Amaroui [28] | 2013 | 1 |
| NA | aTP N, aTG N | IgG ↑ IgG4 ↑ | NA |
| retroperitoneum | no | no |
| Takahashi [29] | 2023 | 1 |
| NA | aTG ↑ aTSH-R ↑ | IgG N IgG4 ↑ | yes |
| NA | yes | Grave’s disease |
| Yu [32] | 2021 | 5 | NA | NA | NA | NA | yes (3) no (2) | NA | NA | NA | NA |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| APRIL | A Proliferation-Inducing LigandaTG-anti-thyroglobulin antibody |
| aTPO | antithyroid peroxidase antibody |
| BMI | body mass index |
| CCL | chemokine C-C motif ligand |
| CRP | C-reactive protein |
| ESR | erythrocyte sedimentation rate |
| FasL | Fas ligand |
| FasR | Fas receptor |
| FNAC | Fine-needle aspiration cytology |
| FT4 | free T4 |
| HPF | high-power field |
| IFN-γ | Interferon Gamma |
| IgG4-RD | Immunoglobulin 4-related disease |
| IL | interleukin |
| LOXL2 | Lysyl oxidase-like 2 |
| MHC | Major Histocompatibility Complex |
| PDGF | Platelet-derived growth factor |
| RT | Riedel thyroiditis |
| TCR | T cell r |
| TGF-β | Transforming Growth Factor Beta |
| TI-RADS | Thyroid Imaging Report and Data System |
| TNF-α | Tumor Necrosis Factor Alpha |
| TSH | thyroid-stimulating hormone |
References
- Lopes-Pinto, M.; López-Presa, D.; Lacerda-Nobre, E. Riedel’s Thyroiditis with Life-Threatening Presentation: Diagnosis and Therapeutic Challenges. Endocrinol. Diabetes Nutr. 2023, 70, 288–290. [Google Scholar] [CrossRef]
- Czarnywojtek, A.; Pietrończyk, K.; Thompson, L.D.R.; Triantafyllou, A.; Florek, E.; Sawicka-Gutaj, N.; Ruchała, M.; Płazinska, M.T.; Nixon, I.J.; Shaha, A.R.; et al. IgG4-Related Sclerosing Thyroiditis (Riedel-Struma): A Review of Clinicopathological Features and Management. Virchows Arch. 2023, 483, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Pandev, R.; Khan, M.; Ratheesh, V. Riedel’s Thyroiditis: Pitfalls in Diagnosis and Subsequent Complications. Case Rep. Endocrinol. 2023, 2023, 9989953. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-J.; Wu, T.-J.; Lee, C.-T.; Huang, S.-M. A Misdiagnosed Riedel’s Thyroiditis Successfully Treated by Thyroidectomy and Tamoxifen. J. Formos. Med. Assoc. 2012, 111, 719–723. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Falhammar, H.; Juhlin, C.C.; Barner, C.; Catrina, S.-B.; Karefylakis, C.; Calissendorff, J. Riedel’s Thyroiditis: Clinical Presentation, Treatment and Outcomes. Endocrine 2018, 60, 185–192. [Google Scholar] [CrossRef]
- Stan, M.N.; Sonawane, V.; Sebo, T.J.; Thapa, P.; Bahn, R.S. Riedel’s Thyroiditis Association with IgG4-Related Disease. Clin. Endocrinol. 2017, 86, 425–430. [Google Scholar] [CrossRef]
- Dahlgren, M.; Khosroshahi, A.; Nielsen, G.P.; Deshpande, V.; Stone, J.H. Riedel’s Thyroiditis and Multifocal Fibrosclerosis Are Part of the IgG4-Related Systemic Disease Spectrum. Arthritis Care Res. 2010, 62, 1312–1318. [Google Scholar] [CrossRef]
- Won, Y.S.; Lee, H.H.; Lee, Y.S.; Kim, J.S.; Jeon, H.M.; Jung, S.S.; Lee, J.H.; Park, W.-C. A Case of Riedel’s Thyroiditis Associated with Benign Nodule: Mimic of Anaplastic Transformation. Int. J. Surg. 2008, 6, e24–e27. [Google Scholar] [CrossRef][Green Version]
- Shahi, N.; Abdelhamid, M.F.; Jindall, M.; Awad, R.W. Riedel’s Thyroiditis Masquerading as Anaplastic Thyroid Carcinoma: A Case Report. J. Med. Case Rep. 2010, 4, 15. [Google Scholar] [CrossRef]
- Shafi, A.A.; Saad, N.B.; AlHarthi, B. Riedel’s Thyroiditis as a Diagnostic Dilemma—A Case Report and Review of the Literature. Ann. Med. Surg. 2020, 52, 5–9. [Google Scholar] [CrossRef]
- Hennessey, J.V. Riedel’s Thyroiditis: A Clinical Review. J. Clin. Endocrinol. Metab. 2011, 96, 3031–3041. [Google Scholar] [CrossRef] [PubMed]
- Hunt, L.; Harrison, B.; Bull, M.; Stephenson, T.; Allahabadia, A. Rituximab: A Novel Treatment for Refractory Riedel’s Thyroiditis. Endocrinol. Diabetes Metab. Case Rep. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Sadacharan, D.; Ahmed, A.; Smitha, S.; Mahadevan, S.; Vimala, R.; Prasad, H. Our Uncommon Experience with 6 Cases of Riedel’s Thyroiditis (Woody Thyroiditis). Indian J. Otolaryngol. Head. Neck Surg. 2022, 74, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Fatourechi, M.M.; Hay, I.D.; McIver, B.; Sebo, T.J.; Fatourechi, V. Invasive Fibrous Thyroiditis (Riedel Thyroiditis): The Mayo Clinic Experience, 1976–2008. Thyroid® 2011, 21, 765–772. [Google Scholar] [CrossRef]
- Lee, D.Y.; Moon, J.S.; Kim, G.-E.; Kim, H.K.; Kang, H.-C. Riedel Thyroiditis in a Patient with Graves Disease. Endocrinol. Metab. 2013, 28, 138–143. [Google Scholar] [CrossRef][Green Version]
- Schwaegerle, S.M.; Bauer, T.W.; Esselstyn, C.B. Riedel’s Thyroiditis. Am. J. Clin. Pathol. 1988, 90, 715–722. [Google Scholar] [CrossRef]
- Boakye, J.; Danielle, M.; Maheshwari, N. Riedel’s Thyroiditis Presenting as Rapidly Progressive Hypothyroidism: A Case Report and Literature Review. Int. J. Phys. Med. Rehabil. 2023, 13, 742. [Google Scholar] [CrossRef]
- Ezanolue, B.C. Riedel Thyroiditis: A Novel Case Report from Nigeria. Am. J. Surg. Case Rep. 2021, 2, 1020. [Google Scholar]
- McIver, B.; Fatourechi, M.M.; Hay, I.D.; Fatourechi, V. Graves’ Disease after Unilateral Riedel’s Thyroiditis. J. Clin. Endocrinol. Metab. 2010, 95, 2525–2526. [Google Scholar] [CrossRef]
- Salhi, S.; Oueslati, I.; Ayari, S.; Kamoun, E.; Yazidi, M.; Chihaoui, M. A Case of Reversible Hypoparathyroidism in a Patient with Riedel’s Thyroiditis Treated with Glucocorticoids. Clin. Case Rep. 2023, 11, e7085. [Google Scholar] [CrossRef]
- Carsote, M.; Nistor, C. Reshaping the Concept of Riedel’s Thyroiditis into the Larger Frame of IgG4-Related Disease (Spectrum of IgG4-Related Thyroid Disease). Biomedicines 2023, 11, 1691. [Google Scholar] [CrossRef]
- Navarro-Sánchez, V.; Marín-Castañeda, L.A.; Gallegos, C.A.; Quiroz, O.; Ahumada-Ayala, M. IgG4-Related Fibrous Thyroiditis (Riedel’s Thyroiditis): A Case Report. Am. J. Case Rep. 2020, 21, e928046-1–e928046-5. [Google Scholar] [CrossRef]
- Góralska, M.; Podlewska, M.; Żach, M. Riedel’s Thyroiditis—Difficulties in Differentiating from Thyroid Cancer. Endokrynol. Pol. 2021, 72, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Canpolat, A.G.; Cinel, M.; Sak, S.D.; Taşkaldıran, I.; Korkmaz, H.; Demir, Ö.; Ersoy, R.; Dağdelen, S.; Berker, D.; Dalva, K.; et al. Long-Term Outcomes of Tamoxifen Citrate Therapy and Histo- and Immunopathological Properties in Riedel Thyroiditis. Eur. Thyroid. J. 2021, 10, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.E.; Hecht, P.; Krogdahl, A.S.; Andersen, P.B.; Hegedüs, L. A Rare Case of Orbital Involvement in Riedel’s Thyroiditis. J. Endocrinol. Investig. 2003, 26, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Pusztaszeri, M.; Triponez, F.; Pache, J.-C.; Bongiovanni, M. Riedel’s Thyroiditis with Increased IgG4 Plasma Cells: Evidence for an Underlying IgG4-Related Sclerosing Disease? Thyroid® 2012, 22, 964–968. [Google Scholar] [CrossRef]
- Jin, M.; Kim, B.; Jang, A.; Jeon, M.J.; Choi, Y.J.; Lee, Y.-M.; Song, D.E.; Kim, W.G. Immunoglobulin G4-Related Thyroid Disease: A Single-Center Experience and Literature Review. Endocrinol. Metab. 2022, 37, 312–322. [Google Scholar] [CrossRef]
- Amraouia, A.; Rousseau, E.; Malvaux, P.; Oriot, P. Riedel’s Fibrosing Thyroiditis Associated with Elevated Serum IgG4 Levels. IJCRM 2013, 2013, 501877. [Google Scholar] [CrossRef][Green Version]
- Takahashi, H.; Kajita, S.; Katoh, H.; Matsumoto, T.; Inoue, A.; Sangai, T.; Saegusa, M. Immunoglobulin G4-Related Thyroiditis Associated with Graves’ Disease: A Case Report. Heliyon 2024, 10, e25843. [Google Scholar] [CrossRef]
- Ataya, A.; Harman, E. Case 35. In Rare and Interesting Cases in Pulmonary Medicine; Ataya, A., Harman, E., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 125–127. ISBN 978-0-12-809590-4. [Google Scholar]
- Jakobiec, F.A.; Stacy, R.C.; Hatton, M.P. Clinical Characterization and Immunopathologic Features of Sclerosing Dacryoadenitis and Riedel Thyroiditis. Arch. Ophthalmol. 2010, 128, 1626–1628. [Google Scholar] [CrossRef][Green Version]
- Yu, Y.; Liu, J.; Yu, N.; Zhang, Y.; Zhang, S.; Li, T.; Gao, Y.; Lu, G.; Zhang, J.; Guo, X. IgG4 Immunohistochemistry in Riedel’s Thyroiditis and the Recommended Criteria for Diagnosis: A Case Series and Literature Review. Clin. Endocrinol. 2021, 94, 851–857. [Google Scholar] [CrossRef]
- Yasmeen, F.; Pirzada, R.H.; Ahmad, B.; Choi, B.; Choi, S. Understanding Autoimmunity: Mechanisms, Predisposing Factors, and Cytokine Therapies. Int. J. Mol. Sci. 2024, 25, 7666. [Google Scholar] [CrossRef]
- Cai, S.; Hu, Z.; Chen, Y.; Zhong, J.; Dong, L. Potential Roles of Non-Lymphocytic Cells in the Pathogenesis of IgG4-Related Disease. Front. Immunol. 2022, 13, 940581. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, J.; Wu, Y. Evolving Understanding of Autoimmune Mechanisms and New Therapeutic Strategies of Autoimmune Disorders. Signal Transduct. Target. Ther. 2024, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Perugino, C.A.; Stone, J.H. IgG4-Related Disease: An Update on Pathophysiology and Implications for Clinical Care. Nat. Rev. Rheumatol. 2020, 16, 702–714. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, J.R.; Mattes, J.; Dent, L.A.; Foster, P.S. Eosinophils Promote Allergic Disease of the Lung by Regulating CD4+ Th2 Lymphocyte Function1. J. Immunol. 2001, 167, 3146–3155. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.; Ravasi, T.; Hume, D.A. Interferon-γ: An Overview of Signals, Mechanisms and Functions. J. Leucoc. Biol. 2004, 75, 163–189. Available online: https://jlb.onlinelibrary.wiley.com/doi/full/10.1189/jlb.0603252 (accessed on 26 May 2025). [CrossRef]
- Long, H.; Lichtnekert, J.; Andrassy, J.; Schraml, B.U.; Romagnani, P.; Anders, H.-J. Macrophages and Fibrosis: How Resident and Infiltrating Mononuclear Phagocytes Account for Organ Injury, Regeneration or Atrophy. Front. Immunol. 2023, 14, 1194988. [Google Scholar] [CrossRef]
- Mackay, F.; Schneider, P.; Rennert, P.; Browning, J. BAFF and APRIL: A Tutorial on B Cell Survival. Annu. Rev. Immunol. 2003, 21, 231–264. [Google Scholar] [CrossRef]
- Barros, M.H.M.; Hauck, F.; Dreyer, J.H.; Kempkes, B.; Niedobitek, G. Macrophage Polarisation: An Immunohistochemical Approach for Identifying M1 and M2 Macrophages. PLoS ONE 2013, 8, e80908. [Google Scholar] [CrossRef]
- Desgeorges, T.; Caratti, G.; Mounier, R.; Tuckermann, J.; Chazaud, B. Glucocorticoids Shape Macrophage Phenotype for Tissue Repair. Front. Immunol. 2019, 10, 1591. [Google Scholar] [CrossRef]
- Liu, J.; Yin, W.; Westerberg, L.S.; Lee, P.; Gong, Q.; Chen, Y.; Dong, L.; Liu, C. Immune Dysregulation in IgG4-Related Disease. Front. Immunol. 2021, 12, 738540. Available online: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2021.738540/full (accessed on 28 April 2025). [CrossRef] [PubMed]
- Gratchev, A.; Kzhyshkowska, J.; Utikal, J.; Goerdt, S. Interleukin-4 and Dexamethasone Counterregulate Extracellular Matrix Remodelling and Phagocytosis in Type-2 Macrophages. Scand. J. Immunol. 2005, 61, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, M.; Jia, S. Macrophage: Key Player in the Pathogenesis of Autoimmune Diseases. Front. Immunol. 2023, 14, 1080310. [Google Scholar] [CrossRef] [PubMed]
- Akinori, H.; Norihiko, S.; Kengo, F.; Yoshio, S.; Motohiro, T.; Richard, B.; Naofumi, M.; Yoh, T.; Kouji, M.; Shuichi, K.; et al. CCL2/CCR2 Augments the Production of Transforming Growth Factor-Beta1, Type 1 Collagen and CCL2 by Human CD45-/Collagen 1-Positive Cells under High Glucose Concentrations. Clin. Exp. Nephrol. 2013, 17, 793–804. Available online: https://link.springer.com/article/10.1007/s10157-013-0796-6 (accessed on 28 April 2025).[Green Version]
- Wynn, T. Cellular and Molecular Mechanisms of Fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Della-Torre, E.; Rigamonti, E.; Perugino, C.; Baghai-Sain, S.; Sun, N.; Kaneko, N.; Maehara, T.; Rovati, L.; Ponzoni, M.; Milani, R.; et al. B Lymphocytes Directly Contribute to Tissue Fibrosis in Patients with IgG4-Related Disease. J. Allergy Clin. Immunol. 2020, 145, 968–981.e14. [Google Scholar] [CrossRef]
- Davoine, F.; Lacy, P. Eosinophil Cytokines, Chemokines, and Growth Factors: Emerging Roles in Immunity. Front. Immunol. 2014, 5, 570. [Google Scholar] [CrossRef]


| Year | Laboratory Test | Patient Values | Normal Range |
|---|---|---|---|
| 2023 | TSH | 0.4 μUI/mL | 0.35–4.94 |
| FT4 | 12.8 pmol/L | 12–22 | |
| aTPO | negative | negative * | |
| aTG | positive | negative * | |
| 2024 | ESR | 25 mm/h | 2–25 |
| fibrinogen | 480 mg/dL | 200–400 | |
| CRP | 2.3 mg/dL | 0–0.5 | |
| TSH | 2.02 μUI/mL | 0.35–4.94 | |
| FT4 | 18 pmol/L | 12–22 | |
| 2025 before surgery | ESR | 19 mm/h | 2–25 |
| fibrinogen | 681 mg/dL | 200–400 | |
| CRP | 3.18 mg/dL | 0–0.5 | |
| TSH | 1.53 μUI/mL | 0.35–4.94 | |
| FT4 | 16.23 pmol/L | 12–22 | |
| aTPO | 41.47 UI/mL | 0–5.6 | |
| aTG | 55.26 UI/mL | 0–115 | |
| 2025 after surgery | IgG1 | 3.9 g/L | 4.05–10.11 |
| IgG2 | 1.24 g/L | 1.69–7.86 | |
| IgG3 | 0.32 g/L | 0.11–0.85 | |
| IgG4 | 0.00 g/L | 0.03–2.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioachim, D.; Publik, M.A.; Terzea, D.; Cristea, C.A.; Ghemigian, A.M.; Dumitrascu, A.; Petrova, E.; Voinea, A.; Smarandache, R.; Ceausu, M. IgG4-Mediated Sclerosing Riedel Thyroiditis: A Multidisciplinary Case Study and Literature Review. Int. J. Mol. Sci. 2025, 26, 7786. https://doi.org/10.3390/ijms26167786
Ioachim D, Publik MA, Terzea D, Cristea CA, Ghemigian AM, Dumitrascu A, Petrova E, Voinea A, Smarandache R, Ceausu M. IgG4-Mediated Sclerosing Riedel Thyroiditis: A Multidisciplinary Case Study and Literature Review. International Journal of Molecular Sciences. 2025; 26(16):7786. https://doi.org/10.3390/ijms26167786
Chicago/Turabian StyleIoachim, Dumitru, Mihai Alin Publik, Dana Terzea, Carmen Adina Cristea, Adina Mariana Ghemigian, Anda Dumitrascu, Eugenia Petrova, Alexandra Voinea, Romeo Smarandache, and Mihail Ceausu. 2025. "IgG4-Mediated Sclerosing Riedel Thyroiditis: A Multidisciplinary Case Study and Literature Review" International Journal of Molecular Sciences 26, no. 16: 7786. https://doi.org/10.3390/ijms26167786
APA StyleIoachim, D., Publik, M. A., Terzea, D., Cristea, C. A., Ghemigian, A. M., Dumitrascu, A., Petrova, E., Voinea, A., Smarandache, R., & Ceausu, M. (2025). IgG4-Mediated Sclerosing Riedel Thyroiditis: A Multidisciplinary Case Study and Literature Review. International Journal of Molecular Sciences, 26(16), 7786. https://doi.org/10.3390/ijms26167786







