Euphorbia hypericifolia Attenuates Citrinin-Induced Oxidative Stress and Maintains Tight Junction Integrity in Porcine Intestinal Epithelial Cells
Abstract
1. Introduction
2. Results
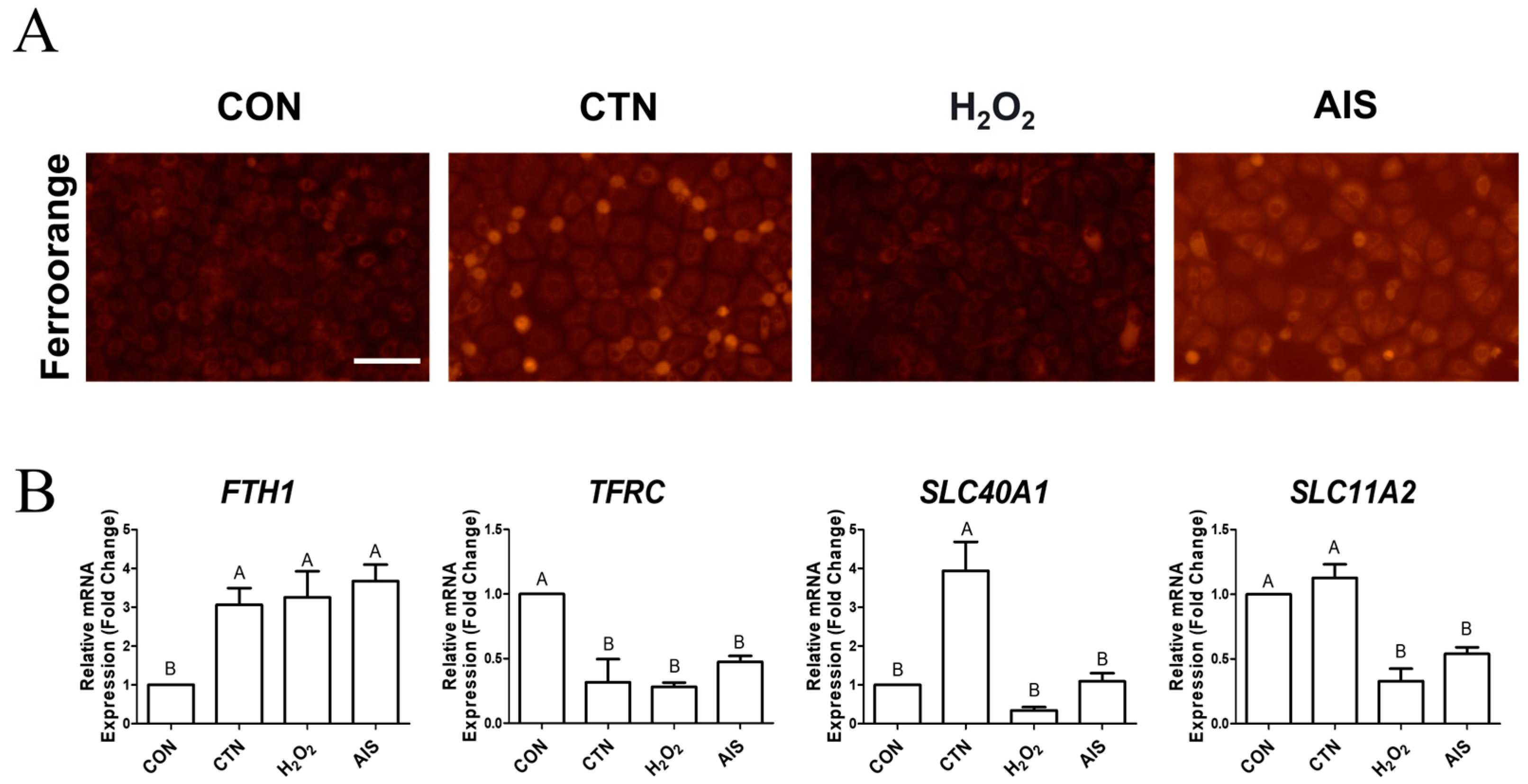
2.1. CTN Induces Intracellular Iron Accumulation
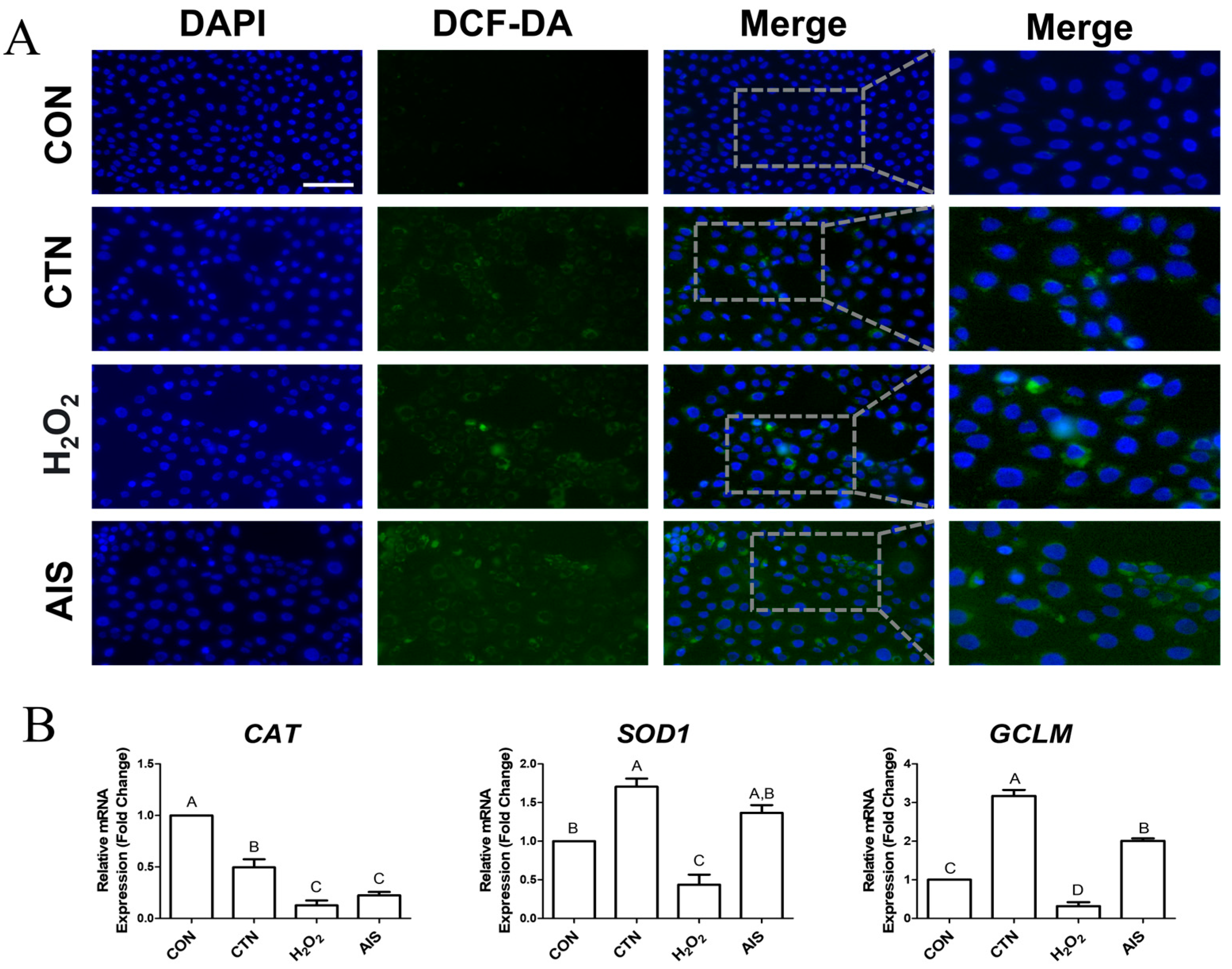
2.2. CTN Induces the Production of Intracellular ROS
2.3. CTN Interferes with Tight Junctions
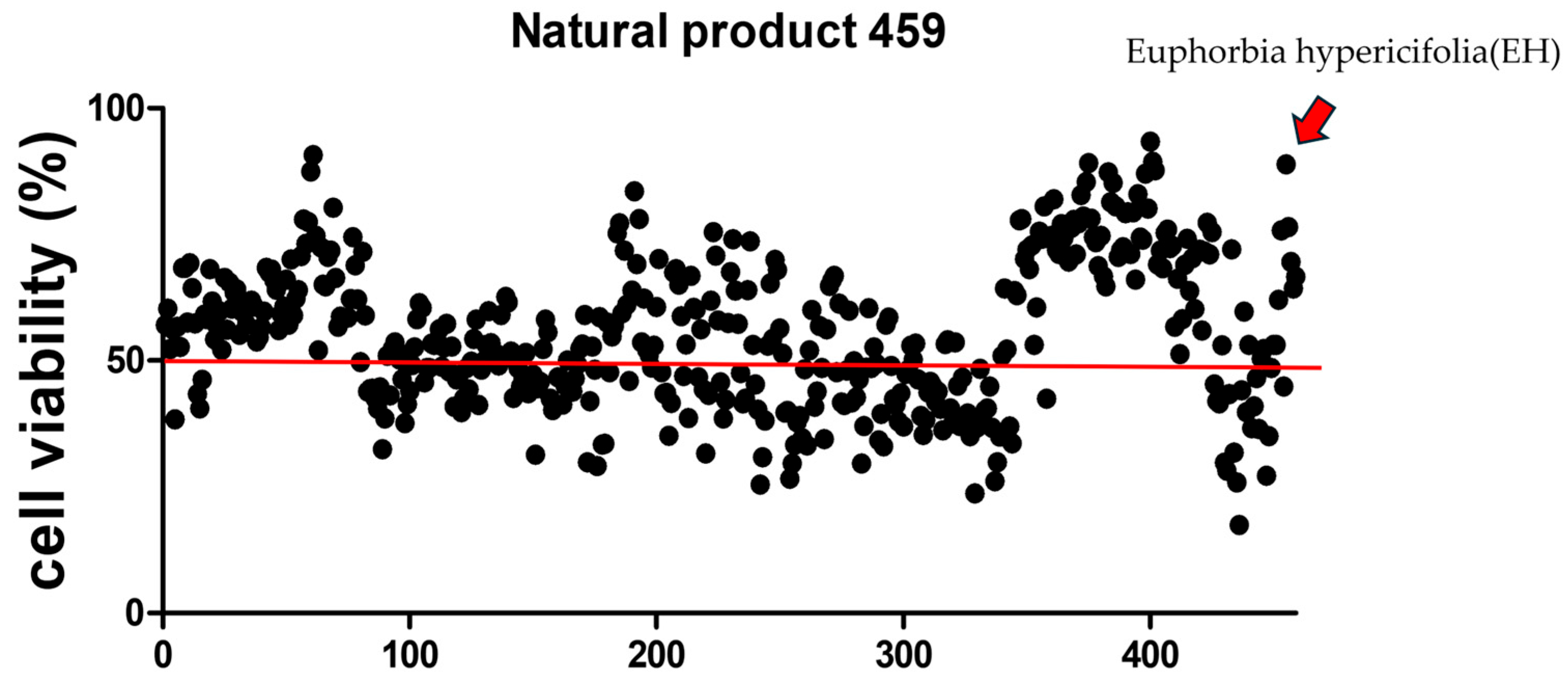
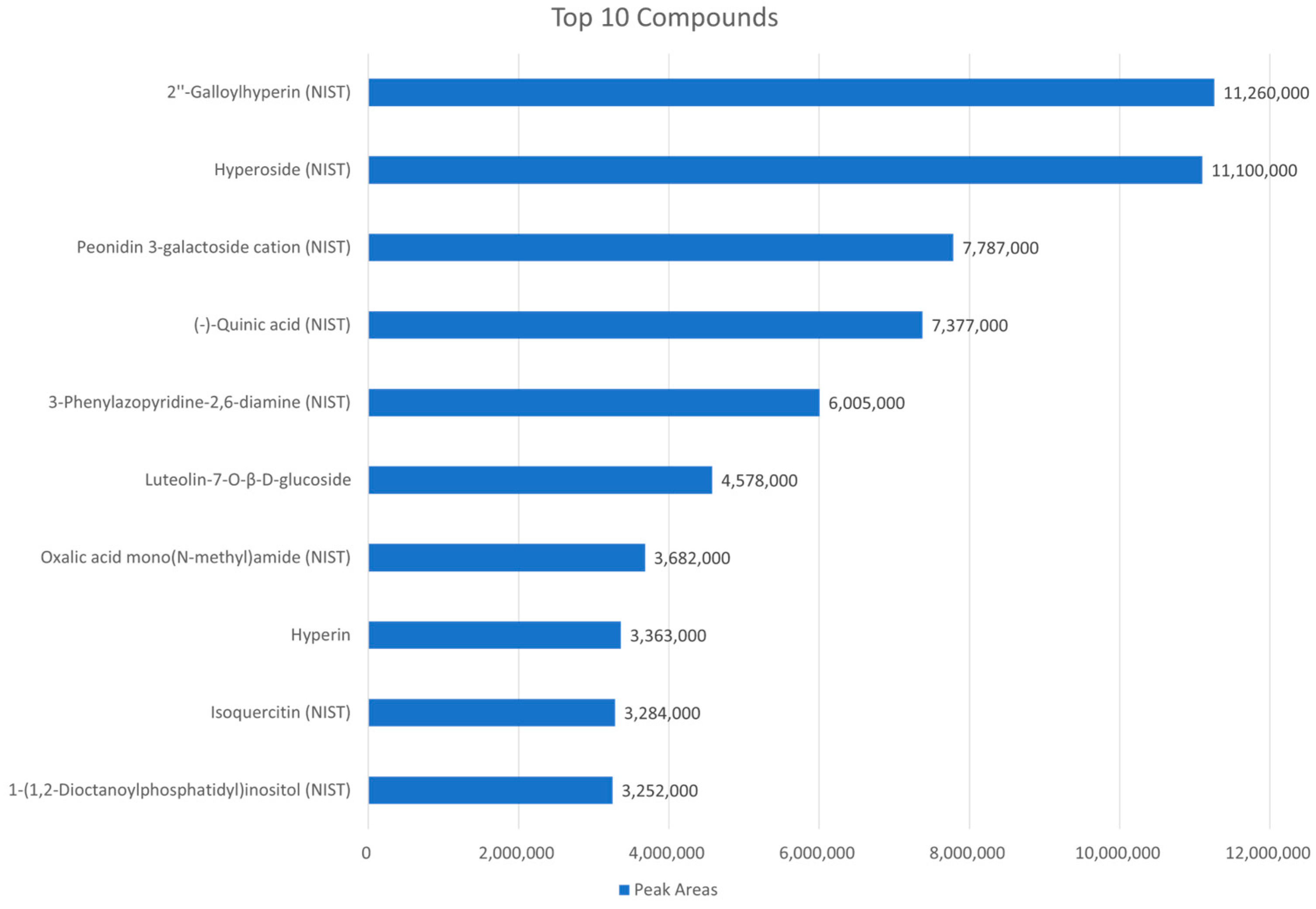
2.4. High-Throughput Screening of Natural Products to Assess Their Capacity to Alleviate CTN Toxicity
2.5. EH Alleviates CTN-Induced Intracellular Iron Accumulation
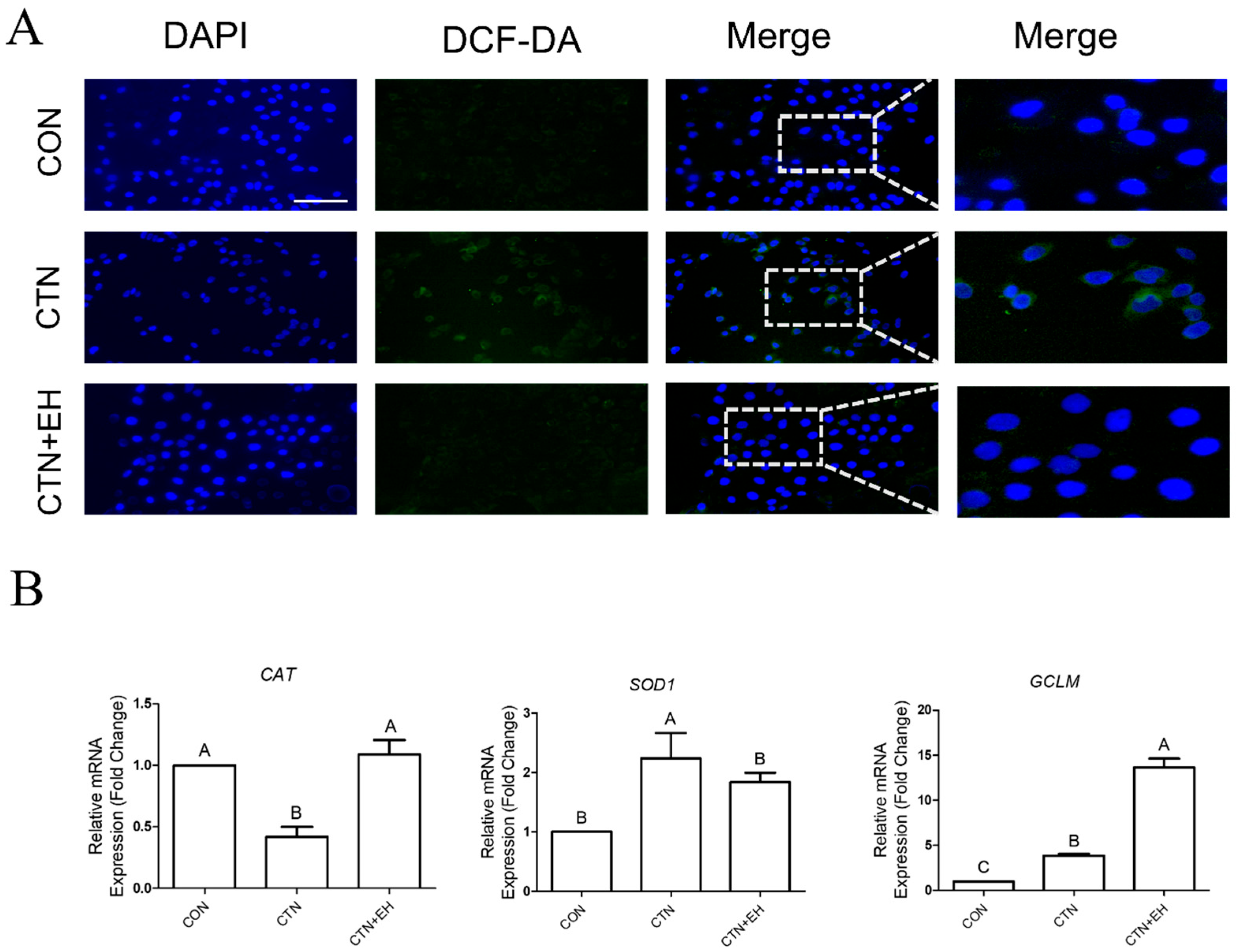
2.6. EH Alleviates the CTN-Induced ROS Accumulation
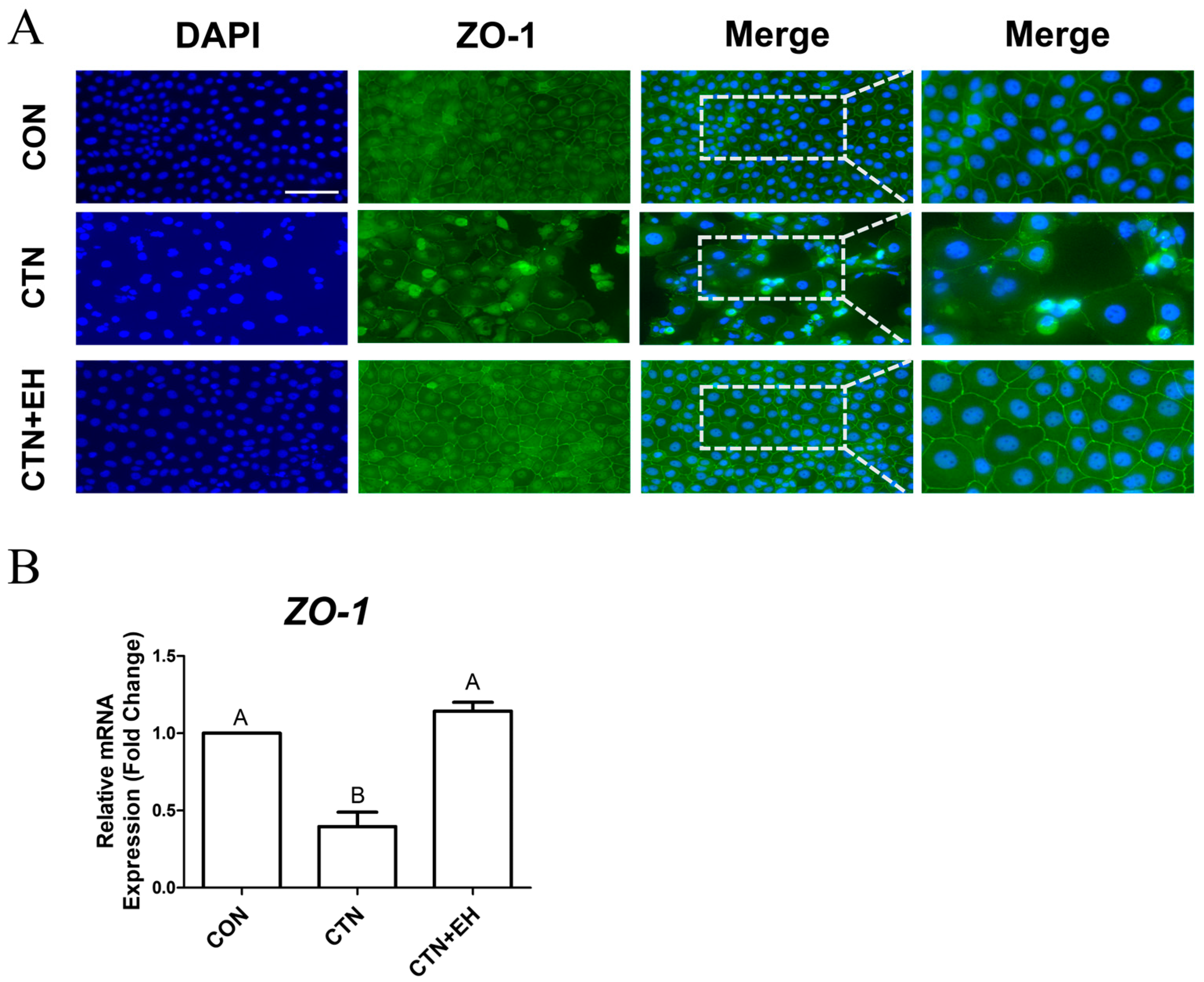
2.7. EH Alleviates the Disruption of Tight Junctions Induced by CTN
3. Discussion
4. Materials and Methods
4.1. Cell Culturing
4.2. RT-qPCR
4.3. Measurement of Fe2+ Levels
4.4. Intracellular ROS Detection
4.5. Immunofluorescence
4.6. Cell Viability Assay
4.7. High-Throughput Screening
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTN | Citrinin |
| EH | Euphorbia hypericifolia |
| TJ | Tight junction |
| ROS | Reactive Oxygen Species |
| Fe2+ | Ferrous Ion |
| DCF-DA | 2′,7′-Dichlorodihydrofluorescein Diacetate |
| IPEC-J2 | Intestinal Porcine Epithelial Cells-J2 |
| H2O2 | hydrogen peroxide |
| SIA | ammonium iron(II) sulfate |
References
- Pleadin, J.; Frece, J.; Markov, K. Mycotoxins in food and feed. Adv. Food Nutr. Res. 2019, 89, 297–345. [Google Scholar] [CrossRef]
- Akbari, P.; Braber, S.; Varasteh, S.; Alizadeh, A.; Garssen, J.; Fink-Gremmels, J. The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins. Arch. Toxicol. 2017, 91, 1007–1029. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.; Bao, X.; Luo, C.; Yang, H.; Wang, J.; Zhao, S.; Zheng, N. Transcriptional and Proteomic Analysis Revealed a Synergistic Effect of Aflatoxin M1 and Ochratoxin A Mycotoxins on the Intestinal Epithelial Integrity of Differentiated Human Caco-2 Cells. J. Proteome Res. 2018, 17, 3128–3142. [Google Scholar] [CrossRef]
- Huang, B.; Wang, J.; Gu, A.; Wang, T.; Li, J.; Shan, A. Zearalenone-Induced Mechanical Damage of Intestinal Barrier via the RhoA/ROCK Signaling Pathway in IPEC-J2 Cells. Int. J. Mol. Sci. 2022, 23, 12550. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zou, L.; Zhao, J.; Zhu, Y. Resveratrol alleviates fumonisin-induced intestinal cytotoxicity by modulating apoptosis, tight junction, and inflammation in IPEC-J2 porcine intestinal epithelial cells. Environ. Toxicol. 2024, 39, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Chen, L.; Peng, Z.; Wang, D.; Song, Y.; Wang, H.; Yao, P.; Yan, H.; Nüssler, A.K.; Liu, L.; et al. Embryotoxicity Caused by DON-Induced Oxidative Stress Mediated by Nrf2/HO-1 Pathway. Toxins 2017, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Zhu, H.; Li, J.; Chen, L.; Zhang, R.; Fu, Q.; Li, X.; Cao, C. Fumonisin B(1) promotes germ cells apoptosis associated with oxidative stress-related Nrf2 signaling in mice testes. Chem. Biol. Interact. 2022, 363, 110009. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Shin, S.; Park, J.; Lee, B.R.; Lee, S.I. TLR/MyD88-Mediated Inflammation Induced in Porcine Intestinal Epithelial Cells by Ochratoxin A Affects Intestinal Barrier Function. Toxics 2023, 11, 437. [Google Scholar] [CrossRef]
- Wu, J.; Yang, C.; Yang, M.; Liang, Z.; Wu, Y.; Kong, X.; Fan, H.; Wang, S.; Ning, C.; Xiao, W.; et al. The role of ER stress and ATP/AMPK in oxidative stress meditated hepatotoxicity induced by citrinin. Ecotoxicol. Environ. Saf. 2022, 237, 113531. [Google Scholar] [CrossRef]
- Abudayyak, M.; Karaman, E.F.; Ozden, S. Mechanisms underlying citrinin-induced toxicity via oxidative stress and apoptosis-mediated by mitochondrial-dependent pathway in SH-SY5Y cells. Drug Chem. Toxicol. 2023, 46, 944–954. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public. Health 2021, 18, 12836. [Google Scholar] [CrossRef]
- Nakayama, H.; Kitagawa, N.; Otani, T.; Iida, H.; Anan, H.; Inai, T. Ochratoxin A, citrinin and deoxynivalenol decrease claudin-2 expression in mouse rectum CMT93-II cells. Microscopy 2018, 67, 99–111. [Google Scholar] [CrossRef]
- Silva, L.J.G.; Pereira, A.; Pena, A.; Lino, C.M. Citrinin in Foods and Supplements: A Review of Occurrence and Analytical Methodologies. Foods 2020, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, L.; Oiao, X.; Ye, L.; Wang, H.; Wang, M.; Sang, J.; Tian, F.; Ge, R.S.; Wang, Y. Citrinin inhibits the function of Leydig cells in male rats in prepuberty. Ecotoxicol. Environ. Saf. 2023, 252, 114568. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Wu, Y.; Wu, A.; Xiao, B.; Liu, X.; Zhang, Q.; Feng, Y.; Yuan, Z.; Yi, J.; et al. Endoplasmic reticulum stress promotes oxidative stress, inflammation, and apoptosis: A novel mechanism of citrinin-induced renal injury and dysfunction. Ecotoxicol. Environ. Saf. 2024, 284, 116946. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2022, 24, 449. [Google Scholar] [CrossRef]
- Liu, J.; Kang, R.; Tang, D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022, 289, 7038–7050. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Dwivedi, P.; Sharma, A.K.; Sankar, M.; Patil, R.D.; Singh, N.D. Apoptosis and lipid peroxidation in ochratoxin A- and citrinin-induced nephrotoxicity in rabbits. Toxicol. Ind. Health 2014, 30, 90–98. [Google Scholar] [CrossRef]
- Niu, R.; Lan, J.; Liang, D.; Xiang, L.; Wu, J.; Zhang, X.; Li, Z.; Chen, H.; Geng, L.; Xu, W.; et al. GZMA suppressed GPX4-mediated ferroptosis to improve intestinal mucosal barrier function in inflammatory bowel disease. Cell Commun. Signal 2024, 22, 474. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Dwivedi, P.D.; Dhawan, A.; Das, M.; Ansari, K.M. Citrinin-generated reactive oxygen species cause cell cycle arrest leading to apoptosis via the intrinsic mitochondrial pathway in mouse skin. Toxicol. Sci. 2011, 122, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Stoev, S.D. Natural feed additives and bioactive supplements versus chemical additives as a safe and practical approach to combat foodborne mycotoxicoses. Front. Nutr. 2024, 11, 1335779. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Benjamaa, R.; Moujanni, A.; Kaushik, N.; Choi, E.H.; Essamadi, A.K.; Kaushik, N.K. Euphorbia species latex: A comprehensive review on phytochemistry and biological activities. Front. Plant Sci. 2022, 13, 1008881. [Google Scholar] [CrossRef]
- Lim, S.J.; Shin, S.; Lee, S.I. 4’-Hydroxydehydrokawain Mitigate the Cytotoxicity of Citrinin in Porcine Intestinal Epithelial Cells. Toxics 2025, 13, 315. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.S.; Yang, J.J.; Yu, F.Y.; Liu, B.H. Evaluation of nephrotoxic effects of mycotoxins, citrinin and patulin, on zebrafish (Danio rerio) embryos. Food Chem. Toxicol. 2012, 50, 4398–4404. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, N.; Li, Y.H.; Zhao, L.; Yang, M.; Jin, Y.; Xu, Y.N.; Guo, H. Citrinin exposure affects oocyte maturation and embryo development by inducing oxidative stress-mediated apoptosis. Oncotarget 2017, 8, 34525–34533. [Google Scholar] [CrossRef]
- Wu, D.; Yang, C.; Yang, M.; Wu, Y.; Mao, Y.; Zhou, X.; Wang, J.; Yuan, Z.; Wu, J. Citrinin-Induced Hepatotoxicity in Mice Is Regulated by the Ca2+/Endoplasmic Reticulum Stress Signaling Pathway. Toxins 2022, 14, 259. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Liu, X.; Wang, Y.; Yang, C.; Wu, Y.; Xiao, B.; Feng, Y.; Wu, A.; Yi, J.; et al. Citrinin-Induced Intestinal Onset of Pyroptosis via the IP3R1-GRP75-VDAC1 Complex-Mediated Mitochondrial Oxidative Stress. J. Agric. Food Chem. 2025, 73, 5803–5815. [Google Scholar] [CrossRef]
- Diaz de Barboza, G.; Guizzardi, S.; Moine, L.; Tolosa de Talamoni, N. Oxidative stress, antioxidants and intestinal calcium absorption. World J. Gastroenterol. 2017, 23, 2841–2853. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Zhang, Q.; Jiang, Z.; Zhang, W.; Yang, C.; Ni, J.; Deng, S.; Yi, J.; Wu, J.; et al. Koumine mediates the generation of mtROS through the IP3R1-GRP75-VDAC1 complex to improve Citrinin induced intestinal inflammation. Phytomedicine 2025, 142, 156803. [Google Scholar] [CrossRef]
- Sugino, Y.; Uchiyama, R.; Shibasaki, C.; Kugawa, F. Regulation of Iron-Ion Transporter SLC11A2 by Three Identical miRNAs. Biol. Pharm. Bull. 2022, 45, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Oliveras-Cañellas, N.; Latorre, J.; Santos-González, E.; Lluch, A.; Ortega, F.; Mayneris-Perxachs, J.; Fernández-Real, J.M.; Moreno-Navarrete, J.M. Inflammatory response to bacterial lipopolysaccharide drives iron accumulation in human adipocytes. Biomed. Pharmacother. 2023, 166, 115428. [Google Scholar] [CrossRef]
- Gebicka, L.; Krych-Madej, J. The role of catalases in the prevention/promotion of oxidative stress. J. Inorg. Biochem. 2019, 197, 110699. [Google Scholar] [CrossRef]
- Kajarabille, N.; Latunde-Dada, G.O. Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators. Int. J. Mol. Sci. 2019, 20, 4968. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ma, L.; Fang, X.; Du, S.; Mauck, J.; Loor, J.J.; Sun, X.; Jia, H.; Xu, C.; Xu, Q. Role of hypoxia-inducible-factor-1α (HIF-1α) in ferroptosis of adipose tissue during ketosis. J. Dairy Sci. 2024, 107, 10611–10627. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, X.; Ding, B.; Zi, M.; Ma, Y. Resveratrol attenuated high intensity exercise training-induced inflammation and ferroptosis via Nrf2/FTH1/GPX4 pathway in intestine of mice. Turk. J. Med. Sci. 2023, 53, 446–454. [Google Scholar] [CrossRef]
- Wang, P.; Gao, Y.M.; Sun, X.; Guo, N.; Li, J.; Wang, W.; Yao, L.P.; Fu, Y.J. Hepatoprotective effect of 2’-O-galloylhyperin against oxidative stress-induced liver damage through induction of Nrf2/ARE-mediated antioxidant pathway. Food Chem. Toxicol. 2017, 102, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lu, S.; Shi, K.; Ali, M.H.; Liu, J.; Yin, F.; Yin, W. Hyperoside attenuates sepsis-induced acute lung injury by Nrf2 activation and ferroptosis inhibition. Int. Immunopharmacol. 2025, 145, 113734. [Google Scholar] [CrossRef]
- Herrera-Bravo, J.; Beltrán, J.F.; Huard, N.; Saavedra, K.; Saavedra, N.; Alvear, M.; Lanas, F.; Salazar, L.A. Anthocyanins Found in Pinot Noir Waste Induce Target Genes Related to the Nrf2 Signalling in Endothelial Cells. Antioxidants 2022, 11, 1239. [Google Scholar] [CrossRef]
- Shariati, S.; Mohtadi, S.; Khodayar, M.J.; Salehcheh, M.; Azadnasab, R.; Mansouri, E.; Moosavi, M. Quinic acid alleviates liver toxicity induced by acetaminophen in mice via anti-oxidative and anti-inflammatory effects. Naunyn Schmiedebergs Arch. Pharmacol. 2025. [Google Scholar] [CrossRef]
- Ghasemi-Dehnoo, M.; Lorigooini, Z.; Amini-Khoei, H.; Sabzevary-Ghahfarokhi, M.; Rafieian-Kopaei, M. Quinic acid ameliorates ulcerative colitis in rats, through the inhibition of two TLR4-NF-κB and NF-κB-INOS-NO signaling pathways. Immun. Inflamm. Dis. 2023, 11, e926. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Lee, H.D.; Lee, S.; Lee, A.Y. Taraxacum coreanum Nakai extract attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier dysfunction in Caco-2 cells. J. Ethnopharmacol. 2024, 319, 117105. [Google Scholar] [CrossRef] [PubMed]



| Target Gene | Description | Accession No. | Sequence (5′–3′) | |
|---|---|---|---|---|
| FTH1 | Ferritin heavy chain 1 | XM_005660803.3 | Forward | AAC AGT GCT TGG ACG GAA C |
| Reverse | AAG TGG GGG TCA TTT TTG TC | |||
| TFRC | Transferrin receptor | NM_214001.1 | Forward | GCT TAT TTT GGG CAG ACC TC |
| Reverse | TCA CCG AGT TTT CAG CAT TG | |||
| SLC40A1 | Solute carrier family 40 member 1 | XM_003483701.4 | Forward | TCA TTG GCT GTG GTT TCA TT |
| Reverse | CAA GGG TTT TGG CTC AGT TT | |||
| SLC11A2 | Solute carrier family 11 member 2 | NM_001128440.1 | Forward | CCA CCA CAT ACA ACA CCA CA |
| Reverse | CAC TTG AGC ATC CAA CAT CG | |||
| SOD1 | Superoxide dismutase 1 | NM_001190422 | Forward | GAG GGA ATG TTT ACT GGG TGA |
| Reverse | GCA CGC AAA TAA AAC TGC TC | |||
| CAT | Catalase | NM_214301 | Forward | GGC TTT TGG CTA CTT TGA GG |
| Reverse | AGG GTC ACG AAC TGT GTC AG | |||
| GCLM | Glutamate-cysteine ligase modifier subunit | XM_001926378 | Forward | CTT GCC TCT TGC TGT GTG AT |
| Reverse | CGA TGT CAG GGA TGC TTT C | |||
| ZO-1 | Zonula occludens 1 | XM_021098856 | Forward | GAT CCT GAC CCG GTG TCT GA |
| Reverse | TTG GTG GGT TTG GTG GGT TG | |||
| GABDH | Glyceraldehyde-3-phosphate dehydrogenase | NM_001206359 | Forward | ACA CCG AGC ATC TCC TGA CT |
| Reverse | GAC GAG GCA GGT CTC CCT AA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.J.; Shin, S.; Kim, T.H.; Lee, S.I. Euphorbia hypericifolia Attenuates Citrinin-Induced Oxidative Stress and Maintains Tight Junction Integrity in Porcine Intestinal Epithelial Cells. Int. J. Mol. Sci. 2025, 26, 7773. https://doi.org/10.3390/ijms26167773
Lim SJ, Shin S, Kim TH, Lee SI. Euphorbia hypericifolia Attenuates Citrinin-Induced Oxidative Stress and Maintains Tight Junction Integrity in Porcine Intestinal Epithelial Cells. International Journal of Molecular Sciences. 2025; 26(16):7773. https://doi.org/10.3390/ijms26167773
Chicago/Turabian StyleLim, Seung Joon, Sangsu Shin, Tae Hyun Kim, and Sang In Lee. 2025. "Euphorbia hypericifolia Attenuates Citrinin-Induced Oxidative Stress and Maintains Tight Junction Integrity in Porcine Intestinal Epithelial Cells" International Journal of Molecular Sciences 26, no. 16: 7773. https://doi.org/10.3390/ijms26167773
APA StyleLim, S. J., Shin, S., Kim, T. H., & Lee, S. I. (2025). Euphorbia hypericifolia Attenuates Citrinin-Induced Oxidative Stress and Maintains Tight Junction Integrity in Porcine Intestinal Epithelial Cells. International Journal of Molecular Sciences, 26(16), 7773. https://doi.org/10.3390/ijms26167773






