Base-Catalyzed Pathway Towards Isocyanate Derivatives of Silsesquioxanes
Abstract
1. Introduction
2. Results and Discussion
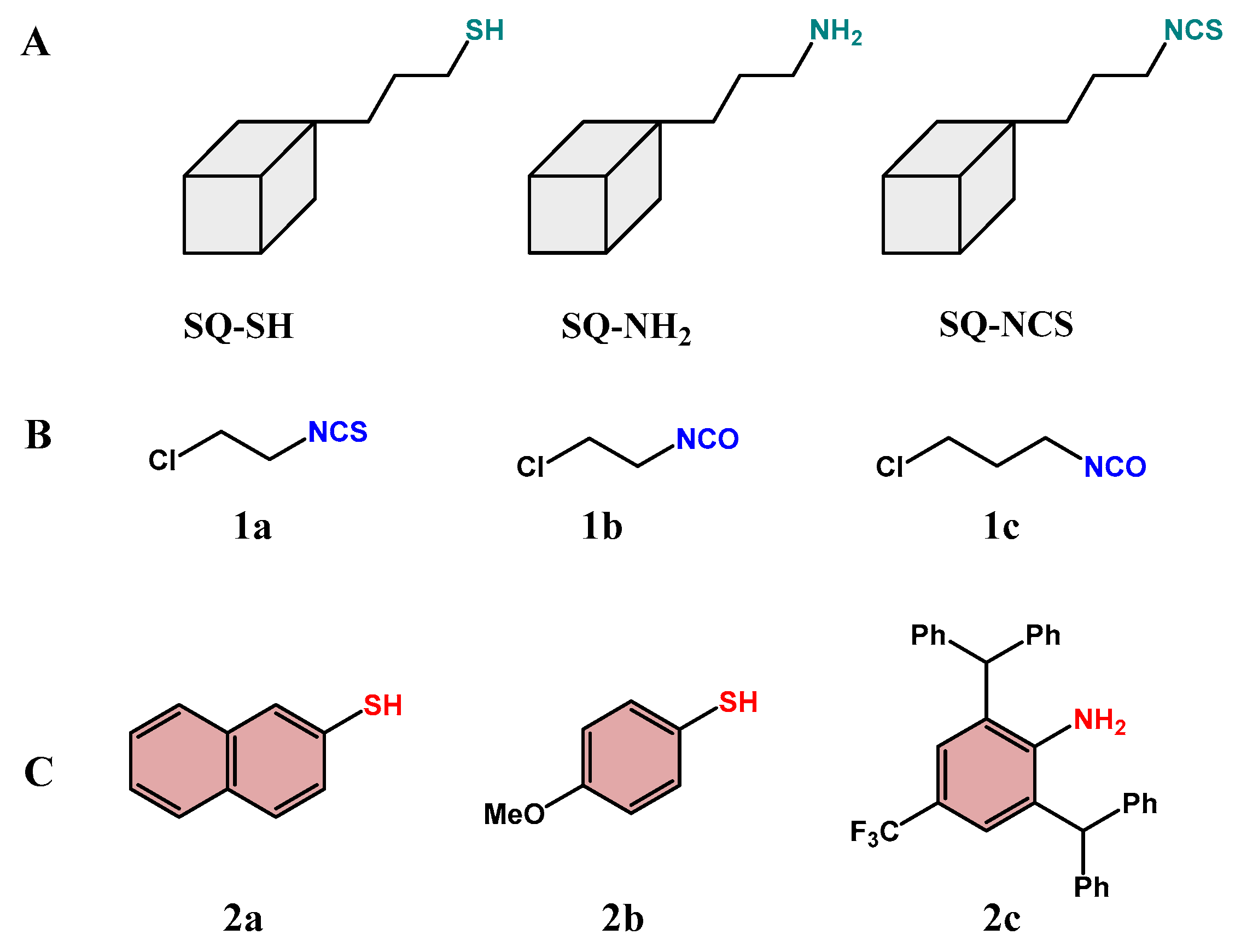
2.1. Design and Optimization of the Reaction System
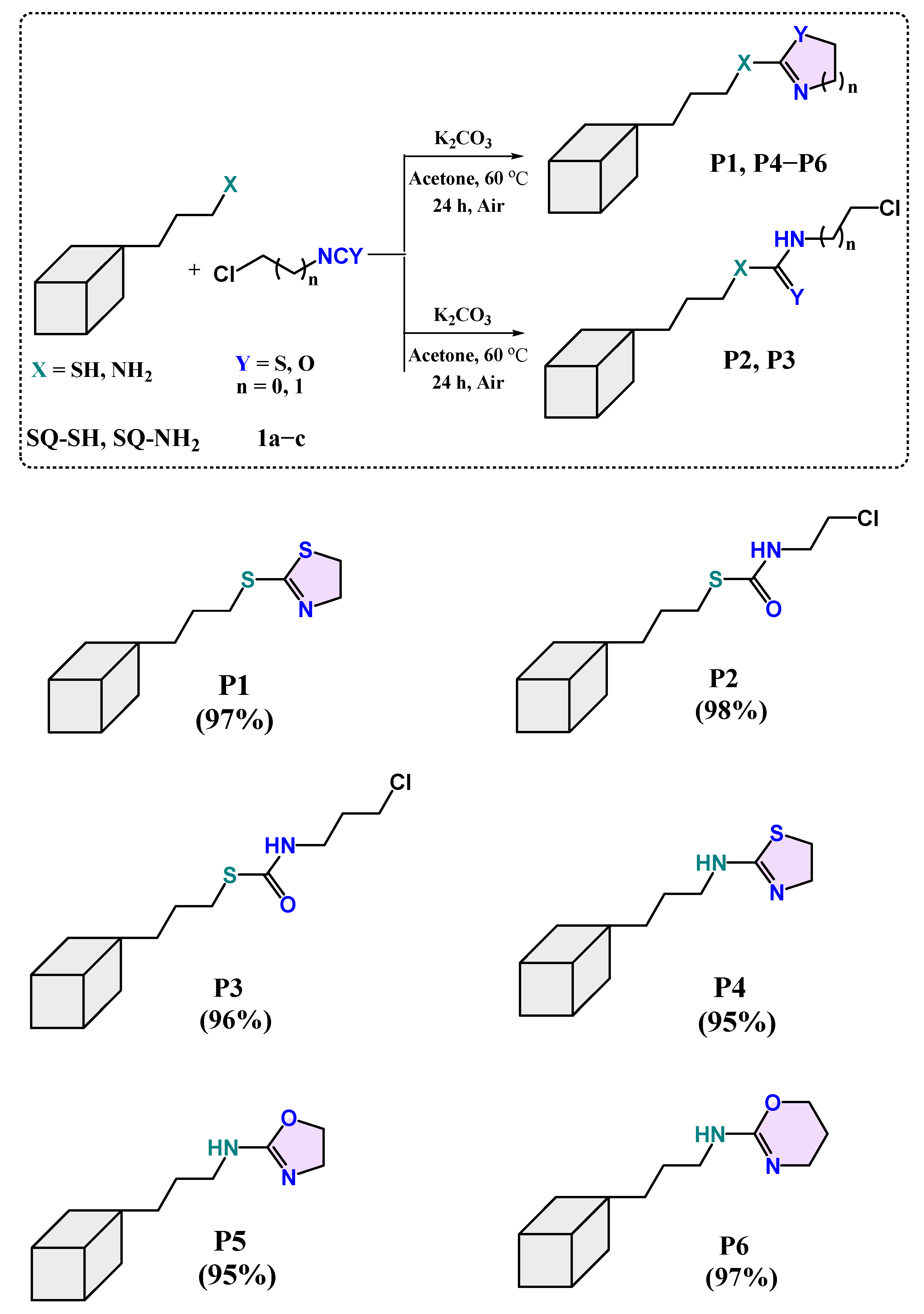
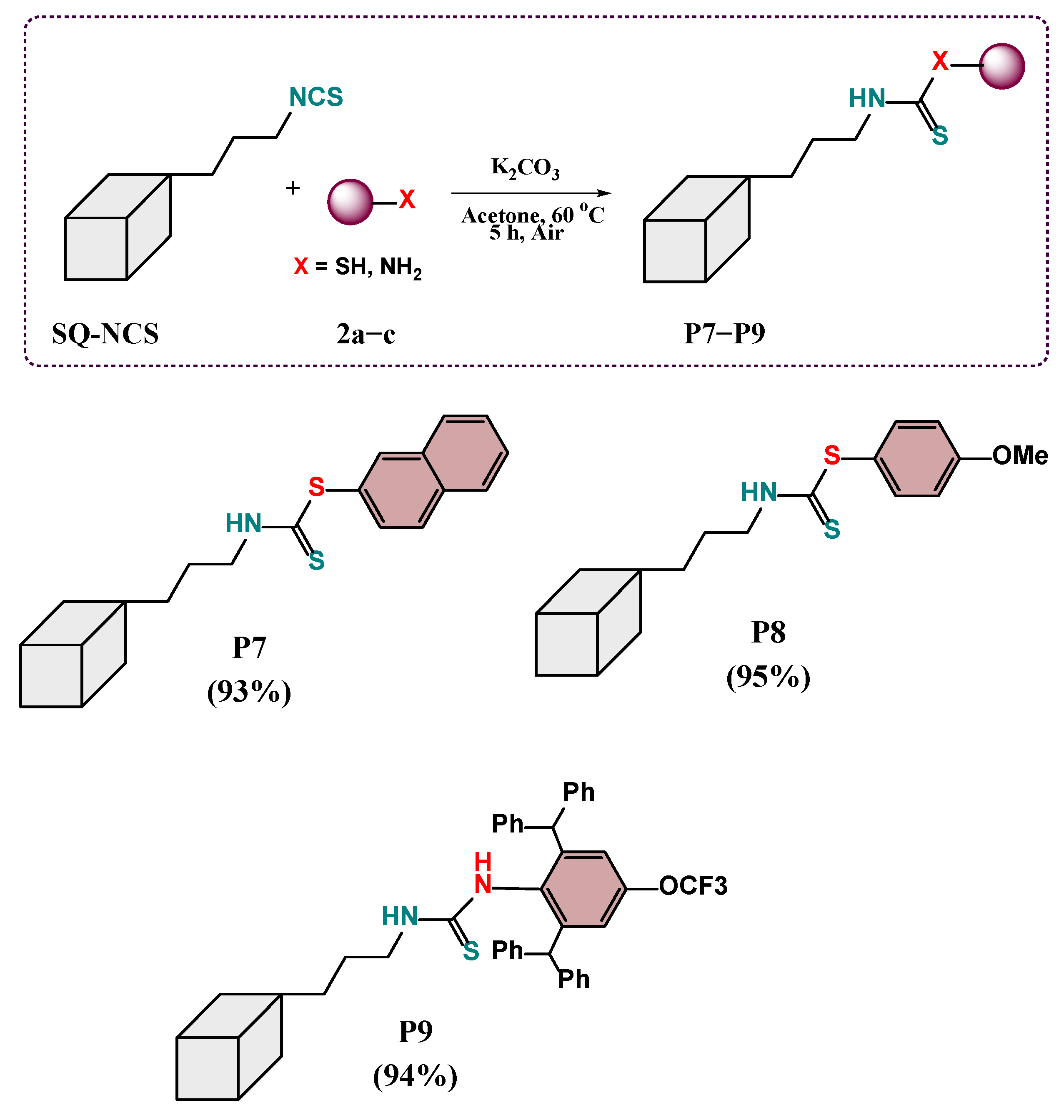
2.2. Scope of the Reaction
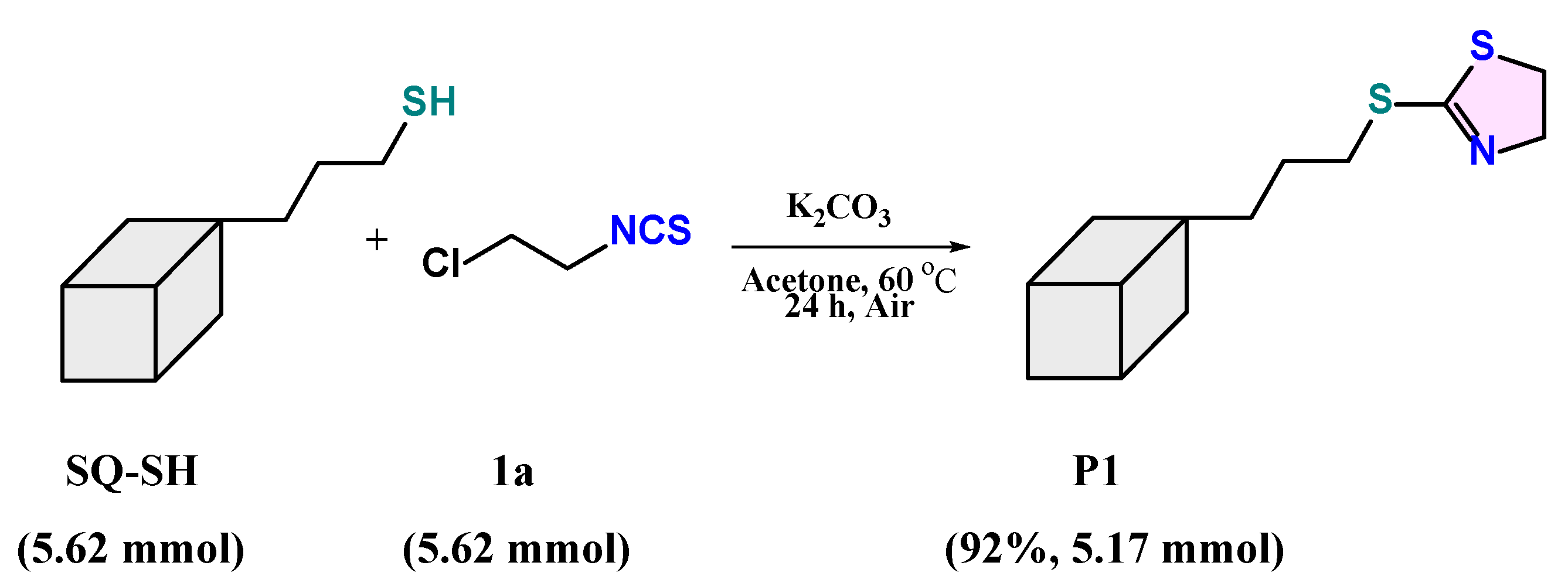
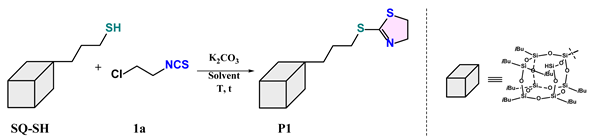
2.3. The Preparative Scale of Synthesis of Product P1
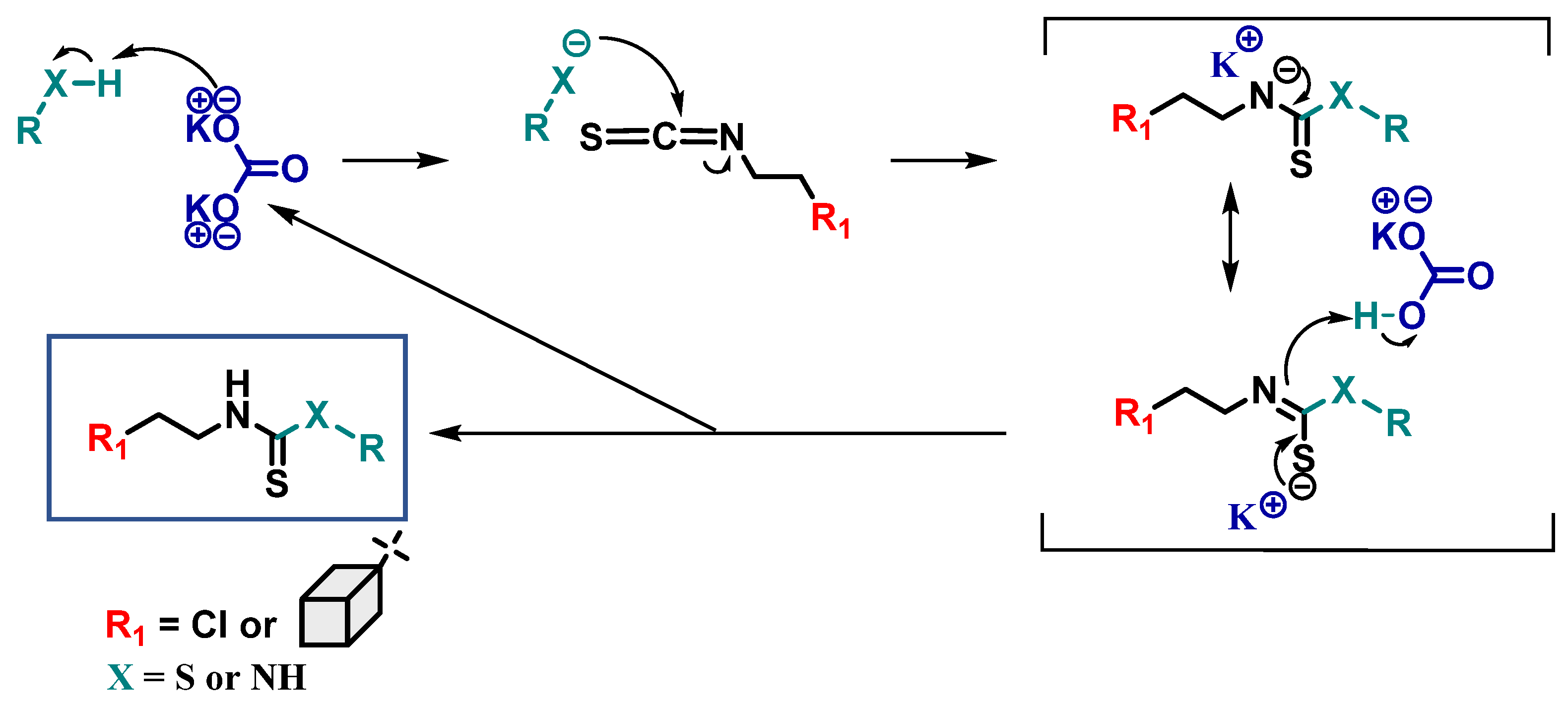
2.4. Proposed Mechanisms
2.5. Thermal Stability
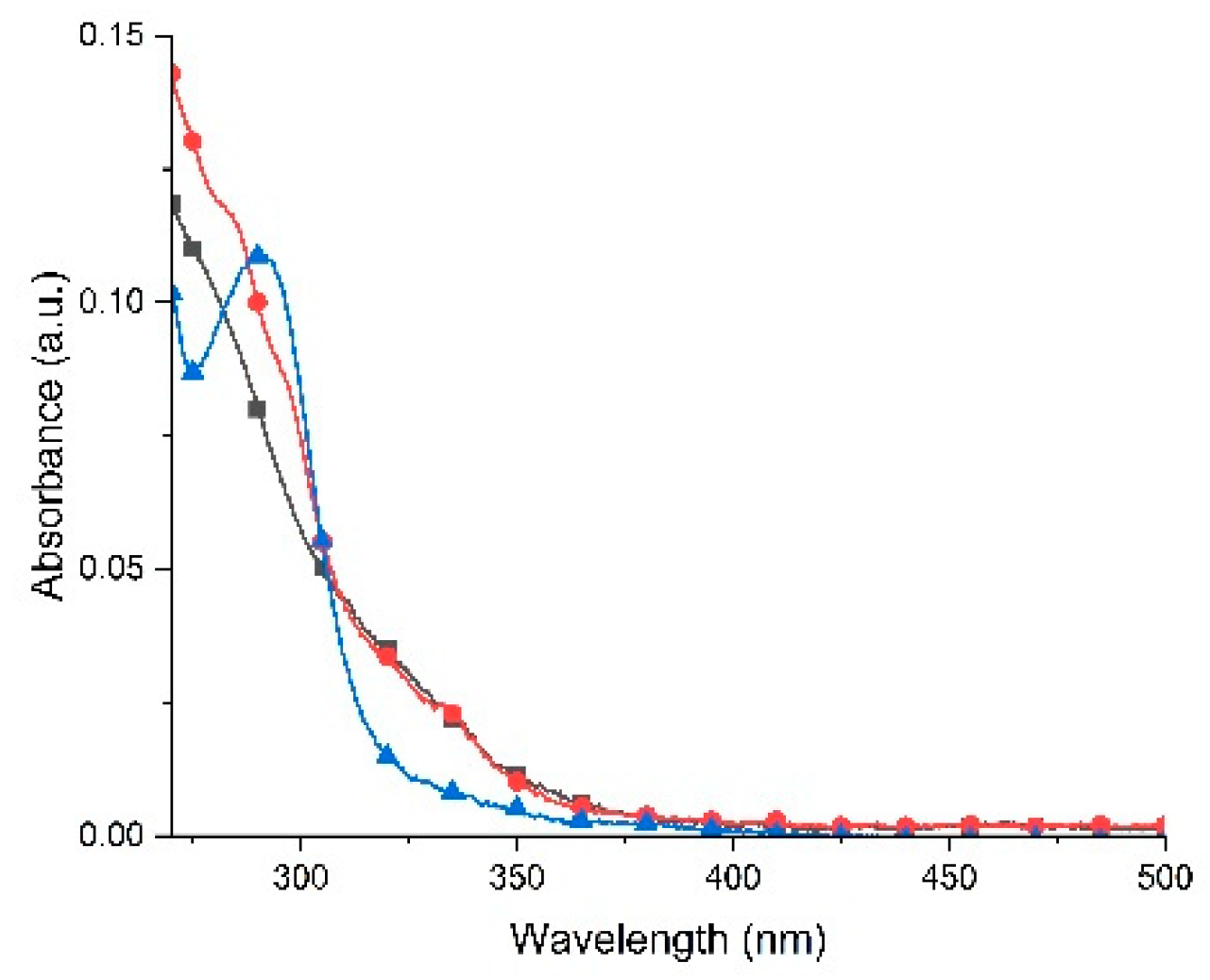
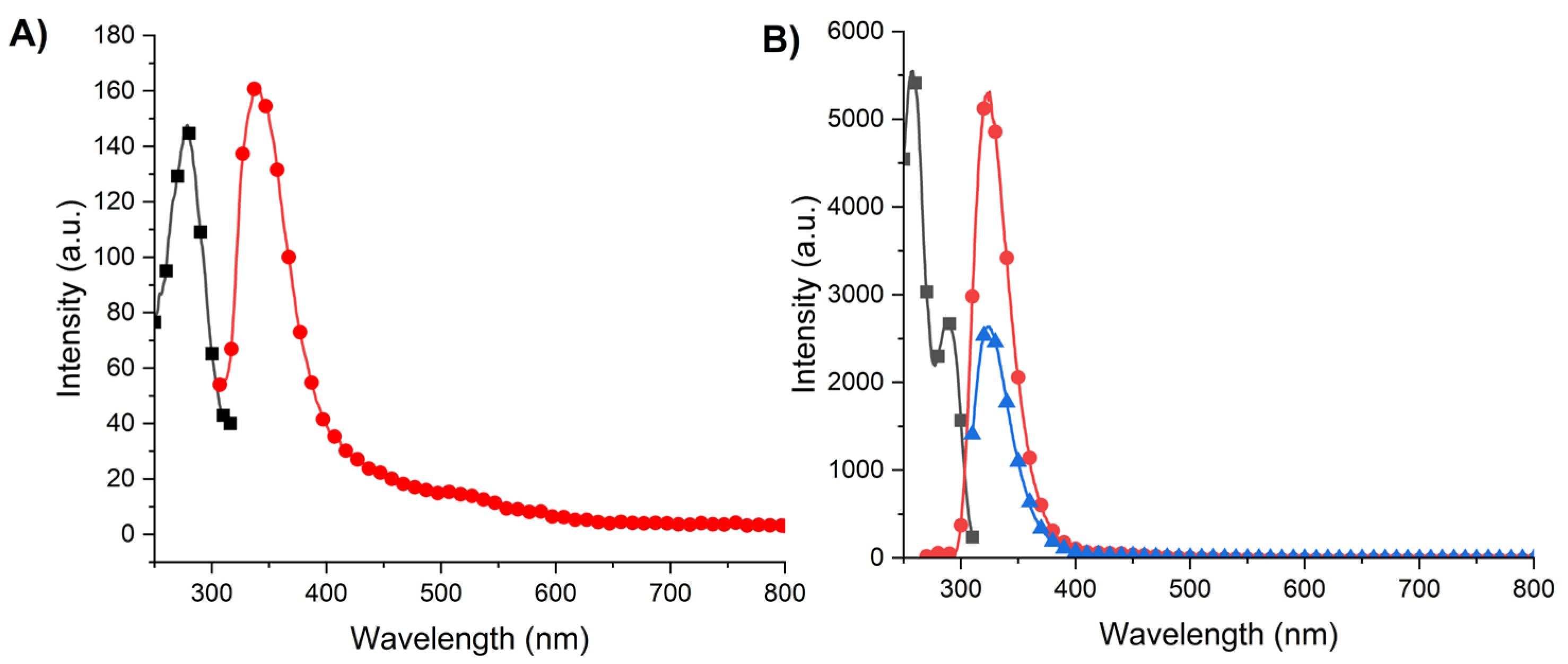
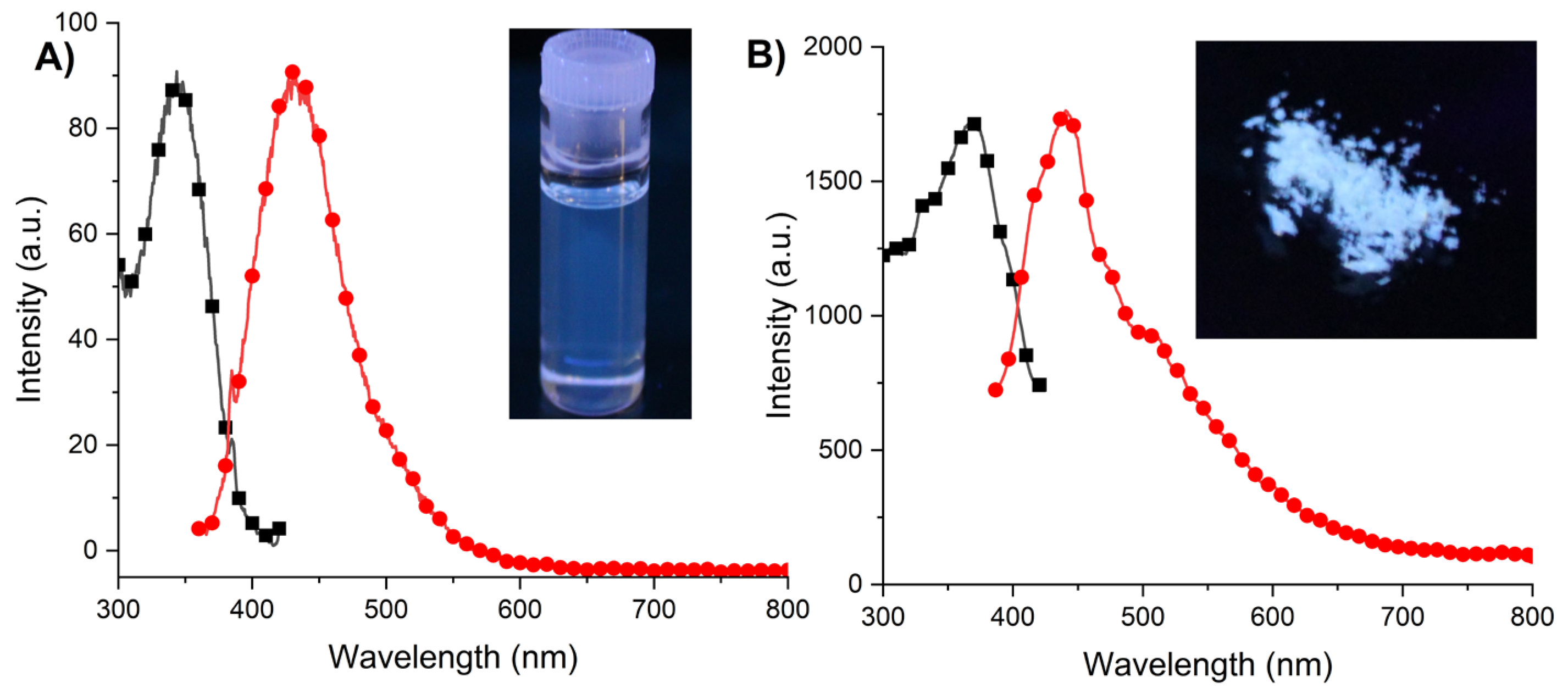
2.6. Photophysical Properties
3. Materials and Methods
3.1. General Methods and Chemicals
3.2. General Procedure for Catalytic Tests
3.3. General Procedure for the Synthesis of Products P1–P3
3.4. General Procedure for the Synthesis of Products P4–P6
3.5. General Procedure for the Synthesis of Products P7–P9
3.6. Synthesis of Product P1 on a Preparative Scale
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakayama, Y. Water-borne Two-pack Isocyanate-free Paint for Plastic Coatings. J. Color Mater. Assoc. 2000, 73, 234–241. [Google Scholar][Green Version]
- Maurya, A.K.; de Souza, F.M.; Gupta, R.K. Polyurethane and Its Composites: Synthesis to Application. In Polyurethanes: Preparation, Properties, and Applications Volume 1: Fundamentals; Gupta, R.K., Ed.; ACS: Washington, DC, USA, 2023; pp. 1–20. [Google Scholar]
- Hong, J.; Feng, G. Isothiocyanate can be used as a highly specific recognition site for fluorescent cysteine probes. Sens. Actuators B-Chem. 2021, 326, 129016. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Cao, A.; Tang, X.; Chen, X.; Fang, W.; Li, Y.; Yan, D.; Wang, Q. Effects of fertilizers and soil amendments on the degradation rate of allyl isothiocyanate in two typical soils of China. Pest Manag. Sci. 2022, 78, 5191–5202. [Google Scholar] [CrossRef]
- Stamm, R.; Johnson, R.; Clarity, C.; McGrath, T.; Hasson, M.; Singla, V. Chemical and Environmental Justice Impacts in the Life Cycle of Building Insulation: Case Study on Isocyanates in Spray Polyurethane Foam. Tech. Rep. 2022. [Google Scholar]
- Roure, B.; Alonso, M.; Lonardi, G.; Yildiz, D.B.; Buettner, C.S.; dos Santos, T.; Xu, Y.; Bossart, M.; Dredau, V.; Méndez, M.; et al. Photochemical permutation of thiazoles, isothiazoles and other azoles. Nature 2025, 637, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.R.; Yallur, B.C.; Chinnam, S.; Ananthnag, G.S.; Santhosh, C.R.; Pant, G.; Kumar, S.G.P.; Hadagali, M.D. Pharmaceutical perspectives of thiazole analogues: An overview. Results Chem. 2024, 12, 101820. [Google Scholar] [CrossRef]
- Guo, J.; Xie, Z.; Ruan, W.; Tang, Q.; Qiao, D.; Zhu, W. Thiazole-based analogues as potential antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA) and their SAR elucidation. Eur. J. Med. Chem. 2023, 259, 115689. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, Y. Dietary isothiocyanates inhibit the growth of human bladder carcinoma cells. J. Nutr. 2004, 134, 2004–2010. [Google Scholar] [CrossRef]
- Traka, M.; Mithen, R. Glucosinolates, Isothiocyanates and human health. Phytochem. Rev. 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Wu, G.; Cheng, Q.; Ding, Z.; Xie, J. Hybrid polymer dots with isothiocyanate functional groups for rapid sensing tyramine in aquatic products. J. Food Compos. Anal. 2024, 128, 106058. [Google Scholar] [CrossRef]
- Zahara, F.N.; Keshavayya, J.; Krishnamurthy, C.; Pallavi, K.M. Live Cell Imaging Studies on Orange Emitting Thiazole-Pyridone Azo Fluorophore and Its Latent Fingerprints, Computational, Electrochemical Sensing for Dopamine Detection. Luminescence 2024, 39, e70003. [Google Scholar] [CrossRef]
- Zong, Q.; Li, J.; Xu, Q.; Wang, K.; Yuan, Y. Self-immolative poly(thiocarbamate) with localized H2S signal amplification for precise cancer imaging and therapy. Nat. Commun. 2024, 15, 7558. [Google Scholar] [CrossRef]
- Ghani, U.; Ashraf, S.; Ul-Haq, Z.; Mujamammi, A.H.; Özkay, Y.; Demirci, F.; Kaplancikli, Z.A. Dithiocarbamate derivatives inhibit α-glucosidase through an apparent allosteric site on the enzyme. Chem. Biol. Drug Des. 2021, 98, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.D.; Abe, M. Sulfur atom effect on the photochemical release of benzylamine from caged amines. Chem. Lett. 2024, 53, upae123. [Google Scholar] [CrossRef]
- Ghiazza, C.; Damond, A.; Marrot, J.; Moreau, X. Improving the Photochemical Skeletal Enlargement of Pyridines to 1,2-Diazepines with Isocyanates. Adv. Synth. Catal. 2025, 367, e202401201. [Google Scholar] [CrossRef]
- Alhamami, M.A.M.; Algethami, J.S.; Khan, S. A Review on Thiazole Based Colorimetric and Fluorimetric Chemosensors for the Detection of Heavy Metal Ions. Crit. Rev. Anal. Chem. 2024, 54, 2689–2713. [Google Scholar] [CrossRef]
- Feng, R.; Wu, Z.; Zeng, X. Synthesis, conformation, and photochemistry of difluoroacetyl isocyanate CF2HC(O)NCO and isothiocyanate CF2HC(O)NCS. J. Mol. Struct. 2018, 1172, 25–32. [Google Scholar] [CrossRef]
- Leonov, A.P.; Wei, A. Photolithography of Dithiocarbamate-Anchored Monolayers and Polymers on Gold. J. Mater. Chem. 2011, 21, 4371–4376. [Google Scholar] [CrossRef]
- Baily, P.T.; Del Castillo, H.P.; Vinales, I.; Urbay, J.E.M.; Paez, A.; Weaver, M.R.; Iturralde, R.; Estevao, I.L.; Jankuru, S.R.; Almeida, I.C.; et al. Synthesis and Photoreactivity of 7-Nitroindoline-S-thiocarbamates. ACS Omega 2023, 8, 9486–9498. [Google Scholar] [CrossRef]
- Zhu, D.; Peng, X.; Wagner, P.; Xiao, P. Symmetrically-substituted Thiazolo[5,4-d]thiazole derivatives as both photoinitiators and dyes for 3D printing under violet LED. Dye. Pigment. 2022, 206, 110638. [Google Scholar] [CrossRef]
- Pivovarova, E.; Climova, A.; Świątkowski, M.; Staszewski, M.; Walczyński, K.; Dzięgielewski, M.; Bauer, M.; Kamysz, W.; Krześlak, A.; Jóźwiak, P.; et al. Synthesis and Biological Evaluation of Thiazole-Based Derivatives with Potential against Breast Cancer and Antimicrobial Agents. Int. J. Mol. Sci. 2022, 23, 9844. [Google Scholar] [CrossRef] [PubMed]
- Uğursoy, O.; Gülfen, M. Synthesis of fluorescence poly(phenylenethiazolo [5,4-d]thiazole) copolymer dye: Spectroscopy, cyclic voltammetry and thermal analysis. Dye. Pigment. 2014, 102, 189–195. [Google Scholar]
- Prager, F.H.; Rosteck, H. Polyurethane and Fire: Fire Performance Testing Under Real Conditions; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 1–511. [Google Scholar]
- Macova, D.; Čáslavský, J.; Bolechova, M.; Vavrova, M. Photo-degradation products of polyurethane foam with enhanced biodegradability. Fresenius Environ. Bull. 2013, 22, 2362–2370. [Google Scholar]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081. [Google Scholar] [CrossRef]
- Dong, F.; Lu, L.; Ha, C.S. Silsesquioxanes-Containing Hybrid Nanomaterials: Fascinating Platforms for Advanced Applications. Chem. Phys. 2019, 220, 1800324. [Google Scholar] [CrossRef]
- Li, L.; Feng, S.; Liu, H. Novel hybrid luminescent materials derived from multicarboxy cage silsesquioxanes and terbium ion. J. Ceram. Soc Jpn. 2015, 123, 719–724. [Google Scholar] [CrossRef]
- Li, L.; Liu, H. Rapid Preparation of Silsesquioxane-Based Ionic Liquids. Chem. Eur. J. 2016, 22, 4713–4716. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H. Cage-like silsesquioxanes-based hybrid materials. Dalton Trans. 2020, 49, 5396–5405. [Google Scholar] [CrossRef]
- Soldatov, M.; Liu, H. Hybrid porous polymers based on cage-like organosiloxanes: Synthesis, properties and applications. Polym. Sci. 2021, 119, 101419. [Google Scholar] [CrossRef]
- De Barros, M.R.; Bittencourt, O.R.; Crocomo, P.Z.; Magra, G.; Carasek, E.; Magoss, H.A.; Jost, C.J.; Winiarski, J.P. Adsorption of hazardous and noxious 4-nitrophenol by a silsesquioxanes organic-inorganic hybrid material. J. Sol-Gel Sci. Technol. 2021, 99, 402–412. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D.; Li, L.; Yang, W.; Feng, S.; Liu, H. Hybrid porous polymers constructed from octavinylsilsesquioxane and benzene via Friedel–Crafts reaction: Tunable porosity, gas sorption, and postfunctionalization. J. Mater. Chem. A 2014, 2, 2160–2167. [Google Scholar] [CrossRef]
- Bołt, M.; Hanek, K.; Frąckowiak, D.; Żak, P. Metal-free functionalization of SQs: A case of chemoselectivity and what ball-milling has got to do with it? Inorg. Chem. Front. 2023, 10, 4190–4196. [Google Scholar] [CrossRef]
- Liu, S.; Guo, R.; Li, C.; Lu, C.; Yang, G.; Wang, F.; Nie, J.; Ma, C.; Gao, M. Anticalcification Potential of POSS-PEG Hybrid Hydrogel as a Scaffold Material for the Development of Synthetic Heart Valve Leaflets. ACS Appl. Bio Mater. 2021, 4, 2534–2543. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, Y.; Liu, Y.; Cai, H.; Zhang, W.; Tan, W.S. POSS-enhanced thermosensitive hybrid hydrogels for cell adhesion and detachment. RSC Adv. 2018, 8, 13813–13819. [Google Scholar] [CrossRef]
- Cui, L.; Hong, J.; Zhu, Z.; Wang, Z.; Liu, Z.; Zheng, W.; Hao, Y.; He, J.; Ni, P. Injectable and Degradable POSS–Polyphosphate–Polysaccharide Hybrid Hydrogel Scaffold for Cartilage Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 20625–20637. [Google Scholar] [CrossRef]
- Choi, J.H.; Balamurugan, G.; Pande, G.K.; Eom, Y.S.; Kim, H.-K.; Cha, D.E.; Park, J.S. Fully spray-coated electrochromic devices containing octa-viologen substituted polyhedral oligomeric silsesquioxane. Thin Solid Film. 2022, 743, 139067. [Google Scholar] [CrossRef]
- Mihelčič, M.; Gaberšček, M.; Di Carlo, G.; Giuliani, C.; de Luna, M.S.; Lavorgna, M.; Surca, A.K. Influence of silsesquioxane addition on polyurethane-based protective coatings for bronze surfaces. Appl. Surf. Sci. 2019, 467–468, 912–925. [Google Scholar] [CrossRef]
- Madhuranthakam, C.M.R.; Pandiyan, S.; Chaalal, O.; Elkamel, A. Study of Water Sorption in Methacryl-Based Polyhedral Oligomeric Silsesquioxane (POSS) Dental Composites Using Molecular Dynamics Simulations. Polymers 2023, 15, 4161. [Google Scholar] [CrossRef] [PubMed]
- Schöneich, C. Thiyl radicals and induction of protein degradation. Free Radic Res. 2016, 50, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Burmistrova, D.A.; Smolyaninov, I.V.; Berberova, N.T. The transformations of thiols and their dimers in the redox-mediated thiol-disulfide exchange reaction. Chim. Techno Acta 2023, 10, 202310418. [Google Scholar] [CrossRef]
- Canudo-Barreras, G.; Herrera, R.P.; Gimeno, M.C. New Twists in the Chemistry of Thioureas: 1,3-Thiazolidines as a Vector of Sustainability. Chem. Eur. J. 2024, 30, e202402812. [Google Scholar] [CrossRef]
- Citi, V.; Passerini, M.; Calderone, V.; Testai, L. Plants and Mushrooms as Possible New Sources of H2S Releasing Sulfur Compounds. Int. J. Mol. Sci. 2023, 24, 11886. [Google Scholar] [CrossRef]
- Hanek, K.; Pietras, N.; Frąckowiak, D.; Żak, P. Solvent- and Transition Metal-Free Mechanochemical Synthesis of 2-Thiazolines and S-Thiocarbamates. Adv. Synth. Catal. 2025, 367, e9595. [Google Scholar] [CrossRef]
- Shin, J.; Matsushima, H.; Comer, C.M.; Bowman, C.N.; Hoyle, C.E. Thiol−Isocyanate−Ene Ternary Networks by Sequential and Simultaneous Thiol Click Reactions. Chem. Mater. 2010, 22, 2616–2625. [Google Scholar] [CrossRef]
- Dyer, E.; Glenn, J.F.; Lendrat, E.G. The Kinetics of the Reactions of Phenyl Isocyanate with Thiols. Org. Chem. 1961, 26, 2919–2925. [Google Scholar] [CrossRef]
- Tan, J.; Li, C.; Li, H.; Zhang, H.; Gu, J.; Zhang, B.; Zhang, H.; Zhang, Q. Water-borne thiol–isocyanate click chemistry in microfluidics: Rapid and energy-efficient preparation of uniform particles. Polym. Chem. 2015, 6, 4366–4373. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F. (Eds.) Polyurethane: An Introduction. In Polyurethane; INTECH Open Access Publisher: London, UK, 2012; Chapter 1. [Google Scholar]
- Camino, G.; Lomakin, S.M.; Lazzari, M. Polydimethylsiloxane thermal degradation Part 1. Kinetic aspects. Polymer 2001, 42, 2395–2402. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lageard, M. Thermal polydimethylsiloxane degradation. Part 2. The degradation mechanisms. Polymer 2002, 43, 2011–2015. [Google Scholar] [CrossRef]
- Balfour, W.J. The 275-nm absorption system of anisole. J. Mol. Spectrosc. 1985, 109, 60–72. [Google Scholar] [CrossRef]
- Maeda, H.; Maeda, T.; Mizuno, K. Absorption and Fluorescence Spectroscopic Properties of 1- and 1,4-Silyl-Substituted Naphthalene Derivatives. Molecules 2012, 17, 5108–5125. [Google Scholar] [CrossRef]
- Hanek, K.; Wałęsa-Chorab, M.; Żak, P. Highly effective synthesis of mercapto-functionalized cubic silsesquioxanes as the first step in designing advanced nano-delivery systems. Inorg. Chem. Front. 2024, 11, 470–477. [Google Scholar] [CrossRef]
- Bayrakçeken, F.; Demir, O.J.; Tunçyürek, L.; Karaaslan, İ.Ş. Triplet–triplet energy transfer from naphthalene to biacetyl in the vapor phase. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 65, 27–31. [Google Scholar] [CrossRef]
- Irshad, R.; Asim, S.; Mansha, A.; Arooj, I. Naphthalene and its Derivatives: Efficient Fluorescence Probes for Detecting and Imaging Purposes. J. Fluoresc. 2023, 33, 1273–1303. [Google Scholar] [CrossRef]
- Zhu, S.-J.; Li, J.-F. A novel synthesis of isothiocyanates from amines and phenyl isothiocyanate via replacement reaction. Chem. Pap. 2021, 75, 4543–4547. [Google Scholar] [CrossRef]
- Hans, M.; Lorkowski, J.; Demonceau, A.; Delaude, L. Efficient synthetic protocols for the preparation of common N-heterocyclic carbene precursors. Beilstein J. Org. Chem. 2015, 11, 2318–2325. [Google Scholar] [CrossRef]
- Vetha, B.S.S.; Oh, P.-S.; Kim, S.H.; Jeong, H.-J. Curcuminoids encapsulated liposome nanoparticles as a blue light emitting diode induced photodynamic therapeutic system for cancer treatment. J. Photochem. Photobiol. B-Biol. 2020, 205, 111840. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Shimada, M.; Tokunaga, T.; Nakao, T.; Nishi, M.; Takasu, C.; Kashihara, H.; Wada, Y.; Okikawa, S.; Yoshikawa, K. Blue light irradiation inhibits the growth of colon cancer and activation of cancer-associated fibroblasts. Oncol. Rep. 2022, 47, 104. [Google Scholar] [CrossRef]
- Xu, Z.; Tang, B.Z.; Wang, Y.; Ma, D. Recent advances in high performance blue organic light-emitting diodes based on fluorescence emitters. J. Mater. Chem. C 2020, 8, 2614–2642. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Y.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; Dai, T. Antimicrobial blue light inactivation of pathogenic microbes: State of the art. Drug Resist. Updates 2017, 33–35, 1–22. [Google Scholar] [CrossRef]
- Tadesse, E.E.; Kulawik, P.; Szymkowiak, A.; Šimat, V. Application of UV-A and blue light emitting diodes within the range of 320–480 nm on quality and shelf-life extension of food products. Food Bioprod. Process. 2024, 148, 436–455. [Google Scholar] [CrossRef]

 | ||||||
|---|---|---|---|---|---|---|
| Entry | Solvent | Temp. [°C] | K2CO3 [equiv.] | Time [h] | Aerobic Conditions | Conv. of 1a [a] [%] |
| 1 | Toluene | 120 | 0.6 | 24 | - | 85 |
| 2 | Toluene | 100 | 0.6 | 24 | - | 95 |
| 3 | Toluene | 80 | 0.6 | 24 | - | 95 |
| 4 | Toluene | 60 | 0.6 | 24 | - | 100 |
| 5 | Toluene | 40 | 0.6 | 48 | - | 80 |
| 6 | Toluene | 25 | 0.6 | 72 | - | 62 |
| 7 | Toluene | 60 | 0.6 | 24 | + | 100 |
| 8 | Acetone | 60 | 0.6 | 12 | + | 100 |
| 9 | Acetone | 40 | 0.6 | 48 | + | 78 |
| 10 | Acetone | 60 | 0.3 | 24 | + | 75 |
| 11 | Acetone | 60 | 0.1 | 48 | + | 42 |
| 12 | Acetone | 60 | 0.6 | 5 | + | 100 |
| 13 | Acetone | 60 | 0.6 | 2 | + | 36 |
| 14 | Acetone | 60 | 0.6 | 1 | + | 11 |
| 15 | Acetone | 60 | - | 72 | + | 0 |
| 16 | iPrOH | 60 | 0.6 | 5 | + | 92 |
| 17 | MIBK | 60 | 0.6 | 5 | + | 100 |
| 18 | EtOAc | 60 | 0.6 | 5 | + | 10 |
| Entry | Compound | Temp. of Mass Lost [°C] | Residue [%] | ||
|---|---|---|---|---|---|
| 5% | 50% | 300 °C | 1000 °C | ||
| 1 | 1a | 47 | 127 | 1.2 | 1.4 |
| 2 | 1b | 28 | 79 | 1.6 | 2.0 |
| 3 | 1c | 29 | 88 | 2.8 | 2.6 |
| 4 | P1 | 290 | 342 | 92 | 13 |
| 5 | P2 | 236 | 333 | 74 | 16 |
| 6 | P3 | 256 | 336 | 74 | 20 |
| 7 | P4 | 291 | 362 | 93 | 15 |
| 8 | P5 | 269 | 344 | 86 | 11 |
| 9 | P6 | 274 | 353 | 88 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanek, K.; Wałęsa-Chorab, M.; Żak, P. Base-Catalyzed Pathway Towards Isocyanate Derivatives of Silsesquioxanes. Int. J. Mol. Sci. 2025, 26, 7769. https://doi.org/10.3390/ijms26167769
Hanek K, Wałęsa-Chorab M, Żak P. Base-Catalyzed Pathway Towards Isocyanate Derivatives of Silsesquioxanes. International Journal of Molecular Sciences. 2025; 26(16):7769. https://doi.org/10.3390/ijms26167769
Chicago/Turabian StyleHanek, Kamil, Monika Wałęsa-Chorab, and Patrycja Żak. 2025. "Base-Catalyzed Pathway Towards Isocyanate Derivatives of Silsesquioxanes" International Journal of Molecular Sciences 26, no. 16: 7769. https://doi.org/10.3390/ijms26167769
APA StyleHanek, K., Wałęsa-Chorab, M., & Żak, P. (2025). Base-Catalyzed Pathway Towards Isocyanate Derivatives of Silsesquioxanes. International Journal of Molecular Sciences, 26(16), 7769. https://doi.org/10.3390/ijms26167769







