1. Introduction
Microbial infections remain a worrying, still unresolved problem associated with the use of biomaterials in orthopedics, as well as in other fields of medicine where prostheses and indwelling devices are largely and increasingly used [
1]. Perioperative antibiotics have proved capable of preventing a significant proportion of infective events. Nonetheless, multiresistant strains have become frequent, and the spread of antibiotic resistance among bacterial strains represents a serious threat for the prevention and cure of prosthetic infections in orthopedics. Indeed, antibiotic-based prophylaxis measures, such as perioperative antibiotics and anti-infective biomaterials, as well as antibiotic-based treatments, encounter reduced efficacy. Moreover, the majority of opportunistic pathogens causing biomaterial-associated infections, once adhered to implant surfaces, form protective biofilms, become tolerant to common antibiotics, and skew host immune defenses [
2]. Current preventive and therapeutic strategies to deal with the phenomenon of implant infections are increasingly focusing on the use of new bioactive molecules that are alternative to current antibiotics. Depending on the clinical application and anatomic site of insertion, they were initially directed towards the development of antifouling surfaces, controlled local delivery of well-characterized antibiotic drugs (for instance, aminoglycosides, glycopeptides such as vancomycin, or combinations of antibiotics such as clindamycin/rifampicin and minocycline/rifampicin), and long-established antibacterial substances (ranging from metal ions, such as Ag
+, Cu
2+, and Ga
3+, and metal nanoparticles to disinfectants such as chlorhexidine and triclosan) [
3]. With time, the range of strategies to counteract biomaterial-associated infections has significantly broadened, with scientists starting to contemplate multifront approaches and even considering the possibility of immunomodulating the host response and strengthening the immune defenses against bacterial biofilms [
4]. An interesting category of antibacterial molecules that, with time, has drawn growing attention is that of antimicrobial peptides (AMPs) [
5]. Initially identified from natural sources, AMPs take part in the innate defense systems of a broad variety of organisms, including plants, fungi, and animals [
6]. Most natural AMPs are polycationic peptides capable of interacting with and disrupting the integrity of the cell membranes of fungi, bacteria, and viruses, determining the lysis of the microorganism. However, others exhibit diverse mechanisms of action and different cellular targets [
7], such as, for instance, enzymatic activities causing the lysis of the bacterial cell wall. Nonetheless, the enormous possibilities of permuting the sequences of amino acids of the peptides offer infinite combinations and real chances to identify new powerful molecules with selective activity against pathogenic microorganisms. Moreover, the selection of improved peptide sequences helps to minimize some shortcomings of the current AMPs, such as thermal instability, vulnerability to the enzymic action of proteases, or inactivation following interaction/complexation with components of the physiologic fluids or defense mechanisms developed by bacteria. Thus, starting from the known sequences of natural peptides, algorithms can be applied to identify new artificial AMPs that optimize antimicrobial and other biological activities [
8,
9,
10].
In 2021, aiming at identifying AMPs for a possible application in the prevention or treatment of prosthetic orthopedic infections, our group conducted an extensive systematic review on known short-chain AMPs, primarily based on the Database of Antimicrobial Activity and Structure of Peptides (DBAASP) [
11]. The choice of considering short-chain peptides was dictated by the potentially lower allergenic risk offered by small peptides. The query included all monomeric AMPs with a length spanning from 7 to 13 amino acids. The research results provided a list of 415 entries, which were then extended by consultations of further information sources (e.g., PubMed, Web of Science and Scholar Google). The top-ranking AMPs emerging from the survey included, among a few others, the new antibiotic teixobactin [
12] and the antimicrobial peptide KSL [
13,
14]. Described for the first time in 2015 [
15], teixobactin damages only membranes that contain lipid II, which is absent in eukaryotes, and thus, it does not produce damage to the membranes of mammalian cells [
12]. The questionable use of important antibiotics in delivery systems for the prevention of infections, the difficult synthesis of the peptide, and a lower potency of teixobactin against Gram-negative pathogens guided us to instead focus our attention on alternative choices, i.e., the decapeptide KSL [
16] and its derivative KSL-W [
17], which were considered some of the most interesting AMPs in terms of the scope of our research. KSL was initially discovered from the screening of combinatorial libraries consisting of simplified amino acid sequences for peptides active against the
Candida albicans membrane [
13,
14,
18]. KSL is a polycationic peptide (net charge of +5) which contains five Lys residues, and the primary target of its bactericidal mechanism is the bacterial membrane. Lipid membrane perturbation by KSL occurs without pore formation. KSL may adopt a β sheet structure on lipid membranes, and this seems to be a critical requirement for its lipid perturbation activity [
19]. KSL-W is a KSL analog with a net charge of +4 in which the Lys
6 residue is replaced by Trp [
17] and whose main strength is a greater stability to enzymic activity such as trypsin-catalyzed cleavage in human saliva. Both KSL and its derivative KSL-W have previously been investigated, with studies proving their activity against oral bacteria and supporting their clinical use as antiplaque agents [
16,
17,
18,
20,
21,
22].
The aim of this study was to provide a further, in-depth exploration of the potential of these two decapeptides and of a third, more recently discovered, AMP consisting of 23 amino acids, namely Dadapin-1 [
23], as potential candidates for the production of novel anti-infective coatings for resurfacing orthopedic implants. Dadapin-1 was initially developed following a process of simultaneous automatic selection of natural peptides of anuran origin (from the dedicated Database of Anuran Defense Peptides, DADP) and mutation of multiple sequences by means of the Mutator software [
23]. The modification strategy consisted in replacing a Val with a Lys residue, which enhanced the net positive charge of the peptide (+5) while decreasing its hemolytic activity. In aqueous solutions, Dadapin-1 exhibits a mostly disordered structure, but it shows a transition to a partly helical structure in the presence of membrane-like environments and has been found to damage bacterial membranes at sub-MICs [
23].
Earlier, Dadapin-1 underwent some preliminary investigations [
24] and was included in the present work mainly as a useful term of comparison for the two decapeptides. Here, the characterization of Dadapin-1 has been further extended to four new bacterial strains, two of which belong to
Enterococcus faecalis, a species on which this peptide was never tested before.
Figure 1 reports the 3D structure prediction for all three peptides.
To characterize the peptides’ antibacterial properties, microbiological tests were performed using two different culture conditions for the reference
S. aureus strain, as well as for other bacterial species involved in orthopedic prosthetic infections. Müller–Hinton Broth II (MHB II) is a culture medium known to be optimized for bacterial growth and antibiotic testing. However, different works have highlighted some chemical components of MHB II medium that have been found to form complexes with cationic peptides and quench their antimicrobial activity [
25,
26,
27]. This interference results in higher MIC, MBC, and MBIC values. Taking into consideration this potential interference, the antibacterial activity of the selected AMPs was therefore tested under two distinct culture conditions, i.e., in undiluted and in diluted MHB II [
24]. Further, noticing a different susceptibility of the peptides to the medium composition, we tried using a quantitative approach to measure the interference of the culture broth on the AMPs. Intriguingly, the loss of activity of the peptides in undiluted medium depended not only on the specific chemistry of the peptide but also on the bacterial species/strain used in the test.
3. Discussion
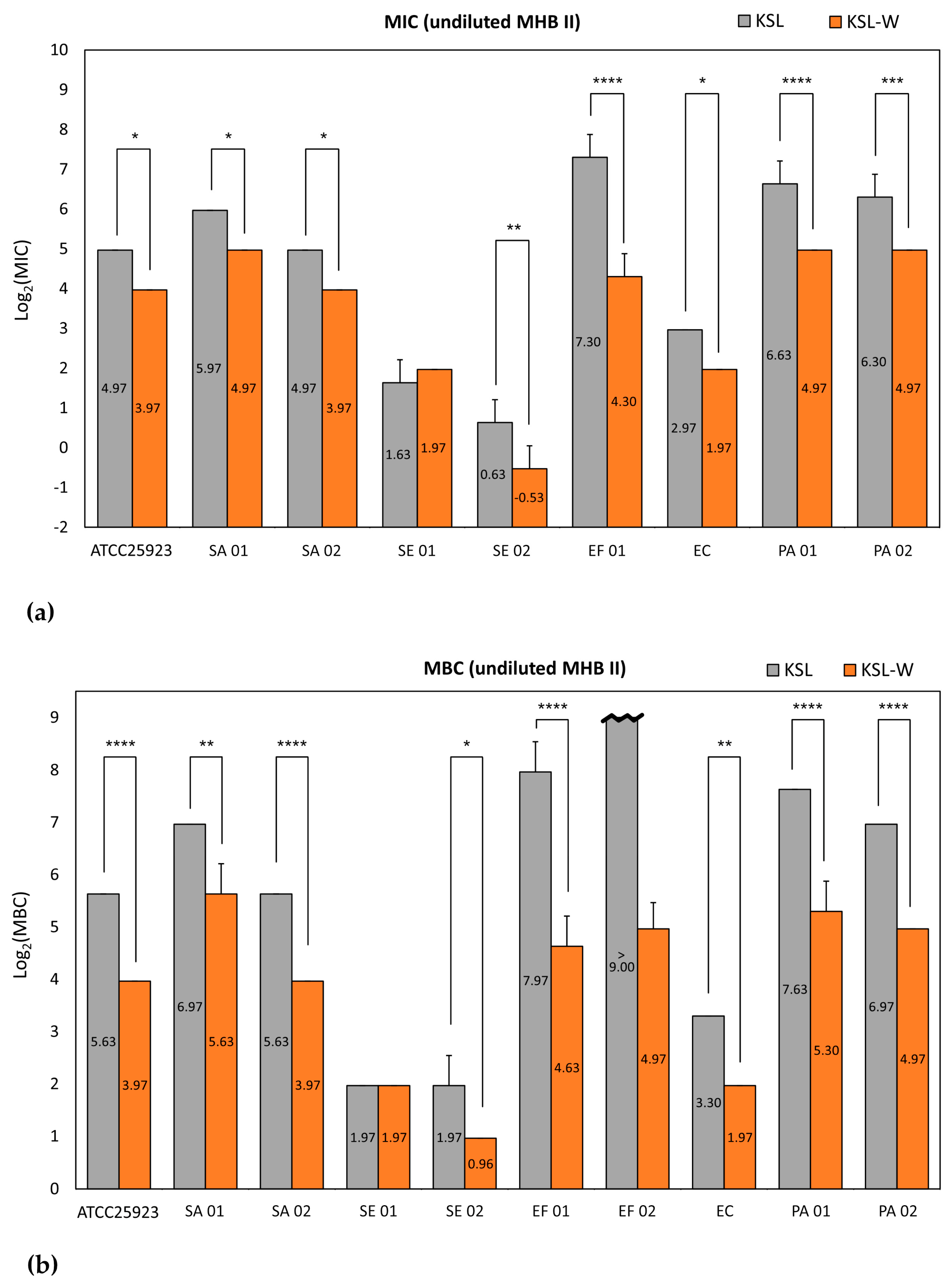
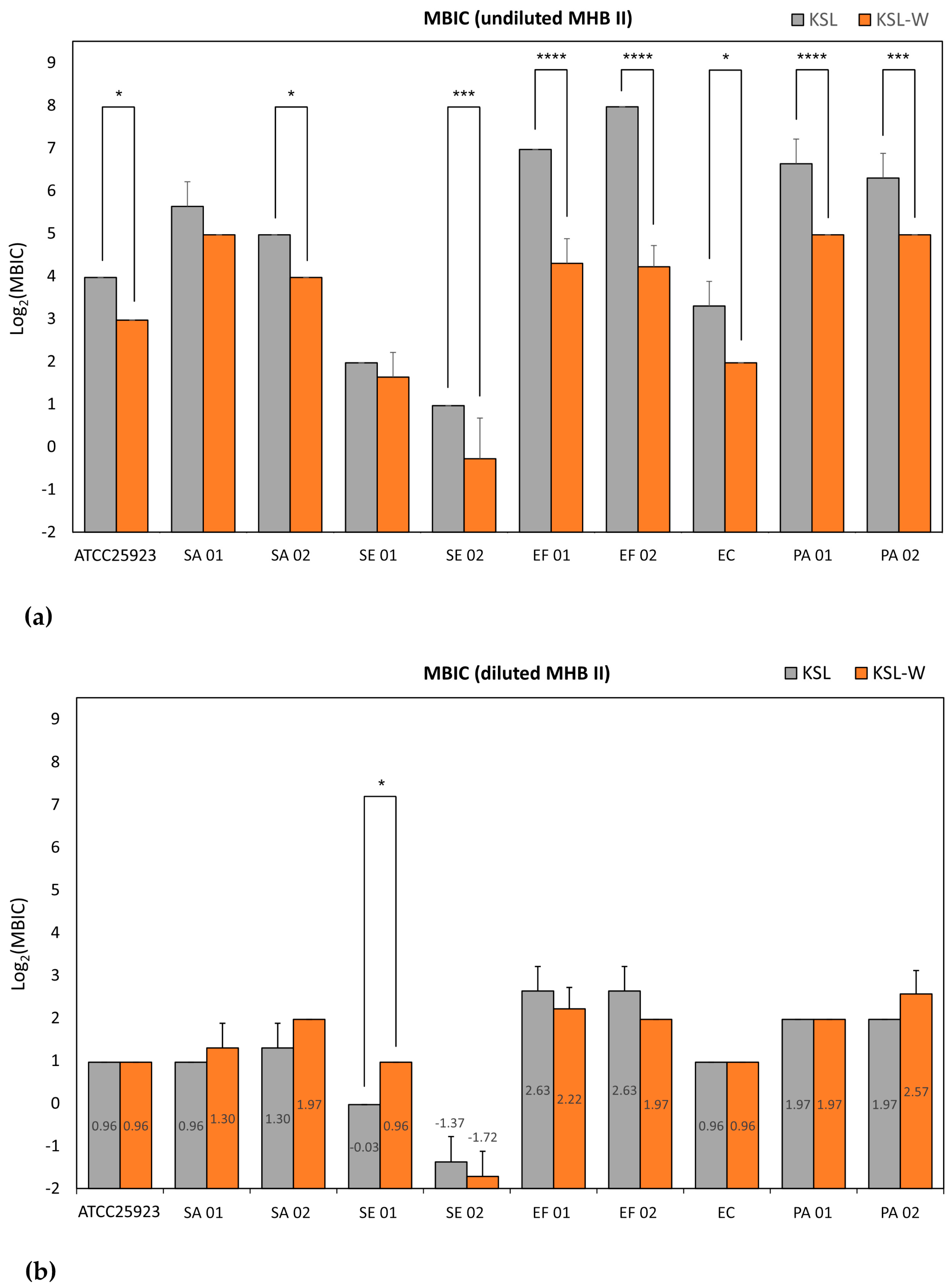
The microbiological properties of three promising antimicrobial peptides, KSL, KSL-W, and Dadapin-1, were extensively analyzed on a panel of relevant and finely characterized clinical bacterial isolates from orthopedic infections. The use of different testing conditions enabled a series of interesting observations. Our findings demonstrate that KSL, KSL-W, and Dadapin-1 have a broad-spectrum antibacterial activity on relevant Gram-positive and Gram-negative bacterial species that are frequently isolated from orthopedic prosthetic infections. Moreover, all test peptides, in particular KSL and KSL-W, turned out to be very active even on multidrug-resistant (MDR) strains, such as SA 02 and EC, which exhibit a high multiplicity of antibiotic resistance (M.A.R. = sum of each single antibiotic resistance) of 9 and 8, respectively. In diluted medium, the differences between the three peptides were subtle, the two decapeptides exhibited equivalent antibacterial activity and all three antimicrobial substances manifested significant antibacterial and bactericidal properties. Conversely, in undiluted medium, the antibacterial activity of the AMPs underwent pronounced variations, with KSL-W emerging as the best performing peptide. In the case of Dadapin-1, the diverging behavior was even more obvious, as its activity was negligible in undiluted medium.
Most known AMPs are amphiphilic polycationic molecules with a positive charge > 3 and whose bactericidal mechanism depends on electrostatic interactions with negatively charged components expressed on the bacterial envelop (e.g., phosphatidylglycerol, lipopolysaccharides and lipoteichoic acids) [
16]. Experimental studies have shown that the use of culture media such as MHB may be not ideal for testing cationic peptides. Turner et al. [
25] investigated the MIC of two AMPs, i.e., LL-37 and PG-1, against four microorganisms using two diverse culture broth formulations, respectively: standard MHB and a customized broth, which they named “refined” MHB. In the latter formulation, MHB was subjected to anion-exchange chromatography to deplete it of possible (poly)anionic inhibitors. The authors found that MIC determinations performed with standard MHB yielded values that were up to 20-fold higher than those obtained with refined MHB. The major component of MHB is an acid hydrolysate of casein, consisting in a significant proportion of dicarboxylic or phosphorocarboxylic acids (generated by total hydrolysis) and polyanionic peptides (derived from incomplete hydrolysis and composed of negatively charged glutamate and phosphoserine residues). The authors concluded that the use of standard MHB in a NCCLS broth microdilution assay vastly underestimates the activity of LL-37, it is suboptimal for the testing of antimicrobial peptides, and its utility can be improved substantially by anion depletion. The rich component of anionic amino acids would cause precipitation of cationic AMPs, in turn resulting in a pronounced loss of antibacterial activity. More in general, in the presence of certain polyanionic substances (among them even phosphates), electrostatic complexation could take place, sequestering/inactivating part of the free active peptide, and thus markedly diminishing its antibacterial activity [
26]. More recently, when the MIC was tested in unmodified standard MHB, a loss of LL-37 activity against Gram-positive and Gram-negative strains was reported also by Mechesso et al. [
28]. The authors reported that the antibacterial activity of the peptide was restored when using diluted MHB (12.5%), which roughly corresponds to the real final dilution of MHB II (10%) used in the present study. Curiously, the same effect was not observed with diluted tryptic soy broth (TSB) or Luria−Bertani (LB). These authors finally underscored the usefulness of the diluted broth for antimicrobial screening of peptides.
Certainly, the current knowledge of how and to what extent the many variables of culture conditions are capable of influencing the activity of positively charged peptides is still limited. Among others, the protein source, cationic salts (e.g., NaCl, CaCl
2, and MgCl
2), anionic components, and pH are known to play a potential role. In any case, this role varies depending on the molecular structure and the antibacterial mechanism of the specific antimicrobial substance, but also on the microorganism under examination, as also observed in our study. The importance of the use of sensitive conditions and the various environmental conditions capable of influencing the results of antibacterial activity testing have recently been reviewed in [
29].
In the present study, we opted to use MHB II as a starting broth, which differs from standard MHB for its balanced cationic formulation. Besides a proportionally reduced concentration of the polyanionic components [
25], its use in a ten-time diluted form implies a proportional reduction of cations as well as of the nutrients. Mechesso et al. [
28] emphasized the importance of not diluting the media to the extent where bacteria do not grow or differences in bacterial growth are no longer clear. We earlier pinpointed the same limitation rising from the use of diluted broth and excogitated the use of alternative techniques such as the ATP luminescence assay for more sensitive, quantitative and objective measurement of MIC and MBIC [
24]. Poor growth was rarely observed. Whenever a reduction in the bacterial growth or an abnormal metabolic activity hindered a correct assessment of the antibacterial activity, the results of the tests were classified as ND.
The structure of the decapeptide KSL-W differs from KSL just for the replacement of the Lys amino acid in sixth position with a Trp residue. This simple substitution was earlier associated with a greater resistance of this peptide to salivary proteases [
18]. Our results further indicate that this single substitution in the primary sequence of KSL-W would confer additional desirable properties that have never been reported before as far as we are aware. The minimal loss of activity in undiluted MHB II proves a greater resilience of this peptide to additional factors such as the presence of polyanionic substances, which were found capable of mightily quenching the bacteriostatic, bactericidal, and biofilm-inhibitory activity of the other two polycationic AMPs [
23]. These results underscore the strong effect a single amino acid substitution can have on peptide behavior and provide new insights.
Trp is an amino acid with unique physicochemical properties [
30] and is capable of both hydrophobic and electrostatic interactions (e.g., cation-π, anion-π, ion pair-π interactions, hydrogen bonds, and hydrophobic contacts) with various molecular components of biological membranes (lipids, glycoconjugates, GAGs, polysaccharides and so on). The role of Trp in membranotropic activity could likely confer to KSL-W an additional functionality with respect to KSL, facilitating its insertion into membranes and its positioning at the membrane–water interface even in conditions where the mechanisms dependable on electrostatic charges are hindered, as in the case of undiluted broth. However, the molecular mechanisms determining the different properties of AMPs under different bioenvironments have still not been completely unveiled. To explain the pronounced LoA of Dadapin-1, whose antibacterial activity in undiluted broth was nearly suppressed, variables are even greater and of more difficult interpretation.
In the attempt to quantitatively express the different susceptibility of KSL-W antibacterial activity to MHB II quenching, we have proposed two types of approaches. LoA emerged as a very versatile measure that warrants further investigation. It potentially enables statistical comparison of the susceptibility of AMPs to interfering substances or physical factors and could be broadly adopted for screening the most stable and suitable candidate peptides. Even further, LoA could also be used to identify and compare the activity of relevant natural molecules that are present in physiologic fluids, which could more critically hinder the efficacy of antimicrobial treatments in the different anatomic sites and interstitial milieux.
It is easily conceivable that AMPs interact differently with the polyanionic components of the culture medium, based on their specific cationic charge and structure. More counterintuitive is that bacterial strains that were found more susceptible to peptides in diluted broth, such as E. coli and S. epidermidis, were the ones less advantaged in undiluted broth, with MICs undergoing no or limited shift. Indeed, it could be expected that lower concentrations of peptides can more easily be completely sequestered. Regarding the role of the microbial strain, we speculate that the zeta potential of bacterial cells could not only condition the susceptibility to cationic peptides, by attracting a greater number of AMP molecules to the surfaces of more electronegative bacteria but could also generate a competition with the polyanionic components responsible for peptide sequestration. Therefore, the impact of peptide complexation with the polyanionic component of the undiluted broth could be weakened or even abolished by bacterial surfaces with higher affinity for polycationic agents.
Early studies by Hong et al. [
13,
14,
27] assessed the antibacterial activity of KSL on a series of pathogens using antibiotic medium 3 (AM3) (
Table S25). Unfortunately, experimental work uniquely concerned testing MIC and not MBC or MBIC. MIC values for
S. aureus ATCC 6538 and 1550 were, respectively, 3.12 to 22 μg/mL. Thus, they fell in a range lower than that found by us with undiluted MHB II and marginally overlapped with that of diluted MHB II, of 1.95–3.91 μg/mL, which was found for three distinct strains in the present work. In AM3, the MIC on
E. coli ATCC 2592 ranged from 3.12 to 10 μg/mL, more closely matching our results with EC strain in undiluted broth (7.81 μg/mL), while, with
P. aeruginosa ATCC 9027, KSL exhibited a MIC of 1.56–5 μg/mL, i.e., very close to that found by us in diluted medium (3.91 μg/mL). More recently, Kosikowska et al. [
22] investigated just the MIC of KSL-W in undiluted non-cation-adjusted MHB (
Table S26). In the absence of supplemental cations, the MIC of KSL-W was 7.8 μg/mL with
Escherichia coli PCM 2057, 25 μg/mL with
S. aureus PCM 2054, 7.8 μg/mL with
S. epidermidis PCM 2118, and 25 μg/mL with
P. aeruginosa PCM 499. Thus, MIC values were mostly similar than those found by us with undiluted MHB II, with
E. coli exhibiting exactly the same value. It is known that even cationic salts in culture broths such as cation-adjusted MHB (MHB II) could potentially interfere with the results of antibacterial activity testing. In fact, they could partly neutralize the negative charges expressed on the outer surfaces of bacteria, having a protective effect. The saturation of the negative charges present on the surface of viable bacteria could compromise the electrostatic interaction between cationic peptides and their anionic targets. However, at least under these circumstances, the supplementation of cations did not appear to cause relevant variations in the results.
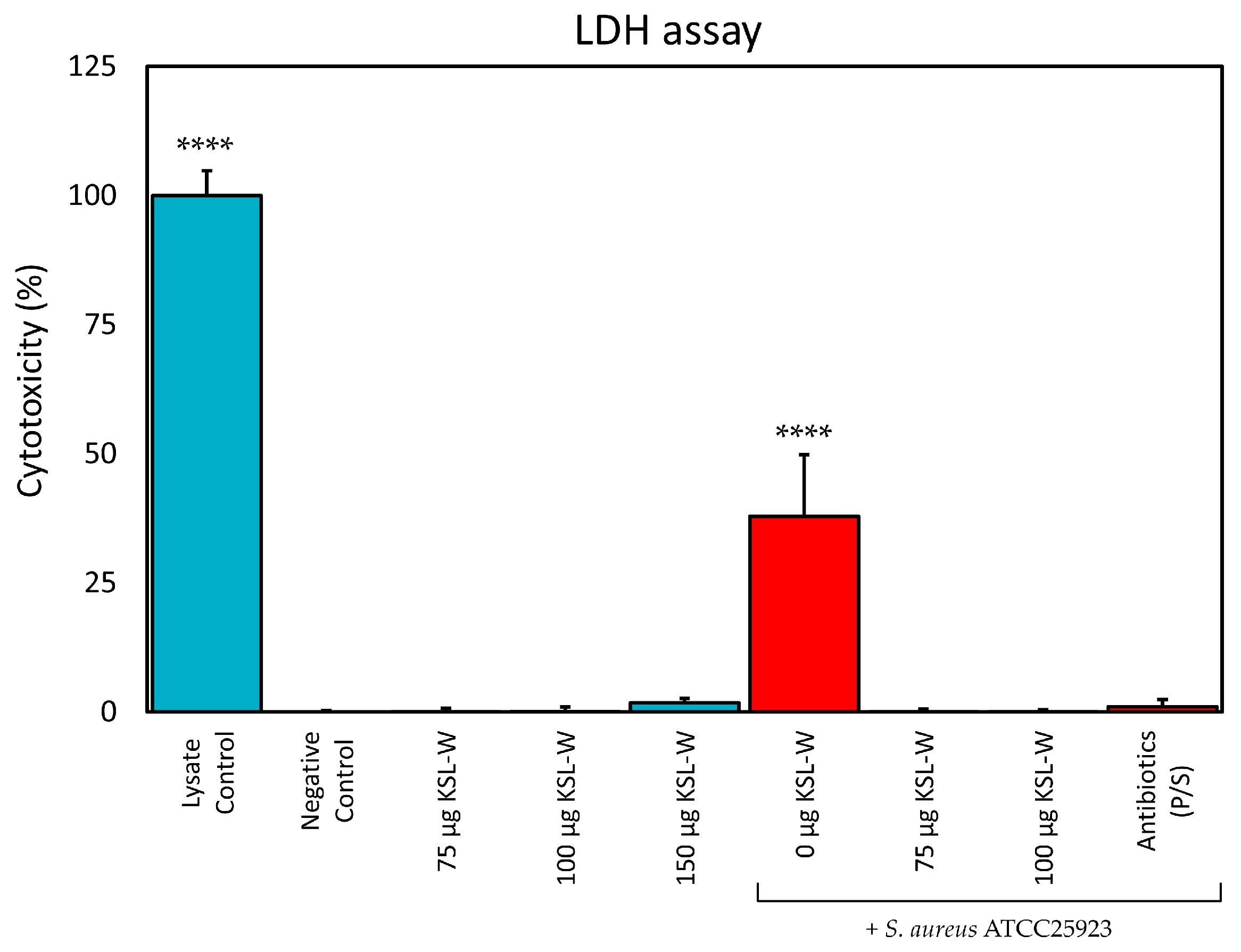
The present findings appear promising, particularly in view of our recent data that attest the bland levels of cytotoxicity of these three peptides, which were recently tested on a panel of cell lines including murine fibroblast-like L929 cells, human osteoblast-like MG63 cells and human bone marrow-derived mesenchymal stem cells (hMSCs) [
24,
31]. As far as KSL-W is concerned, lack of cytotoxicity [
16,
32,
33] or even pro-regenerative properties [
34] have been documented also on a series of other primary and secondary cell lines. Furthermore, a Phase 1 and 2a clinical trial recently demonstrated KSL-W’s safety when used as an anti-plaque agent [
35]. In the present study, we have provided further evidence that concentrations of KSL-W of up to 150 μg/mL do not cause significant release of LDH and, thus, do not cause cytotoxicity by disrupting membrane integrity of human osteoblast-like cells. Even further, our findings indicate that, although in the presence of serum proteases, KSL-W at a concentration as low as 75 μg/mL totally abolished the toxicity of
S. aureus in co-culture with MG63 cells, resulting selectively toxic and bactericidal for
S. aureus.
Along with natural amino acid substitution as the one described for KSL-W, new advancing strategies are being implemented to address the intrinsic limitations of stability and selectivity of peptides. These strategies involve the possibility to empower short peptides activity and stability through various types of chemical modifications such as peptide cyclization [
36], lipidation/alkylation [
37], nanoparticle conjugation, substitution of natural L-amino acids with synthetic D-amino acids, and peptidomimetics [
38].
We do not want to ignore the suggestion that comes from other articles, which is to look not only for single successful AMPs but also for cocktails of AMPs or combinations of AMPs and other antimicrobials. In this regard, Maron et al. studied the effects against
Staphylococcus aureus of single AMPs, pairs of AMPs, and a library of antimicrobial peptides. The authors found that the treatment with combined pairs of AMPs hindered the evolution of resistance compared to treatments based on single AMPs and that the lowest level of resistance was observed with a random antimicrobial peptide mixture (RPM) that contained more than one million different peptides [
39]. Antunes et al. [
40] found that RPMs reduce the risk of resistance in
P. aeruginosa more than single AMPs [
41]. Bauer et al. [
41], using an RPM entrapped in a copper metal matrix, found an increased antimicrobial activity toward methicillin-resistant
S. aureus than either copper or RPM alone.
Nanotechnologies lend themselves to enhancing the properties of AMPs. The encapsulation of AMPs in nanoparticles could improve their stability and protect them from protease degradation in the physiological environments [
42]. Moreover, functionalizing nanoparticles with AMPs could enable the development of antimicrobial coatings for biomaterials [
43,
44].
Two very recent and interesting papers focused on the AMP–protease interaction. Kim et al. presented two novel-designed AMPs, DAP-7 and DAP-10, which are able to remain stable for up to 12 h after exposure to proteases [
45], while Zhu et al. focused on protease inhibitors as molecular scaffolds in the creation of highly stable peptide-based biomaterials [
46].
In this study, an original model including bone cells is proposed to better mimic the complexity of the in vivo environment, with potential future expansion to incorporate immune system components. Key advantages of this approach are the following: a more accurate simulation of the physiological environment by including serum proteins and tissue cells usually absent in standard tests; simultaneous assessment of antibacterial activity and cytotoxicity, verifying bactericidal effects without damage to human cells; and insights into the peptide’s ability to protect human cells from damage caused by pathogens or their toxins.
4. Materials and Methods
4.1. AMPs
The synthetic peptides KSL (aminoacidic sequence: KKVVFKVKFK), KSL-W (aminoacidic sequence: KKVVFWVKFK), and Dadapin-1, consisting of 23 amino acids (aminoacidic sequence: GLLRASSKWGRKYYVDLAGCAKA), were purchased from GenScript (Shanghai, China) and from Proteogenix Sas (Schiltigheim, France) with a degree of purity > 98%. Mass spectra and HPLC chromatograms of all the different lots of peptides used for the experimental work are reported at the end of the document in the
Supplementary Materials (Section: Spectra and chromatograms of the different lots of peptides). Upon arrival, the peptides were placed at −80 °C for long-term storage. For the experimental work, the peptides were diluted to a concentration of 1 mg/mL in sterile deionized water, as recommended by the producers. Peptide aliquots were stored at −80 °C and, once thawed, entirely used within a day.
4.2. Strains
The antibacterial properties of the antimicrobial peptides (AMPs) were evaluated using the commercial reference strain
S.
aureus ATCC 25923 Seattle 1945, a human clinical isolate (ATCC
® Catalog No. 25923™), simply referred to as 25923 or SA 00, along with additional strains representing the most common Gram-positive and Gram-negative pathogens involved in implant-related orthopedic infections:
S.
aureus (cra4030 [
23] and cra2198), hereafter referred to as SA 01 and SA 02, respectively;
S.
epidermidis (cra4034 [
23,
31] and cra4029 [
23]), referred to as SE 01 and SE 02, respectively;
E.
coli (cra4038 [
23,
31]), referred to as EC;
P.
aeruginosa (cra4004 [
23] and cra3840 [
31]), referred to as PA 01 and PA 02, respectively; and
E.
faecalis (cra4024 and cra4033), referred to as EF 01 and EF 02, respectively.
To assess the potential of the AMP KSL-W in suppressing the cytotoxic effects of S. aureus on osteoblasts, the S. aureus ATCC 25923 strain and osteoblast-like MG63 cells (ATCC, Rockville, MD, USA) were utilized.
4.3. Bacterial Strain Ribotyping
Each bacterial strain was thawed from frozen stock and seeded on Mueller–Hinton agar (MHA, PO50007A, ThermoScientific, Thermo Fisher Scientific, Segrate, Italy) plates for 24 h at 37 °C. Pure colonies on the MHA were resuspended and processed by RiboPrinter® microbial characterization system (Qualicon, Wilmington, DE, USA), following the manufacturer’s instructions. EcoRI was the restriction enzyme used for the analysis.
4.4. Antibiotic Resistance Characterization of Bacterial Strains
All clinical isolates were assayed to determine their resistance to a panel of antibiotic substances by using the technique of the MIC test strips (MTSs) on MHA. MTSs were purchased from Liofilchem (Roseto degli Abruzzi, Italy) and were as follows: gentamicin 0.016–256 μg/mL; imipenem 0.002–32 μg/mL; oxacillin 0.016–256 μg/mL; amoxicillin 0.016–256 μg/mL; ciprofloxacin 0.002–32 μg/mL; vancomycin 0.016–256 μg/mL; clindamycin 0.016–256 μg/mL; penicillin G 0.016–256 μg/mL; penicillin G 0.002–32 μg/mL; piperacillin 0.016–256 μg/mL; tobramycin 0.016–256 μg/mL; trimethoprim*—sulfamethoxazole (1/19) 0.002–32* μg/mL; ceftazidime 0.016–256 μg/mL; ceftobiprole 0.002–32 μg/mL; and tigecycline 0.016–256 μg/mL. Once the MIC values had been obtained, EUCAST breakpoints (“The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 10.0, 2020”) were applied to classify bacterial strains as clinically resistant/intermediate/sensitive.
4.5. Characterization of the Antibacterial Properties of AMPs
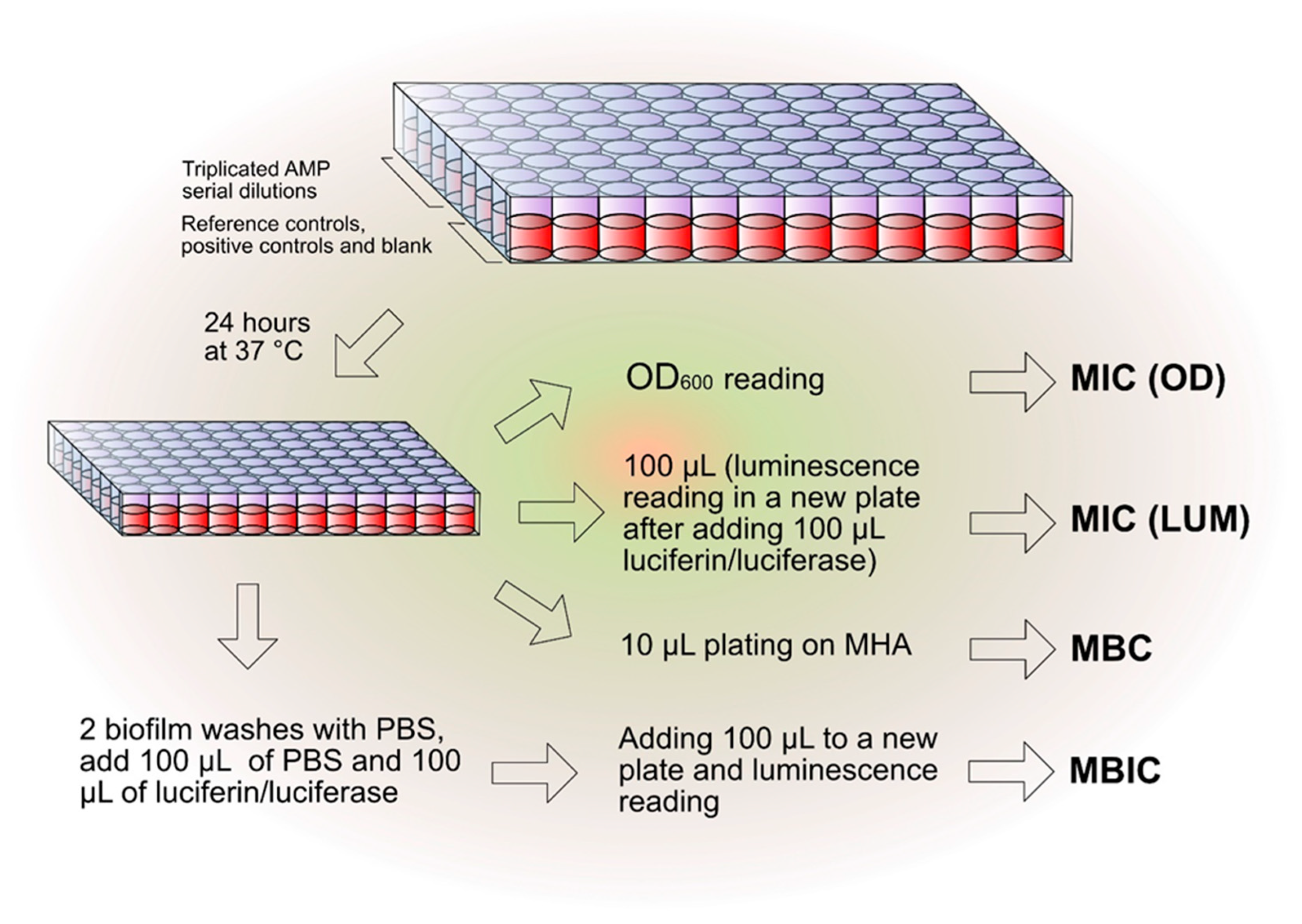
The experimental scheme reported in
Figure 6 helped to simplify and optimize the in vitro testing of the antibacterial activity of the different AMPs. The initial transparent microtiter plate for the determination of the minimum inhibitory concentration (MIC) by optical density (OD) measurement was conveniently used as a starting point not only for the alterative determination of the MIC by luminescence but also for the minimum bactericidal concentration (MBC) and for the minimum biofilm inhibitory concentration (MBIC), as explained below. For every test peptide, at least three independent experiments were performed on each bacterial strain per test condition (100% MHB and 20% MHB). The sole exception to this rule was when MIC values were on rare occasions found to be greater than 500 µg/mL. Under these circumstances, two consecutive experiments were performed to confirm the results. In this case, the antibacterial activity was considered insignificant and the third experiment was generally considered unnecessary.
4.6. Minimum Inhibitory Concentration (MIC)
The antibacterial properties of the test AMPs were assayed under two distinct conditions of bacterial culture: (i) with bacteria cultured in undiluted Mueller–Hinton II Broth (MHB II) and (ii) with bacteria cultured in diluted MHB II as earlier described in Campoccia et al. [
24]. Briefly, a few colonies of each bacterial strain, taken from overnight cultures on Mueller–Hinton Agar plates (MHA, PO50007A, ThermoScientific, Thermo Fisher Scientific, Segrate, Italy), were resuspended in Mueller–Hinton II Broth (MHB II, 24107, Liofilchem, Roseto degli Abruzzi, Italy), either undiluted or at a 20% dilution in deionized water. The concentration of bacteria was adjusted to approximately 10
8 CFU/mL, as estimated by optical density reading at 625 nm, using a Hewlett Packard G1103A spectrophotometer (Waldbronn, Germany). The bacterial suspension was further diluted 1:100 in 100% or 20% MHB II.
Serial 1:2 dilutions of all the test peptides were prepared in sterile deionized water starting from a stock solution of 1 mg/mL. Microtiter plates (96-Well CytoOne® Plate, CC7672-7596, Starlab S.r.l., Milano, Italy) were prepared by adding a volume of 100 µL of inoculum to 100 µL of treatment. Each AMP dilution was assayed in triplicate; each microplate included triplicate wells for the blank (100 µL of sterile distilled water and 100 µL of sterile MHB II), and 3 to 6 wells for reference control of 100% growth (100 µL of sterile deionized water and 100 µL of MHB II inoculated with the bacterium). Positive controls were performed in triplicate wells with 100 µL of a 10 mg/mL gentamicin solution (G1272, Sigma Aldrich, Milan, Italy) and with 100 µL of a solution containing 10,000 units/mL of penicillin and 10,000 µg/mL of streptomycin (penicillin–streptomycin, 15140-122, Life Technologies, Monza, Italy).
After a 1-day incubation at 37 °C, the MIC was determined by two distinct methods: (1) optical density measurement and (2) bioluminescence reading. In the first case, the optical density of the microtiter plates was read directly at a wavelength of 600 nm and the MIC was estimated from the curves obtained by plotting the OD measurements. For the bioluminescence reading, after gentle resuspension, 100 µL of each treated culture was transferred to a new 96-well non-treated plate (236105/237105, Life Technologies, Monza, Italy) and the metabolic activity was determined by the kit Bac-Titer Glo Microbial Cell Viability Assay (G8231, Promega Italia S.r.l, Milan, Italy). Optical density and bioluminescence were read by a Modulus II multifunction plate reader (Turner BioSystems, Sunnyvale, CA, USA) or, alternatively, by an Infinite M Plex (Tecan Group Ltd., Männedorf, Switzerland). As earlier described in [
24], in the case of luminescence, the MIC was identified from the curves of growth inhibition as the first concentration leading to a 1-log reduction in bacterial growth (i.e., 90% growth inhibition). A close match was generally observed between MIC estimated by optical reading and luminescence. Nonetheless, when using 20% MHB II, the growth of some bacterial strains was exceptionally low, and OD reading was not as sensitive as luminescence in detecting the bacterial growth. In rare occasions, even the curve obtained by luminescence reading did not allow the extrapolation of valid MIC values. For instance, in the experiments performed with undiluted medium, both strains of
P. aeruginosa showed an unexpected burst in metabolic activity just in proximity of the MIC value assessed by OD reading.
4.7. Minimum Bactericidal Concentration (MBC)
For assessing the MBC, 10 μL of bacterial suspensions was taken from wells corresponding to MIC, 2× MIC, and 4× MIC and then plated on MHA plates. After one day of incubation at 37 °C, the agar plates were examined for bacterial growth. The MBC was determined as the lowest dilution causing absence of bacterial colonies growth on the plate.
4.8. Minimum Biofilm Inhibitory Concentration (MBIC)
The wells of the microplate, initially used for the determination of the MIC, were rinsed twice with 220 µL of phosphate-buffered solution (PBS) to remove loosely attached and planktonic bacteria. A total of 100 µL of fresh PBS and 100 µL of Bac-Titer Glo solution (G8231, Promega Italia S.r.l., Milan, Italy) were added to each well. After a 10 min incubation, a volume of 100 µL was transferred to a new microplate for luminescence determination as described above. As a general rule, the MBIC value was identified as the concentration at which a reduction of at least a log in the metabolic activity was observed.
4.9. AMPs Susceptibility to the Interference by MHB II
In order to assess the extent of the interference of the culture medium composition with the antibacterial activity of the three peptides, a “Rate of Abatement” of the bactericidal properties (RoA) was calculated using the following formula (Equation (1)):
RoA indicates the fold increases in the MBC when tested in undiluted medium (MBC
20% MHB), i.e., with maximal interference of broth polyanionic substances, with respect to diluted medium (MBC
MHB). A limitation of the rate of abatement is that, under some circumstances, the MBC does not correspond to a single value but rather to a range of values, thus RoA must be expressed as a range of values as well. A more versatile measure to express the loss of activity was calculated as follows. MBC values from each single test were Log
2-transformed and the mean of the three to five values obtained from the replicated experiments was calculated per each peptide, per each strain, and per each condition (leading to a single Log
2 (MBC) mean value). The loss of activity (LoA) was therefore measured in terms of Log
2-reduction with the following formula (Equation (2)):
In case of no MBC variation between undiluted and diluted medium, LoA assumes the value of 0 (i.e., no loss of activity), while a LoA of 1 corresponds to a doubled MBC in undiluted medium (as easily calculated by the formula 2LoA).
4.10. Cell Culture of Osteoblast-like MG63 Cells
MG63 cells (ATCC, Rockville, MD, USA) were thawed from a frozen stock and cultured in DMEM (11885-084, Thermo Fisher Scientific, Segrate, Italy) supplemented with non-inactivated 10% fetal bovine serum (FBS, F7524, Sigma-Aldrich, Milan, Italy) and 100× Penicillin/Streptomycin solution (P/S, 10,000 units penicillin and 10 mg streptomycin/mL, P4333, Sigma-Aldrich, Milan, Italy), under standard culture conditions (i.e., at 37 °C, in a humidified atmosphere with 5% CO2). Cells were regularly subcultured 2–3 times a week by using TrypLE Select 1× (GIBCO, Thermo Fisher Scientific, Segrate, Italy).
4.11. Assessment of KSL-W Suppression of S. aureus Cytotoxicity
KSL-W was the peptide selected to investigate the efficacy in suppressing the cytotoxicity of S. aureus ATCC 25923 on osteoblast-like MG63 cells. S. aureus ATCC 25923 was thawed from the frozen stock and plated on MHA and incubated at 37 °C for 24 h.
A suspension of MG63 cells was prepared in complete DMEM at a concentration of 5 × 104 cells/mL. A total of 100 µL of cell suspension was added to each well of a 96-well cell culture-treated plate (137101, Nunclon Delta Black Microwell, ThermoScientific, Thermo Fisher Scientific, Segrate, Italy). After cell seeding, the plate was incubated for a day at 37 °C under standard culture conditions. A single colony of S. aureus ATCC 25923 was taken from the MHA plate and inoculated in 9 mL of Tryptic Soy Broth (TSB, 24513, Liofilchem, Roseto degli Abruzzi, Italy). The inoculated broth was then incubated at 37 °C overnight.
The following day, the cultured broth was centrifuged at 4000 rpm for 15 min using a Biofuge Fresco (Heraeus Instruments, Hanau, Germany) and the pellet was resuspended in DMEM. The new bacterial suspension was sonicated for 10 min and diluted to obtain an optical density of 0.1 at 625 nm. A further 1:50 dilution was performed using DMEM. Before adding the bacterial inoculum to the MG63 cell, the cell culture medium was removed from the wells of the 96-well plate and replaced with 50 µL of fresh DMEM with 10% FBS but without antibiotics. Then, the following treatments were added to the wells cultured with MG63 cells: 50 µL of DMEM w/o FBS and w/o antibiotics (Negative Control and Lysate Control); 50 µL of DMEM containing 2% Tween 20 (P7949, Merck KGaA, Darmstadt, Germany) (Positive Control); 25 µL DMEM + 25 µL of a solution of 600 µg/mL of KSL-W in DMEM (150 µg/mL KSL-W); 25 µL DMEM + 25 µL of a solution of 400 µg/mL of KSL-W in DMEM (100 µg/mL KSL-W); 25 µL DMEM + 25 µL of a solution of 300 µg/mL of KSL-W in DMEM (75 µg/mL KSL-W); 25 µL of bacterial inoculum + 25 µL of DMEM (0 µg/mL KSL-W + S. aureus); 25 µL of bacterial inoculum + 25 µL of a solution of 300 µg/mL of KSL-W in DMEM (75 µg/mL KSL-W + S. aureus); 25 µL of bacterial inoculum + 25 µL of a solution of 400 µg/mL of KSL-W in DMEM (100 µg/mL KSL-W + S. aureus); 25 µL of bacterial inoculum + 25 µL of DMEM with 4× concentration of P/S (Antibiotics + S. aureus). The plate was incubated at 37 °C under standard culture conditions for 24 h. All treatments were performed in triplicate wells.
Cytotoxicity was assessed by measuring the release of lactate dehydrogenase (LDH) from damaged cells by the LDH Cytotoxicity Assay (ab197004, Abcam, Cambridge, UK), strictly following the instructions of the manufacturer. For all treatments, each well was assayed in duplicate by transferring 5 µL aliquots of the supernatants (5.5 µL in the case of the Lysate Control as per manufacturer’s indications) to the wells of a new 96-well white plate with clear bottom (165306, Thermo Fisher Scientific, Rochester, NY, USA). Then, 95 μL of working reagent was added to each well. After 10 min shaking, the plate was read in fluorescence (excitation: 535 nm; emission 587 nm) by an Infinite 200 PRO mod. M PLEX multifunction plate reader (Tecan Group Ltd., Männedorf, Switzerland). The experiments were independently performed three times. Furthermore, the bactericidal effectiveness of KSL-W treatment on co-cultures was verified by plating 10 μL of the culture medium on MHA and incubating the agar plates for a day at 37 °C.
4.12. Statistical Analysis
For the statistical analysis of the differences between AMPs under the different testing conditions, MIC, MBC, and MBIC values of each single experiment were Log
2-normalized (see [
47]). Differences in Log
2-normalized data were analyzed by two-way ANOVA followed by post hoc Tukey’s multiple comparisons test using the statistical software GraphPad (ver. 10.4). Values obtained for Dadapin-1, especially in undiluted medium, generally exhibited consistent differences with respect to KSL and KSL-W and did not require statistical analysis.
Data in relative fluorescence units (RFU) from the investigation on KSL-W suppression of S. aureus cytotoxicity were processed as follows. The mean of the duplicate readings from each single treated well was calculated. Within each plate, the mean of the Lysate Control was equated to 100% cytotoxicity and all measurements were expressed as percentage of the Lysate Control. After this transformation, the statistical difference between treatments was analyzed by one-way ANOVA, followed by Bonferroni/Dunn test (significance level: 5%) using StatView (version 5.0.1, Sas Institute Inc., Cary, NC, USA).