Mild Mitochondrial Uncoupling for True Ectopic Lipid Disposal
Abstract
1. Introduction
2. Pathophysiological Role of Ectopic Lipids in Metabolic Disorders
2.1. Physiological Routes of Dietary Lipid Processing
2.2. Lipid Spillover to Non-Adipose Tissues
2.3. Ectopic Lipid Accumulations in Skeletal Muscle, Liver and Pancreas
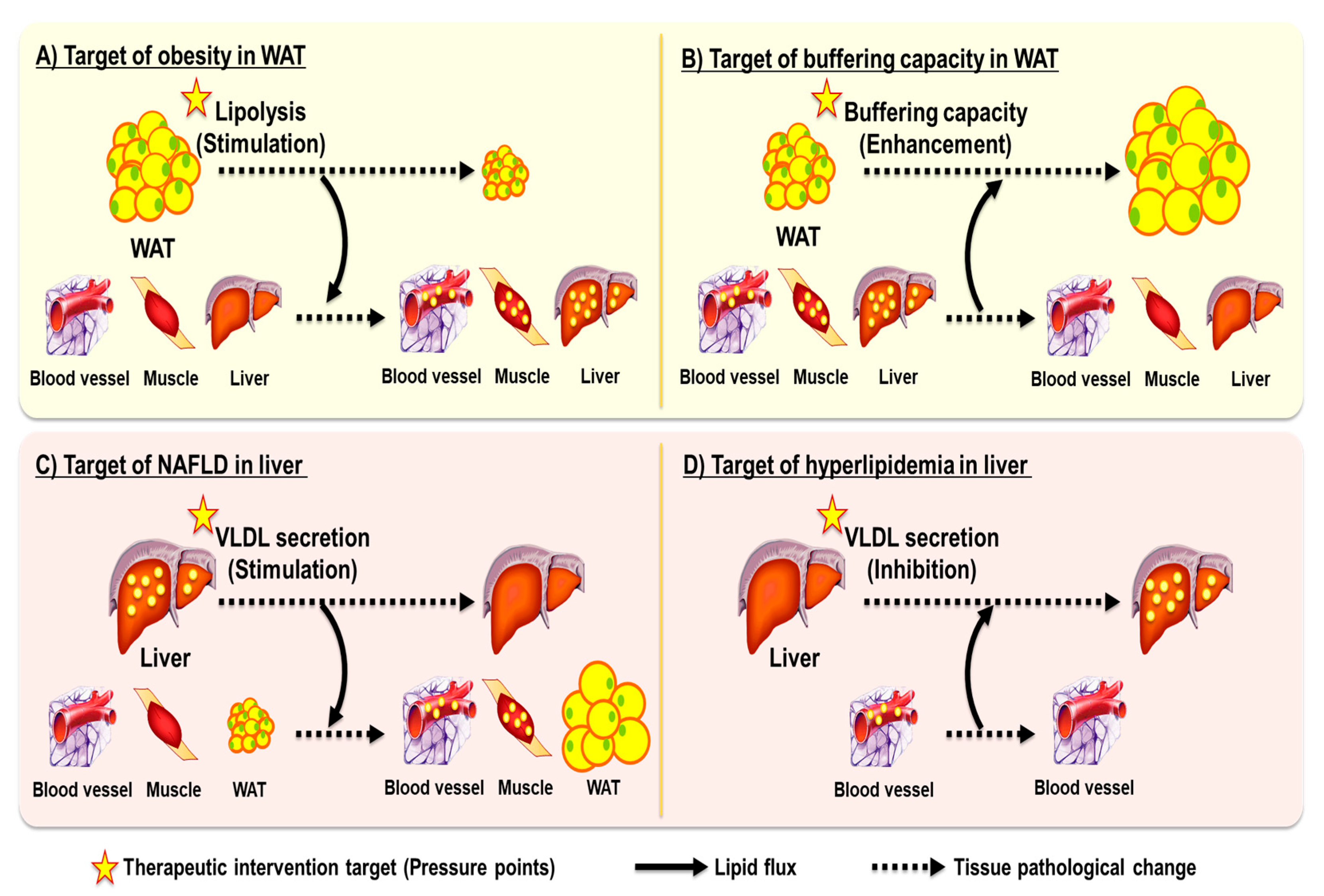
3. Ballooning Effect When Targeting Single Metabolic Disease
3.1. Obesity and Adipose Tissue Lipolysis
3.2. Dyslipidemia, NAFLD, and Hepatic Lipid Export
4. Strategies Toward True Disposal of Excess Lipids: Beyond Redistribution
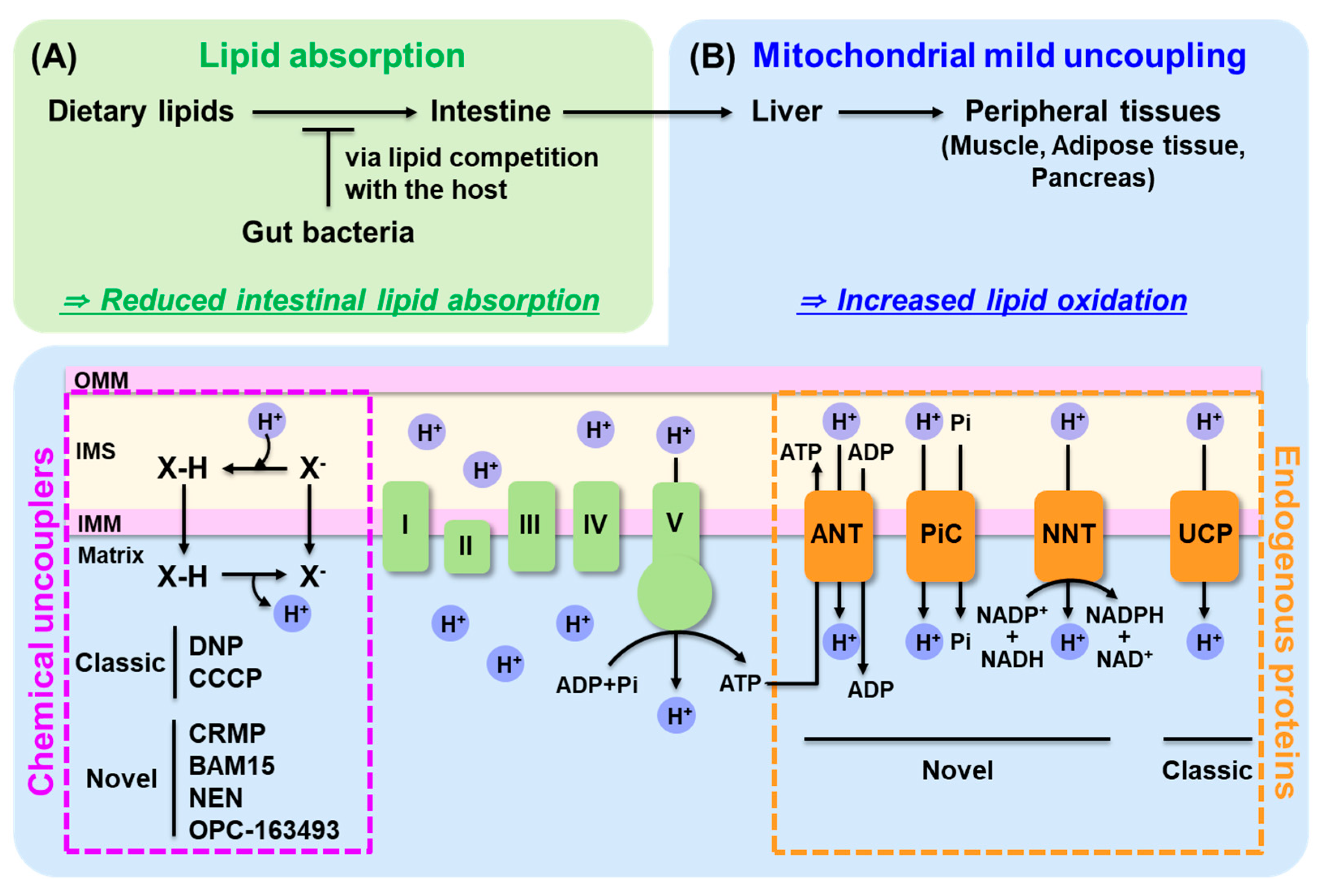
4.1. Decreasing Dietary Lipid Absorption
4.2. Enhance Lipid Oxidation: Mitochondrial Uncoupling
4.2.1. Classic and Novel Mitochondrial Uncouplers
4.2.2. Novel Endogenous Uncoupling Proteins
4.2.3. From Traditional to Mild Uncoupling for Safety and Beyond
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 2237–2238. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Invest. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Choi, C.S.; Birkenfeld, A.L.; Alves, T.C.; Jornayvaz, F.R.; Jurczak, M.J.; Zhang, D.; Woo, D.K.; Shadel, G.S.; Ladiges, W.; et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010, 12, 668–674. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Savage, D.B.; Bilz, S.; Solomon, G.; Yonemitsu, S.; Cline, G.W.; Befroy, D.; Zemany, L.; Kahn, B.B.; et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 12587–12594. [Google Scholar] [CrossRef]
- Tamura, Y. Ectopic fat, insulin resistance and metabolic disease in non-obese Asians: Investigating metabolic gradation. Endocr. J. 2019, 66, 1–9. [Google Scholar] [CrossRef]
- Laurens, C.; de Glisezinski, I.; Larrouy, D.; Harant, I.; Moro, C. Influence of Acute and Chronic Exercise on Abdominal Fat Lipolysis: An Update. Front. Physiol. 2020, 11, 575363. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Guo, L. Exercise-regulated lipolysis: Its role and mechanism in health and diseases. J. Adv. Res. 2024, 28, S2090-1232(24)00550-2. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A. The chylomicron saga: Time to focus on postprandial metabolism. Front. Endocrinol. 2023, 14, 1322869. [Google Scholar] [CrossRef]
- Mu, H.; Høy, C.E. The digestion of dietary triacylglycerols. Prog. Lipid Res. 2004, 43, 105–133. [Google Scholar] [CrossRef]
- Wang, T.Y.; Liu, M.; Portincasa, P.; Wang, D.Q. New insights into the molecular mechanism of intestinal fatty acid absorption. Eur. J. Clin. Investig. 2013, 43, 1203–1223. [Google Scholar] [CrossRef]
- Hokkanen, K.; Tirronen, A.; Ylä-Herttuala, S. Intestinal lymphatic vessels and their role in chylomicron absorption and lipid homeostasis. Curr. Opin. Lipidol. 2019, 30, 370–376. [Google Scholar] [CrossRef]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, bnaa004. [Google Scholar] [CrossRef]
- Moon, J.; Kim, P. Intravital Two-photon Imaging of Dynamic Alteration of Hepatic Lipid Droplets in Fasted and Refed State. J. Lipid Atheroscler. 2021, 10, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.C.; Meyer, J.M. Transient elevation of triacylglycerol content in the liver: A fundamental component of the acute response to exercise. J Appl Physiol (1985) 2021, 130, 1293–1303. [Google Scholar] [CrossRef]
- Pienkowska, J.; Brzeska, B.; Kaszubowski, M.; Kozak, O.; Jankowska, A.; Szurowska, E. The correlation between the MRI-evaluated ectopic fat accumulation and the incidence of diabetes mellitus and hypertension depends on body mass index and waist circumference ratio. PLoS ONE 2020, 15, e0226889. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Newcomer, B.R.; Ravussin, E.; Volaufova, J.; Bennett, B.; Chalew, S.; Cefalu, W.T.; Sothern, M. Intrahepatic and intramyocellular lipids are determinants of insulin resistance in prepubertal children. Diabetologia 2011, 54, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Erion, D.M.; Park, H.J.; Lee, H.Y. The role of lipids in the pathogenesis and treatment of type 2 diabetes and associated co-morbidities. BMB Rep. 2016, 49, 139–148. [Google Scholar] [CrossRef]
- An, S.M.; Cho, S.H.; Yoon, J.C. Adipose Tissue and Metabolic Health. Diabetes Metab. J. 2023, 47, 595–611. [Google Scholar] [CrossRef]
- Patel, P.; Abate, N. Role of subcutaneous adipose tissue in the pathogenesis of insulin resistance. J. Obes. 2013, 2013, 489187. [Google Scholar] [CrossRef]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Invest. 2020, 130, 1453–1460. [Google Scholar] [CrossRef]
- Wagner, R.; Jaghutriz, B.A.; Gerst, F.; Barroso Oquendo, M.; Machann, J.; Schick, F.; Loffler, M.W.; Nadalin, S.; Fend, F.; Konigsrainer, A.; et al. Pancreatic Steatosis Associates With Impaired Insulin Secretion in Genetically Predisposed Individuals. J. Clin. Endocrinol. Metab. 2020, 105, 3518–3525. [Google Scholar] [CrossRef]
- Tunaru, S.; Bonnavion, R.; Brandenburger, I.; Preussner, J.; Thomas, D.; Scholich, K.; Offermanns, S. 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nat. Commun. 2018, 9, 177. [Google Scholar] [CrossRef]
- Szczepaniak, L.S.; Victor, R.G.; Mathur, R.; Nelson, M.D.; Szczepaniak, E.W.; Tyer, N.; Chen, I.; Unger, R.H.; Bergman, R.N.; Lingvay, I. Pancreatic steatosis and its relationship to beta-cell dysfunction in humans: Racial and ethnic variations. Diabetes Care 2012, 35, 2377–2383. [Google Scholar] [CrossRef]
- Mahyoub, M.A.; Elhoumed, M.; Maqul, A.H.; Almezgagi, M.; Abbas, M.; Jiao, Y.; Wang, J.; Alnaggar, M.; Zhao, P.; He, S. Fatty infiltration of the pancreas: A systematic concept analysis. Front. Med. 2023, 10, 1227188. [Google Scholar] [CrossRef]
- Ye, R.; Onodera, T.; Scherer, P.E. Lipotoxicity and beta Cell Maintenance in Obesity and Type 2 Diabetes. J. Endocr. Soc. 2019, 3, 617–631. [Google Scholar] [CrossRef]
- Ross, S.R.; Graves, R.A.; Spiegelman, B.M. Targeted expression of a toxin gene to adipose tissue: Transgenic mice resistant to obesity. Genes. Dev. 1993, 7, 1318–1324. [Google Scholar] [CrossRef]
- Moitra, J.; Mason, M.M.; Olive, M.; Krylov, D.; Gavrilova, O.; Marcus-Samuels, B.; Feigenbaum, L.; Lee, E.; Aoyama, T.; Eckhaus, M.; et al. Life without white fat: A transgenic mouse. Genes Dev. 1998, 12, 3168–3181. [Google Scholar] [CrossRef]
- Cortés, V.A.; Curtis, D.E.; Sukumaran, S.; Shao, X.; Parameswara, V.; Rashid, S.; Smith, A.R.; Ren, J.; Esser, V.; Hammer, R.E.; et al. Molecular mechanisms of hepatic steatosis and insulin resistance in the AGPAT2-deficient mouse model of congenital generalized lipodystrophy. Cell Metab. 2009, 9, 165–176. [Google Scholar] [CrossRef]
- Simha, V.; Szczepaniak, L.S.; Wagner, A.J.; DePaoli, A.M.; Garg, A. Effect of leptin replacement on intrahepatic and intramyocellular lipid content in patients with generalized lipodystrophy. Diabetes Care 2003, 26, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.A.; Choi, C.S.; Wang, Y.; Kim, S.; Hwang, Y.J.; Kim, Y.B.; Cline, G.; Shulman, G.I.; Sul, H.S. Resistance to high-fat diet-induced obesity but exacerbated insulin resistance in mice overexpressing preadipocyte factor-1 (Pref-1): A new model of partial lipodystrophy. Diabetes 2008, 57, 3258–3266. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, H.Y.; Song, J.H.; Kim, G.T.; Jeon, S.; Song, Y.J.; Lee, J.S.; Hur, J.H.; Oh, H.H.; Park, S.Y.; et al. Adipocyte-Specific Deficiency of De Novo Sphingolipid Biosynthesis Leads to Lipodystrophy and Insulin Resistance. Diabetes 2017, 66, 2596–2609. [Google Scholar] [CrossRef]
- van Zwol, W.; van de Sluis, B.; Ginsberg, H.N.; Kuivenhoven, J.A. VLDL Biogenesis and Secretion: It Takes a Village. Circ. Res. 2024, 134, 226–244. [Google Scholar] [CrossRef] [PubMed]
- Tietge, U.J.; Bakillah, A.; Maugeais, C.; Tsukamoto, K.; Hussain, M.; Rader, D.J. Hepatic overexpression of microsomal triglyceride transfer protein (MTP) results in increased in vivo secretion of VLDL triglycerides and apolipoprotein B. J. Lipid Res. 1999, 40, 2134–2139. [Google Scholar] [CrossRef]
- Cuchel, M.; Bloedon, L.T.; Szapary, P.O.; Kolansky, D.M.; Wolfe, M.L.; Sarkis, A.; Millar, J.S.; Ikewaki, K.; Siegelman, E.S.; Gregg, R.E.; et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N. Engl. J. Med. 2007, 356, 148–156. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Pavanello, C.; Bertolini, S. Microsomal transfer protein (MTP) inhibition-a novel approach to the treatment of homozygous hypercholesterolemia. Ann. Med. 2014, 46, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.E.; Wagener, G.; Baker, B.F.; Geary, R.S.; Donovan, J.M.; Beuers, U.H.; Nederveen, A.J.; Verheij, J.; Trip, M.D.; Basart, D.C.; et al. Mipomersen, an apolipoprotein B synthesis inhibitor, lowers low-density lipoprotein cholesterol in high-risk statin-intolerant patients: A randomized, double-blind, placebo-controlled trial. Eur. Heart J. 2012, 33, 1142–1149. [Google Scholar] [CrossRef]
- Lee, H.Y.; Birkenfeld, A.L.; Jornayvaz, F.R.; Jurczak, M.J.; Kanda, S.; Popov, V.; Frederick, D.W.; Zhang, D.; Guigni, B.; Bharadwaj, K.G.; et al. Apolipoprotein CIII overexpressing mice are predisposed to diet-induced hepatic steatosis and hepatic insulin resistance. Hepatology 2011, 54, 1650–1660. [Google Scholar] [CrossRef]
- Norata, G.D.; Tsimikas, S.; Pirillo, A.; Catapano, A.L. Apolipoprotein C-III: From Pathophysiology to Pharmacology. Trends Pharmacol. Sci. 2015, 36, 675–687. [Google Scholar] [CrossRef]
- Sacks, F.M.; Stanesa, M.; Hegele, R.A. Severe hypertriglyceridemia with pancreatitis: Thirteen years’ treatment with lomitapide. JAMA Intern. Med. 2014, 174, 443–447. [Google Scholar] [CrossRef]
- Wing, R.R.; Bolin, P.; Brancati, F.L.; Bray, G.A.; Clark, J.M.; Coday, M.; Crow, R.S.; Curtis, J.M.; Egan, C.M.; Espeland, M.A.; et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369, 145–154. [Google Scholar]
- Weinsier, R.L.; Ullmann, D.O. Gallstone formation and weight loss. Obes. Res. 1993, 1, 51–56. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Zhao, Y.; Zhang, X.; Li, B.; Cui, R. The Effects of Calorie Restriction in Depression and Potential Mechanisms. Curr. Neuropharmacol. 2015, 13, 536–542. [Google Scholar] [CrossRef]
- Johansson, K.; Neovius, K.; DeSantis, S.M.; Rössner, S.; Neovius, M. Discontinuation due to adverse events in randomized trials of orlistat, sibutramine and rimonabant: A meta-analysis. Obes. Rev. An. Off. J. Int. Assoc. Study Obes. 2009, 10, 564–575. [Google Scholar] [CrossRef]
- Heck, A.M.; Yanovski, J.A.; Calis, K.A. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy 2000, 20, 270–279. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, H.Y. Revisiting the Bacterial Phylum Composition in Metabolic Diseases Focused on Host Energy Metabolism. Diabetes Metab. J. 2020, 44, 658–667. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Peng, L.; Feng, P.; Han, R.; Khan, A.; Kulshreshtha, S.; Ling, Z.; Liu, P.; Li, X. Gut microbes consume host energy and reciprocally provide beneficial factors to sustain a symbiotic relationship with the host. Sci. Total Environ. 2023, 904, 166773. [Google Scholar] [CrossRef]
- Corbin, K.D.; Carnero, E.A.; Dirks, B.; Igudesman, D.; Yi, F.; Marcus, A.; Davis, T.L.; Pratley, R.E.; Rittmann, B.E.; Krajmalnik-Brown, R.; et al. Host-diet-gut microbiome interactions influence human energy balance: A randomized clinical trial. Nat. Commun. 2023, 14, 3161. [Google Scholar] [CrossRef]
- Jang, H.R.; Park, H.J.; Kang, D.; Chung, H.; Nam, M.H.; Lee, Y.; Park, J.H.; Lee, H.Y. A protective mechanism of probiotic Lactobacillus against hepatic steatosis via reducing host intestinal fatty acid absorption. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Veerkamp, J.H. Fatty Acid Composition of Bifidobacterium and Lactobacillus Strains. J. Bacteriol. 1971, 108, 861–867. [Google Scholar] [CrossRef]
- Polacheck, J.W.; Tropp, B.E.; Law, J.H. Biosynthesis of cyclopropane compounds. 8. The conversion of oleate to dihydrosterculate. J. Biol. Chem. 1966, 241, 3362–3364. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Growth of probiotic lactobacilli in the presence of oleic acid enhances subsequent survival in gastric juice. Microbiology 2007, 153, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Demine, S.; Renard, P.; Arnould, T. Mitochondrial Uncoupling: A Key Controller of Biological Processes in Physiology and Diseases. Cells 2019, 8, 795. [Google Scholar] [CrossRef]
- Kotova, E.A.; Antonenko, Y.N. Fifty Years of Research on Protonophores: Mitochondrial Uncoupling As a Basis for Therapeutic Action. Acta Naturae 2022, 14, 4–13. [Google Scholar] [CrossRef]
- Georgakopoulos, N.D.; Wells, G.; Campanella, M. The pharmacological regulation of cellular mitophagy. Nat. Chem. Biol. 2017, 13, 136–146. [Google Scholar] [CrossRef]
- Shrestha, R.; Johnson, E.; Byrne, F.L. Exploring the therapeutic potential of mitochondrial uncouplers in cancer. Mol. Metab. 2021, 51, 101222. [Google Scholar] [CrossRef]
- Sousa, D.; Carmo, H.; Roque Bravo, R.; Carvalho, F.; Bastos, M.L.; Guedes de Pinho, P.; Dias da Silva, D. Diet aid or aid to die: An update on 2,4-dinitrophenol (2,4-DNP) use as a weight-loss product. Arch. Toxicol. 2020, 94, 1071–1083. [Google Scholar] [CrossRef]
- Samuel, V.T.; Liu, Z.X.; Qu, X.; Elder, B.D.; Bilz, S.; Befroy, D.; Romanelli, A.J.; Shulman, G.I. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 2004, 279, 32345–32353. [Google Scholar] [CrossRef]
- Perry, R.J.; Kim, T.; Zhang, X.M.; Lee, H.Y.; Pesta, D.; Popov, V.B.; Zhang, D.; Rahimi, Y.; Jurczak, M.J.; Cline, G.W.; et al. Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targeted mitochondrial uncoupler. Cell Metab. 2013, 18, 740–748. [Google Scholar] [CrossRef]
- Perry, R.J.; Zhang, D.; Zhang, X.M.; Boyer, J.L.; Shulman, G.I. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science 2015, 347, 1253–1256. [Google Scholar] [CrossRef]
- Goedeke, L.; Peng, L.; Montalvo-Romeral, V.; Butrico, G.M.; Dufour, S.; Zhang, X.M.; Perry, R.J.; Cline, G.W.; Kievit, P.; Chng, K.; et al. Controlled-release mitochondrial protonophore (CRMP) reverses dyslipidemia and hepatic steatosis in dysmetabolic nonhuman primates. Sci. Transl. Med. 2019, 11, eaay0284. [Google Scholar] [CrossRef]
- Bando, Y.; Geisler, J.G. Disease modifying mitochondrial uncouplers, MP101, and a slow release ProDrug, MP201, in models of Multiple Sclerosis. Neurochem. Int. 2019, 131, 104561. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Johnson, J.; Fang, W.; Halpern, J.; Marosi, K.; Liu, D.; Geisler, J.G.; Mattson, M.P. A mitochondrial uncoupler prodrug protects dopaminergic neurons and improves functional outcome in a mouse model of Parkinson’s disease. Neurobiol. Aging 2020, 85, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Zhang, K.; Ma, Y.; Song, Y.; Zhang, W.; Qi, T.; Qiu, H.; Shi, J.; Kan, C.; Zhang, J.; et al. BAM15 as a mitochondrial uncoupler: A promising therapeutic agent for diverse diseases. Front. Endocrinol. 2023, 14, 1252141. [Google Scholar] [CrossRef] [PubMed]
- Kenwood, B.M.; Weaver, J.L.; Bajwa, A.; Poon, I.K.; Byrne, F.L.; Murrow, B.A.; Calderone, J.A.; Huang, L.; Divakaruni, A.S.; Tomsig, J.L.; et al. Identification of a novel mitochondrial uncoupler that does not depolarize the plasma membrane. Mol. Metab. 2014, 3, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, S.J.; Chen, S.Y.; Brandon, A.E.; Salamoun, J.M.; Byrne, F.L.; Garcia, C.J.; Beretta, M.; Olzomer, E.M.; Shah, D.P.; Philp, A.M.; et al. Mitochondrial uncoupler BAM15 reverses diet-induced obesity and insulin resistance in mice. Nat. Commun. 2020, 11, 2397. [Google Scholar] [CrossRef]
- Tao, H.; Zhang, Y.; Zeng, X.; Shulman, G.I.; Jin, S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat. Med. 2014, 20, 1263–1269. [Google Scholar] [CrossRef]
- Han, P.; Shao, M.; Guo, L.; Wang, W.; Song, G.; Yu, X.; Zhang, C.; Ge, N.; Yi, T.; Li, S.; et al. Niclosamide ethanolamine improves diabetes and diabetic kidney disease in mice. Am. J. Transl. Res. 2018, 10, 1071–1084. [Google Scholar]
- Alasadi, A.; Chen, M.; Swapna, G.V.T.; Tao, H.; Guo, J.; Collantes, J.; Fadhil, N.; Montelione, G.T.; Jin, S. Effect of mitochondrial uncouplers niclosamide ethanolamine (NEN) and oxyclozanide on hepatic metastasis of colon cancer. Cell Death Dis. 2018, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Kanemoto, N.; Okamoto, T.; Tanabe, K.; Shimada, T.; Minoshima, H.; Hidoh, Y.; Aoyama, M.; Ban, T.; Kobayashi, Y.; Ando, H.; et al. Antidiabetic and cardiovascular beneficial effects of a liver-localized mitochondrial uncoupler. Nat. Commun. 2019, 10, 2172. [Google Scholar] [CrossRef]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef]
- Nesci, S.; Rubattu, S. UCP2, a Member of the Mitochondrial Uncoupling Proteins: An Overview from Physiological to Pathological Roles. Biomedicines 2024, 12, 1307. [Google Scholar] [CrossRef]
- Choi, C.S.; Fillmore, J.J.; Kim, J.K.; Liu, Z.X.; Kim, S.; Collier, E.F.; Kulkarni, A.; Distefano, A.; Hwang, Y.J.; Kahn, M.; et al. Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. J. Clin. Investig. 2007, 117, 1995–2003. [Google Scholar] [CrossRef] [PubMed]
- Toime, L.J.; Brand, M.D. Uncoupling protein-3 lowers reactive oxygen species production in isolated mitochondria. Free. Radic. Biol. Med. 2010, 49, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Yu, X.X.; Zhong, A.; Li, W.; Brush, J.; Sherwood, S.W.; Adams, S.H.; Pan, G. UCP4, a novel brain-specific mitochondrial protein that reduces membrane potential in mammalian cells. FEBS Lett. 1999, 443, 326–330. [Google Scholar] [CrossRef]
- Kreiter, J.; Rupprecht, A.; Škulj, S.; Brkljača, Z.; Žuna, K.; Knyazev, D.G.; Bardakji, S.; Vazdar, M.; Pohl, E.E. ANT1 Activation and Inhibition Patterns Support the Fatty Acid Cycling Mechanism for Proton Transport. Int. J. Mol. Sci. 2021, 22, 2490. [Google Scholar] [CrossRef]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.S.; Soukas, A.A. Identity, structure, and function of the mitochondrial permeability transition pore: Controversies, consensus, recent advances, and future directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef]
- Kwong, J.Q.; Molkentin, J.D. Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metab. 2015, 21, 206–214. [Google Scholar] [CrossRef]
- Morrow, R.M.; Picard, M.; Derbeneva, O.; Leipzig, J.; McManus, M.J.; Gouspillou, G.; Barbat-Artigas, S.; Dos Santos, C.; Hepple, R.T.; Murdock, D.G.; et al. Mitochondrial energy deficiency leads to hyperproliferation of skeletal muscle mitochondria and enhanced insulin sensitivity. Proc. Natl. Acad. Sci. USA 2017, 114, 2705–2710. [Google Scholar] [CrossRef]
- Cho, J.; Zhang, Y.; Park, S.Y.; Joseph, A.M.; Han, C.; Park, H.J.; Kalavalapalli, S.; Chun, S.K.; Morgan, D.; Kim, J.S.; et al. Mitochondrial ATP transporter depletion protects mice against liver steatosis and insulin resistance. Nat. Commun. 2017, 8, 14477. [Google Scholar] [CrossRef]
- Brand, M.D.; Pakay, J.L.; Ocloo, A.; Kokoszka, J.; Wallace, D.C.; Brookes, P.S.; Cornwall, E.J. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem. J. 2005, 392, 353–362. [Google Scholar] [CrossRef]
- Sparks, L.M.; Gemmink, A.; Phielix, E.; Bosma, M.; Schaart, G.; Moonen-Kornips, E.; Jorgensen, J.A.; Nascimento, E.B.; Hesselink, M.K.; Schrauwen, P.; et al. ANT1-mediated fatty acid-induced uncoupling as a target for improving myocellular insulin sensitivity. Diabetologia 2016, 59, 1030–1039. [Google Scholar] [CrossRef]
- Moon, J.S.; da Cunha, F.F.; Huh, J.Y.; Andreyev, A.Y.; Lee, J.; Mahata, S.K.; Reis, F.C.; Nasamran, C.A.; Lee, Y.S. ANT2 drives proinflammatory macrophage activation in obesity. JCI Insight 2021, 6, e147033. [Google Scholar] [CrossRef]
- Seo, J.B.; Riopel, M.; Cabrales, P.; Huh, J.Y.; Bandyopadhyay, G.K.; Andreyev, A.Y.; Murphy, A.N.; Beeman, S.C.; Smith, G.I.; Klein, S.; et al. Knockdown of Ant2 Reduces Adipocyte Hypoxia And Improves Insulin Resistance in Obesity. Nat. Metab. 2019, 1, 86–97. [Google Scholar] [CrossRef]
- Rial, E.; Zardoya, R. Oxidative stress, thermogenesis and evolution of uncoupling proteins. J. Biol. 2009, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Jia, Y.; Zhang, S.; Liu, N.; Zhang, X.; Wang, T.; Qiao, J.; Yang, G.; Che, X.; Chen, K.; et al. SLC25A3 negatively regulates NLRP3 inflammasome activation by restricting the function of NLRP3. J. Biol. Chem. 2024, 300, 107233. [Google Scholar] [CrossRef] [PubMed]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Baseler, W.A.; Dabkowski, E.R.; Williamson, C.L.; Croston, T.L.; Thapa, D.; Powell, M.J.; Razunguzwa, T.T.; Hollander, J.M. Proteomic alterations of distinct mitochondrial subpopulations in the type 1 diabetic heart: Contribution of protein import dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R186–R200. [Google Scholar] [CrossRef]
- Mayr, J.A.; Zimmermann, F.A.; Horvath, R.; Schneider, H.C.; Schoser, B.; Holinski-Feder, E.; Czermin, B.; Freisinger, P.; Sperl, W. Deficiency of the mitochondrial phosphate carrier presenting as myopathy and cardiomyopathy in a family with three affected children. Neuromuscul. Disord. 2011, 21, 803–808. [Google Scholar] [CrossRef]
- Kampjut, D.; Sazanov, L.A. Structure and mechanism of mitochondrial proton-translocating transhydrogenase. Nature 2019, 573, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Lopert, P.; Patel, M. Nicotinamide nucleotide transhydrogenase (Nnt) links the substrate requirement in brain mitochondria for hydrogen peroxide removal to the thioredoxin/peroxiredoxin (Trx/Prx) system. J. Biol. Chem. 2014, 289, 15611–15620. [Google Scholar] [CrossRef] [PubMed]
- Nickel, A.G.; von Hardenberg, A.; Hohl, M.; Löffler, J.R.; Kohlhaas, M.; Becker, J.; Reil, J.C.; Kazakov, A.; Bonnekoh, J.; Stadelmaier, M.; et al. Reversal of Mitochondrial Transhydrogenase Causes Oxidative Stress in Heart Failure. Cell Metab. 2015, 22, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P. Uncoupling: New approaches to an old problem of bioenergetics. Biochim. Et Biophys. Acta 1998, 1363, 100–124. [Google Scholar] [CrossRef]
- Starkov, A.A. “Mild” uncoupling of mitochondria. Biosci. Rep. 1997, 17, 273–279. [Google Scholar] [CrossRef]
- Cunha, F.M.; Caldeira da Silva, C.C.; Cerqueira, F.M.; Kowaltowski, A.J. Mild mitochondrial uncoupling as a therapeutic strategy. Curr. Drug Targets 2011, 12, 783–789. [Google Scholar] [CrossRef]
- Mookerjee, S.A.; Divakaruni, A.S.; Jastroch, M.; Brand, M.D. Mitochondrial uncoupling and lifespan. Mech. Ageing Dev. 2010, 131, 463–472. [Google Scholar] [CrossRef]
- Caldeira da Silva, C.C.; Cerqueira, F.M.; Barbosa, L.F.; Medeiros, M.H.; Kowaltowski, A.J. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell 2008, 7, 552–560. [Google Scholar] [CrossRef]
- Berry, B.J.; Trewin, A.J.; Amitrano, A.M.; Kim, M.; Wojtovich, A.P. Use the Protonmotive Force: Mitochondrial Uncoupling and Reactive Oxygen Species. J. Mol. Biol. 2018, 430, 3873–3891. [Google Scholar] [CrossRef]
- Amara, C.E.; Shankland, E.G.; Jubrias, S.A.; Marcinek, D.J.; Kushmerick, M.J.; Conley, K.E. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Dikov, D.; Aulbach, A.; Muster, B.; Dröse, S.; Jendrach, M.; Bereiter-Hahn, J. Do UCP2 and mild uncoupling improve longevity? Exp. Gerontol. 2010, 45, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Weisová, P.; Anilkumar, U.; Ryan, C.; Concannon, C.G.; Prehn, J.H.; Ward, M.W. ‘Mild mitochondrial uncoupling’ induced protection against neuronal excitotoxicity requires AMPK activity. Biochim. Biophys. Acta 2012, 1817, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Curtin, N.A.; Clapham, J.C.; Barclay, C.J. Excess recovery heat production by isolated muscles from mice overexpressing uncoupling protein-3. J. Physiol. 2002, 542, 231–235. [Google Scholar] [CrossRef]
- Schlagowski, A.I.; Singh, F.; Charles, A.L.; Gali Ramamoorthy, T.; Favret, F.; Piquard, F.; Geny, B.; Zoll, J. Mitochondrial uncoupling reduces exercise capacity despite several skeletal muscle metabolic adaptations. J. Appl. Physiol. (1985) 2014, 116, 364–375. [Google Scholar] [CrossRef]
- Hausenloy, D.; Wynne, A.; Duchen, M.; Yellon, D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation 2004, 109, 1714–1717. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; Castilho, R.F.; Vercesi, A.E. Opening of the mitochondrial permeability transition pore by uncoupling or inorganic phosphate in the presence of Ca2+ is dependent on mitochondrial-generated reactive oxygen species. FEBS Lett. 1996, 378, 150–152. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-Y. Mild Mitochondrial Uncoupling for True Ectopic Lipid Disposal. Int. J. Mol. Sci. 2025, 26, 7740. https://doi.org/10.3390/ijms26167740
Lee H-Y. Mild Mitochondrial Uncoupling for True Ectopic Lipid Disposal. International Journal of Molecular Sciences. 2025; 26(16):7740. https://doi.org/10.3390/ijms26167740
Chicago/Turabian StyleLee, Hui-Young. 2025. "Mild Mitochondrial Uncoupling for True Ectopic Lipid Disposal" International Journal of Molecular Sciences 26, no. 16: 7740. https://doi.org/10.3390/ijms26167740
APA StyleLee, H.-Y. (2025). Mild Mitochondrial Uncoupling for True Ectopic Lipid Disposal. International Journal of Molecular Sciences, 26(16), 7740. https://doi.org/10.3390/ijms26167740





