Gastric Inflammation Impacts Serotonin Secretion in a Mouse Model of Helicobacter pylori Vaccination †
Abstract
1. Introduction
2. Results
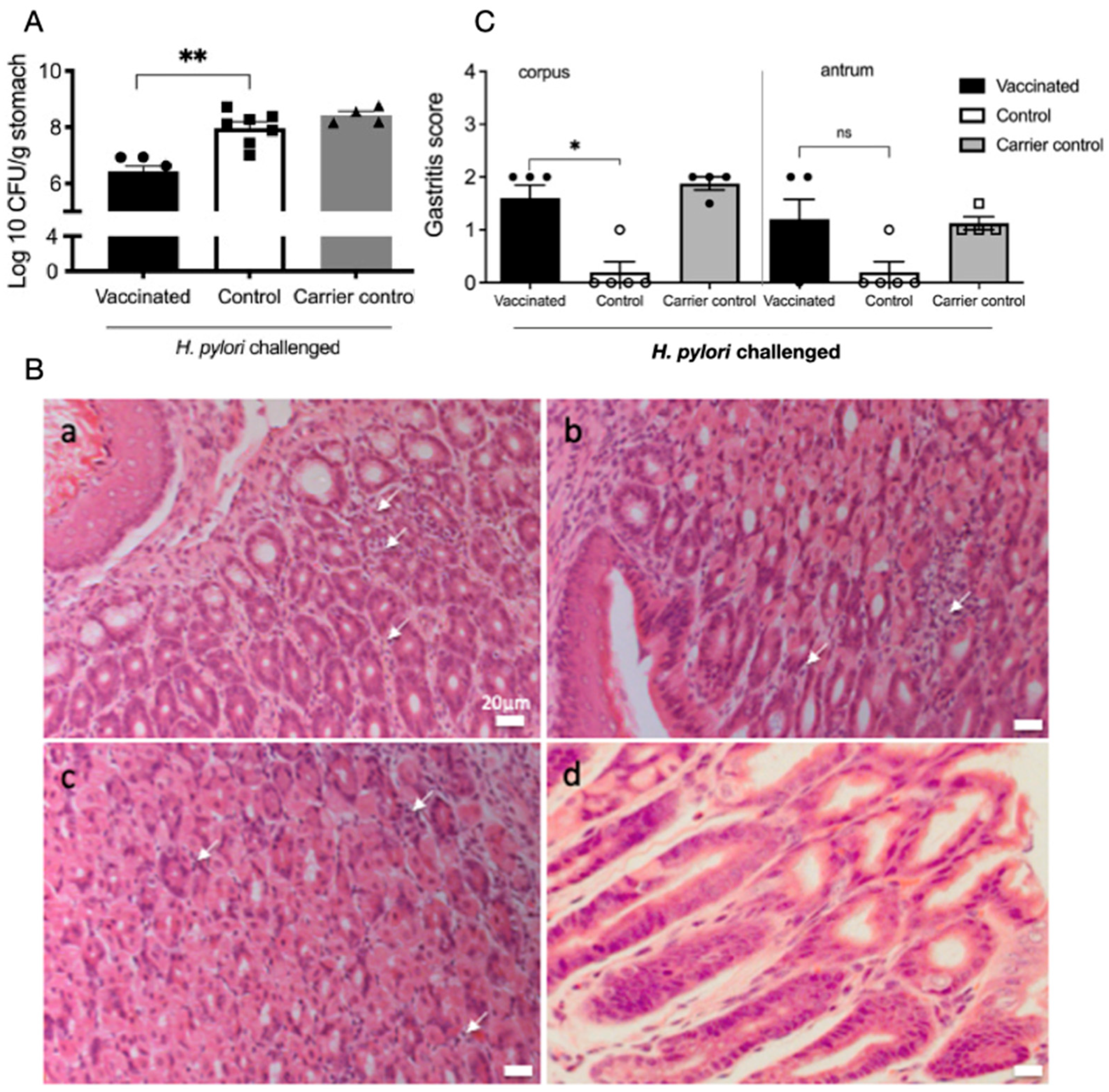
2.1. Reduced H. pylori Colonisation and Increased Inflammatory Infiltrates in Gastric Mucosa of Vaccinated Mice
2.2. Increased 5-HT Release in Corpus Region of Vaccinated Mice
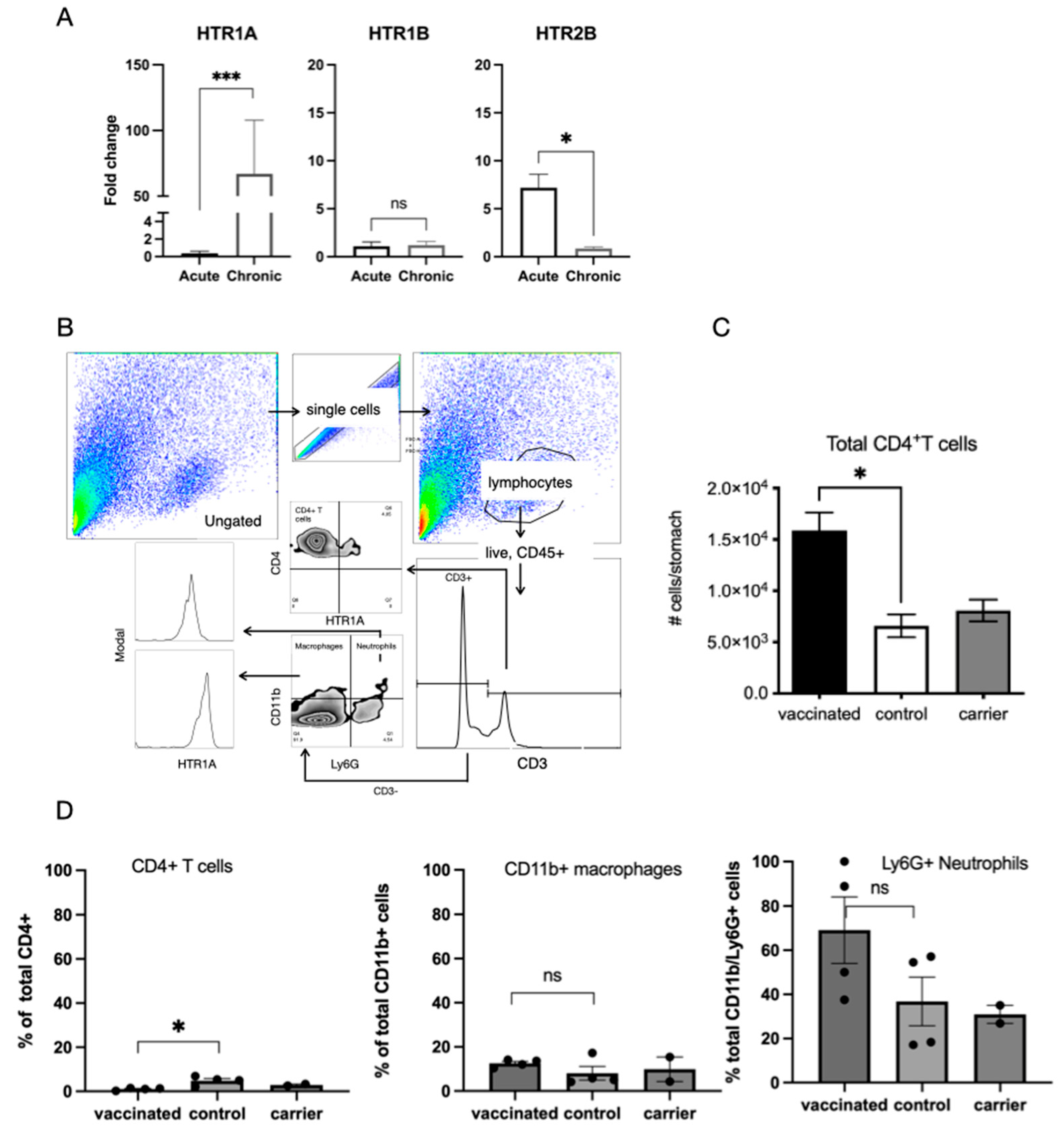
2.3. H. pylori Infection Impacts Expression of 5-HT Receptors on Immune Cell Populations in the Gastric Mucosa
2.4. Effect of H. pylori Infection on Gastric Epithelial Cell Responses to 5-HT
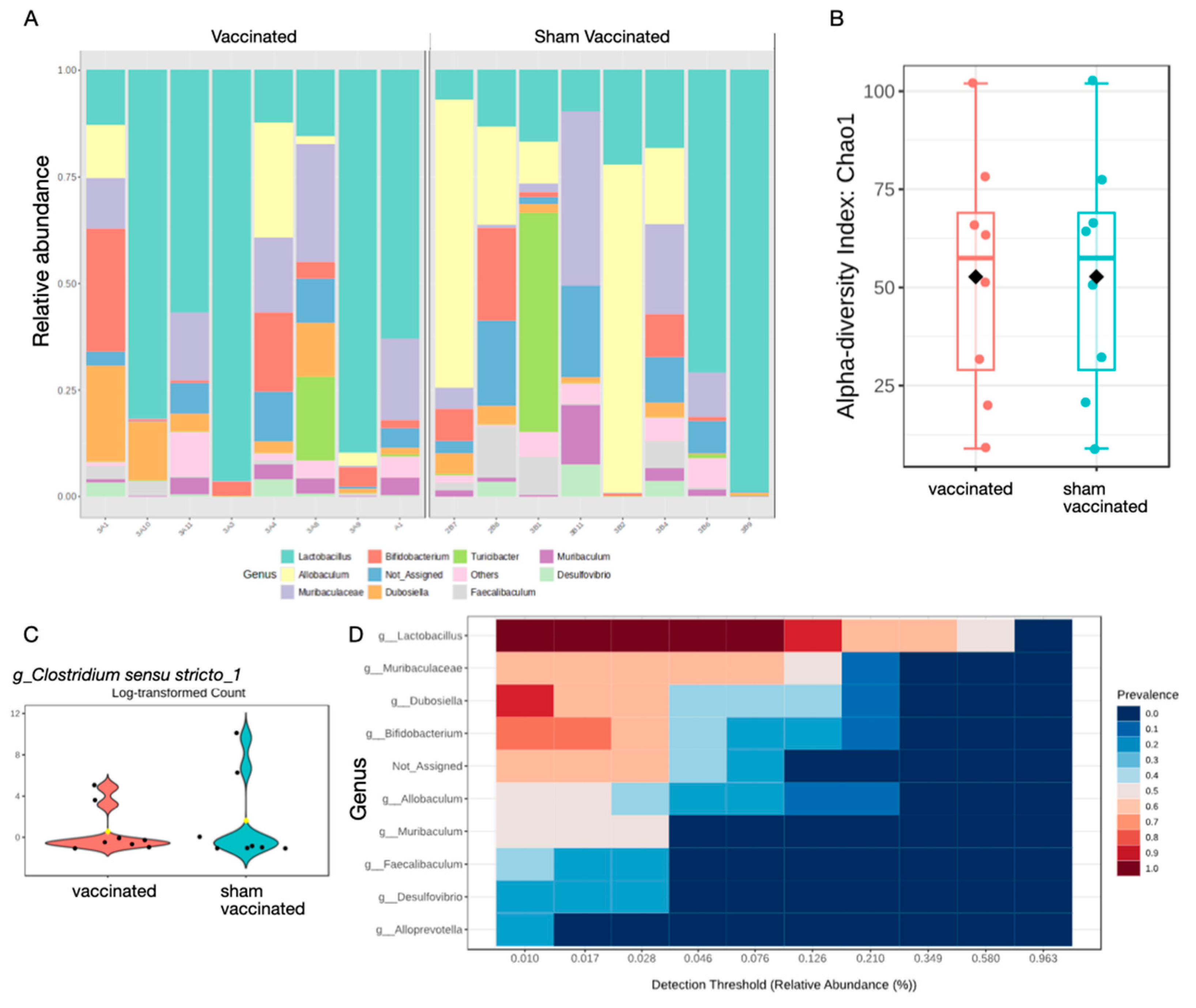
2.5. Vaccination Does Not Significantly Affect Composition of Gastric Microbiota
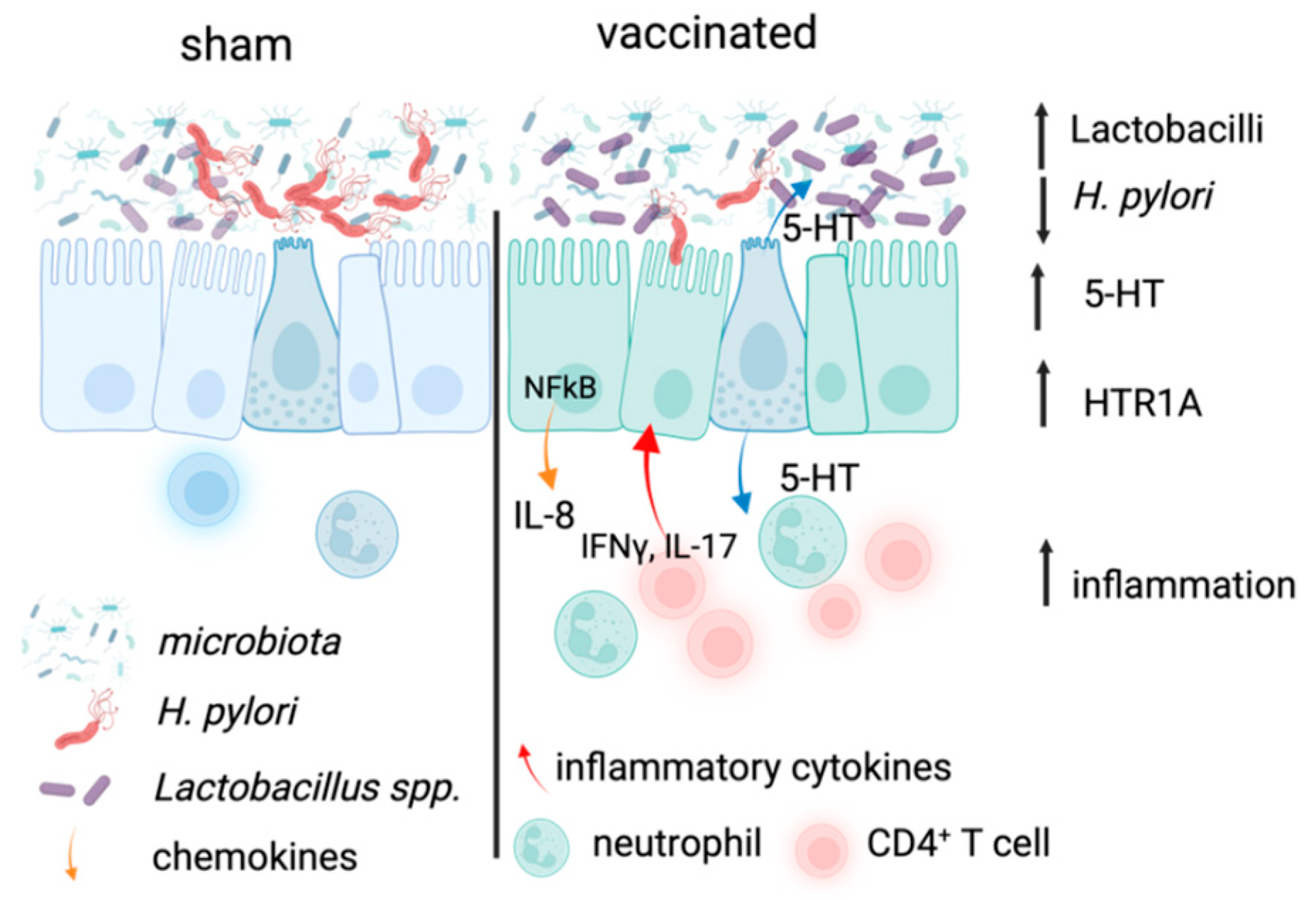
3. Discussion
4. Materials and Methods
4.1. Mouse Model of H. pylori Infection
4.2. AGS Model of H. pylori Infection
4.3. Carbon Fibre Amperometry
4.4. 16S rRNA Sequencing Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine |
| ERK | Extracellular signal-regulated kinase 1 |
| NFκB | Nuclear factor-kappa B |
| IL-8 | Interleukin 8 |
| HTR1A | 5-HT receptor 1A |
| AGS | Human gastric cancer cell line |
| MOI | Multiplicity of infection |
| FCS | Foetal calf serum |
| GC | Gastric cancer |
| PBS | Phosphate buffered saline |
References
- Cadamuro, A.C.T.; Rossi, A.F.T.; Maniezzo, N.M.; Silva, A.E. Helicobacter pylori Infection: Host Immune Response, Implications on Gene Expression and MicroRNAs. World J. Gastroenterol. 2014, 20, 1424. [Google Scholar] [CrossRef]
- Walduck, A.K.; Becher, D. Leptin, CD4+ Treg and the Prospects for Vaccination against H. pylori Infection. Front. Immunol. 2012, 3, 316. [Google Scholar] [CrossRef]
- Sutton, P.; Danon, S.J.; Walker, M.; Thompson, L.J.; Wilson, J.; Kosaka, T.; Lee, A. Post-Immunisation Gastritis and Helicobacter Infection in the Mouse: A Long Term Study. Gut 2001, 49, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Becher, D.; Deutscher, M.E.; Simpfendorfer, K.R.; Wijburg, O.L.; Pederson, J.S.; Lew, A.M.; Strugnell, R.A.; Walduck, A.K. Local Recall Responses in the Stomach Involving Reduced Regulation and Expanded Help Mediate Vaccine-Induced Protection against Helicobacter pylori in Mice. Eur. J. Immunol. 2010, 40, 2778–2790. [Google Scholar] [CrossRef]
- Walduck, A.K.; Raghavan, S. Immunity and Vaccine Development against Helicobacter pylori. In Helicobacter pylori in Human Diseases; Backert, S., Ed.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2019; Volume 11, pp. 257–275. ISBN 9783030219154. [Google Scholar]
- Dixon, B.R.E.A.; Lee, T.J.; Healey, D.C.C.; Li, J.; Goettel, J.A.; Piazuelo, M.B.; Algood, H.M.S. IL-17 Receptor Signaling through IL-17A or IL-17F Is Sufficient to Maintain Innate Response and Control of Helicobacter pylori Immunopathogenesis. ImmunoHorizons 2022, 6, 116–129. [Google Scholar] [CrossRef]
- Khan, W.I.; Ghia, J.E. Gut Hormones: Emerging Role in Immune Activation and Inflammation. Clin. Exp. Immunol. 2010, 161, 19–27. [Google Scholar] [CrossRef]
- Banskota, S.; Ghia, J.-E.; Khan, W.I. Serotonin in the Gut: Blessing or a Curse. Biochimie 2019, 161, 56–64. [Google Scholar] [CrossRef]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A Role for Ghrelin in the Central Regulation of Feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef]
- Mackey-Lawrence, N.M.; Petri, W.A. Leptin and Mucosal Immunity. Mucosal Immunol. 2012, 5, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P.; Canetti, C.; Gottschalk, A.; Tithof, P.K.; Peters-Golden, M. Leptin Augments Alveolar Macrophage Leukotriene Synthesis by Increasing Phospholipase Activity and Enhancing Group IVC IPLA2 (CPLA2gamma) Protein Expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L497–L502. [Google Scholar] [CrossRef]
- Paoluzi, O.A.; Blanco, D.V.G.; Caruso, R.; Monteleone, I.; Monteleone, G.; Pallone, F. Impairment of Ghrelin Synthesis in Helicobacter pylori-Colonized Stomach: New Clues for the Pathogenesis of H. pylori-Related Gastric Inflammation. World J. Gastroenterol. 2014, 20, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Gainsford, T.; Willson, T.A.; Metcalf, D.; Handman, E.; McFarlane, C.; Ng, A.; Nicola, N.A.; Alexander, W.S.; Hilton, D.J. Leptin Can Induce Proliferation, Differentiation, and Functional Activation of Hemopoietic Cells. Proc. Natl. Acad. Sci. USA 1996, 93, 14564–14568. [Google Scholar] [CrossRef]
- Loffreda, S.; Yang, S.Q.; Lin, H.Z.; Karp, C.L.; Brengman, M.L.; Wang, D.J.; Klein, A.S.; Bulkley, G.B.; Bao, C.; Noble, P.W.; et al. Leptin Regulates Proinflammatory Immune Responses. FASEB J. 1998, 12, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R.; Lam, C. An Update on Post-Infectious Irritable Bowel Syndrome: Role of Genetics, Immune Activation, Serotonin and Altered Microbiome. J. Neurogastroenterol. Motil. 2012, 18, 258. [Google Scholar] [CrossRef]
- Dembinski, A.; Warzecha, Z.; Ceranowicz, P.; Tomaszewska, R.; Stachura, J.; Konturek, S.J.; Konturek, P.C. Ghrelin Attenuates the Development of Acute Pancreatitis in Rat. J. Physiol. Pharmacol. 2003, 54, 561–573. [Google Scholar]
- Gonzalez–Rey, E.; Chorny, A.; Delgado, M. Therapeutic Action of Ghrelin in a Mouse Model of Colitis. Gastroenterology 2006, 130, 1707–1720. [Google Scholar] [CrossRef]
- Ghia, J.; Blennerhassett, P.; Deng, Y.; Verdu, E.F.; Khan, W.I.; Collins, S.M. Reactivation of Inflammatory Bowel Disease in a Mouse Model of Depression. Gastroenterology 2009, 136, 2280–2288.e4. [Google Scholar] [CrossRef]
- Warzecha, Z.; Ceranowicz, P.; Dembinski, A.; Cieszkowski, J.; Kusnierz-Cabala, B.; Tomaszewska, R.; Kuwahara, A.; Kato, I. Therapeutic Effect of Ghrelin in the Course of Cerulein-Induced Acute Pancreatitis in Rats. J. Physiol. Pharmacol. 2009, 61, 419–427. [Google Scholar]
- Keates, S.; Hitti, Y.; Upton, M.; Kelly, C. Helicobacter pylori Infection Activates NF-Kappa B in Gastric Epithelial Cells. Gastroenterology 1997, 113, 1099–1109. [Google Scholar] [CrossRef]
- Isomoto, H.; Miyazaki, M.; Mizuta, Y.; Takeshima, F.; Murase, K.; Inoue, K.; Yamasaki, K.; Murata, I.; Koji, T.; Kohno, S. Expression of Nuclear Factor-? B in Helicobacter pylori-Infected Gastric Mucosa Detected with Southwestern Histochemistry. Scand. J. Gastroenterol. 2000, 35, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Brink, G.R.v.D.; Kate, F.J.T.; Ponsioen, C.Y.; Rive, M.M.; Tytgat, G.N.; van Deventer, S.J.H.; Peppelenbosch, M.P. Expression and Activation of NF-ΚB in the Antrum of the Human Stomach. J. Immunol. 2000, 164, 3353–3359. [Google Scholar] [CrossRef]
- Ferrero, R.L.; Avé, P.; Ndiaye, D.; Bambou, J.-C.; Huerre, M.R.; Philpott, D.J.; Mémet, S. NF-ΚB Activation during Acute Helicobacter pylori Infection in Mice. Infect. Immun. 2008, 76, 551–561. [Google Scholar] [CrossRef][Green Version]
- Sharma, S.A.; Tummuru, M.K.; Miller, G.G.; Blaser, M.J. Interleukin-8 Response of Gastric Epithelial Cell Lines to Helicobacter pylori Stimulation In Vitro. Infect. Immun. 1995, 63, 1681–1687. [Google Scholar] [CrossRef]
- Meng, W.-P.; Wang, Z.-Q.; Deng, J.-Q.; Liu, Y.; Deng, M.-M.; Lü, M.-H. The Role of H. pylori CagA in Regulating Hormones of Functional Dyspepsia Patients. Gastroenterol. Res. Pract. 2016, 2016, 7150959. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kwon, Y.H.; Dewan, V.; Vahedi, F.; Syed, S.; Fontes, M.E.; Ashkar, A.A.; Surette, M.G.; Khan, W.I. TLR2 Plays a Pivotal Role in Mediating Mucosal Serotonin Production in the Gut. J. Immunol. 2019, 202, 3041–3052. [Google Scholar] [CrossRef] [PubMed]
- Hannon, J.; Hoyer, D. Molecular Biology of 5-HT Receptors. Behav. Brain Res. 2008, 195, 198–213. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin Signalling in the Gut—Functions, Dysfunctions and Therapeutic Targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef]
- Koopman, N.; Katsavelis, D.; Hove, A.S.t.; Brul, S.; de Jonge, W.J.; Seppen, J. The Multifaceted Role of Serotonin in Intestinal Homeostasis. Int. J. Mol. Sci. 2021, 22, 9487. [Google Scholar] [CrossRef] [PubMed]
- Freire-Garabal, M.; Núñez, M.J.; Balboa, J.; López-Delgado, P.; Gallego, R.; García-Caballero, T.; Fernández-Roel, M.D.; Brenlla, J.; Rey-Méndez, M. Serotonin Upregulates the Activity of Phagocytosis through 5-HT1A Receptors. Br. J. Pharmacol. 2003, 139, 457–463. [Google Scholar] [CrossRef]
- León-Ponte, M.; Ahern, G.P.; O’COnnell, P.J. Serotonin Provides an Accessory Signal to Enhance T-Cell Activation by Signaling through the 5-HT7 Receptor. Blood 2007, 109, 3139–3146. [Google Scholar] [CrossRef]
- Chen, Y.; Leon-Ponte, M.; Pingle, S.C.; O’Connell, P.J.; Ahern, G.P. T Lymphocytes Possess the Machinery for 5-HT Synthesis, Storage, Degradation and Release. Acta Physiol. 2015, 213, 860–867. [Google Scholar] [CrossRef]
- Slomiany, B.L.; Slomiany, A. Interference by Leptin with Helicobacter pylori Lipopolysaccharide-Induced Cytosolic Phospholipase A2 Activation in Gastric Mucosal Cells. J. Physiol. Pharmacol. 2006, 58, 117–130. [Google Scholar]
- Waseem, T.; Duxbury, M.; Ito, H.; Ashley, S.W.; Robinson, M.K. Exogenous Ghrelin Modulates Release of Pro-Inflammatory and Anti-Inflammatory Cytokines in LPS-Stimulated Macrophages through Distinct Signaling Pathways. Surgery 2008, 143, 334–342. [Google Scholar] [CrossRef]
- Wehrens, A.; Aebischer, T.; Meyer, T.F.; Walduck, A.K. Leptin Receptor Signaling Is Required for Vaccine-Induced Protection against Helicobacter pylori. Helicobacter 2008, 13, 94–102. [Google Scholar] [CrossRef]
- Bik, E.M.; Eckburg, P.B.; Gill, S.R.; Nelson, K.E.; Purdom, E.A.; Francois, F.; Perez-Perez, G.; Blaser, M.J.; Relman, D.A. Molecular Analysis of the Bacterial Microbiota in the Human Stomach. Proc. Natl. Acad. Sci. USA 2006, 103, 732–737. [Google Scholar] [CrossRef]
- Tan, M.P.; Kaparakis, M.; Galic, M.; Pedersen, J.; Pearse, M.; Wijburg, O.L.C.; Janssen, P.H.; Strugnell, R.A. Chronic Helicobacter pylori Infection Does Not Significantly Alter the Microbiota of the Murine Stomach. Appl. Environ. Microbiol. 2007, 73, 1010–1013. [Google Scholar] [CrossRef][Green Version]
- Aebischer, T.; Fischer, A.; Walduck, A.; Schlötelburg, C.; Lindig, M.; Schreiber, S.; Meyer, T.F.; Bereswill, S.; Göbel, U.B. Vaccination Prevents Helicobacter pylori-Induced Alterations of the Gastric Flora in Mice. FEMS Immunol. Med. Microbiol. 2006, 46, 221–229. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 163, 258. [Google Scholar] [CrossRef]
- Yunle, K.; Tong, W.; Jiyang, L.; Guojun, W. Advances in Helicobacter pylori Vaccine Research: From Candidate Antigens to Adjuvants—A Review. Helicobacter 2024, 29, e13034. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Mao, X.-H.; Li, J.-X.; Tong, W.-D.; Wang, B.; Zhang, Y.-J.; Guo, G.; Zhao, Z.-J.; Li, L.; Wu, D.-L.; et al. Efficacy, Safety, and Immunogenicity of an Oral Recombinant Helicobacter pylori Vaccine in Children in China: A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2015, 386, 1457–1464. [Google Scholar] [CrossRef]
- Koesling, J.; Lucas, B.; Develioglou, L.; Aebischer, T.; Meyer, T.F. Vaccination of Mice with Live Recombinant Salmonella Typhimurium AroA against H. pylori: Parameters Associated with Prophylactic and Therapeutic Vaccine Efficacy. Vaccine 2001, 20, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Bumann, D.; Metzger, W.G.; Mansouri, E.; Palme, O.; Wendland, M.; Hurwitz, R.; Haas, G.; Aebischer, T.; Specht, B.-U.V.; Meyer, T.F. Safety and Immunogenicity of Live Recombinant Salmonella Enterica Serovar Typhi Ty21a Expressing Urease A and B from Helicobacter pylori in Human Volunteers. Vaccine 2001, 20, 845–852. [Google Scholar] [CrossRef]
- Aebischer, T.; Bumann, D.; Epple, H.J.; Metzger, W.; Schneider, T.; Cherepnev, G.; Walduck, A.K.; Kunkel, D.; Moos, V.; Loddenkemper, C.; et al. Correlation of T Cell Response and Bacterial Clearance in Human Volunteers Challenged with Helicobacter pylori Revealed by Randomised Controlled Vaccination with Ty21a-Based Salmonella Vaccines. Gut 2008, 57, 1065. [Google Scholar] [CrossRef]
- Kaparakis, M.; Walduck, A.K.; Price, J.D.; Pedersen, J.S.; van Rooijen, N.; Pearse, M.J.; Wijburg, O.L.C.; Strugnell, R.A. Macrophages Are Mediators of Gastritis in Acute Helicobacter pylori Infection in C57BL/6 Mice. Infect. Immun. 2008, 76, 2235–2239. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Saruuljavkhlan, B.; Alfaray, R.I.; Linz, B. Helicobacter pylori and Gastric Cancer. Curr. Top. Microbiol. Immunol. 2024, 444, 117–155. [Google Scholar] [CrossRef]
- Spiller, R. Serotonin and GI Clinical Disorders. Neuropharmacology 2008, 55, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Russell, R.M.; Pifer, R.; Menezes-Garcia, Z.; Cuesta, S.; Narayanan, S.; MacMillan, J.B.; Sperandio, V. The Serotonin Neurotransmitter Modulates Virulence of Enteric Pathogens. Cell Host Microbe 2020, 28, 41–53.e8. [Google Scholar] [CrossRef] [PubMed]
- Lucas, B.; Bumann, D.; Walduck, A.; Koesling, J.; Develioglu, L.; Meyer, T.F.; Aebischer, T. Adoptive Transfer of CD4+ T Cells Specific for Subunit A of Helicobacter pylori Urease Reduces H. pylori Stomach Colonization in Mice in the Absence of Interleukin-4 (IL-4)/IL-13 Receptor Signaling. Infect. Immun. 2001, 69, 1714–1721. [Google Scholar] [CrossRef]
- Mohammadi, M.; Nedrud, J.; Redline, R.; Lycke, N.; Czinn, S.J. Murine CD4 T-Cell Response to Helicobacter Infection: TH1 Cells Enhance Gastritis and TH2 Cells Reduce Bacterial Load. Gastroenterology 1997, 113, 1848–1857. [Google Scholar] [CrossRef]
- Wu, H.; Herr, D.; MacIver, N.J.; Rathmell, J.C.; Gerriets, V.A. CD4 T Cells Differentially Express Cellular Machinery for Serotonin Signaling, Synthesis, and Metabolism. Int. Immunopharmacol. 2020, 88, 106922. [Google Scholar] [CrossRef] [PubMed]
- Higashi, H.; Nakaya, A.; Tsutsumi, R.; Yokoyama, K.; Fujii, Y.; Ishikawa, S.; Higuchi, M.; Takahashi, A.; Kurashima, Y.; Teishikata, Y.; et al. Helicobacter pylori CagA Induces Ras-Independent Morphogenetic Response through SHP-2 Recruitment and Activation. J. Biol. Chem. 2004, 279, 17205–17216. [Google Scholar] [CrossRef]
- Jin, L.-X.; Fang, Y.-P.; Xia, C.-M.; Cai, T.-W.; Li, Q.-Q.; Wang, Y.-Y.; Yan, H.-F.; Chen, X. Helicobacter pylori Infection Alters Gastric Microbiota Structure and Biological Functions in Patients with Gastric Ulcer or Duodenal Ulcer. World J. Gastroenterol. 2024, 30, 3076–3085. [Google Scholar] [CrossRef]
- Miao, R.; Wan, C.; Wang, Z. The Relationship of Gastric Microbiota and Helicobacter pylori Infection in Pediatrics Population. Helicobacter 2020, 25, e12676. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Peng, C.; Wang, H.; Ouyang, Y.; Zhu, Z.; Shu, X.; Zhu, Y.; Lu, N. The Eradication of Helicobacter pylori Restores Rather than Disturbs the Gastrointestinal Microbiota in Asymptomatic Young Adults. Helicobacter 2019, 24, e12590. [Google Scholar] [CrossRef] [PubMed]
- Canducci, F.; Armuzzi, A.; Cremonini, F.; Cammarota, G.; Bartolozzi, F.; Pola, P.; Gasbarrini, G.; Gasbarrini, A. A Lyophilized and Inactivated Culture of Lactobacillus Acidophilus Increases Helicobacter pylori Eradication Rates. Aliment. Pharmacol. Ther. 2000, 14, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Sýkora, J.; Valeková, K.; Amlerová, J.; Siala, K.; Ddek, P.; Watkins, S.; Varvaovská, J.; Stoický, F.; Pazdiora, P.; Schwarz, J. Effects of a Specially Designed Fermented Milk Product Containing Probiotic Lactobacillus Casei DN-114 001 and the Eradication of H. pylori in Children. J. Clin. Gastroenterol. 2005, 39, 692–698. [Google Scholar] [CrossRef]
- Martin, A.M.; Sun, E.W.; Rogers, G.B.; Keating, D.J. The Influence of the Gut Microbiome on Host Metabolism Through the Regulation of Gut Hormone Release. Front. Physiol. 2019, 10, 428. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., III; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut Microbes Promote Colonic Serotonin Production through an Effect of Short-chain Fatty Acids on Enterochromaffin Cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Lertpiriyapong, K.; Whary, M.T.; Muthupalani, S.; Lofgren, J.L.; Gamazon, E.R.; Feng, Y.; Ge, Z.; Wang, T.C.; Fox, J.G. Gastric Colonisation with a Restricted Commensal Microbiota Replicates the Promotion of Neoplastic Lesions by Diverse Intestinal Microbiota in the Helicobacter pylori INS-GAS Mouse Model of Gastric Carcinogenesis. Gut 2014, 63, 54. [Google Scholar] [CrossRef]
- Everett, B.A.; Tran, P.; Prindle, A. Toward Manipulating Serotonin Signaling via the Microbiota–Gut–Brain Axis. Curr. Opin. Biotechnol. 2022, 78, 102826. [Google Scholar] [CrossRef]
- Lyte, J.M.; Shrestha, S.; Wagle, B.R.; Liyanage, R.; Martinez, D.A.; Donoghue, A.M.; Daniels, K.M.; Lyte, M. Serotonin Modulates Campylobacter jejuni Physiology and In Vitro Interaction with the Gut Epithelium. Poult. Sci. 2021, 100, 100944. [Google Scholar] [CrossRef] [PubMed]
- Walduck, A.; Schmitt, A.; Lucas, B.; Aebischer, T.; Meyer, T.F. Transcription Profiling Analysis of the Mechanisms of Vaccine-Induced Protection against H. pylori. FASEB J. 2004, 18, 1955–1957. [Google Scholar] [CrossRef][Green Version]
- Metzger, W.G.; Mansouri, E.; Kronawitter, M.; Diescher, S.; Soerensen, M.; Hurwitz, R.; Bumann, D.; Aebischer, T.; Specht, B.-U.V.; Meyer, T.F. Impact of Vector-Priming on the Immunogenicity of a Live Recombinant Salmonella Enterica Serovar Typhi Ty21a Vaccine Expressing Urease A and B from Helicobacter pylori in Human Volunteers. Vaccine 2004, 22, 2273–2277. [Google Scholar] [CrossRef]
- Skakic, I.; Francis, J.E.; Dekiwadia, C.; Aibinu, I.; Huq, M.; Taki, A.C.; Walduck, A.; Smooker, P.M. An Evaluation of Urease A Subunit Nanocapsules as a Vaccine in a Mouse Model of Helicobacter pylori Infection. Vaccines 2023, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Ruijter, J.M.; Pfaffl, M.W.; Zhao, S.; Spiess, A.N.; Boggy, G.; Blom, J.; Rutledge, R.G.; Sisti, D.; Lievens, A.; Preter, K.D.; et al. Evaluation of QPCR Curve Analysis Methods for Reliable Biomarker Discovery: Bias, Resolution, Precision, and Implications. Methods 2013, 59, 32–46. [Google Scholar] [CrossRef]
- Stavely, R.; Fraser, S.; Sharma, S.; Rahman, A.A.; Stojanovska, V.; Sakkal, S.; Apostolopoulos, V.; Bertrand, P.; Nurgali, K. The Onset and Progression of Chronic Colitis Parallels Increased Mucosal Serotonin Release via Enterochromaffin Cell Hyperplasia and Downregulation of the Serotonin Reuptake Transporter. Inflamm. Bowel Dis. 2018, 24, 1021–1034. [Google Scholar] [CrossRef]
- Bertrand, P.P.; Bertrand, R.L.; Camello, P.J.; Pozo, M.J. Simultaneous Measurement of Serotonin and Melatonin from the Intestine of Old Mice: The Effects of Daily Melatonin Supplementation. J. Pineal Res. 2010, 49, 23–34. [Google Scholar] [CrossRef]
- Joat, N.; Van, T.T.H.; Stanley, D.; Moore, R.J.; Chousalkar, K. Temporal Dynamics of Gut Microbiota in Caged Laying Hens: A Field Observation from Hatching to End of Lay. Appl. Microbiol. Biotechnol. 2021, 105, 4719–4730. [Google Scholar] [CrossRef]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An Improved Dual-Indexing Approach for Multiplexed 16S RRNA Gene Sequencing on the Illumina MiSeq Platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for Comprehensive Statistical, Functional, and Meta-Analysis of Microbiome Data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idowu, S.; Polglaze, K.; Van, T.T.H.; Moore, R.J.; Ramsland, P.A.; Bertrand, P.P.; Walduck, A.K. Gastric Inflammation Impacts Serotonin Secretion in a Mouse Model of Helicobacter pylori Vaccination. Int. J. Mol. Sci. 2025, 26, 7735. https://doi.org/10.3390/ijms26167735
Idowu S, Polglaze K, Van TTH, Moore RJ, Ramsland PA, Bertrand PP, Walduck AK. Gastric Inflammation Impacts Serotonin Secretion in a Mouse Model of Helicobacter pylori Vaccination. International Journal of Molecular Sciences. 2025; 26(16):7735. https://doi.org/10.3390/ijms26167735
Chicago/Turabian StyleIdowu, Sulaimon, Kate Polglaze, Thi Thu Hao Van, Robert J. Moore, Paul A. Ramsland, Paul P. Bertrand, and Anna K. Walduck. 2025. "Gastric Inflammation Impacts Serotonin Secretion in a Mouse Model of Helicobacter pylori Vaccination" International Journal of Molecular Sciences 26, no. 16: 7735. https://doi.org/10.3390/ijms26167735
APA StyleIdowu, S., Polglaze, K., Van, T. T. H., Moore, R. J., Ramsland, P. A., Bertrand, P. P., & Walduck, A. K. (2025). Gastric Inflammation Impacts Serotonin Secretion in a Mouse Model of Helicobacter pylori Vaccination. International Journal of Molecular Sciences, 26(16), 7735. https://doi.org/10.3390/ijms26167735






