Pawpaw (Asimina triloba) Seed Extract Suppresses High-Fat Diet-Induced Obesity in Mice
Abstract
1. Introduction
2. Results
2.1. Body Weight and Food Intake
2.2. Weight of Organs and Visceral Fat Tissues
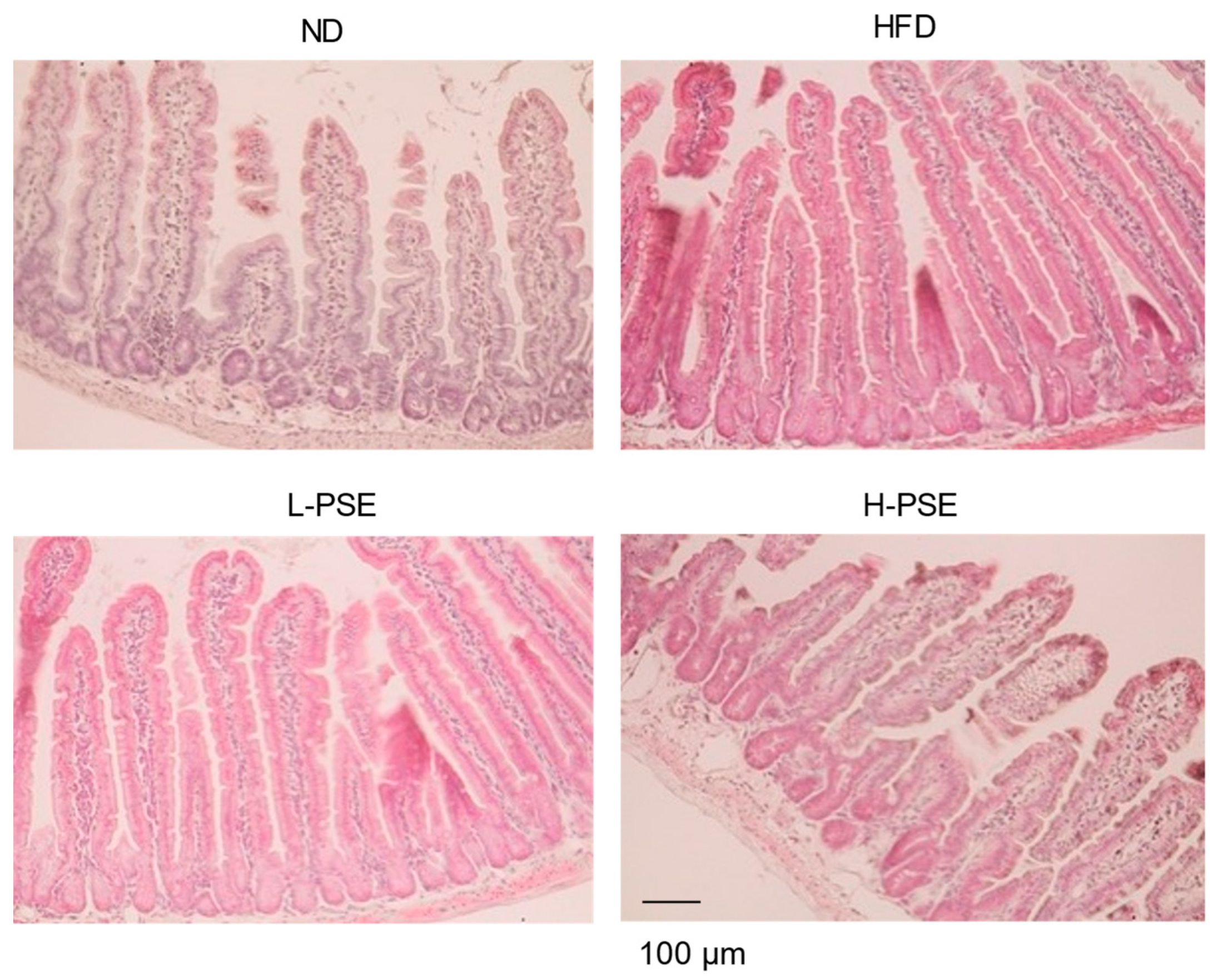
2.3. Histological Analysis of Small Intestine Tissue
2.4. Blood Glucose, Plasma Lipids, and Total Liver Lipid Concentrations
2.5. Expression of Genes Involved in Triglyceride Metabolism in Adipose Tissue
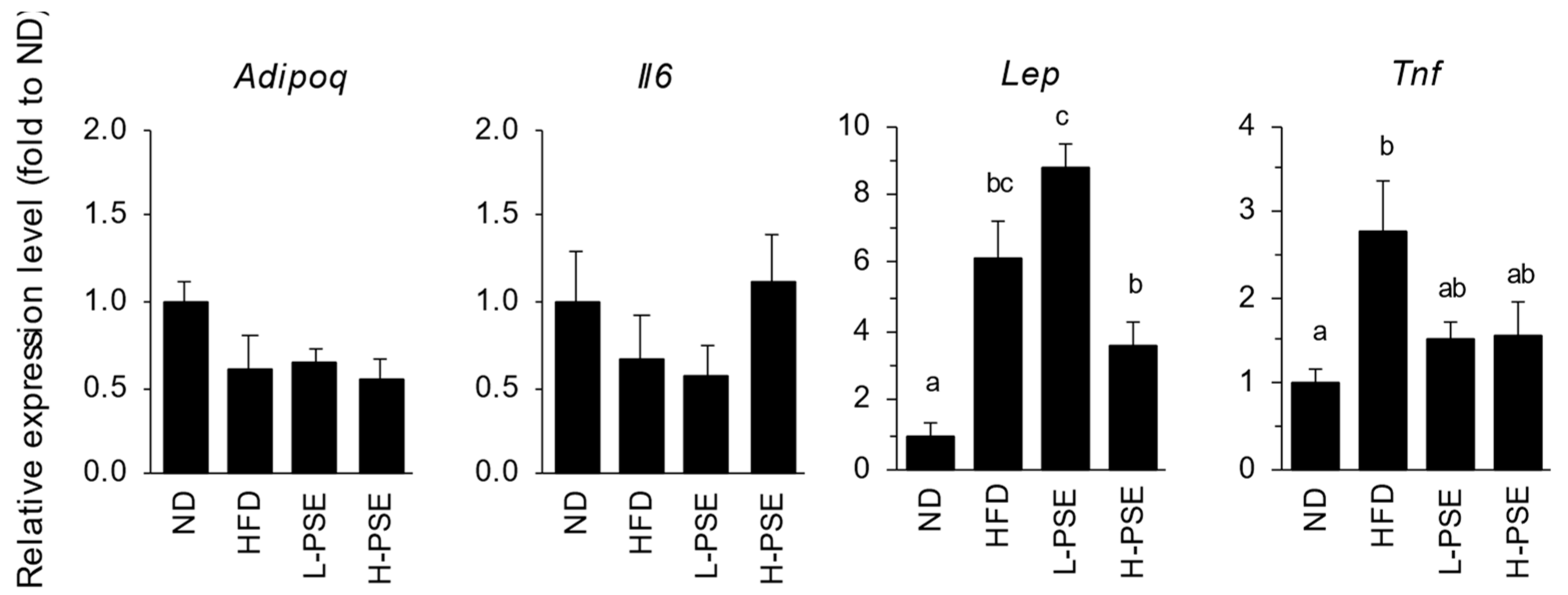
2.6. Expression of Adipocytokine Genes in Adipose Tissue
3. Discussion
4. Materials and Methods
4.1. Preparation of PSE
4.2. Animals and Experimental Diet
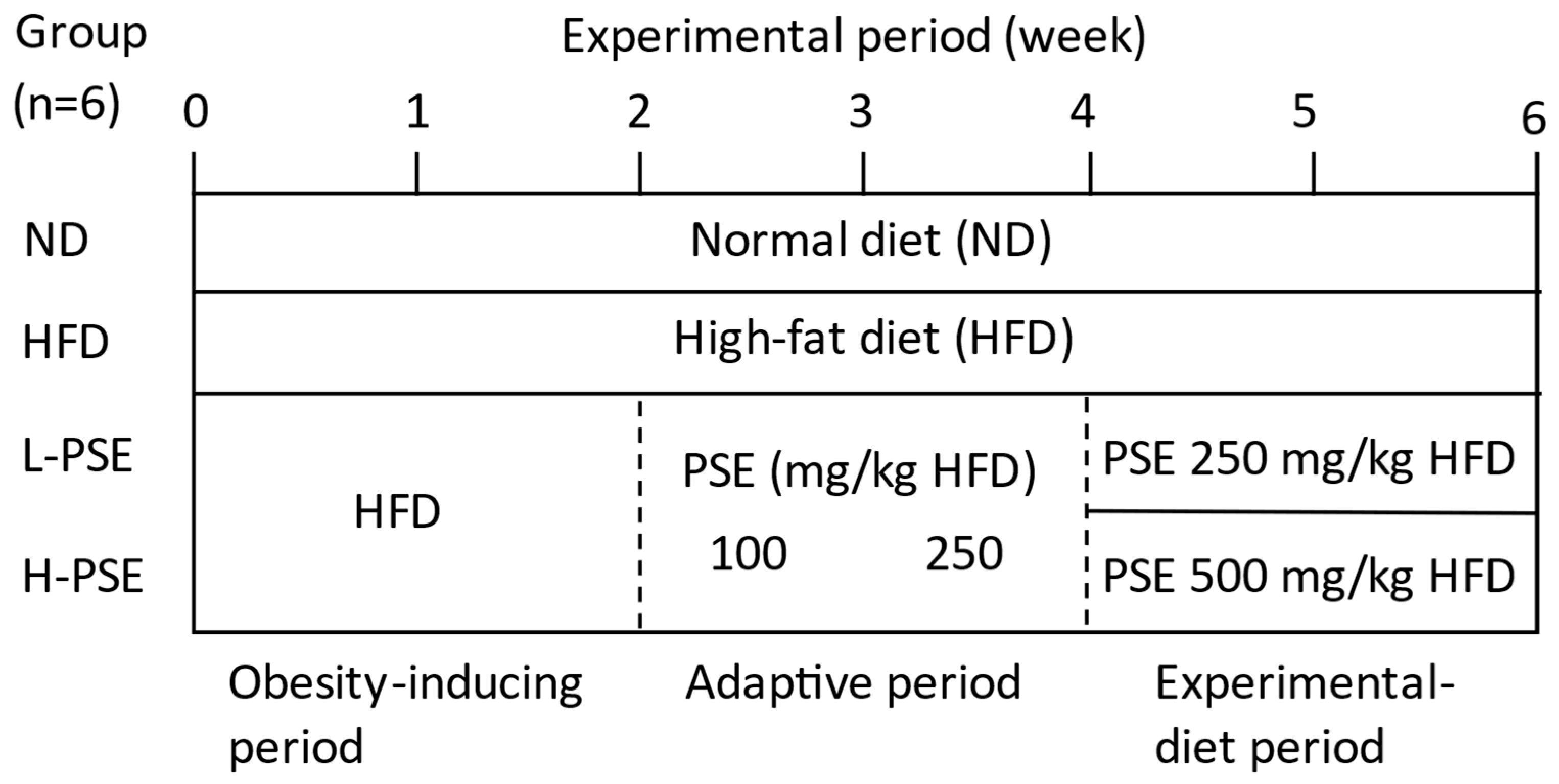
4.3. Experimental Design
4.4. Determination of Blood Glucose, Plasma Lipids, and Total Liver Lipid Concentrations
4.5. Preparation of cDNA Samples
4.6. Quantitative Analysis of Gene Expression Using Real-Time PCR
4.7. Histological Analysis of Small Intestinal Tissue
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| C/EBP | CCTTA/enhancer-binding protein |
| DGAT2 | diglyceride acyltransferase 2 |
| FAS | fatty acid synthase |
| H&E | hematoxylin and eosin |
| HFD | high-fat diet |
| HSL | hormone-sensitive lipase |
| NEFA | non-esterified fatty acids |
| ND | normal diet |
| PSE | pawpaw seed extract |
| PPAR | proliferator-activated receptor |
| SREBP | sterol regulatory element-binding protein |
| TNF | tumor necrosis factor |
References
- Zhao, G.X.; Rieser, M.J.; Hui, Y.H.; Miesbauer, L.R.; Smith, D.L.; McLaughlin, J.L. Biologically active acetogenins from stem bark of Asimina triloba. Phytochemistry 1993, 33, 1065–1073. [Google Scholar] [CrossRef]
- Nam, J.S.; Jang, H.L.; Rhee, Y.H. Antioxidant Activities and Phenolic Compounds of Several Tissues of Pawpaw (Asimina triloba [L.] Dunal) Grown in Korea. J. Food Sci. 2017, 82, 1827–1833. [Google Scholar] [CrossRef]
- Kobayashi, H.; Changzheng Wang, C.; Pomper, K.W. Phenolic content and antioxidant capacity of pawpaw fruit (Asimina triloba L.) at different ripening stages. HortScience 2008, 43, 268–270. [Google Scholar] [CrossRef]
- Nam, J.S.; Park, S.Y.; Oh, H.J.; Jang, H.L.; Rhee, Y.H. Phenolic Profiles, Antioxidant and Antimicrobial Activities of Pawpaw Pulp (Asimina triloba [L.] Dunal) at Different Ripening Stages. J. Food Sci. 2019, 84, 174–182. [Google Scholar] [CrossRef]
- Nam, J.S.; Park, S.Y.; Lee, S.O.; Lee, H.J.; Jang, H.L.; Rhee, Y.H. The growth-inhibitory effects of pawpaw (Asimina triloba [L.] Dunal) roots, twigs, leaves, and fruit against human gastric (AGS) and cervical (HeLa) cancer cells and their anti-inflammatory activities. Mol. Biol. Rep. 2021, 48, 2173–2181. [Google Scholar] [CrossRef]
- Ratnayake, S.; Rupprecht, J.K.; Potter, W.M.; McLaughlin, J.L. Evaluation of various parts of the paw paw tree, Asimina triloba (Annonaceae), as commercial sources of the pesticidal annonaceous acetogenins. J. Econ. Entomol. 1992, 85, 2353–2356. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.L. Paw paw and cancer: Annonaceous acetogenins from discovery to commercial products. J. Nat. Prod. 2008, 71, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Pomper, K.W.; Lowe, J.D.; Crabtree, S.B.; Keller, W. Identification of annonaceous acetogenins in the ripe fruit of the North American pawpaw (Asimina triloba). J. Agric. Food Chem. 2009, 57, 8339–8343. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, J.K.; Hui, Y.H.; McLaughlin, J.L. Annonaceous acetogenins: A review. J. Nat. Prod. 1990, 53, 237–278. [Google Scholar] [CrossRef]
- Oberlies, N.H.; Jones, J.L.; Corbett, T.H.; Fotopoulos, S.S.; McLaughlin, J.L. Tumor cell growth inhibition by several Annonaceous acetogenins in an in vitro disk diffusion assay. Cancer Lett. 1995, 96, 55–62. [Google Scholar] [CrossRef]
- Morré, D.J.; de Cabo, R.; Farley, C.; Oberlies, N.H.; McLaughlin, J.L. Mode of action of bullatacin, a potent antitumor acetogenin: Inhibition of NADH oxidase activity of HeLa and HL-60, but not liver, plasma membranes. Life Sci. 1995, 56, 343–348. [Google Scholar] [CrossRef]
- Guadaño, A.; Gutiérrez, C.; de La Peña, E.; Cortes, D.; González-Coloma, A. Insecticidal and mutagenic evaluation of two annonaceous acetogenins. J. Nat. Prod. 2000, 63, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.C.; Wu, T.Y.; Chang, F.R.; Wu, Y.C. Historic perspectives on Annonaceous acetogenins from the chemical bench to preclinical trials. Planta. Med. 2010, 76, 1390–1404. [Google Scholar] [CrossRef] [PubMed]
- Ahammadsahib, K.I.; Hollingworth, R.M.; McGovren, J.P.; Hui, Y.H.; McLaughlin, J.L. Mode of action of bullatacin: A potent antitumor and pesticidal annonaceous acetogenin. Life Sci. 1993, 53, 1113–1120. [Google Scholar] [CrossRef]
- Gavamukulya, Y.; Abou-Elella, F.; Wamunyokoli, F.; AEl-Shemy, H. Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac. J. Trop. Med. 2014, 7S1, S355–S363. [Google Scholar] [CrossRef]
- Gavamukulya, Y.; Wamunyokoli, F.; El-Shemy, H.A. Annona muricata: Is the natural therapy to most disease conditions including cancer growing in our backyard? A systematic review of its research history and future prospects. Asian Pac. J. Trop. Med. 2017, 10, 835–848. [Google Scholar] [CrossRef]
- Quílez, A.M.; Fernández-Arche, M.A.; García-Giménez, M.D.; De la Puerta, R. Potential therapeutic applications of the genus Annona: Local and traditional uses and pharmacology. J. Ethnopharmacol. 2018, 225, 244–270. [Google Scholar] [CrossRef] [PubMed]
- Justino, A.B.; Miranda, N.C.; Franco, R.R.; Martins, M.M.; Silva, N.M.D.; Espindola, F.S. Annona muricata Linn. leaf as a source of antioxidant compounds with in vitro antidiabetic and inhibitory potential against α-amylase, α-glucosidase, lipase, non-enzymatic glycation and lipid peroxidation. Biomed. Pharmacother. 2018, 100, 83–92. [Google Scholar] [CrossRef]
- Cercato, L.M.; White, P.A.S.; Nampo, F.K.; Santos, M.R.V.; Camargo, E.A. A systematic review of medicinal plants used for weight loss in Brazil: Is there potential for obesity treatment? J. Ethnopharmacol. 2015, 176, 286–296. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.M.; Kadir, H.A. Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int. J. Mol. Sci. 2015, 16, 15625–15658. [Google Scholar] [CrossRef]
- Leite, D.O.D.; de F A Nonato, C.; Camilo, C.J.; de Carvalho, N.K.G.; da Nobrega, M.G.L.A.; Pereira, R.C.; da Costa, J.G.M. Annona Genus: Traditional Uses, Phytochemistry and Biological Activities. Curr. Pharm. Des. 2020, 26, 4056–4091. [Google Scholar] [CrossRef]
- Florence, N.T.; Benoit, M.Z.; Jonas, K.; Alexandra, T.; Désiré, D.D.P.; Pierre, K.; Théophile, D. Antidiabetic and antioxidant effects of Annona muricata (Annonaceae), aqueous extract on streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2014, 151, 784–790. [Google Scholar] [CrossRef]
- Saraiva, A.L.; Justino, A.B.; Franco, R.R.; Silva, H.C.G.; Arruda, F.D.S.; Klein, S.G.; Celes, M.R.N.; Goulart, L.R.; Espindola, F.S. Polyphenols-Rich Fraction from Annona muricata Linn. Leaves Attenuates Oxidative and Inflammatory Responses in Neutrophils, Macrophages, and Experimental Lung Injury. Pharmaceutics 2022, 14, 1182. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Fuentes, G.A.; Delgado-Enciso, O.G.; Larios-Cedeño, E.G.; Sánchez-Galindo, J.M.; Ceballos-Magaña, S.G.; Pineda-Urbina, K.; Alcalá-Pérez, M.A.; Magaña-Vergara, N.E.; Delgado-Enciso, J.; Díaz-Llerenas, U.; et al. Comparative Analysis of Infusions and Ethanolic Extracts of Annona muricata Leaves from Colima, Mexico: Phytochemical Profile and Antioxidant Activity. Life 2024, 14, 1702. [Google Scholar] [CrossRef] [PubMed]
- Sasso, S.; Sampaio, E.; Souza, P.C.; Santana, L.F.; Cardoso, C.A.L.; Alves, F.M.; Portugal, L.C.; de Faria, B.B.; da Silva, A.F.; Motta-Castro, A.R.C.; et al. Use of an Extract of Annona muricata Linn to Prevent High-Fat Diet Induced Metabolic Disorders in C57BL/6 Mice. Nutrients 2019, 11, 1509. [Google Scholar] [CrossRef] [PubMed]
- Brannan, R.G.; Peters, T.E.; Black, K.J.; Kukor, B.J. Valorization of underutilized North American pawpaw (Asimina triloba): Investigation as a lipid oxidation inhibitor in turkey homogenate model system. J. Sci. Food Agric. 2018, 98, 2210–2214. [Google Scholar] [CrossRef]
- Iobe, H.; Koike, A.; Takeda, S.; Watanabe, K.; Saito-Matsuzawa, Y.; Sone, H.; Kamiyama, S. Effects of Pawpaw (Asimina triloba) Seed Extract on the Differentiation and Fat Accumulation of 3T3-L1 Cells under Different Glucose Conditions. J. Nutr. Sci. Vitaminol. 2023, 69, 53–61. [Google Scholar] [CrossRef]
- Lee, C.J.; Kim, Y.S.; Hur, J.; Yoo, G.; Choi, S.Y. Asimina triloba (pawpaw) fruit extract suppresses adipocyte differentiation and lipogenesis-related protein expression in 3T3-L1 cells. J. Food Sci. 2019, 84, 174–182. [Google Scholar] [CrossRef]
- Degli Esposti, M.; Ghelli, A.; Ratta, M.; Cortes, D.; Estornell, E. Natural substances (acetogenins) from the family Annonaceae are powerful inhibitors of mitochondrial NADH dehydrogenase (Complex I). Biochem. J. 1994, 301, 161–167. [Google Scholar] [CrossRef]
- Friedrich, T.; Ohnishi, T.; Forche, E.; Kunze, B.; Jansen, R.; Trowitzsch, W.; Höfle, G.; Reichenbach, H.; Weiss, H. Two binding sites for naturally occurring inhibitors in mitochondrial and bacterial NADH:ubiquinone oxidoreductase (complex I). Biochem. Soc. Trans. 1994, 22, 226–230. [Google Scholar] [CrossRef]
- Landolt, J.L.; Ahammadsahib, K.I.; Hollingworth, R.M.; Barr, R.; Crane, F.L.; Buerck, N.L.; McCabe, G.P.; McLaughlin, J.L. Determination of structure-activity relationships of Annonaceous acetogenins by inhibition of oxygen uptake in rat liver mitochondria. Chem. Biol. Interact. 1995, 98, 1–13. [Google Scholar] [CrossRef]
- Lewis, M.A.; Arnason, J.T.; Philogene, B.J.R.; Rupprecht, J.K.; Mclaughlin, J.L. Inhibition of respiration at site I by asimicin, an insecticidal acetogenin of the pawpaw, Asimina triloba (Annonaceae). Pestic. Biochem. Physiol. 1993, 45, 15–23. [Google Scholar] [CrossRef]
- Champy, P.; Höglinger, G.U.; Féger, J.; Gleye, C.; Hocquemiller, R.; Laurens, A.; Guérineau, V.; Laprévote, O.; Medja, F.; Lombès, A.; et al. Annonacin, a lipophilic inhibitor of mitochondrial complex I, induces nigral and striatal neurodegeneration in rats: Possible relevance for atypical parkinsonism in Guadeloupe. J. Neurochem. 2004, 88, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Champy, P.; Melot, A.; Guérineau Eng, V.; Gleye, C.; Fall, D.; Höglinger, G.U.; Ruberg, M.; Lannuzel, A.; Laprévote, O.; Laurens, A.; et al. Quantification of acetogenins in Annona muricata linked to atypical parkinsonism in guadeloupe. Mov. Disord. 2005, 20, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Champy, P.; Guérineau, V.; Laprévote, O. MALDI-TOF MS profiling of annonaceous acetogenins in Annona muricata products for human consumption. Molecules 2009, 14, 5235–5246. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kataria, M.A.; Saini, V.; Yadav, A. Role of leptin and adiponectin in insulin resistance. Clin. Chim. Acta 2013, 417, 80–84. [Google Scholar] [CrossRef]
- Ahima, R.S.; Flier, J.S. Adipose tissue as an endocrine organ. Trends Endocrinol. Metab. 2000, 11, 327–332. [Google Scholar] [CrossRef]
- Woods, S.C.; D’Alessio, D.A. Central control of body weight and appetite. J. Clin. Endocrinol. Metab. 2008, 93, S37–S50. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Spiegelman, B.M. Tumor necrosis factor alpha: A key component of the obesity-diabetes link. Diabetes 1994, 43, 1271–1278. [Google Scholar] [CrossRef]
- Sonnenberg, G.E.; Krakower, G.R.; Kissebah, A.H. A novel pathway to the manifestations of metabolic syndrome. Obes. Res. 2004, 12, 180–186. [Google Scholar] [CrossRef]
- Rydén, M.; Arner, P. Tumour necrosis factor-alpha in human adipose tissue–From signalling mechanisms to clinical implications. J. Intern. Med. 2007, 262, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]


| ND | HFD | L-PSE | H-PSE | |
|---|---|---|---|---|
| Body weight (g) | 25.3 ± 0.28 a | 34.0 ± 1.31 c | 32.4 ± 0.81 bc | 29.2 ± 0.59 b |
| Liver (g) | 0.92 ± 0.03 | 1.02 ± 0.08 | 0.90 ± 0.06 | 1.06 ± 0.15 |
| Kidney (g) | 0.29 ± 0.01 a | 0.34 ± 0.01 b | 0.34 ± 0.01 b | 0.33 ± 0.01 b |
| Gastrointestinal tract (g) | 1.31 ± 0.04 | 1.40 ± 0.07 | 1.39 ± 0.07 | 1.25 ± 0.06 |
| Mesenteric fat (g) | 0.18 ± 0.01 a | 0.58 ± 0.07 bc | 0.67 ± 0.06 c | 0.43 ± 0.03 b |
| Perirenal fat (g) | 0.05 ± 0.01 a | 0.57 ± 0.07 c | 0.46 ± 0.04 c | 0.26 ± 0.05 b |
| Testicular fat (g) | 0.25 ± 0.01 a | 1.45 ± 0.13 c | 1.18 ± 0.11 bc | 0.78 ± 0.13 b |
| Total visceral fat (g) | 0.47 ± 0.02 a | 2.60 ± 0.23 c | 2.30 ± 0.19 c | 1.46 ± 0.20 b |
| ND | HFD | L-PSE | H-PSE | |
|---|---|---|---|---|
| Blood glucose (mg/100 mL) | 103.8 ± 8.9 a | 181.0 ± 15.8 b | 178.7 ± 20.6 b | 190.2 ± 13.5 b |
| Plasma cholesterol (mg/100 mL) | 55.3 ± 2.5 a | 127.8 ± 10.1 b | 113.6 ± 14.7 b | 125.7 ± 6.4 b |
| Plasma triglyceride (mg/100 mL) | 81.8 ± 7.5 b | 63.9 ± 3.2 ab | 49.2 ± 2.1 a | 62.4 ± 4.4 ab |
| Plasma non-esterified fatty acid (mEq/L) | 1.19 ± 0.13 | 1.15 ± 0.16 | 0.99 ± 0.10 | 1.05 ± 0.06 |
| Plasma norepinephrine (ng/mL) | 3.35 ± 0.61 b | 0.82 ± 0.11 ab | 0.72 ± 0.12 a | 2.37 ± 1.12 ab |
| Liver triglyceride (mg/g wet tissue) | 68.7 ± 5.2 b | 60.1 ± 10.8 ab | 48.4 ± 5.4 ab | 35.2 ± 5.7 a |
| Liver cholesterol (mg/g wet tissue) | 5.2 ± 0.4 b | 5.4 ± 0.4 b | 4.7 ± 0.4 ab | 3.5 ± 0.3 a |
| Total liver lipid (mg/g wet tissue) | 147.0 ± 7.3 b | 139.7 ± 14.6 b | 117.0 ± 6.8 ab | 92.4 ± 6.0 a |
| Gene | Forward Primer (5’–3’) | Reverse Primer (5’–3’) | Accession Number |
|---|---|---|---|
| Actb | cttgggtatggaatcctgtgg | gtacttgcgctcaggaggag | NM_007393 |
| Acaca | gcaactgacagaggaagatgg | tggaaggggaatccatagtg | NM_133360 |
| Adipoq | cccagtcatgccgaagatga | agtgccatctctgccatcac | NM_009605 |
| Acsl1 | agatggctggttacacacgg | taggctctcaacgtcgggta | NM_001302163 |
| Cd36 | gtgcaaaacccagatgacgt | tccaacagacagtgaaggct | NM_001159558 |
| Dgat1 | atatccccgtgcacaagtgg | agaatcggcccacaatccag | NM_010046 |
| Dgat2 | ggcgctacttccgagactac | tccggaagttaccagccaac | NM_026384 |
| Fasn | tctgtgcccgtcgtctatac | ggaggtatgctcgcttctct | NM_007988 |
| Il6 | cggccttccctacttcacaa | caagtgcatcatcgttgttca | NM_031168 |
| Lep | gacatttcacacacgcagtcg | agcccaggaatgaagtccaa | NM_008493 |
| Lipe | tgagattgaggtgctgtcgt | gtaccttgctgtcctgtcct | NM_010719 |
| Pnpla2 | caacgccactcacatctacg | accaggttgaaggagggatg | NM_001163689 |
| Pparg | agggcgatcttgacaggaaa | cgaaactggcacccttgaaa | NM_001127330 |
| Srebf1 | cccacctcaaacctggatct | aagcagcaagatgtcctcct | NM_011480 |
| Tnf | cacagaaagcatgatccgcg | actgatgagagggaggccat | NM_001278601 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takano, S.; Kaneko, S.; Midorikawa, R.; Nara, H.; Sato, Y.; Uchiyama, M.; Iobe, H.; Saito-Matsuzawa, Y.; Sone, H.; Kamiyama, S. Pawpaw (Asimina triloba) Seed Extract Suppresses High-Fat Diet-Induced Obesity in Mice. Int. J. Mol. Sci. 2025, 26, 7719. https://doi.org/10.3390/ijms26167719
Takano S, Kaneko S, Midorikawa R, Nara H, Sato Y, Uchiyama M, Iobe H, Saito-Matsuzawa Y, Sone H, Kamiyama S. Pawpaw (Asimina triloba) Seed Extract Suppresses High-Fat Diet-Induced Obesity in Mice. International Journal of Molecular Sciences. 2025; 26(16):7719. https://doi.org/10.3390/ijms26167719
Chicago/Turabian StyleTakano, Shiori, Sakura Kaneko, Ryo Midorikawa, Honoka Nara, Yurie Sato, Minori Uchiyama, Haruka Iobe, Yuki Saito-Matsuzawa, Hideyuki Sone, and Shin Kamiyama. 2025. "Pawpaw (Asimina triloba) Seed Extract Suppresses High-Fat Diet-Induced Obesity in Mice" International Journal of Molecular Sciences 26, no. 16: 7719. https://doi.org/10.3390/ijms26167719
APA StyleTakano, S., Kaneko, S., Midorikawa, R., Nara, H., Sato, Y., Uchiyama, M., Iobe, H., Saito-Matsuzawa, Y., Sone, H., & Kamiyama, S. (2025). Pawpaw (Asimina triloba) Seed Extract Suppresses High-Fat Diet-Induced Obesity in Mice. International Journal of Molecular Sciences, 26(16), 7719. https://doi.org/10.3390/ijms26167719







