Role of Phosphorylation of Serotonin and Norepinephrine Transporters in Animal Behavior: Relevance to Neuropsychiatric Disorders
Abstract
1. Introduction
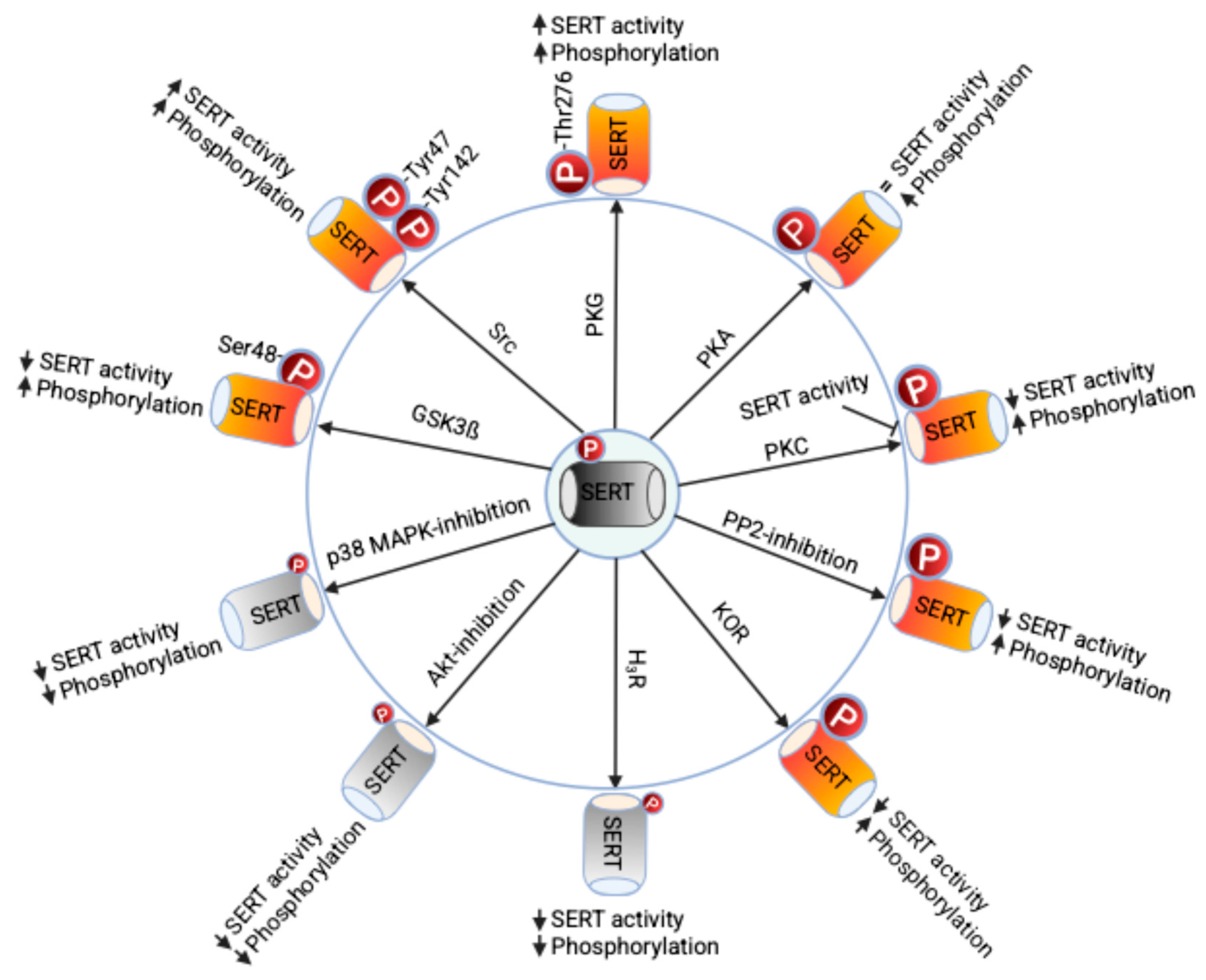
2. The Serotonin Transporter Phosphorylation and Its Significance
2.1. Regulation of SERT by Phosphorylation
2.2. Role of SERT Phosphorylation in Mood Disorders
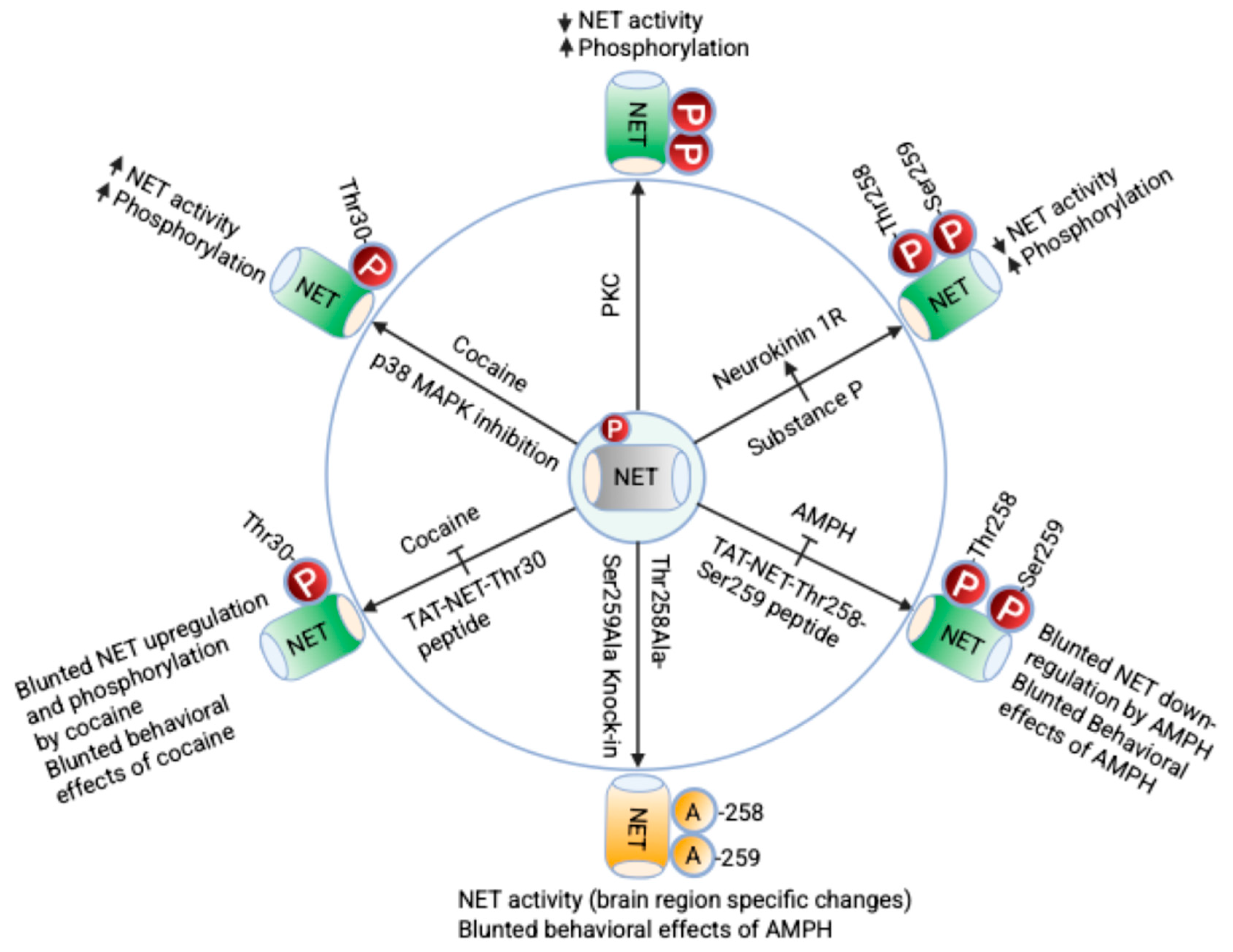
3. The Norepinephrine Transporter Phosphorylation and Its Significance
3.1. Regulation of NET by Phosphorylation
3.2. Role of NET Phosphorylation in Psychostimulant Use Disorders
4. Discussion and Conclusions
| Regulators | Effects on Transporter Function and Trafficking | Effects on Transporter Phosphorylation | References |
|---|---|---|---|
| Protein kinases: | SERT: Reduces 5-HT uptake, SERT Vmax, and surface SERT while increasing SERT endocytosis. It exhibits biphasic effects in platelets: In the initial phase (5 min), it inhibits 5-HT uptake, reduces Vmax, and lowers 5-HT affinity without affecting surface SERT. The later phase (30 min) continues to inhibit 5-HT uptake and reduce Vmax without altering 5-HT affinity, while enhancing SERT internalization. | Increases SERT phosphorylation During the initial phase, SERT is phosphorylated on Ser residues, followed by phosphorylation on both serine and threonine residues at later phase. Phosphorylates Ser149, Ser277 and Thr603 sites in vitro hSERT-peptide phosphorylation assay. | [41,47,64] |
| PKC-activation | NET: Decreases NE uptake, NET Vmax, and surface NET while increasing NET endocytosis. | Increases NET phosphorylation. Phosphorylates Thr258 Ser259 sites. | [93] |
| p38 MAPK-inhibition | SERT: Decreases 5-HT uptake, SERT Vmax, and Km. with or without changes in surface SERT proteins. | Decreases SERT phosphorylation. Phosphorylation of Thr616 by in vitro hSERT-peptide phosphorylation assay. | [46,64] |
| NET: Increases NE uptake and NET surface expression. p38 MAPK associates with NET in the presence of cocaine. | Involved in basal phosphorylation of NET. Blocks cocaine-induced NET phosphorylation. | [101,102] | |
| PKG-activation | SERT: Increases 5-HT uptake, SERT Vmax with no effect on 5-HT affinity Km. Trafficking dependent and/or independent. | Phosphorylation of theThr276 site in hSERT is required for PKG to stimulate SERT. | [45] |
| CaMKII- Inhibition | SERT: Decreases 5-HT uptake. | Decreases SERT phosphorylation Phosphorylated Ser13 in in vitro hSERT-peptide phosphorylation. | [41,59,64] |
| Src-tyrosine kinase-activation/inhibition | SERT: Increases 5-HT uptake, SERT Vmax, surface expression, and stability, while inhibition produces the opposite effect. | Phosphorylation of Tyr47 and Tyr142 in hSERT is required for Src-Induced increases in 5-HT uptake and SERT stability. | [66] |
| GSK3ß-activation/inhibition | SERT: Reduces SERT function, Vmax, surface density, and the opposite with inhibition. | Phosphorylation of Ser48 in hSERT is required for GSK3ß-mediated regulation of SERT function and trafficking. | [53] |
| PKA activation | SERT: No effect on SERT activity. | Increases SERT phosphorylation. | [41] |
| Akt/PKB inhibition | SERT: Reduces SERT function, Vmax, surface density, SERT exocytosis. | Decreases SERT phosphorylation. | [44] |
| Phosphatases: PP2Ac-inhibition | SERT: Decreases 5-HT uptake. | Increases SERT phosphorylation. Associates with SERT. | [41] |
| Receptor ligands: H3R-agonists | SERT: Reduces 5-HT uptake, SERT Vmax, with no effect on Km, and decrease surface expression. | Decreases SERT phosphorylation. | [52] |
| NGF | SERT: Increases 5-HT uptake. | Increases Ser phosphorylation of SERT. | [60] |
| KOR-agonists | SERT Reduces 5-HT uptake, SERT Vmax with no effect on Km, and surface expression.by reducing exocytosis and increasing endocytosis | Increases SERT phosphorylation. | [67] |
| NK1R-agonists | NET: Decreases NE uptake, NET Vmax, and surface NET while increasing NET endocytosis. | Increases NET phosphorylation via PKC activation. Thr258 and Ser259 sites are required for NK1R-mediated NET phosphorylation and raft-mediated subcellular translocation. | [94,104] |
| Transporter substrates: 5-HT | SERT: Upregulates surface SERT and attenuates PKC-dependent surface down regulation. | Attenuates PKC-dependent SERT phosphorylation. | [43] |
| AMPH | SERT: Not known. | Increases SERT phosphorylation through p38 MAPK pathway. | [46] |
| NET: Downregulates NET and Thr258/Ser259 PKC site is required. | Not known. | [95,127] | |
| Fenfluramine | SERT: Attenuates PKC-dependent surface down regulation. | Attenuates PKC-dependent SERT phosphorylation. | [43] |
| Transporter inhibitors: Paroxetine, Citalopram, Imipramine, and Cocaine | SERT: Attenuate PKC-dependent surface down regulation. | Attenuates PKC-dependent SERT phosphorylation. | [43] |
| Cocaine | NET: Increases NE uptake and upregulates surface NET and is blocked by p38 MAPK inhibition. | Increases NET phosphorylation which is blocked by p38 MAPK inhibition. Thr30 is required for cocaine-mediated NET phosphorylation. | [101,102] |
5. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine (serotonin) |
| 8-Br-cGMP | 8-bromoguanosine 3′:5′-cyclic monophosphate |
| Akt | protein kinase B |
| AMPH | D-amphetamine |
| Asp | aspartic acid |
| CaMK | calcium/calmodulin- dependent protein kinase |
| CPP | conditioned place preference |
| CPu | caudate-putamen |
| DA | dopamine |
| DAT | dopamine transporter |
| ERK1/2 | extracellular signal-regulated kinase |
| GSK3ß | glycogen synthase kinase 3ß |
| hSERT | human serotonin transporter |
| H3R | histamine receptor 3 |
| HEK-293 cells | human embryonic kidney-293 cells |
| KI | knock-in |
| KOR | kappa opioid receptor |
| MA | monoamines |
| MDMA | 3,4-methylenedioxymethamphetamine |
| METH | methamphetamine |
| mPFC | medial prefrontal cortex |
| NAc | nucleus accumbens |
| NE | norepinephrine |
| NET | norepinephrine transporter |
| NK1R | neurokinin-1 receptor |
| OCD | Obsessive–Compulsive Disorder |
| p38 MAPK | p38 mitogen-activated protein kinase |
| PI-3 kinase | phosphoinositide 3 kinase |
| PKC | protein kinase C |
| PKG | protein kinase G |
| PP2Ac | protein phosphatase 2A catalytic subunit |
| Ser | serine |
| SERT | serotonin transporter |
| SSRI | selective serotonin reuptake inhibitor |
| ß-PMA | phorbol 12-myristate 13 acetate |
| SUD | substance use disorder |
| Thr | threonine |
| Tyr | tyrosine |
References
- Coccaro, E.F. Central serotonin and impulsive aggression. Br. J. Psychiatry 1989, 155, 52–62. [Google Scholar] [CrossRef]
- Owens, M.J.; Nemeroff, C.B. Role of serotonin in the pathophysiology of depression: Focus on the serotonin transporter. Clin. Chem. 1994, 40, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Sellers, E.M.; Higgins, G.A.; Tompkins, D.M.; Romach, M.K. Serotonin and alcohol drinking. NIDA Res. Monogr. 1992, 119, 141–145. [Google Scholar]
- Compagnon, P.; Ernouf, D.; Narcisse, G.; Daoust, M. Serotonin in animal models of alcoholism. Alcohol Alcohol. 1993, 2, 215–219. [Google Scholar]
- Schildkraut, J.J. The catecholamine hypothesis of affective disorders: A review of supporting evidence. Am. J. Psychiatry 1965, 122, 509–522. [Google Scholar] [CrossRef]
- Ressler, K.J.; Nemeroff, C.B. Role of norepinephrine in the pathophysiology and treatment of mood disorders. Biol. Psychiatry 1999, 46, 1219–1233. [Google Scholar] [CrossRef]
- Leonard, B.E. The role of noradrenaline in depression: A review. J. Psychopharmacol. 1997, 11, S39–S47. [Google Scholar]
- Klimek, V.; Stockmeier, C.; Overholser, J.; Meltzer, H.Y.; Kalka, S.; Dilley, G.; Ordway, G.A. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J. Neurosci. 1997, 17, 8451–8458. [Google Scholar] [CrossRef]
- Bohm, M.; La Rosse, K.; Schwinger, R.H.G.; Erdmann, E. Evidence for reduction norepinephrine uptake sites in the failing human heart. J. Am. Coll. Cardiol. 1995, 25, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Fan, T.-H.M.; Sullebarger, J.T.; Sakamoto, S. Decreased adrenergic neuronal uptake activity in experimental right heart failure. J. Clin. Investig. 1989, 84, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Merlet, P.; Dubois-Rande, J.-L.; Adnot, S.; Bourguignon, M.H.; Benvenuti, C.; Loisance, D.; Valette, H.; Castaigne, A.; Syrota, A. Myocardial b-adrenergic desensitization and neuronal norepinephrine uptake function in idiopathic dilated cardiomyopathy. J. Cardiovasc. Pharmacol. 1992, 19, 10–16. [Google Scholar] [CrossRef]
- Schafers, M.; Dutka, D.; Rhodes, C.G.; Lammertsma, A.A.; Hermansen, F.; Schober, O.; Camici, P.G. Myocardial presynaptic and postsynaptic autonomic dysfunction in hypertrophic cardiomyopathy. Circ. Res. 1998, 82, 57–62. [Google Scholar] [CrossRef]
- Esler, M.; Jackman, G.; Bobik, A.; Leonard, P.; Kelleher, D.; Skews, H.; Jennings, G.; Korner, P. Norepinephrine kinetics in essential hypertension. Defective neuronal uptake of norepinephrine in some patients. Hypertension 1981, 3, 149–156. [Google Scholar] [CrossRef]
- Imamura, M.; Lander, H.M.; Levi, R. Activation of histamine H3-receptors inhibits carrier-mediated norepinephrine release during protracted myocardial ischemia. Comparison with adenosine A1-receptors and alpha2-adrenoceptors. Circ. Res. 1996, 78, 475–481. [Google Scholar] [CrossRef]
- Coleman, J.A.; Green, E.M.; Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 2016, 532, 334–339. [Google Scholar] [CrossRef]
- Hu, T.; Yu, Z.; Zhao, J.; Meng, Y.; Salomon, K.; Bai, Q.; Wei, Y.; Zhang, J.; Xu, S.; Dai, Q.; et al. Transport and inhibition mechanisms of the human noradrenaline transporter. Nature 2024, 632, 930–937. [Google Scholar] [CrossRef]
- Yamashita, A.; Singh, S.K.; Kawate, T.; Jin, Y.; Gouaux, E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature 2005, 437, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Barker, E.L.; Blakely, R.D. Norepinephrine and serotonin transporters: Molecular targets of antidepressant drugs. In Psychopharmacology: The Fourth Generation of Progress; Bloom, F.E., Kupfer, D.J., Eds.; Raven Press: New York, NY, USA, 1995; pp. 321–333. [Google Scholar]
- Jayanthi, L.D.; Ramamoorthy, S. Regulation of monoamine transporters: Influence of psychostimulants and therapeutic antidepressants. AAPS J. 2005, 7, E728–E738. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R.; Caron, M.G. Monoamine transporters: From genes to behavior. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 261–284. [Google Scholar] [CrossRef]
- Hall, F.S.; Li, X.F.; Sora, I.; Xu, F.; Caron, M.; Lesch, K.P.; Murphy, D.L.; Uhl, G.R. Cocaine mechanisms: Enhanced cocaine, fluoxetine and nisoxetine place preferences following monoamine transporter deletions. Neuroscience 2002, 115, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Adriani, W.; Boyer, F.; Gioiosa, L.; Macri, S.; Dreyer, J.L.; Laviola, G. Increased impulsive behavior and risk proneness following lentivirus-mediated dopamine transporter over-expression in rats’ nucleus accumbens. Neuroscience 2009, 159, 47–58. [Google Scholar] [CrossRef]
- Sora, I.; Hall, F.S.; Andrews, A.M.; Itokawa, M.; Li, X.F.; Wei, H.B.; Wichems, C.; Lesch, K.P.; Murphy, D.L.; Uhl, G.R. Molecular mechanisms of cocaine reward: Combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc. Natl. Acad. Sci. USA 2001, 98, 5300–5305. [Google Scholar] [CrossRef]
- Li, Q.; Ma, L.; Innis, R.B.; Seneca, N.; Ichise, M.; Huang, H.; Laruelle, M.; Murphy, D.L. Pharmacological and genetic characterization of two selective serotonin transporter ligands: 2-[2-(dimethylaminomethylphenylthio)]-5-fluoromethylphenylamine (AFM) and 3-amino-4-[2-(dimethylaminomethyl-phenylthio)]benzonitrile (DASB). J. Pharmacol. Exp. Ther. 2004, 308, 481–486. [Google Scholar] [CrossRef]
- Rioux, A.; Fabre, V.; Lesch, K.P.; Moessner, R.; Murphy, D.L.; Lanfumey, L.; Hamon, M.; Martres, M.P. Adaptive changes of serotonin 5-HT2A receptors in mice lacking the serotonin transporter. Neurosci. Lett. 1999, 262, 113–116. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Wetsel, W.C.; Jones, S.R.; Levin, E.D.; Jaber, M.; Caron, M.G. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 1999, 283, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Bengel, D.; Murphy, D.L.; Andrews, A.M.; Wichems, C.H.; Feltner, D.; Heils, A.; Mossner, R.; Westphal, H.; Lesch, K.P. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymetamphetamine (“ecstasy”) in serotonin transporter-deficient mice. Mol. Pharmacol. 1998, 53, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, J.; Jorgensen, T.N.; Gether, U. Regulation of dopamine transporter function by protein-protein interactions: New discoveries and methodological challenges. J. Neurochem. 2010, 113, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Zahniser, N.R.; Doolen, S. Chronic and acute regulation of Na+/Cl−-dependent neurotransmitter transporters: Drugs, substrates, presynaptic receptors, and signaling systems. Pharmacol. Ther. 2001, 92, 21–55. [Google Scholar] [CrossRef]
- Zahniser, N.R.; Sorkin, A. Rapid regulation of the dopamine transporter: Role in stimulant addiction? Neuropharmacology 2004, 47 (Suppl. 1), 80–91. [Google Scholar] [CrossRef]
- Steiner, J.A.; Carneiro, A.M.; Blakely, R.D. Going with the flow: Trafficking-dependent and -independent regulation of serotonin transport. Traffic 2008, 9, 1393–1402. [Google Scholar] [CrossRef]
- Torres, G.E.; Amara, S.G. Glutamate and monoamine transporters: New visions of form and function. Curr. Opin. Neurobiol. 2007, 17, 304–312. [Google Scholar] [CrossRef]
- Torres, G.E.; Gainetdinov, R.R.; Caron, M.G. Plasma membrane monoamine transporters: Structure, regulation and function. Nat. Rev. Neurosci. 2003, 4, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A. Phosphorylation and regulation of psychostimulant-sensitive neurotransmitter transporters. J. Pharmacol. Exp. Ther. 2004, 310, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Melikian, H.E. Neurotransmitter transporter trafficking: Endocytosis, recycling, and regulation. Pharmacol. Ther. 2004, 104, 17–27. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Shippenberg, T.S.; Jayanthi, L.D. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol. Ther. 2011, 129, 220–238. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Bauman, A.L.; Moore, K.R.; Han, H.; Yang-Feng, T.; Chang, A.S.; Ganapathy, V.; Blakely, R.D. Antidepressant- and cocaine-sensitive human serotonin transporter: Molecular cloning, expression, and chromosomal localization. Proc. Natl. Acad. Sci. USA 1993, 90, 2542–2546. [Google Scholar] [CrossRef]
- Lesch, K.P.; Wolozin, B.L.; Estler, H.C.; Murphy, D.L.; Riederer, P. Isolation of a cDNA encoding the human brain serotonin transporter. J. Neural Transm. 1993, 91, 67–72. [Google Scholar] [CrossRef]
- Hoffman, B.J.; Mezey, E.; Brownstein, M.J. Cloning of a serotonin transporter affected by antidepressants. Science 1991, 254, 579–580. [Google Scholar] [CrossRef]
- Miller, K.J.; Hoffman, B.J. Adenosine A3 receptors regulate serotonin transoprt via nitric oxide and cGMP. J. Biol. Chem. 1994, 269, 27351–27356. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Giovanetti, E.; Qian, Y.; Blakely, R.D. Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J. Biol. Chem. 1998, 273, 2458–2466. [Google Scholar] [CrossRef]
- Blakely, R.D.; Ramamoorthy, S.; Schroeter, S.; Qian, Y.; Apparsundaram, S.; Galli, A.; DeFelice, L.J. Regulated phosphorylation and trafficking of antidepressant-sensitive serotonin transporter proteins. Biol. Psychiatry 1998, 44, 169–178. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Blakely, R.D. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science 1999, 285, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Rajamanickam, J.; Annamalai, B.; Rahbek-Clemmensen, T.; Sundaramurthy, S.; Gether, U.; Jayanthi, L.D.; Ramamoorthy, S. Akt-mediated regulation of antidepressant-sensitive serotonin transporter function, cell-surface expression and phosphorylation. Biochem. J. 2015, 468, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Samuvel, D.J.; Buck, E.R.; Rudnick, G.; Jayanthi, L.D. Phosphorylation of threonine residue 276 is required for acute regulation of serotonin transporter by cyclic GMP. J. Biol. Chem. 2007, 282, 11639–11647. [Google Scholar] [CrossRef]
- Samuvel, D.J.; Jayanthi, L.D.; Bhat, N.R.; Ramamoorthy, S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: Evidence for distinct cellular mechanisms involved in transporter surface expression. J. Neurosci. 2005, 25, 29–41. [Google Scholar] [CrossRef]
- Jayanthi, L.D.; Samuvel, D.J.; Blakely, R.D.; Ramamoorthy, S. Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol. Pharmacol. 2005, 67, 2077–2087. [Google Scholar] [CrossRef]
- Qian, Y.; Galli, A.; Ramamoorthy, S.; Risso, S.; DeFelice, L.J.; Blakely, R.D. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J. Neurosci. 1997, 17, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, L.D.; Ramamoorthy, S.; Mahesh, V.B.; Leibach, F.H.; Ganapathy, V. Calmodulin-dependent regulation of the catalytic function of the human serotonin transporter in placental choriocarcinoma cells. J. Biol. Chem. 1994, 269, 14424–14429. [Google Scholar] [CrossRef]
- Hoffman, B.J.; Miller, K.J. The N-Terminal Domain of the 5HT Transporter Partially Mediates the Regulation of Uptake by Protein Kinase C (PKC); Society of Neuroscience: Washington, DC, USA, 1994. [Google Scholar]
- Zhu, C.B.; Carneiro, A.M.; Dostmann, W.R.; Hewlett, W.A.; Blakely, R.D. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J. Biol. Chem. 2005, 280, 15649–15658. [Google Scholar] [CrossRef]
- Annamalai, B.; Ragu Varman, D.; Horton, R.E.; Daws, L.C.; Jayanthi, L.D.; Ramamoorthy, S. Histamine Receptors Regulate the Activity, Surface Expression, and Phosphorylation of Serotonin Transporters. ACS Chem. Neurosci. 2020, 11, 466–476. [Google Scholar] [CrossRef]
- Ragu Varman, D.; Jayanthi, L.D.; Ramamoorthy, S. Glycogen synthase kinase-3ß supports serotonin transporter function and trafficking in a phosphorylation-dependent manner. J. Neurochem. 2021, 156, 445–464. [Google Scholar] [CrossRef]
- Ruan, Q.T.; Yazdani, N.; Blum, B.C.; Beierle, J.A.; Lin, W.; Coelho, M.A.; Fultz, E.K.; Healy, A.F.; Shahin, J.R.; Kandola, A.K.; et al. A Mutation in Hnrnph1 That Decreases Methamphetamine-Induced Reinforcement, Reward, and Dopamine Release and Increases Synaptosomal hnRNP H and Mitochondrial Proteins. J. Neurosci. 2020, 40, 107–130. [Google Scholar] [CrossRef]
- Zarpellon, A.; Donella-Deana, A.; Folda, A.; Turetta, L.; Pavanetto, M.; Deana, R. Serotonin (5-HT) transport in human platelets is modulated by Src-catalysed Tyr-phosphorylation of the plasma membrane transporter SERT. Cell. Physiol. Biochem. 2008, 21, 87–94. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Gesmonde, J.; Ramamoorthy, S.; Rudnick, G. Serotonin transporter phosphorylation by cGMP-dependent protein kinase is altered by a mutation associated with obsessive compulsive disorder. J. Neurosci. 2007, 27, 10878–10886. [Google Scholar] [CrossRef]
- Zhu, C.B.; Hewlett, W.A.; Feoktistov, I.; Biaggioni, I.; Blakely, R.D. Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol. Pharmacol. 2004, 65, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.B.; Steiner, J.A.; Munn, J.L.; Daws, L.C.; Hewlett, W.A.; Blakely, R.D. Rapid stimulation of presynaptic serotonin transport by A(3) adenosine receptors. J. Pharmacol. Exp. Ther. 2007, 322, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.A.; Timmons, M.; Phillips, A.; Quick, M.W. Calcium/calmodulin-dependent kinase II regulates the interaction between the serotonin transporter and syntaxin 1A. Neuropharmacology 2008, 55, 763–770. [Google Scholar] [CrossRef]
- Gil, C.; Najib, A.; Aguilera, J. Serotonin transport is modulated differently by tetanus toxin and growth factors. Neurochem. Int. 2003, 42, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Ragu Varman, D.; Jayanthi, L.D.; Ramamoorthy, S. Kappa Opioid Receptor Mediated Differential Regulation of Serotonin and Dopamine Transporters in Mood and Substance Use Disorder. Handb. Exp. Pharmacol. 2022, 271, 97–112. [Google Scholar] [CrossRef]
- Rudnick, G. Active transport of 5-hydroxytryptamine by plasma membrane vesicles isolated from human blood platelets. The Journal of Biological Chemistry. 1977, 252, 2170–2174. [Google Scholar] [CrossRef]
- Iny, L.J.; Pecknold, J.; Suranyi-Cadotte, B.E.; Bernier, B.; Luthe, L.; Nair, N.P.; Meaney, M.J. Studies of a neurochemical link between depression, anxiety, and stress from [3H]imipramine and [3H]paroxetine binding on human platelets. Biol. Psychiatry. 1994, 36, 281–291. [Google Scholar] [CrossRef]
- Sorensen, L.; Stromgaard, K.; Kristensen, A.S. Characterization of intracellular regions in the human serotonin transporter for phosphorylation sites. ACS Chem. Biol. 2014, 9, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Helmeste, D.M.; Tang, S.W. Tyrosine kinase inhibitors regulate serotonin uptake in platelets. Eur. J. Pharmacol. 1995, 280, R5–R7. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, B.; Mannangatti, P.; Arapulisamy, O.; Shippenberg, T.S.; Jayanthi, L.D.; Ramamoorthy, S. Tyrosine phosphorylation of the human serotonin transporter: A role in the transporter stability and function. Mol. Pharmacol. 2012, 81, 73–85. [Google Scholar] [CrossRef]
- Sundaramurthy, S.; Annamalai, B.; Samuvel, D.J.; Shippenberg, T.S.; Jayanthi, L.D.; Ramamoorthy, S. Modulation of serotonin transporter function by kappa-opioid receptor ligands. Neuropharmacology 2017, 113, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.-P.; Bengel, D.; Heils, A.; Sabol, S.Z.; Greenberg, B.D.; Petri, S.; Benjamin, J.; Müller, C.R.; Hamer, D.H.; Murphy, D.L. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996, 274, 1527–1531. [Google Scholar] [CrossRef]
- Greenberg, B.D.; McMahon, F.J.; Murphy, D.L. Serotonin transporter candidate gene studies in affective disorders and personality: Promises and potential pitfalls [editorial]. Mol. Psychiatry 1998, 3, 186–189. [Google Scholar] [CrossRef][Green Version]
- Campbell, S.; Marriott, M.; Nahmias, C.; MacQueen, G.M. Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am. J. Psychiatry 2004, 161, 598–607. [Google Scholar] [CrossRef]
- Kambeitz, J.P.; Howes, O.D. The serotonin transporter in depression: Meta-analysis of in vivo and post mortem findings and implications for understanding and treating depression. J. Affect. Disord. 2015, 186, 358–366. [Google Scholar] [CrossRef]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.H. Effect of ginsenoside Re on depression- and anxiety-like behaviors and cognition memory deficit induced by repeated immobilization in rats. J. Microbiol. Biotechnol. 2012, 22, 708–720. [Google Scholar] [CrossRef]
- Airaksinen, E.; Larsson, M.; Forsell, Y. Neuropsychological functions in anxiety disorders in population-based samples: Evidence of episodic memory dysfunction. J. Psychiatr. Res. 2005, 39, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Delgado, P.L.; Price, L.H.; Miller, H.L.; Salomon, R.M.; Aghajanian, G.K.; Heninger, G.R.; Charney, D.S. Serotonin and the neurobiology of depression. Effects of tryptophan depletion in drug-free depressed patients. Arch. Gen. Psychiatry 1994, 51, 865–874. [Google Scholar] [CrossRef]
- Barr, L.C.; Goodman, W.K.; McDougle, C.J.; Delgado, P.L.; Heninger, G.R.; Charney, D.S.; Price, L.H. Tryptophan depletion in patients with obsessive-compulsive disorder who respond to serotonin reuptake inhibitors. Arch. Gen. Psychiatry 1994, 51, 309–317. [Google Scholar] [CrossRef]
- Borgkvist, A.; Malmlof, T.; Feltmann, K.; Lindskog, M.; Schilstrom, B. Dopamine in the hippocampus is cleared by the norepinephrine transporter. Int. J. Neuropsychopharmacol. 2012, 15, 531–540. [Google Scholar] [CrossRef]
- Holmes, A.; Murphy, D.L.; Crawley, J.N. Abnormal behavioral phenotypes of serotonin transporter knockout mice: Parallels with human anxiety and depression. Biol. Psychiatry 2003, 54, 953–959. [Google Scholar] [CrossRef]
- Holmes, A.; Yang, R.J.; Lesch, K.P.; Crawley, J.N.; Murphy, D.L. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology 2003, 28, 2077–2088. [Google Scholar] [CrossRef]
- Pardo, M.; Abrial, E.; Jope, R.S.; Beurel, E. GSK3beta isoform-selective regulation of depression, memory and hippocampal cell proliferation. Genes Brain Behav. 2016, 15, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Hong, B.; Fan, L.; Zhou, L.; Liu, Y.; Wu, Q.; Zhang, X.; Dong, M. Protective effect of puerarin against beta-amyloid-induced oxidative stress in neuronal cultures from rat hippocampus: Involvement of the GSK-3beta/Nrf2 signaling pathway. Free Radic. Res. 2013, 47, 55–63. [Google Scholar] [CrossRef]
- Li, X.H.; Lv, B.L.; Xie, J.Z.; Liu, J.; Zhou, X.W.; Wang, J.Z. AGEs induce Alzheimer-like tau pathology and memory deficit via RAGE-mediated GSK-3 activation. Neurobiol. Aging 2012, 33, 1400–1410. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Q.; Dong, Y.; Zhou, W.; Song, H.; Liu, Y.; Liu, M.; Yuan, Y.; Ding, F.; Gu, X.; et al. Inhibition of gecko GSK-3beta promotes elongation of neurites and oligodendrocyte processes but decreases the proliferation of blastemal cells. J. Cell. Biochem. 2012, 113, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Serretti, A.; Benedetti, F.; Mandelli, L.; Calati, R.; Caneva, B.; Lorenzi, C.; Fontana, V.; Colombo, C.; Smeraldi, E. Association between GSK-3beta -50T/C polymorphism and personality and psychotic symptoms in mood disorders. Psychiatry Res. 2008, 158, 132–140. [Google Scholar] [CrossRef]
- Meinke, C.; Quinlan, M.A.; Paffenroth, K.C.; Harrison, F.E.; Fenollar-Ferrer, C.; Katamish, R.M.; Stillman, I.; Ramamoorthy, S.; Blakely, R.D. Serotonin Transporter Ala276 Mouse: Novel Model to Assess the Neurochemical and Behavioral Impact of Thr276 Phosphorylation In Vivo. Neurochem. Res. 2022, 47, 37–60. [Google Scholar] [CrossRef]
- Prasad, H.C.; Zhu, C.B.; McCauley, J.L.; Samuvel, D.J.; Ramamoorthy, S.; Shelton, R.C.; Hewlett, W.A.; Sutcliffe, J.S.; Blakely, R.D. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc. Natl. Acad. Sci. USA 2005, 102, 11545–11550. [Google Scholar] [CrossRef]
- Veenstra-VanderWeele, J.; Muller, C.L.; Iwamoto, H.; Sauer, J.E.; Owens, W.A.; Shah, C.R.; Cohen, J.; Mannangatti, P.; Jessen, T.; Thompson, B.J.; et al. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc. Natl. Acad. Sci. USA 2012, 109, 5469–5474. [Google Scholar] [CrossRef]
- Siemann, J.K.; Muller, C.L.; Forsberg, C.G.; Blakely, R.D.; Veenstra-VanderWeele, J.; Wallace, M.T. An autism-associated serotonin transporter variant disrupts multisensory processing. Transl. Psychiatry 2017, 7, e1067. [Google Scholar] [CrossRef]
- Robson, M.J.; Quinlan, M.A.; Margolis, K.G.; Gajewski-Kurdziel, P.A.; Veenstra-VanderWeele, J.; Gershon, M.D.; Watterson, D.M.; Blakely, R.D. p38alpha MAPK signaling drives pharmacologically reversible brain and gastrointestinal phenotypes in the SERT Ala56 mouse. Proc. Natl. Acad. Sci. USA 2018, 115, E10245–E10254. [Google Scholar] [CrossRef]
- Chan, M.C.; Procko, E.; Shukla, D. Structural Rearrangement of the Serotonin Transporter Intracellular Gate Induced by Thr276 Phosphorylation. ACS Chem. Neurosci. 2022, 13, 933–945. [Google Scholar] [CrossRef]
- Kilic, F.; Murphy, D.L.; Rudnick, G. A human serotonin transporter mutation causes constitutive activation of transport activity. Mol. Pharmacol. 2003, 64, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Pacholczyk, T.; Blakely, R.D.; Amara, S.G. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature 1991, 350, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.D.; Jayanthi, L.D.; Thoreson, M.A.; Blakely, R.D. Cloning and chromosomal mapping of the murine norepinephrine transporter. J. Neurochem. 1998, 70, 2241–2251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jayanthi, L.D.; Samuvel, D.J.; Ramamoorthy, S. Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters. Evidence for localization in lipid rafts and lipid raft-mediated internalization. J. Biol. Chem. 2004, 279, 19315–19326. [Google Scholar] [CrossRef]
- Jayanthi, L.D.; Annamalai, B.; Samuvel, D.J.; Gether, U.; Ramamoorthy, S. Phosphorylation of the norepinephrine transporter at threonine 258 and serine 259 is linked to protein kinase C-mediated transporter internalization. J. Biol. Chem. 2006, 281, 23326–23340. [Google Scholar] [CrossRef]
- Annamalai, B.; Mannangatti, P.; Arapulisamy, O.; Ramamoorthy, S.; Jayanthi, L.D. Involvement of threonine 258 and serine 259 motif in amphetamine-induced norepinephrine transporter endocytosis. J. Neurochem. 2010, 115, 23–35. [Google Scholar] [CrossRef]
- Apparsundaram, S.; Schroeter, S.; Blakely, R.D. Acute regulation of norepinephrine transport: II. PKC-modulated surface expression of human norepinephrine transporter proteins. J. Pharmacol. Exp. Ther. 1998, 287, 744–751. [Google Scholar] [CrossRef]
- Apparsundaram, S.; Galli, A.; DeFelice, L.J.; Hartzell, H.C.; Blakely, R.D. Acute regulation of norepinephrine transport: I. PKC-linked muscarinic receptors influence transport capacity and transporter density in SK-N-SH cells. J. Pharmacol. Exp. Ther. 1998, 287, 733–743. [Google Scholar] [CrossRef]
- Ding, Y.S.; Singhal, T.; Planeta-Wilson, B.; Gallezot, J.D.; Nabulsi, N.; Labaree, D.; Ropchan, J.; Henry, S.; Williams, W.; Carson, R.E.; et al. PET imaging of the effects of age and cocaine on the norepinephrine transporter in the human brain using (S,S)-[(11)C]O-methylreboxetine and HRRT. Synapse 2010, 64, 30–38. [Google Scholar] [CrossRef]
- Macey, D.J.; Smith, H.R.; Nader, M.A.; Porrino, L.J. Chronic cocaine self-administration upregulates the norepinephrine transporter and alters functional activity in the bed nucleus of the stria terminalis of the rhesus monkey. J. Neurosci. 2003, 23, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.J.; Smith, H.R.; Nader, M.A.; Porrino, L.J. Effects of chronic cocaine self-administration on norepinephrine transporters in the nonhuman primate brain. Psychopharmacology 2005, 180, 781–788. [Google Scholar] [CrossRef]
- Mannangatti, P.; Arapulisamy, O.; Shippenberg, T.S.; Ramamoorthy, S.; Jayanthi, L.D. Cocaine up-regulation of the norepinephrine transporter requires threonine 30 phosphorylation by p38 mitogen-activated protein kinase. J. Biol. Chem. 2011, 286, 20239–20250. [Google Scholar] [CrossRef] [PubMed]
- Mannangatti, P.; NarasimhaNaidu, K.; Damaj, M.I.; Ramamoorthy, S.; Jayanthi, L.D. A Role for p38 Mitogen-activated Protein Kinase-mediated Threonine 30-dependent Norepinephrine Transporter Regulation in Cocaine Sensitization and Conditioned Place Preference. J. Biol. Chem. 2015, 290, 10814–10827. [Google Scholar] [CrossRef] [PubMed]
- Bauman, A.L.; Apparsundaram, S.; Ramamoorthy, S.; Wadzinski, B.E.; Vaughan, R.A.; Blakely, R.D. Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J. Neurosci. 2000, 20, 7571–7578. [Google Scholar] [CrossRef] [PubMed]
- Arapulisamy, O.; Mannangatti, P.; Jayanthi, L.D. Regulated norepinephrine transporter interaction with the neurokinin-1 receptor establishes transporter subcellular localization. J. Biol. Chem. 2013, 288, 28599–28610. [Google Scholar] [CrossRef] [PubMed]
- Sung, U.; Apparsundaram, S.; Galli, A.; Kahlig, K.M.; Savchenko, V.; Schroeter, S.; Quick, M.W.; Blakely, R.D. A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity. J. Neurosci. 2003, 23, 1697–1709. [Google Scholar] [CrossRef]
- Samuvel, D.J.; Jayanthi, L.D.; Manohar, S.; Kaliyaperumal, K.; See, R.E.; Ramamoorthy, S. Dysregulation of dopamine transporter trafficking and function after abstinence from cocaine self-administration in rats: Evidence for differential regulation in caudate putamen and nucleus accumbens. J. Pharmacol. Exp. Ther. 2008, 325, 293–301. [Google Scholar] [CrossRef]
- Rickhag, M.; Owens, W.A.; Winkler, M.T.; Strandfelt, K.N.; Rathje, M.; Sorensen, G.; Andresen, B.; Madsen, K.L.; Jorgensen, T.N.; Wortwein, G.; et al. Membrane-permeable C-terminal dopamine transporter peptides attenuate amphetamine-evoked dopamine release. J. Biol. Chem. 2013, 288, 27534–27544. [Google Scholar] [CrossRef]
- Mergy, M.A.; Gowrishankar, R.; Gresch, P.J.; Gantz, S.C.; Williams, J.; Davis, G.L.; Wheeler, C.A.; Stanwood, G.D.; Hahn, M.K.; Blakely, R.D. The rare DAT coding variant Val559 perturbs DA neuron function, changes behavior, and alters in vivo responses to psychostimulants. Proc. Natl. Acad. Sci. USA 2014, 111, E4779–E4788. [Google Scholar] [CrossRef]
- Siciliano, C.A.; Calipari, E.S.; Jones, S.R. Amphetamine potency varies with dopamine uptake rate across striatal subregions. J. Neurochem. 2014, 131, 348–355. [Google Scholar] [CrossRef]
- Kaye, D.M.; Wiviott, S.D.; Kobzik, L.; Kelly, R.A.; Smith, T.W. S-nitrosothiols inhibit neuronal norepinephrine transport. Am. J. Physiol. 1997, 272, H875–H883. [Google Scholar] [CrossRef]
- Dipace, C.; Sung, U.; Binda, F.; Blakely, R.D.; Galli, A. Amphetamine induces a calcium/calmodulin-dependent protein kinase II-dependent reduction in norepinephrine transporter surface expression linked to changes in syntaxin 1A/transporter complexes. Mol. Pharmacol. 2007, 71, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Matthies, H.J.; Moore, J.L.; Saunders, C.; Matthies, D.S.; Lapierre, L.A.; Goldenring, J.R.; Blakely, R.D.; Galli, A. Rab11 supports amphetamine-stimulated norepinephrine transporter trafficking. J. Neurosci. 2010, 30, 7863–7877. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.R.; Robbins, T.W. Noradrenergic modulation of cognition: Therapeutic implications. J. Psychopharmacol. 2013, 27, 694–718. [Google Scholar] [CrossRef]
- Cummins Jacklin, E.; Boughner, E.; Kent, K.; Kwiatkowski, D.; MacDonald, T.; Leri, F. Memory of a drug lapse: Role of noradrenaline. Neuropharmacology 2015, 99, 98–105. [Google Scholar] [CrossRef]
- Hester, R.; Garavan, H. Executive dysfunction in cocaine addiction: Evidence for discordant frontal, cingulate, and cerebellar activity. J. Neurosci. 2004, 24, 11017–11022. [Google Scholar] [CrossRef]
- Woicik, P.A.; Urban, C.; Alia-Klein, N.; Henry, A.; Maloney, T.; Telang, F.; Wang, G.J.; Volkow, N.D.; Goldstein, R.Z. A pattern of perseveration in cocaine addiction may reveal neurocognitive processes implicit in the Wisconsin Card Sorting Test. Neuropsychologia 2011, 49, 1660–1669. [Google Scholar] [CrossRef]
- Volkow, N.D.; Morales, M. The Brain on Drugs: From Reward to Addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef]
- Wiers, C.E.; Cabrera, E.; Skarda, E.; Volkow, N.D.; Wang, G.J. PET imaging for addiction medicine: From neural mechanisms to clinical considerations. Prog. Brain Res. 2016, 224, 175–201. [Google Scholar] [CrossRef]
- Howell, L.L.; Negus, S.S. Monoamine transporter inhibitors and substrates as treatments for stimulant abuse. Adv. Pharmacol. 2014, 69, 129–176. [Google Scholar] [CrossRef]
- dela Pena, I.; Gevorkiana, R.; Shi, W.X. Psychostimulants affect dopamine transmission through both dopamine transporter-dependent and independent mechanisms. Eur. J. Pharmacol. 2015, 764, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Tanda, G.; Pontieri, F.E.; Frau, R.; Di Chiara, G. Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. Eur. J. Neurosci. 1997, 9, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Moron, J.A.; Brockington, A.; Wise, R.A.; Rocha, B.A.; Hope, B.T. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: Evidence from knock-out mouse lines. J. Neurosci. 2002, 22, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Weinshenker, D.; Schroeder, J.P. There and back again: A tale of norepinephrine and drug addiction. Neuropsychopharmacology 2007, 32, 1433–1451. [Google Scholar] [CrossRef]
- Zaniewska, M.; Filip, M.; Przegalinski, E. The Involvement of Norepinephrine in Behaviors Related to Psychostimulant Addiction. Curr. Neuropharmacol. 2015, 13, 407–418. [Google Scholar] [CrossRef]
- Howell, L.L.; Kimmel, H.L. Monoamine transporters and psychostimulant addiction. Biochem. Pharmacol. 2008, 75, 196–217. [Google Scholar] [CrossRef]
- Carboni, E.; Tanda, G.L.; Frau, R.; Chiara, G.D. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: Evidence that dopamine is taken up in vivo by noradrenergic terminals. J. Neurochem. 1990, 55, 1067–1070. [Google Scholar] [CrossRef]
- Mannangatti, P.; Ramamoorthy, S.; Jayanthi, L.D. Interference of norepinephrine transporter trafficking motif attenuates amphetamine-induced locomotor hyperactivity and conditioned place preference. Neuropharmacology 2018, 128, 132–141. [Google Scholar] [CrossRef]
- Moorman, D.E.; James, M.H.; McGlinchey, E.M.; Aston-Jones, G. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res. 2015, 1628, 130–146. [Google Scholar] [CrossRef]
- Janak, P.H.; Bowers, M.S.; Corbit, L.H. Compound stimulus presentation and the norepinephrine reuptake inhibitor atomoxetine enhance long-term extinction of cocaine-seeking behavior. Neuropsychopharmacology 2012, 37, 975–985. [Google Scholar] [CrossRef]
- Carey, R.J.; DePalma, G.; Shanahan, A.; Damianopoulos, E.N.; Muller, C.P.; Huston, J.P. Effects on spontaneous and cocaine-induced behavior of pharmacological inhibition of noradrenergic and serotonergic systems. Pharmacol. Biochem. Behav. 2008, 89, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Ragu Varman, D.; Mannangatti, P.; Subler, M.A.; Windle, J.J.; Ramamoorthy, S.; Jayanthi, L.D. Blunted Amphetamine-induced Reinforcing Behaviors and Transporter Downregulation in Knock-in Mice Carrying Alanine Mutations at Threonine-258 and Serine-259 of Norepinephrine Transporter. J. Mol. Neurosci. 2022, 72, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, C.A.; Calipari, E.S.; Ferris, M.J.; Jones, S.R. Biphasic mechanisms of amphetamine action at the dopamine terminal. J. Neurosci. 2014, 34, 5575–5582. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.L.; Zahniser, N.R. Rapid substrate-induced down-regulation in function and surface localization of dopamine transporters: Rat dorsal striatum versus nucleus accumbens. J. Neurochem. 2009, 108, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.X.; Ma, Q.; Spealman, R.D.; Yao, W.D. Amphetamine modulation of long-term potentiation in the prefrontal cortex: Dose dependency, monoaminergic contributions, and paradoxical rescue in hyperdopaminergic mutant. J. Neurochem. 2010, 115, 1643–1654. [Google Scholar] [CrossRef]
- Becker, J.B.; Hu, M. Sex differences in drug abuse. Front. Neuroendocrinol. 2008, 29, 36–47. [Google Scholar] [CrossRef]
- Becker, J.B.; Koob, G.F. Sex Differences in Animal Models: Focus on Addiction. Pharmacol. Rev. 2016, 68, 242–263. [Google Scholar] [CrossRef]
- Becker, J.B.; McClellan, M.L.; Reed, B.G. Sex differences, gender and addiction. J. Neurosci. Res. 2017, 95, 136–147. [Google Scholar] [CrossRef]
- Lynch, W.J.; Roth, M.E.; Carroll, M.E. Biological basis of sex differences in drug abuse: Preclinical and clinical studies. Psychopharmacology 2002, 164, 121–137. [Google Scholar] [CrossRef]
- Roth, M.E.; Cosgrove, K.P.; Carroll, M.E. Sex differences in the vulnerability to drug abuse: A review of preclinical studies. Neurosci. Biobehav. Rev. 2004, 28, 533–546. [Google Scholar] [CrossRef]
- Cornish, J.L.; Prasad, A.A. Sex Differences in Substance Use Disorders: A Neurobiological Perspective. Front. Glob. Women’s Health 2021, 2, 778514. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.J.; Carroll, M.E. Females are more vulnerable to drug abuse than males: Evidence from preclinical studies and the role of ovarian hormones. Curr. Top Behav. Neurosci. 2011, 8, 73–96. [Google Scholar] [CrossRef]
- Ragu Varman, D.; Subler, M.A.; Windle, J.J.; Jayanthi, L.D.; Ramamoorthy, S. Novelty-induced hyperactivity and suppressed cocaine induced locomotor activation in mice lacking threonine 53 phosphorylation of dopamine transporter. Behav. Brain Res. 2021, 408, 113267. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, R.V.; Ramamoorthy, S.; Jayanthi, L.D. Threonine-53 Phosphorylation of Dopamine Transporter Dictates kappa-Opioid Receptor-Mediated Locomotor Suppression, Aversion, and Cocaine Reward. J. Neurosci. 2025, 45, e0171252025. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayanthi, L.D.; Ramamoorthy, S. Role of Phosphorylation of Serotonin and Norepinephrine Transporters in Animal Behavior: Relevance to Neuropsychiatric Disorders. Int. J. Mol. Sci. 2025, 26, 7713. https://doi.org/10.3390/ijms26167713
Jayanthi LD, Ramamoorthy S. Role of Phosphorylation of Serotonin and Norepinephrine Transporters in Animal Behavior: Relevance to Neuropsychiatric Disorders. International Journal of Molecular Sciences. 2025; 26(16):7713. https://doi.org/10.3390/ijms26167713
Chicago/Turabian StyleJayanthi, Lankupalle D., and Sammanda Ramamoorthy. 2025. "Role of Phosphorylation of Serotonin and Norepinephrine Transporters in Animal Behavior: Relevance to Neuropsychiatric Disorders" International Journal of Molecular Sciences 26, no. 16: 7713. https://doi.org/10.3390/ijms26167713
APA StyleJayanthi, L. D., & Ramamoorthy, S. (2025). Role of Phosphorylation of Serotonin and Norepinephrine Transporters in Animal Behavior: Relevance to Neuropsychiatric Disorders. International Journal of Molecular Sciences, 26(16), 7713. https://doi.org/10.3390/ijms26167713




