Iron-Doped Hydroxyapatite Nanoparticles for Magnetic Guided siRNA Delivery
Abstract
1. Introduction
2. Results
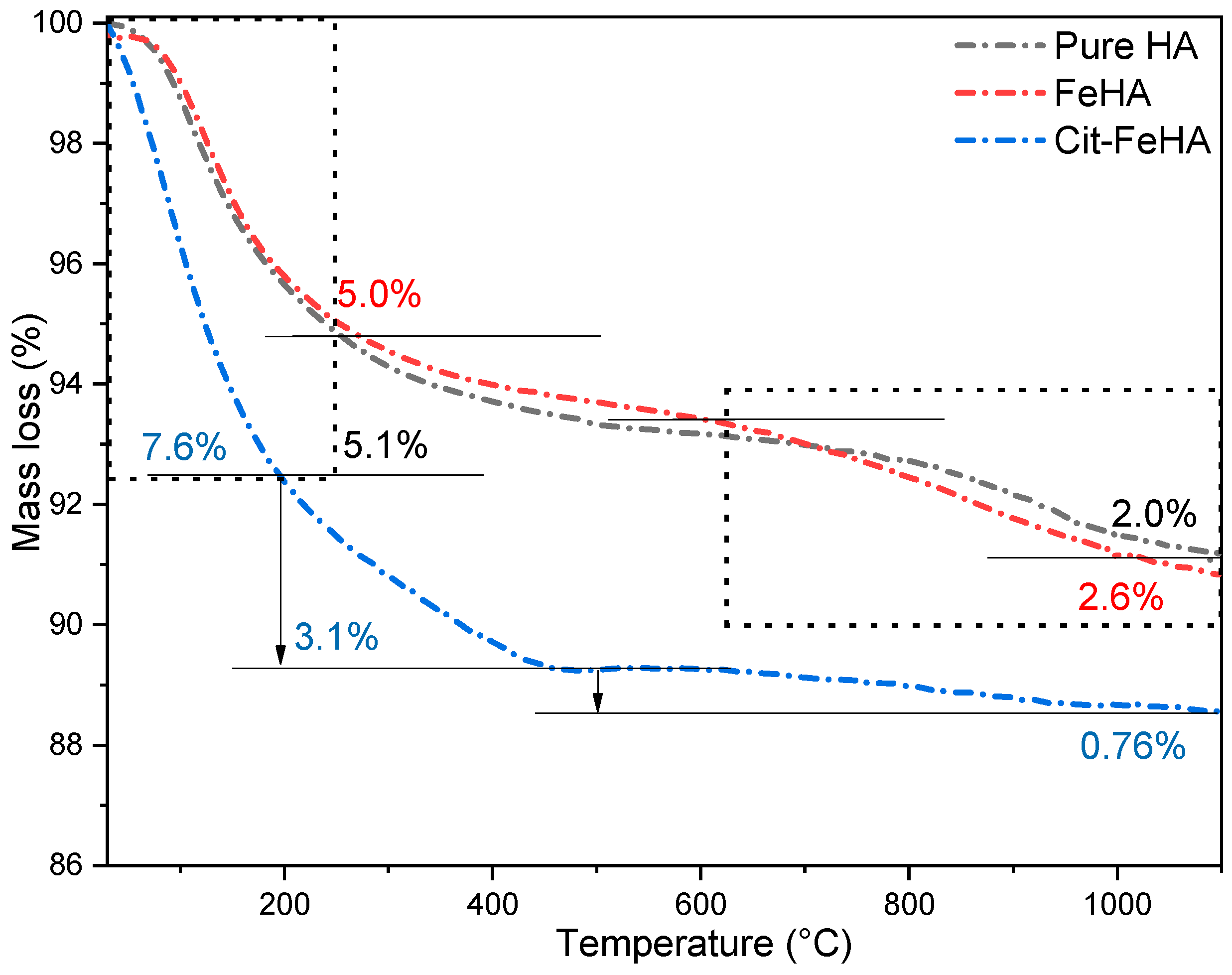
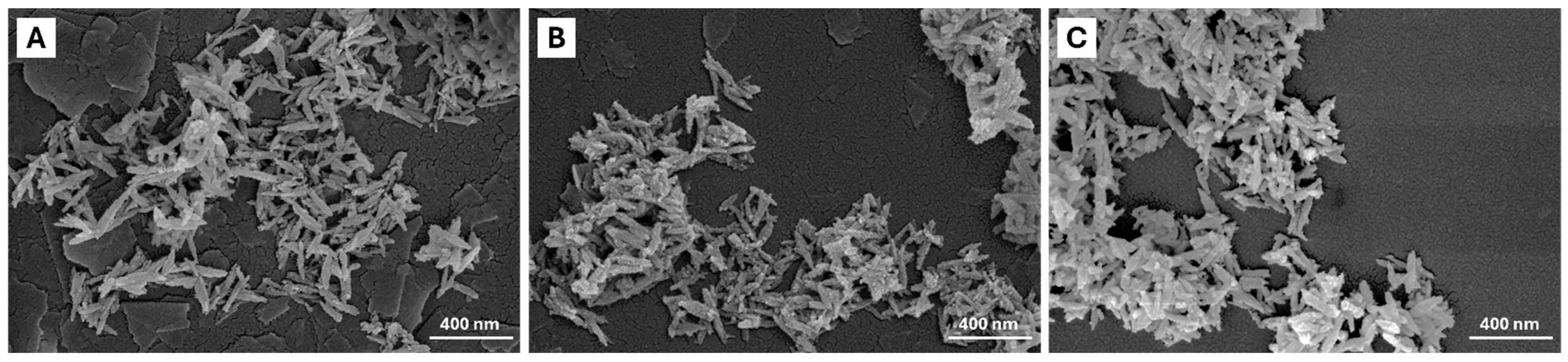
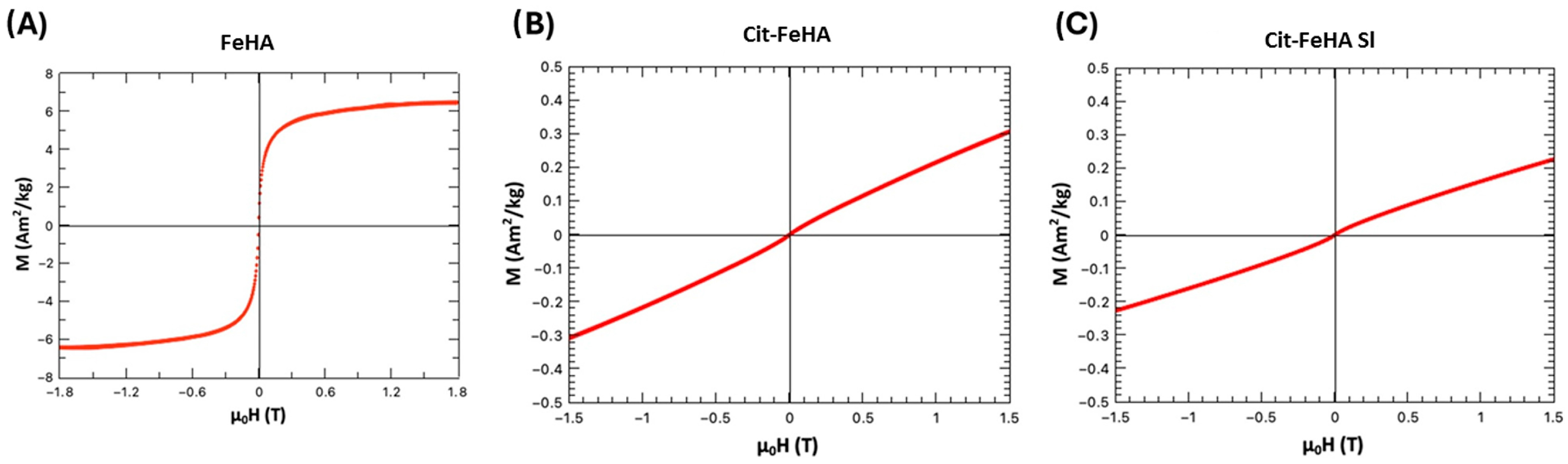
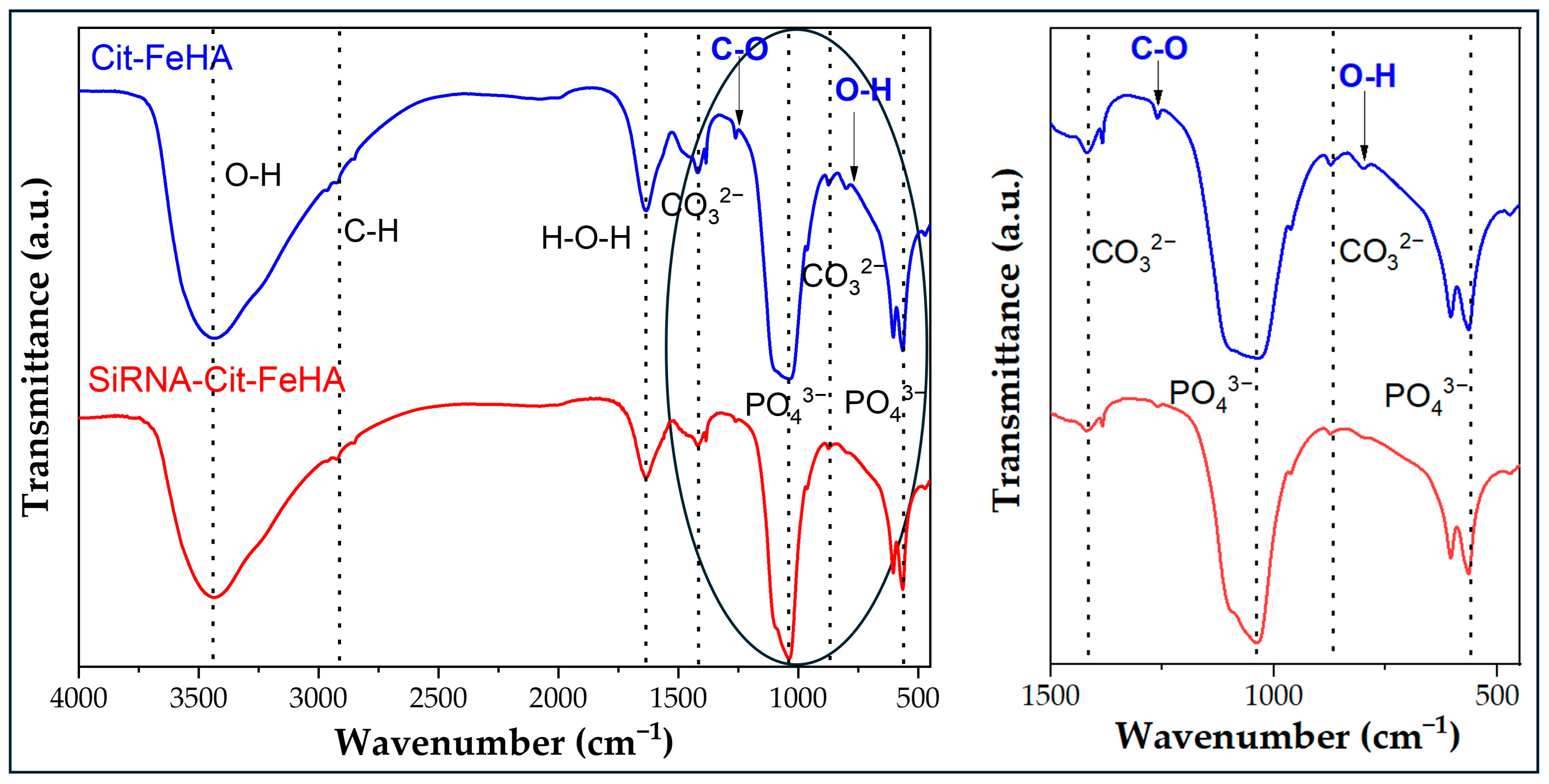
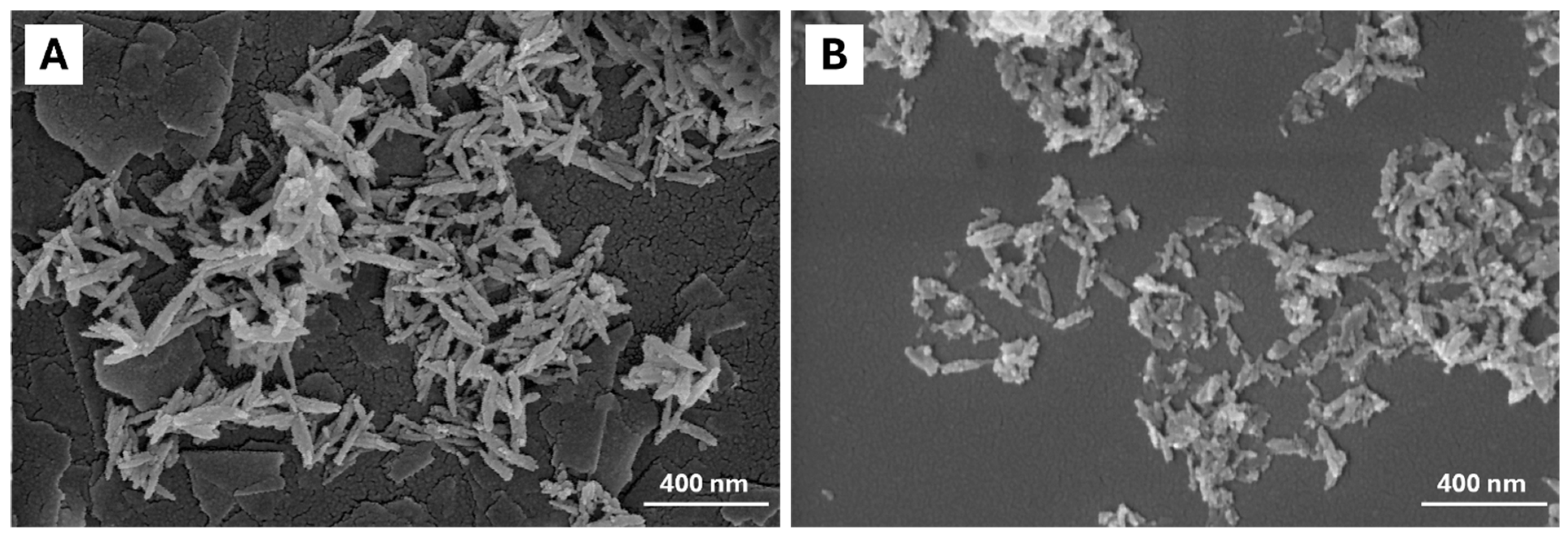
2.1. Characterization of Pure Hydroxyapatite, FeHA, and Citrate-FeHA Nanoparticles
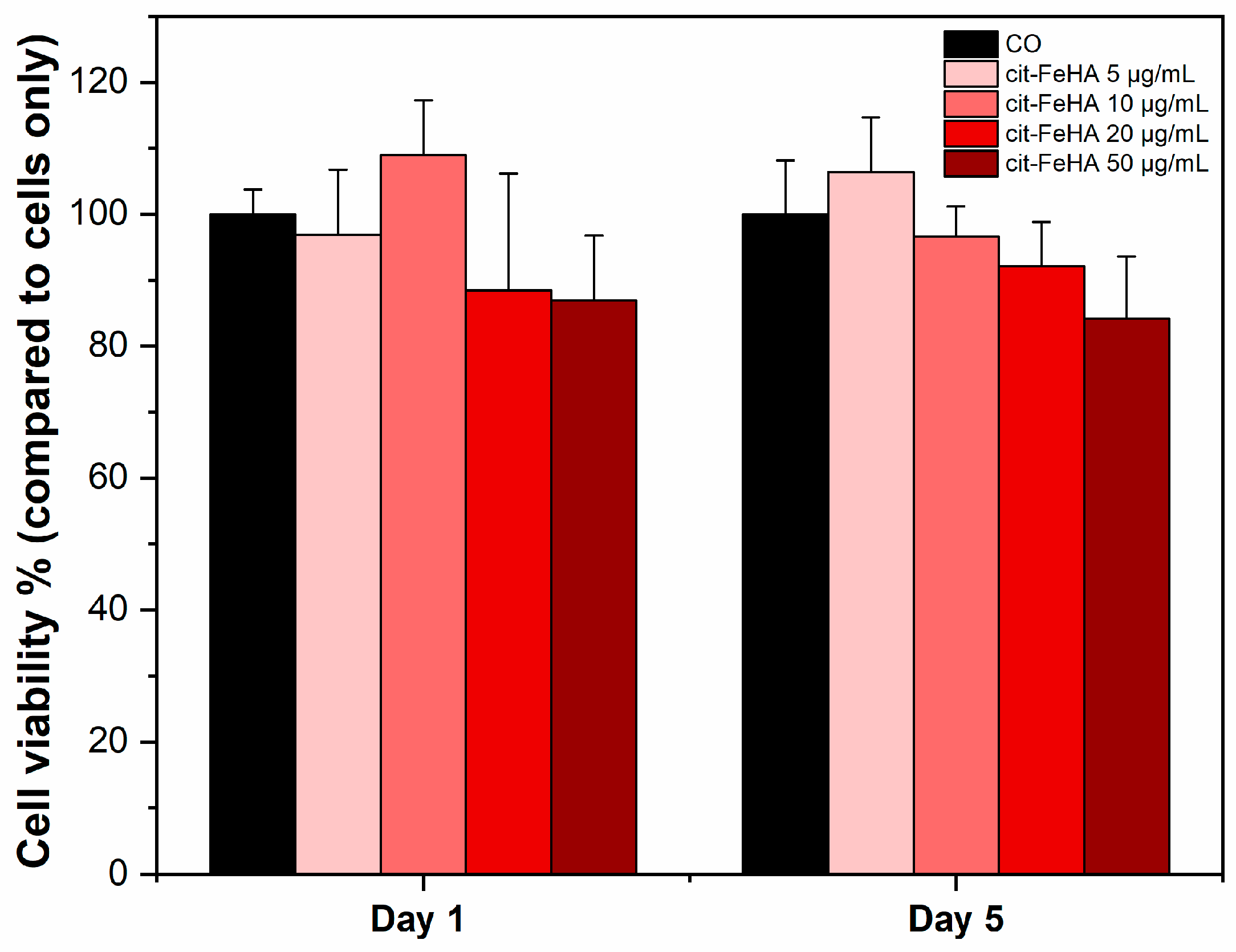
2.2. Cit-FeHA Nanoparticle In Vitro Cyocompatibility Assay
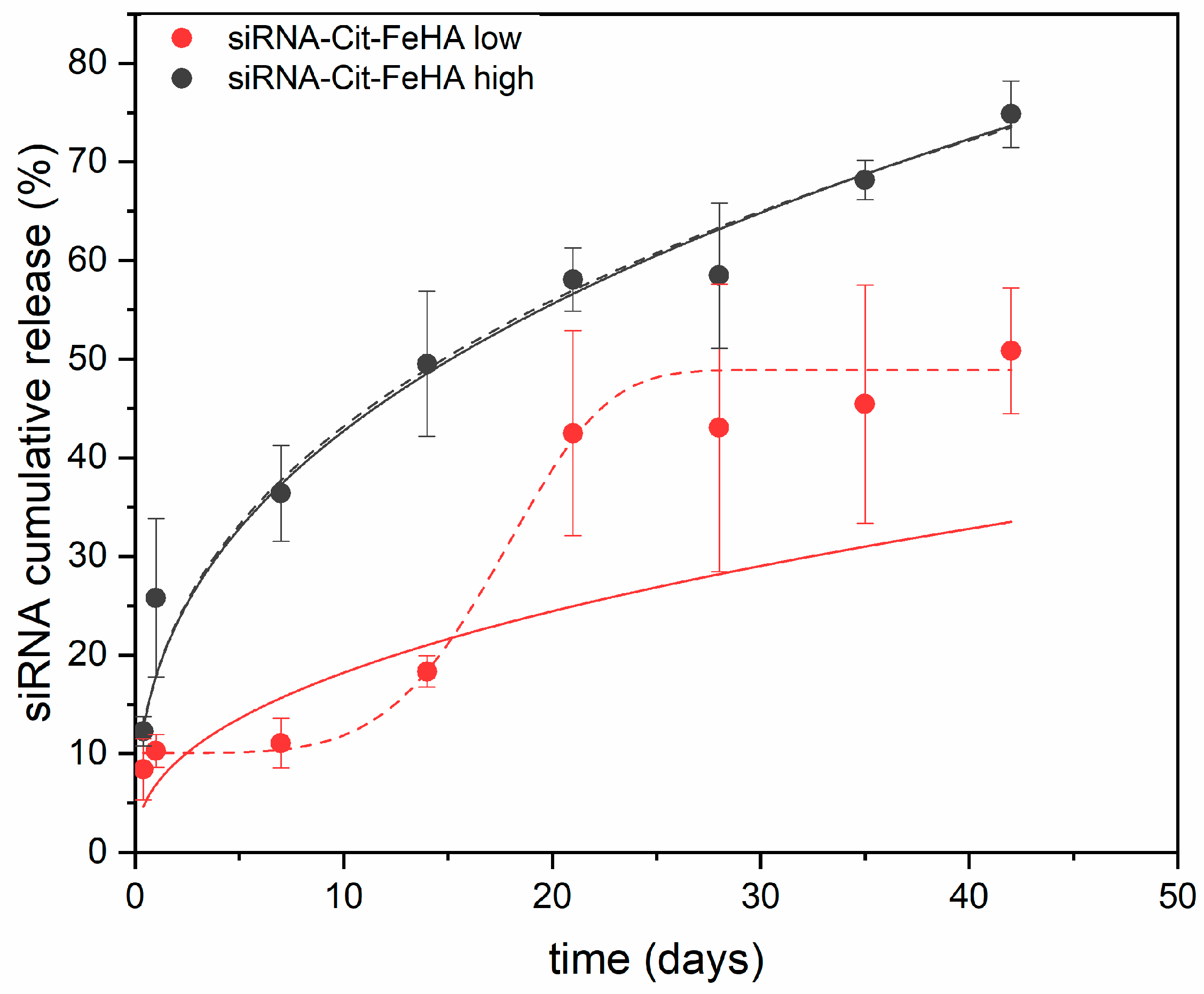
2.3. Characterization of siRNA-Loaded Cit-FeHA NPs and Release Properties
3. Materials and Methods
3.1. Materials
3.2. FeHA NPs Synthesis and Citrate Functionalization
3.3. FeHA and Cit-FeHA Characterization
3.4. In Vitro Cyocompatibility Assay
3.5. GAPDH siRNA Adsorption on Cit-FeHA
3.6. Characterization of siRNA-Cit-FeHA and siRNA Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef]
- Davidson, B.L.; McCray, P.B., Jr. Current prospects for RNA interference-based therapies. Nat. Rev. Genet. 2011, 12, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Sah, D.W. Therapeutic potential of RNA interference for neurological disorders. Life Sci. 2006, 79, 1773–1780. [Google Scholar] [CrossRef]
- Friedrich, M.; Aigner, A. Therapeutic siRNA: State-of-the-art and future perspectives. BioDrugs 2022, 36, 549–571. [Google Scholar] [CrossRef]
- Jebelli, A.; Baradaran, B.; Mosafer, J.; Baghbanzadeh, A.; Mokhtarzadeh, A.; Tayebi, L. Recent developments in targeting genes and pathways by RNAi-based approaches in colorectal cancer. Med. Res. Rev. 2021, 41, 395–434. [Google Scholar] [CrossRef]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef]
- Kesharwani, P.; Gajbhiye, V.; Jain, N.K. A review of nanocarriers for the delivery of small interfering RNA. Biomaterials 2012, 33, 7138–7150. [Google Scholar] [CrossRef]
- Chen, X.; Mangala, L.S.; Rodriguez-Aguayo, C.; Kong, X.; Lopez-Berestein, G.; Sood, A.K. RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. 2018, 37, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Duguet, E.; Vasseur, S.; Mornet, S.; Devoisselle, J.-M. Magnetic nanoparticles and their applications in medicine. Nanomedicine 2006, 1, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Pisanic II, T.R.; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Medarova, Z.; Pham, W.; Farrar, C.; Petkova, V.; Moore, A. In vivo imaging of siRNA delivery and silencing in tumors. Nat. Med. 2007, 13, 372–377. [Google Scholar] [CrossRef]
- Mykhaylyk, O.; Vlaskou, D.; Tresilwised, N.; Pithayanukul, P.; Möller, W.; Plank, C. Magnetic nanoparticle formulations for DNA and siRNA delivery. J. Magn. Magn. Mater. 2007, 311, 275–281. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Liu, T.; Zhang, D.S.Z.; Wang, Y.; Gu, H.; Di, W. Highly effective antiangiogenesis via magnetic mesoporous silica-based siRNA vehicle targeting the VEGF gene for orthotopic ovarian cancer therapy. Int. J. Nanomed. 2015, 10, 2579–2594. [Google Scholar]
- Veiseh, O.; Kievit, F.M.; Fang, C.; Mu, N.; Jana, S.; Leung, M.C.; Zhang, M. Chlorotoxin bound magnetic nanovector tailored for cancer cell targeting, imaging, and siRNA delivery. Biomaterials 2010, 31, 8032–8042. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Moore, A. In vivo magnetic resonance imaging of small interfering RNA nanodelivery to pancreatic islets. In RNA Imaging: Methods and Protocols; Springer: New York, NY, USA, 2016; pp. 25–36. [Google Scholar]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.-S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.-R.; Hsiao, C.-D. Potential toxicity of iron oxide magnetic nanoparticles: A review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef]
- Tampieri, A.; D’Alessandro, T.; Sandri, M.; Sprio, S.; Landi, E.; Bertinetti, L.; Panseri, S.; Pepponi, G.; Goettlicher, J.; Bañobre-López, M. Intrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatite. Acta Biomater. 2012, 8, 843–851. [Google Scholar] [CrossRef]
- Iannotti, V.; Adamiano, A.; Ausanio, G.; Lanotte, L.; Aquilanti, G.; Coey, J.M.D.; Lantieri, M.; Spina, G.; Fittipaldi, M.; Margaris, G. Fe-doping-induced magnetism in nano-hydroxyapatites. Inorg. Chem. 2017, 56, 4446–4458. [Google Scholar] [CrossRef]
- Iafisco, M.; Drouet, C.; Adamiano, A.; Pascaud, P.; Montesi, M.; Panseri, S.; Sarda, S.; Tampieri, A. Superparamagnetic iron-doped nanocrystalline apatite as a delivery system for doxorubicin. J. Mater. Chem. B 2016, 4, 57–70. [Google Scholar] [CrossRef]
- Adamiano, A.; Iafisco, M.; Sandri, M.; Basini, M.; Arosio, P.; Canu, T.; Sitia, G.; Esposito, A.; Iannotti, V.; Ausanio, G. On the use of superparamagnetic hydroxyapatite nanoparticles as an agent for magnetic and nuclear in vivo imaging. Acta Biomater. 2018, 73, 458–469. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Adamiano, A.; Siliqi, D.; Giannini, C.; Iafisco, M. The effect of chemical structure of carboxylate molecules on hydroxyapatite nanoparticles. A structural and morphological study. Bioact. Mater. 2021, 6, 2360–2371. [Google Scholar] [CrossRef] [PubMed]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef]
- Ren, F.Z.; Leng, Y. Carbonated Apatite, Type-A or Type-B? Key Eng. Mater. 2011, 493–494, 293–297. [Google Scholar] [CrossRef]
- Foley, B.; Guibert, C.; Selmane, M.; Mezzetti, A.; Lefebvre, C.; El Kirat, K.; Landoulsi, J. Tunable Enzyme-Assisted Mineralization of Apatitic Calcium Phosphate by Homogeneous Catalysis. Int. J. Mol. Sci. 2022, 24, 43. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Adamiano, A.; Tampieri, A.; Ramirez-Rodriguez, G.B.; Siliqi, D.; Giannini, C.; Ivanchenko, P.; Martra, G.; Lin, F.-H.; Delgado-López, J.M. Combined effect of citrate and fluoride ions on hydroxyapatite nanoparticles. Cryst. Growth Des. 2020, 20, 3163–3172. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Markovic, S.; Ignjatovic, N.; Panseri, S.; Montesi, M.; Adamiano, A.; Fosca, M.; Rau, J.V.; Uskoković, V.; Iafisco, M. Thermal crystallization of amorphous calcium phosphate combined with citrate and fluoride doping: A novel route to produce hydroxyapatite bioceramics. J. Mater. Chem. B 2021, 9, 4832–4845. [Google Scholar] [CrossRef]
- Bettinsoli, V.; Melzi, G.; Crea, A.; Degli Esposti, L.; Iafisco, M.; Catalucci, D.; Corsini, E. A novel approach for in vitro testing and hazard evaluation of nanoformulated RyR2-targeting siRNA drugs using human PBMCs. Life 2025, 15, 95. [Google Scholar] [CrossRef]
- Paarakh, M.P.; Jose, P.A.; Setty, C.; Peterchristoper, G. Release kinetics–concepts and applications. Int. J. Pharm. Res. Technol. 2018, 8, 12–20. [Google Scholar]
- Luo, C.; Wu, S.; Li, J.; Li, X.; Yang, P.; Li, G. Chitosan/calcium phosphate flower-like microparticles as carriers for drug delivery platform. Int. J. Biol. Macromol. 2020, 155, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Prokopowicz, M.; Szewczyk, A.; Skwira, A.; Sądej, R.; Walker, G. Biphasic composite of calcium phosphate-based mesoporous silica as a novel bone drug delivery system. Drug Deliv. Transl. Res. 2020, 10, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Heredia, N.S.; Vizuete, K.; Flores-Calero, M.; Pazmiño, V.K.; Pilaquinga, F.; Kumar, B.; Debut, A. Comparative statistical analysis of the release kinetics models for nanoprecipitated drug delivery systems based on poly (lactic-co-glycolic acid). PLoS ONE 2022, 17, e0264825. [Google Scholar] [CrossRef]
- Panseri, S.; Montesi, M.; Sandri, M.; Iafisco, M.; Adamiano, A.; Ghetti, M.; Cenacchi, G.; Tampieri, A. Magnetic Labelling of Mesenchymal Stem Cells with Iron-Doped Hydroxyapatite Nanoparticles as Tool for Cell Therapy. J. Biomed. Nanotechnol. 2016, 12, 909–921. [Google Scholar] [CrossRef] [PubMed]
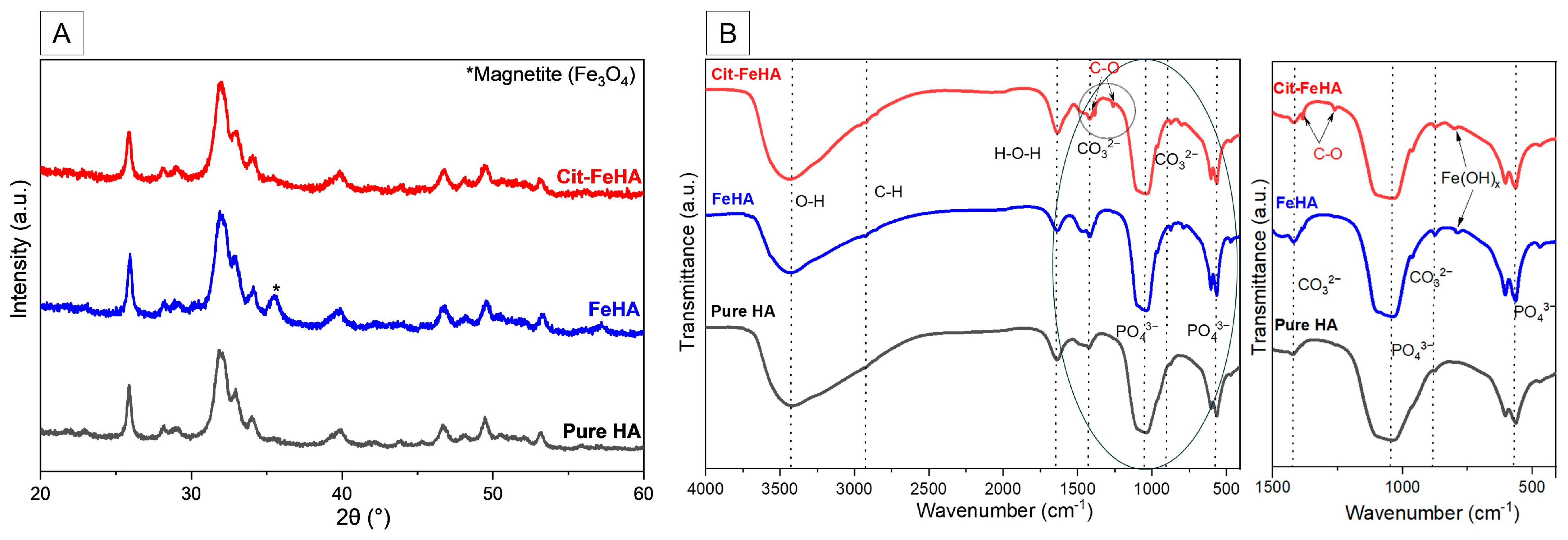
| Sample | Ca (wt.%) a | P (wt.%) a | Fetot (wt.%) a | Fe2+ in HA (wt.%) b | Fe3+/Fe2+ in HA (wt.%) b | Ca/P (mol) a | (Ca+FeHA)/P (mol) a | SSABET (m2/g) |
|---|---|---|---|---|---|---|---|---|
| HA | 35 ± 1 | 15.6 ± 0.2 | - | - | - | 1.62 ± 0.01 | 1.62 ± 0.01 | 97 ± 10 |
| FeHA | 44 ± 1 | 22.9 ± 2.5 | 13.0 ± 1.8 | 0.74 ± 0.01 | 10.6 | 1.47 ± 0.01 | 1.69 ± 0.02 | 102 ± 10 |
| Cit-FeHA | 30 ± 1 | 14.9 ± 0.6 | 7.0 ± 0.3 | 0.71 ± 0.02 | 8.8 | 1.53 ± 0.03 | 1.66 ± 0.05 | 204 ± 20 |
| Sample | Water Loss (20–250 °C) (wt.%) | Citrate Loss (250–500 °C) (wt.%) | Carbonate Loss (600–1100 °C) (wt.%) | Total Loss (20–1100 °C) (wt.%) |
|---|---|---|---|---|
| HA | 5.1 ± 0.5 | N/A | 2.0 ± 0.3 | 7.1 ± 0.7 |
| FeHA | 5.0 ± 0.5 | N/A | 2.6 ± 0.3 | 7.5 ± 0.8 |
| Cit-FeHA | 7.6 ± 0.8 | 3.1 ± 0.3 | 0.8 ± 0.1 | 11.5 ± 1.0 |
| Sample | Length (nm) | Width (nm) | Aspect Ratio | Z-Average (nm) | PdI | ζ-Potential (mV) |
|---|---|---|---|---|---|---|
| HA | 177 ± 5 | 37 ± 1 | 4.6 ± 0.2 | 220 ± 3 | 0.20 ± 0.01 | −18 ± 2 |
| FeHA | 186 ± 3 | 40 ± 1 | 4.8 ± 0.1 | 195 ± 1 | 0.20 ± 0.03 | −24 ± 1 |
| Cit-FeHA | 180 ± 2 | 43 ± 1 | 4.0 ± 0.1 | 170 ± 2 | 0.12 ± 0.01 | −35 ± 2 |
| Sample | siRNA Payload (nmol/mg) | siRNA Adsorption Efficiency (%) | Z-Average (nm) | PdI | ζ-Potential (mV) |
|---|---|---|---|---|---|
| Cit-FeHA-Sl | 0.59 ± 0.02 | 87.0 ± 0.5 | 188 ± 1 | 0.20 ± 0.01 | −33 ± 3 |
| Cit-FeHA-Sh | 0.18 ± 0.01 | 82.0 ± 0.2 | 179 ± 3 | 0.25 ± 0.05 | −34 ± 5 |
| Sample | KKP (Release Constant) | n (Release Exponent) | R2 (COD) | KW (Release Constant) | β (Release Exponent) | R2 (COD) |
|---|---|---|---|---|---|---|
| Cit-FeHA Sl | 0.07 ± 0.03 | 0.4 ± 0.1 | 0.6373 | 0.053 ± 0.003 | 4.8 ± 0.9 | 0.9895 |
| Cit-FeHA Sh | 0.18 ± 0.01 | 0.38 ± 0.01 | 0.9973 | 0.002 ± 0.001 | 0.39 ± 0.07 | 0.9973 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inam, H.; Degli Esposti, L.; Pupilli, F.; Tavoni, M.; Casoli, F.; Sprio, S.; Tampieri, A. Iron-Doped Hydroxyapatite Nanoparticles for Magnetic Guided siRNA Delivery. Int. J. Mol. Sci. 2025, 26, 7712. https://doi.org/10.3390/ijms26167712
Inam H, Degli Esposti L, Pupilli F, Tavoni M, Casoli F, Sprio S, Tampieri A. Iron-Doped Hydroxyapatite Nanoparticles for Magnetic Guided siRNA Delivery. International Journal of Molecular Sciences. 2025; 26(16):7712. https://doi.org/10.3390/ijms26167712
Chicago/Turabian StyleInam, Hina, Lorenzo Degli Esposti, Federico Pupilli, Marta Tavoni, Francesca Casoli, Simone Sprio, and Anna Tampieri. 2025. "Iron-Doped Hydroxyapatite Nanoparticles for Magnetic Guided siRNA Delivery" International Journal of Molecular Sciences 26, no. 16: 7712. https://doi.org/10.3390/ijms26167712
APA StyleInam, H., Degli Esposti, L., Pupilli, F., Tavoni, M., Casoli, F., Sprio, S., & Tampieri, A. (2025). Iron-Doped Hydroxyapatite Nanoparticles for Magnetic Guided siRNA Delivery. International Journal of Molecular Sciences, 26(16), 7712. https://doi.org/10.3390/ijms26167712






