Transcriptomic Signatures and Molecular Pathways in Hidradenitis Suppurativa—A Narrative Review
Abstract
1. Introduction
2. Results
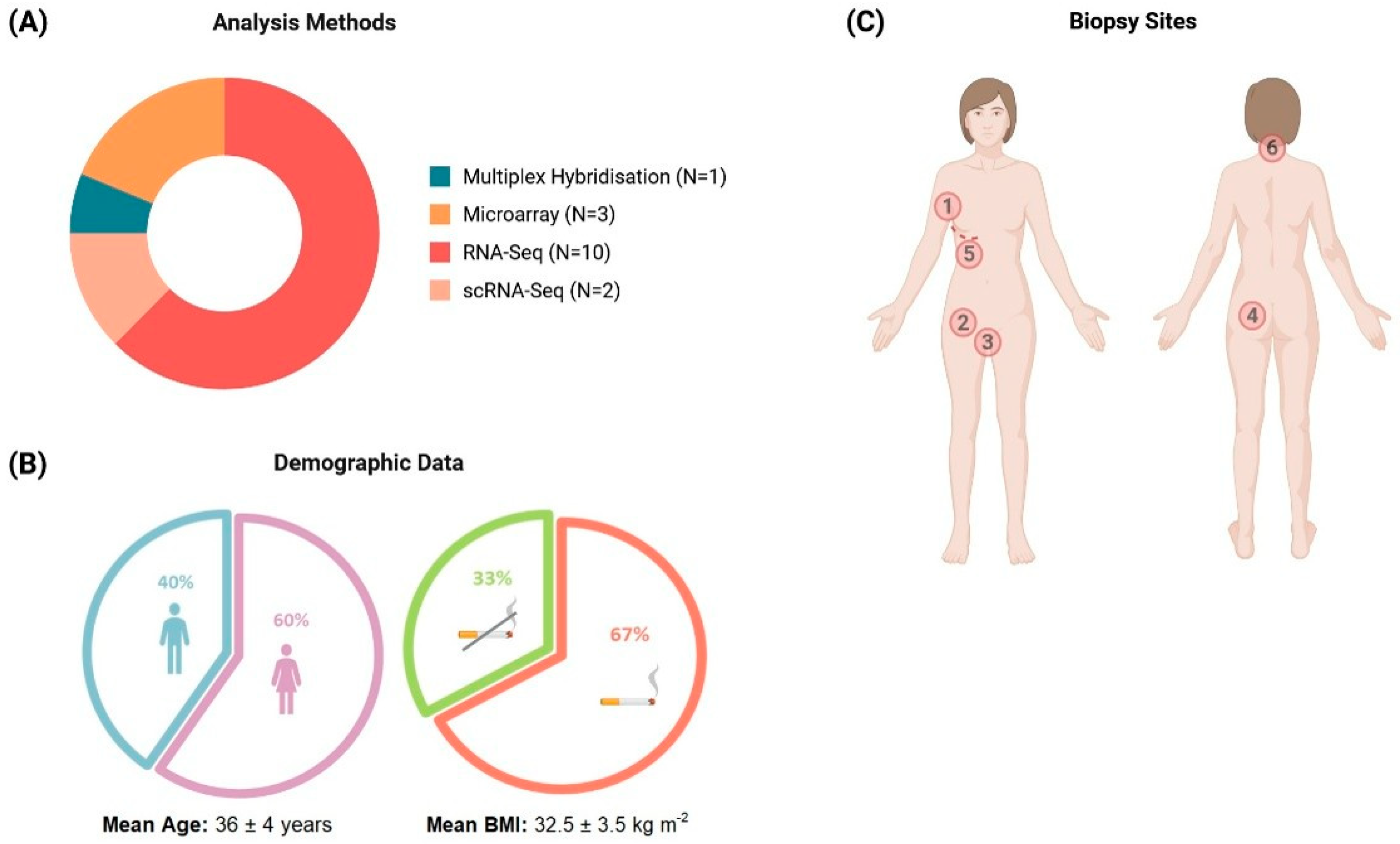
2.1. Study Characteristics and Data Extraction
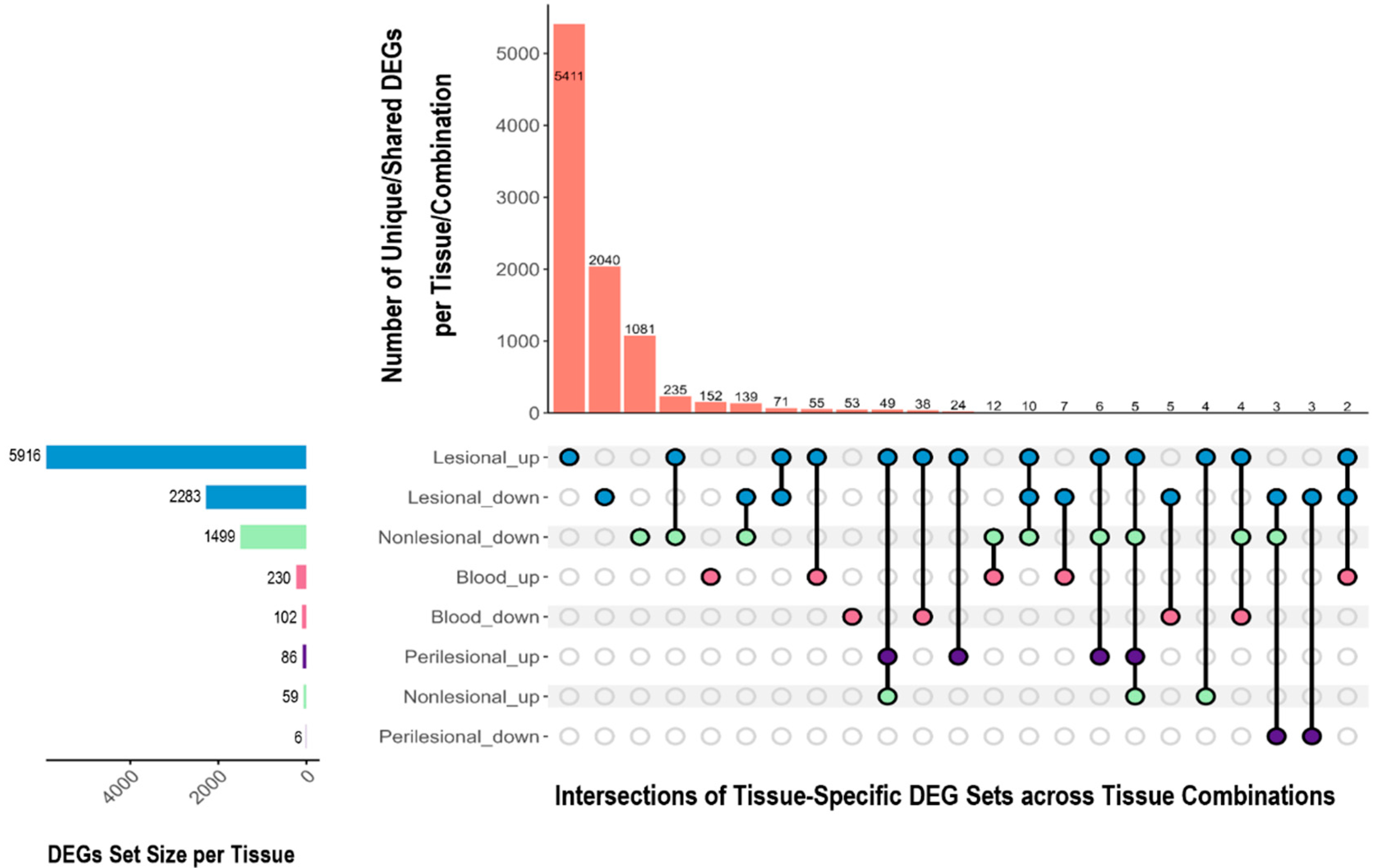
2.2. Differentially Expressed Genes Across Tissues
2.2.1. Overlapping and Unique DEGs Across Tissues
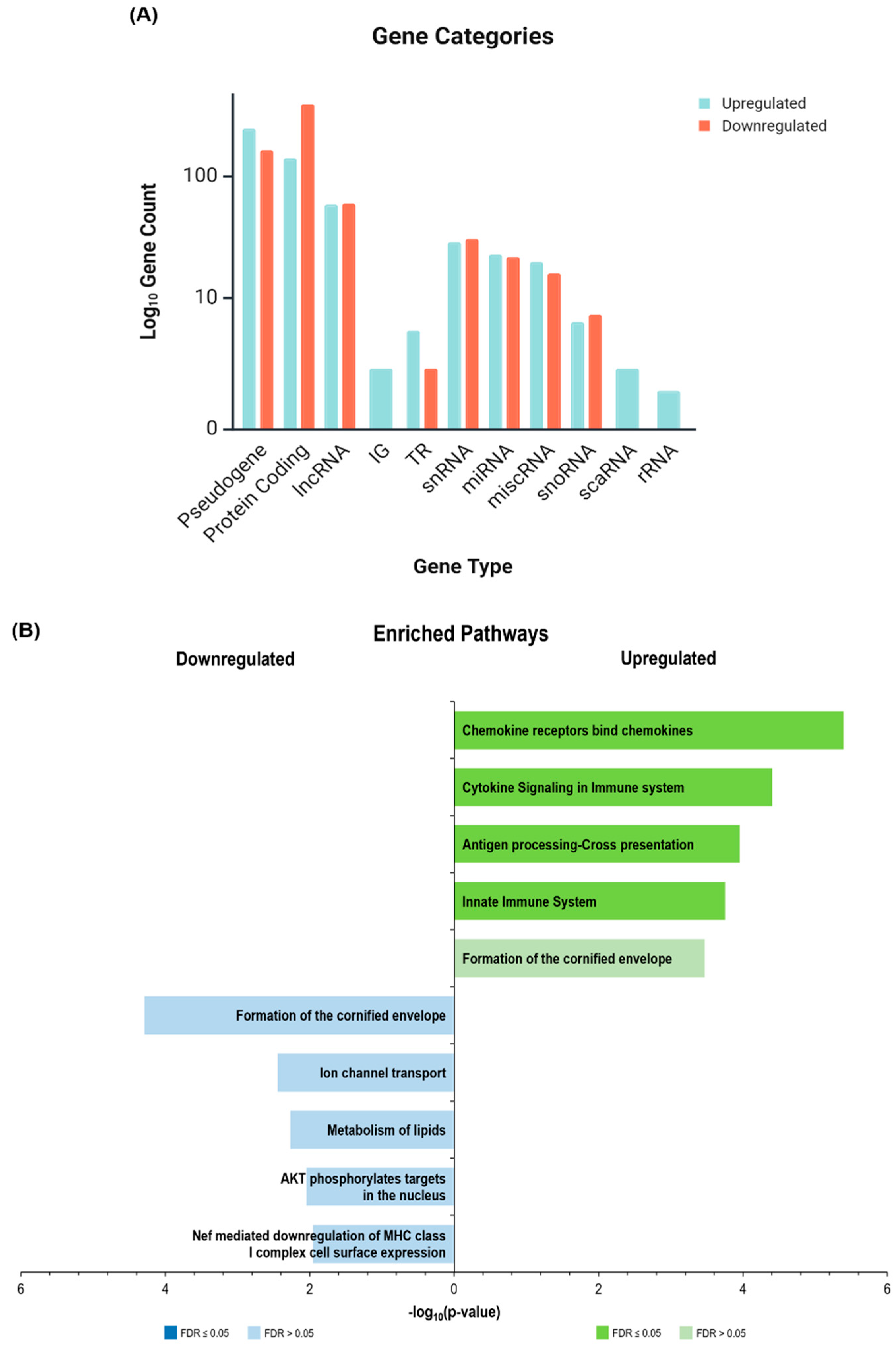
2.2.2. Gene Categories and Immune Signatures
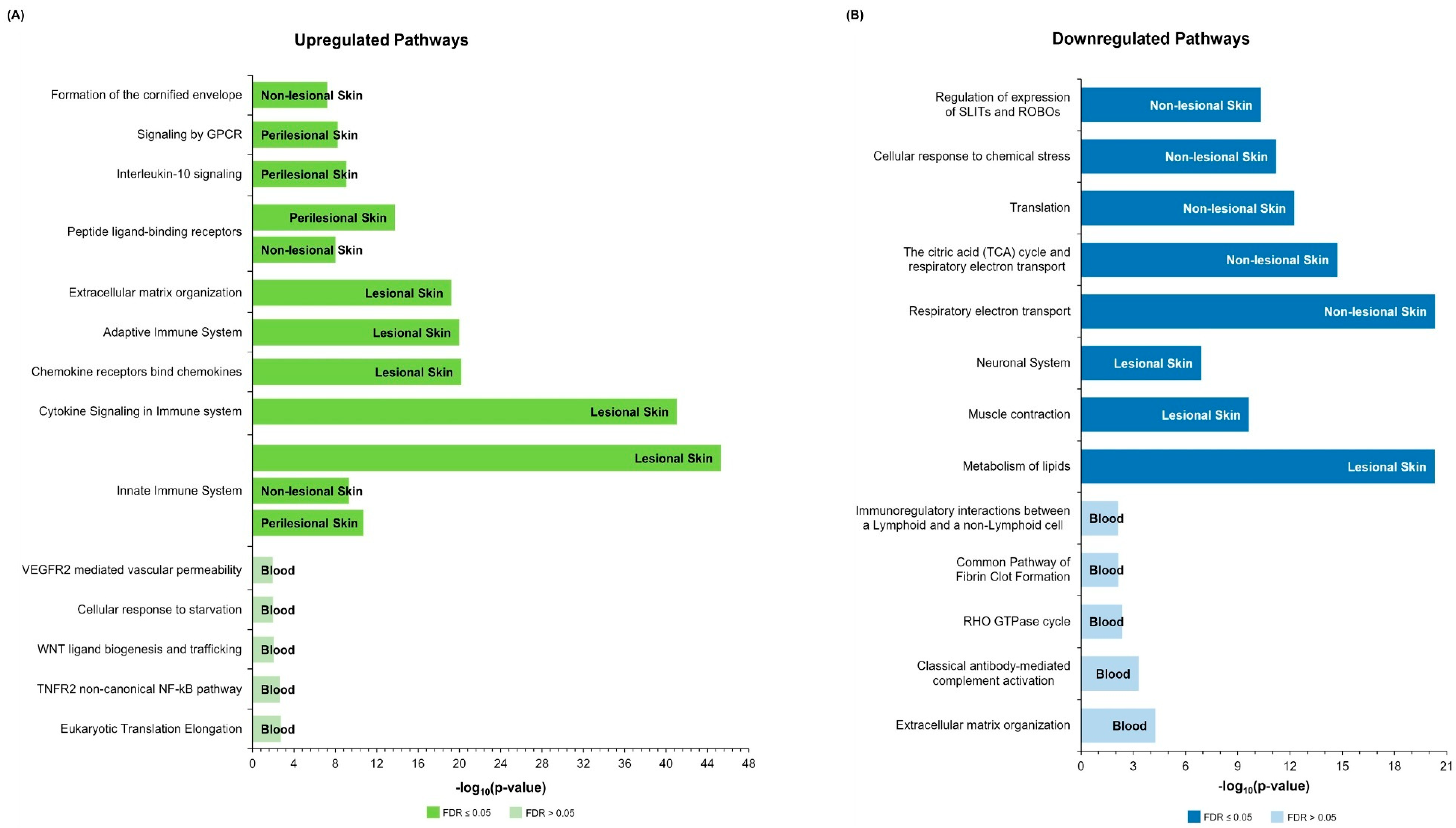
2.3. Over-Representation Analysis
2.3.1. Upregulated Pathways
2.3.2. Downregulated Pathways
2.4. Insights from Single-Cell Transcriptomics
3. Discussion
3.1. Immune Dysregulation as a Central Feature
3.2. Epidermal Barrier Dysfunction
3.2.1. Keratinocyte Involvement in Barrier Dysregulation
3.2.2. Microbial Dysbiosis: A Contributor to Chronic Inflammation in HS
3.3. Comorbidities and Systemic Involvement
3.3.1. Metabolic Alterations and Associated Comorbid Disorders
3.3.2. HS Blood Transcriptome
3.4. Comparison with Other Inflammatory Dermatoses and Implications for HS Treatment
3.5. Methodological Considerations
4. Materials and Methods
4.1. Study Selection and Inclusion Criteria
4.2. Exclusion Criteria
4.3. Data Extraction
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HS | Hidradenitis suppurativa |
| qPCR | Quantitative polymerase chain reaction |
| RNA-seq | RNA sequencing |
| scRNA-seq | Single-cell RNA sequencing |
| DEG/s | Differentially expressed gene/s |
| IG | Immunoglobulin |
| TR | T-cell receptor |
| lncRNA | Long non-coding RNA |
| miRNA | Micro RNA |
| snoRNA | Small nucleolar RNA |
| snRNA | Small nuclear RNA |
| miscRNA | Miscellaneous RNA |
| scaRNA | Small Cajal body-specific RNA |
| rRNA | Ribosomal RNA |
| IL | Interleukin |
| Th | T helper cell |
| FDR | False discovery rate |
| TNF/TNFR | Tumor necrosis factor/Tumor necrosis factor receptor |
| IFN | Interferon |
| NK | Natural killer cell |
| GWAS | Genome wide association study |
| MetS | Metabolic syndrome |
| AD | Atopic dermatitis |
| FC | Fold change |
| BMI | Body mass index |
| AHR | Aryl hydrocarbon receptor |
References
- Zouboulis, C.C.; Bechara, F.G.; Fritz, K.; Goebeler, M.; Hetzer, F.H.; Just, E.; Kirsten, N.; Kokolakis, G.; Kurzen, H.; Nikolakis, G.; et al. S2k guideline for the treatment of hidradenitis suppurativa/acne inversa—Short version. J. Dtsch. Dermatol. Ges. 2024, 22, 868–889. [Google Scholar] [CrossRef]
- Kirby, J.; Kim, K.; Zivkovic, M.; Wang, S.; Garg, V.; Danavar, A.; Li, C.; Chen, N.; Garg, A. Uncovering the burden of hidradenitis suppurativa misdiagnosis and underdiagnosis: A machine learning approach. Front. Med. Technol. 2024, 6, 1200400. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, K.; Emtestam, L.; Jemec, G.B.E.; Lapins, J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br. J. Dermatol. 2009, 161, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Nogueira da Costa, A.; Makrantonaki, E.; Hou, X.X.; Almansouri, D.; Dudley, J.T.; Edwards, H.; Readhead, B.; Balthasar, O.; Jemec, G.B.E.; et al. Alterations in innate immunity and epithelial cell differentiation are the molecular pillars of hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 846–861. [Google Scholar] [CrossRef]
- Bouazzi, D.; Andersen, R.K.; Vinding, G.R.; Medianfar, C.E.; Nielsen, S.M.; Saunte, D.M.L.; Chandran, N.S.; van der Zee, H.H.; Zouboulis, C.C.; Benhadou, F.; et al. The Global Hidradenitis Suppurativa Atlas Methodology: Combining Global Proportions in a Pooled Analysis. Dermatology 2024, 240, 369–375. [Google Scholar] [CrossRef]
- Jfri, A.; Nassim, D.; O’Brien, E.; Gulliver, W.; Nikolakis, G.; Zouboulis, C.C. Prevalence of Hidradenitis Suppurativa: A Systematic Review and Meta-regression Analysis. JAMA Dermatol. 2021, 157, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Goyal, M.; Byrd, A.S. Hidradenitis suppurativa in skin of colour. Exp. Dermatol. 2021, 30, 27–30. [Google Scholar] [CrossRef]
- Garg, A.; Geissbühler, Y.; Houchen, E.; Choudhary, N.; Arora, D.; Vellanki, V.; Srivastava, A.; Priyanka; Darcy, J.; Richardson, C.; et al. Disease Burden and Treatment Patterns Among US Patients with Hidradenitis Suppurativa: A Retrospective Cohort Study. Am. J. Clin. Dermatol. 2023, 24, 977–990. [Google Scholar] [CrossRef]
- Garg, A.; Lavian, J.; Lin, G.; Strunk, A.; Alloo, A. Incidence of hidradenitis suppurativa in the United States: A sex- and age-adjusted population analysis. J. Am. Acad. Dermatol. 2017, 77, 118–122. [Google Scholar] [CrossRef]
- Sachdeva, M.; Shah, M.; Alavi, A. Race-Specific Prevalence of Hidradenitis Suppurativa. J. Cutan. Med. Surg. 2021, 25, 177–187. [Google Scholar] [CrossRef]
- Byrd, A.S.; Rosenberg, A.Z.; Shipman, W.D.; Okoh, U.J.; Mazhar, M.; Okoye, G.A.; Bragazzi, N.L.; Mortellaro, C.; Jemec, G.B.E.; Damiani, G. Hidradenitis suppurativa in Black and White patients—A clinical study. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Clark, A.K.; Shi, V.Y. Hidradenitis Suppurativa: Disease Burden and Etiology in Skin of Color. Dermatology 2018, 233, 456–461. [Google Scholar] [CrossRef]
- Krueger, J.G.; Frew, J.; Jemec, G.B.E.; Kimball, A.B.; Kirby, B.; Bechara, F.G.; Navrazhina, K.; Prens, E.; Reich, K.; Cullen, E.; et al. Hidradenitis suppurativa: New insights into disease mechanisms and an evolving treatment landscape. Br. J. Dermatol. 2024, 190, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Frew, J.W.; Vekic, D.A.; Woods, J.; Cains, G.D. Phenotypic heterogeneity implies heterogeneous pathogenic pathways in hidradenitis suppurativa. Exp. Dermatol. 2015, 24, 338–339. [Google Scholar] [CrossRef] [PubMed]
- Kozera, E.K.; Frew, J.W. The pathogenesis of hidradenitis suppurativa: Evolving paradigms in a complex disease. Dermatol. Rev. 2022, 3, 39–49. [Google Scholar] [CrossRef]
- Szukala, W.; Lichawska-Cieslar, A.; Krajewski, P.K.; Kulecka, M.; Rumienczyk, I.; Mikula, M.; Matusiak, Ł.; Jura, J.; Szepietowski, J.C. An Atlas of the Hidradenitis Suppurativa Transcriptome. Dermatol. Ther. 2024, 14, 409–420. [Google Scholar] [CrossRef]
- Blok, J.L.; Li, K.; Brodmerkel, C.; Jonkman, M.F.; Horváth, B. Gene expression profiling of skin and blood in hidradenitis suppurativa. Br. J. Dermatol. 2016, 174, 1392–1394. [Google Scholar] [CrossRef]
- Pink, A.E.; Simpson, M.A.; Desai, N.; Dafou, D.; Hills, A.; Mortimer, P.; Smith, C.H.; Trembath, R.C.; Barker, J.N.W. Mutations in the γ-Secretase Genes NCSTN, PSENEN, and PSEN1 Underlie Rare Forms of Hidradenitis Suppurativa (Acne Inversa). J. Investig. Dermatol. 2012, 132, 2459–2461. [Google Scholar] [CrossRef]
- Takeichi, T.; Matsumoto, T.; Nomura, T.; Takeda, M.; Niwa, H.; Kono, M.; Shimizu, H.; Ogi, T.; Akiyama, M. A novel NCSTN missense mutation in the signal peptide domain causes hidradenitis suppurativa, which has features characteristic of an autoinflammatory keratinization disease. Br. J. Dermatol. 2020, 182, 491–493. [Google Scholar] [CrossRef]
- de Oliveira, A.S.L.E.; de Siqueira, R.C.; Nait-Meddour, C.; Tricarico, P.M.; Moura, R.; Agrelli, A.; d’Adamo, A.P.; Jamain, S.; Crovella, S.; de Fátima Medeiros Brito, M.; et al. A loss-of-function NCSTN mutation associated with familial Dowling Degos disease and hidradenitis suppurativa. Exp. Dermatol. 2023, 32, 1935–1945. [Google Scholar] [CrossRef]
- Freudenberg, J.M.; Liu, Z.; Singh, J.; Thomas, E.; Traini, C.; Rajpal, D.K.; Sayed, C.J. A Hidradenitis Suppurativa molecular disease signature derived from patient samples by high-throughput RNA sequencing and re-analysis of previously reported transcriptomic data sets. PLoS ONE 2023, 18, e0284047. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, H.; Bregnhøj, A.; Iversen, L.; Johansen, C. Transcriptomic Analysis of Hidradenitis Suppurativa: A Unique Molecular Signature with Broad Immune Activation. Int. J. Mol. Sci. 2023, 24, 17014. [Google Scholar] [CrossRef]
- Navrazhina, K.; Garcet, S.; Frew, J.W.; Zheng, X.; Coats, I.; Guttman-Yassky, E.; Krueger, J.G. The inflammatory proteome of hidradenitis suppurativa skin is more expansive than that of psoriasis vulgaris. J. Am. Acad. Dermatol. 2022, 86, 322–330. [Google Scholar] [CrossRef]
- Navrazhina, K.; Frew, J.W.; Gilleaudeau, P.; Sullivan-Whalen, M.; Garcet, S.; Krueger, J.G. Epithelialized Tunnels are a Source of Inflammation in Hidradenitis Suppurativa. J. Allergy Clin. Immunol. 2021, 147, 2213–2224. [Google Scholar] [CrossRef]
- Mariottoni, P.; Jiang, S.W.; Prestwood, C.A.; Jain, V.; Suwanpradid, J.; Whitley, M.J.; Coates, M.; Brown, D.A.; Erdmann, D.; Corcoran, D.L.; et al. Single-Cell RNA Sequencing Reveals Cellular and Transcriptional Changes Associated With M1 Macrophage Polarization in Hidradenitis Suppurativa. Front. Med. 2021, 8, 665873. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Li, X.; Lee, H.S.; Kim, K.; Chaparala, V.; Murphy, W.; Zhou, W.; Cao, J.; Lowes, M.A.; et al. Single-cell transcriptomics suggest distinct upstream drivers of IL-17A/F in hidradenitis versus psoriasis. J. Allergy Clin. Immunol. 2023, 152, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Nebo, I.D.; Frew, J.W.; Gudjonsson, J.E.; Petukhova, L. Tissue comparability and bias in hidradenitis suppurativa transcriptomic studies. Proc. Natl. Acad. Sci. USA 2024, 121, e2404503121. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsson, J.E.; Tsoi, L.C.; Ma, F.; Billi, A.C.; van Straalen, K.R.; Vossen, A.R.J.V.; van der Zee, H.H.; Harms, P.W.; Wasikowski, R.; Yee, C.M.; et al. Contribution of plasma cells and B cells to hidradenitis suppurativa pathogenesis. JCI Insight 2020, 5, e139930. [Google Scholar] [CrossRef]
- Shanmugam, V.K.; Jones, D.; McNish, S.; Bendall, M.L.; Crandall, K.A. Transcriptome patterns in hidradenitis suppurativa: Support for the role of antimicrobial peptides and interferon pathways in disease pathogenesis. Clin. Exp. Dermatol. 2019, 44, 882–892. [Google Scholar] [CrossRef]
- Rumberger, B.E.; Boarder, E.L.; Owens, S.L.; Howell, M.D. Transcriptomic analysis of hidradenitis suppurativa skin suggests roles for multiple inflammatory pathways in disease pathogenesis. Inflamm. Res. 2020, 69, 967–973. [Google Scholar] [CrossRef]
- Lowe, M.M.; Naik, H.B.; Clancy, S.; Pauli, M.; Smith, K.M.; Bi, Y.; Dunstan, R.; Gudjonsson, J.E.; Paul, M.; Harris, H.; et al. Immunopathogenesis of hidradenitis suppurativa and response to anti–TNF-α therapy. JCI Insight 2020, 5, e139932. [Google Scholar] [CrossRef]
- Wolk, K.; Brembach, T.-C.; Šimaitė, D.; Bartnik, E.; Cucinotta, S.; Pokrywka, A.; Irmer, M.L.; Triebus, J.; Witte-Händel, E.; Salinas, G.; et al. Activity and components of the granulocyte colony-stimulating factor pathway in hidradenitis suppurativa. Br. J. Dermatol. 2021, 185, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Navrazhina, K.; Garcet, S.; Zheng, X.; Hur, H.B.; Frew, J.W.; Krueger, J.G. High inflammation in hidradenitis suppurativa extends to perilesional skin and can be subdivided by lipocalin-2 expression. J. Allergy Clin. Immunol. 2022, 149, 135–144.e12. [Google Scholar] [CrossRef]
- Krajewski, P.K.; Szukała, W.; Szepietowski, J.C. The NLRP3 Inflammasome Gene Is Overexpressed in Hidradenitis Suppurativa Lesions: A Preliminary Study on the Role of Pyroptosis in Disease Pathogenesis. Curr. Issues Mol. Biol. 2024, 46, 2544–2552. [Google Scholar] [CrossRef] [PubMed]
- Frings, V.G.; Jopp, L.; Srivastava, M.; Presser, D.; Goebeler, M.; Schmidt, M. Stress signalling and STAT1 activation characterize the keratinocytic gene expression pattern in Hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 2488–2498. [Google Scholar] [CrossRef]
- Dajnoki, Z.; Somogyi, O.; Medgyesi, B.; Jenei, A.; Szabó, L.; Gáspár, K.; Hendrik, Z.; Gergely, P.; Imre, D.; Póliska, S.; et al. Primary alterations during the development of hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Flora, A.; Jepsen, R.; Kozera, E.K.; Woods, J.A.; Cains, G.D.; Radzieta, M.; Jensen, S.O.; Malone, M.; Frew, J.W. Human dermal fibroblast subpopulations and epithelial mesenchymal transition signals in hidradenitis suppurativa tunnels are normalized by spleen tyrosine kinase antagonism in vivo. PLoS ONE 2023, 18, e0282763. [Google Scholar] [CrossRef]
- Jin, L.; Kashyap, M.P.; Chen, Y.; Khan, J.; Guo, Y.; Chen, J.Q.; Lee, M.B.; Weng, Z.; Oak, A.; Patcha, P.; et al. Mechanism underlying follicular hyperproliferation and oncogenesis in hidradenitis suppurativa. iScience 2023, 26, 106896. [Google Scholar] [CrossRef]
- Mintoff, D.; Borg, I.; Pace, N.P. Serum Immunoglobulin G Is a Marker of Hidradenitis Suppurativa Disease Severity. Int. J. Mol. Sci. 2022, 23, 13800. [Google Scholar] [CrossRef]
- Witte-Händel, E.; Wolk, K.; Tsaousi, A.; Irmer, M.L.; Mößner, R.; Shomroni, O.; Lingner, T.; Witte, K.; Kunkel, D.; Salinas, G.; et al. The IL-1 Pathway Is Hyperactive in Hidradenitis Suppurativa and Contributes to Skin Infiltration and Destruction. J. Investig. Dermatol. 2019, 139, 1294–1305. [Google Scholar] [CrossRef]
- Kelly, G.; Hughes, R.; McGarry, T.; van den Born, M.; Adamzik, K.; Fitzgerald, R.; Lawlor, C.; Tobin, A.M.; Sweeney, C.M.; Kirby, B. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br. J. Dermatol. 2015, 173, 1431–1439. [Google Scholar] [CrossRef]
- Van Der Zee, H.H.; De Ruiter, L.; Van Den Broecke, D.G.; Dik, W.A.; Laman, J.D.; Prens, E.P. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: A rationale for targeting TNF-α and IL-1β. Br. J. Dermatol. 2011, 164, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Warszawska, K.; Hoeflich, C.; Witte, E.; Schneider-Burrus, S.; Witte, K.; Kunz, S.; Buss, A.; Roewert, H.J.; Krause, M.; et al. Deficiency of IL-22 Contributes to a Chronic Inflammatory Disease: Pathogenetic Mechanisms in Acne Inversa. J. Immunol. 2011, 186, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Kurzen, H.; Jung, E.G.; Hartschuh, W.; Moll, I.; Franke, W.W.; Moll, R. Forms of epithelial differentiation of draining sinus in acne inversa (hidradenitis suppurativa). Br. J. Dermatol. 1999, 141, 231–239. [Google Scholar] [CrossRef]
- Johnston, D.G.W.; Kirby, B.; Tobin, D.J. Hidradenitis suppurativa: A folliculotropic disease of innate immune barrier dysfunction? Exp. Dermatol. 2021, 30, 1554–1568. [Google Scholar] [CrossRef]
- Kjærsgaard Andersen, R.; Stefansdottir, L.; Riis, P.T.; Halldorsson, G.; Ferkingstad, E.; Oddsson, A.; Walters, B.; Olafsdottir, T.A.; Rutsdottir, G.; Zachariae, C.; et al. A genome-wide association meta-analysis links hidradenitis suppurativa to common and rare sequence variants causing disruption of the Notch and Wnt/β-catenin signaling pathways. J. Am. Acad. Dermatol. 2025, 92, 761–772. [Google Scholar] [CrossRef]
- Liu, M.; Degner, J.; Georgantas, R.W.; Nader, A.; Mostafa, N.M.; Teixeira, H.D.; Williams, D.A.; Kirsner, R.S.; Nichols, A.J.; Davis, J.W.; et al. A Genetic Variant in the BCL2 Gene Associates with Adalimumab Response in Hidradenitis Suppurativa Clinical Trials and Regulates Expression of BCL2. J. Investig. Dermatol. 2020, 140, 574–582.e2. [Google Scholar] [CrossRef]
- He, Y.; Wang, W.; Ma, X.; Duan, Z.; Wang, B.; Li, M.; Xu, H. Discovery and Potential Functional Characterization of Long Noncoding RNAs Associated with Familial Acne Inversa with NCSTN Mutation. Dermatology 2024, 240, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Dany, M.; Elston, D. Gene expression of sphingolipid metabolism pathways is altered in hidradenitis suppurativa. J. Am. Acad. Dermatol. 2017, 77, 268–273.e6. [Google Scholar] [CrossRef]
- Fincher, J.A.; Jones, D.R.; Korte, A.R.; Dyer, J.E.; Parlanti, P.; Popratiloff, A.; Brantner, C.A.; Morris, N.J.; Pirlo, R.K.; Shanmugam, V.K.; et al. Mass Spectrometry Imaging of Lipids in Human Skin Disease Model Hidradenitis Suppurativa by Laser Desorption Ionization from Silicon Nanopost Arrays. Sci. Rep. 2019, 9, 17508. [Google Scholar] [CrossRef]
- Penno, C.A.; Jäger, P.; Laguerre, C.; Hasler, F.; Hofmann, A.; Gass, S.K.; Wettstein-Ling, B.; Schaefer, D.J.; Avrameas, A.; Raulf, F.; et al. Lipidomics Profiling of Hidradenitis Suppurativa Skin Lesions Reveals Lipoxygenase Pathway Dysregulation and Accumulation of Proinflammatory Leukotriene B4. J. Investig. Dermatol. 2020, 140, 2421–2432.e10. [Google Scholar] [CrossRef]
- Hotz, C.; Boniotto, M.; Guguin, A.; Surenaud, M.; Jean-Louis, F.; Tisserand, P.; Ortonne, N.; Hersant, B.; Bosc, R.; Poli, F.; et al. Intrinsic Defect in Keratinocyte Function Leads to Inflammation in Hidradenitis Suppurativa. J. Investig. Dermatol. 2016, 136, 1768–1780. [Google Scholar] [CrossRef]
- Lima, A.L.; Karl, I.; Giner, T.; Poppe, H.; Schmidt, M.; Presser, D.; Goebeler, M.; Bauer, B. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br. J. Dermatol. 2016, 174, 514–521. [Google Scholar] [CrossRef]
- Jenei, A.; Dajnoki, Z.; Medgyesi, B.; Gáspár, K.; Béke, G.; Kinyó, Á.; Méhes, G.; Hendrik, Z.; Dinya, T.; Törőcsik, D.; et al. Apocrine Gland–Rich Skin Has a Non-Inflammatory IL-17–Related Immune Milieu, that Turns to Inflammatory IL-17–Mediated Disease in Hidradenitis Suppurativa. J. Investig. Dermatol. 2019, 139, 964–968. [Google Scholar] [CrossRef]
- Williams, S.C.; Garcet, S.; Hur, H.; Miura, S.; Gonzalez, J.; Navrazhina, K.; Yamamura-Murai, M.; Yamamura, K.; Li, X.; Frew, J.; et al. Gram-negative anaerobes elicit a robust keratinocytes immune response with potential insights into HS pathogenesis. Exp. Dermatol. 2024, 33, e15087. [Google Scholar] [CrossRef]
- Riverain-Gillet, É.; Guet-Revillet, H.; Jais, J.-P.; Ungeheuer, M.-N.; Duchatelet, S.; Delage, M.; Lam, T.; Hovnanian, A.; Nassif, A.; Join-Lambert, O. The Surface Microbiome of Clinically Unaffected Skinfolds in Hidradenitis Suppurativa: A Cross-Sectional Culture-Based and 16S rRNA Gene Amplicon Sequencing Study in 60 Patients. J. Investig. Dermatol. 2020, 140, 1847–1855.e6. [Google Scholar] [CrossRef] [PubMed]
- Guet-Revillet, H.; Jais, J.-P.; Ungeheuer, M.-N.; Coignard-Biehler, H.; Duchatelet, S.; Delage, M.; Lam, T.; Hovnanian, A.; Lortholary, O.; Nassif, X.; et al. The Microbiological Landscape of Anaerobic Infections in Hidradenitis Suppurativa: A Prospective Metagenomic Study. Clin. Infect. Dis. 2017, 65, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Guenin-Macé, L.; Morel, J.-D.; Doisne, J.-M.; Schiavo, A.; Boulet, L.; Mayau, V.; Goncalves, P.; Duchatelet, S.; Hovnanian, A.; Bondet, V.; et al. Dysregulation of tryptophan catabolism at the host-skin microbiota interface in hidradenitis suppurativa. JCI Insight 2020, 5, e140598. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.C.; Frew, J.W.; Krueger, J.G. A systematic review and critical appraisal of metagenomic and culture studies in hidradenitis suppurativa. Exp. Dermatol. 2021, 30, 1388–1397. [Google Scholar] [CrossRef]
- Lelonek, E.; Bouazzi, D.; Jemec, G.B.E.; Szepietowski, J.C. Skin and Gut Microbiome in Hidradenitis Suppurativa: A Systematic Review. Biomedicines 2023, 11, 2277. [Google Scholar] [CrossRef]
- Świerczewska, Z.; Lewandowski, M.; Surowiecka, A.; Barańska-Rybak, W. Microbiome in Hidradenitis Suppurativa—What We Know and Where We Are Heading. Int. J. Mol. Sci. 2022, 23, 11280. [Google Scholar] [CrossRef] [PubMed]
- Sodagar, S.; Ghane, Y.; Heidari, A.; Heidari, N.; Khodadust, E.; Ahmadi, S.A.Y.; Seirafianpour, F.; Baradaran, H.; Goodarzi, A. Association between metabolic syndrome and prevalent skin diseases: A systematic review and meta-analysis of case-control studies. Health Sci. Rep. 2023, 6, e1576. [Google Scholar] [CrossRef]
- Miller, I.M.; Ellervik, C.; Vinding, G.R.; Zarchi, K.; Ibler, K.S.; Knudsen, K.M.; Jemec, G.B.E. Association of metabolic syndrome and hidradenitis suppurativa. JAMA Dermatol. 2014, 150, 1273–1280. [Google Scholar] [CrossRef]
- Shalom, G.; Freud, T.; Harman-Boehm, I.; Polishchuk, I.; Cohen, A.D. Hidradenitis suppurativa and metabolic syndrome: A comparative cross-sectional study of 3207 patients. Br. J. Dermatol. 2015, 173, 464–470. [Google Scholar] [CrossRef]
- Omari, N.; Simonsen, S.; Gluud, L.L.; Martin, H.M.; Trelle, M.B.; Jemec, G.B.; Skov, L.; Näslund-Koch, C. Assessment of metabolic dysfunction-associated steatotic liver disease in patients with hidradenitis suppurativa: A cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2025, 39, e67–e70. [Google Scholar] [CrossRef] [PubMed]
- Durán-Vian, C.; Arias-Loste, M.T.; Hernández, J.L.; Fernández, V.; González, M.; Iruzubieta, P.; Rasines, L.; González-Vela, C.; Vaqué, J.P.; Blanco, R.; et al. High prevalence of non-alcoholic fatty liver disease among hidradenitis suppurativa patients independent of classic metabolic risk factors. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2131–2136. [Google Scholar] [CrossRef]
- Kaleta, K.P.; Nikolakis, G.; Hossini, A.M.; Balthasar, O.; Almansouri, D.; Vaiopoulos, A.; Knolle, J.; Boguslawska, A.; Wojas-Pelc, A.; Zouboulis, C.C. Metabolic Disorders/Obesity Is a Primary Risk Factor in Hidradenitis Suppurativa: An Immunohistochemical Real-World Approach. Dermatology 2022, 238, 251–259. [Google Scholar] [CrossRef]
- Luo, X.; Ruan, Z.; Liu, L. Causal relationship between metabolic syndrome and hidradenitis suppurativa: A two-sample bidirectional Mendelian randomization study. J. Dermatol. 2024, 51, 1335–1349. [Google Scholar] [CrossRef] [PubMed]
- Fabbrocini, G.; De Vita, V.; Donnarumma, M.; Russo, G.; Monfrecola, G. South Italy: A Privileged Perspective to Understand the Relationship between Hidradenitis Suppurativa and Overweight/Obesity. Ski. Appendage Disord. 2016, 2, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Lindsø Andersen, P.; Kromann, C.; Fonvig, C.E.; Theut Riis, P.; Jemec, G.B.E.; Holm, J.-C. Hidradenitis suppurativa in a cohort of overweight and obese children and adolescents. Int. J. Dermatol. 2020, 59, 47–51. [Google Scholar] [CrossRef]
- Balgobind, A.; Finelt, N.; Strunk, A.; Garg, A. Association between obesity and hidradenitis suppurativa among children and adolescents: A population-based analysis in the United States. J. Am. Acad. Dermatol. 2020, 82, 502–504. [Google Scholar] [CrossRef]
- Miller, I.M.; Rytgaard, H.; Mogensen, U.B.; Miller, E.; Ring, H.C.; Ellervik, C.; Jemec, G.B. Body composition and basal metabolic rate in Hidradenitis Suppurativa: A Danish population-based and hospital-based cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 980–988. [Google Scholar] [CrossRef]
- Vossen, A.R.J.V.; van der Zee, H.H.; Onderdijk, A.J.; Boer, J.; Prens, E.P. Hidradenitis suppurativa is not associated with the metabolic syndrome based on body type: A cross-sectional study. J. Dermatol. 2017, 44, 154–159. [Google Scholar] [CrossRef]
- van Straalen, K.R.; Vanlaerhoven, A.M.J.D.; Ardon, C.B.; van der Zee, H.H. Body mass index at the onset of hidradenitis suppurativa. J. Dtsch. Dermatol. Ges. 2021, 19, 437–439. [Google Scholar] [CrossRef]
- Mintoff, D.; Pace, N. Causal association between body fat percentage and hidradenitis suppurativa: A two-sample Mendelian randomization study. J. Eur. Acad. Dermatol. Venereol. 2025, 39, e713–e715. [Google Scholar] [CrossRef]
- Choi, E.; Mir, S.A.; Ji, S.; Ooi, X.T.; Chua, E.W.L.; Yi Wei, Y.; Wenk, M.R.; Bendt, A.K.; Chandran, N.S. Understanding the systemic burden of disease in hidradenitis suppurativa from plasma lipidomic analysis. J. Dermatol. Sci. 2022, 107, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Boer, J.; Jemec, G.B.E. Mechanical stress and the development of pseudo-comedones and tunnels in Hidradenitis suppurativa/Acne inversa. Exp. Dermatol. 2016, 25, 396–397. [Google Scholar] [CrossRef] [PubMed]
- Boer, J.; Jemec, G.B.E. Mechanical forces and Hidradenitis Suppurativa. Exp. Dermatol. 2021, 30, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, P.K.; Matusiak, Ł.; Ständer, S.; Thaçi, D.; Szepietowski, J.C.; Zirpel, H. Risk of cardiovascular disorders in hidradenitis suppurativa patients: A large-scale, propensity-matched global retrospective cohort study. Int. J. Dermatol. 2024, 63, 799–805. [Google Scholar] [CrossRef]
- Zhou, P.; Jiang, X.; Wang, D. Hidradenitis suppurativa and cardiovascular diseases: A bidirectional Mendelian randomization study. Ski. Res. Technol. 2024, 30, e13853. [Google Scholar] [CrossRef]
- Cuenca-Barrales, C.; Montero-Vilchez, T.; Salvador-Rodríguez, L.; Sánchez-Díaz, M.; Arias-Santiago, S.; Molina-Leyva, A. Implications of Hidradenitis Suppurativa Phenotypes in Cardiovascular Risk and Treatment Decisions: A Retrospective Cohort Study. Dermatology 2021, 237, 727–732. [Google Scholar] [CrossRef]
- Nielsen, V.W.; Bundgaard Vad, O.; Holgersen, N.; Paludan-Müller, C.; Meseguer Monfort, L.; Beyer, A.F.; Jemec, G.B.E.; Kjærsgaard Andersen, R.; Egeberg, A.; Thyssen, J.P.; et al. Genetic Susceptibility to Hidradenitis Suppurativa and Predisposition to Cardiometabolic Disease. JAMA Dermatol. 2025, 161, 22–30. [Google Scholar] [CrossRef]
- Brydges, H.T.; Onuh, O.C.; Friedman, R.; Barrett, J.; Betensky, R.A.; Lu, C.P.; Caplan, A.S.; Alavi, A.; Chiu, E.S. Autoimmune, Autoinflammatory Disease and Cutaneous Malignancy Associations with Hidradenitis Suppurativa: A Cross-Sectional Study. Am. J. Clin. Dermatol. 2024, 25, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Almuhanna, N.; Finstad, A.; Alhusayen, R. Association between Hidradenitis Suppurativa and Inflammatory Arthritis: A Systematic Review and Meta-Analysis. Dermatology 2021, 237, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Burrus, S.; Witte-Haendel, E.; Christou, D.; Rigoni, B.; Sabat, R.; Diederichs, G. High Prevalence of Back Pain and Axial Spondyloarthropathy in Patients with Hidradenitis Suppurativa. Dermatology 2017, 232, 606–612. [Google Scholar] [CrossRef]
- Sy, S.M.T.; Eder, L.; Jerome, D.; Obetta, C.; McKee, H.; Mirza, R.; Pek, E.; Piguet, V.; Alhusayen, R. Prevalence and Predictors of Inflammatory Arthritis in Hidradenitis Suppurativa. Exp. Dermatol. 2024, 33, e15194. [Google Scholar] [CrossRef]
- Kridin, K.; Shavit, E.; Damiani, G.; Cohen, A.D. Hidradenitis suppurativa and rheumatoid arthritis: Evaluating the bidirectional association. Immunol. Res. 2021, 69, 533–540. [Google Scholar] [CrossRef] [PubMed]
- D’Onghia, M.; Ciaffi, J.; Calabrese, L.; Tognetti, L.; Cinotti, E.; Rubegni, P.; Frediani, B.; Ursini, F. Fibromyalgia and Skin Disorders: A Systematic Review. J. Clin. Med. 2024, 13, 4404. [Google Scholar] [CrossRef]
- Prens, L.M.; Bouwman, K.; Troelstra, L.D.; Prens, E.P.; Alizadeh, B.Z.; Horváth, B. New insights in hidradenitis suppurativa from a population-based Dutch cohort: Prevalence, smoking behaviour, socioeconomic status and comorbidities. Br. J. Dermatol. 2022, 186, 814–822. [Google Scholar] [CrossRef]
- Patel, K.R.; Lee, H.H.; Rastogi, S.; Vakharia, P.P.; Hua, T.; Chhiba, K.; Singam, V.; Silverberg, J.I. Association between hidradenitis suppurativa, depression, anxiety, and suicidality: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2020, 83, 737–744. [Google Scholar] [CrossRef]
- Machado, M.O.; Stergiopoulos, V.; Maes, M.; Kurdyak, P.A.; Lin, P.-Y.; Wang, L.-J.; Shyu, Y.-C.; Firth, J.; Koyanagi, A.; Solmi, M.; et al. Depression and Anxiety in Adults With Hidradenitis Suppurativa: A Systematic Review and Meta-analysis. JAMA Dermatol. 2019, 155, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Fabrazzo, M.; Cipolla, S.; Signoriello, S.; Camerlengo, A.; Calabrese, G.; Giordano, G.M.; Argenziano, G.; Galderisi, S. A systematic review on shared biological mechanisms of depression and anxiety in comorbidity with psoriasis, atopic dermatitis, and hidradenitis suppurativa. Eur. Psychiatry 2021, 64, e71. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.K.; Tomalin, L.E.; Schultz, G.; Howell, M.D.; Anandasabapathy, N.; Alavi, A.; Suárez-Fariñas, M.; Lowes, M.A. Integrating the skin and blood transcriptomes and serum proteome in hidradenitis suppurativa reveals complement dysregulation and a plasma cell signature. PLoS ONE 2018, 13, e0203672. [Google Scholar] [CrossRef]
- Lowe, M.M.; Cohen, J.N.; Moss, M.I.; Clancy, S.; Adler, J.P.; Yates, A.E.; Naik, H.B.; Yadav, R.; Pauli, M.; Taylor, I.; et al. Tertiary lymphoid structures sustain cutaneous B cell activity in hidradenitis suppurativa. JCI Insight 2024, 9, e169870. [Google Scholar] [CrossRef]
- Petrasca, A.; Hambly, R.; Kearney, N.; Smith, C.M.; Pender, E.K.; Mac Mahon, J.; O’Rourke, A.M.; Ismaiel, M.; Boland, P.A.; Almeida, J.P.; et al. Metformin has anti-inflammatory effects and induces immunometabolic reprogramming via multiple mechanisms in hidradenitis suppurativa. Br. J. Dermatol. 2023, 189, 730–740. [Google Scholar] [CrossRef]
- Rode, M.; Nenoff, K.; Wirkner, K.; Horn, K.; Teren, A.; Regenthal, R.; Loeffler, M.; Thiery, J.; Aigner, A.; Pott, J.; et al. Impact of medication on blood transcriptome reveals off-target regulations of beta-blockers. PLoS ONE 2022, 17, e0266897. [Google Scholar] [CrossRef]
- Srour, J.; Marsela, E.; Fiocco, Z.; Kendziora, B.; Gürtler, A.; French, L.E.; Reinholz, M. Serum levels of serum amyloid A, interleukin-6 and C-reactive protein correlate with severity of hidradenitis suppurativa. Ital. J. Dermatol. Venerol. 2023, 158, 341–346. [Google Scholar] [CrossRef]
- Singh, K.P.; Miaskowski, C.; Dhruva, A.A.; Flowers, E.; Kober, K.M. Mechanisms and Measurement of Changes in Gene Expression. Biol. Res. Nurs. 2018, 20, 369–382. [Google Scholar] [CrossRef]
- Blok, J.L.; Li, K.; Brodmerkel, C.; Horvátovich, P.; Jonkman, M.F.; Horváth, B. Ustekinumab in hidradenitis suppurativa: Clinical results and a search for potential biomarkers in serum. Br. J. Dermatol. 2016, 174, 839–846. [Google Scholar] [CrossRef]
- Dimitrion, P.M.; Krevh, R.; Veenstra, J.; Ge, J.; Siddiqui, A.; Ferguson, D.; Hans, A.; Zuniga, B.; Sidhu, K.; Daveluy, S.; et al. High-throughput proteomics identifies inflammatory proteins associated with disease severity and genetic ancestry in patients with hidradenitis suppurativa. Br. J. Dermatol. 2025, 192, 1063–1071. [Google Scholar] [CrossRef]
- Navrazhina, K.; Garcet, S.; Gonzalez, J.; Grand, D.; Frew, J.W.; Krueger, J.G. In-Depth Analysis of the Hidradenitis Suppurativa Serum Proteome Identifies Distinct Inflammatory Subtypes. J. Investig. Dermatol. 2021, 141, 2197–2207. [Google Scholar] [CrossRef]
- Flora, A.; Pham, J.; Jepsen, R.; Frew, J.W. The serum proteome of pyoderma gangrenosum is more expansive than that of hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2024, 38, e348–e350. [Google Scholar] [CrossRef]
- de Oliveira, A.S.L.E.; Bloise, G.; Moltrasio, C.; Coelho, A.; Agrelli, A.; Moura, R.; Tricarico, P.M.; Jamain, S.; Marzano, A.V.; Crovella, S.; et al. Transcriptome Meta-Analysis Confirms the Hidradenitis Suppurativa Pathogenic Triad: Upregulated Inflammation, Altered Epithelial Organization, and Dysregulated Metabolic Signaling. Biomolecules 2022, 12, 1371. [Google Scholar] [CrossRef]
- Li, L.; Hajam, I.; McGee, J.S.; Tang, Z.; Zhang, Y.; Badey, N.; Mintzer, E.; Zhang, Z.; Liu, G.Y.; Church, G.M.; et al. Comparative Transcriptome Analysis of Acne vulgaris, Rosacea, and Hidradenitis Suppurativa Supports High Dose Dietary Zinc as a Therapeutic Agent. Exp. Dermatol. 2024, 33, e15145. [Google Scholar] [CrossRef] [PubMed]
- Bukvić Mokos, Z.; Miše, J.; Balić, A.; Marinović, B. Understanding the Relationship Between Smoking and Hidradenitis Suppurativa: Acta dermatovenerologica Croatica: ADC. Acta Dermatovenerol. Croat. 2020, 28, 9–13. [Google Scholar] [PubMed]
- Hrvatin Stancic, B.; Falabella, P.; Dolenc Voljč, M. The influence of body mass index and smoking on the severity of hidradenitis suppurativa in the Slovenian population. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1305–e1306. [Google Scholar] [CrossRef]
- Molinelli, E.; Simonetti, O. Bimekizumab: Dual inhibition as a promising tool in the management of hidradenitis suppurativa. Lancet 2024, 403, 2457–2459. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, D.; Shah, A.; Yumeen, S.; Saliba, E. The use of biologics and JAK inhibitors in the management of moderate to severe Hidradenitis Suppurativa treatment: A scoping review. Arch. Dermatol. Res. 2024, 316, 259. [Google Scholar] [CrossRef]
- Garbayo-Salmons, P.; Saus, E.; Exposito-Serrano, V.; Moreno, M.; Sàbat, M.; Calvet, J. Hidradenitis Suppurativa from a Multi-Omic Scope. J. Cutan. Med. Surg. 2024, 29, 159–166. [Google Scholar] [CrossRef]
- Petukhova, L.; Colvin, A.; Koerts, N.D.K.; Horváth, B. Leveraging genotypes and phenotypes to implement precision medicine in hidradenitis suppurativa management. Br. J. Dermatol. 2025, 192, i22–i29. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP 2018. Available online: https://bioinformatics.sdstate.edu/idep/ (accessed on 5 January 2025).
- Elizarraras, J.; Yuxing, L.; Shi, Z.; Zhang, B. WEB-Based GEne SeT AnaLysis Toolkit 2024. Available online: https://www.webgestalt.org/ (accessed on 23 February 2025).
- Evangelista, J.E.; Xie, Z.; Marino, G.B.; Nguyen, N.; Clarke, D.J.B.; Ma’ayan, A. Enrichr-KG: Bridging enrichment analysis across multiple libraries. Nucleic Acids Res. 2023, 51, W168–W179. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiteri, J.; Mintoff, D.; Grech, L.; Pace, N.P. Transcriptomic Signatures and Molecular Pathways in Hidradenitis Suppurativa—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 7704. https://doi.org/10.3390/ijms26167704
Spiteri J, Mintoff D, Grech L, Pace NP. Transcriptomic Signatures and Molecular Pathways in Hidradenitis Suppurativa—A Narrative Review. International Journal of Molecular Sciences. 2025; 26(16):7704. https://doi.org/10.3390/ijms26167704
Chicago/Turabian StyleSpiteri, Jasmine, Dillon Mintoff, Laura Grech, and Nikolai P. Pace. 2025. "Transcriptomic Signatures and Molecular Pathways in Hidradenitis Suppurativa—A Narrative Review" International Journal of Molecular Sciences 26, no. 16: 7704. https://doi.org/10.3390/ijms26167704
APA StyleSpiteri, J., Mintoff, D., Grech, L., & Pace, N. P. (2025). Transcriptomic Signatures and Molecular Pathways in Hidradenitis Suppurativa—A Narrative Review. International Journal of Molecular Sciences, 26(16), 7704. https://doi.org/10.3390/ijms26167704




