Comparative Pain Expression and Its Association to Intestinal Microbiota Through the MI-RAT© Osteoarthritis Model Induced in LOU/C/Jall and Sprague-Dawley Aged Rats
Abstract
1. Introduction
2. Results
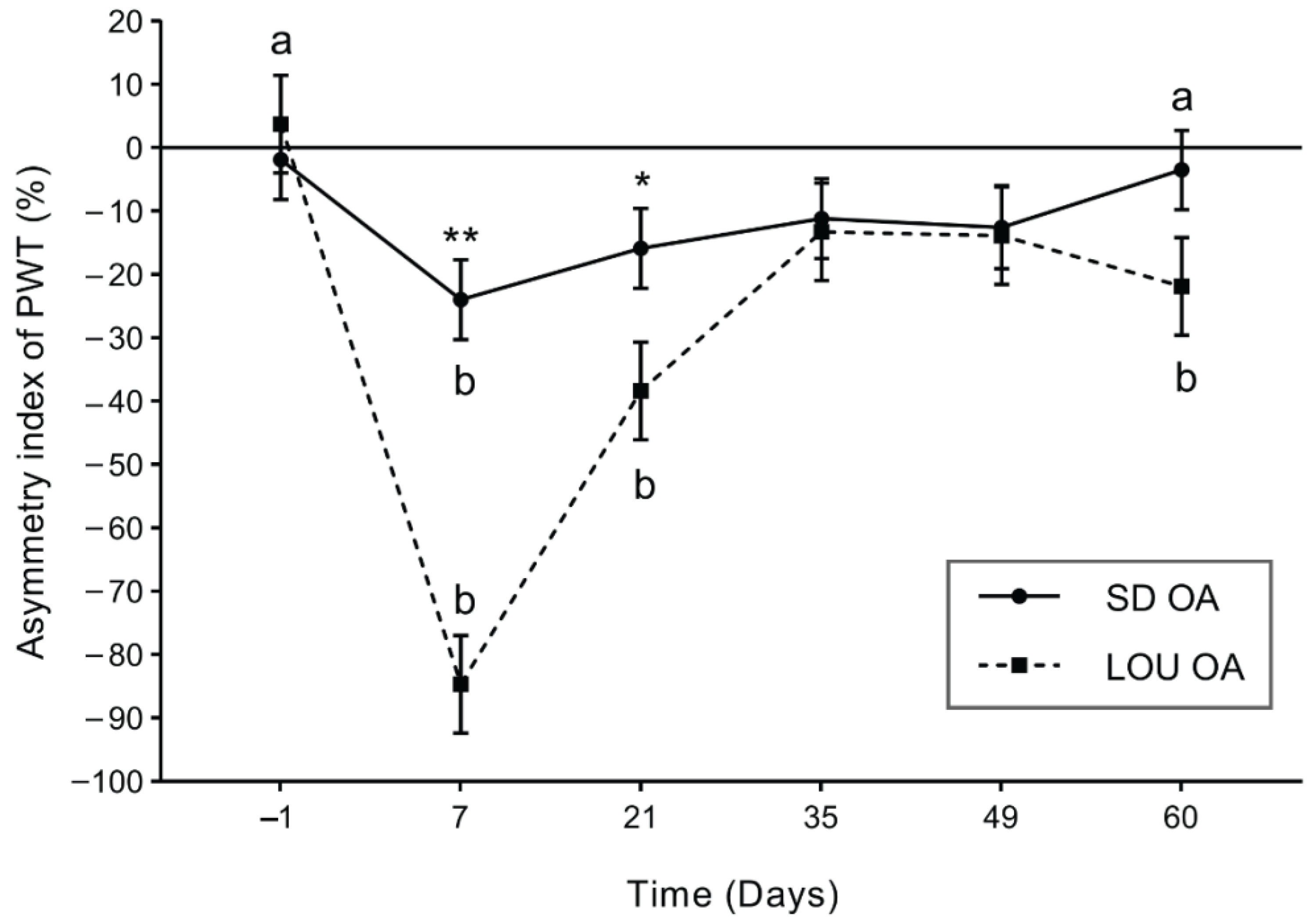
2.1. LOU Rats Exhibit Stronger and Longer-Lasting Mechanical Hypersensitivity After OA Induction
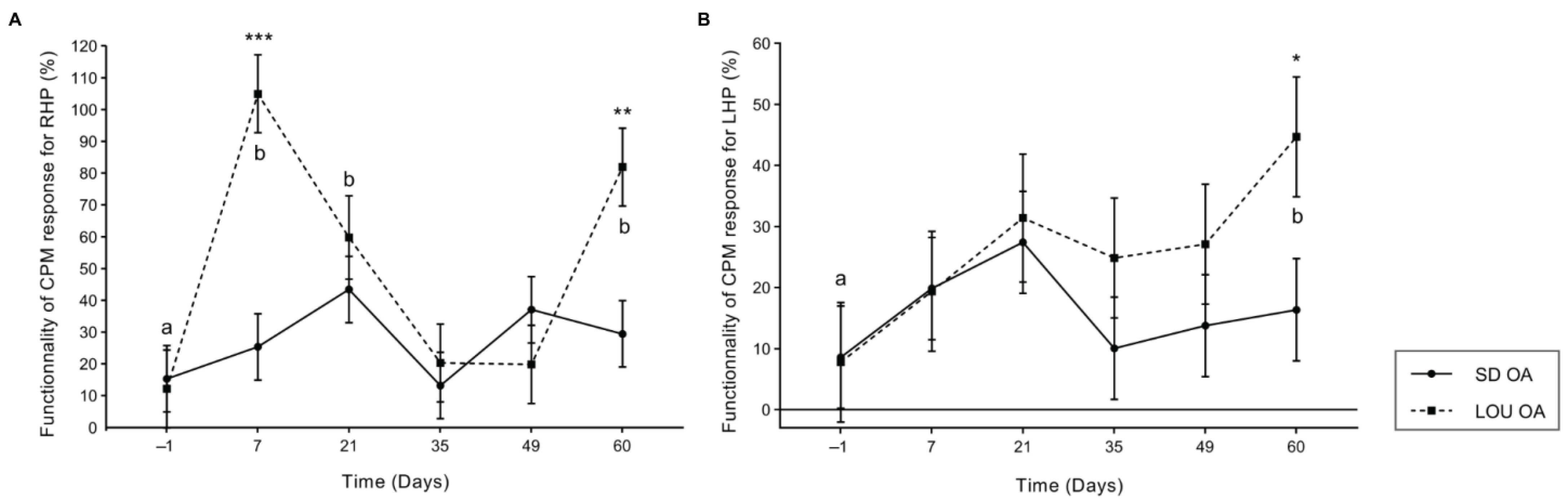
2.2. Enhanced Conditioned Pain Modulation in LOU OA Rats Suggests More Efficient Endogenous Inhibitory Control
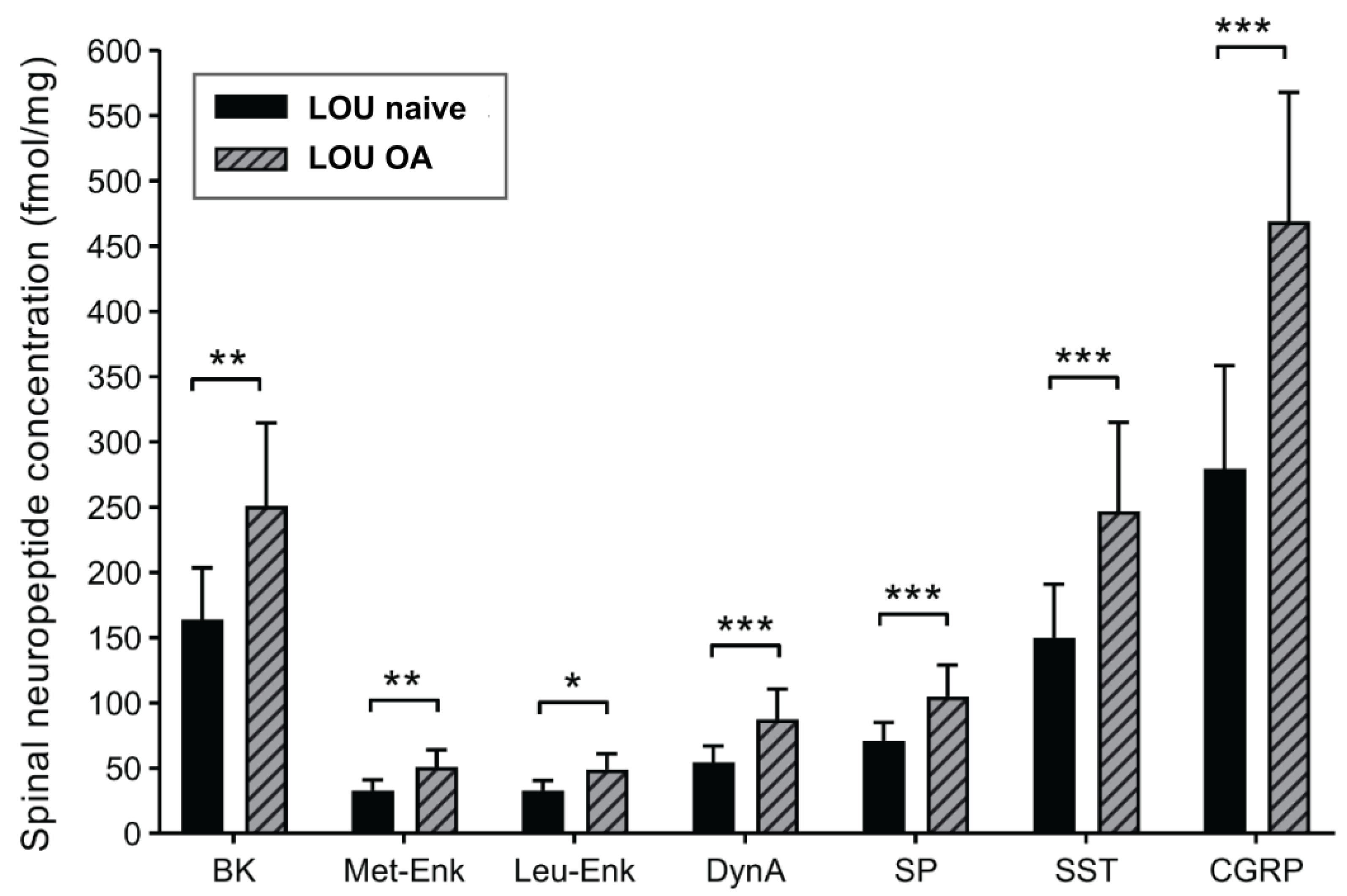
2.3. Spinal Neuropeptide Changes in LOU OA Rats Reflect Global Sensitization but Do Not Distinguish Strain Differences
2.4. LOU and SD OA Rats Show Comparable Cognitive Search Strategies in Morris Water Maze Despite Locomotor Differences
2.5. Comparable OA-Induced Cartilage Damage Between Rat Strains
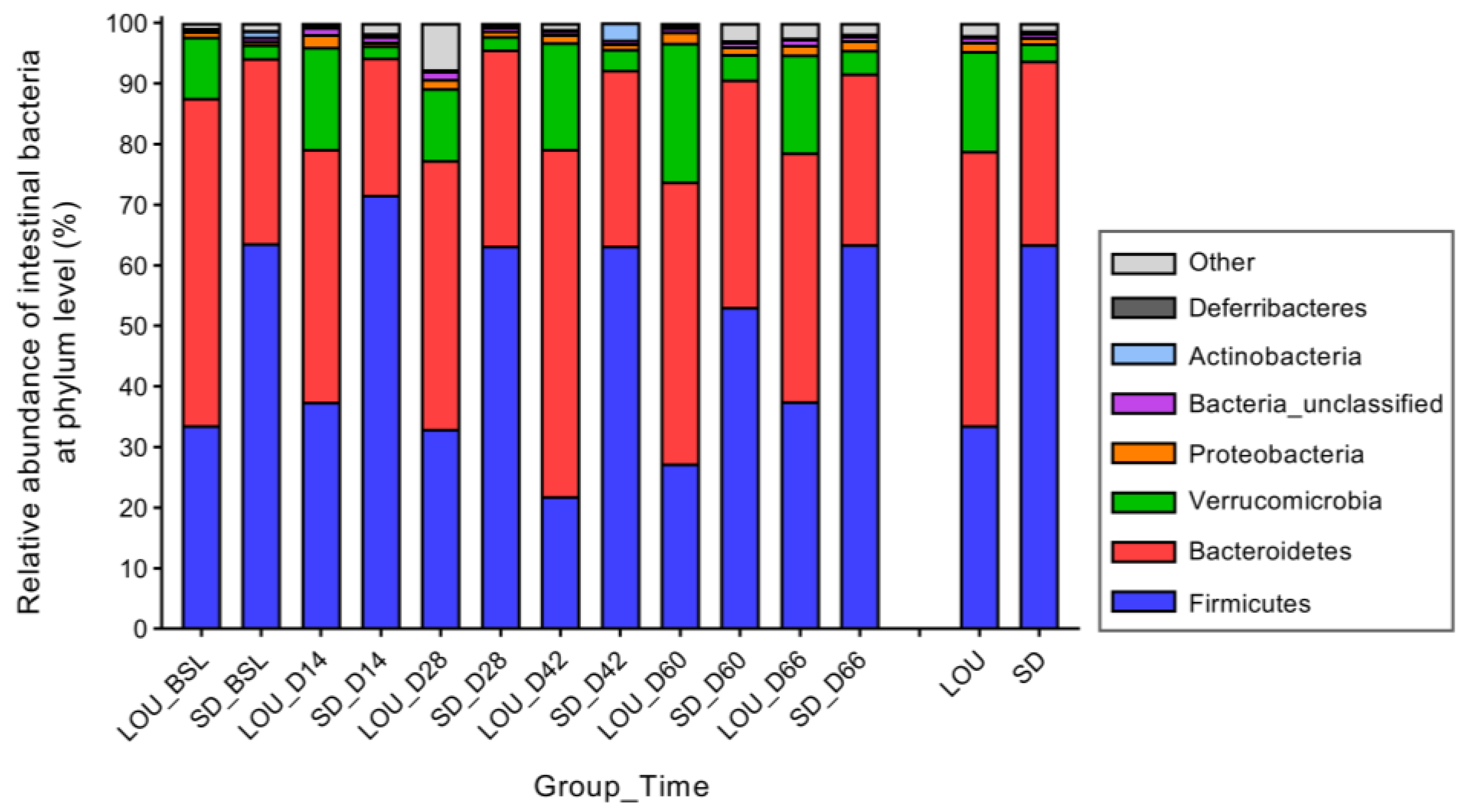
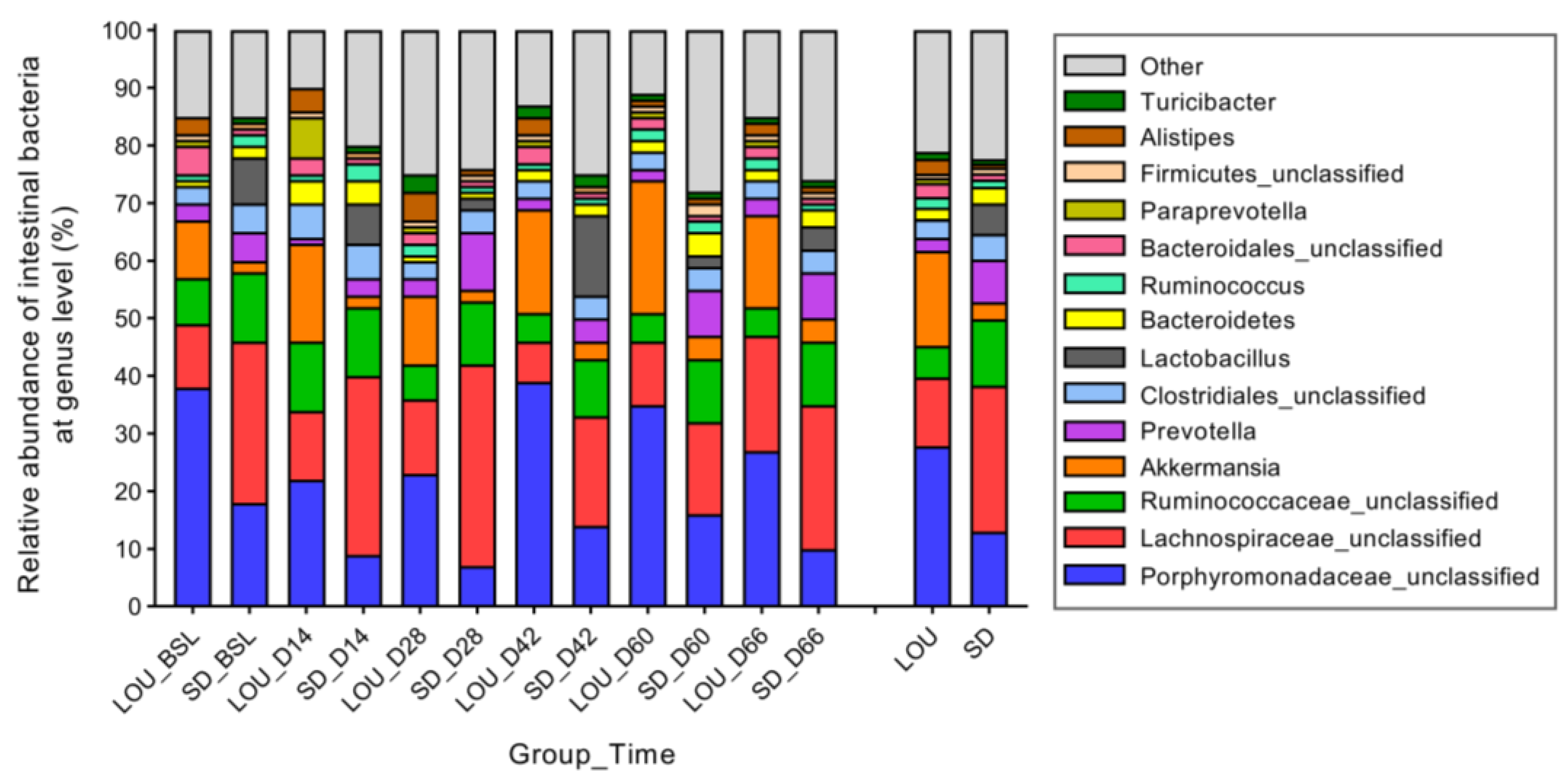
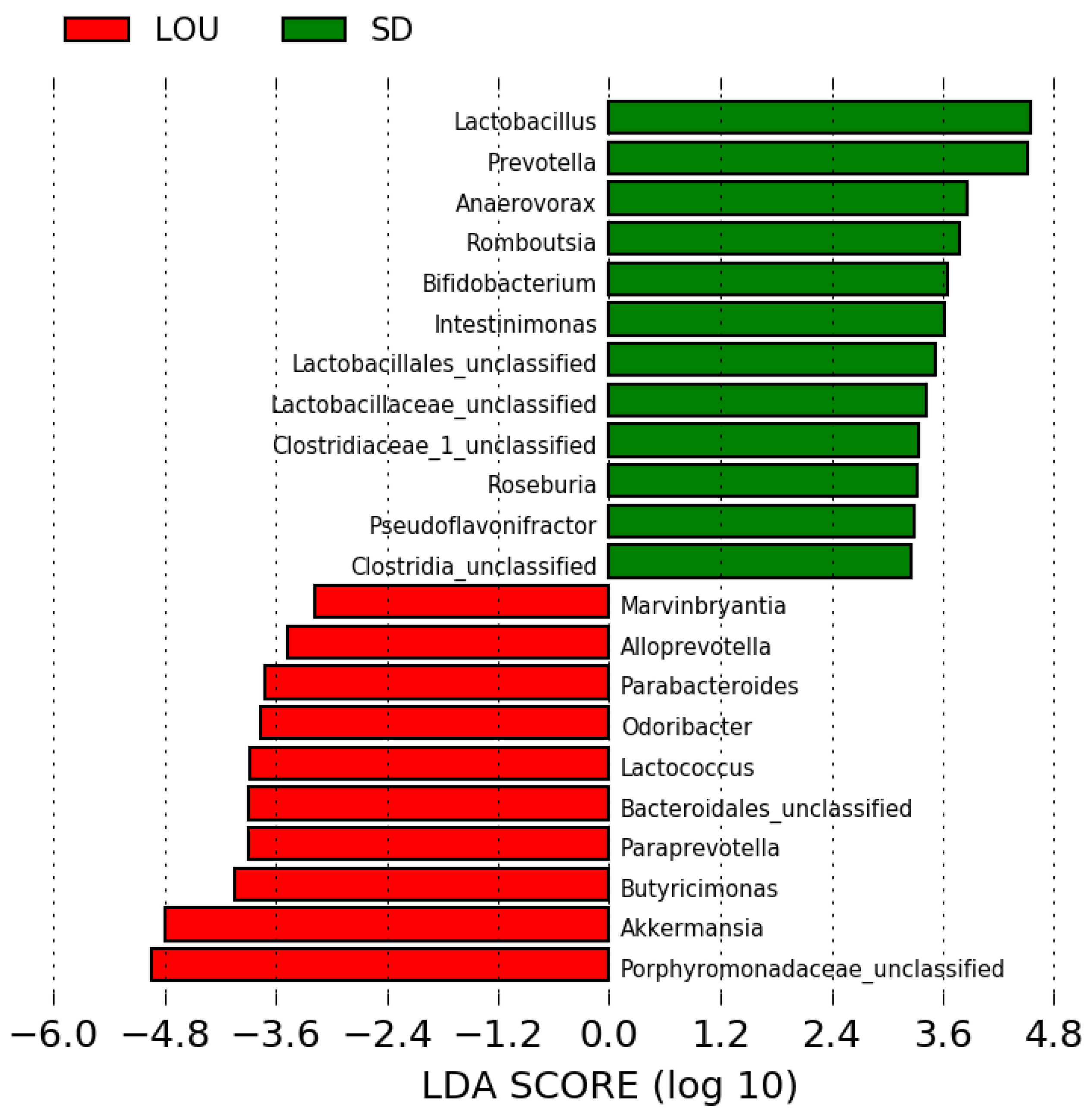
2.6. LOU and SD OA Rats Exhibit Distinct Gut Microbial Profiles with Divergent Diversity and Dominant Taxa

3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Montreal Induction of Rat Arthritis Testing (MI-RAT©) Model
4.3. Experimental Design
4.4. Mechanical Paw Withdrawal Threshold (PWT)
4.5. Conditioned Pain Modulation (CPM)
4.6. Neuropeptidomic Analysis
4.7. Cognitive Evaluation
4.8. Structural Histological Joint Analysis
4.9. Gut Microbiota Analyses
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GREPAQ | Groupe de recherche en pharmacologie animale du Québec |
| CRCHUM | University of Montreal hospital research center |
| OA | Osteoarthritis |
| SD | Sprague-Dawley |
| LOU | LOU/C/Jall |
| QST | Quantitative sensory testing |
| MI-RAT© | Montreal induction of rat arthritis testing |
| NP | Neuropeptides |
| PWT | Paw withdrawal threshold |
| CPM | Conditioned pain modulation |
| SP | Substance P |
| CGRP | Calcitonin gene-related peptide |
| SST | Somatostatin |
| Met-Enk | Methionine-enkephalin |
| Leu-Enk | Leucine-enkephalin |
| GABA | Gamma-aminobutyric acid |
| D | Day |
| RHP | Right hind paw |
| LHP | Left hind paw |
| BSL | Baseline |
| CS | Conditioning stimulus |
| BK | Bradykinin |
| DynA | Dynorphin A |
| MWM | Morris water maze |
| PCoA | Principal coordinate analysis |
| AMOVA | Analysis of molecular variance |
| F/B | Firmicutes/Bacteroidetes |
| LDA | Linear discriminant analysis |
| LSM | Least squares mean |
| SEM | Standard error of the mean |
Appendix A
| I. Structural changes (0–10) | |
| Normal 0 | 0 |
| Surface irregularities (Undulating articular surface but no fibrillation) | 1 |
| Minimal mild superficial fibrillation (less than 10% of articular cartilage thickness) < 50% | 2 |
| Minimal mild superficial fibrillation (less than 10% of articular cartilage thickness) > 50% | 3 |
| Fibrillation/clefts/fissure/loss of articular cartilage involving superficial 1/3 of articular cartilage < 50% | 4 |
| Fibrillation/clefts/fissure/loss of articular cartilage involving superficial 1/3 of articular cartilage > 50% | 5 |
| Fibrillation/clefts/fissure/loss of articular cartilage involving superficial 1/3 to 2/3 of articular cartilage < 50% | 6 |
| Fibrillation/clefts/fissure/loss of articular cartilage involving superficial 1/3 to 2/3 of articular cartilage > 50% | 7 |
| Fibrillation/clefts/fissure/loss of articular cartilage involving superficial > 2/3 of articular cartilage < 50% | 8 |
| Fibrillation/clefts/fissure/loss of articular cartilage involving superficial > 2/3 of articular cartilage > 50% | 9 |
| Fibrillation/clefts/fissure/loss of articular cartilage to subchondral bone | 10 |
| II. Safranin O staning (0–6) | |
| Normal | 0 |
| Loss of staining in superficial zone of articular cartilage involving <50% | 1 |
| Loss of staining in superficial zone of articular cartilage involving ≥50% | 2 |
| Loss of staining in upper 2/3 of articular cartilage involving <50% | 3 |
| Loss of staining in upper 2/3 of articular cartilage involving ≥50% | 4 |
| Loss of staining in all the articular cartilage involving <50% | 5 |
| Loss of staining in all the articular cartilage involving >50% | 6 |
| III. Cluster formation (0–3) | |
| None | 0 |
| <4 clusters | 1 |
| ≥4 but <8 clusters | 2 |
| ≥8 clusters | 3 |
| IV. Loss of chondrocytes (0–6) | |
| Normal | 0 |
| Focal chondrocyte loss | 1 |
| Loss of chondrocytes in superficial zone < 50% | 2 |
| Loss of chondrocytes in superficial zone > 50% | 3 |
| Loss of chondrocytes in mid-zone < 50% | 4 |
| Loss of chondrocytes in mid-zone > 50% | 5 |
| Diffuse loss of chondrocytes | 6 |
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Arendt-Nielsen, L. Pain sensitisation in osteoarthritis. Clin. Exp. Rheumatol. 2017, 35 (Suppl. S107), 68–74. [Google Scholar]
- Apkarian, V.A.; Hashmi, J.A.; Baliki, M.N. Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain 2011, 152, S49–S64. [Google Scholar] [CrossRef]
- Baliki, M.N.; Apkarian, A.V. Nociception, pain, negative moods, and behavior selection. Neuron 2015, 87, 474–491. [Google Scholar] [CrossRef]
- Buldys, K.; Gornicki, T.; Kalka, D.; Szuster, E.; Biernikiewicz, M.; Markuszewski, L.; Sobieszczanska, M. What do we know about nociplastic pain? Healthcare 2023, 11, 1794. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, M.C.; Ceko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef]
- Weaver, K.R.; Griffioen, M.A.; Klinedinst, N.J.; Galik, E.; Duarte, A.C.; Colloca, L.; Resnick, B.; Dorsey, S.G.; Renn, C.L. Quantitative sensory testing across chronic pain conditions and use in special populations. Front. Pain Res. 2021, 2, 779068. [Google Scholar] [CrossRef]
- Monteiro, B.P.; Otis, C.; Del Castillo, J.R.E.; Nitulescu, R.; Brown, K.; Arendt-Nielsen, L.; Troncy, E. Quantitative sensory testing in feline osteoarthritic pain—A systematic review and meta-analysis. Osteoarthr. Cartil. 2020, 28, 885–896. [Google Scholar] [CrossRef]
- Monteiro, B.P.; Otis, C.; Nitulescu, R.; Troncy, E. Quantitative sensory testing in canine musculoskeletal pain: Findings from a systematic review, meta-analysis feasibility assessment, and limitations. Vet. J. 2024, 304, 106102. [Google Scholar] [CrossRef]
- Gervais, J.A.; Otis, C.; Lussier, B.; Guillot, M.; Martel-Pelletier, J.; Pelletier, J.P.; Beaudry, F.; Troncy, E. Osteoarthritic pain model influences functional outcomes and spinal neuropeptidomics: A pilot study in female rats. Can. J. Vet. Res. 2019, 83, 133–141. [Google Scholar]
- Keita-Alassane, S.; Otis, C.; Bouet, E.; Guillot, M.; Frezier, M.; Delsart, A.; Moreau, M.; Bedard, A.; Gaumond, I.; Pelletier, J.P.; et al. Estrogenic impregnation alters pain expression: Analysis through functional neuropeptidomics in a surgical rat model of osteoarthritis. Naunyn Schmiedeberg’s Arch. Pharmacol. 2022, 395, 703–715. [Google Scholar] [CrossRef]
- Otis, C.; Bouet, E.; Keita-Alassane, S.; Frezier, M.; Delsart, A.; Guillot, M.; Bedard, A.; Pelletier, J.P.; Martel-Pelletier, J.; Lussier, B.; et al. Face and predictive validity of MI-RAT (Montreal Induction of rat Arthritis Testing), a surgical model of osteoarthritis pain in rodents combined with calibrated exercise. Int. J. Mol. Sci. 2023, 24, 16341. [Google Scholar] [CrossRef]
- Otis, C.; Gervais, J.; Guillot, M.; Gervais, J.A.; Gauvin, D.; Pethel, C.; Authier, S.; Dansereau, M.A.; Sarret, P.; Martel-Pelletier, J.; et al. Concurrent validity of different functional and neuroproteomic pain assessment methods in the rat osteoarthritis monosodium iodoacetate (MIA) model. Arthritis Res. Ther. 2016, 18, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Otis, C.; Guillot, M.; Moreau, M.; Martel-Pelletier, J.; Pelletier, J.P.; Beaudry, F.; Troncy, E. Spinal neuropeptide modulation, functional assessment and cartilage lesions in a monosodium iodoacetate rat model of osteoarthritis. Neuropeptides 2017, 65, 56–62. [Google Scholar] [CrossRef]
- Otis, C.; Guillot, M.; Moreau, M.; Pelletier, J.P.; Beaudry, F.; Troncy, E. Sensitivity of functional targeted neuropeptide evaluation in testing pregabalin analgesic efficacy in a rat model of osteoarthritis pain. Clin. Exp. Pharmacol. Physiol. 2019, 46, 723–733. [Google Scholar] [CrossRef]
- Otis, C.; Cristofanilli, K.-A.; Frezier, M.; Delsart, A.; Martel-Pelletier, J.; Pelletier, J.-P.; Beaudry, F.; Lussier, B.; Boyer, A.; Troncy, E. Predictive and concurrent validity of pain sensitivity phenotype, neuropeptidomics and neuroepigenetics in the MI-RAT osteoarthritic surgical model in rats. Front. Cell Dev. Biol. 2024, 12, 1400650. [Google Scholar] [CrossRef]
- Wang, S.; Albers, K.M. Behavioral and cellular level changes in the aging somatosensory system. Ann. N. Y. Acad. Sci. 2009, 1170, 745–749. [Google Scholar] [CrossRef]
- Weyer, A.D.; Zappia, K.J.; Garrison, S.R.; O’Hara, C.L.; Dodge, A.K.; Stucky, C.L. Nociceptor sensitization depends on age and pain chronicity(1,2,3). eNeuro 2016, 3, ENEURO.0115-15.2015. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.; Clauw, D.J. Central pain mechanisms in chronic pain states–maybe it is all in their head. Best Pract. Res. Clin. Rheumatol. 2011, 25, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Bannister, K.; Dickenson, A.H. The plasticity of descending controls in pain: Translational probing. J. Physiol. 2017, 595, 4159–4166. [Google Scholar] [CrossRef]
- Chen, Q.; Heinricher, M.M. Descending control mechanisms and chronic pain. Curr. Rheumatol. Rep. 2019, 21, 13. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Morimura, K.; Porreca, F. Descending pain modulation and chronification of pain. Curr. Opin. Support. Palliat. Care 2014, 8, 143–151. [Google Scholar] [CrossRef]
- Ro, J.Y.; Zhang, Y.; Tricou, C.; Yang, D.; da Silva, J.T.; Zhang, R. Age and sex differences in acute and osteoarthritis-Like pain responses in rats. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1465–1472. [Google Scholar] [CrossRef]
- Zhang, R.X.; Lao, L.; Qiao, J.T.; Ruda, M.A. Effects of aging on hyperalgesia and spinal dynorphin expression in rats with peripheral inflammation. Brain Res. 2004, 999, 135–141. [Google Scholar] [CrossRef]
- Edwards, R.R.; Fillingim, R.B.; Ness, T.J. Age-related differences in endogenous pain modulation: A comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain 2003, 101, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Guindon, J.; Mody, P.H.; Ashworth, G.; Kopel, J.; Chilakapati, S.; Adogwa, O.; Neugebauer, V.; Burton, M.D. Pain and aging: A unique challenge in neuroinflammation and behavior. Mol. Pain 2023, 19, 17448069231203090. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.T.; Tricou, C.; Zhang, Y.; Seminowicz, D.A.; Ro, J.Y. Brain networks and endogenous pain inhibition are modulated by age and sex in healthy rats. Pain 2020, 161, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.; Naugle, K.E.; Naugle, K.M. The decline of endogenous pain modulation with aging: A meta-analysis of temporal summation and conditioned pain modulation. J. Pain 2020, 21, 514–528. [Google Scholar] [CrossRef]
- Gagliese, L.; Melzack, R. Age differences in nociception and pain behaviours in the rat. Neurosci. Biobehav. Rev. 2000, 24, 843–854. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Gaspar, M.G.; Nunez-Carro, C.; Blanco-Blanco, M.; Blanco, F.J.; de Andres, M.C. Inflammaging contributes to osteoarthritis development and human microbiota variations and vice versa: A systematic review. Osteoarthr. Cartil. 2025, 33, 218–230. [Google Scholar] [CrossRef]
- Kollen, M.; Stephan, A.; Faivre-Bauman, A.; Loudes, C.; Sinet, P.M.; Alliot, J.; Billard, J.M.; Epelbaum, J.; Dutar, P.; Jouvenceau, A. Preserved memory capacities in aged Lou/C/Jall rats. Neurobiol. Aging 2010, 31, 129–142. [Google Scholar] [CrossRef]
- Pickering, G.; Jourdan, D.; Millecamps, M.; Chapuy, E.; Alliot, J.; Eschalier, A. Age-related impact of neuropathic pain on animal behaviour. Eur. J. Pain 2006, 10, 749–755. [Google Scholar] [CrossRef]
- Menard, C.; Quirion, R.; Vigneault, E.; Bouchard, S.; Ferland, G.; El Mestikawy, S.; Gaudreau, P. Glutamate presynaptic vesicular transporter and postsynaptic receptor levels correlate with spatial memory status in aging rat models. Neurobiol. Aging 2015, 36, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Menard, C.; Quirion, R.; Bouchard, S.; Ferland, G.; Gaudreau, P. Glutamatergic signaling and low prodynorphin expression are associated with intact memory and reduced anxiety in rat models of healthy aging. Front. Aging Neurosci. 2014, 6, 81. [Google Scholar] [CrossRef][Green Version]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. The gut microbiome as a modulator of healthy ageing. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 565–584. [Google Scholar] [CrossRef]
- Borre, Y.E.; Moloney, R.D.; Clarke, G.; Dinan, T.G.; Cryan, J.F. The impact of microbiota on brain and behavior: Mechanisms & therapeutic potential. Adv. Exp. Med. Biol. 2014, 817, 373–403. [Google Scholar] [CrossRef]
- Costa, M.C.; Weese, J.S. Understanding the intestinal microbiome in health and disease. Vet. Clin. N. Am. Equine Pract. 2018, 34, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, L.H.; Xing, C.; Liu, T. Pain regulation by gut microbiota: Molecular mechanisms and therapeutic potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef]
- Chen, J.; Wang, A.; Wang, Q. Dysbiosis of the gut microbiome is a risk factor for osteoarthritis in older female adults: A case control study. BMC Bioinform. 2021, 22, 299. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, Y.; Li, S.; Yang, L.; Wang, H.; Wang, T.; Bin, S.; Gai, Z.; Heng, X.; Zhang, C.; et al. Variations in oral microbiome profiles in rheumatoid arthritis and osteoarthritis with potential biomarkers for arthritis screening. Sci. Rep. 2018, 8, 17126. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, C.; Ren, Y.N.; Ye, Z.J.; Jiang, C.; Wu, Z.B. Alterations in the gut microbiota and metabolite profiles in the context of neuropathic pain. Mol. Brain 2021, 14, 50. [Google Scholar] [CrossRef]
- Vitetta, L.; Coulson, S.; Linnane, A.W.; Butt, H. The gastrointestinal microbiome and musculoskeletal diseases: A beneficial role for probiotics and prebiotics. Pathogens 2013, 2, 606–626. [Google Scholar] [CrossRef] [PubMed]
- Molnar, V.; Matisic, V.; Kodvanj, I.; Bjelica, R.; Jelec, Z.; Hudetz, D.; Rod, E.; Cukelj, F.; Vrdoljak, T.; Vidovic, D.; et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Tong, L.; Yu, H.; Huang, X.; Shen, J.; Xiao, G.; Chen, L.; Wang, H.; Xing, L.; Chen, D. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022, 10, 60. [Google Scholar] [CrossRef]
- Kumar, M.; Babaei, P.; Ji, B.; Nielsen, J. Human gut microbiota and healthy aging: Recent developments and future prospective. Nutr. Healthy Aging 2016, 4, 3–16. [Google Scholar] [CrossRef]
- Saraswati, S.; Sitaraman, R. Aging and the human gut microbiota-from correlation to causality. Front. Microbiol. 2014, 5, 764. [Google Scholar] [CrossRef]
- Brandon-Mong, G.J.; Shaw, G.T.; Chen, W.H.; Chen, C.C.; Wang, D. Correction to: A network approach to investigating the key microbes and stability of gut microbial communities in a mouse neuropathic pain model. BMC Microbiol. 2020, 20, 377. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, N.; Yoon, H.; Nam, R.H.; Lee, D.H. Microbial changes and host response in F344 rat colon depending on sex and age following a high-fat diet. Front. Microbiol. 2018, 9, 2236. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhao, F.; Sun, J.; Lin, B.; Zhao, L.; Liu, Y.; Jin, Y.; Li, S.; Li, A.; Wei, Y. Alterations in the gut microbiota and metabolite profiles of thyroid carcinoma patients. Int. J. Cancer 2019, 144, 2728–2745. [Google Scholar] [CrossRef]
- Tremaroli, V.; Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Lee, J.Y.; Mannaa, M.; Kim, Y.; Kim, J.; Kim, G.T.; Seo, Y.S. Comparative analysis of fecal microbiota composition between rheumatoid arthritis and osteoarthritis patients. Genes 2019, 10, 748. [Google Scholar] [CrossRef]
- Fooks, L.J.; Fuller, R.; Gibson, G.R. Prebiotics, probiotics and human gut microbiology. Int. Dairy J. 1999, 9, 53–61. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Johnson, C.; Luk, B.; Uribe, G.; Fu, Y.; Oezguen, N.; Matsunami, R.K.; Lugo, M.; Major, A.; Mori-Akiyama, Y.; et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 2017, 29, e12904. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Wang, Y.; Zhang, P.; Yuan, Y.; Zhang, Y.; Chen, G. Gut microbiota regulates neuropathic pain: Potential mechanisms and therapeutic strategy. J. Headache Pain 2020, 21, 103. [Google Scholar] [CrossRef]
- Collins, K.H.; Paul, H.A.; Reimer, R.A.; Seerattan, R.A.; Hart, D.A.; Herzog, W. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: Studies in a rat model. Osteoarthr. Cartil. 2015, 23, 1989–1998. [Google Scholar] [CrossRef]
- Moyseos, M.; Michael, J.; Ferreira, N.; Sophocleous, A. The effect of probiotics on the management of pain and inflammation in osteoarthritis: A systematic review and meta-analysis of clinical studies. Nutrients 2024, 16, 2243. [Google Scholar] [CrossRef]
- Costa, M.C.; Arroyo, L.G.; Allen-Vercoe, E.; Stampfli, H.R.; Kim, P.T.; Sturgeon, A.; Weese, J.S. Comparison of the fecal microbiota of healthy horses and horses with colitis by high throughput sequencing of the V3-V5 region of the 16S rRNA gene. PLoS ONE 2012, 7, e41484. [Google Scholar] [CrossRef]
- Honneffer, J.B.; Minamoto, Y.; Suchodolski, J.S. Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. World J. Gastroenterol. 2014, 20, 16489–16497. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Li, M.; Li, X.; Long, X.; Zuo, X.L.; Hou, X.H.; Cong, Y.Z.; Li, Y.Q. Visceral hypersensitive rats share common dysbiosis features with irritable bowel syndrome patients. World J. Gastroenterol. 2016, 22, 5211–5227. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, Y.; Chen, L.; Liu, W.; Lin, C.; Chen, Y.; Wang, X. HGF aggravated periodontitis-associated gut barrier and microbial dysfunction: Implications for oral-gut axis regulation. Biology 2025, 14, 496. [Google Scholar] [CrossRef] [PubMed]
- Bodogai, M.; O’Connell, J.; Kim, K.; Kim, Y.; Moritoh, K.; Chen, C.; Gusev, F.; Vaughan, K.; Shulzhenko, N.; Mattison, J.A.; et al. Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci. Transl. Med. 2018, 10, eaat4271. [Google Scholar] [CrossRef] [PubMed]
- van der Lugt, B.; van Beek, A.A.; Aalvink, S.; Meijer, B.; Sovran, B.; Vermeij, W.P.; Brandt, R.M.C.; de Vos, W.M.; Savelkoul, H.F.J.; Steegenga, W.T.; et al. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1 (−/Delta7) mice. Immun. Ageing 2019, 16, 6. [Google Scholar] [CrossRef]
- Li, S.; Hua, D.; Wang, Q.; Yang, L.; Wang, X.; Luo, A.; Yang, C. The role of bacteria and its derived metabolites in chronic pain and depression: Recent findings and research progress. Int. J. Neuropsychopharmacol. 2020, 23, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Qu, Y.; Fujita, Y.; Ren, Q.; Ma, M.; Dong, C.; Hashimoto, K. Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl. Psychiatry 2017, 7, 1294. [Google Scholar] [CrossRef]
- Hiippala, K.; Barreto, G.; Burrello, C.; Diaz-Basabe, A.; Suutarinen, M.; Kainulainen, V.; Bowers, J.R.; Lemmer, D.; Engelthaler, D.M.; Eklund, K.K.; et al. Novel Odoribacter splanchnicus strain and Its outer membrane vesicles exert immunoregulatory effects in vitro. Front. Microbiol. 2020, 11, 575455. [Google Scholar] [CrossRef]
- Lai, Z.L.; Tseng, C.H.; Ho, H.J.; Cheung, C.K.Y.; Lin, J.Y.; Chen, Y.J.; Cheng, F.C.; Hsu, Y.C.; Lin, J.T.; El-Omar, E.M.; et al. Fecal microbiota transplantation confers beneficial metabolic effects of diet and exercise on diet-induced obese mice. Sci. Rep. 2018, 8, 15625. [Google Scholar] [CrossRef]
- Russo, R.; De Caro, C.; Avagliano, C.; Cristiano, C.; La Rana, G.; Mattace Raso, G.; Berni Canani, R.; Meli, R.; Calignano, A. Sodium butyrate and its synthetic amide derivative modulate nociceptive behaviors in mice. Pharmacol. Res. 2016, 103, 279–291. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Yarnitsky, D.; Arendt-Nielsen, L.; Bouhassira, D.; Edwards, R.R.; Fillingim, R.B.; Granot, M.; Hansson, P.; Lautenbacher, S.; Marchand, S.; Wilder-Smith, O. Recommendations on terminology and practice of psychophysical DNIC testing. Eur. J. Pain 2010, 14, 339. [Google Scholar] [CrossRef] [PubMed]
- Le Bars, D.; Dickenson, A.H.; Besson, J.M. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain 1979, 6, 283–304. [Google Scholar] [CrossRef] [PubMed]
- Mackey, I.G.; Dixon, E.A.; Johnson, K.; Kong, J.T. Dynamic quantitative sensory testing to characterize central pain processing. J. Vis. Exp. 2017, 120, e54452. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, B.P.; de Lorimier, L.P.; Moreau, M.; Beauchamp, G.; Blair, J.; Lussier, B.; Pelletier, J.P.; Troncy, E. Pain characterization and response to palliative care in dogs with naturally-occurring appendicular osteosarcoma: An open label clinical trial. PLoS ONE 2018, 13, e0207200. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Colombo, C.; Butler, M.; Hickman, L.; Selwyn, M.; Chart, J.; Steinetz, B. A new model of osteoarthritis in rabbits. II. Evaluation of anti-osteoarthritic effects of selected antirheumatic drugs administered systemically. Arthritis Rheum. 1983, 26, 1132–1139. [Google Scholar] [CrossRef]
- Gerwin, N.; Bendele, A.M.; Glasson, S.S.; Carlson, C.S. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S24–S34. [Google Scholar] [CrossRef] [PubMed]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, Y.; Luo, B.; Li, L.; Zhang, Y.; Yan, F. Non-surgical periodontal treatment restored the gut microbiota and intestinal barrier in apolipoprotein E(−/−) mice with periodontitis. Front. Cell. Infect. Microbiol. 2020, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, E.; Di Pilato, V.; Parisio, C.; Micheli, L.; Toti, A.; Pacini, A.; Bartolucci, G.; Baldi, S.; Niccolai, E.; Amedei, A.; et al. Visceral sensitivity modulation by faecal microbiota transplantation: The active role of gut bacteria in pain persistence. Pain 2022, 163, 861–877. [Google Scholar] [CrossRef] [PubMed]

| MWM Test Parameters | LOU OA (n = 8) | SD OA (n = 8) |
|---|---|---|
| Time in quadrant 1 (s) | 43.23 (9.79) a | 40.04 (8.17) a |
| Time in quadrant 3 (s) | 12.60 (4.51) b | 12.81 (6.96) b |
| Distance in quadrant 1 (cm) | 1118.00 (220.60) c | 898.80 (190.40) c |
| Distance in quadrant 3 (cm) | 385.00 (152.40) d | 333.20 (136.80) d |
| Distance from the platform (cm) | 54.62 (9.78) | 51.11 (6.57) |
| Total distance swimming (cm) | 2488.00 (189.80) ** | 2140.00 (257.10) ** |
| Average speed in activity (cm/s) | 34.68 (0.78) | 34.64 (1.13) |
| Number of times the rats enter the area without platform (count) | 2.75 (2.25) | 2.88 (2.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frézier, M.; Otis, C.; Labelle, E.; Lussier, B.; Gaudreau, P.; Authier, S.; Costa, M.C.; Beaudry, H.; Troncy, E. Comparative Pain Expression and Its Association to Intestinal Microbiota Through the MI-RAT© Osteoarthritis Model Induced in LOU/C/Jall and Sprague-Dawley Aged Rats. Int. J. Mol. Sci. 2025, 26, 7698. https://doi.org/10.3390/ijms26167698
Frézier M, Otis C, Labelle E, Lussier B, Gaudreau P, Authier S, Costa MC, Beaudry H, Troncy E. Comparative Pain Expression and Its Association to Intestinal Microbiota Through the MI-RAT© Osteoarthritis Model Induced in LOU/C/Jall and Sprague-Dawley Aged Rats. International Journal of Molecular Sciences. 2025; 26(16):7698. https://doi.org/10.3390/ijms26167698
Chicago/Turabian StyleFrézier, Marilyn, Colombe Otis, Emilie Labelle, Bertrand Lussier, Pierrette Gaudreau, Simon Authier, Marcio Carvalho Costa, Hélène Beaudry, and Eric Troncy. 2025. "Comparative Pain Expression and Its Association to Intestinal Microbiota Through the MI-RAT© Osteoarthritis Model Induced in LOU/C/Jall and Sprague-Dawley Aged Rats" International Journal of Molecular Sciences 26, no. 16: 7698. https://doi.org/10.3390/ijms26167698
APA StyleFrézier, M., Otis, C., Labelle, E., Lussier, B., Gaudreau, P., Authier, S., Costa, M. C., Beaudry, H., & Troncy, E. (2025). Comparative Pain Expression and Its Association to Intestinal Microbiota Through the MI-RAT© Osteoarthritis Model Induced in LOU/C/Jall and Sprague-Dawley Aged Rats. International Journal of Molecular Sciences, 26(16), 7698. https://doi.org/10.3390/ijms26167698





