The Sweet Side of IVF: Biological Role and Diagnostic Potential of Galectin-9 in Female Infertility
Abstract
1. Introduction
2. Results
2.1. Galectin-9 Expression in the Human Ovary
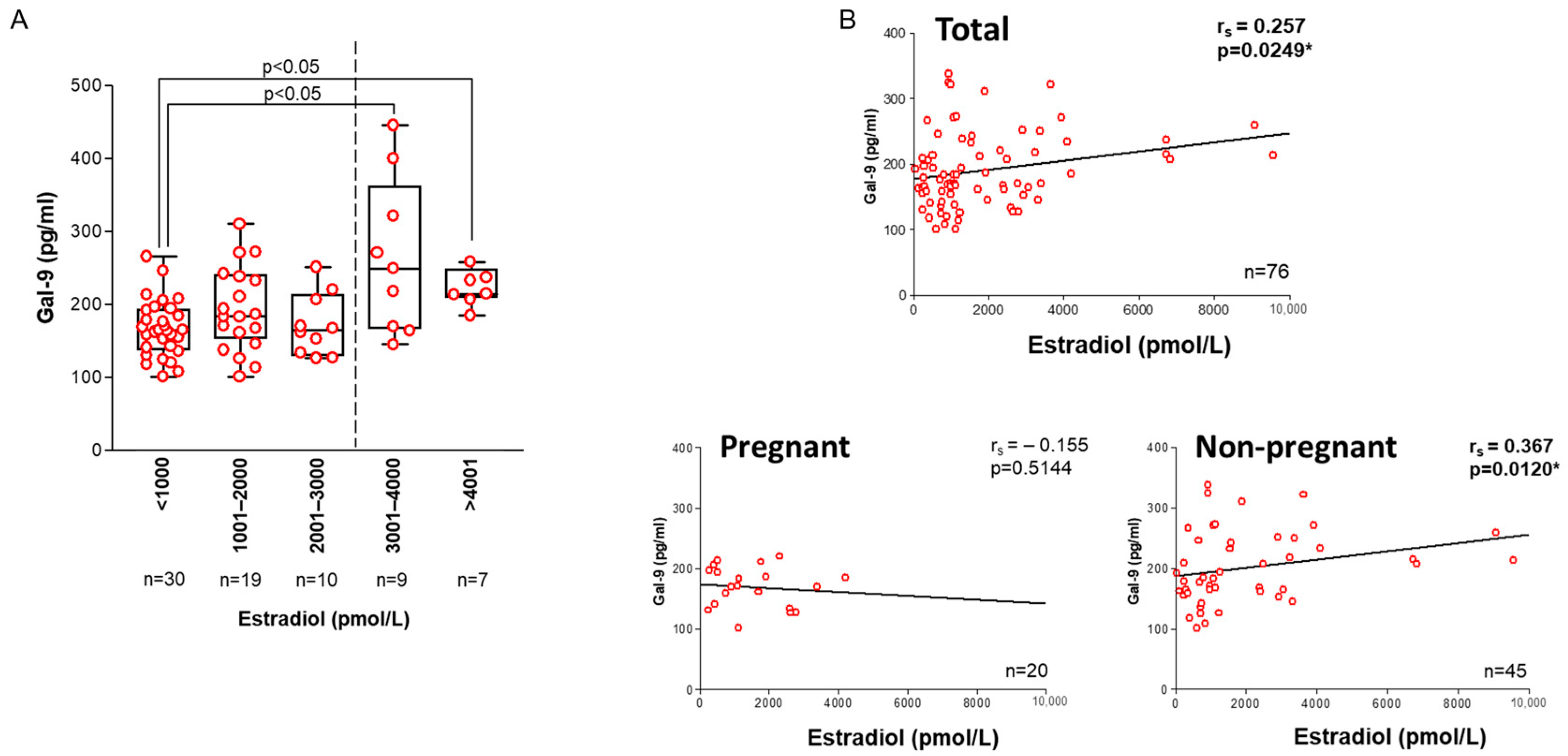
2.2. Association with IVF-Related Parameters
2.3. Association with Laboratory Parameters
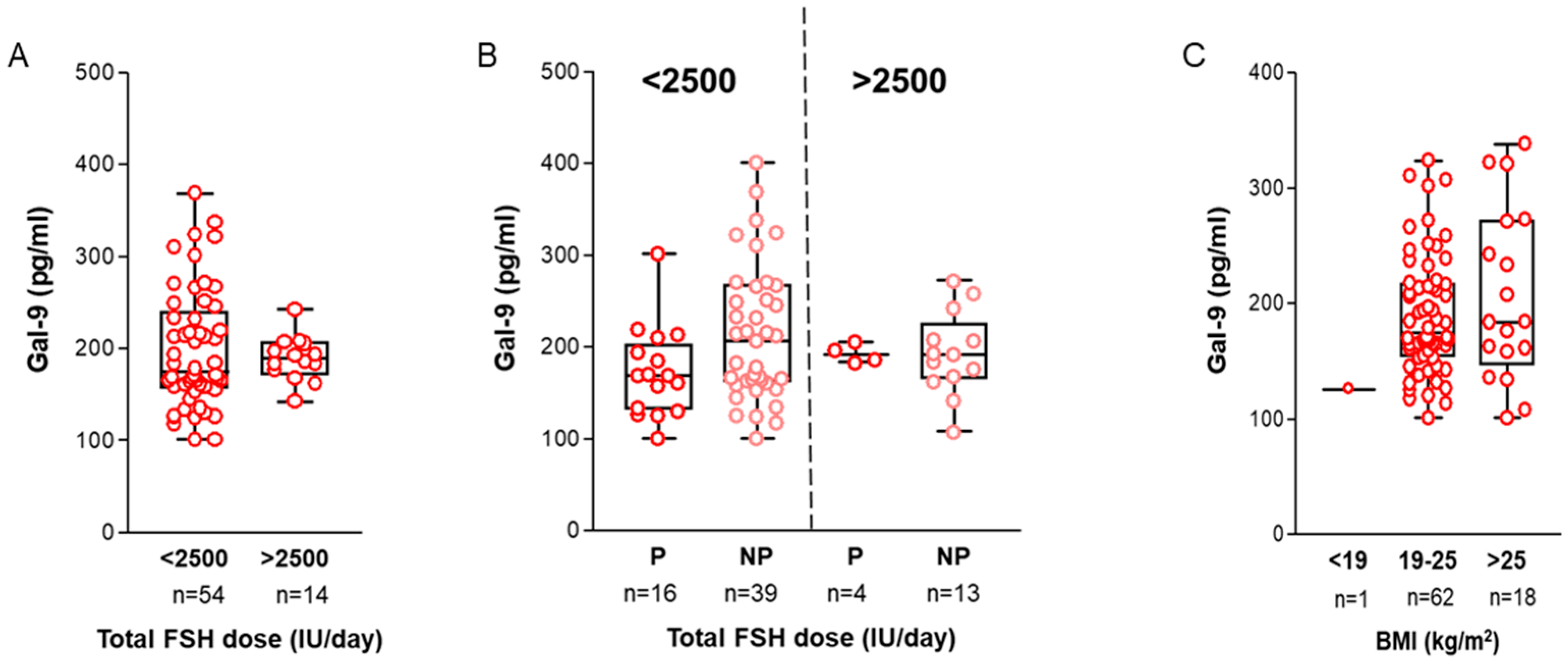
2.4. Association with Treatment-Related Data and Clinical Parameters
2.5. Interactive Effect of Multiple Clinical Parameters
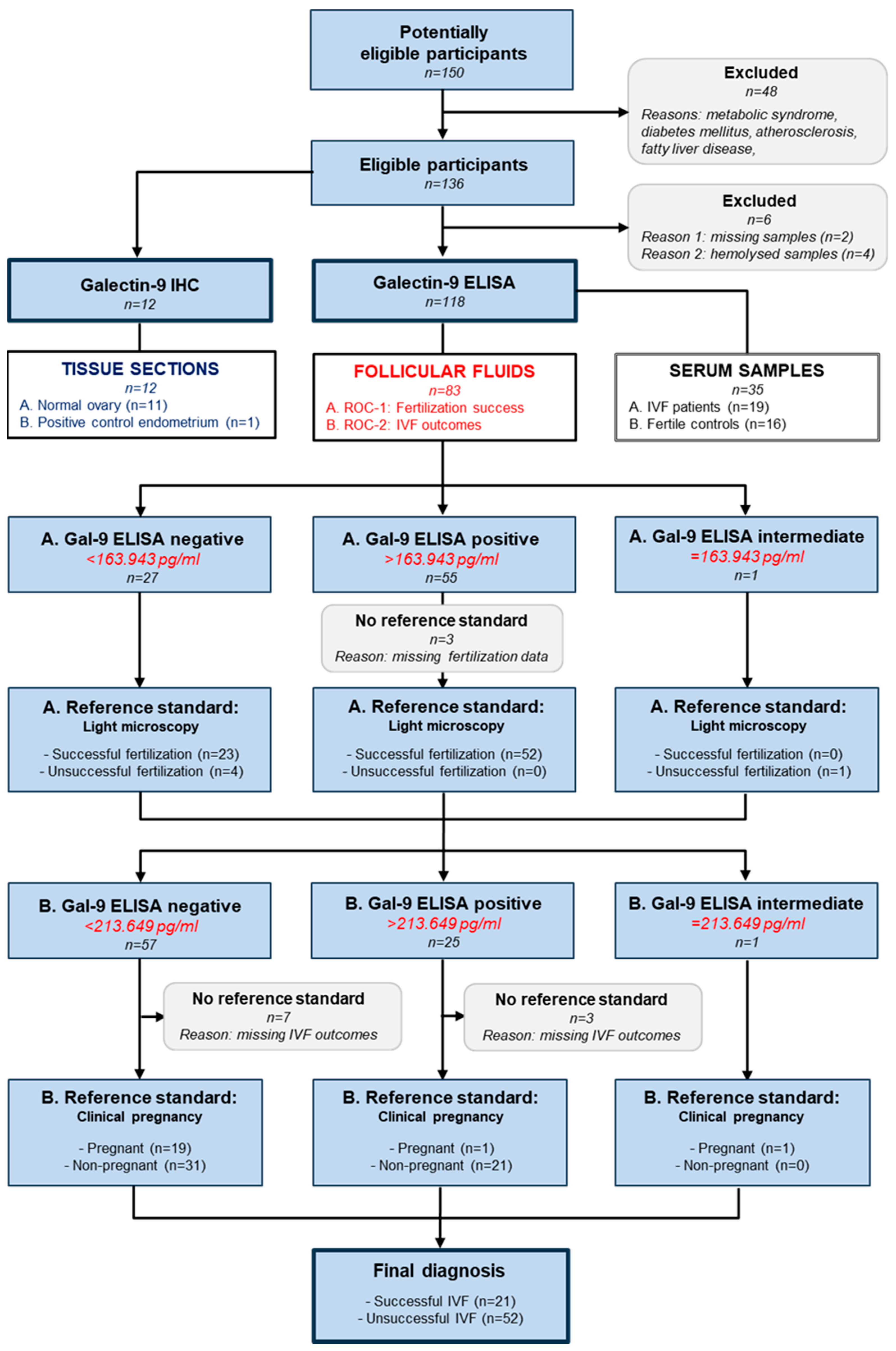
2.6. Follicular and Serum Galectin-9 Levels in IVF Patients and Controls
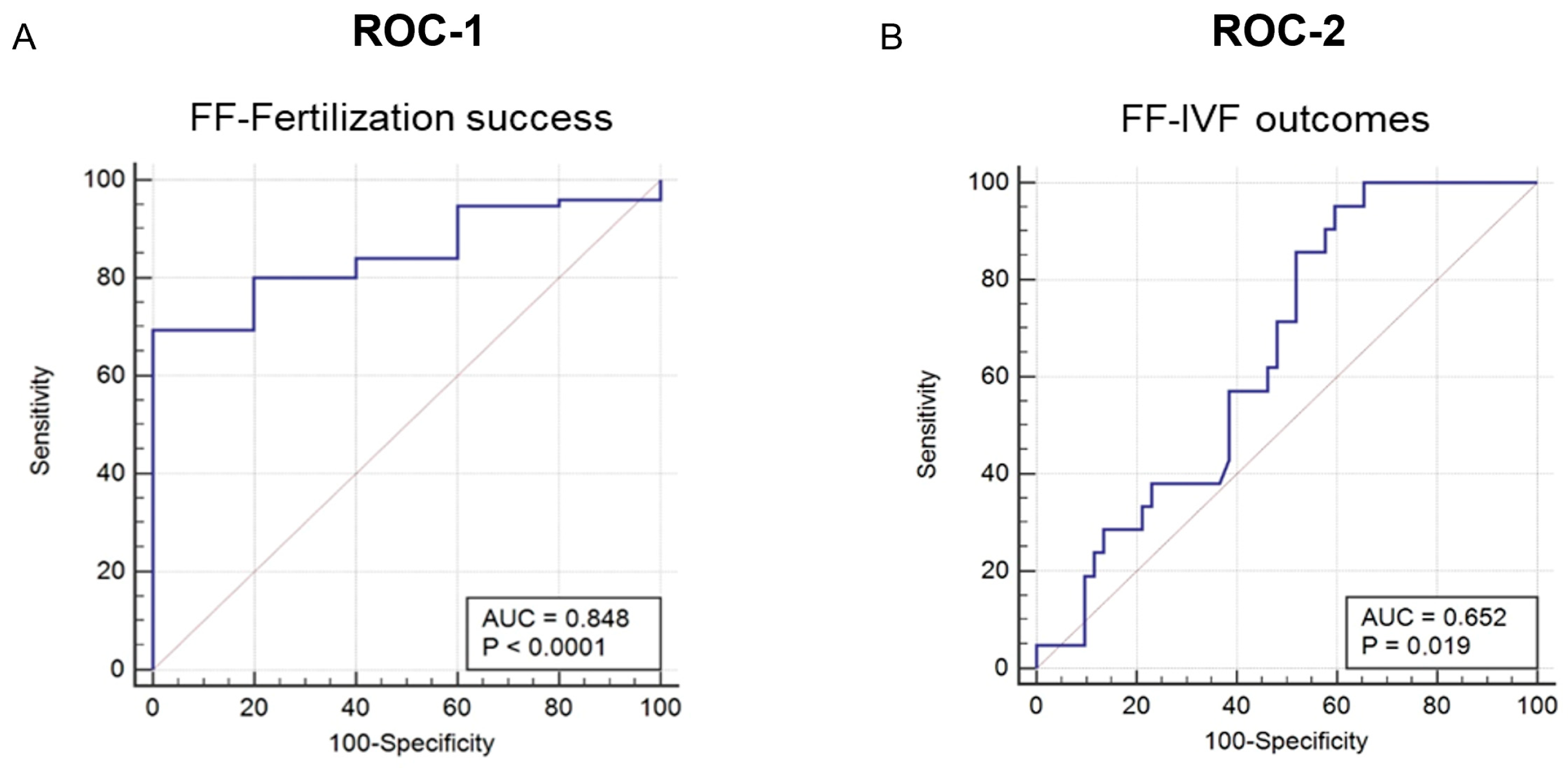
2.7. Diagnostic Value of Intrafollicular Gal-9 Measurement
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Controlled Ovarian Hyperstimulation (COH)
4.3. Collection of Biological Samples
4.4. In Vitro Fertilization (IVF) Procedures
4.5. Immunohistochemistry (IHC)
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFC | Antral Follicle Count |
| AMH | Anti-Müllerian hormone |
| ART | Assisted Reproductive Technology |
| AUC | Area Under the Curve |
| BMI | Body Mass Index |
| BSA | Bovine Serum Albumin |
| CG | Cumulus granulosa |
| cIVF | Conventional IVF |
| COH | Controlled ovarian hyperstimulation |
| D3 | Day 3 |
| DHT | Dihydrotestosterone |
| E2 | Estradiol |
| ELISA | Enzyme Linked Immunosorbent Assay |
| ET | Embryo transfer |
| FF | Follicular fluid |
| FFPE | Formalin-fixed paraffin-embedded |
| FSH | Follicle-stimulating hormone |
| Gal | Galectin |
| hCG | Human choriogonadotropin alfa |
| H&E | Hematoxylin and eosin |
| ICSI | Intracytoplasmic sperm injection |
| IHC | Immunohistochemistry |
| IL | Interleukin |
| IGF1 | Insulin-like growth factor-1 |
| IU | International Unit |
| i.v. | Intravenous |
| IVF | In vitro fertilization |
| LH | Luteinizing hormone |
| MG | Mural Granulosa |
| min | Minutes |
| NoET | No Embryo Transfer |
| NP | Non pregnant |
| ns | Not significant |
| OHSS | Ovarian hyperstimulation syndrome |
| P | Pregnant |
| PM | Plasma membrane |
| PMSG | Pregnant mare serum gonadotropin |
| miRNA | MicroRNA |
| ROC | Receiver Operating Characteristic |
| RT | Room temperature |
| SENS | Sensitivity |
| SF | Successful fertilization |
| sHLA-G | Soluble human leukocyte antigen-G |
| SPEC | Specificity |
| TBS | Tris buffered saline |
| TBST | TBS-Tween |
| TE | Theca externa |
| TI | Theca interna |
| UF | Unsuccessful fertilization |
Appendix A
| Demographic Characteristics | 1. NoET (n = 6) | 2. Pregnant (n = 22) | 3. Non Pregnant (n = 55) | p-Values |
|---|---|---|---|---|
| Demographic Variables | ||||
| Age (years) [mean (SD)] | 37.00 (2.83) | 34.58 (5.62) | 36.91 (4.91) | ns |
| BMI (kg/m2) [mean (SD)] | 22.1 (1.23) | 24.10 (2.91) | 24.18 (4.02) | ns |
| Nulligravida [n (%)] | 2/6 (33.33) | 9/22 (40.91) | 35/55 (60.00) | ns |
| Clinical background data | ||||
| Cause of infertility [n (%)] | ||||
| Female factor | 4/6 (66.67) | 18/22 (81.82) | 41/55 (74.55) | ns |
| Male factor | 1/6 (16.67) | 3/22 (13.64) | 4/55 (7.27) | ns |
| Combined | 0/6 (0) | 0/22 (0) | 3/55 (5.45) | ns |
| Unexplained | 1/6 (16.66) | 1/22 (4.54) | 7/55 (12.73) | ns |
| Infertility duration (years) [mean (SD)] | 3.33 (1.21) | 3.05 (1.53) | 3.73 (2.03) | ns |
| Previous pregnancy [mean (SD)] | 1.5 (1.52) | 1.00 (1.19) | 0.91 (1.37) | ns |
| Previous IVF cycles [mean (SD)] | 2.17 (1.33) | 1.41 (0.67) | 1.69 (1.09) | ns |
| Total of retrieved oocyte [mean (SD)] | 18.83 (15.11) | 8.82 (5.41) | 7.22 (5.26) | p = 0.0413 * (1–2), (1–3) |
| Total of created embryo [mean (SD)] | 5.83 (5.27) | 4.38 (3.12) | 3.49 (3.85) | ns |
| Number of blastocysts per transfer [mean (SD)] | 0 (0) | 1.96 (0.49) | 1.68 (0.59) | p < 0.0001 * (1–2), (1–3) |
| Laboratory data | ||||
| Serum Estradiol (pmol/L) [mean (SD)] | 2948.2 (2632.8) | 1530.2 (1139.7) | 1923.1 (2116.6) | ns |
| FSH (IU/L)—day3 [mean (SD)] | 6.13 (1.43) | 7.39 (3.45) | 6.88 (3.05) | ns |
| LH (IU/L)—day3 [mean (SD)] | 6.36 (4.66) | 3.98 (1.36) | 4.72 (4.57) | ns |
| Total FSH dose/day (IU) [mean (SD)] | 1383.3 (561.8) | 1866.7 (784.7) | 1871.8 (739.4) | ns |
| Demographic Characteristics | 1. IVF Patients (n = 19) | 2. Fertile Controls (n = 16) | p-Values |
|---|---|---|---|
| Age (years) [mean (SD)] | 33.58 (5.10) | 33.06 (8.93) | ns |
| BMI (kg/m2) [mean (SD)] | 25.02 (5.43) | 23.43 (4.46) | ns |
| Nulligravida [n (%)] | 7/19 (36.84) | 7/16 (43.75) | ns |
| Cause of infertility [n (%)] | |||
| Female factor | 8/19 (42.10) | - | - |
| Male factor | 9/19 (47.37) | - | - |
| Combined | 2/19 (10.53) | - | - |
| Unexplained | 0/19 (0.00) | - | - |
| Previous pregnancy [mean (SD)] | 0.95 (1.08) | 1.25 (1.29) | ns |
References
- Harrison, J.E.; Weber, S.; Jakob, R.; Chute, C.G. ICD-11: An International Classification of Diseases for the Twenty-First Century. BMC Med. Inform. Decis. Mak. 2021, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A Unique View on Male Infertility around the Globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.A.; Crosignani, P.G. Unexplained Infertility: A Review of Diagnosis, Prognosis, Treatment Efficacy and Management. Int. J. Gynaecol. Obstet. 1992, 39, 267–275. [Google Scholar] [CrossRef]
- Walker, M.H.; Tobler, K.J. Female Infertility; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Revelli, A.; Piane, L.D.; Casano, S.; Molinari, E.; Massobrio, M.; Rinaudo, P. Follicular Fluid Content and Oocyte Quality: From Single Biochemical Markers to Metabolomics. Reprod. Biol. Endocrinol. 2009, 7, 40. [Google Scholar] [CrossRef]
- Angelucci, S.; Ciavardelli, D.; Di Giuseppe, F.; Eleuterio, E.; Sulpizio, M.; Tiboni, G.M.; Giampietro, F.; Palumbo, P.; Di Ilio, C. Proteome Analysis of Human Follicular Fluid. Biochim. Biophys. Acta 2006, 1764, 1775–1785. [Google Scholar] [CrossRef]
- Lazzarino, G.; Pallisco, R.; Bilotta, G.; Listorti, I.; Mangione, R.; Saab, M.W.; Caruso, G.; Amorini, A.M.; Brundo, M.V.; Lazzarino, G.; et al. Altered Follicular Fluid Metabolic Pattern Correlates with Female Infertility and Outcome Measures of In Vitro Fertilization. Int. J. Mol. Sci. 2021, 22, 8735. [Google Scholar] [CrossRef]
- Bianchi, L.; Gagliardi, A.; Landi, C.; Focarelli, R.; De Leo, V.; Luddi, A.; Bini, L.; Piomboni, P. Protein Pathways Working in Human Follicular Fluid: The Future for Tailored IVF? Expert. Rev. Mol. Med. 2016, 18, e9. [Google Scholar] [CrossRef]
- Da Broi, M.G.; Giorgi, V.S.I.; Wang, F.; Keefe, D.L.; Albertini, D.; Navarro, P.A. Influence of Follicular Fluid and Cumulus Cells on Oocyte Quality: Clinical Implications. J. Assist. Reprod. Genet. 2018, 35, 735–751. [Google Scholar] [CrossRef]
- Van Blerkom, J. Molecular Mining of Follicular Fluid for Reliable Biomarkers of Human Oocyte and Embryo Developmental Competence. In In Vitro Fertilization; Springer International Publishing: Cham, Switzerland, 2019; pp. 929–937. [Google Scholar] [CrossRef]
- Asimakopoulos, B.; Nikolettos, N.; Papachristou, D.N.; Simopoulou, M.; Al-Hasani, S.; Diedrich, K. Follicular Fluid Levels of Vascular Endothelial Growth Factor and Leptin Are Associated with Pregnancy Outcome of Normal Women Participating in Intracytoplasmic Sperm Injection Cycles. Physiol. Res. 2005, 54, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Kucera, R.; Babuska, V.; Ulcova-Gallova, Z.; Kulda, V.; Topolcan, O. Follicular Fluid Levels of Anti-Müllerian Hormone, Insulin-like Growth Factor 1 and Leptin in Women with Fertility Disorders. Syst. Biol. Reprod. Med. 2018, 64, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Wang, T.T.; Chen, X.J.; Zhu, X.M.; Dong, M.Y.; Sheng, J.Z.; Xu, C.M.; Huang, H.F. Bone Morphogenetic Protein-15 in Follicle Fluid Combined with Age May Differentiate between Successful and Unsuccessful Poor Ovarian Responders. Reprod. Biol. Endocrinol. 2012, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Jarkovska, K.; Skalnikova, H.K.; Halada, P.; Hrabakova, R.; Moos, J.; Rezabek, K.; Gadher, S.J.; Kovarova, H. Development of Ovarian Hyperstimulation Syndrome: Interrogation of Key Proteins and Biological Processes in Human Follicular Fluid of Women Undergoing in Vitro Fertilization. Mol. Hum. Reprod. 2011, 17, 679–692. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.; Esko, J.; Freeze, H.; Hart, G.; Marth, J. Essentials of Glycobiology. In Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 1999. [Google Scholar]
- Than, N.G.; Romero, R.; Balogh, A.; Karpati, E.; Mastrolia, S.A.; Staretz-Chacham, O.; Hahn, S.; Erez, O.; Papp, Z.; Kim, C.J. Galectins: Double-Edged Swords in the Cross-Roads of Pregnancy Complications and Female Reproductive Tract Inflammation and Neoplasia. J. Pathol. Transl. Med. 2015, 49, 181–208. [Google Scholar] [CrossRef]
- Than, N.G.; Romero, R.; Goodman, M.; Weckle, A.; Xing, J.; Dong, Z.; Xu, Y.; Tarquini, F.; Szilagyi, A.; Gal, P.; et al. A Primate Subfamily of Galectins Expressed at the Maternal-Fetal Interface That Promote Immune Cell Death. Proc. Natl. Acad. Sci. USA 2009, 106, 9731–9736. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, U.; Hutter, S.; Heublein, S.; Vrekoussis, T.; Andergassen, U.; Unverdorben, L.; Papadakis, G.; Makrigiannakis, A. Expression and Function of Galectins in the Endometrium and at the Human Feto-Maternal Interface. Placenta 2013, 34, 863–872. [Google Scholar] [CrossRef] [PubMed]
- von Wolff, M.; Wang, X.; Gabius, H.J.; Strowitzki, T. Galectin Fingerprinting in Human Endometrium and Decidua during the Menstrual Cycle and in Early Gestation. Mol. Hum. Reprod. 2005, 11, 189–194. [Google Scholar] [CrossRef]
- Blois, S.M.; Verlohren, S.; Wu, G.; Clark, G.; Dell, A.; Haslam, S.M.; Barrientos, G. Role of Galectin-Glycan Circuits in Reproduction: From Healthy Pregnancy to Preterm Birth (PTB). Semin. Immunopathol. 2020, 42, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Popovici, R.M.; Krause, M.S.; Germeyer, A.; Strowitzki, T.; Von Wolff, M. Galectin-9: A New Endometrial Epithelial Marker for the Mid- and Late-Secretory and Decidual Phases in Humans. J. Clin. Endocrinol. Metab. 2005, 90, 6170–6176. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Kabir-Salmani, M.; Azadbakht, M.; Sugihara, K.; Sakai, K.; Iwashita, M. Expression and Localization of Galectin-9 in the Human Uterodome. Endocr. J. 2008, 55, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Heusschen, R.; Freitag, N.; Tirado-González, I.; Barrientos, G.; Moschansky, P.; Muñoz-Fernández, R.; Leno-Durán, E.; Klapp, B.F.; Thijssen, V.L.J.L.; Blois, S.M. Profiling Lgals9 Splice Variant Expression at the Fetal-Maternal Interface: Implications in Normal and Pathological Human Pregnancy. Biol. Reprod. 2013, 88, 22. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhou, W.H.; Tao, Y.; Wang, S.C.; Jiang, Y.L.; Zhang, D.; Piao, H.L.; Fu, Q.; Li, D.J.; Du, M.R. The Galectin-9/Tim-3 Pathway Is Involved in the Regulation of NK Cell Function at the Maternal-Fetal Interface in Early Pregnancy. Cell Mol. Immunol. 2016, 13, 73–81. [Google Scholar] [CrossRef]
- He, M.; Jiang, M.; Zhou, Y.; Li, F.; Yang, M.; Fan, Y.; Xie, Y.; Beejadhursing, R.; Feng, L.; Deng, D. Impaired Gal-9 Dysregulates the PBMC-Induced Th1/Th2 Imbalance in Abortion-Prone Matings. J. Immunol. Res. 2018, 2018, 9517842. [Google Scholar] [CrossRef]
- Meggyes, M.; Miko, E.; Polgar, B.; Bogar, B.; Farkas, B.; Illes, Z.; Szereday, L. Peripheral Blood TIM-3 Positive NK and CD8+ T Cells throughout Pregnancy: TIM-3/Galectin-9 Interaction and Its Possible Role during Pregnancy. PLoS ONE 2014, 9, e92371. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, L.L.; Liu, H.; Muyayalo, K.P.; Huang, X.B.; Mor, G.; Liao, A.H. Galectin-9 Alleviates LPS-Induced Preeclampsia-Like Impairment in Rats via Switching Decidual Macrophage Polarization to M2 Subtype. Front. Immunol. 2019, 9, 3142. [Google Scholar] [CrossRef]
- Li, M.; Peng, X.; Qian, J.; Sun, F.; Chen, C.; Wang, S.; Zhang, J.; Du, M. Galectin-9 Regulates HTR8/SVneo Function via JNK Signaling. Reproduction 2021, 161, 1–10. [Google Scholar] [CrossRef]
- Thijssen, V.L.; Hulsmans, S.; Griffioen, A.W. The Galectin Profile of the Endothelium: Altered Expression and Localization in Activated and Tumor Endothelial Cells. Am. J. Pathol. 2008, 172, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, G.; Freitag, N.; Tirado-González, I.; Unverdorben, L.; Jeschke, U.; Thijssen, V.L.J.L.; Blois, S.M. Involvement of Galectin-1 in Reproduction: Past, Present and Future. Hum. Reprod. Update 2014, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Vergetaki, A.; Jeschke, U.; Vrekoussis, T.; Taliouri, E.; Sabatini, L.; Papakonstanti, E.A.; Makrigiannakis, A. Galectin-1 Overexpression in Endometriosis and Its Regulation by Neuropeptides (CRH, UCN) Indicating Its Important Role in Reproduction and Inflammation. PLoS ONE 2014, 9, e114229. [Google Scholar] [CrossRef]
- Yang, H.; Yin, J.; Ficarrotta, K.; Hsu, S.H.; Zhang, W.; Cheng, C. Aberrant Expression and Hormonal Regulation of Galectin-3 in Endometriosis Women with Infertility. J. Endocrinol. Invest. 2016, 39, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Brubel, R.; Bokor, A.; Pohl, A.; Schilli, G.K.; Szereday, L.; Bacher-Szamuel, R.; Rigo, J.; Polgar, B. Serum Galectin-9 as a Noninvasive Biomarker for the Detection of Endometriosis and Pelvic Pain or Infertility-Related Gynecologic Disorders. Fertil. Steril. 2017, 108, 1016–1025.e2. [Google Scholar] [CrossRef]
- Labrie, M.; De Araujo, L.O.F.; Communal, L.; Mes-Masson, A.M.; St-Pierre, Y. Tissue and Plasma Levels of Galectins in Patients with High Grade Serous Ovarian Carcinoma as New Predictive Biomarkers. Sci. Rep. 2017, 7, 13244. [Google Scholar] [CrossRef]
- von Wolff, M.; Schwartz, A.K.; Bitterlich, N.; Stute, P.; Fäh, M. Only Women’s Age and the Duration of Infertility Are the Prognostic Factors for the Success Rate of Natural Cycle IVF. Arch. Gynecol. Obstet. 2019, 299, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Pan, C.; Zhang, C. Unraveling the Complexity of Follicular Fluid: Insights into Its Composition, Function, and Clinical Implications. J. Ovarian Res. 2024, 17, 237. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, L.; Zosmer, A.; Hennessy, E.M.; Tozer, A.; Al-Shawaf, T. Relevance of Basal Serum FSH to IVF Outcome Varies with Patient Age. Reprod. Biomed. Online 2008, 17, 10–19. [Google Scholar] [CrossRef]
- Amanvermez, R.; Tosun, M. An Update on Ovarian Aging and Ovarian Reserve Tests. Int. J. Fertil. Steril. 2016, 9, 411–415. [Google Scholar] [CrossRef]
- Pasqualotto, E.B.; Agarwal, A.; Sharma, R.K.; Izzo, V.M.; Pinotti, J.A.; Joshi, N.J.; Rose, B.I. Effect of Oxidative Stress in Follicular Fluid on the Outcome of Assisted Reproductive Procedures. Fertil. Steril. 2004, 81, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.E.; Greenblatt, E.M. Elevated Day 3 Follicle-Stimulating Hormone/Luteinizing Hormone Ratio >or= 2 Is Associated with Higher Rates of Cancellation in in Vitro Fertilization-Embryo Transfer Cycles. Fertil. Steril. 2008, 90, 297–301. [Google Scholar] [CrossRef]
- Munch, E.M.; Sparks, A.E.; Zimmerman, M.B.; Van Voorhis, B.J.; Duran, E.H. High FSH Dosing Is Associated with Reduced Live Birth Rate in Fresh but Not Subsequent Frozen Embryo Transfers. Hum. Reprod. 2017, 32, 1402–1409. [Google Scholar] [CrossRef]
- Baker, V.L.; Brown, M.B.; Luke, B.; Smith, G.W.; Ireland, J.J. Gonadotropin Dose Is Negatively Correlated with Live Birth Rate: Analysis of More than 650,000 Assisted Reproductive Technology Cycles. Fertil. Steril. 2015, 104, 1145–1152.e5. [Google Scholar] [CrossRef]
- Medenica, S.; Spoltore, M.E.; Ormazabal, P.; Marina, L.V.; Sojat, A.S.; Faggiano, A.; Gnessi, L.; Mazzilli, R.; Watanabe, M. Female Infertility in the Era of Obesity: The Clash of Two Pandemics or Inevitable Consequence? Clin. Endocrinol. 2023, 98, 141–152. [Google Scholar] [CrossRef]
- Zakaria, A.M.; Saeed, A.M.; Roshdy, A.A. The Relation between Follicular Fluid Anti-Müllerian Hormone (AMH), Oocyte Maturation and Embryo Development in Intracytoplasmic Sperm Injection (ICSI) Cycles. Al-Azhar Int. Med. J. 2023, 4, 14. [Google Scholar] [CrossRef]
- Wyse, B.A.; Fuchs Weizman, N.; Defer, M.; Montbriand, J.; Szaraz, P.; Librach, C. The Follicular Fluid Adipocytokine Milieu Could Serve as a Prediction Tool for Fertility Treatment Outcomes. Reprod. Biomed. Online 2021, 43, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liu, M.; Xu, S.; Wang, Z.; Liu, T.; Zhou, J.; Zhang, D.; Dong, X.; Pan, B.; Wang, B.; et al. Follicular Fluid Steroid and Gonadotropic Hormone Levels and Mitochondrial Function from Exosomes Predict Embryonic Development. Front. Endocrinol 2022, 13, 1025523. [Google Scholar] [CrossRef] [PubMed]
- Jee, B.C.; Suh, C.S.; Kim, S.H.; Moon, S.Y. Soluble Human Leukocyte Antigen G Level in Fluid from Single Dominant Follicle and the Association with Oocyte Competence. Yonsei Med. J. 2011, 52, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Artini, P.G.; Scarfò, G.; Marzi, I.; Fusi, J.; Obino, M.E.; Franzoni, F.; Zappelli, E.; Chelucci, E.; Martini, C.; Cela, V.; et al. Oxidative Stress-Related Signaling Pathways Predict Oocytes’ Fertilization In Vitro and Embryo Quality. Int. J. Mol. Sci. 2022, 23, 13442. [Google Scholar] [CrossRef]
- Ilhan, G.; Bacanakgil, B.H.; Vuruşkan, A.K.; Eken, M.K.; Karasu, A.F.G.; Bilgiç, B.E.; Küçükyurt, A.K. The Effect of Individual Oocyte Matched Follicular Fluid Oxidant, Antioxidant Status, and pro- and Anti-Inflammatory Cytokines on IVF Outcomes of Patients with Diminished Ovarian Reserve. Medicine 2023, 102, e32757. [Google Scholar] [CrossRef]
- Elsabaa, H.A.; El Mandoh Mohamed, M.; Koura, A.M.S.; Elsayed, T.H. Follicular Fluid Leptin as a Marker for Pregnancy Outcomes in Women in General Subfertility Population Undergoing IVF/ICSI Treatment. Ginekol. I Poloz. 2023, 18, 1–7. [Google Scholar]
- Lv, Y.; Du, S.; Huang, X.; Hao, C. Follicular Fluid Estradiol Is an Improved Predictor of in Vitro Fertilization/Intracytoplasmic Sperm Injection and Embryo Transfer Outcomes. Exp. Ther. Med. 2020, 20, 131. [Google Scholar] [CrossRef]
- Faraj, N.; Alhalabi, M.; Al-Quobaili, F. Predictive Value of Follicular Fluid Insulin like Growth Factor-1 in IVF Outcome of Normo-Ovulatory Women. Middle East Fertil. Soc. J. 2017, 22, 101–104. [Google Scholar] [CrossRef]
- Mostafa, M.H.; Faisal, M.M.; Mohamed, N.R.; Idle, F.H.; Mostafa, M.H.; Faisal, M.M.; Mohamed, N.R.; Idle, F.H. Effect of Follicular Fluid Lactoferrin Level on Oocytes Quality and Pregnancy Rate in Intracytoplasmic Sperm Injection Cycles. Open J. Obstet. Gynecol. 2019, 9, 745–754. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, C.; Li, Y.; Li, H.; Zhang, W.; Liu, J.; Wang, L.; Sun, C. Galectin-9 in Cancer Therapy: From Immune Checkpoint Ligand to Promising Therapeutic Target. Front. Cell Dev. Biol. 2024, 11, 1332205. [Google Scholar] [CrossRef]
- Sunkara, S.K.; Rittenberg, V.; Raine-Fenning, N.; Bhattacharya, S.; Zamora, J.; Coomarasamy, A. Association between the Number of Eggs and Live Birth in IVF Treatment: An Analysis of 400 135 Treatment Cycles. Hum. Reprod. 2011, 26, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Liang, X.; Ren, X.; Shi, Y.; Su, H.; Li, Y.; Du, K.; Wang, J.; Jia, X.; Chen, S.; et al. Integrated Analysis of mRNA and miRNA Expression Profiles in the Ovary of Oryctolagus Cuniculus in Response to Gonadotrophic Stimulation. Front. Endocrinol. 2019, 10, 744. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Tanaka, S.; Hisamatsu, Y.; Tsubokura, H.; Hashimoto, Y.; Kitada, M.; Okada, H. Transcriptional Regulation of LGALS9 by HAND2 and FOXO1 in Human Endometrial Stromal Cells in Women with Regular Cycles. Mol. Hum. Reprod. 2021, 27, gaab063. [Google Scholar] [CrossRef] [PubMed]
- Naciff, J.M.; Jump, M.L.; Torontali, S.M.; Carr, G.J.; Tiesman, J.P.; Overmann, G.J.; Daston, G.P. Gene Expression Profile Induced by 17alpha-Ethynyl Estradiol, Bisphenol A, and Genistein in the Developing Female Reproductive System of the Rat. Toxicol. Sci. 2002, 68, 184–199. [Google Scholar] [CrossRef]
- Anifandis, G.; Koutselini, E.; Louridas, K.; Liakopoulos, V.; Leivaditis, K.; Mantzavinos, T.; Sioutopoulou, D.; Vamvakopoulos, N. Estradiol and Leptin as Conditional Prognostic IVF Markers. Reproduction 2005, 129, 531–534. [Google Scholar] [CrossRef]
- Rienzi, L.; Vajta, G.; Ubaldi, F. Predictive Value of Oocyte Morphology in Human IVF: A Systematic Review of the Literature. Hum. Reprod. Update 2010, 17, 34. [Google Scholar] [CrossRef]




| Variables | Bivariate Correlation | Multiple Regression | |||
|---|---|---|---|---|---|
| R2 = 0.137, p = 0.148 | |||||
| r | p | B | SE | p | |
| (Constant) | - | - | 153.666 | 65.500 | 0.022 * |
| BMI | 0.056 | 0.613 | 2.872 | 2.417 | 0.239 |
| E2 | 0.201 | 0.072 | 0.003 | 0.002 | 0.216 |
| FSH | −0.011 | 0.916 | 2.441 | 3.194 | 0.447 |
| Total FSH dose/day | −0.247 | 0.022 * | −0.030 | 0.013 | 0.021 * |
| LH | 0.151 | 0.173 | 2.845 | 2.866 | 0.324 |
| Infertility duration | 0.005 | 0.963 | −4.183 | 5.284 | 0.434 |
| IVF cycles | 0.048 | 0.657 | 9.727 | 9.509 | 0.310 |
| Fertilization | AUC | SENS | SPEC | IVF Outcome | AUC | SENS | SPEC |
|---|---|---|---|---|---|---|---|
| Gal-9 | 0.848 | 63.3 | 100 | Gal-9 | 0.652 | 95.2 | 40.4 |
| AMH | 0.813 | 98.4 | 62.5 | E2 | 0.283 | 27.3 | 37.5 |
| Prolactin | 0.760 | 77.0 | 78.0 | IGF1 | 0.629 | 87.9 | 40.0 |
| DHT | 0.756 | 81.7 | 65.4 | Leptin | 0.856 | 80.8 | 77.3 |
| IL-10 | 0.910 | 87.0 | 74.0 | IL-8 | 0.920 | 100.0 | 78.6 |
| sHLA-G | 0.676 | 71.4 | 75.6 | Lactoferrin | 0.513 | 45.0 | 65.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polgar, B.; Meggyes, M.; Godony, K.; Varnagy, A.; Kovacs, K.; Mauchart, P.; Matrai, P.; Kovacs, K.; Semjen, D.; Tornoczki, T.; et al. The Sweet Side of IVF: Biological Role and Diagnostic Potential of Galectin-9 in Female Infertility. Int. J. Mol. Sci. 2025, 26, 7672. https://doi.org/10.3390/ijms26167672
Polgar B, Meggyes M, Godony K, Varnagy A, Kovacs K, Mauchart P, Matrai P, Kovacs K, Semjen D, Tornoczki T, et al. The Sweet Side of IVF: Biological Role and Diagnostic Potential of Galectin-9 in Female Infertility. International Journal of Molecular Sciences. 2025; 26(16):7672. https://doi.org/10.3390/ijms26167672
Chicago/Turabian StylePolgar, Beata, Matyas Meggyes, Krisztina Godony, Akos Varnagy, Kalman Kovacs, Peter Mauchart, Peter Matrai, Krisztina Kovacs, David Semjen, Tamas Tornoczki, and et al. 2025. "The Sweet Side of IVF: Biological Role and Diagnostic Potential of Galectin-9 in Female Infertility" International Journal of Molecular Sciences 26, no. 16: 7672. https://doi.org/10.3390/ijms26167672
APA StylePolgar, B., Meggyes, M., Godony, K., Varnagy, A., Kovacs, K., Mauchart, P., Matrai, P., Kovacs, K., Semjen, D., Tornoczki, T., & Szereday, L. (2025). The Sweet Side of IVF: Biological Role and Diagnostic Potential of Galectin-9 in Female Infertility. International Journal of Molecular Sciences, 26(16), 7672. https://doi.org/10.3390/ijms26167672






