Substance Abuse and Cognitive Decline: The Critical Role of Tau Protein as a Potential Biomarker
Abstract
1. Introduction
2. Results
2.1. Tau and Depressants (Alcohol)
2.2. Tau and Stimulants (Cocaine and Methamphetamine)
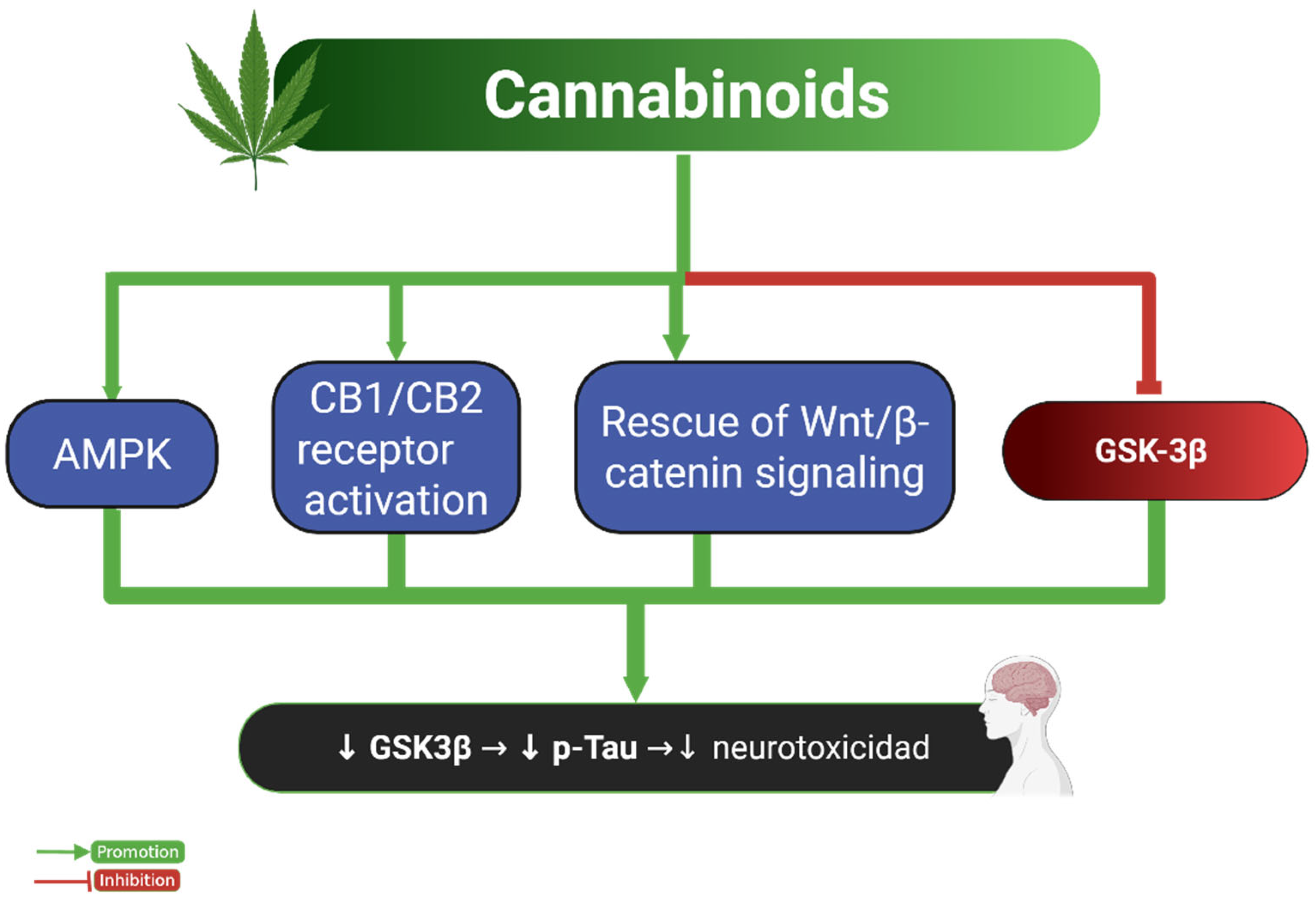
2.3. Tau and Cannabinoids
2.4. Tau and Opioids
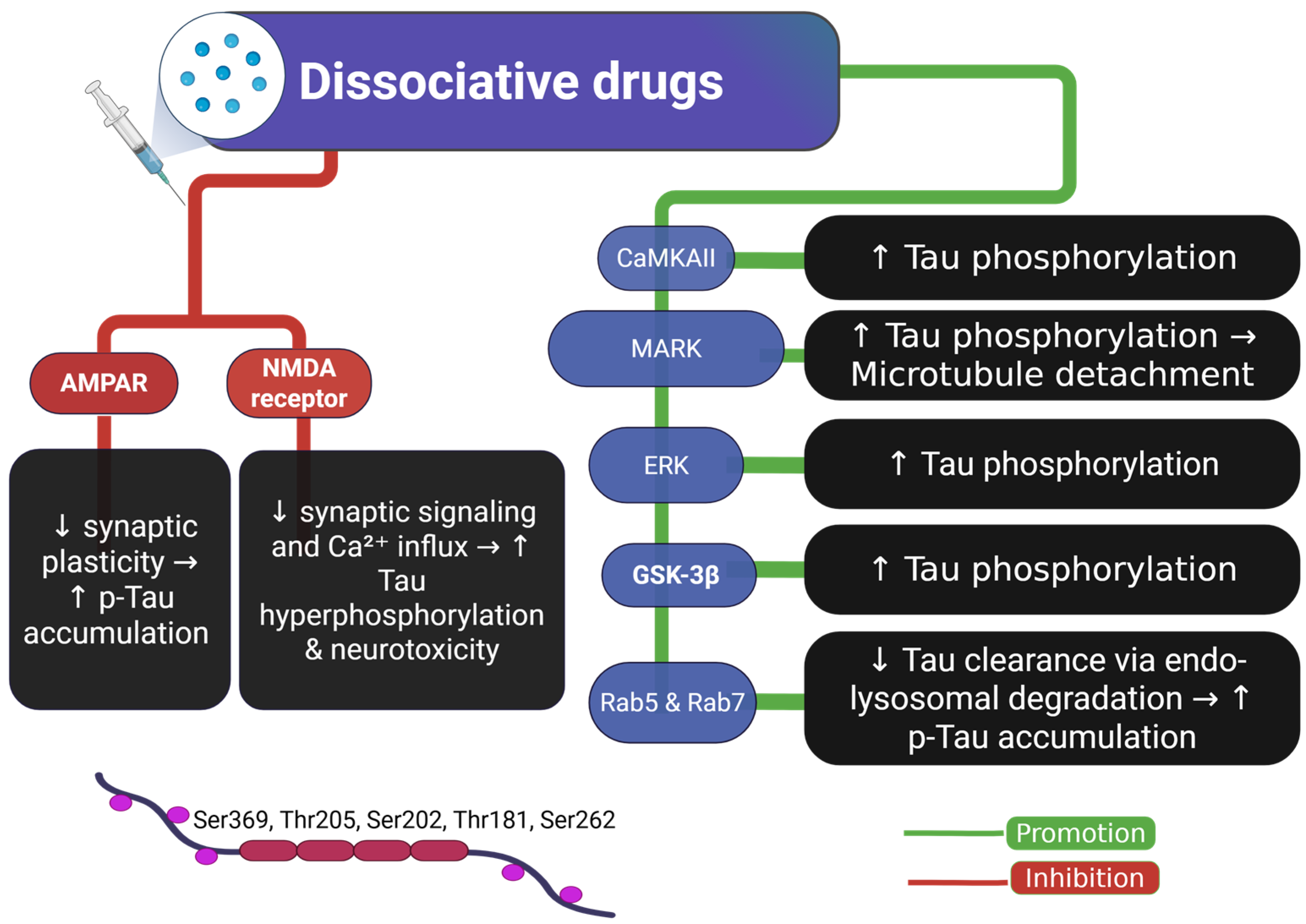
2.5. Tau and Dissociative Drugs
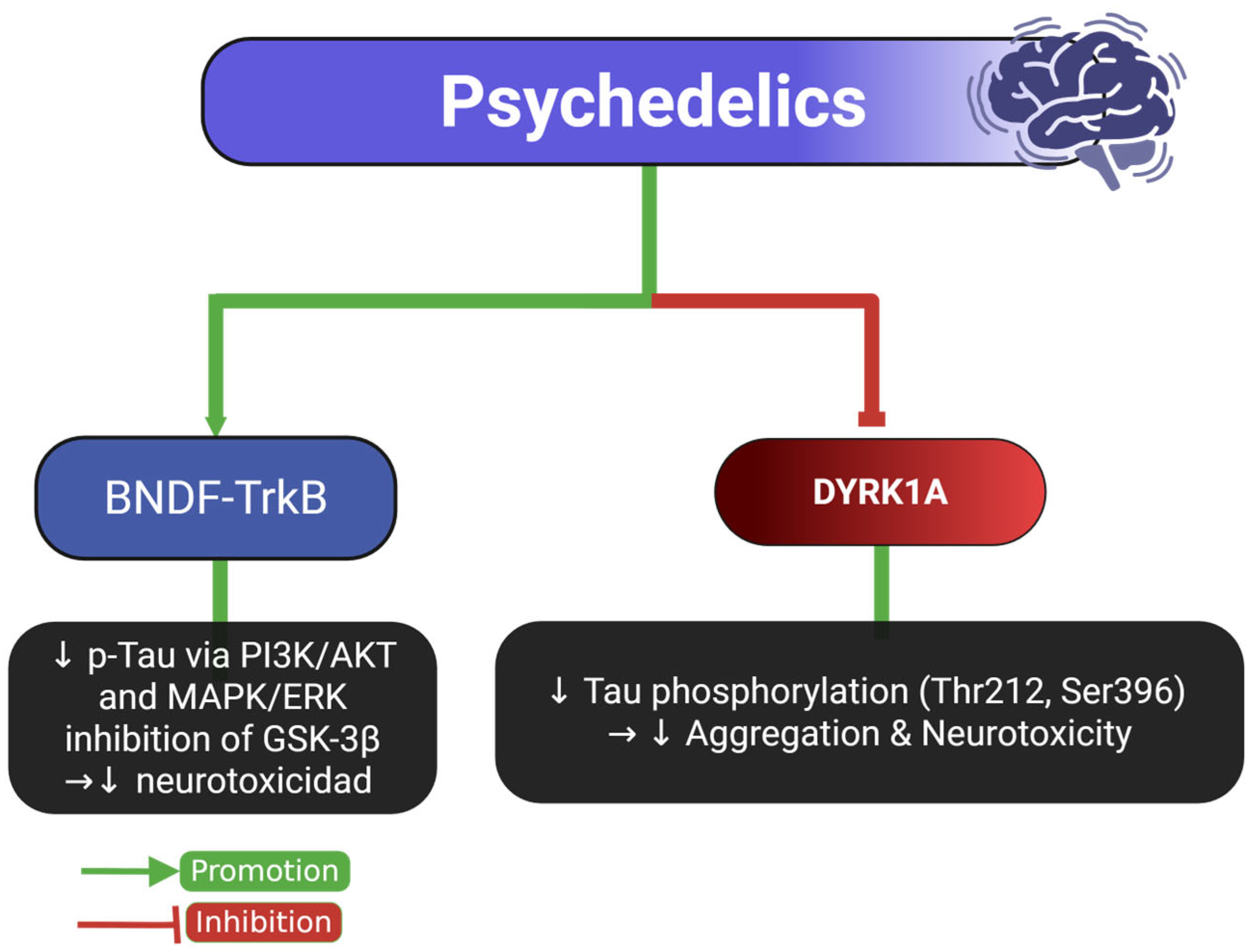
2.6. Tau and Psychedelic Drugs
3. General Considerations
4. Materials and Methods
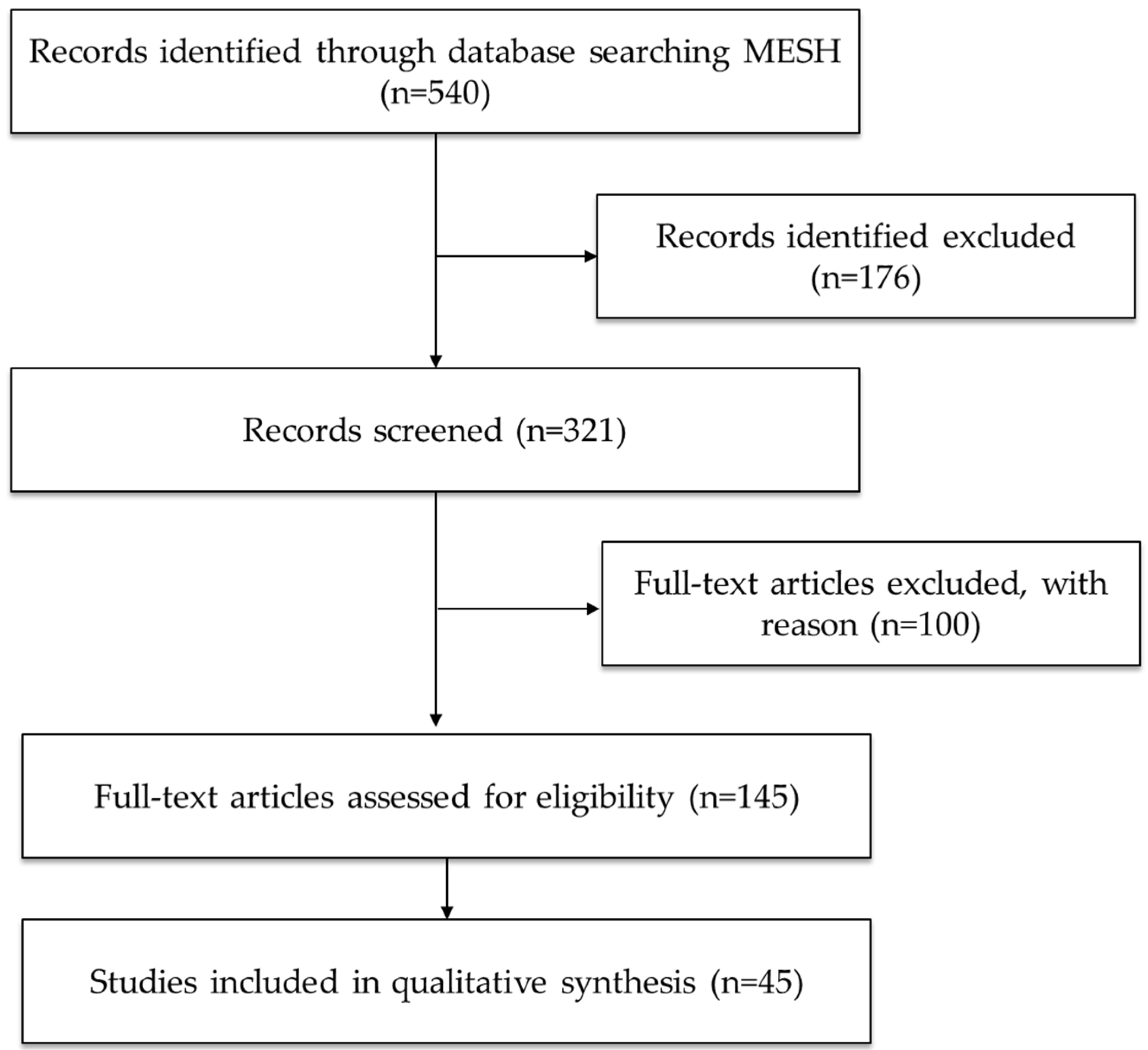
4.1. Search Strategy
4.2. Study Selection and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- MacKillop, J.; Agabio, R.; Feldstein Ewing, S.W.; Heilig, M.; Kelly, J.F.; Leggio, L.; Lingford-Hughes, A.; Palmer, A.A.; Parry, C.D.; Ray, L.; et al. Hazardous Drinking and Alcohol Use Disorders. Nat. Rev. Dis. Primers 2022, 8, 80. [Google Scholar] [CrossRef]
- World Health Organization: Global Status Report on Alcohol and Health 2018. Available online: https://scholar.google.com/scholar_lookup?title=Global%20status%20report%20on%20alcohol%20and%20health%202018&publication_year=2018& (accessed on 23 June 2025).
- Volkow, N.D.; Blanco, C. Substance Use Disorders: A Comprehensive Update of Classification, Epidemiology, Neurobiology, Clinical Aspects, Treatment and Prevention. World Psychiatry 2023, 22, 203–229. [Google Scholar] [CrossRef]
- World Drug Report 2021. Available online: https://www.unodc.org/unodc/en/data-and-analysis/wdr2021.html (accessed on 23 June 2025).
- Substance Abuse and Mental Health Services Administration (US). Substance Use Disorders. In Impact of the DSM-IV to DSM-5 Changes on the National Survey on Drug Use and Health; Substance Abuse and Mental Health Services Administration (US): Rockville, MD, USA, 2016. [Google Scholar]
- Gould, T.J. Addiction and Cognition. Addict. Sci. Clin. Pract. 2010, 5, 4–14. [Google Scholar]
- Medeiros, R.; Baglietto-Vargas, D.; LaFerla, F.M. The Role of Tau in Alzheimer’s Disease and Related Disorders. CNS Neurosci. Ther. 2011, 17, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Ramage, S.N.; Anthony, I.C.; Carnie, F.W.; Busuttil, A.; Robertson, R.; Bell, J.E. Hyperphosphorylated Tau and Amyloid Precursor Protein Deposition Is Increased in the Brains of Young Drug Abusers. Neuropathol. Appl. Neurobiol. 2005, 31, 439–448. [Google Scholar] [CrossRef]
- Cao, M.; Liu, F.; Ji, F.; Liang, J.; Liu, L.; Wu, Q.; Wang, T. Effect of C-Jun N-Terminal Kinase (JNK)/P38 Mitogen-Activated Protein Kinase (P38 MAPK) in Morphine-Induced Tau Protein Hyperphosphorylation. Behav. Brain Res. 2013, 237, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Frost, D.; Meechoovet, B.; Wang, T.; Gately, S.; Giorgetti, M.; Shcherbakova, I.; Dunckley, T. β-Carboline Compounds, Including Harmine, Inhibit DYRK1A and Tau Phosphorylation at Multiple Alzheimer’s Disease-Related Sites. PLoS ONE 2011, 6, e19264. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Horvath, M.C.; Majtenyi, K.; Lutz, M.I.; Hurd, Y.L.; Keller, E. Heroin Abuse Exaggerates Age-Related Deposition of Hyperphosphorylated Tau and P62-Positive Inclusions. Neurobiol. Aging 2015, 36, 3100–3107. [Google Scholar] [CrossRef]
- Liu, Q.-R.; Zhang, P.-W.; Zhen, Q.; Walther, D.; Wang, X.-B.; Uhl, G.R. KEPI, a PKC-Dependent Protein Phosphatase 1 Inhibitor Regulated by Morphine. J. Biol. Chem. 2002, 277, 13312–13320. [Google Scholar] [CrossRef] [PubMed]
- Ohene-Nyako, M.; Nass, S.R.; Hahn, Y.K.; Knapp, P.E.; Hauser, K.F. Morphine and HIV-1 Tat Interact to Cause Region-Specific Hyperphosphorylation of Tau in Transgenic Mice. Neurosci. Lett. 2021, 741, 135502. [Google Scholar] [CrossRef]
- Lew, G.M. D-Lysergic Acid Reduces Microtubule-Associated Tau Protein in SH-SY5Y Human Neuroblastoma Cells. General. Pharmacol. Vasc. Syst. 1995, 26, 1045–1048. [Google Scholar] [CrossRef]
- Brengel, E.K.; Axe, B.; Maheswari, A.; Abeer, M.I.; Ortiz, R.J.; Woodward, T.J.; Walhof, R.; Utama, R.; Sawada, C.; Balaji, S.; et al. Psilocybin as a Treatment for Repetitive Mild Head Injury: Evidence from Neuroradiology and Molecular Biology. bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Harada, C.N.; Natelson Love, M.C.; Triebel, K. Normal Cognitive Aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, S.; Amjad, Z.; Abaza, A.; Vasavada, A.M.; Sadhu, A.; Valencia, C.; Fatima, H.; Nwankwo, I.; Anam, M.; Mohammed, L. Executive Dysfunction in Patients with Alcohol Use Disorder: A Systematic Review. Cureus 2022, 14, e29207. [Google Scholar] [CrossRef] [PubMed]
- Ramey, T.; Regier, P.S. Cognitive Impairment in Substance Use Disorders. CNS Spectr. 2019, 24, 102–113. [Google Scholar] [CrossRef]
- Bruijnen, C.J.W.H.; Dijkstra, B.A.G.; Walvoort, S.J.W.; Markus, W.; VanDerNagel, J.E.L.; Kessels, R.P.C.; DE Jong, C.A.J. Prevalence of Cognitive Impairment in Patients with Substance Use Disorder. Drug Alcohol. Rev. 2019, 38, 435–442. [Google Scholar] [CrossRef]
- Tolomeo, S.; Davey, F.; Steele, J.D.; Baldacchino, A.M. Effects of Opioid Dependence on Visuospatial Memory and Its Associations with Depression and Anxiety. Front. Psychiatry 2019, 10, 743. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Yamada, K. Methamphetamine Use Causes Cognitive Impairment and Altered Decision-Making. Neurochem. Int. 2019, 124, 106–113. [Google Scholar] [CrossRef]
- Hall, M.G.; Hauson, A.O.; Wollman, S.C.; Allen, K.E.; Connors, E.J.; Stern, M.J.; Kimmel, C.L.; Stephan, R.A.; Sarkissians, S.; Barlet, B.D.; et al. Neuropsychological Comparisons of Cocaine versus Methamphetamine Users: A Research Synthesis and Meta-Analysis. Am. J. Drug Alcohol. Abus. 2018, 44, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Velit-Salazar, M.R.; Shiroma, P.R.; Cherian, E. A Systematic Review of the Neurocognitive Effects of Psychedelics in Healthy Populations: Implications for Depressive Disorders and Post-Traumatic Stress Disorder. Brain Sci. 2024, 14, 248. [Google Scholar] [CrossRef]
- Bates, M.L.S.; Trujillo, K.A. Use and Abuse of Dissociative and Psychedelic Drugs in Adolescence. Pharmacol. Biochem. Behav. 2021, 203, 173129. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.M.; Halliday, G.M. Neurof Ibrillary Tangles in Chronic Alcoholics. Neuropathol. Appl. Neurobiol. 1995, 21, 312–318. [Google Scholar] [CrossRef]
- Bailey, C.S.; Jagielo-Miller, J.E.; Keller, P.S.; Glaser, E.P.; Wilcox, A.L.; Prendergast, M.A. Ethanol Sustains Phosphorylated Tau Protein in the Cultured Neonatal Rat Hippocampus: Implications for Fetal Alcohol Spectrum Disorders. Alcohol. 2022, 103, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wolf, T.; Moss, L.; Hudson, C.; Winters, A.M.; Abdelmaboud, S.S.; Avlas, M.; Wohlfahrt, J.; Guergues, J.; Bickford, P.C.; Stevens, S.M., Jr. Chronic Alcohol Exposure during Young Adulthood Attenuates Microglial Reactivity and Downstream Immune Response Pathways in a Mouse Model of Tauopathy Later in Life. Alcohol Clin. Exp. Res. 2025, 49, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.L.; Faccidomo, S.; Kim, M.; Taylor, S.M.; Agoglia, A.E.; May, A.M.; Smith, E.N.; Wong, L.C.; Hodge, C.W. Alcohol Drinking Exacerbates Neural and Behavioral Pathology in the 3xTg-AD Mouse Model of Alzheimer’s Disease. Int. Rev. Neurobiol. 2019, 148, 169–230. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, Y.; Tang, X.; Jing, C.; Qiu, S.; Li, B.; Li, Y. IL-6 and IL-1β Upregulation and Tau Protein Phosphorylation in Response to Chronic Alcohol Exposure in the Mouse Hippocampus. Neuroreport 2021, 32, 851–857. [Google Scholar] [CrossRef]
- Tucker, A.E.; Alicea Pauneto, C.D.M.; Barnett, A.M.; Coleman, L.G. Chronic Ethanol Causes Persistent Increases in Alzheimer’s Tau Pathology in Female 3xTg-AD Mice: A Potential Role for Lysosomal Impairment. Front. Behav. Neurosci. 2022, 16, 886634. [Google Scholar] [CrossRef] [PubMed]
- Drouka, A.; Ntetsika, K.-D.; Brikou, D.; Mamalaki, E.; Ntanasi, E.; Chatzipanagiotou, S.; Gu, Y.; Scarmeas, N.; Yannakoulia, M. Associations of Moderate Alcohol Intake with Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease: Data from the ALBION Study. Eur. J. Nutr. 2025, 64, 142. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Chen, R.; Xiao, Y.; Zhou, T. The Src-Kinase Fyn Is Required for Cocaine-Associated Memory Through Regulation of Tau. Front. Pharmacol. 2022, 13, 769827. [Google Scholar] [CrossRef]
- Liu, S.-J.; Fang, Z.-Y.; Yang, Y.; Deng, H.-M.; Wang, J.-Z. Alzheimer-like Phosphorylation of Tau and Neurofilament Induced by Cocaine in Vivo. Acta Pharmacol. Sin. 2003, 24, 512–518. [Google Scholar]
- Lew, G.M. Microtubular Tau Protein after Cocaine in Cultured SH-SY5Y Human Neuroblastoma. Gen. Pharmacol. 1992, 23, 1111–1113. [Google Scholar] [CrossRef]
- Xu, H.; Chen, X.; Wang, J.; Yang, T.; Liu, N.; Cheng, J.; Gao, R.; Liu, J.; Xiao, H. Involvement of Insulin Signalling Pathway in Methamphetamine-Induced Hyperphosphorylation of Tau. Toxicology 2018, 408, 88–94. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, L.; Yu, P.; Fang, F.; Jiang, L.; Fei, J.; Xiao, H.; Wang, J. Methamphetamine Exposure Upregulates the Amyloid Precursor Protein and Hyperphosphorylated Tau Expression: The Roles of Insulin Signaling in SH-SY5Y Cell Line. J. Toxicol. Sci. 2019, 44, 493–503. [Google Scholar] [CrossRef]
- Alali, S.; Riazi, G.; Ashrafi-Kooshk, M.R.; Meknatkhah, S.; Ahmadian, S.; Hooshyari Ardakani, M.; Hosseinkhani, B. Cannabidiol Inhibits Tau Aggregation In Vitro. Cells 2021, 10, 3521. [Google Scholar] [CrossRef]
- Wang, L.; Liu, B.-J.; Cao, Y.; Xu, W.-Q.; Sun, D.-S.; Li, M.-Z.; Shi, F.-X.; Li, M.; Tian, Q.; Wang, J.-Z.; et al. Deletion of Type-2 Cannabinoid Receptor Induces Alzheimer’s Disease-Like Tau Pathology and Memory Impairment Through AMPK/GSK3β Pathway. Mol. Neurobiol. 2018, 55, 4731–4744. [Google Scholar] [CrossRef]
- Esposito, G.; De Filippis, D.; Carnuccio, R.; Izzo, A.A.; Iuvone, T. The Marijuana Component Cannabidiol Inhibits β-Amyloid-Induced Tau Protein Hyperphosphorylation through Wnt/β-Catenin Pathway Rescue in PC12 Cells. J. Mol. Med. 2006, 84, 253–258. [Google Scholar] [CrossRef]
- Aso, E.; Palomer, E.; Juvés, S.; Maldonado, R.; Muñoz, F.J.; Ferrer, I. CB1 Agonist ACEA Protects Neurons and Reduces the Cognitive Impairment of AβPP/PS1 Mice. J. Alzheimers Dis. 2012, 30, 439–459. [Google Scholar] [CrossRef] [PubMed]
- Casarejos, M.J.; Perucho, J.; Gomez, A.; Muñoz, M.P.; Fernandez-Estevez, M.; Sagredo, O.; Fernandez Ruiz, J.; Guzman, M.; de Yebenes, J.G.; Mena, M.A. Natural Cannabinoids Improve Dopamine Neurotransmission and Tau and Amyloid Pathology in a Mouse Model of Tauopathy. J. Alzheimers Dis. 2013, 35, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Anthony, I.C.; Norrby, K.E.; Dingwall, T.; Carnie, F.W.; Millar, T.; Arango, J.C.; Robertson, R.; Bell, J.E. Predisposition to Accelerated Alzheimer-Related Changes in the Brains of Human Immunodeficiency Virus Negative Opiate Abusers. Brain 2010, 133, 3685–3698. [Google Scholar] [CrossRef] [PubMed]
- Hector, A.; McAnulty, C.; Piché-Lemieux, M.-É.; Alves-Pires, C.; Buée-Scherrer, V.; Buée, L.; Brouillette, J. Tau Hyperphosphorylation Induced by the Anesthetic Agent Ketamine/Xylazine Involved the Calmodulin-Dependent Protein Kinase II. FASEB J. 2020, 34, 2968–2977. [Google Scholar] [CrossRef]
- Li, Y.; Ding, R.; Ren, X.; Wen, G.; Dong, Z.; Yao, H.; Tan, Y.; Yu, H.; Wang, X.; Zhan, X.; et al. Long-Term Ketamine Administration Causes Tau Protein Phosphorylation and Tau Protein-Dependent AMPA Receptor Reduction in the Hippocampus of Mice. Toxicol. Lett. 2019, 315, 107–115. [Google Scholar] [CrossRef]
- Yeung, L.Y.; Wai, M.S.M.; Fan, M.; Mak, Y.T.; Lam, W.P.; Li, Z.; Lu, G.; Yew, D.T. Hyperphosphorylated Tau in the Brains of Mice and Monkeys with Long-Term Administration of Ketamine. Toxicol. Lett. 2010, 193, 189–193. [Google Scholar] [CrossRef]
- Sachdeva, A.; Chandra, M.; Choudhary, M.; Dayal, P.; Anand, K.S. Alcohol-Related Dementia and Neurocognitive Impairment: A Review Study. Int. J. High. Risk Behav. Addict. 2016, 5, e27976. [Google Scholar] [CrossRef] [PubMed]
- McKerracher, L.; Essagian, C.; Aguayo, A. Marked Increase in Beta-Tubulin mRNA Expression during Regeneration of Axotomized Retinal Ganglion Cells in Adult Mammals. J. Neurosci. 1993, 13, 5294–5300. [Google Scholar] [CrossRef]
- Jiang, M.; Tang, X.; Wang, P.; Yang, L.; Du, R. Association between Daily Alcohol Consumption and Serum Alpha Klotho Levels among U.S. Adults over 40 Years Old: A Cross-Sectional Study. BMC Public. Health 2023, 23, 1901. [Google Scholar] [CrossRef] [PubMed]
- González-Reimers, E.; Romero-Acevedo, L.; Espelosín-Ortega, E.; Martín-González, M.C.; Quintero-Platt, G.; Abreu-González, P.; José de-la-Vega-Prieto, M.; Martínez-Martínez, D.; Santolaria-Fernández, F. Soluble Klotho and Brain Atrophy in Alcoholism. Alcohol. Alcohol. 2018, 53, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, M.; Yoshimura, K.; Shibata, M.; Koike, M.; Matsuura, N.; Uchiyama, Y.; Gotow, T. Morphological and Biochemical Signs of Age-Related Neurodegenerative Changes in Klotho Mutant Mice. Neuroscience 2008, 152, 924–941. [Google Scholar] [CrossRef]
- Grøntvedt, G.R.; Sando, S.B.; Lauridsen, C.; Bråthen, G.; White, L.R.; Salvesen, Ø.; Aarsland, D.; Hessen, E.; Fladby, T.; Waterloo, K.; et al. Association of Klotho Protein Levels and KL-VS Heterozygosity with Alzheimer Disease and Amyloid and Tau Burden. JAMA Netw. Open 2022, 5, e2243232. [Google Scholar] [CrossRef]
- Carlen, P.L.; Wilkinson, D.A. Reversibility of Alcohol-Related Brain Damage: Clinical and Experimental Observations. Acta Med. Scand. Suppl. 1987, 717, 19–26. [Google Scholar] [CrossRef]
- Smith, D.M.; Atkinson, R.M. Alcoholism and Dementia. Int. J. Addict. 1995, 30, 1843–1869. [Google Scholar] [CrossRef]
- Saito, M.; Chakraborty, G.; Mao, R.-F.; Paik, S.-M.; Vadasz, C.; Saito, M. Tau Phosphorylation and Cleavage in Ethanol-Induced Neurodegeneration in the Developing Mouse Brain. Neurochem. Res. 2010, 35, 651–659. [Google Scholar] [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Mathew, B.; Das, P.K.; Perveen, A.; Ashraf, G.M. Emerging Promise of Immunotherapy for Alzheimer’s Disease: A New Hope for the Development of Alzheimer’s Vaccine. Curr. Top. Med. Chem. 2020, 20, 1214–1234. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y. Tau and Neuroinflammation in Alzheimer’s Disease: Interplay Mechanisms and Clinical Translation. J. Neuroinflammation 2023, 20. [Google Scholar] [CrossRef] [PubMed]
- Panda, C.; Voelz, C.; Habib, P.; Mevissen, C.; Pufe, T.; Beyer, C.; Gupta, S.; Slowik, A. Aggregated Tau-PHF6 (VQIVYK) Potentiates NLRP3 Inflammasome Expression and Autophagy in Human Microglial Cells. Cells 2021, 10, 1652. [Google Scholar] [CrossRef] [PubMed]
- Stancu, I.C.; Cremers, N.; Vanrusselt, H.; Couturier, J.; Vanoosthuyse, A.; Kessels, S.; Lodder, C.; Brône, B.; Huaux, F.; Octave, J.N.; et al. Aggregated Tau Activates NLRP3-ASC Inflammasome Exacerbating Exogenously Seeded and Non-Exogenously Seeded Tau Pathology in Vivo. Acta Neuropathol. 2019, 137, 599–617. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 Inflammasome Activation Drives Tau Pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Zhou, M.; Feng, S.; Peng, X.; Wang, Y. Microglial TLR4/NLRP3 Inflammasome Signaling in Alzheimer’s Disease. J. Alzheimers Dis. 2024, 97, 75–88. [Google Scholar] [CrossRef]
- Barnett, A.; David, E.; Rohlman, A.; Nikolova, V.D.; Moy, S.S.; Vetreno, R.P.; Coleman, L.G., Jr. Adolescent Binge Alcohol Enhances Early Alzheimer’s Disease Pathology in Adulthood Through Proinflammatory Neuroimmune Activation. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef]
- Grüsser, S.M.; Wrase, J.; Klein, S.; Hermann, D.; Smolka, M.N.; Ruf, M.; Weber-Fahr, W.; Flor, H.; Mann, K.; Braus, D.F.; et al. Cue-Induced Activation of the Striatum and Medial Prefrontal Cortex Is Associated with Subsequent Relapse in Abstinent Alcoholics. Psychopharmacology 2004, 175, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Salinas, A.G.; Mateo, Y.; Carlson, V.C.C.; Stinnett, G.S.; Luo, G.; Seasholtz, A.F.; Grant, K.A.; Lovinger, D.M. Long-Term Alcohol Consumption Alters Dorsal Striatal Dopamine Release and Regulation by D2 Dopamine Receptors in Rhesus Macaques. Neuropsychopharmacology 2021, 46, 1432–1441. [Google Scholar] [CrossRef]
- Dannenhoffer, C.A.; Robertson, M.M.; Macht, V.A.; Mooney, S.M.; Boettiger, C.A.; Robinson, D.L. Chronic Alcohol Exposure during Critical Developmental Periods Differentially Impacts Persistence of Deficits in Cognitive Flexibility and Related Circuitry. Int. Rev. Neurobiol. 2021, 160. [Google Scholar] [CrossRef]
- Gass, J.T.; Glen, W.B.; McGonigal, J.T.; Trantham-Davidson, H.; Lopez, M.F.; Randall, P.K.; Yaxley, R.; Floresco, S.B.; Chandler, L.J. Adolescent Alcohol Exposure Reduces Behavioral Flexibility, Promotes Disinhibition, and Increases Resistance to Extinction of Ethanol Self-Administration in Adulthood. Neuropsychopharmacology 2014, 39, 2570–2583. [Google Scholar] [CrossRef]
- Langlais, P.J. Alcohol-Related Thiamine Deficiency. Alcohol. Health Res. World 1995, 19, 113–121. [Google Scholar]
- Ding, J.; Lian, Y.; Meng, Y.; He, Y.; Fan, H.; Li, C.; Qiu, P. The Effect of α-Synuclein and Tau in Methamphetamine Induced Neurotoxicity in Vivo and in Vitro. Toxicol. Lett. 2020, 319, 213–224. [Google Scholar] [CrossRef]
- Sengupta, U.; Kayed, R. Amyloid β, Tau, and α-Synuclein Aggregates in the Pathogenesis, Prognosis, and Therapeutics for Neurodegenerative Diseases. Progress. Neurobiol. 2022, 214, 102270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, N.-Q.; Yan, F.; Jin, H.; Zhou, S.-Y.; Shi, J.-S.; Jin, F. Diabetes Mellitus and Alzheimer’s Disease: GSK-3β as a Potential Link. Behav. Brain Res. 2018, 339, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Zhang, F.; Zhu, B.; Liu, C.; Lin, Z.; Wang, H.; Xie, W.-B. CDK5-Mediated Tau Accumulation Triggers Methamphetamine-Induced Neuronal Apoptosis via Endoplasmic Reticulum-Associated Degradation Pathway. Toxicol. Lett. 2018, 292, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.C.; Tseng, H.-C.; Goldman, J.A.; Shih, H.; Tsai, L.-H. Aberrant Cdk5 Activation by P25 Triggers Pathological Events Leading to Neurodegeneration and Neurofibrillary Tangles. Neuron 2003, 40, 471–483. [Google Scholar] [CrossRef]
- Al-Khalil, K.; Bell, R.P.; Towe, S.L.; Gadde, S.; Burke, E.; Meade, C.S. Cortico-Striatal Networking Deficits Associated with Advanced HIV Disease and Cocaine Use. J. Neurovirol. 2023, 29, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Ceceli, A.O.; Huang, Y.; Kronberg, G.; Malaker, P.; Miller, P.; King, S.G.; Gaudreault, P.-O.; McClain, N.; Gabay, L.; Vasa, D.; et al. Common and Distinct Fronto-Striatal Volumetric Changes in Heroin and Cocaine Use Disorders. Brain 2023, 146, 1662–1671. [Google Scholar] [CrossRef]
- Wingert, J.C.; Ramos, J.D.; Reynolds, S.X.; Gonzalez, A.E.; Rose, R.M.; Hegarty, D.M.; Aicher, S.A.; Bailey, L.G.; Brown, T.E.; Abbas, A.I.; et al. Perineuronal Nets in the Rat Medial Prefrontal Cortex Alter Hippocampal–Prefrontal Oscillations and Reshape Cocaine Self-Administration Memories. J. Neurosci. 2024, 44. [Google Scholar] [CrossRef]
- Aso, E.; Ferrer, I. Cannabinoids for Treatment of Alzheimer’s Disease: Moving toward the Clinic. Front. Pharmacol. 2014, 5, 37. [Google Scholar] [CrossRef]
- Esposito, G.; De Filippis, D.; Steardo, L.; Scuderi, C.; Savani, C.; Cuomo, V.; Iuvone, T. CB1 Receptor Selective Activation Inhibits β-Amyloid-Induced iNOS Protein Expression in C6 Cells and Subsequently Blunts Tau Protein Hyperphosphorylation in Co-Cultured Neurons. Neurosci. Lett. 2006, 404, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.J.; Xi, Z.-X. Progress in Brain Cannabinoid CB2 Receptor Research: From Genes to Behavior. Neurosci. Biobehav. Rev. 2019, 98, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, J.; He, J.; Wen, G.; Wu, X. Mechanism of Psychoactive Substance-Induced Cognitive Disorders: Does Tau Protein Play a Role? Front. Biosci. (Landmark Ed) 2022, 27, 6. [Google Scholar] [CrossRef]
- Campbell, V.A.; Gowran, A. Alzheimer’s Disease; Taking the Edge off with Cannabinoids? British J. Pharmacol. 2007, 152, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Li, X. Advances in the Relationship between Tau Protein and Morphine De-Pendence in Cognitive Dysfunction. Adv. Emerg. Med. 2019, 8, 96–101. [Google Scholar] [CrossRef]
- Cai, Z.; Ratka, A. Opioid System and Alzheimer’s Disease. Neuromol. Med. 2012, 14, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Kálmán, J.; Bjelik, A.; Hugyecz, M.; Tímár, J.; Gyarmati, Z.; Zana, M.; Fürst, Z.; Janka, Z.; Rakonczay, Z.; Horváth, Z.; et al. 3,4-Methylenedioxymethamphetamine (MDMA), but Not Morphine, Alters APP Processing in the Rat Brain. Int. J. Neuropsychopharmacol. 2007, 10, 183–190. [Google Scholar] [CrossRef]
- Herlinger, K.; Lingford-Hughes, A. Opioid Use Disorder and the Brain: A Clinical Perspective. Addiction 2022, 117, 495–505. [Google Scholar] [CrossRef]
- Murnane, K.S.; Edinoff, A.N.; Cornett, E.M.; Kaye, A.D. Updated Perspectives on the Neurobiology of Substance Use Disorders Using Neuroimaging. SAR 2023, 14, 99–111. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Balboni, G.; Xia, Y. A New Pathway for Neuroprotection against Tau Hyperphosphorylation via δ-Opioid Receptor Initiated Inhibition of CDK5 and AMPK Signaling. Front. Aging Neurosci. 2025, 17. [Google Scholar] [CrossRef]
- Moore, T.J.; Alami, A.; Alexander, G.C.; Mattison, D.R. Safety and Effectiveness of NMDA Receptor Antagonists for Depression: A Multidisciplinary Review. Pharmacotherapy 2022, 42, 567–579. [Google Scholar] [CrossRef]
- Strous, J.F.M.; Weeland, C.J.; van der Draai, F.A.; Daams, J.G.; Denys, D.; Lok, A.; Schoevers, R.A.; Figee, M. Brain Changes Associated with Long-Term Ketamine Abuse, A Systematic Review. Front. Neuroanat. 2022, 16, 795231. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.-G.; He, J.-G.; Lu, L.-L.; Song, S.-J.; Chen, M.-M.; Wang, F.; Chen, J.-G. Enhanced TARP-Γ8-PSD-95 Coupling in Excitatory Neurons Contributes to the Rapid Antidepressant-like Action of Ketamine in Male Mice. Nat. Commun. 2023, 14, 7971. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kobayashi, S.; Nakao, K.; Dong, C.; Han, M.; Qu, Y.; Ren, Q.; Zhang, J.; Ma, M.; Toki, H.; et al. AMPA Receptor Activation–Independent Antidepressant Actions of Ketamine Metabolite (S)-Norketamine. Biol. Psychiatry 2018, 84, 591–600. [Google Scholar] [CrossRef]
- Zaytseva, A.; Bouckova, E.; Wiles, M.J.; Wustrau, M.H.; Schmidt, I.G.; Mendez-Vazquez, H.; Khatri, L.; Kim, S. Ketamine’s Rapid Antidepressant Effects Are Mediated by Ca2+-Permeable AMPA Receptors. eLife 2023, 12, e86022. [Google Scholar] [CrossRef]
- Li, Y.; Wen, G.; Ding, R.; Ren, X.; Jing, C.; Liu, L.; Yao, J.; Zhang, G.; Lu, Y.; Li, B.; et al. Effects of Single-Dose and Long-Term Ketamine Administration on Tau Phosphorylation–Related Enzymes GSK-3β, CDK5, PP2A, and PP2B in the Mouse Hippocampus. J. Mol. Neurosci. 2020, 70, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- De Montigny, A.; Elhiri, I.; Allyson, J.; Cyr, M.; Massicotte, G. NMDA Reduces Tau Phosphorylation in Rat Hippocampal Slices by Targeting NR2A Receptors, GSK3β, and PKC Activities. Neural Plast. 2013, 2013, 261593. [Google Scholar] [CrossRef]
- Ali, F.; Gerhard, D.M.; Sweasy, K.; Pothula, S.; Pittenger, C.; Duman, R.S.; Kwan, A.C. Ketamine Disinhibits Dendrites and Enhances Calcium Signals in Prefrontal Dendritic Spines. Nat. Commun. 2020, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Boese, M.; Berman, R.; Spencer, H.; Rujan, O.; Metz, E.; Radford, K.; Choi, K. Effects of Subanesthetic Intravenous Ketamine Infusion on Stress Hormones and Synaptic Density in Rats with Mild Closed-Head Injury. Biomedicines 2025, 13, 787. [Google Scholar] [CrossRef]
- Lopes-Aguiar, C.; Ruggiero, R.N.; Rossignoli, M.T.; Esteves, I.M.; Peixoto-Santos, J.E.; Romcy-Pereira, R.N.; Leite, J.P. Long-Term Potentiation Prevents Ketamine-Induced Aberrant Neurophysiological Dynamics in the Hippocampus-Prefrontal Cortex Pathway In Vivo. Sci. Rep. 2020, 10, 7167. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Minkowicz, S.; Dumrongprechachan, V.; Hamilton, P.; Kozorovitskiy, Y. Ketamine Rapidly Enhances Glutamate-Evoked Dendritic Spinogenesis in Medial Prefrontal Cortex through Dopaminergic Mechanisms. Biol. Psychiatry 2021, 89, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, S.; Yang, Y.; Song, A.; Liang, F.; Zhang, Y.; Dong, Y.; Wu, X.; Xie, Z. Ketamine Induces Delirium-Like Behavior and Interferes with Endosomal Tau Trafficking. Anesth. Analg. 2023, 136, 779–788. [Google Scholar] [CrossRef]
- Xu, W.; Fang, F.; Ding, J.; Wu, C. Dysregulation of Rab5-Mediated Endocytic Pathways in Alzheimer’s Disease. Traffic 2018, 19, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Varidaki, A.; Hong, Y.; Coffey, E.T. Repositioning Microtubule Stabilizing Drugs for Brain Disorders. Front. Cell Neurosci. 2018, 12, 226. [Google Scholar] [CrossRef]
- Cario, A.; Berger, C.L. Tau, Microtubule Dynamics, and Axonal Transport: New Paradigms for Neurodegenerative Disease. Bioessays 2023, 45, e2200138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Du, Y.; Yao, Y.; Dai, W.; Yin, Y.; Wang, G.; Li, Y.; Zhang, L. Psilocybin Promotes Neuroplasticity and Induces Rapid and Sustained Antidepressant-like Effects in Mice. J. Psychopharmacol. 2024, 38, 489–499. [Google Scholar] [CrossRef]
- Duchon, A.; Herault, Y. DYRK1A, a Dosage-Sensitive Gene Involved in Neurodevelopmental Disorders, Is a Target for Drug Development in Down Syndrome. Front. Behav. Neurosci. 2016, 10, 104. [Google Scholar] [CrossRef]
- Medina, M.; Garrido, J.J.; Wandosell, F.G. Modulation of GSK-3 as a Therapeutic Strategy on Tau Pathologies. Front. Mol. Neurosci. 2011, 4. [Google Scholar] [CrossRef]
- Kitagishi, Y.; Kobayashi, M.; Kikuta, K.; Matsuda, S. Roles of PI3K/AKT/GSK3/mTOR Pathway in Cell Signaling of Mental Illnesses. Depress. Res. Treat. 2012, 2012, 752563. [Google Scholar] [CrossRef] [PubMed]
- Kitagishi, Y.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Dietary Regulation of PI3K/AKT/GSK-3β Pathway in Alzheimer’s Disease. Alzheimers Res. Ther. 2014, 6, 35. [Google Scholar] [CrossRef] [PubMed]



| Substance | Model | Effects on Tau | Cognitive Impact | Proposed Mechanism | References |
|---|---|---|---|---|---|
| Depressants (Alcohol) | Cell culture (Human neurons) | ↑ p-Tau, ↑ Neurofibrillary tangles in nucleus basalis | Memory dysfunction | Disturbance in phosphorylation/dephosphorylation pathways | [25] |
| Depressants (Alcohol) | Cell culture (neonatal rat hippocampus) | ↑ p-Thr231-Tau | N/A | GSK-β activation, PP2A inhibition | [26] |
| Depressants (Alcohol) | Animal (C57BL/6J, 3xTg-AD mice) | ↑ Total Tau, ↑ p-Tau (Ser199/Ser202) | Spatial memory deficits, impaired gating | Dysregulation of mTOR, CRMP2, HSPs, AMPH, GSK | [27,28,29,30] |
| Depressants (Alcohol) | Cerebrospinal fluid (Humans) | ↑ Tau/Aβ42 and p-Tau/Aβ42 ratios in individuals with high adherence to the Mediterranean-alcohol dietary pattern | Preclinical AD risk | Associated with Aβ positivity, Tau/Aβ imbalance | [31] |
| Stimulants (Cocaine) | Animal (rats, mice) | ↑ p-Tau (PHF-1), ↓ Tau-1, ↑ total Tau | Neurocytoskeletal changes; cocaine-cue memory | Alternative kinases, Fyn kinase activation, PI3K-AKT suppression | [32,33] |
| Stimulants (Cocaine) | Human-derived neuronal cell culture | ↓ Tau (50 kDa) | N/A | Cytoskeletal instability | [34] |
| Stimulants (Methamphetamine) | Human cells (SH-SY5Y human neuroblastoma) | ↑ p-Tau (Ser396, Thr231), ↑ total Tau; ER stress markers (p-PERK, caspase-12); disrupted insulin signaling (↓ IRS-1, AKT, ↑ GSK3β); ↓ autophagy | Neuronal apoptosis, not directly | CDK5 activation; α-syn interaction; ER stress; insulin signaling disruption; autophagy impairment | [35,36] |
| Stimulants (Methamphetamine) | Animal cell (Primary rat hippocampal neurons; Neuro2A mouse neuronal cells) | ↑ p-Tau (Ser396, Thr231), ↑ total Tau; disrupted autophagy | Neurodegeneration, not directly | Tau hyperphosphorylation via CDK5 and α-syn; autophagy dysfunction | [36] |
| Cannabinoids | Human (recombinant human Tau protein 1N/4R; aggregation assays in vitro | CBD inhibits Tau fibril formation, prevents conformational changes | Neuroprotective potential | Direct interaction with Tau; competes with heparin at VQIINK/VQIVYK motifs | [37] |
| Cannabinoids | Human cells (HEK293) | HEK293 Tau (human embryonic kidney cells) | HEK293 Tau (human embryonic kidney cells) | HEK293 Tau (human embryonic kidney cells) | [38] |
| Cannabinoids | Animal cells (PC12 cells + Aβ) | CBD ↓ Tau hyperphosphorylation | Neuroprotection (in vitro) | Rescue of Wnt/β-catenin signaling | [39] |
| Cannabinoids | Animal models | ↓ p-Tau (Thr181) in AβPP/PS1 transgenic mice | Prevented cognitive impairment | CB1 activation → ↓ GSK3β → ↓ Tau p | [40] |
| ↓ Tau and Aβ deposition; ↑ autophagy in PK-/-/TauVLW mice (FTD, Parkinsonism) | ↓ Abnormal behaviors | CB1/CB2 activation → ↓ oxidative stress, ↑ mitochondrial function and autophagy | [41] | ||
| Opioids | Human | ↑ p-Tau (AT8, AT100), NFTs in frontal/temporal cortex and locus coeruleus, and ↑ GSK3β in postmortem human brains | Cognitive impairment; overlaps with early AD pathology | ↑ GSK3β activity; neuroinflammation; microglial activation correlates with p-Tau levels | [42] |
| ↑ p-Tau in AD-related regions (e.g., hippocampus) of postmortem brain tissue from heroin users | Potential predisposition to accelerated AD-like neurodegeneration | Microglial activation; p-Tau independent of duration of drug use | [11] | ||
| ↑ KEPI expression → PP1 inhibition in morphine-treated human brain tissue | Not specified | μ-opioid receptor activation → ↑ PKC → ↑ KEPI → PP1 inhibition → ↑ Tau phosphorylation | [12] | ||
| Opioids | Mouse model (Tat-transgenic) with morphine co-exposure | ↑ pSer396 (striatum, PFC); Tat ↑ pSer404 and pThr205 | Cognitive/sensory dysfunction (suggested) | ↑ CDK5/p35 activity; region-specific Tau hyperphosphorylation | [13] |
| Opioids | Cell (rat embryo cortical neurons treated with morphine) | ↑ Tau hyperphosphorylation | Not assessed | Opioid receptor-dependent activation of JNK/p38 MAPK pathway | [9] |
| Dissociative drugs (ketamine) | Animal models | ↑ p-Tau (Ser396, Ser262, Thr181, Ser202/Thr205) dose-dependent in mouse | N/A | CaMKII activation → ↑ p-Tau; also affects MARK, ERK, GSK3 pathways | [43] |
| ↑ p-Tau (Ser202/Thr205, Ser396) in mice | ↓ AMPA receptor levels, ↓ synaptic efficiency | Tau-dependent reduction in AMPA receptors; Tau phosphorylation mediates synaptic dysfunction | [44] | ||
| ↑ p-Tau in prefrontal and entorhinal cortex; TUNEL+ neurons in mice and monkeys | Memory impairment (linked to aging/Alzheimer-like neurodegeneration) | NMDA receptor antagonism → Tau hyperphosphorylation and potential apoptosis | [45] | ||
| Psychedelics | Human cell (SH-SY5Y neuroblastoma cells) | ↓ Microtubule-associated Tau in both cytoplasmic and membrane fractions | N/A | Promotes Tau dissociation from microtubules, increasing soluble Tau levels | [14] |
| Psychedelics | Animal model (Female Wistar rats | ↓ Phosphorylated Tau (PHF-1 epitope) to control levels in soluble fraction; ↓ trend in insoluble aggregated Tau | Improved cognitive and motor behaviors post-injury | Psilocybin enhances neuroplasticity, reduces neuroinflammation, restores vascular reactivity, and modulates BDNF-TrkB signaling | [15] |
| Psychedelics | Cell culture and in vitro assays (harmine and β-carbolines) | ↓ Tau phosphorylation at S396, S262/S356, and T231 | N/A | Inhibition of DYRK1A kinase by harmine and related β-carboline compounds | [10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebolledo-Pérez, L.; Hernández-Bello, J.; Martínez-Ramos, A.; Castañeda-Arellano, R.; Fernández-Quezada, D.; Sandoval-García, F.; Aguilar-García, I.G. Substance Abuse and Cognitive Decline: The Critical Role of Tau Protein as a Potential Biomarker. Int. J. Mol. Sci. 2025, 26, 7638. https://doi.org/10.3390/ijms26157638
Rebolledo-Pérez L, Hernández-Bello J, Martínez-Ramos A, Castañeda-Arellano R, Fernández-Quezada D, Sandoval-García F, Aguilar-García IG. Substance Abuse and Cognitive Decline: The Critical Role of Tau Protein as a Potential Biomarker. International Journal of Molecular Sciences. 2025; 26(15):7638. https://doi.org/10.3390/ijms26157638
Chicago/Turabian StyleRebolledo-Pérez, Liliana, Jorge Hernández-Bello, Alicia Martínez-Ramos, Rolando Castañeda-Arellano, David Fernández-Quezada, Flavio Sandoval-García, and Irene Guadalupe Aguilar-García. 2025. "Substance Abuse and Cognitive Decline: The Critical Role of Tau Protein as a Potential Biomarker" International Journal of Molecular Sciences 26, no. 15: 7638. https://doi.org/10.3390/ijms26157638
APA StyleRebolledo-Pérez, L., Hernández-Bello, J., Martínez-Ramos, A., Castañeda-Arellano, R., Fernández-Quezada, D., Sandoval-García, F., & Aguilar-García, I. G. (2025). Substance Abuse and Cognitive Decline: The Critical Role of Tau Protein as a Potential Biomarker. International Journal of Molecular Sciences, 26(15), 7638. https://doi.org/10.3390/ijms26157638







