Phytochemical Analysis, Antioxidant Activity, and Anticancer Potential of Afzelia quanzensis Welw—Bark Extract: A Traditional Remedy Utilized by Indigenous Communities in KwaZulu-Natal and Eastern Cape Provinces of South Africa
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis
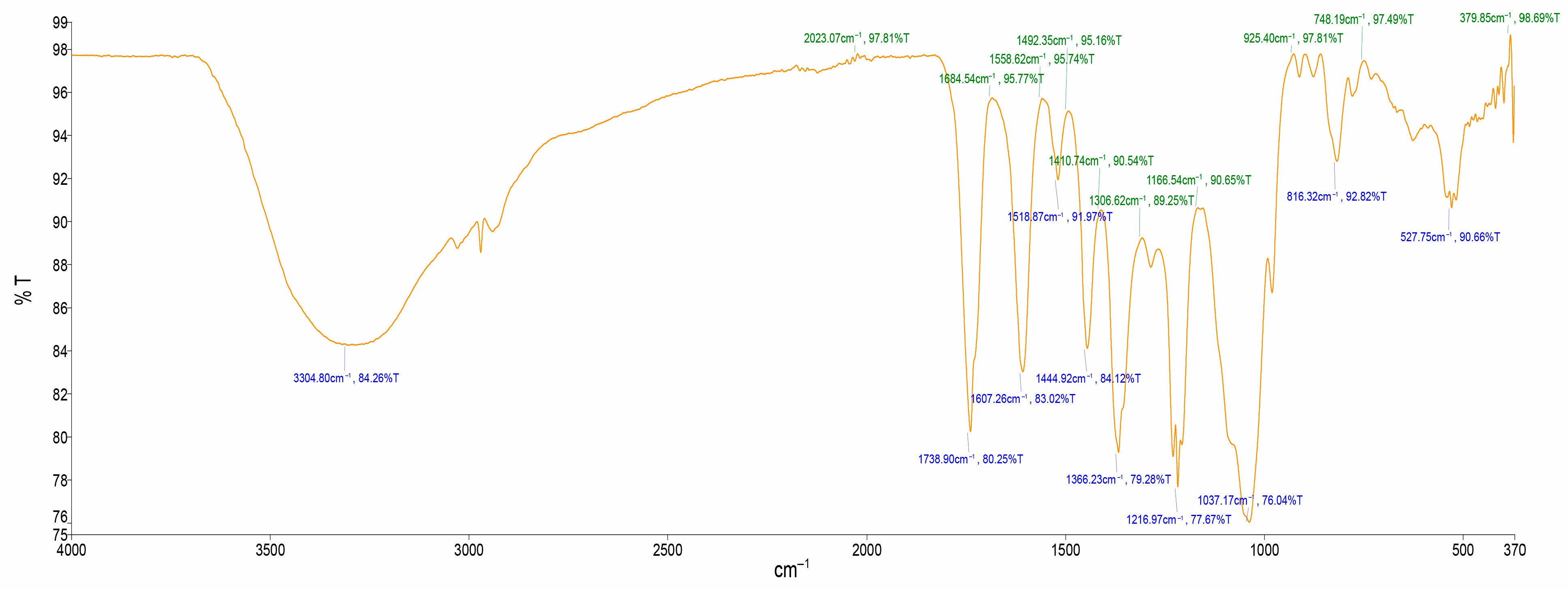
2.2. FTIR Spectroscopic Analysis
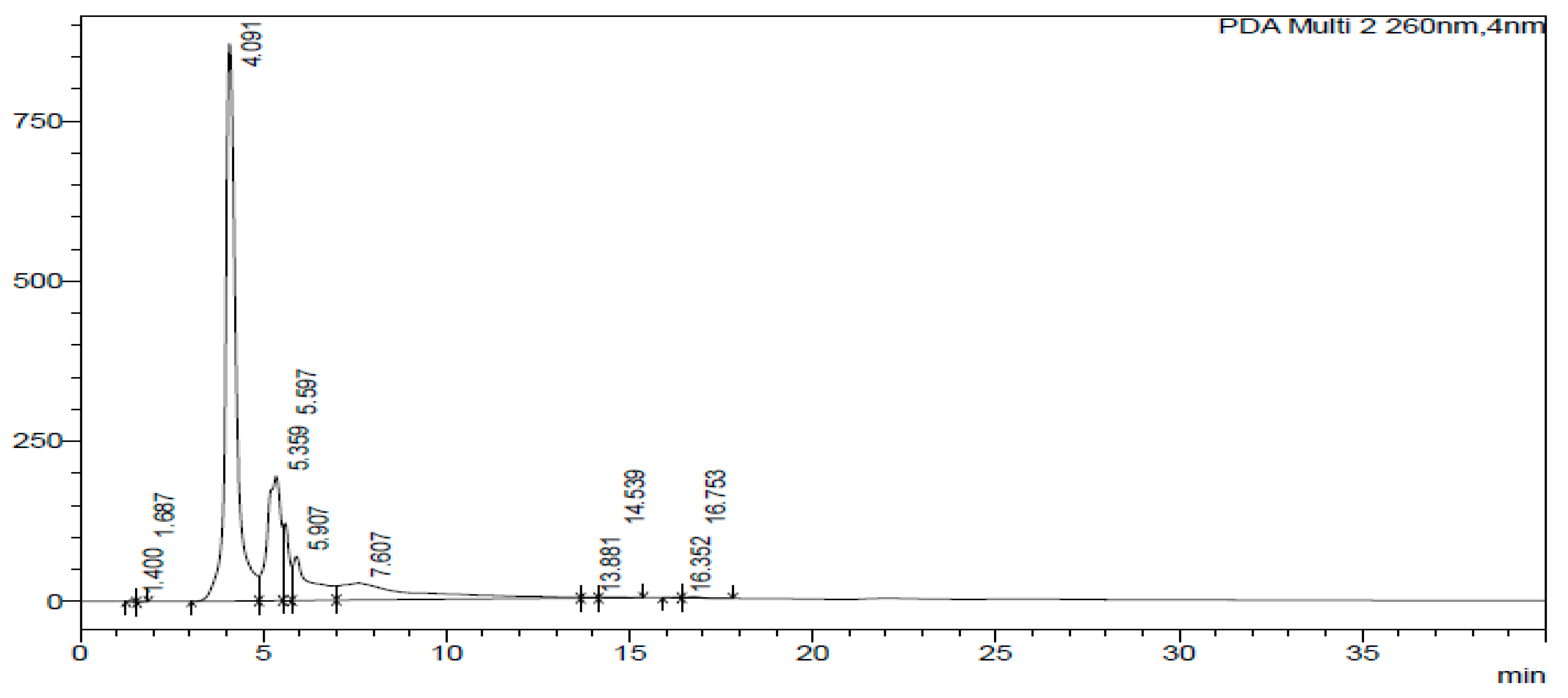
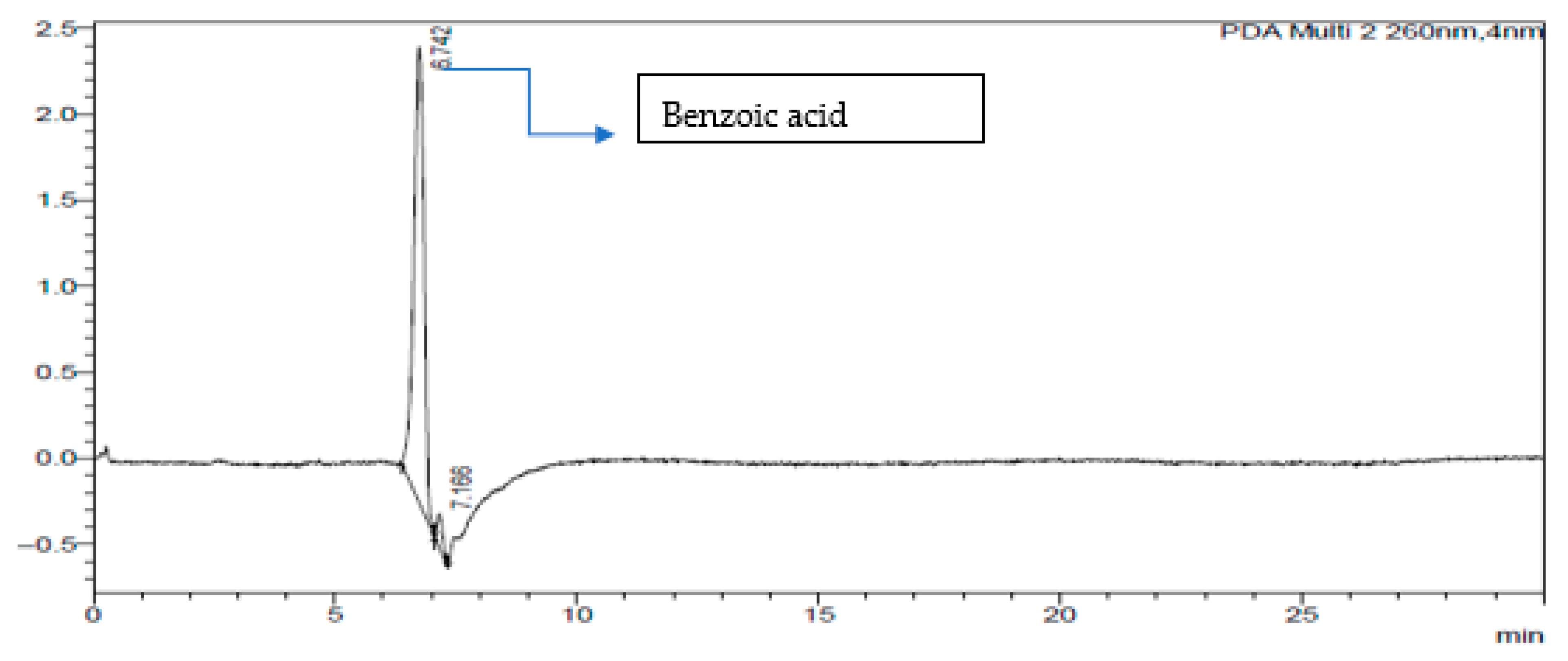
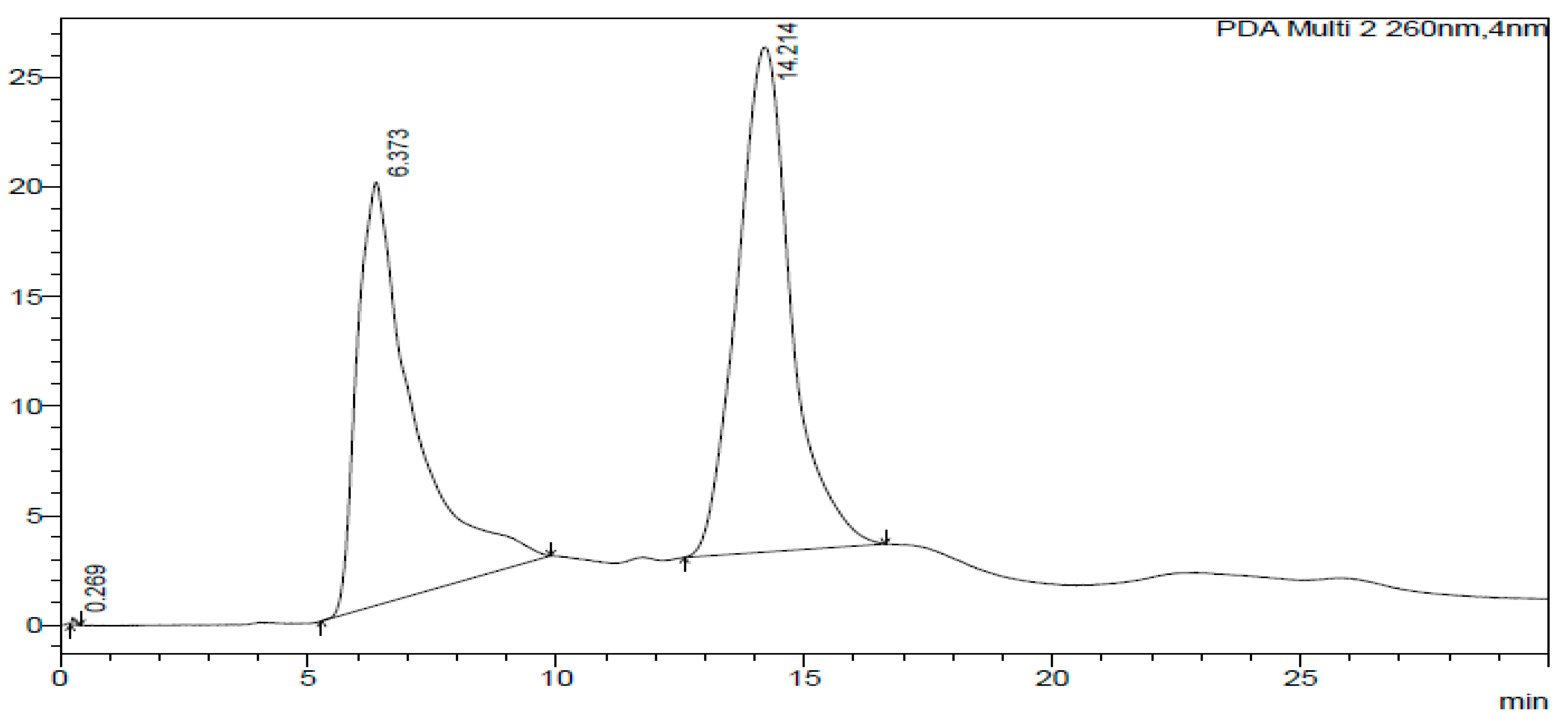
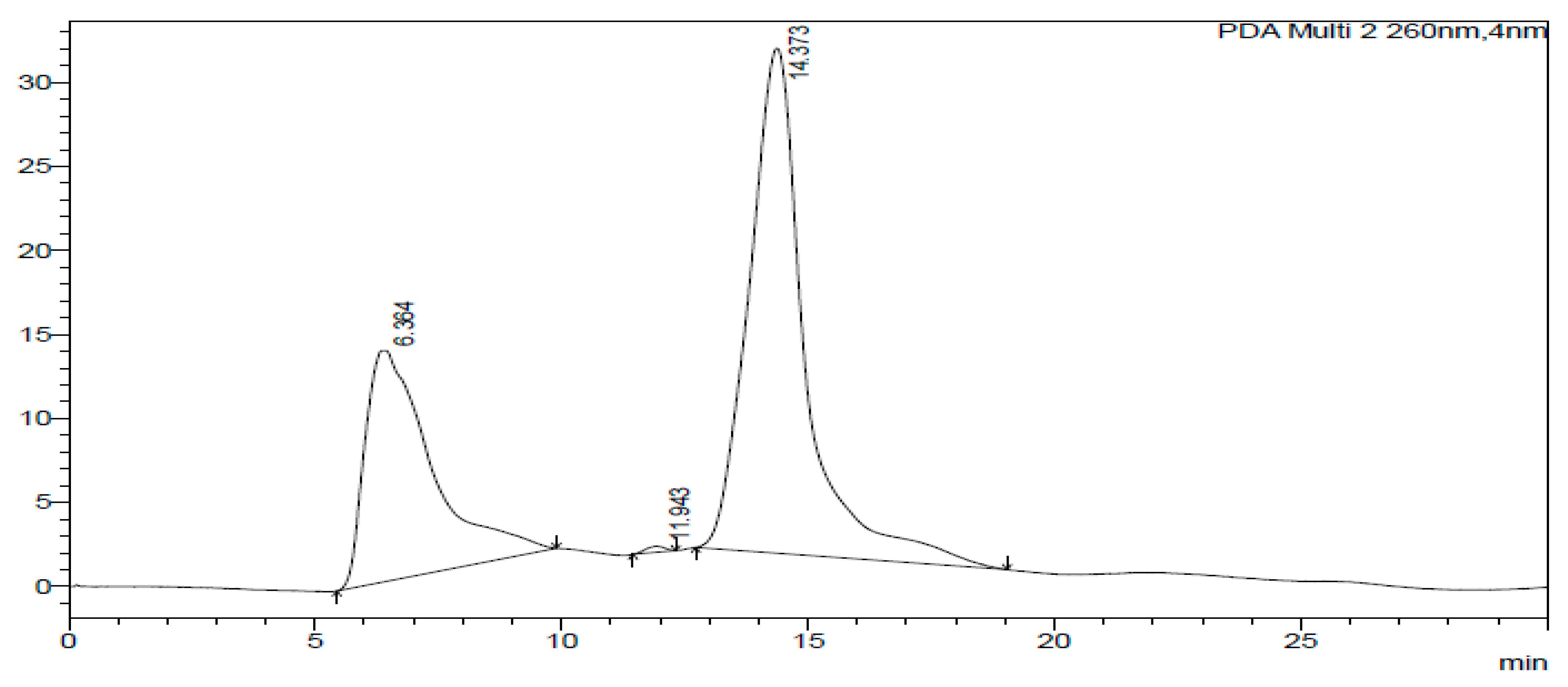
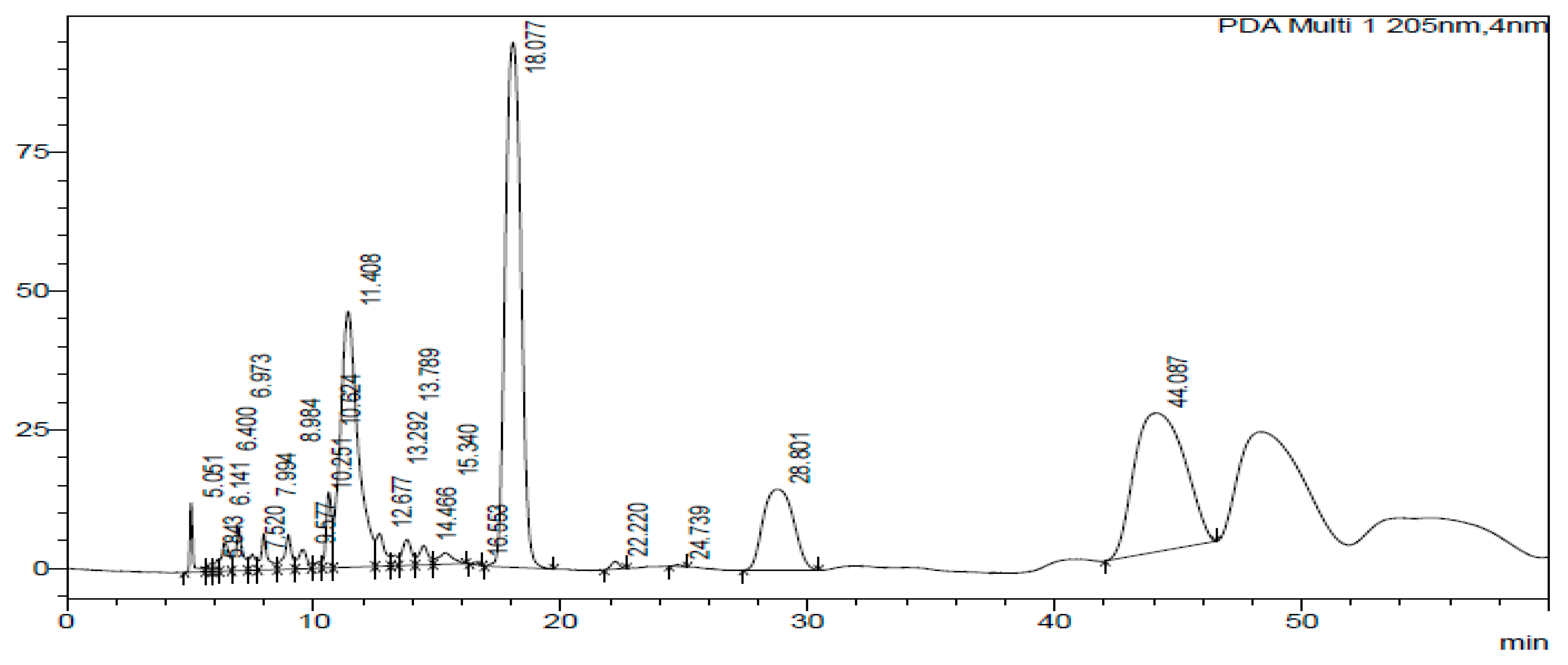
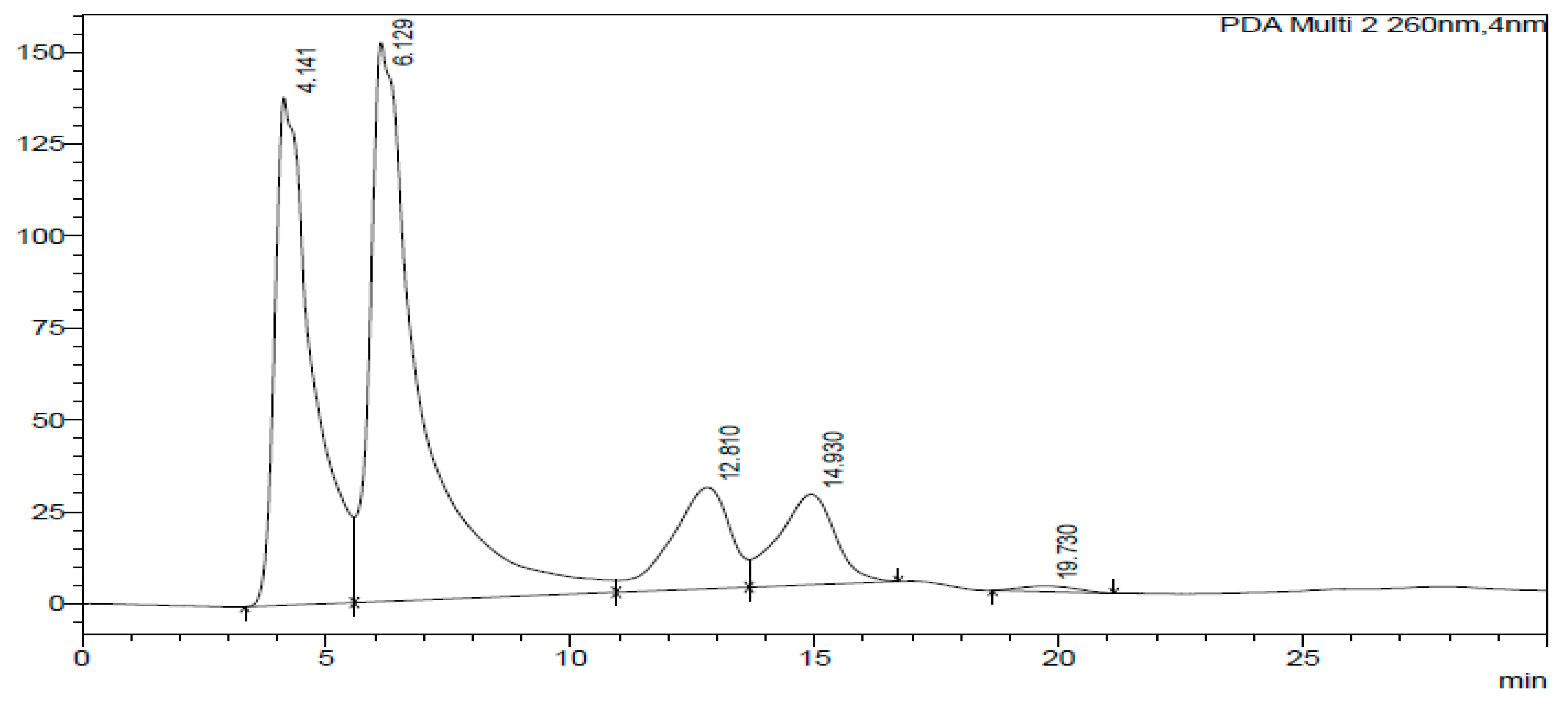
2.3. HPLC-DAD of A. quanzensis Bark
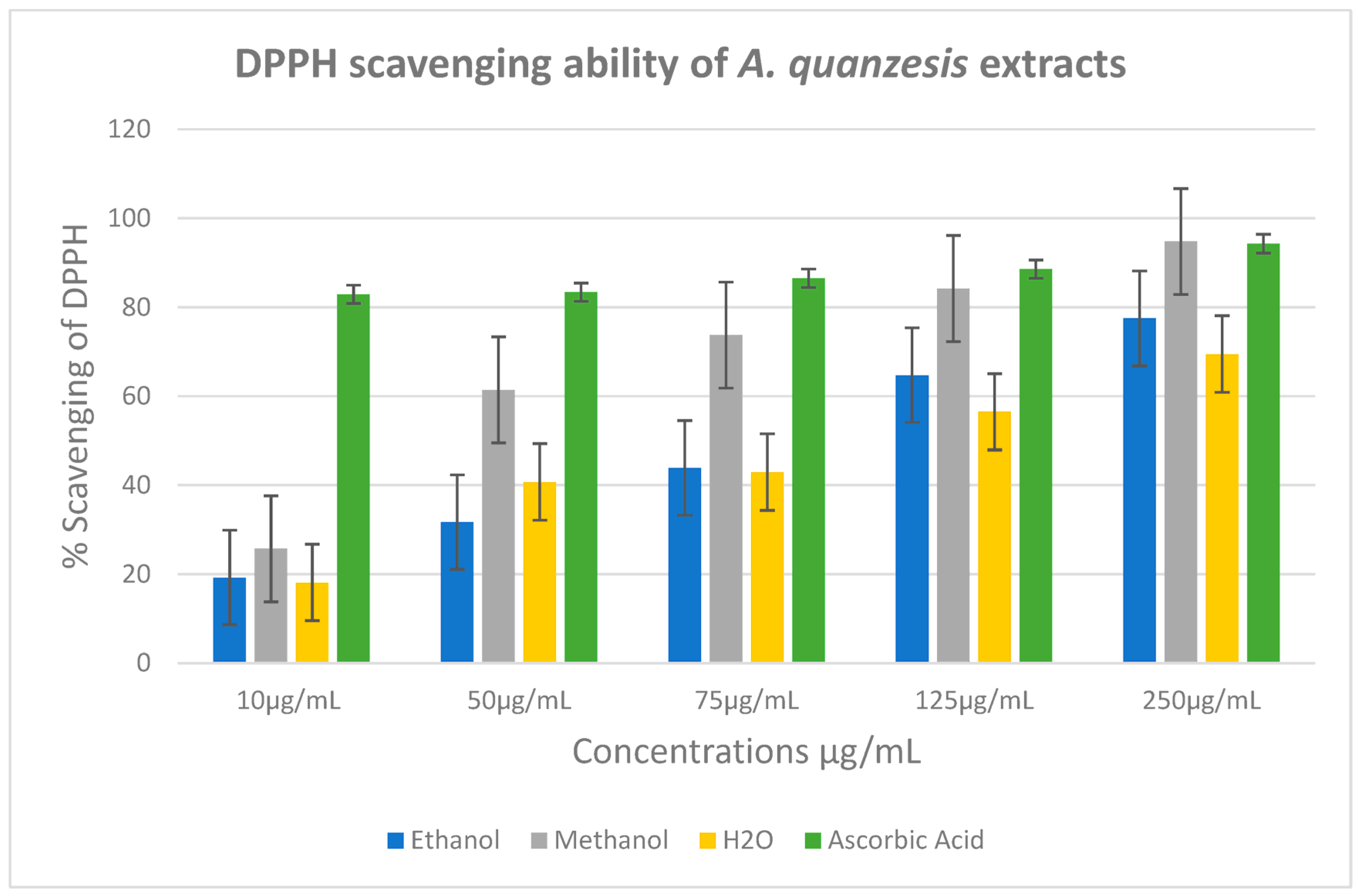
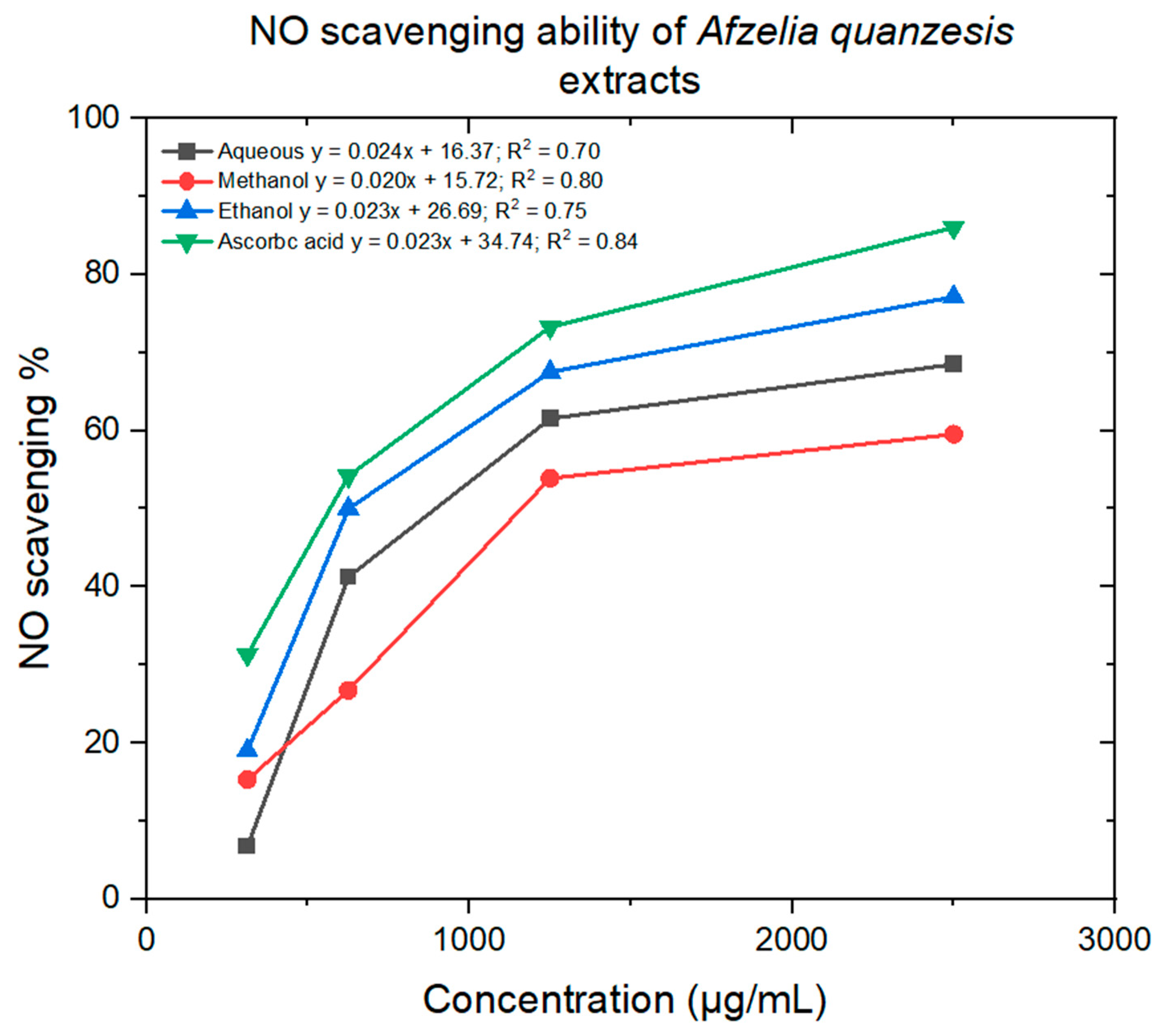
2.4. Antioxidant Assay
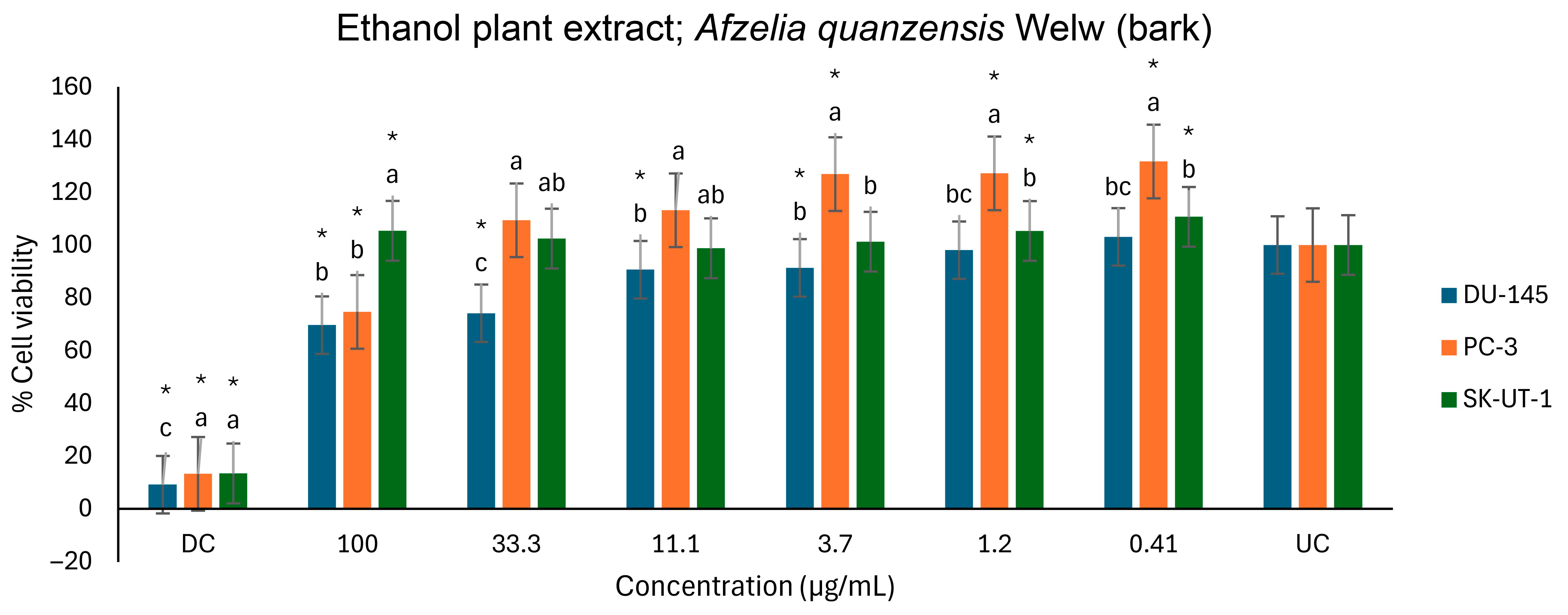
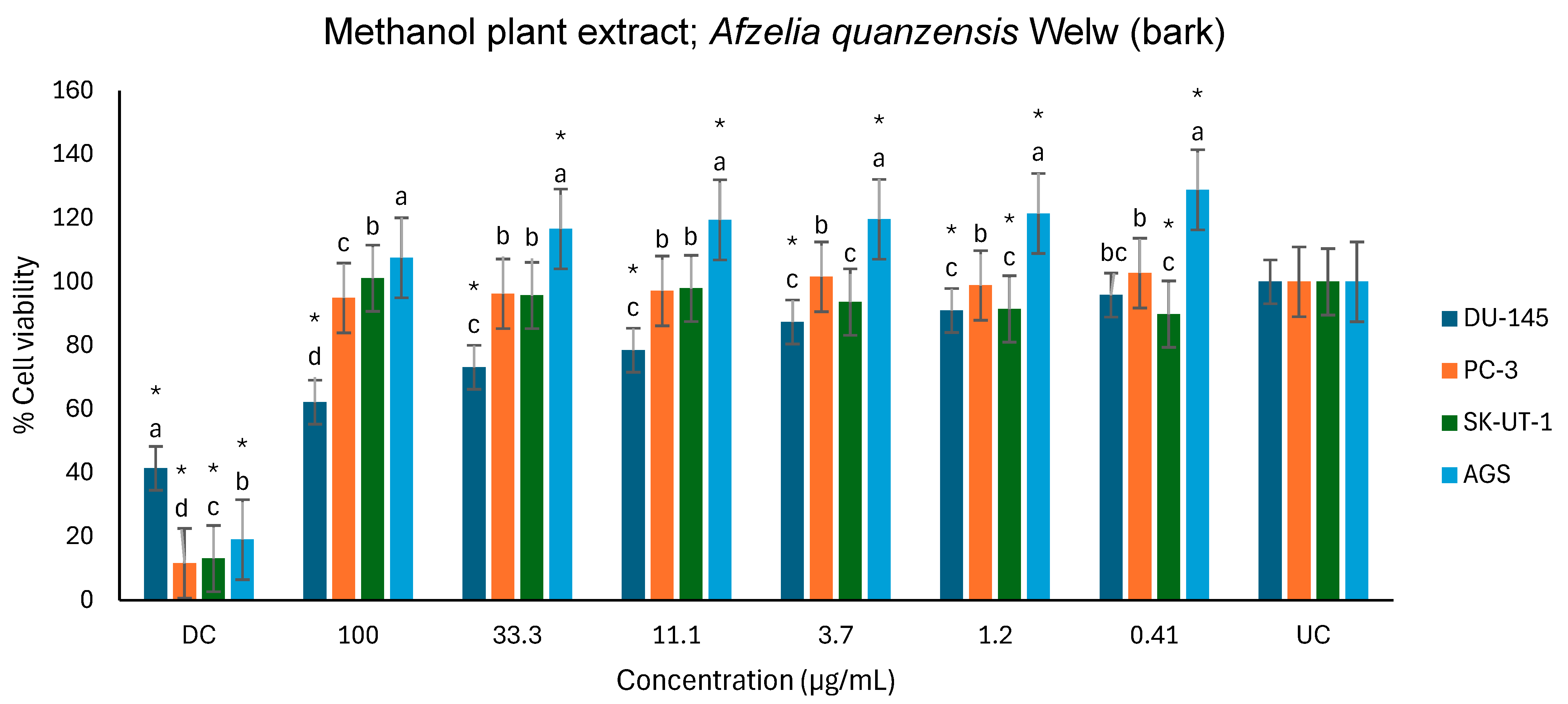
2.5. Anticancer Activity
3. Discussion
3.1. Phytochemical Analysis
3.2. FTIR Spectroscopic Analysis
3.3. HPLC-DAD Analysis of A. quanzensis Bark Extracts
3.4. Antioxidant Assay
3.5. Anticancer Activity
4. Materials and Methods
4.1. Collection of Plant Material
4.2. Preparation of Extracts
4.3. Qualitative Phytochemical Screening
4.4. Fourier-Transform Infrared Spectroscopy Analysis
4.5. HPLC-DAD Analysis of A. quanzensis Bark Extracts
4.6. Antioxidant Assay
4.6.1. DPPH Radicals Scavenging Assay
Absorbance of Blank) × 100
4.6.2. Nitric Oxide (NO) Scavenging Activity
control − Absorbance of Blank) × 100
4.7. Anticancer Activity
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masumbu, F.F.F.; Mwamatope, B.; Tembo, D.; Mwakikunga, A.; Kamanula, J. Ethnobotanical Survey of Medicinal Plants Claimed by Traditional Herbal Practitioners to Manage Cancers in Malawi. J. Herb. Med. 2023, 42, 100796. [Google Scholar] [CrossRef]
- Alabi, M.A.; Muthusamy, A.; Kabekkodu, S.P.; Adebawo, O.O.; Satyamoorthy, K. Anticancer Properties of Recipes Derived from Nigeria and African Medicinal Plants on Breast Cancer Cells in Vitro. Sci. Afr. 2020, 8, e00446. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, S.; Khan, F.; Noor, S.; Sajid, H.; Yar, S.; Rasheed, I. Prevalence and Significance of Potential Drug-Drug Interactions among Cancer Patients Receiving Chemotherapy. BMC Cancer 2020, 20, 335. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, B.S.; Parang, K. Chemical Biology LETTERS Global Cancer Statistics 2022. In The Trends Projection Analysis; American Cancer Society: Atlanta, GA, USA, 2023; Volume 2023. [Google Scholar]
- Twilley, D.; Rademan, S.; Lall, N. A Review on Traditionally Used South African Medicinal Plants, Their Secondary Metabolites and Their Potential Development into Anticancer Agents. J. Ethnopharmacol. 2020, 261, 113101. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, A.; Bayat, M.; Asemani, Y.; Amirghofran, Z. A Review of Potential Anti-Cancer Properties of Some Selected Medicinal Plants Grown in Iran. J. Herb. Med. 2022, 33, 100557. [Google Scholar] [CrossRef]
- Fouche, G.; Cragg, G.M.; Pillay, P.; Kolesnikova, N.; Maharaj, V.J.; Senabe, J. In Vitro Anticancer Screening of South African Plants. J. Ethnopharmacol. 2008, 119, 455–461. [Google Scholar] [CrossRef]
- Finestone, E.; Wishnia, J. Estimating the burden of cancer in South Africa. S. Afr. J. Oncol. 2022, 6, 220. [Google Scholar] [CrossRef]
- Kandawa-Schulz, M.; El-Sayed Lofty, H.; Lyantagaye, S. Anticancer, Antioxidant and Antimicrobial Screening of Extracts from Selected Medicinal Plants from Oshikoto, Namibia. Asian J. Trop. Biotechnol. 2018, 15, 51–65. [Google Scholar] [CrossRef]
- Mlilo, S.; Sibanda, S. An Ethnobotanical Survey of the Medicinal Plants Used in the Treatment of Cancer in Some Parts of Matebeleland, Zimbabwe. S. Afr. J. Bot. 2022, 146, 401–408. [Google Scholar] [CrossRef]
- McFarlane, C. South Africa: The Rise of Traditional Medicine. Insight Afr. 2015, 7, 60–70. [Google Scholar] [CrossRef]
- Hughes, G.D.; Aboyade, O.M.; Okonji, C.O.; Clark, B.; Mabweazara, S.Z. Comparison of the Prevalence of Non-Communicable Diseases and Traditional Herbal Medicine Use in Urban and Rural Communities in South Africa. Adv. Integr. Med. 2021, 8, 136–143. [Google Scholar] [CrossRef]
- Juárez, P. Plant-Derived Anticancer Agents: A Promising Treatment for Bone Metastasis. BoneKEy Rep. 2014, 3, 599. [Google Scholar] [CrossRef]
- Williams, V.L.; Witkowski, E.T.F.; Balkwill, K. Relationship between Bark Thickness and Diameter at Breast Height for Six Tree Species Used Medicinally in South Africa. S. Afr. J. Bot. 2007, 73, 449–465. [Google Scholar] [CrossRef][Green Version]
- Grace, O.M.; Prendergast, H.; Van Staden, J.; Jäger, A.K. The status of bark in South African traditional health care. S. Afr. J. Bot. 2002, 68, 21–30. [Google Scholar] [CrossRef][Green Version]
- Cunningham, T. Herbal medicine trade: A hidden economy. Indic. S. Afr. 1989, 6, 51–54. [Google Scholar]
- Gerhardt, K.; Todd, C. Natural Regeneration and Population Dynamics of the Tree Afzelia quanzensis in Woodlands in Southern Africa. Afr. J. Ecol. 2009, 47, 583–591. [Google Scholar] [CrossRef]
- PlantZAfrica. Available online: https://pza.sanbi.org/afzelia-quanzensis (accessed on 11 June 2025).
- van Wyk, B.-E. Medicinal Plants of South Africa; Land Reform and Rural Development (DALRRD): Pretoria, South Africa, 1997; p. 304. [Google Scholar]
- Moyo, M.; Gomba, M.; Nharingo, T. Afzelia quanzensis Bark Extract for Green Synthesis of Silver Nanoparticles and Study of Their Antibacterial Activity. Int. J. Ind. Chem. 2015, 6, 329–338. [Google Scholar] [CrossRef]
- Burlacu, E.; Tanase, C.; Coman, N.A.; Berta, L. A Review of Bark-Extract-Mediated Green Synthesis of Metallic Nanoparticles and Their Applications. Molecules 2019, 24, 4354. [Google Scholar] [CrossRef]
- Ahmed, S.I.; Hayat, M.Q.; Tahir, M.; Mansoor, Q.; Ismail, M.; Keck, K.; Bates, R.B. Pharmacologically Active Flavonoids from the Anticancer, Antioxidant and Antimicrobial Extracts of Cassia angustifolia Vahl. BMC Complement. Altern. Med. 2016, 16, 460. [Google Scholar] [CrossRef]
- Raina, H.; Soni, G.; Jauhari, N.; Sharma, N.; Bharadvaja, N. Phytochemical Importance of Medicinal Plants as Potential Sources of Anticancer Agents. Turk. J. Bot. 2014, 38, 1027–1035. [Google Scholar] [CrossRef]
- Xaba, V.M.; Adeniran, A.L.; Lamula, S.Q.N.; Buwa-Komoreng, L.V. In Vitro Bioactivities of Plants Used against Skin Diseases in the Eastern Free State, South Africa. Int. J. Plant Biol. 2024, 15, 13–31. [Google Scholar] [CrossRef]
- Kalaichelvi, K.; Dhivya, S.M. Screening of Phytoconstituents, UV-VIS Spectrum and FTIR Analysis of Micrococca mercurialis (L.) Benth. Int. J. Herb. Med. 2017, 5, 40–44. [Google Scholar]
- Durak, T.; Depciuch, J. Effect of Plant Sample Preparation and Measuring Methods on ATR-FTIR Spectra Results. Environ. Exp. Bot. 2020, 169, 103915. [Google Scholar] [CrossRef]
- Bhawsar, J.; Jain, P.K.; Soni, A.; Jain, P. Phytochemical Analysis of Mentha spicata Plant Extract Using UV-VIS, FTIR and GC/MS Technique. J. Chem. Pharm. Res. 2016, 8, 1–6. [Google Scholar]
- Chaudhry, A.W.; Memon, A.A.; Mangi, J.U.; Gorar, M.; Zaman, N.; Mahar, Z.A.; Soomro, S.A.; Qureshi, A.U.; Sidhu, A.R. An Efficient Determination of Phenolic Compounds by HPLC-Dad and Their Bioactivity Assay from Aerial Parts of Eucalyptus tereticornis. Pak. J. Bot. 2024, 56, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, V.; Fernandes, A.C.; Van Rensburg, C.E.J. Screening of Venda Medicinal Plants for Antifungal Activity against Candida albicans. S. Afr. J. Bot. 2007, 73, 256–258. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Baskar, A.A.; Al Numair, K.S.; Alsaif, M.A.; Ignacimuthu, S. In Vitro Antioxidant and Antiproliferative Potential of Medicinal Plants Used in Traditional Indian Medicine to Treat Cancer. Redox Rep. 2012, 17, 145–156. [Google Scholar] [CrossRef]
- Turan, I.; Demir, S.; Kilinc, K.; Burnaz, N.A.; Yaman, S.O.; Akbulut, K.; Mentese, A.; Aliyazicioglu, Y.; Deger, O. Antiproliferative and Apoptotic Effect of Morus nigra Extract on Human Prostate Cancer Cells. Saudi Pharm. J. 2017, 25, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gong, S.; Roy-Burman, P.; Lee, P.; Culig, Z. Current Mouse and Cell Models in Prostate Cancer Research. Endocr. Relat. Cancer 2013, 20, R155–R170. [Google Scholar] [CrossRef]
- Aghaei, M.; Karami-Tehrani, F.; Panjehpour, M.; Salami, S.; Fallahian, F. Adenosine Induces Cell-Cycle Arrest and Apoptosis in Androgen-Dependent and-Independent Prostate Cancer Cell Lines, LNcap-FGC-10, DU-145, and PC3. Prostate 2012, 72, 361–375. [Google Scholar] [CrossRef]
- Roomi, M.W.; Bhanap, B.; Niedzwiecki, A.; Rath, M. A Nutrient Mixture Reduced Tumor Growth of SK-UT-1 Human Leiomyosarcoma Cells in Vivo and in Vitro by Inhibiting Mmps and Inducing Apoptosis. Exp. Oncol. 2021, 43, 209–216. [Google Scholar] [CrossRef]
- Guggenheim, D.E.; Shah, M.A. Gastric Cancer Epidemiology and Risk Factors. J. Surg. Oncol. 2013, 107, 230–236. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New Insights of the Application of Water or Ethanol-Water Plant Extract Rich in Active Compounds in Food. Front. Nutr. 2023, 10, 118761. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Althwanay, A.; Alharthi, M.M.; Aljumaan, M.; Almubarak, Y.; Alamri, A. Methanol, Paracetamol Toxicities and Acute Blindness. Cureus 2020, 12, e8179. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.A.; Albinhassan, T.H.; Al-Ghazzawi, A.M.; Mohaya, A.; Shati, A.A.; Ayoub, H.J.; Abdallah, Q.M. Anticancer Property of Hexane Extract of Suaeda fruticose Plant Leaves against Different Cancer Cell Lines. Trop. J. Pharm. Res. 2020, 19, 129–136. [Google Scholar] [CrossRef]
- Somaida, A.; Tariq, I.; Ambreen, G.; Abdelsalam, A.M.; Ayoub, A.M.; Wojcik, M.; Dzoyem, J.P.; Bakowsky, U. Potent Cytotoxicity of Four Cameroonian Plant Extracts on Different Cancer Cell Lines. Pharmaceuticals 2020, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis Book; Springer: Berlin/Heidelberg, Germany, 1973. [Google Scholar]
- Trease, G.E.; Evans, W.C. Pharmacognsy, 11th ed.; Bailliere Tindall: Kidlington, UK, 1989. [Google Scholar]
- Sofowora, A. Phytochemical Screening of Medicinal Plants and Traditional Medicine in Africa; IGI Global: Hershey, PN, USA, 1993; pp. 150–156. [Google Scholar]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical Constituents of Some Nigerian Medicinal Plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Mashile, G.P.; Mpupa, A.; Nomngongo, P.N. In-Syringe Micro Solid-Phase Extraction Method for the Separation and Preconcentration of Parabens in Environmental Water Samples. Molecules 2018, 23, 1450. [Google Scholar] [CrossRef]
- Madikizela, B.; McGaw, L.J. In Vitro Cytotoxicity, Antioxidant and Anti-Inflammatory Activities of Pittosporum viridiflorum Sims and Hypoxis colchicifolia Baker Used Traditionally against Cancer in Eastern Cape, South Africa. S. Afr. J. Bot. 2019, 126, 250–255. [Google Scholar] [CrossRef]
- Wintola, O.A.; Olajuyigbe, A.A.; Afolayan, A.J.; Coopoosamy, R.M.; Olajuyigbe, O.O. Chemical Composition, Antioxidant Activities and Antibacterial Activities of Essential Oil from Erythrina caffra Thunb. Growing in South Africa. Heliyon 2021, 7, e07244. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]


| S/No. | Compounds | Colour Change | D(+)/ND(−) |
|---|---|---|---|
| 1 | Alkaloids | Yellowish–creamish | ++ |
| 2 | Steroids | Violet to blue | + |
| 3 | Terpenoid | Reddish brown | + |
| 4 | Flavonoids | Orange | ++ |
| 5 | Saponins | Frothing | + |
| 6 | Phlobatannins | Dirty green precipitate | ++ |
| 7 | Tannins | Red precipitate | ++ |
| 8 | Cardiac glycosides | Brown ring | + |
| Spec. No | Wave Number cm−1 (Test Samples) | Wave Number cm−1 (Hemmalakshmi et al., 2017) | Functional Group |
|---|---|---|---|
| 1 | 3304.80 | 3570–3200 | O–H strech |
| 2 | 1738.90 | 1820–1670 | C=O stretch |
| 3 | 1607.26 | 1650–1600 | C=O stretch |
| 4 | 1518.87 | 1600–1400 | C=C stretch |
| 5 | 1444.92 | 1600–1400 | C=C stretch |
| 6 | 1366.23 | 1410–1310 | O–H stretch, alcoholic group |
| 7 | 1216.97 | 1360–1210 | C–N stretch |
| 8 | 1037.17 | 1100–1000 | PO3 stretch |
| 9 | 816.32 | 1000–675 | =C–H |
| 10 | 527.75 | 730–500 | C–Cl |
| Samples | DPPH (lC50 μg/mL) | NO (lC50 μg/mL) |
|---|---|---|
| Aqueous | 4 ± 17 | 1401 ± 10 |
| Ethanol | 3 ± 21 | 1014 ± 14 |
| Methanol | 2 ± 24 | 1714 ± 16 |
| Ascorbic acid | 2 ± 4 | 664 ± 10 |
| Cell Lines | ||||
|---|---|---|---|---|
| DU-145 | PC-3 | SK-UT-1 | AGS | |
| Plant Extracts | % Inhibition at 100 µg/mL | |||
| Aqueous | 36.3 | 26.1 | - | 33.4 |
| Ethanol | 30.4 | 25.3 | - | Not tested |
| Methanol | 37.8 | 5.1 | - | |
| Hexane | - | - | - | Not tested |
| (−); No activity | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamula, S.Q.N.; Bhanisa, T.; Wium, M.; Paccez, J.D.; Zerbini, L.F.; Buwa-Komoreng, L.V. Phytochemical Analysis, Antioxidant Activity, and Anticancer Potential of Afzelia quanzensis Welw—Bark Extract: A Traditional Remedy Utilized by Indigenous Communities in KwaZulu-Natal and Eastern Cape Provinces of South Africa. Int. J. Mol. Sci. 2025, 26, 7623. https://doi.org/10.3390/ijms26157623
Lamula SQN, Bhanisa T, Wium M, Paccez JD, Zerbini LF, Buwa-Komoreng LV. Phytochemical Analysis, Antioxidant Activity, and Anticancer Potential of Afzelia quanzensis Welw—Bark Extract: A Traditional Remedy Utilized by Indigenous Communities in KwaZulu-Natal and Eastern Cape Provinces of South Africa. International Journal of Molecular Sciences. 2025; 26(15):7623. https://doi.org/10.3390/ijms26157623
Chicago/Turabian StyleLamula, Siphamandla Qhubekani Njabuliso, Thando Bhanisa, Martha Wium, Juliano Domiraci Paccez, Luiz Fernando Zerbini, and Lisa V. Buwa-Komoreng. 2025. "Phytochemical Analysis, Antioxidant Activity, and Anticancer Potential of Afzelia quanzensis Welw—Bark Extract: A Traditional Remedy Utilized by Indigenous Communities in KwaZulu-Natal and Eastern Cape Provinces of South Africa" International Journal of Molecular Sciences 26, no. 15: 7623. https://doi.org/10.3390/ijms26157623
APA StyleLamula, S. Q. N., Bhanisa, T., Wium, M., Paccez, J. D., Zerbini, L. F., & Buwa-Komoreng, L. V. (2025). Phytochemical Analysis, Antioxidant Activity, and Anticancer Potential of Afzelia quanzensis Welw—Bark Extract: A Traditional Remedy Utilized by Indigenous Communities in KwaZulu-Natal and Eastern Cape Provinces of South Africa. International Journal of Molecular Sciences, 26(15), 7623. https://doi.org/10.3390/ijms26157623






