Glucocorticoid-Mediated Skeletal Muscle Atrophy: Molecular Mechanisms and Potential Therapeutic Targets
Abstract
1. Introduction
2. Glucocorticoids
3. Effects of Glucocorticoids on Muscle Tissues
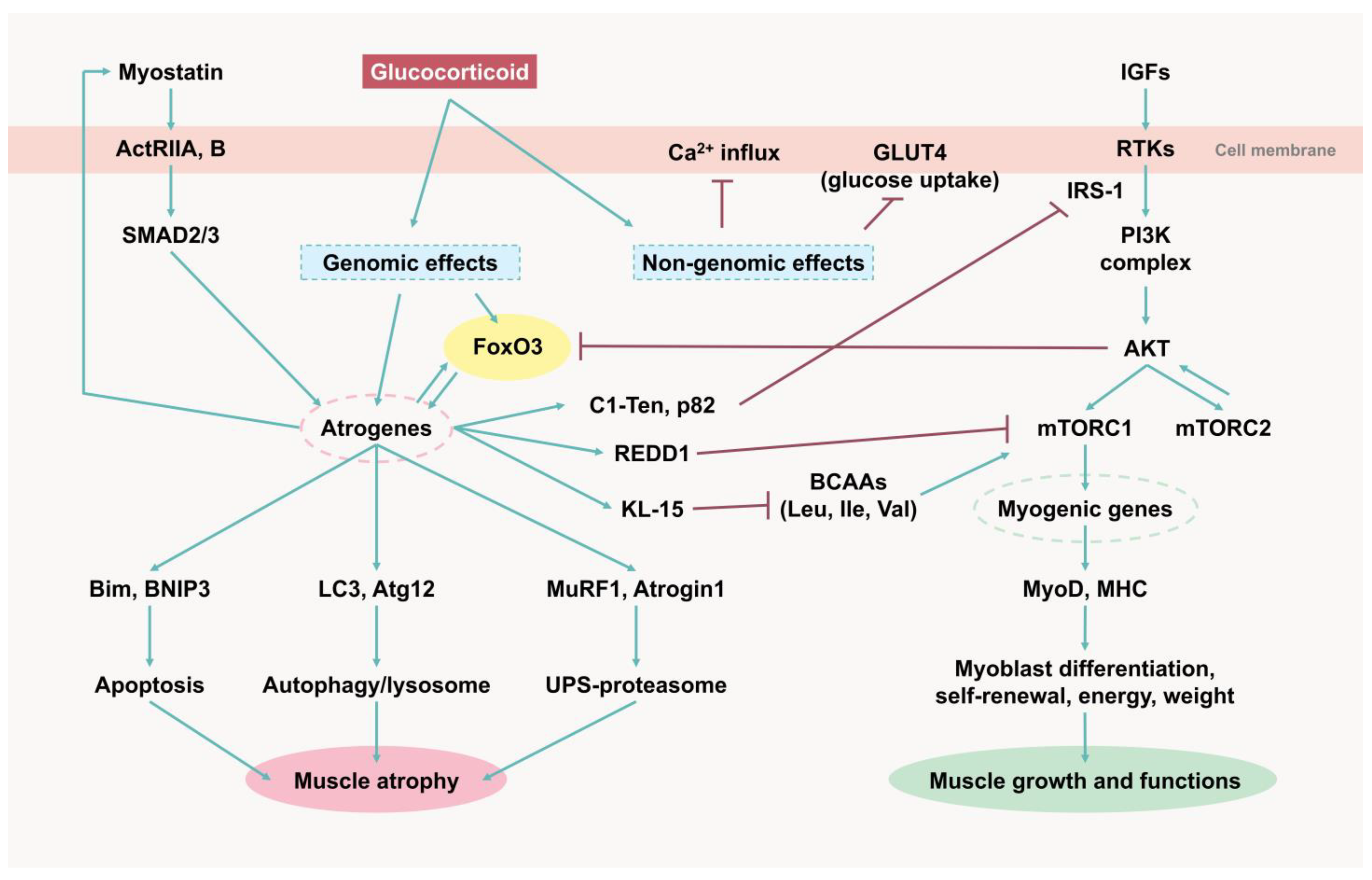
4. Molecular Mechanisms of Glucocorticoid-Induced Muscle Atrophy
4.1. GLUT4
4.2. PI3K/AKT/mTOR
4.3. Myostatin
4.4. Forkhead Box O
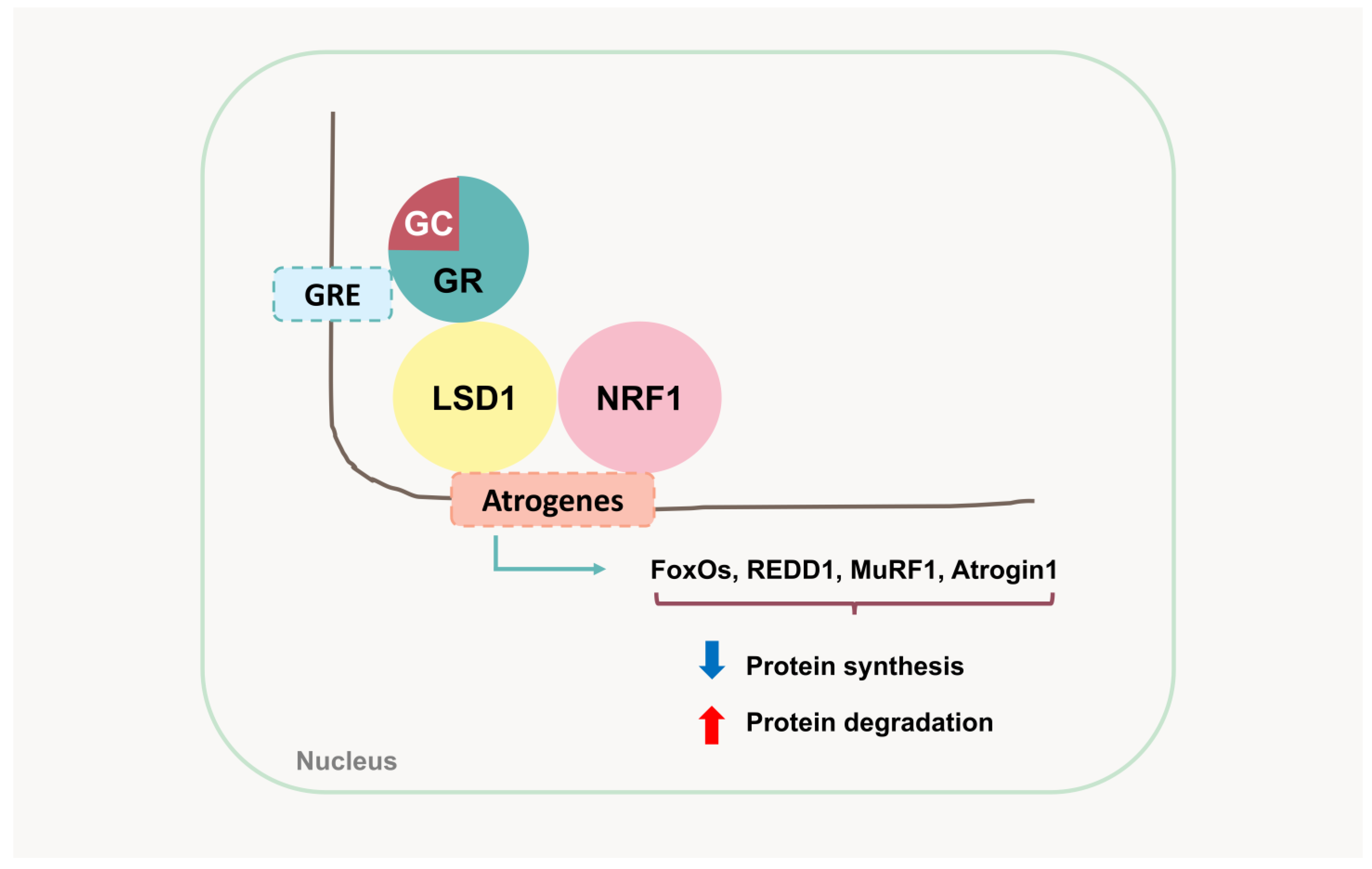
4.5. Atrogenes and UPS-Mediated Protein Degradation
5. Emerging Targets for Drug Discovery
5.1. SIRT6
5.2. LSD1
5.3. Kynurenine Pathway and IDO-1
6. Discussion and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Chun, H.J.; Ahmad, K.; Shaikh, S.; Lim, J.H.; Ali, S.; Han, S.S.; Hur, S.J.; Sohn, J.H.; Lee, E.J.; et al. The roles of growth factors and hormones in the regulation of muscle satellite cells for cultured meat production. J. Anim. Sci. Technol. 2023, 65, 16–31. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jun, H.S. Role of myokines in regulating skeletal muscle mass and function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qi, G.; Wang, K.; Yang, J.; Shen, Y.; Yang, X.; Chen, X.; Yao, X.; Gu, X.; Qi, L.; et al. Oxidative stress: Roles in skeletal muscle atrophy. Biochem. Pharmacol. 2023, 214, 115664. [Google Scholar] [CrossRef]
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing 2022, 51, afac220. [Google Scholar] [CrossRef]
- El Assar, M.; Álvarez-Bustos, A.; Sosa, P.; Angulo, J.; Rodríguez-Mañas, L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8713. [Google Scholar] [CrossRef]
- Macfarlane, E.; Zhou, H.; Seibel, M.J. Endogenous glucocorticoids during skeletal ageing. Explor. Endocr. Metab. Dis. 2024, 1, 191–212. [Google Scholar] [CrossRef]
- Reincke, M. Cushing Syndrome Associated Myopathy: It Is Time for a Change. Endocrinol. Metab. 2021, 36, 564–571. [Google Scholar] [CrossRef]
- Braun, T.P.; Marks, D.L. The regulation of muscle mass by endogenous glucocorticoids. Front. Physiol. 2015, 6, 12. [Google Scholar] [CrossRef]
- Morgan, S.A.; Sherlock, M.; Gathercole, L.L.; Lavery, G.G.; Lenaghan, C.; Bujalska, I.J.; Laber, D.; Yu, A.; Convey, G.; Mayers, R.; et al. 11β-hydroxysteroid dehydrogenase type 1 regulates glucocorticoid- induced insulin resistance in skeletal muscle. Diabetes 2009, 58, 2506–2515. [Google Scholar] [CrossRef]
- Shimizu, N.; Yoshikawa, N.; Ito, N.; Maruyama, T.; Suzuki, Y.; Takeda, S.; Nakae, J.; Tagata, Y.; Nishitani, S.; Takehana, K.; et al. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 2011, 13, 170–182. [Google Scholar] [CrossRef]
- Qin, J.; Du, R.; Yang, Y.Q.; Zhang, H.Q.; Li, Q.; Liu, L.; Guan, H.; Hou, J.; An, X.R. Dexamethasone-induced skeletal muscle atrophy was associated with upregulation of myostatin promoter activity. Res. Vet. Sci. 2013, 94, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Ohkawa, S.; Li, H.; Roberts-Wilson, T.K.; Price, S.R. FOXO3a mediates signaling crosstalk that coordinates ubiquitin and atrogin-1/MAFbx expression during glucocorticoid-induced skeletal muscle atrophy. FASEB J. 2010, 24, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.W.; Miao, C.Y.; Liu, L.; Zhou, J.; Su, D.F.; Wang, Y.X.; Jiang, C.L. Rapid inhibitory effect of glucocorticoids on airway smooth muscle contractions in guinea pigs. Steroids 2006, 71, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.; Leighton, B.; Parry-Billings, M.; Sasson, S.; Young, M.; Krause, U.; Bevan, S.; Piva, T.; Wegener, G.; Newsholme, E.A. Effects of Glucocorticoid Excess on the Sensitivity of Glucose Transport and Metabolism to Insulin in Rat Skeletal Muscle. Biochem. J. 1997, 321, 707–712. [Google Scholar] [CrossRef]
- Mishra, S.; Cosentino, C.; Tamta, A.K.; Khan, D.; Srinivasan, S.; Ravi, V.; Abbotto, E.; Arathi, B.P.; Kumar, S.; Jain, A.; et al. Sirtuin 6 inhibition protects against glucocorticoid-induced skeletal muscle atrophy by regulating IGF/PI3K/AKT signaling. Nat. Commun. 2022, 13, 5415. [Google Scholar] [CrossRef]
- Cai, Q.; Sahu, R.; Ueberschlag-Pitiot, V.; Souali-Crespo, S.; Charvet, C.; Silem, I.; Cottard, F.; Ye, T.; Taleb, F.; Metzger, E.; et al. LSD1 inhibition circumvents glucocorticoid-induced muscle wasting of male mice. Nat. Commun. 2024, 15, 3563. [Google Scholar] [CrossRef]
- Kaiser, H.; Ding, K.H.; Isales, C.; Hamrick, M. The effects of kynurenine metabolites on skeletal muscle in vivo and in vitro. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Dastidar, R.G.; Ong, J.F.; Levy, O.; Karp, J.M. Performance-enhanced mesenchymal stem cells via intracellular delivery of steroids. Sci. Rep. 2014, 4, 4645. [Google Scholar] [CrossRef]
- Gjerstad, J.K.; Lightman, S.L.; Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 2018, 21, 403–416. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues. Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Lucassen, E.A.; Cizza, G. The Hypothalamic-Pituitary-Adrenal Axis, Obesity, and Chronic Stress Exposure: Sleep and the HPA Axis in Obesity. Curr. Obes. Rep. 2012, 1, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Akalestou, E.; Genser, L.; Rutter, G.A. Glucocorticoid Metabolism in Obesity and Following Weight Loss. Front. Endocrinol. 2020, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Nakagawa, Y.; Ohzeki, T. Gene Expression of 11β-Hydroxysteroid Dehydrogenase Type 1 and Type 2 in the Kidneys of Insulin-Dependent Diabetic Rats. Hypertension 1998, 31, 885–889. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tomas, C.; Newton, J.; Watson, S. A Review of Hypothalamic-Pituitary-Adrenal Axis Function in Chronic Fatigue Syndrome. Int. Sch. Res. Not. 2013, 2013, 784520. [Google Scholar] [CrossRef]
- Demorrow, S. Role of the hypothalamic–pituitary–adrenal axis in health and disease. Int. J. Mol. Sci. 2018, 19, 986. [Google Scholar] [CrossRef]
- Lockett, J.; Inder, W.J.; Clifton, V.L. The Glucocorticoid Receptor: Isoforms, Functions, and Contribution to Glucocorticoid Sensitivity. Endocr. Rev. 2024, 45, 593–624. [Google Scholar] [CrossRef]
- Quatrini, L.; Ugolini, S. New insights into the cell- and tissue-specificity of glucocorticoid actions. Cell. Mol. Immunol. 2021, 18, 269–278. [Google Scholar] [CrossRef]
- De Bosscher, K.; Haegeman, G. Minireview: Latest Perspectives on Antiinflammatory Actions of Glucocorticoids. Mol. Endocrinol. 2009, 23, 281–291. [Google Scholar] [CrossRef]
- Franco, L.M.; Gadkari, M.; Howe, K.N.; Sun, J.; Kardava, L.; Kumar, P.; Kumari, S.; Hu, Z.; Fraser, I.D.C.; Moir, S.; et al. Immune regulation by glucocorticoids can be linked to cell type–dependent transcriptional responses. J. Exp. Med. 2019, 216, 384–406. [Google Scholar] [CrossRef]
- Grose, R.; Werner, S.; Kessler, D.; Tuckermann, J.; Huggel, K.; Durka, S.; Reichardt, H.M.; Werner, S. A role for endogenous glucocorticoids in wound repair. EMBO Rep. 2002, 3, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Gerber, A.N.; Newton, R.; Sasse, S.K. Repression of transcription by the glucocorticoid receptor: A parsimonious model for the genomics era. J. Biol. Chem. 2021, 296, 100687. [Google Scholar] [CrossRef] [PubMed]
- Hudson, W.H.; de Vera, I.M.S.; Nwachukwu, J.C.; Weikum, E.R.; Herbst, A.G.; Yang, Q.; Bain, D.L.; Nettles, K.W.; Kojetin, D.J.; Ortlund, E.A. Cryptic glucocorticoid receptor-binding sites pervade genomic NF-κB response elements. Nat. Commun. 2018, 9, 1337. [Google Scholar] [CrossRef]
- Conn, D.L. The Story Behind the Use of Glucocorticoids in the Treatment of Rheumatoid Arthritis. Semin. Arthritis. Rheum. 2021, 51, 15–19. [Google Scholar] [CrossRef]
- Reichardt, S.D.; Amouret, A.; Muzzi, C.; Vettorazzi, S.; Tuckermann, J.P.; Lühder, F.; Reichardt, H.M. The role of glucocorticoids in inflammatory diseases. Cells 2021, 10, 2921. [Google Scholar] [CrossRef]
- Porta, S.; Danza, A.; Arias, S.M.; Carlomagno, A.; Goizueta, M.C.; Vivero, F.; Ruiz-Irastorza, G. Glucocorticoids in Systemic Lupus Erythematosus. Ten Questions and Some Issues. J. Clin. Med. 2020, 9, 2709. [Google Scholar] [CrossRef]
- Tavares, L.C.P.; Caetano, L.d.V.N.; Ianhez, M. Side effects of chronic systemic glucocorticoid therapy: What dermatologists should know. An. Bras. Dermatol. 2024, 99, 259–268. [Google Scholar] [CrossRef]
- Fardet, L.; Nazareth, I.; Petersen, I. Long-term systemic glucocorticoid therapy and weight gain: A population-based cohort study. Rheumatology 2021, 60, 1502–1511. [Google Scholar] [CrossRef]
- Beaupere, C.; Liboz, A.; Fève, B.; Blondeau, B.; Guillemain, G. Molecular Mechanisms of Glucocorticoid-Induced Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 623. [Google Scholar] [CrossRef]
- Caplan, A.; Fett, N.; Rosenbach, M.; Werth, V.P.; Micheletti, R.G. Prevention and management of glucocorticoid-induced side effects: A comprehensive review: Ocular, cardiovascular, muscular, and psychiatric side effects and issues unique to pediatric patients. J. Am. Acad. Dermatol. 2017, 76, 201–207. [Google Scholar] [CrossRef]
- Morgan, S.A.; McCabe, E.L.; Gathercole, L.L.; Hassan-Smith, Z.K.; Larner, D.P.; Bujalska, I.J.; Stewart, P.M.; Tomlinson, J.W.; Lavery, G.G. 11β-HSD1 is the major regulator of the tissue-specific effects of circulating glucocorticoid excess. Proc. Nat. Acad. Sci. USA 2014, 111, E2482–E2491. [Google Scholar] [CrossRef]
- Hassan-Smith, Z.K.; Morgan, S.A.; Sherlock, M.; Hughes, B.; Taylor, A.E.; Lavery, G.G.; Tomlinson, J.W.; Stewart, P.M. Gender-Specific Differences in Skeletal Muscle 11β-HSD1 Expression Across Healthy Aging. J. Clin. Endocrinol. Metab. 2015, 100, 2673–2681. [Google Scholar] [CrossRef]
- Hardy, R.S.; Doig, C.L.; Hussain, Z.; O’Leary, M.; Morgan, S.A.; Pearson, M.J.; Naylor, A.; Jones, S.W.; Filer, A.; Stewart, P.M.; et al. 11β-Hydroxysteroid dehydrogenase type 1 within muscle protects against the adverse effects of local inflammation. J. Pathol. 2016, 240, 472–483. [Google Scholar] [CrossRef]
- Markey, K.A.; Ottridge, R.; Mitchell, J.L.; Rick, C.; Woolley, R.; Ives, N.; Nightingale, P.; Sinclair, A.J. Assessing the Efficacy and Safety of an 11β-Hydroxysteroid Dehydrogenase Type 1 Inhibitor (AZD4017) in the Idiopathic Intracranial Hypertension Drug Trial, IIH:DT: Clinical Methods and Design for a Phase II Randomized Controlled Trial. JMIR Res. Protoc. 2017, 6, e181. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; An, G. Characterizing the Nonlinear Pharmacokinetics and Pharmacodynamics of BI 187004, an 11β-Hydroxysteroid Dehydrogenase Type 1 Inhibitor, in Humans by a Target-Mediated Drug Disposition Model. J. Clin. Pharmacol. 2024, 64, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.S.; Botfield, H.; Markey, K.; Mitchell, J.L.; Alimajstorovic, Z.; Westgate, C.S.J.; Sagmeister, M.; Fairclough, R.J.; Ottridge, R.S.; Yiangou, A.; et al. 11βHSD1 Inhibition with AZD4017 Improves Lipid Profiles and Lean Muscle Mass in Idiopathic Intracranial Hypertension. J. Clin. Endocrinol. Metab. 2021, 106, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Othonos, N.; Pofi, R.; Arvaniti, A.; White, S.; Bonaventura, I.; Nikolaou, N.; Moolla, A.; Marjot, T.; Stimson, R.H.; van Beek, A.P.; et al. 11β-HSD1 inhibition in men mitigates prednisolone-induced adverse effects in a proof-of-concept randomised double-blind placebo-controlled trial. Nat. Commun. 2023, 14, 1025. [Google Scholar] [CrossRef]
- Vignjević, P.S.; Milošević, M.S.; Marković, D.; Momčilović, S. Interplay between stress and cancer-A focus on inflammation. Front. Physiol. 2023, 14, 1119095. [Google Scholar] [CrossRef]
- Sato, A.Y.; Richardson, D.; Cregor, M.; Davis, H.M.; Au, E.D.; McAndrews, K.; Zimmers, T.A.; Organ, J.M.; Peacock, M.; Plotkin, L.I.; et al. Glucocorticoids Induce Bone and Muscle Atrophy by Tissue-Specific Mechanisms Upstream of E3 Ubiquitin Ligases. Endocrinology 2017, 158, 664–677. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, Y. Glucocorticoid-induced myopathy: Pathophysiology, diagnosis, and treatment. Indian. J. Endocrinol. Metab. 2013, 17, 913–916. [Google Scholar] [CrossRef]
- Schakman, O.; Kalista, S.; Barbé, C.; Loumaye, A.; Thissen, J.P. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell. Biol. 2013, 45, 2163–2172. [Google Scholar] [CrossRef]
- Kuo, T.; Harris, C.A.; Wang, J.C. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol. Cell. Endocrinol. 2013, 380, 79–88. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Julien, I.B.; Sephton, C.F.; Dutchak, P.A. Metabolic networks influencing skeletal muscle fiber composition. Front. Cell Dev. Biol. 2018, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Baylor, S.M.; Hollingworth, S. Intracellular calcium movements during excitation–contraction coupling in mammalian slow-twitch and fast-twitch muscle fibers. J. Gen. Physiol. 2012, 139, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Li, J.L. Role of PGC-1α signaling in skeletal muscle health and disease. Ann. N. Y. Acad. Sci. 2012, 1271, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Komiya, Y.; Iseki, S.; Ochiai, M.; Takahashi, Y.; Yokoyama, I.; Suzuki, T.; Tatsumi, R.; Sawano, S.; Mizunoya, W.; Arihara, K. Dietary oleic acid intake increases the proportion of type 1 and 2X muscle fibers in mice. Sci. Rep. 2024, 14, 755. [Google Scholar] [CrossRef]
- Song, P.; Zhao, J.; Li, F.; Zhao, X.; Feng, J.; Su, Y.; Wang, B.; Zhao, J. Vitamin A regulates mitochondrial biogenesis and function through p38 MAPK-PGC-1α signaling pathway and alters the muscle fiber composition of sheep. J. Anim. Sci. Biotechnol. 2024, 15, 18. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Zhang, P.; Liu, H.; He, J.; Zhang, C.; Fan, M.; Chen, X. PGC-1α over-expression suppresses the skeletal muscle atrophy and myofiber-type composition during hindlimb unloading. Biosci. Biotechnol. Biochem. 2017, 81, 500–513. [Google Scholar] [CrossRef]
- Servais, L.; Lair, L.L.; Connolly, A.M.; Byrne, B.J.; Chen, K.S.; Coric, V.; Qureshi, I.; Durham, S.; Campbell, D.J.; Maclaine, G.; et al. Taldefgrobep Alfa and the Phase 3 RESILIENT Trial in Spinal Muscular Atrophy. Int. J. Mol. Sci. 2024, 25, 10273. [Google Scholar] [CrossRef]
- Muntoni, F.; Byrne, B.J.; McMillan, H.J.; Ryan, M.M.; Wong, B.L.; Dukart, J.; Bansal, A.; Cosson, V.; Dreghici, R.; Guridi, M.; et al. The Clinical Development of Taldefgrobep Alfa: An Anti-Myostatin Adnectin for the Treatment of Duchenne Muscular Dystrophy. Neurol. Ther. 2024, 13, 183–219. [Google Scholar] [CrossRef]
- Pirruccello-Straub, M.; Jackson, J.; Wawersik, S.; Webster, M.T.; Salta, L.; Long, K.; McConaughy, W.; Capili, A.; Boston, C.; Carven, G.J.; et al. Blocking extracellular activation of myostatin as a strategy for treating muscle wasting. Sci. Rep. 2018, 8, 2292. [Google Scholar] [CrossRef]
- Crawford, T.O.; Darras, B.T.; Day, J.W.; Dunaway Young, S.; Duong, T.; Nelson, L.L.; Barrett, D.; Song, G.; Bilic, S.; Cote, S.; et al. Safety and Efficacy of Apitegromab in Patients With Spinal Muscular Atrophy Types 2 and 3: The Phase 2 TOPAZ Study. Neurology 2024, 103, e209519. [Google Scholar] [CrossRef]
- Kaur, M.; Misra, S. Bimagrumab: An investigational human monoclonal antibody against activin type II receptors for treating obesity. J. Basic. Clin. Physiol. Pharmacol. 2024, 35, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Lach-Trifilieff, E.; Minetti, G.C.; Sheppard, K.; Ibebunjo, C.; Feige, J.N.; Hartmann, S.; Brachat, S.; Rivet, H.; Koelbing, C.; Morvan, F.; et al. An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol. Cell. Biol. 2014, 34, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Vilchinskaya, N.; Altaeva, E.; Lomonosova, Y. Gaining insight into the role of FoxO1 in the progression of disuse-induced skeletal muscle atrophy. Adv. Biol. Regul. 2022, 85, 100903. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Diaz, B.; Deroose, M.; Lee, S.X.; Belvedere, S.; Accili, D.; Leibel, R.L.; Lin, H.V. FOXO1 inhibition synergizes with FGF21 to normalize glucose control in diabetic mice. Mol. Metab. 2021, 49, 101187. [Google Scholar] [CrossRef]
- Salcher, S.; Spoden, G.; Hagenbuchner, J.; Führer, S.; Kaserer, T.; Tollinger, M.; Huber-Cantonati, P.; Gruber, T.; Schuster, D.; Gust, R.; et al. A drug library screen identifies Carbenoxolone as novel FOXO inhibitor that overcomes FOXO3-mediated chemoprotection in high-stage neuroblastoma. Oncogene 2020, 39, 1080–1097. [Google Scholar] [CrossRef]
- Sato, A.Y.; Cregor, M.; McAndrews, K.; Schurman, C.A.; Schaible, E.; Shutter, J.; Vyas, P.; Adhikari, B.; Willis, M.S.; Boerma, M.; et al. Pharmacologic or genetic interference with atrogene signaling protects from glucocorticoid-induced musculoskeletal and cardiac disease. JCI Insight 2024, 9, e182664. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes. Care 2009, 32, S157–S163. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; McQueen, A.; Chen, T.C.; Wang, J.C. Regulation of glucose homeostasis by glucocorticoids. Adv. Exp. Med. Biol. 2015, 872, 99–126. [Google Scholar] [PubMed]
- Swarbrick, M.; Zhou, H.; Seibel, M. Local and systemic effects of glucocorticoids on metabolism: New lessons from animal models. Eur. J. Endocrinol. 2021, 185, R113–R129. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Liu, L.; Ni, C.X.; Zhang, Y.; Su, W.J.; Lian, Y.J.; Peng, W.; Zhang, J.P.; Jiang, C.L. Dexamethasone rapidly inhibits glucose uptake via non-genomic mechanisms in contracting myotubes. Arch. Biochem. Biophys. 2016, 603, 102–109. [Google Scholar] [CrossRef]
- Glass, D.J. PI3 Kinase Regulation of Skeletal Muscle Hypertrophy and Atrophy. In Phosphoinositide 3-Kinase in Health and Disease; Rommel, C., Vanhaesebroeck, B., Vogt, P.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 1, pp. 267–278. [Google Scholar]
- Yoon, M.S. mTOR as a key regulator in maintaining skeletal muscle mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef]
- Imanaga, H.; Semba, Y.; Sasaki, K.; Setoguchi, K.; Maniriho, H.; Yamauchi, T.; Terasaki, T.; Hirabayashi, S.; Nakao, F.; Nogami, J.; et al. Central role of the mTORC1 pathway in glucocorticoid activity against B-ALL cells. Blood Neoplasia 2024, 1, 100015. [Google Scholar] [CrossRef]
- Shi, W.; Wang, D.; Yuan, X.; Liu, Y.; Guo, X.; Li, J.; Song, J. Glucocorticoid receptor-IRS-1 axis controls EMT and the metastasis of breast cancers. J. Mol. Cell. Biol. 2019, 11, 1042–1055. [Google Scholar] [CrossRef]
- Koh, A.; Lee, M.N.; Yang, Y.R.; Jeong, H.; Ghim, J.; Noh, J.; Kim, J.; Ryu, D.; Park, S.; Song, P.; et al. C1-Ten Is a Protein Tyrosine Phosphatase of Insulin Receptor Substrate 1 (IRS-1), Regulating IRS-1 Stability and Muscle Atrophy. Mol. Cell. Biol. 2013, 33, 1608–1620. [Google Scholar] [CrossRef]
- Giorgino, F.; Pedrini, M.T.; Matera, L.; Smith, R.J. Specific Increase in p85α Expression in Response to Dexamethasone Is Associated with Inhibition of Insulin-like Growth Factor-I Stimulated Phosphatidylinositol 3-Kinase Activity in Cultured Muscle Cells. J. Biol. Chem. 1997, 272, 7455–7463. [Google Scholar] [CrossRef]
- Kuo, T.; Lew, M.J.; Mayba, O.; Harris, C.A.; Speed, T.P.; Wang, J.C. Genome-wide analysis of glucocorticoid receptor-binding sites in myotubes identifies gene networks modulating insulin signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 11160–11165. [Google Scholar] [CrossRef]
- Kukreti, H.; Amuthavalli, K.; Harikumar, A.; Sathiyamoorthy, S.; Feng, P.Z.; Anantharaj, R.; Tan, S.L.; Lokireddy, S.; Bonala, S.; Sriram, S.; et al. Muscle-specific MicroRNA1 (miR1) Targets Heat Shock Protein 70 (HSP70) during Dexamethasone-mediated Atrophy. J. Biol. Chem. 2013, 288, 6663–6678. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kwon, Y.G.; Kim, Y.M. The stress-responsive protein REDD1 and its pathophysiological functions. Exp. Mol. Med. 2023, 55, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.D.; Kimball, S.R.; Fort, P.E.; Jefferson, L.S. Regulated in Development and DNA Damage 1 Is Necessary for Hyperglycemia-induced Vascular Endothelial Growth Factor Expression in the Retina of Diabetic Rodents. J. Biol. Chem. 2015, 290, 3865–3874. [Google Scholar] [CrossRef] [PubMed]
- Sunilkumar, S.; Dennis, M.D. REDD1 is a Promising Therapeutic Target to Combat the Development of Diabetic Complications: A Report on Research Supported by Pathway to Stop Diabetes. Diabetes 2024, 73, 1533–1562. [Google Scholar] [CrossRef]
- Vega-Rubin-de-Celis, S.; Abdallah, Z.; Kinch, L.; Grishin, N.V.; Brugarolas, J.; Zhang, X. Structural analysis and functional implications of the negative mTORC1 regulator REDD1. Biochemistry 2010, 49, 2491–2501. [Google Scholar] [CrossRef]
- Baida, G.; Bhalla, P.; Kirsanov, K.; Lesovaya, E.; Yakubovskaya, M.; Yuen, K.; Guo, S.; Lavker, R.M.; Readhead, B.; Dudley, J.T.; et al. REDD 1 functions at the crossroads between the therapeutic and adverse effects of topical glucocorticoids. EMBO Mol. Med. 2015, 7, 42–58. [Google Scholar] [CrossRef]
- Britto, F.A.; Begue, G.; Rossano, B.; Docquier, A.; Vernus, B.; Sar, C.; Ferry, A.; Bonnieu, A.; Ollendorff, V.; Favier, F.B. REDD1 deletion prevents dexamethasone-induced skeletal muscle atrophy. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E983–E993. [Google Scholar] [CrossRef]
- Lesovaya, E.; Agarwal, S.; Readhead, B.; Vinokour, E.; Baida, G.; Bhalla, P.; Kirsanov, K.; Yakubovskaya, M.; Platanias, L.C.; Dudley, J.T.; et al. Rapamycin Modulates Glucocorticoid Receptor Function, Blocks Atrophogene REDD1, and Protects Skin from Steroid Atrophy HHS Public Access. J. Investig. Dermatol. 2018, 138, 1935–1944. [Google Scholar] [CrossRef]
- Agarwal, S.; Mirzoeva, S.; Readhead, B.; Dudley, J.T.; Budunova, I. PI3K inhibitors protect against glucocorticoid-induced skin atrophy. EBioMedicine 2019, 41, 526–537. [Google Scholar] [CrossRef]
- Lesovaya, E.A.; Savinkova, A.V.; Morozova, O.V.; Lylova, E.S.; Zhidkova, E.M.; Kulikov, E.P.; Kirsanov, K.I.; Klopot, A.; Baida, G.; Yakubovskaya, M.G.; et al. A novel approach to safer glucocorticoid receptor–targeted anti-lymphoma therapy via REDD1 (regulated in development and DNA damage 1) inhibition. Mol. Cancer Ther. 2020, 19, 1898–1908. [Google Scholar] [CrossRef]
- Dittmann, A.; Werner, T.; Chung, C.W.; Savitski, M.M.; Fälth Savitski, M.; Grandi, P.; Hopf, C.; Lindon, M.; Neubauer, G.; Prinjha, R.K.; et al. The Commonly Used PI3-Kinase Probe LY294002 Is an Inhibitor of BET Bromodomains. ACS Chem. Biol. 2014, 9, 495–502. [Google Scholar] [CrossRef]
- Jefferies, H.B.J.; Fumagalli, S.; Dennis, P.B.; Reinhard, C.; Pearson, R.B.; Thomas, G. Rapamycin Suppresses 5′ TOP mRNA Translation through Inhibition of p70S6k. EMBO J. 1997, 16, 3693–3704. [Google Scholar] [CrossRef] [PubMed]
- Patursky-Polischuk, I.; Stolovich-Rain, M.; Hausner-Hanochi, M.; Kasir, J.; Cybulski, N.; Avruch, J.; Rüegg, M.A.; Hall, M.N.; Meyuhas, O. The TSC-mTOR Pathway Mediates Translational Activation of TOP mRNAs by Insulin Largely in a Raptor- or Rictor-Independent Manner. Mol. Cell. Biol. 2009, 29, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.R.; Liao, Y.H.; Doerner III, P.G.; Ding, Z.; Hsieh, M.; Wang, W.; Nelson, J.L.; Ivy, J.L. An amino acid mixture is essential to optimize insulin-stimulated glucose uptake and GLUT4 translocation in perfused rodent hindlimb muscle. J. Appl. Physiol. 2012, 113, 97–104. [Google Scholar] [CrossRef]
- Nishitani, S.; Takehana, K.; Fujitani, S.; Sonaka, I. Branched-chain amino acids improve glucose metabolism in rats with liver cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 288, G1292–G1300. [Google Scholar] [CrossRef]
- Doi, M.; Yamaoka, I.; Fukunaga, T.; Nakayama, M. Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12 myotubes. Biochem. Biophys. Res. Commun. 2003, 312, 1111–1117. [Google Scholar] [CrossRef]
- Anthony, J.C.; Anthony, T.G.; Layman, D.K. Leucine Supplementation Enhances Skeletal Muscle Recovery in Rats Following Exercise12. J. Nutr. 1999, 129, 1102–1106. [Google Scholar] [CrossRef]
- Drummond, M.J.; Rasmussen, B.B. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 222–226. [Google Scholar] [CrossRef]
- Koopman, R.; Wagenmakers, A.J.M.; Manders, R.J.F.; Zorenc, A.H.; Senden, J.M.; Gorselink, M.; Keizer, H.A.; van Loon, L.J. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am. J. Physiol. Endocrinol. Metab. 2005, 288, 645–653. [Google Scholar] [CrossRef]
- Anthony, J.C.; Yoshizawa, F.; Anthony, T.G.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Leucine stimulates translation Initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 2000, 130, 2413–2419. [Google Scholar] [CrossRef]
- Anthony, J.C.; Anthony, T.G.; Kimball, S.R.; Vary, T.C.; Jefferson, L.S. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with Increased eIF4F Formation. J. Nutr. 2000, 130, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chu, L.; Qiao, S.; Mao, X.; Zeng, X. Effects of dietary leucine supplementation in low crude protein diets on performance, nitrogen balance, whole-body protein turnover, carcass characteristics and meat quality of finishing pigs. Anim. Sci. J. 2016, 87, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, M.; Hu, Z.; Zhan, H.; Bu, D.; Xu, L. Meta-analysis of the effects of supplemental leucine alone or in combination with other branched-chain amino acids on lactational performance in dairy cows and the associated influencing factors. J. Dairy Sci. 2025, 108, 7063–7073. [Google Scholar] [CrossRef]
- Santoyo-Suarez, M.G.; Mares-Montemayor, J.D.; Padilla-Rivas, G.R.; Delgado-Gallegos, J.L.; Quiroz-Reyes, A.G.; Roacho-Perez, J.A.; Benitez-Chao, D.F.; Garza-Ocañas, L.; Arevalo-Martinez, G.; Garza-Treviño, E.N.; et al. The Involvement of Krüppel-like Factors in Cardiovascular Diseases. Life 2023, 13, 420. [Google Scholar] [CrossRef]
- Haldar, S.M.; Ibrahim, O.A.; Jain, M.K. Kruppel-like Factors (KLFs) in muscle biology. J. Mol. Cell. Cardiol. 2007, 43, 1–10. [Google Scholar] [CrossRef]
- Leenders, J.J.; Wijnen, W.J.; van der Made, I.; Hiller, M.; Swinnen, M.; Vandendriessche, T.; Chuah, M.; Pinto, Y.M.; Creemers, E.E. Repression of cardiac hypertrophy by KLF15: Underlying mechanisms and therapeutic implications. PLoS ONE 2012, 7, e36754. [Google Scholar] [CrossRef]
- Tanaka, M.; Masuda, S.; Yamakage, H.; Inoue, T.; Ohue-Kitano, R.; Yokota, S.; Kusakabe, T.; Wada, H.; Sanada, K.; Ishii, K.; et al. Role of serum myostatin in the association between hyperinsulinemia and muscle atrophy in Japanese obese patients. Diabetes Res. Clin. Pract. 2018, 142, 195–202. [Google Scholar] [CrossRef]
- Desgeorges, M.M.; Devillard, X.; Toutain, J.; Divoux, D.; Castells, J.; Bernaudin, M.; Touzani, O.; Freyssenet, D.G. Molecular mechanisms of skeletal muscle atrophy in a mouse model of cerebral ischemia. Stroke 2015, 46, 1673–1680. [Google Scholar] [CrossRef]
- Lee, S.J. Myostatin: A Skeletal muscle chalone. Annu. Rev. Physiol. 2023, 85, 269–291. [Google Scholar] [CrossRef]
- Bish, L.T.; Morine, K.J.; Sleeper, M.M.; Sweeney, H.L. Myostatin is upregulated following stress in an Erk-dependent manner and negatively regulates cardiomyocyte growth in culture and in a mouse model. PLoS ONE 2010, 5, e10230. [Google Scholar] [CrossRef]
- Matsakas, A.; Prosdocimo, D.A.; Mitchell, R.; Collins-Hooper, H.; Giallourou, N.; Swann, J.R.; Potter, P.; Epting, T.; Jain, M.K.; Patel, K. Investigating mechanisms underpinning the detrimental impact of a high-fat diet in the developing and adult hypermuscular myostatin null mouse. Skelet. Muscle 2015, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Tando, T.; Hirayama, A.; Furukawa, M.; Sato, Y.; Kobayashi, T.; Funayama, A.; Kanaji, A.; Hao, W.; Watanabe, R.; Morita, M.; et al. Smad2/3 Proteins are required for immobilization-induced skeletal muscle atrophy. J. Biol. Chem. 2016, 291, 12184–12194. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, L.; Chen, A.; Xiang, G.; Wang, Y.; Wu, J.; Mitchelson, K.; Cheng, J.; Zhou, Y. Identification of the gene transcription and apoptosis mediated by TGF-β-Smad2/3-Smad4 signaling. J. Cell. Physiol. 2008, 215, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Garibotto, G.; Esposito, P.; Picciotto, D.; Verzola, D. Activin/myostatin receptor signaling and vascular calcifications in chronic kidney disease: A “liaison dangereuse”? Kidney. Res. Clin. Pract. 2019, 38, 407–410. [Google Scholar] [CrossRef]
- Amirouche, A.; Durieux, A.C.; Banzet, S.; Koulmann, N.; Bonnefoy, R.; Mouret, C.; Bigard, X.; Peinnequin, A.; Freyssenet, D. Down-regulation of AKT/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology 2009, 150, 286–294. [Google Scholar] [CrossRef]
- Hitachi, K.; Nakatani, M.; Tsuchida, K. Myostatin signaling regulates AKT activity via the regulation of miR-486 expression. Int. J. Biochem. Cell. Biol. 2014, 47, 93–103. [Google Scholar] [CrossRef]
- Zhang, F.; Geng, L.; Zhang, J.; Han, S.; Guo, M.; Xu, Y.; Chen, C. miR-486-5p diagnosed atrial fibrillation, predicted the risk of left atrial fibrosis, and regulated angiotensin II-induced cardiac fibrosis via modulating PI3K/AKT signaling through targeting FOXO1. Mol. Cell. Biochem. 2025, 480, 1077–1087. [Google Scholar] [CrossRef]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin reduces AKT/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef]
- Abati, E.; Manini, A.; Comi, G.P.; Corti, S. Inhibition of myostatin and related signaling pathways for the treatment of muscle atrophy in motor neuron diseases. Cell. Mol. Life Sci. 2022, 79, 374. [Google Scholar] [CrossRef]
- Thomas, M.; Langley, B.; Berry, C.; Sharma, M.; Kirk, S.; Bass, J.; Kambadur, R. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J. Biol. Chem. 2000, 275, 40235–40243. [Google Scholar] [CrossRef]
- Ma, K.; Mallidis, C.; Bhasin, S.; Mahabadi, V.; Artaza, J.; Gonzalez-Cadavid, N.; Arias, J.; Salehian, B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E363–E371. [Google Scholar] [CrossRef]
- Gilson, H.; Schakman, O.; Combaret, L.; Lause, P.; Grobet, L.; Attaix, D.; Ketelslegers, J.M.; Thissen, J.P. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 2007, 148, 452–460. [Google Scholar] [CrossRef]
- Wang, R.; Jiao, H.; Zhao, J.; Wang, X.; Lin, H. Glucocorticoids enhance muscle proteolysis through a myostatin-dependent pathway at the early stage. PLoS ONE 2016, 11, e0156225. [Google Scholar] [CrossRef]
- Saitoh, M.; Ishida, J.; Ebner, N.; Anker, S.D.; Springer, J.; Haehling, S.V. Myostatin inhibitors as pharmacological treatment for muscle wasting and muscular dystrophy. JCSM Clin. Rep. 2017, 2, 1–10. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, D.; Kim, N.D. Pathogenesis, intervention, and current status of drug development for sarcopenia: A Review. Biomedicines 2023, 11, 1635. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.R. The elusive promise of myostatin inhibition for muscular dystrophy. Curr. Opin. Neurol. 2020, 33, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Lee, Y.S. Myostatin inhibitors: Panacea or predicament for musculoskeletal misorders? J. Bone. Metab. 2020, 27, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Hammers, D.W.; Hart, C.C.; Patsalos, A.; Matheny, M.K.; Wright, L.A.; Nagy, L.; Sweeney, H.L. Glucocorticoids counteract hypertrophic effects of myostatin inhibition in dystrophic muscle. JCI Insight 2020, 5, e133276. [Google Scholar] [CrossRef]
- Polkey, M.I.; Praestgaard, J.; Berwick, A.; Franssen, F.M.E.; Singh, D.; Steiner, M.C.; Casaburi, R.; Tillmann, H.C.; Lach-Trifilieff, E.; Roubenoff, R.; et al. Activin type II receptor blockade for treatment of muscle depletion in chronic obstructive pulmonary disease: A randomized trial. Am. J. Respir. Crit. Care Med. 2019, 199, 313–320. [Google Scholar] [CrossRef]
- Miranda, A.C.; Cornelio, C.K.; Tran, B.A.C.; Fernandez, J. Sotatercept: A First-In-Class Activin Signaling Inhibitor for Pulmonary Arterial Hypertension. J. Pharm. Technol. 2025, 41, 134–143. [Google Scholar] [CrossRef]
- Orea-Soufi, A.; Paik, J.; Bragança, J.; Donlon, T.A.; Willcox, B.J.; Link, W. FOXO transcription factors as therapeutic targets in human diseases. Trends Pharmacol. Sci. 2022, 43, 1070–1084. [Google Scholar] [CrossRef]
- Asadi, Y.; Moundounga, R.K.; Chakroborty, A.; Pokokiri, A.; Wang, H. FOXOs and their roles in acute and chronic neurological disorders. Front. Mol. Biosci. 2025, 12, 1538472. [Google Scholar] [CrossRef]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L.; et al. Regulation of autophagy and the ubiquitin–proteasome system by the FoxO transcriptional network during muscle atrophy. Nat. Commun. 2015, 6, 6670. [Google Scholar] [CrossRef]
- Zhao, J.; Brault, J.J.; Schild, A.; Cao, P.; Sandri, M.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. FoxO3 Coordinately Activates Protein Degradation by the Autophagic/Lysosomal and Proteasomal Pathways in Atrophying Muscle Cells. Cell Metab. 2007, 6, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Lützner, N.; Kalbacher, H.; Krones-Herzig, A.; Rösl, F. FOXO3 is a glucocorticoid receptor target and regulates LKB1 and its own expression based on cellular AMP levels via a positive autoregulatory loop. PLoS ONE. 2012, 7, e42166. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Pan, J.; Qin, Y.; Lee, D.N.; Bauman, W.A.; Cardozo, C. Identification of functional glucocorticoid response elements in the mouse FoxO1 promoter. Biochem. Biophys. Res. Commun. 2014, 450, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Langenbacher, A.D.; Huang, J.; Wang, K.; Otto, G.; Geisler, R.; Wang, Y.; Chen, J.N. The Calcineurin-FoxO-MuRF1 signaling pathway regulates myofibril integrity in cardiomyocytes. Elife. 2017, 6, e27955. [Google Scholar] [CrossRef]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. AKT Promotes Cell Survival by Phosphorylating and Inhibiting a Forkhead Transcription Factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/AKT pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription fFactors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef]
- Cahill, C.M.; Tzivion, G.; Nasrin, N.; Ogg, S.; Dore, J.; Ruvkun, G.; Alexander-Bridges, M. Phosphatidylinositol 3-kinase signaling inhibits DAF-16 DNA binding and function via 14-3-3-dependent and 14-3-3-independent Pathways. J. Biol. Chem. 2001, 276, 13402–13410. [Google Scholar] [CrossRef]
- Lu, M.; Xu, W.; Gao, B.; Xiong, S. Blunting autoantigen-induced FOXO3a protein phosphorylation and degradation is a novel pathway of glucocorticoids for the treatment of systemic lupus erythematosus. J. Biol. Chem. 2016, 291, 19900–19912. [Google Scholar] [CrossRef]
- Bollinger, L.M.; Witczak, C.A.; Houmard, J.A.; Brault, J.J. SMAD3 augments FoxO3-induced MuRF-1 promoter activity in a DNA-binding-dependent manner. Am. J. Physiol. Cell. Physiol. 2014, 307, C278–C287. [Google Scholar] [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef]
- Senf, S.M.; Dodd, S.L.; Judge, A.R. FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am. J. Physiol. Cell. Physiol. 2010, 298, 38–45. [Google Scholar] [CrossRef]
- Waddell, D.S.; Baehr, L.M.; Van Den Brandt, J.; Johnsen, S.A.; Reichardt, H.M.; Furlow, J.D.; Bodine, S.C. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E785–E797. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Judge, S.M.; Wu, C.L.; Beharry, A.W.; Roberts, B.M.; Ferreira, L.F.; Kandarian, S.C.; Judge, A.R. Genome-wide identification of FoxO-dependent gene networks in skeletal muscle during C26 cancer cachexia. BMC Cancer 2014, 14, 997. [Google Scholar] [CrossRef]
- Mammucari, C.; Milan, G.; Romanello, V.; Masiero, E.; Rudolf, R.; Del Piccolo, P.; Burden, S.J.; Di Lisi, R.; Sandri, C.; Zhao, J.; et al. FoxO3 Controls Autophagy in Skeletal Muscle In Vivo. Cell Metab. 2007, 6, 458–471. [Google Scholar] [CrossRef]
- Nakashima, R.; Hosoda, R.; Tatekoshi, Y.; Iwahara, N.; Saga, Y.; Kuno, A. Transcriptional dysregulation of autophagy in the muscle of a mouse model of Duchenne muscular dystrophy. Sci. Rep. 2024, 14, 1365. [Google Scholar] [CrossRef]
- Sandri, M.; Coletto, L.; Grumati, P.; Bonaldo, P. Misregulation of autophagy and protein degradation systems in myopathies and muscular dystrophies. J. Cell Sci. 2013, 126, 5325–5333. [Google Scholar] [CrossRef]
- Sanchez, A.M.J.; Candau, R.B.; Bernardi, H. FoxO transcription factors: Their roles in the maintenance of skeletal muscle homeostasis. Cell. Mol. Life Sci. 2014, 71, 1657–1671. [Google Scholar] [CrossRef]
- Consolaro, F.; Ghaem-Maghami, S.; Bortolozzi, R.; Zona, S.; Khongkow, M.; Basso, G.; Viola, G.; Lam, E.W. FOXO3a and posttranslational modifications mediate glucocorticoid sensitivity in B-ALL. Mol. Cancer Res. 2015, 13, 1578–1590. [Google Scholar] [CrossRef]
- Pang, X.S.; Zhang, P.; Chen, X.P.; Liu, W.M. Ubiquitin-proteasome pathway in skeletal muscle atrophy. Front. Physiol. 2023, 14, 1289537. [Google Scholar] [CrossRef]
- Foletta, V.C.; White, L.J.; Larsen, A.E.; Léger, B.; Russell, A.P. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflugers Arch. 2011, 461, 325–335. [Google Scholar] [CrossRef]
- Du Bois, P.; Pablo Tortola, C.; Lodka, D.; Kny, M.; Schmidt, F.; Song, K.; Schmidt, S.; Bassel-Duby, R.; Olson, E.N.; Fielitz, J. Angiotensin II induces skeletal muscle atrophy by activating TFEB-mediated MuRF1 expression. Circ. Res. 2015, 117, 424–436. [Google Scholar] [CrossRef]
- Polge, C.; Cabantous, S.; Deval, C.; Claustre, A.; Hauvette, A.; Bouchenot, C.; Aniort, J.; Béchet, D.; Combaret, L.; Attaix, D.; et al. A muscle-specific MuRF1-E2 network requires stabilization of MuRF1-E2 complexes by telethonin, a newly identified substrate. J. Cachexia Sarcopenia Muscle 2018, 9, 129–145. [Google Scholar] [CrossRef]
- Xiong, J.; Le, Y.; Rao, Y.; Zhou, L.; Hu, Y.; Guo, S.; Sun, Y. RANKL mediates muscle atrophy and dysfunction in a cigarette smoke-induced model of chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2021, 64, 617–628. [Google Scholar] [CrossRef]
- Lagirand-Cantaloube, J.; Offner, N.; Csibi, A.; Leibovitch, M.P.; Batonnet-Pichon, S.; Tintignac, L.A.; Segura, C.T.; Leibovitch, S.A. The initiation factor eIF3-f is a major target for Atrogin1/MAFbx function in skeletal muscle atrophy. EMBO J. 2008, 27, 1266–1276. [Google Scholar] [CrossRef]
- Cong, H.; Sun, L.; Liu, C.; Tien, P. Inhibition of Atrogin-1/MAFbx Expression by Adenovirus-Delivered Small Hairpin RNAs Attenuates Muscle Atrophy in Fasting Mice. Hum. Gene Ther. 2010, 22, 313–324. [Google Scholar] [CrossRef]
- Frost, R.A.; Nystrom, G.J.; Jefferson, L.S.; Lang, C.H. Hormone, cytokine, and nutritional regulation of sepsis-induced increases in atrogin-1 and MuRF1 in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E501–E512. [Google Scholar] [CrossRef]
- Gonnella, P.; Alamdari, N.; Tizio, S.; Aversa, Z.; Petkova, V.; Hasselgren, P.O. C/EBPβ regulates dexamethasone-induced muscle cell atrophy and expression of atrogin-1 and MuRF1. J. Cell. Biochem. 2011, 112, 1737–1748. [Google Scholar] [CrossRef]
- Hudson, M.B.; Woodworth-Hobbs, M.E.; Gooch, J.L.; Price, S.R. Calcineurin-NFAT signaling regulates atrogin-1 and MuRF1 via miR-23a during glucocorticoid induced muscle atrophy. Kidney. Res. Clin. Pract. 2012, 31, A92. [Google Scholar] [CrossRef][Green Version]
- Wada, S.; Kato, Y.; Okutsu, M.; Miyaki, S.; Suzuki, K.; Yan, Z.; Schiaffino, S.; Asahara, H.; Ushida, T.; Akimoto, T. Translational suppression of atrophic regulators by miR-23a integrates resistance to skeletal muscle atrophy. J. Biol. Chem. 2012, 286, 38456–38465. [Google Scholar] [CrossRef]
- Ito, Y.; Yamagata, M.; Yamamoto, T.; Hirasaka, K.; Nikawa, T.; Sato, T. The reciprocal regulation between mitochondrial-associated membranes and Notch signaling in skeletal muscle atrophy. Elife 2023, 12, RP89381. [Google Scholar] [CrossRef]
- Almowallad, S.; Alqahtani, L.S.; Mobashir, M. NF-kB in signaling patterns and its temporal dynamics encode/decode human diseases. Life 2022, 12, 2012. [Google Scholar] [CrossRef]
- Welsh, S.; Riggs, D.; Meermeier, E.; Shi, C.X.; Garbitt, V.; Sharik, M.E.; Du, M.; Todd, K.; Hammond, Z.; Brown, S.; et al. Disrupting ectopic super-enhancers to treat multiple myeloma. Blood 2021, 138, 1593. [Google Scholar] [CrossRef]
- Fry, C.S.; Nayeem, S.Z.; Dillon, E.L.; Sarkar, P.S.; Tumurbaatar, B.; Urban, R.J.; Wright, T.J.; Sheffield-Moore, M.; Tilton, R.G.; Choudhary, S. Glucocorticoids increase skeletal muscle NF-κB inducing kinase (NIK): Links to muscle atrophy. Physiol. Rep. 2016, 4, e13014. [Google Scholar] [CrossRef]
- Okamoto, T.; Torii, S.; Machida, S. Differential gene expression of muscle-specific ubiquitin ligase MAFbx/Atrogin-1 and MuRF1 in response to immobilization-induced atrophy of slow-twitch and fast-twitch muscles. J. Physiol. Sci. 2011, 61, 537–546. [Google Scholar] [CrossRef]
- Latham, C.M.; Brightwell, C.R.; Keeble, A.R.; Munson, B.D.; Thomas, N.T.; Zagzoog, A.M.; Fry, C.S.; Fry, J.L. Vitamin D promotes skeletal muscle regeneration and mitochondrial health. Front. Physiol. 2021, 12, 660498. [Google Scholar] [CrossRef]
- Bass, J.J.; Kazi, A.A.; Deane, C.S.; Nakhuda, A.; Ashcroft, S.P.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; et al. The mechanisms of skeletal muscle atrophy in response to transient knockdown of the vitamin D receptor in vivo. J. Physiol. 2021, 599, 963–979. [Google Scholar] [CrossRef]
- Sociali, G.; Magnone, M.; Ravera, S.; Bae, E.J.; Park, B.H. Pharmacological Sirt6 inhibition improves glucose tolerance in a type 2 diabetes mouse model. FASEB J. 2017, 31, 3138–3149. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.S.; Ahn, J.H.; Jang, J.G.; Lee, J.H.; Kim, H.N.; Kim, D.; Lee, W. GSK-LSD1, an LSD1 inhibitor, quashes SARS-CoV-2-triggered cytokine release syndrome in-vitro. Signal. Transduct. Target. Ther. 2020, 5, 267. [Google Scholar] [CrossRef] [PubMed]
- Wirthgen, E.; Leonard, A.K.; Scharf, C.; Domanska, G. The immunomodulator 1-Methyltryptophan drives tryptophan catabolism toward the kynurenic acid branch. Front. Immunol. 2020, 11, 313. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Lv, T.; Zhang, T.; Feng, D.; Zhu, F.; Xu, Y.; Zhang, L.; Gu, L.; Guo, Z.; Ding, C.; et al. Interleukin-6 promotes skeletal muscle catabolism by activating tryptophan–indoleamine 2,3-dioxygenase 1–kynurenine pathway during intra-abdominal sepsis. J. Cachexia Sarcopenia Muscle 2023, 14, 1046–1059. [Google Scholar] [CrossRef]
- Jung, K.H.; LoRusso, P.; Burris, H.; Gordon, M.; Bang, Y.J.; Hellmann, M.D.; Cervantes, A.; Ochoa de Olza, M.; Marabelle, A.; Hodi, F.S.; et al. Phase I Study of the Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitor Navoximod (GDC-0919) Administered with PD-L1 Inhibitor (Atezolizumab) in Advanced Solid Tumors. Clin. Cancer Res. 2019, 25, 3220–3228. [Google Scholar] [CrossRef]
- Fiorentino, F.; Mai, A.; Rotili, D. Emerging Therapeutic Potential of SIRT6 Modulators. J. Med. Chem. 2021, 64, 9732–9758. [Google Scholar] [CrossRef]
- Daveson, A.J.M.; Stubbs, R.; Polasek, T.M.; Isola, J.; Anderson, R.; Tye-Din, J.A.; Schoeman, M.; Lionnet, C.; Mei, S.L.C.Y.; Mihajlović, J.; et al. Safety, clinical activity, pharmacodynamics, and pharmacokinetics of IMU-856, a SIRT6 modulator, in coeliac disease: A first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Gastrooenterol. Hepatol. 2025, 10, 44–54. [Google Scholar] [CrossRef]
- Li, Y.; Jin, J.; Wang, Y. SIRT6 widely regulates aging, immunity, and cancer. Front. Oncol. 2022, 12, 861334. [Google Scholar] [CrossRef]
- Mostoslavsky, R.; Chua, K.F.; Lombard, D.B.; Pang, W.W.; Fischer, M.R.; Gellon, L.; Liu, P.; Mostoslavsky, G.; Franco, S.; Murphy, M.M.; et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 2006, 124, 315–329. [Google Scholar] [CrossRef]
- Kanfi, Y.; Naiman, S.; Amir, G.; Peshti, V.; Zinman, G.; Nahum, L.; Bar-Joseph, Z.; Cohen, H.Y. The sirtuin SIRT6 regulates lifespan in male mice. Nature 2012, 483, 218–221. [Google Scholar] [CrossRef]
- Roichman, A.; Elhanati, S.; Aon, M.A.; Abramovich, I.; Di Francesco, A.; Shahar, Y.; Avivi, M.Y.; Shurgi, M.; Rubinstein, A.; Wiesner, Y.; et al. Restoration of energy homeostasis by SIRT6 extends healthy lifespan. Nat. Commun. 2021, 12, 3208. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Vasudevan, P.; Zhong, L.; Kim, G.; Samant, S.; Parekh, V.; Pillai, V.B.; Ravindra, P.V.; Gupta, M.; Jeevanandam, V.; et al. The sirtuin SIRT6 blocks IGF-AKT signaling and development of cardiac hypertrophy by targeting c-Jun. Nat. Med. 2012, 18, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Parenti, M.D.; Grozio, A.; Bauer, I.; Kim, G.; Samant, S.; Parekh, V.; Pillai, V.B.; Ravindra, P.V.; Gupta, M.; Jeevanandam, V.; et al. Discovery of novel and selective SIRT6 inhibitors. J. Med. Chem. 2014, 57, 4796–4804. [Google Scholar] [CrossRef] [PubMed]
- Rahnasto-Rilla, M.; Tyni, J.; Huovinen, M.; Jarho, E.; Kulikowicz, T.; Ravichandran, S.; A Bohr, V.; Ferrucci, L.; Lahtela-Kakkonen, M.; Moaddel, R. Natural polyphenols as sirtuin 6 modulators. Sci. Rep. 2018, 8, 4163. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Han, C.Y.; Moon, Y.J.; Lee, J.H.; Bae, E.J.; Park, B.H. Sirt6 reprograms myofibers to oxidative type through CREB-dependent Sox6 suppression. Nat. Commun. 2022, 13, 1808. [Google Scholar] [CrossRef]
- Georgieva, A.M.; Guo, X.; Bartkuhn, M.; Günther, S.; Künne, C.; Smolka, C.; Atzberger, A.; Gärtner, U.; Mamchaoui, K.; Bober, E.; et al. Inactivation of Sirt6 ameliorates muscular dystrophy in mdx mice by releasing suppression of utrophin expression. Nat. Commun. 2022, 13, 4184. [Google Scholar] [CrossRef]
- Maiques-Diaz, A.; Somervaille, T.C.P. LSD1: Biologic roles and therapeutic targeting. Epigenomics 2016, 8, 1103–1116. [Google Scholar] [CrossRef]
- Perillo, B.; Tramontano, A.; Pezone, A.; Migliaccio, A. LSD1: More than demethylation of histone lysine residues. Exp. Mol. Med. 2020, 52, 1936–1947. [Google Scholar] [CrossRef]
- Metzger, E.; Wissmann, M.; Yin, N.; Müller, J.M.; Schneider, R.; Peters, A.H.; Günther, T.; Buettner, R.; Schüle, R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 2005, 437, 436–439. [Google Scholar] [CrossRef]
- Zuchegna, C.; Aceto, F.; Bertoni, A.; Romano, A.; Perillo, B.; Laccetti, P.; Gottesman, M.E.; Avvedimento, E.V.; Porcellini, A. Mechanism of retinoic acid-induced transcription: Histone code, DNA oxidation and formation of chromatin loops. Nucleic Acids Res. 2014, 42, 11040–11055. [Google Scholar] [CrossRef][Green Version]
- Ross-Innes, C.S.; Stark, R.; Holmes, K.A.; Schmidt, D.; Spyrou, C.; Russell, R.; Massie, C.E.; Vowler, S.L.; Eldridge, M.; Carroll, J.S. Cooperative interaction between retinoic acid receptor-α and estrogen receptor in breast cancer. Genes Dev. 2010, 24, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Vicent, G.P.; Nacht, A.S.; Zaurin, R.; Font-Mateu, J.; Soronellas, D.; Le Dily, F.; Reyes, D.; Beato, M. Unliganded progesterone receptormediated targeting of an RNA-containing repressive complex silences a subset of hormone-inducible genes. Genes Dev. 2013, 27, 1179–1197. [Google Scholar] [CrossRef] [PubMed]
- Noce, B.; Di Bello, E.; Fioravanti, R.; Mai, A. LSD1 inhibitors for cancer treatment: Focus on multi-target agents and compounds in clinical trials. Front. Pharmacol. 2023, 14, 1120911. [Google Scholar] [CrossRef] [PubMed]
- Sacilotto, N.; Dessanti, P.; Lufino, M.M.P.; Ortega, A.; Rodríguez-Gimeno, A.; Salas, J.; Maes, T.; Buesa, C.; Mascaró, C.; Soliva, R. Comprehensive in Vitro Characterization of the LSD1 Small Molecule Inhibitor Class in Oncology. ACS Pharmacol. Transl. Sci. 2021, 4, 1818–1834. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Liu, X.; Xie, C.; Shi, J. The role of the kynurenine pathway in cardiovascular disease. Front. Cardiovasc. Med. 2024, 11, 1406856. [Google Scholar] [CrossRef]
- Ye, Z.; Yue, L.; Shi, J.; Shao, M.; Wu, T. Role of IDO and TDO in cancers and related diseases and the therapeutic implications. J. Cancer 2019, 10, 2771–2782. [Google Scholar] [CrossRef]
- Bakker, L.; Choe, K.; Eussen, S.J.P.M.; Ramakers, I.H.G.B.; van den Hove, D.L.A.; Kenis, G.; Rutten, B.P.F.; Verhey, F.R.J.; Köhler, S. Relation of the kynurenine pathway with normal age: A systematic review. Mech. Ageing Dev. 2024, 217, 111890. [Google Scholar] [CrossRef]
- Tsuji, A.; Ikeda, Y.; Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Morikawa, S.; Nakashima, M.; Asai, T.; Matsuda, S. The tryptophan and kynurenine pathway involved in the development of immune-related diseases. Int. J. Mol. Sci. 2023, 24, 5742. [Google Scholar] [CrossRef]
- Kaiser, H.; Parker, E.; Hamrick, M.W. Kynurenine signaling through the aryl hydrocarbon receptor: Implications for aging and healthspan. Exp. Gerontol. 2020, 130, 110797. [Google Scholar] [CrossRef]
- Ballesteros, J.; Rivas, D.; Duque, G. The role of the kynurenine pathway in the pathophysiology of frailty, sarcopenia, and osteoporosis. Nutrients 2023, 15, 3132. [Google Scholar] [CrossRef]
- Ritz, J.; Wunderle, C.; Stumpf, F.; Laager, R.; Tribolet, P.; Neyer, P.; Bernasconi, L.; Stanga, Z.; Mueller, B.; Schuetz, P. Association of tryptophan pathway metabolites with mortality and effectiveness of nutritional support among patients at nutritional risk: Secondary analysis of a randomized clinical trial. Front. Nutr. 2024, 11, 1335242. [Google Scholar] [CrossRef]
- Szczygiel, M.; Derewenda, U.; Scheiner, S.; Minor, W.; Derewenda, Z.S. A structural role for tryptophan in proteins, and the ubiquitous Trp Cδ1-H…O C (backbone) hydrogen bond. Biol. Crystallogr. 2024, 80, 551–562. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Fukuwatari, T.; Shibata, K. Nutritional aspect of tryptophan metabolism. Int. J. Tryptophan. Res. 2013, 6, IJTR-S11588. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhou, X.Q.; Wu, X.Y.; Xu, S.X.; Feng, L.; Liu, Y.; Jiang, W.D.; Wu, P.; Zhao, J.; et al. Effects of dietary tryptophan on muscle growth, protein synthesis and antioxidant capacity in hybrid catfish Pelteobagrus vachelli♀ × Leiocassis longirostris♂. Br. J. Nutr. 2022, 127, 1761–1773. [Google Scholar] [CrossRef]
- Dukes, A.; Davis, C.; El Refaey, M.; Upadhyay, S.; Mork, S.; Arounleut, P.; Johnson, M.H.; Hill, W.D.; Isales, C.M.; Hamrick, M.W. The aromatic amino acid tryptophan stimulates skeletal muscle IGF1/p70s6k/mTor signaling in vivo and the expression of myogenic genes in vitro. Nutrition 2015, 31, 1018–1024. [Google Scholar] [CrossRef]
- Ninomiya, S.; Nakamura, N.; Nakamura, H.; Mizutani, T.; Kaneda, Y.; Yamaguchi, K.; Matsumoto, T.; Kitagawa, J.; Kanemura, N.; Shiraki, M.; et al. Low levels of serum tryptophan underlie skeletal muscle atrophy. Nutrients 2020, 12, 978. [Google Scholar] [CrossRef]
- Agulló-Ortuño, M.T.; Mancebo, E.; Grau, M.; Núñez Sobrino, J.A.; Paz-Ares, L.; López-Martín, J.A.; Flández, M. Tryptophan modulation in cancer-associated cachexia mouse models. Int. J. Mol. Sci. 2023, 24, 13005. [Google Scholar] [CrossRef]
- Takeshita, H.; Yamamoto, K. Tryptophan metabolism and COVID-19-induced skeletal muscle damage: Is ACE2 a key regulator? Front. Nutr. 2022, 9, 868845. [Google Scholar] [CrossRef]
- Kaiser, H.; Yu, K.; Pandya, C.; Mendhe, B.; Isales, C.M.; McGee-Lawrence, M.E.; Johnson, M.; Fulzele, S.; Hamrick, M.W. Kynurenine, a Tryptophan metabolite that increases with Age, Induces Muscle Atrophy and Lipid Peroxidation. Oxid. Med. Cell. Longev. 2019, 2019, 9894238. [Google Scholar] [CrossRef] [PubMed]
- Palzkill, V.R.; Thome, T.; Murillo, A.L.; Khattri, R.B.; Ryan, T.E. Increasing plasma L-kynurenine impairs mitochondrial oxidative phosphorylation prior to the development of atrophy in murine skeletal muscle: A pilot study. Front. Physiol. 2022, 13, 992413. [Google Scholar] [CrossRef] [PubMed]
- Shadboorestan, A.; Koual, M.; Dairou, J.; Coumoul, X. The role of the kynurenine/AhR pathway in diseases related to metabolism and cancer. Int. J. Tryptophan. Res. 2023, 16, 11786469231185102. [Google Scholar] [CrossRef]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front. Immunol. 2018, 8, 1957. [Google Scholar] [CrossRef]
- Zhen, D.; Liu, J.; Zhang, X.D.; Song, Z. Kynurenic acid acts as a signaling molecule regulating energy expenditure and is closely associated with metabolic diseases. Front. Endocrinol. 2022, 13, 847611. [Google Scholar] [CrossRef]
- Wyckelsma, V.L.; Lindkvist, W.; Venckunas, T.; Brazaitis, M.; Kamandulis, S.; Pääsuke, M.; Ereline, J.; Westerblad, H.; Andersson, D.C. Kynurenine aminotransferase isoforms display fiber-type specific expression in young and old human skeletal muscle. Exp. Gerontol. 2020, 134, 110880. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Ferreira, D.M.S.; Dadvar, S.; Cervenka, I.; Ketscher, L.; Izadi, M.; Zhengye, L.; Furrer, R.; Handschin, C.; Venckunas, T.; et al. Skeletal muscle PGC-1α1 reroutes kynurenine metabolism to increase energy efficiency and fatigue-resistance. Nat. Commun. 2019, 10, 2767. [Google Scholar] [CrossRef]
- Saran, T.; Turska, M.; Kocki, T.; Zawadka, M.; Zieliński, G.; Turski, W.A.; Gawda, P. Effect of 4-week physical exercises on tryptophan, kynurenine and kynurenic acid content in human sweat. Sci. Rep. 2021, 11, 11092. [Google Scholar] [CrossRef]
- Walzik, D.; Jonas, W.; Joisten, N.; Belen, S.; Wüst, R.C.I.; Guillemin, G.; Zimmer, P. Tissue-specific effects of exercise as NAD+-boosting strategy: Current knowledge and future perspectives. Acta Physiol. 2023, 237, e13921. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, M.; Ding, Y.; Wang, Q.; Zhang, W.; Song, P.; Zou, M.H. Activation of NAD(P)H Oxidase by Tryptophan-Derived 3-Hydroxykynurenine Accelerates Endothelial Apoptosis and Dysfunction In Vivo. Circ. Res. 2014, 114, 480–492. [Google Scholar] [CrossRef]
- Malina, H.Z.; Hess, O.M. Xanthurenic acid translocates proapoptotic Bcl-2 family proteins into mitochondria and impairs mitochondrial function. BMC Cell Biol. 2004, 5, 14. [Google Scholar] [CrossRef][Green Version]
- Lugo-Huitrón, R.; Ugalde Muñiz, P.; Pineda, B.; Pedraza-Chaverrí, J.; Ríos, C.; Pérez-de la Cruz, V. Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxid. Med. Cell. Longev. 2013, 2013, 104024. [Google Scholar] [CrossRef]
- Duque, G.; Vidal, C.; Li, W.; Al Saedi, A.; Khalil, M.; Lim, C.K.; Myers, D.E.; Guillemin, G.J. Picolinic acid, a catabolite of tryptophan, has an anabolic effect on bone in vivo. J. Bone Miner. Res. 2020, 35, 2275–2288. [Google Scholar] [CrossRef]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The role of tryptophan dysmetabolism and quinolinic acid in depressive and neurodegenerative diseases. Biomolecules 2022, 12, 998. [Google Scholar] [CrossRef] [PubMed]
- Hetherington-Rauth, M.; Johnson, E.; Migliavacca, E.; Langsetmo, L.; Hepple, R.T.; Ryan, T.E.; Ferrucci, L.; Breuillé, D.; Corthesy, J.; Lane, N.E.; et al. The mediating role of kynurenine pathway metabolites on the relationship between inflammation and muscle mass in oldest–old Men. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae131. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.B. Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int. J. Tryptophan. Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.S.; Azzolini, M.; Ruas, J.L. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am. J. Physiol. Cell Physiol. 2020, 318, C818–C830. [Google Scholar] [CrossRef]
- Al Saedi, A.; Chow, S.; Vogrin, S.; Guillemin, G.J.; Duque, G. Association between tryptophan metabolites, physical performance, and frailty in older persons. Int. J. Tryptophan. Res. 2022, 15, 11786469211069951. [Google Scholar] [CrossRef]
- Aldajani, W.A.; Salazar, F.; Sewell, H.F.; Knox, A.; Ghaemmaghami, A.M. Expression and regulation of immune-modulatory enzyme indoleamine 2,3-dioxygenase (IDO) by human airway epithelial cells and its effect on T cell activation. Oncotarget 2016, 7, 57606–57617. [Google Scholar] [CrossRef]
- Zhang, S.; Fang, J.; Liu, Z.; Hou, P.; Cao, L.; Zhang, Y.; Liu, R.; Li, Y.; Shang, Q.; Chen, Y.; et al. Inflammatory cytokines-stimulated human muscle stem cells ameliorate ulcerative colitis via the IDO-TSG6 axis. Stem. Cell Res. Ther. 2021, 12, 50. [Google Scholar] [CrossRef]
- Meireson, A.; Devos, M.; Brochez, L. IDO expression in cancer: Different compartment, different functionality? Front. Immunol. 2020, 11, 531491. [Google Scholar] [CrossRef]
- Gibney, S.M.; Fagan, E.M.; Waldron, A.M.; O’Byrne, J.; Connor, T.J.; Harkin, A. Inhibition of stress-induced hepatic tryptophan 2,3-dioxygenase exhibits antidepressant activity in an animal model of depressive behaviour. Int. J. Neuropsychopharmacol. 2014, 17, 917–928. [Google Scholar] [CrossRef]
- Brooks, A.K.; Lawson, M.A.; Smith, R.A.; Janda, T.M.; Kelley, K.W.; McCusker, R.H. Interactions between inflammatory mediators and corticosteroids regulate transcription of genes within the kynurenine pathway in the mouse hippocampus. J. Neuroinflamm. 2016, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Hayashi, S.; Du, X.; Cai, X.; Deng, B.; Zheng, H.; Ishido, S.; Tsutsui, H.; Sheng, J. Caffeine protects against stress-induced murine depression through activation of PPARγC1α-mediated restoration of the kynurenine pathway in the skeletal muscle. Sci. Rep. 2021, 11, 7287. [Google Scholar] [CrossRef] [PubMed]
- Khadka, S.; Druffner, S.R.; Duncan, B.C.; Busada, J.T. Glucocorticoid regulation of cancer development and progression. Front. Endocrinol. 2023, 14, 1161768. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, G.C.; Malachowski, W.P.; DuHadaway, J.B.; Muller, A.J. Discovery of IDO1 inhibitors: From bench to bedside. Cancer Res. 2017, 77, 6795–6811. [Google Scholar] [CrossRef]
- Seo, S.K.; Kwon, B. Immune regulation through tryptophan metabolism. Exp. Mol. Med. 2023, 55, 1371–1379. [Google Scholar] [CrossRef]
- Passarelli, A.; Pisano, C.; Cecere, S.C.; Di Napoli, M.; Rossetti, S.; Tambaro, R.; Ventriglia, J.; Gherardi, F.; Iannacone, E.; Venanzio, S.S.; et al. Targeting immunometabolism mediated by the IDO1 Pathway: A new mechanism of immune resistance in endometrial cancer. Front. Immunol. 2022, 13, 953115. [Google Scholar] [CrossRef]
- Muller, A.J.; Mondal, A.; Dey, S.; Prendergast, G.C. IDO1 and inflammatory neovascularization: Bringing new blood to tumor-promoting inflammation. Front. Oncol. 2023, 13, 1165298. [Google Scholar] [CrossRef]
- Le Naour, J.; Galluzzi, L.; Zitvogel, L.; Kroemer, G.; Vacchelli, E. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology 2020, 9, 1777625. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, M.; Li, M.; Chen, Y.; Ren, M. Indoleamine 2, 3-dioxygenase 1 inhibitory compounds from natural sources. Front. Pharmacol. 2022, 13, 1046818. [Google Scholar] [CrossRef]
- Naclerio, F.; Seijo, M. Whey protein supplementation and muscle mass: Current perspectives. Nutr. Diet. Suppl. 2019, 11, 37–48. [Google Scholar] [CrossRef]
- van Dronkelaar, C.; van Velzen, A.; Abdelrazek, M.; van der Steen, A.; Weijs, P.J.M.; Tieland, M. Minerals and Sarcopenia; The Role of Calcium, Iron, Magnesium, Phosphorus, Potassium, Selenium, Sodium, and Zinc on Muscle Mass, Muscle Strength, and Physical Performance in Older Adults: A Systematic Review. J. Am. Med. Dir. Assoc. 2018, 19, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Bagherniya, M.; Mahdavi, A.; Shokri-Mashhadi, N.; Banach, M.; Von Haehling, S.; Johnston, T.P.; Sahebkar, A. The beneficial therapeutic effects of plant-derived natural products for the treatment of sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 2772–2790. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef]
- Sylow, L.; Tokarz, V.L.; Richter, E.A.; Klip, A. The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab. 2021, 33, 758–780. [Google Scholar] [CrossRef]
- Yeo, D.; Kang, C.; Ji, L. Overexpression of SIRT6 suppresses TNFα-induced muscle cell atrophy via NFκB Inhibition. FASEB J. 2018, 31, 1022-16. [Google Scholar] [CrossRef]
- Zhiyin, L.; Jinliang, C.; Qiunan, C.; Yunfei, Y.; Qian, X. Fucoxanthin rescues dexamethasone induced C2C12 myotubes atrophy. Biomed. Pharmacother. 2021, 139, 111590. [Google Scholar] [CrossRef]
- Tao, W.; Ouyang, Z.; Liao, Z.; Li, L.; Zhang, Y.; Gao, J.; Ma, L.; Yu, S. Ursolic acid alleviates cancer cachexia and prevents muscle wasting via activating SIRT1. Cancers 2023, 15, 2378. [Google Scholar] [CrossRef]
- Anan, K.; Hino, S.; Shimizu, N.; Sakamoto, A.; Nagaoka, K.; Takase, R.; Kohrogi, K.; Araki, H.; Hino, Y.; Usuki, S.; et al. LSD1 mediates metabolic reprogramming by glucocorticoids during myogenic differentiation. Nucleic. Acids. Res. 2018, 46, 5441–5454. [Google Scholar] [CrossRef]


| Targets | Compound | Structure | Activity | Refs. |
|---|---|---|---|---|
| 11β-HSD1 |
| 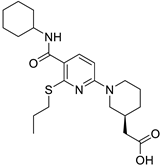 |
| [47,48] |
| Myostatin |
| 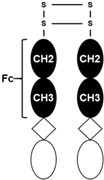 A fusion protein; Adnectin (binds to myostatin) and human IgG1-Fc |
| [61,62] |
|  A full human IgG4 |
| [63,64] | |
| Activin receptor |
| 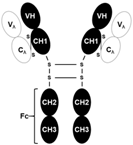 A full human IgG1 |
| [65,66] |
| FoxO1 |
| 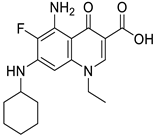 |
| [67,68] |
| 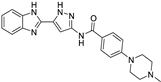 | |||
| FoxO3 |
| 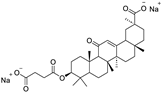 |
| [69] |
| Vitamin D receptor |
| 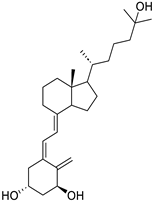 |
| [70] |
| Targets | Compound | Structure | Activity | Refs. |
|---|---|---|---|---|
| SIRT6 |
| 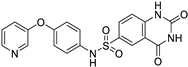 | (↑) IGF2 (↑) GLUT-1,4 (↑) Glycolytic metabolism (↑) p-AKT (↓) FoxOs, Atrogin1, MuRF1 | [17,173] |
| LSD1 |
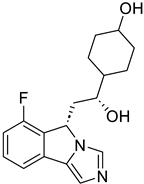
| 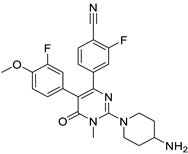 | (↓) Myostatin, REDD1 (↓) FoxO3, Atrogin1 (↓) Polyubiquitin-C (↓) Autophagy (↓) NF-κB activity (↑) Myotube area (↑) Muscle proteins | [18] |
| 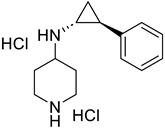 | [174] | ||
| IDO-1 |
| 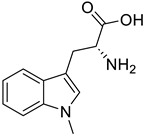 | (↑) Serum tryptophan (↓) KYN (↑) KYNA (↓) ROS, TNF-α (↓) FoxO3, Atrogin1 (↑) p-AKT (↑) MHC | [19,175] |
|  | [176,177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Permpoon, U.; Moon, J.; Kim, C.Y.; Nam, T.-g. Glucocorticoid-Mediated Skeletal Muscle Atrophy: Molecular Mechanisms and Potential Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 7616. https://doi.org/10.3390/ijms26157616
Permpoon U, Moon J, Kim CY, Nam T-g. Glucocorticoid-Mediated Skeletal Muscle Atrophy: Molecular Mechanisms and Potential Therapeutic Targets. International Journal of Molecular Sciences. 2025; 26(15):7616. https://doi.org/10.3390/ijms26157616
Chicago/Turabian StylePermpoon, Uttapol, Jiyeong Moon, Chul Young Kim, and Tae-gyu Nam. 2025. "Glucocorticoid-Mediated Skeletal Muscle Atrophy: Molecular Mechanisms and Potential Therapeutic Targets" International Journal of Molecular Sciences 26, no. 15: 7616. https://doi.org/10.3390/ijms26157616
APA StylePermpoon, U., Moon, J., Kim, C. Y., & Nam, T.-g. (2025). Glucocorticoid-Mediated Skeletal Muscle Atrophy: Molecular Mechanisms and Potential Therapeutic Targets. International Journal of Molecular Sciences, 26(15), 7616. https://doi.org/10.3390/ijms26157616





