Assessing CFTR Function and Epithelial Morphology in Human Nasal Respiratory Cell Cultures: A Combined Immunofluorescence and Electrophysiological Study
Abstract
1. Introduction
2. Results
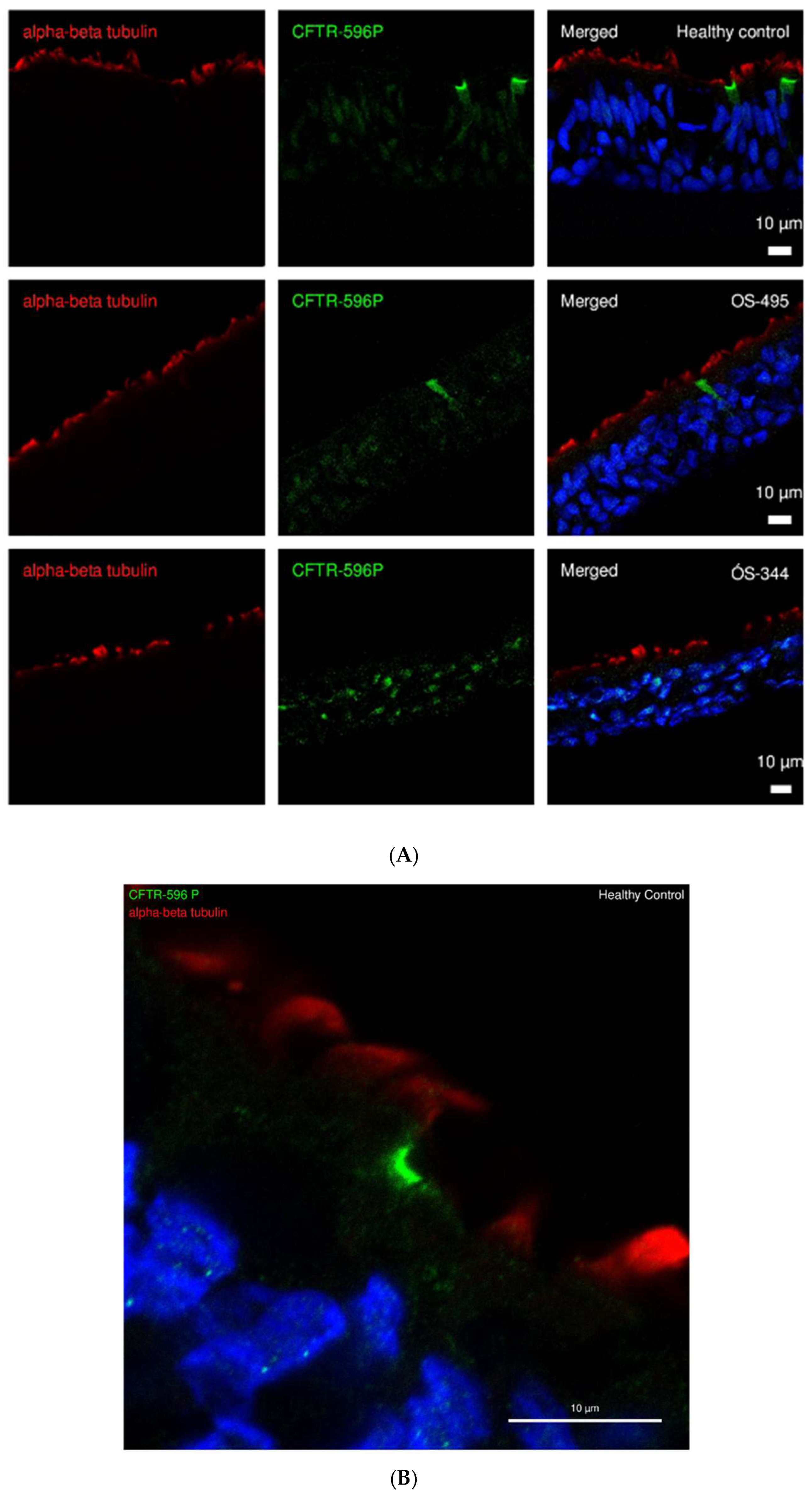
2.1. CFTR Expression and Localization in ALI Cultures of HC and CF
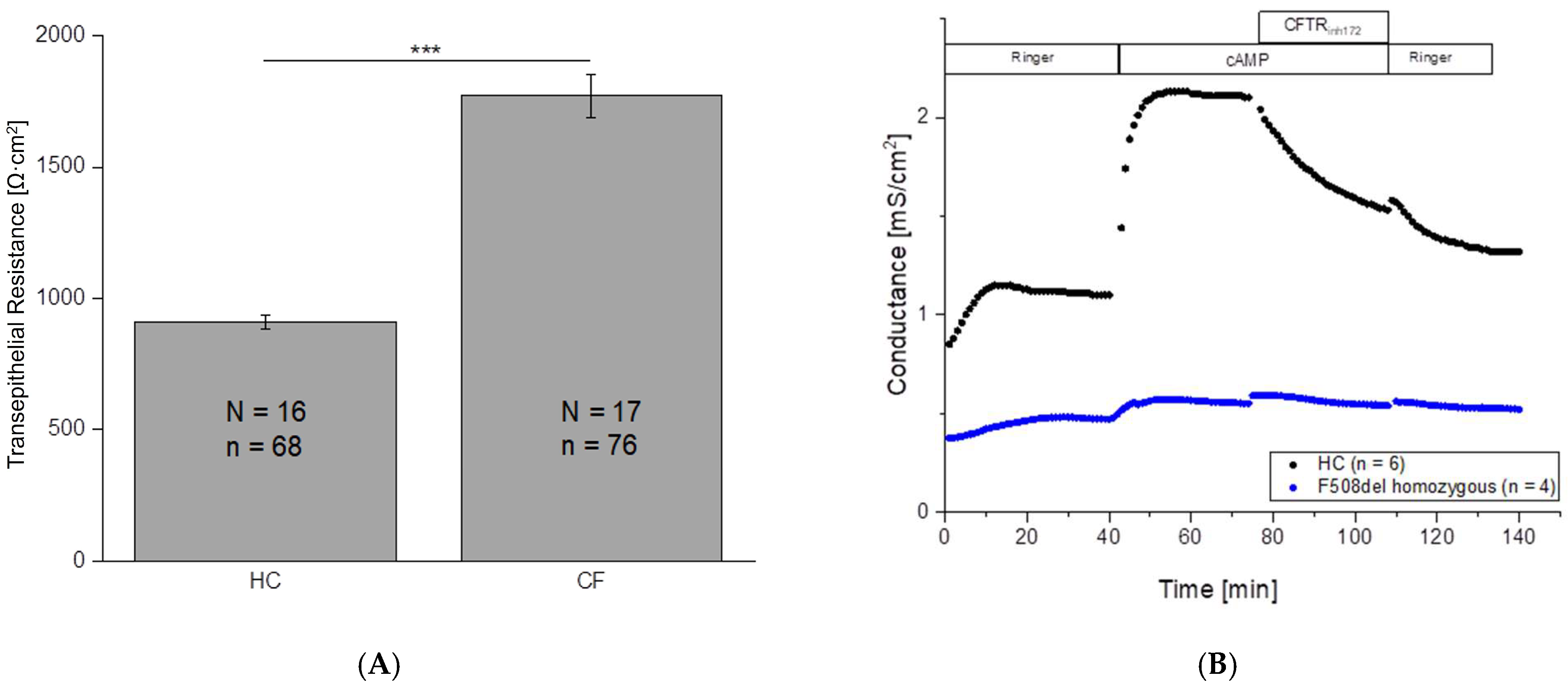
2.2. Significant Differences in Transepithelial Resistance Between HC and CF Cells
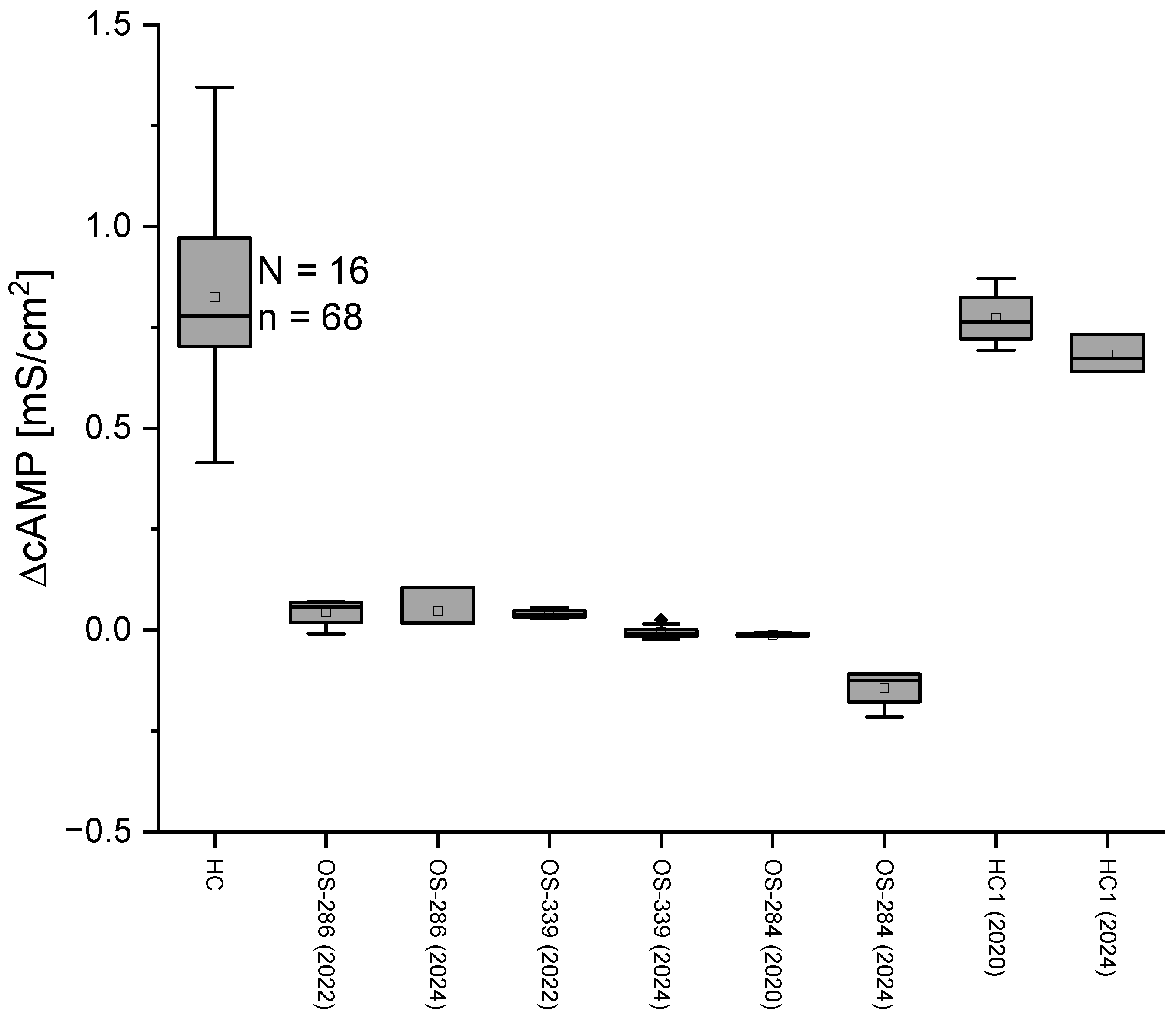
2.3. No Change in Conductance After cAMP Application in CF Patients
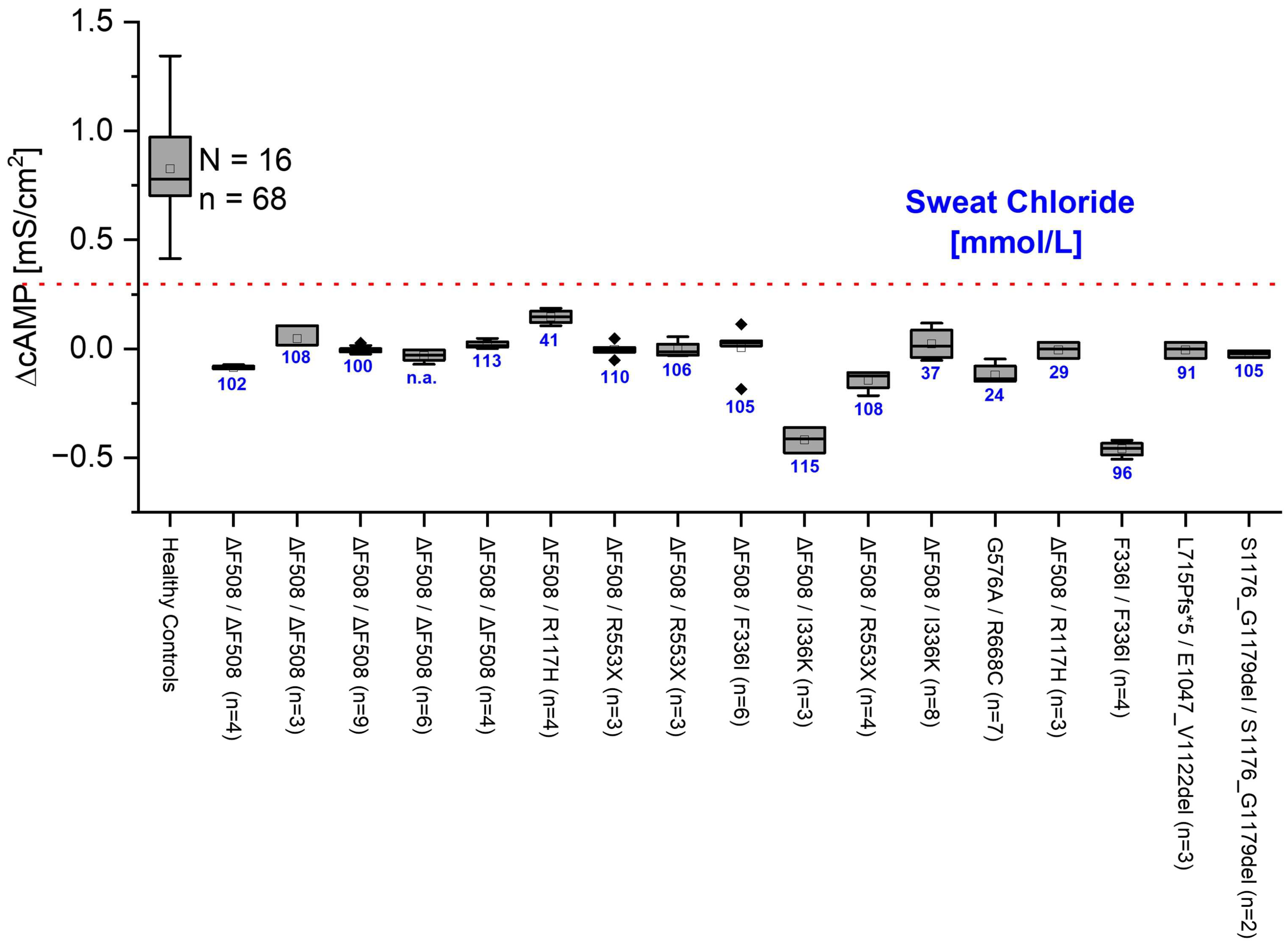
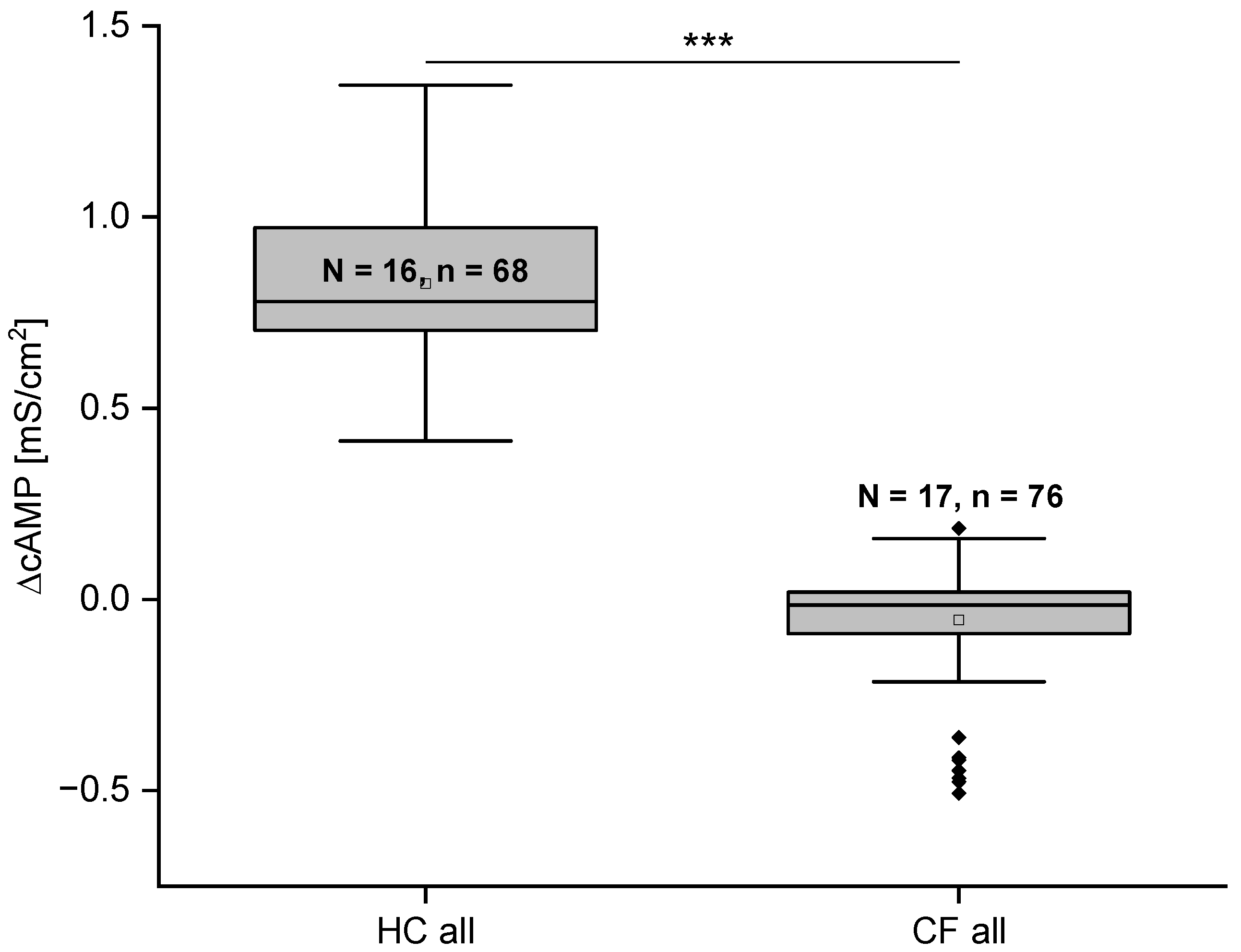
2.4. Gt Is Significantly Different in HC and CF Cells
2.5. Long-Term Reproducibility of the MTECC Method
2.6. Comparison of Electrophysiological Data and Cell Layer Thickness of ALI Cell Cultures
2.7. CFTR Modulators Improve CF Function
3. Discussion
3.1. If Stainings of CFTR Confirm Electrophysiological Findings
3.2. The MTECC Is Suitable for Reliable Diagnosis of CFTR Function In Vitro
4. Limitations
5. Materials and Methods
5.1. Patient Data Acquisition
5.2. Sweat Test
5.3. Cell Culture
5.4. Preparation of Cryosections
5.5. Immunofluorescence Staining and Measurement of Cell Layer Thickness
5.6. Transepithelial Measurements
5.7. Modulator Response Experiments
5.8. Statistical Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mall, M.A.; Burgel, P.-R.; Castellani, C.; Davies, J.C.; Salathe, M.; Taylor-Cousar, J.L. Cystic Fibrosis. Nat. Rev. Dis. Prim. 2024, 10, 53. [Google Scholar] [CrossRef]
- Graeber, S.Y.; Mall, M.A. The future of cystic fibrosis treatment: From disease mechanisms to novel therapeutic approaches. Lancet 2023, 402, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.-S.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.-L.; et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Rommens, J.M.; Iannuzzi, M.C.; Kerem, B.; Drumm, M.L.; Melmer, G.; Dean, M.; Rozmahel, R.; Cole, J.L.; Kennedy, D.; Hidaka, N.; et al. Identification of the cystic fibrosis gene: Chromosome walking and jumping. Science 1989, 245, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Kerem, B.S.; Rommens, J.M.; Buchanan, J.A.; Markiewicz, D.; Cox, T.K.; Chakravarti, A.; Buchwald, M.; Tsui, L.C. Identification of the cystic fibrosis gene: Genetic analysis. Science 1989, 245, 1073–1080. [Google Scholar] [CrossRef]
- Yaakow, Y.; Kerem, E.D.; Yahav, Y.D.; Rivlin, J.M.; Blau, H.M.; Bentur, L.M.; Aviram, M.M.; Picard, E.M.; Bdolah-Abram, T.M.; Wilschonski, M.M. Reproducibility of nasal potential difference measurements in cystic fibrosis. Chest 2007, 132, 1219–1226. [Google Scholar] [CrossRef]
- Zomer-van Ommen, D.D.; de Poel, E.; Kruisselbrink, E.; Oppelaar, H.; Vonk, A.M.; Janssens, H.M.; van der Ent, C.K.; Hagemeijer, M.C.; Beekman, J.M. Comparison of ex vivo and in vitro intestinal cystic fibrosis models to measure CFTR-dependent ion channel activity. J. Cyst. Fibros. 2018, 17, 316–324. [Google Scholar] [CrossRef]
- Clacy, J.P.; Calvin, U.C.; Scotta, H.D.; Geroge, M.S.; Donold, R.V.; Michael, P.B.; Martina, G.; Jerry, A.N.; Beate, I.; John, C.W.; et al. CFTR modulator theratyping: Current status, gaps and future directions. J. Cyst. Fibros. 2019, 18, 22–34. [Google Scholar] [CrossRef]
- Fiedorczuk, K.; Chen, J. Molecular structures reveal synergistic rescue of D508 CFTR by trikafta modulators. Science 2022, 378, 284–290. [Google Scholar] [CrossRef]
- Weber, W.-M.; Clauss, W.; Cuppens, H.; Cassiman, J.J.; Van Driessche, W. Capacitance measurements reveal different pathways for the activation of CFTR. Pflügers Arch. Eur. J. Physiol. 1999, 438, 561–569. [Google Scholar] [CrossRef]
- Dudchenko, O.; Ordovas-Montanes, J.; Bingle, C.D. Respiratory epithelial cell types, states and fates in the era of single-cell RNA-sequencing. Biochem. J. 2023, 480, 921–939. [Google Scholar] [CrossRef]
- Deprez, M.; Zaragosi, L.-E.; Truchi, M.; Becavin, C.; García, S.R.; Arguel, M.-J.; Plaisant, M.; Magnone, V.; Lebrigand, K.; Abelanet, S.; et al. A single-cell atlas of the human airways. Am. J. Respir. Crit. Care Med. 2020, 20, 1636–1645. [Google Scholar] [CrossRef]
- Shah, A.S.; Ben-Shahar, Y.; Moninger, T.O.; Kline, J.N.; Welsh, M.J. Motile cilia of human airway epithelia are chemosensory. Science 2009, 325, 1131–1134. [Google Scholar] [CrossRef]
- Legendre, M.; Zaragosi, L.-E.; Mitchison, H.M. Motile cilia and airway disease. Semin. Cell Dev. Biol. 2021, 110, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Montoro, D.T.; Haber, A.L.; Biton, M.; Vinarsky, V.; Lin, B.; Birket, S.E.; Yuan, F.; Chen, S.; Leung, H.M.; Villoria, J.; et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018, 560, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Plasschaert, L.W.; Žilionis, R.; Choo-Wing, R.; Savova, V.; Knehr, J.; Roma, G.; Klein, A.M.; Jaffe, A.B. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 2018, 560, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Kolonko, A.K.; Efing, J.; González-Espinosa, Y.; Bangel-Ruland, N.; van Driessche, W.; Goycoolea, F.M.; Weber, W.-M. Capsaicin-loaded chitosan nanoparticles for wtCFTR-mRNA delivery to a cystic fibrosis cell line. Biomedicines 2020, 8, 364. [Google Scholar] [CrossRef]
- Pranke, I.M.; Hatton, A.; Simonin, J.; Jais, J.P.; Le Pimpec-Barthes, F.; Carsin, A.; Bonnette, P.; Fayon, M.; Bel, N.S.-L.; Grenet, D.; et al. Correction of CFTR function in nasal epithelial cells from cystic fibrosis patients predicts improvement of respiratory function by CFTR modulators. Sci. Rep. 2017, 7, 7375. [Google Scholar] [CrossRef]
- Davis, P.B. Cystic fibrosis since 1938. Am. J. Respir. Crit. Care Med. 2006, 73, 475–482. [Google Scholar] [CrossRef]
- Delmarco, A.; Pradal, U.; Cabrini, G.; Bonizzato, A.; Mastella, G. Nasal potential difference in cystic fibrosis patients presenting borderline sweat test. Eur. Respir. J. 1997, 10, 1145–1149. [Google Scholar] [CrossRef]
- Ratjen, F.; Bell, S.C.; Rowe, S.M.; Goss, C.H.; Quittner, A.L.; Bush, A. Cystic Fibrosis. Nat. Rev. 2015, 1, 15010. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; Rosenstein, B.J.; White, T.B.; Accurso, F.J.; Castellani, C.; Cutting, G.R.; Durie, P.R.; LeGrys, V.A.; Massie, J.; Parad, R.B.; et al. Guiedelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic fibrosis foundation consensus report. J. Pediatr. 2008, 153, S4–S14. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, K.; Wilschanski, M.; Castellani, C.; Taylor, C.; Cuppens, H.; Dodge, J.; Sinaasappel, M. Cystic fibrosis: Terminology and diagnostic algorithms. Thorax 2006, 61, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Tluczek, A.; Orland, K.M.; Cavanagh, L. Psychosocial consequences of false-positive newborn screens for cystic fibrosis. Qual. Health Res. 2011, 21, 174–186. [Google Scholar] [CrossRef]
- Tluczek, A.; Chevalier McKechnie, A.; Lynam, P.A. When the cystic fibrosis label does not fit: A modified uncertainty theory. Qual. Health Res. 2010, 20, 209–223. [Google Scholar] [CrossRef]
- Taylor, C.J.; Hardcastle, J.; Southern, K.W. Physiological measurements confirming the diagnosis of cystic fibrosis: The sweat test and measurements of transepithelial potential difference. Paediatr. Respir. Rev. 2009, 10, 220–226. [Google Scholar] [CrossRef]
- Fernández-Fernández, E.; Santos-Carballal, B.; Weber, W.-M.; Goycoolea, F.M. Chitosan as a non-viral co-transfection system in a cystic fibrosis cell line. Int. J. Pharm. 2016, 502, 1–9. [Google Scholar] [CrossRef]
- Bangel-Ruland, N.; Tomczak, K.; Fernández, E.F.; Leier, G.; Leciejewski, B.; Rudolph, C.; Rosenecker, J.; Weber, W. Cystic fibrosis transmembrane conductance regulator mRNA delivery: A novel alternative for CF gene therapy. J. Gene Med. 2013, 15, 414–426. [Google Scholar] [CrossRef]
- Deignan, J.L.; Gregg, A.R.; Grody, W.W.; Guo, M.H.; Kearney, H.; Monaghan, K.G.; Raraigh, K.S.; Taylor, J.; Zepeda-Mendoza, C.J.; Ziats, C. Updated recommendations for CFTR carrier screening: A position statement of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2023, 25, 100867. [Google Scholar] [CrossRef]
- LeGrys, V.A.; Yankaskas, J.R.; Quittell, L.M.; Marshall, B.C.; Mogayzel, P.J. Diagnostic sweat testing: The cystic fibrosis foundation guidelines. J. Pediatr. 2007, 151, 85–89. [Google Scholar] [CrossRef]
- Grosse-Onnebrink, J.; Werner, C.; Loges, N.T.; Hörmann, K.; Blum, A.; Schmidt, R.; Olbrich, H.; Omran, H. Effect of TH2 cytokines and interferon gamma on beat frequency of human respiratory cilia. Clin. Investig. 2016, 79, 731–735. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Kolonko, A.K.; Fernández Fernández, E.; Santos-Carballal, B.; Goycoolea, F.M.; Weber, W.M. Functional restoring of defect CFTR by transfection of CFTR-mRNA using chitosan. JSM Genet. Genom. 2016, 3, 1016–1019. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.N.; Mete, V.; van Driessche, W.; Omran, H.; Weber, W.-M.; Grosse-Onnebrink, J. Assessing CFTR Function and Epithelial Morphology in Human Nasal Respiratory Cell Cultures: A Combined Immunofluorescence and Electrophysiological Study. Int. J. Mol. Sci. 2025, 26, 7618. https://doi.org/10.3390/ijms26157618
Singh RN, Mete V, van Driessche W, Omran H, Weber W-M, Grosse-Onnebrink J. Assessing CFTR Function and Epithelial Morphology in Human Nasal Respiratory Cell Cultures: A Combined Immunofluorescence and Electrophysiological Study. International Journal of Molecular Sciences. 2025; 26(15):7618. https://doi.org/10.3390/ijms26157618
Chicago/Turabian StyleSingh, Roshani Narayan, Vanessa Mete, Willy van Driessche, Heymut Omran, Wolf-Michael Weber, and Jörg Grosse-Onnebrink. 2025. "Assessing CFTR Function and Epithelial Morphology in Human Nasal Respiratory Cell Cultures: A Combined Immunofluorescence and Electrophysiological Study" International Journal of Molecular Sciences 26, no. 15: 7618. https://doi.org/10.3390/ijms26157618
APA StyleSingh, R. N., Mete, V., van Driessche, W., Omran, H., Weber, W.-M., & Grosse-Onnebrink, J. (2025). Assessing CFTR Function and Epithelial Morphology in Human Nasal Respiratory Cell Cultures: A Combined Immunofluorescence and Electrophysiological Study. International Journal of Molecular Sciences, 26(15), 7618. https://doi.org/10.3390/ijms26157618






