The Catalyzing Effect of Aggregates on the Fibrillation Pathway of Human Insulin: A Spectroscopic Investigation During the Lag Phase
Abstract
1. Introduction
2. Results and Discussion
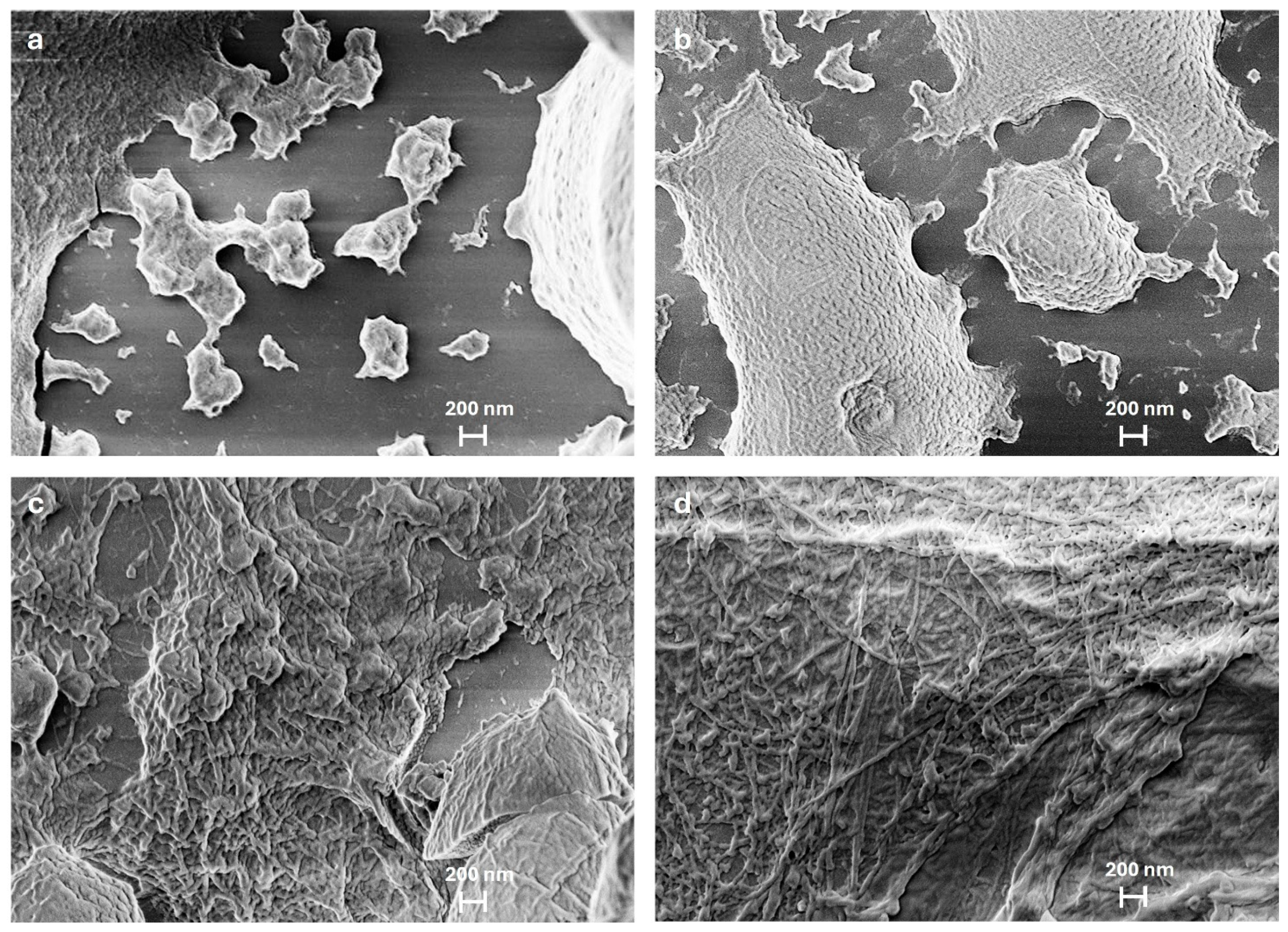
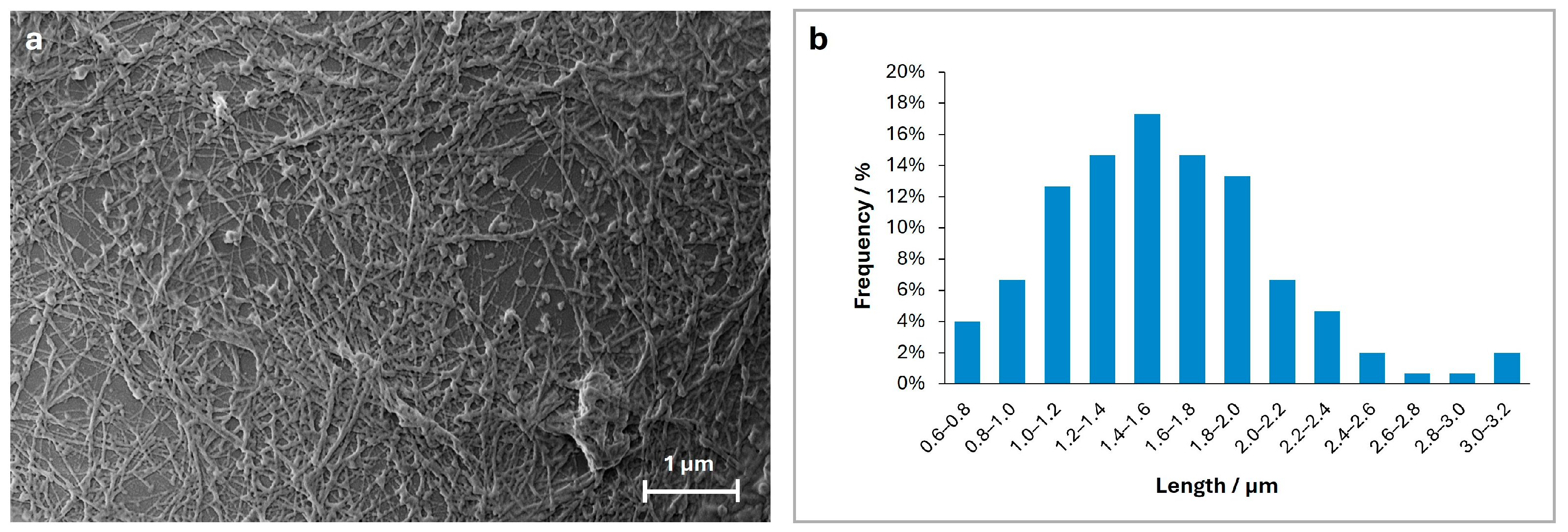
2.1. Morphological Assessment
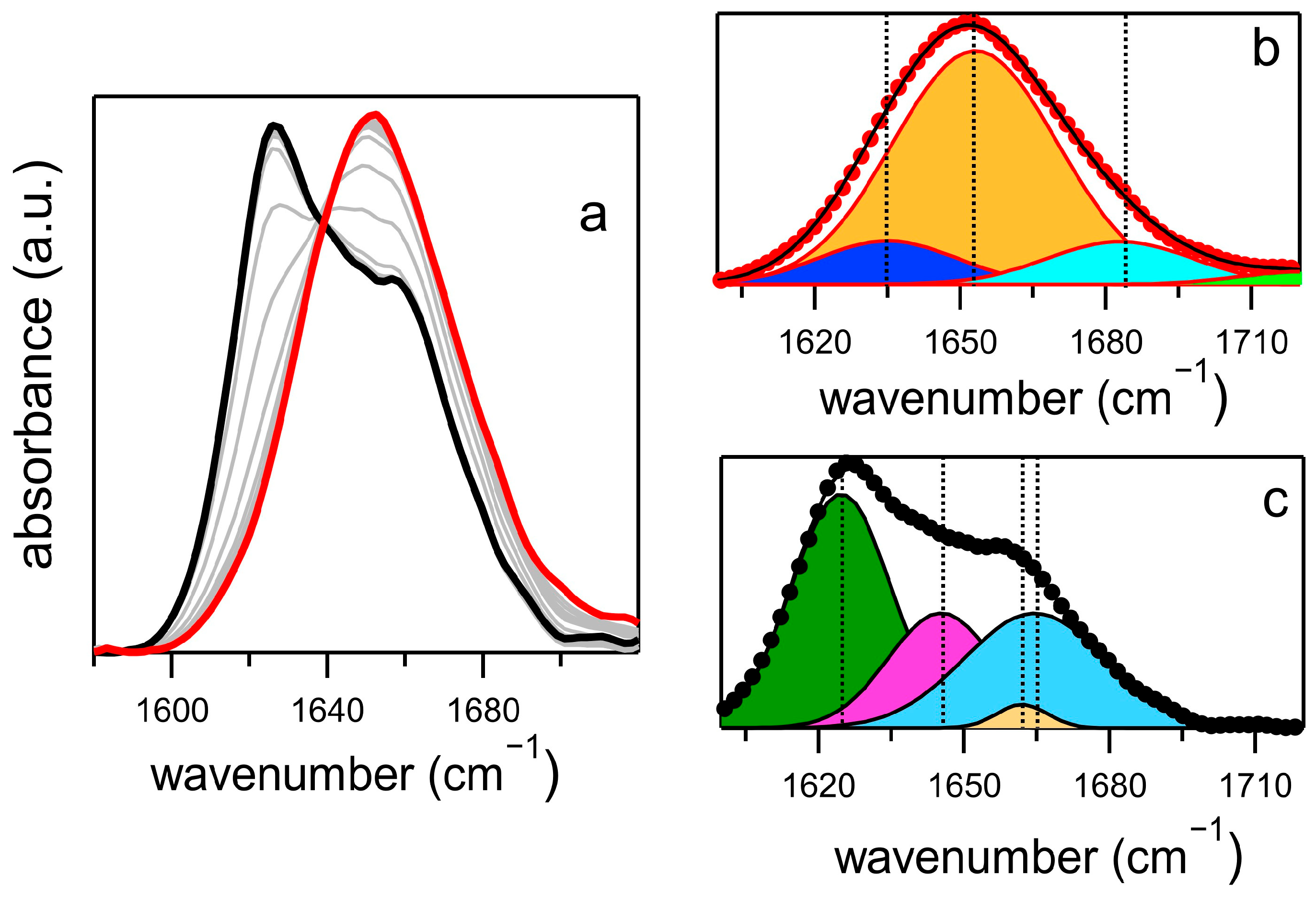
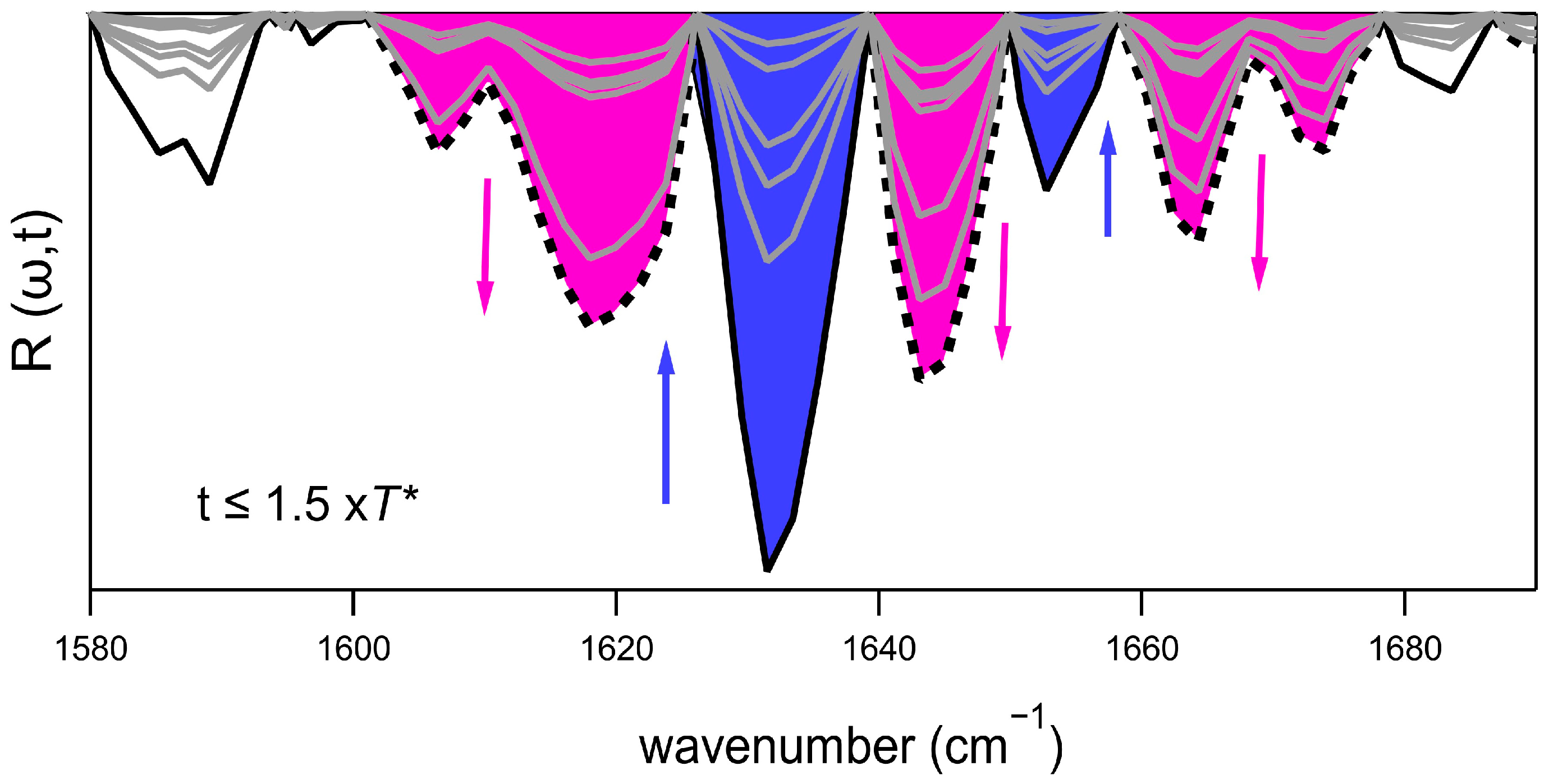
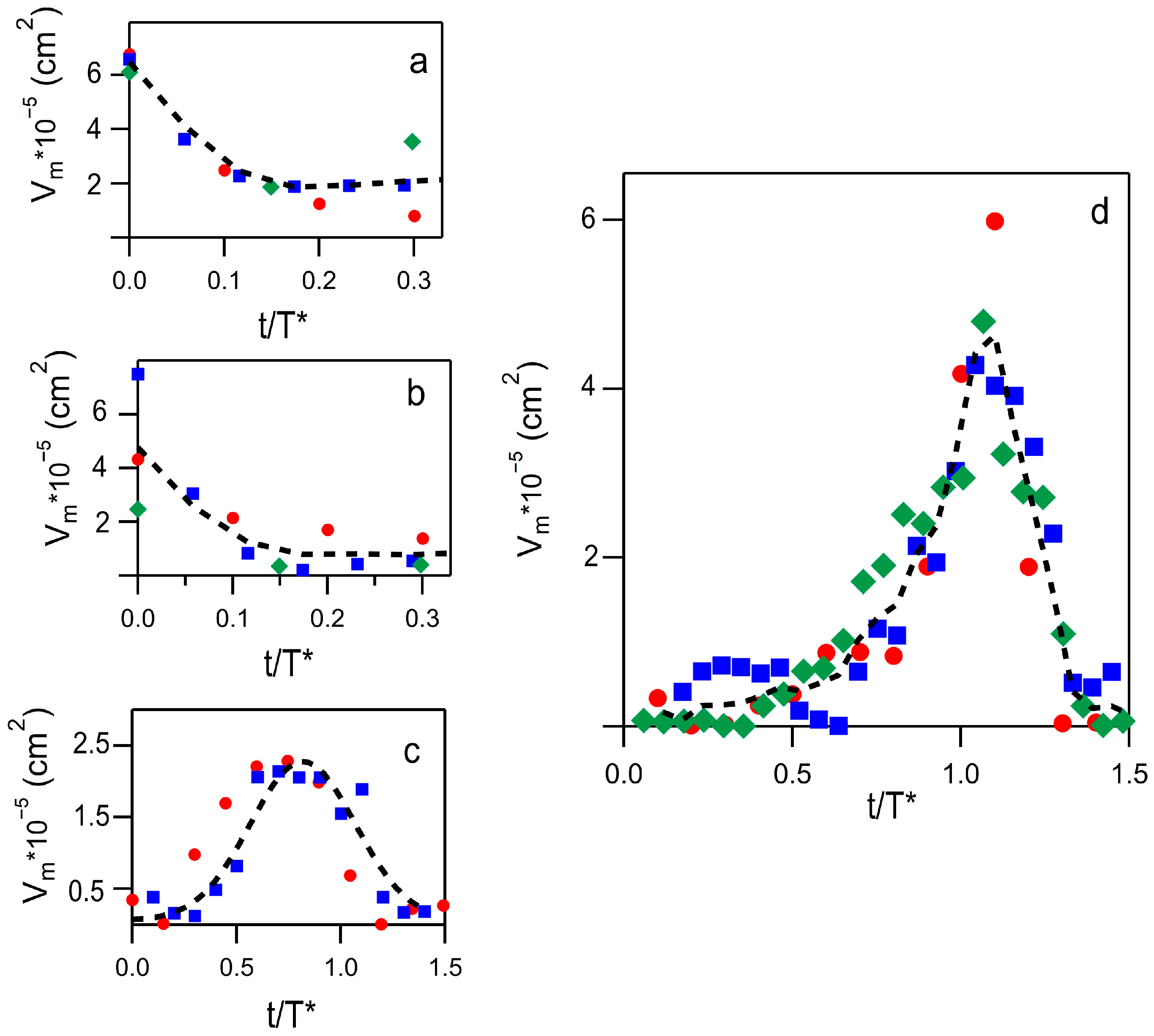
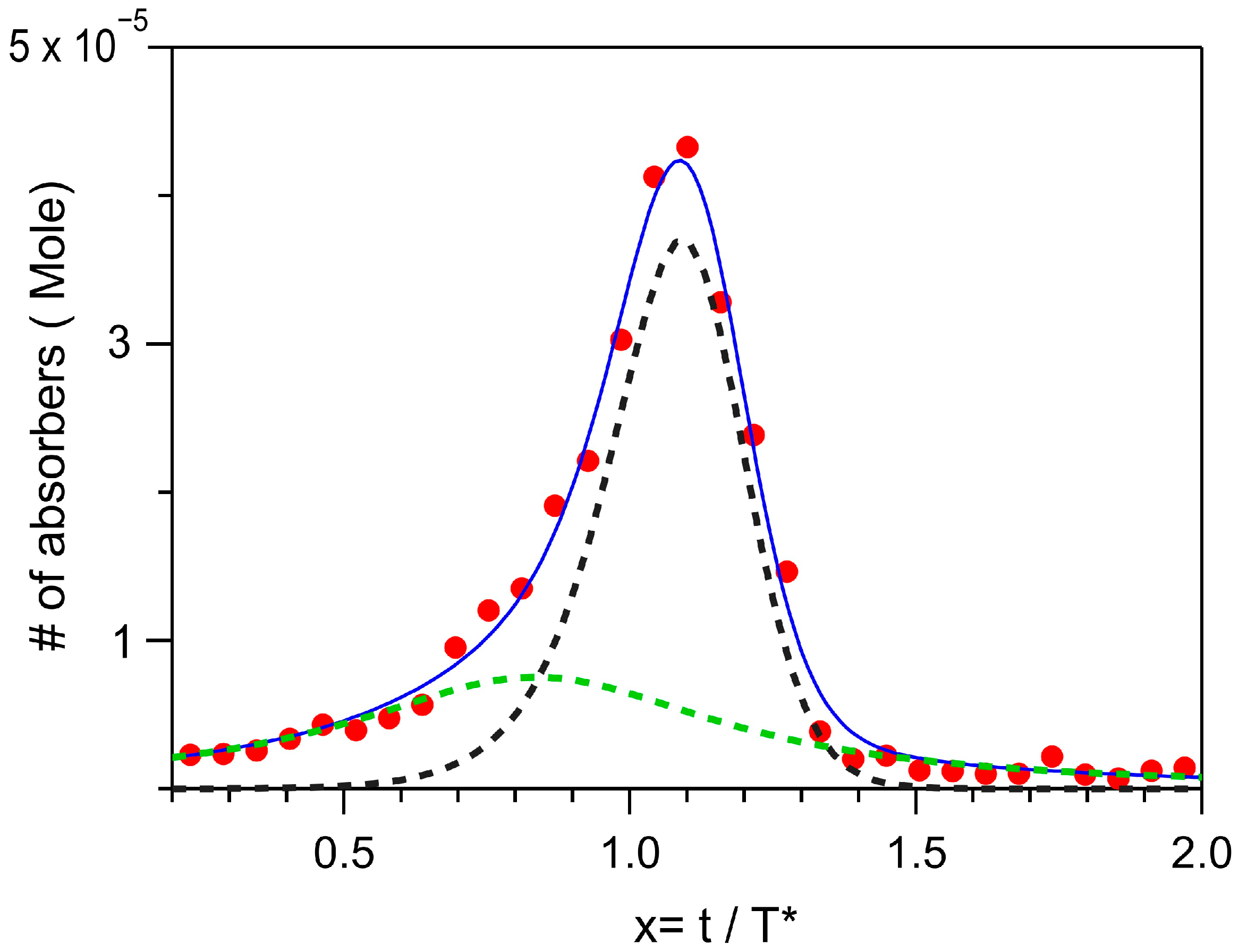
2.2. Phenomenology of the Aggregation Pathways by FTIR
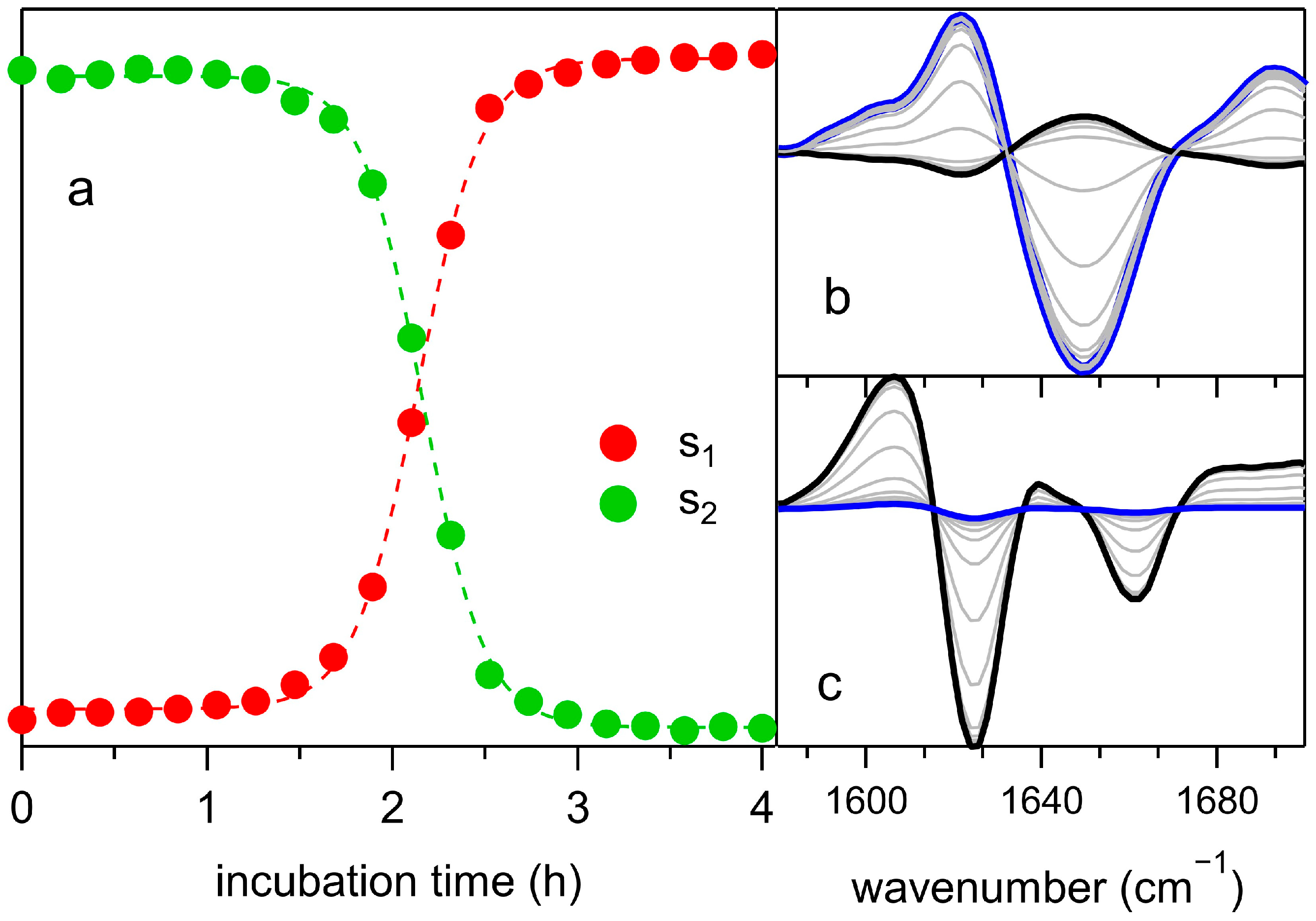
2.3. Principal Components Analysis (PCA) of the FTIR Data Set
3. Materials and Methods
3.1. Sample Preparation
3.2. SEM Imaging
3.3. Dynamic Light Scattering
3.4. FTIR Data Acquisition and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SEM | Scanning Electron Microscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
| PCA | Principal Component Analysis |
| DLS | Dynamic Light Scattering |
| PDI | Polydispersity Index |
References
- Kumar, V.; Sami, N.; Kashav, T.; Islam, A.; Ahmad, F.; Hassan, M.I. Protein Aggregation and Neurodegenerative Diseases: From Theory to Therapy. Eur. J. Med. Chem. 2016, 124, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Kurouski, D.; Van Duyne, R.P.; Lednev, I.K. Exploring the Structure and Formation Mechanism of Amyloid Fibrils by Raman Spectroscopy: A Review. Analyst 2015, 140, 4967–4980. [Google Scholar] [CrossRef]
- Martial, B.; Lefèvre, T.; Auger, M. Understanding Amyloid Fibril Formation Using Protein Fragments: Structural Investigations via Vibrational Spectroscopy and Solid-State NMR. Biophys. Rev. 2018, 10, 1133–1149. [Google Scholar] [CrossRef]
- Zhao, R.; So, M.; Maat, H.; Ray, N.J.; Arisaka, F.; Goto, Y.; Carver, J.A.; Hall, D. Measurement of Amyloid Formation by Turbidity Assay—Seeing through the Cloud. Biophys. Rev. 2016, 8, 445–471. [Google Scholar] [CrossRef]
- Rice, L.J.; Ecroyd, H.; van Oijen, A.M. Illuminating Amyloid Fibrils: Fluorescence-Based Single-Molecule Approaches. Comput. Struct. Biotechnol. J. 2021, 19, 4711–4724. [Google Scholar] [CrossRef]
- Cohen, S.I.A.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P.J. From Macroscopic Measurements to Microscopic Mechanisms of Protein Aggregation. J. Mol. Biol. 2012, 421, 160–171. [Google Scholar] [CrossRef]
- Arosio, P.; Knowles, T.P.J.; Linse, S. On the Lag Phase in Amyloid Fibril Formation. Phys. Chem. Chem. Phys. 2015, 17, 7606–7618. [Google Scholar] [CrossRef]
- Chatani, E.; Yamamoto, N. Recent Progress on Understanding the Mechanisms of Amyloid Nucleation. Biophys. Rev. 2018, 10, 527–534. [Google Scholar] [CrossRef]
- Grudzielanek, S.; Velkova, A.; Shukla, A.; Smirnovas, V.; Tatarek-Nossol, M.; Rehage, H.; Kapurniotu, A.; Winter, R. Cytotoxicity of Insulin within Its Self-Assembly and Amyloidogenic Pathways. J. Mol. Biol. 2007, 370, 372–384. [Google Scholar] [CrossRef]
- Törnquist, M.; Michaels, T.C.T.; Sanagavarapu, K.; Yang, X.; Meisl, G.; Cohen, S.I.A.; Knowles, T.P.J.; Linse, S. Secondary Nucleation in Amyloid Formation. Chem. Commun. 2018, 54, 8667–8684. [Google Scholar] [CrossRef]
- Nayak, A.; Dutta, A.K.; Belfort, G. Surface-Enhanced Nucleation of Insulin Amyloid Fibrillation. Biochem. Biophys. Res. Commun. 2008, 369, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Shah, M.; Saraogi, I. Molecular Aspects of Insulin Aggregation and Various Therapeutic Interventions. ACS Bio. Med. Chem. Au 2022, 2, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Meneghini, L. Insulin Stacking versus Therapeutic Accumulation: Understanding the Differences. Endocr. Pract. 2014, 20, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Crespo, R.; Rocha, F.A.; Damas, A.M.; Martins, P.M. A Generic Crystallization-like Model That Describes the Kinetics of Amyloid Fibril Formation. J. Biol. Chem. 2012, 287, 30585–30594. [Google Scholar] [CrossRef]
- Carbonaro, M.; Ripanti, F.; Filabozzi, A.; Minicozzi, V.; Stellato, F.; Placidi, E.; Morante, S.; Divenere, A.; Nicolai, E.; Postorino, P.; et al. Human Insulin Fibrillogenesis in the Presence of Epigallocatechin Gallate and Melatonin: Structural Insights from a Biophysical Approach. Int. J. Biol. Macromol. 2018, 115, 1157–1164. [Google Scholar] [CrossRef]
- Bencs, F.; Románszki, L.; Farkas, V.; Perczel, A. Structural Insights Into Amyloid Polymorphism: The Impact of Glutamine to Norleucine Substitutions in GNNQQNY Aggregation. Chem. Eur. J. 2025, 31, e202404255. [Google Scholar] [CrossRef]
- White, H.E.; Hodgkinson, J.L.; Jahn, T.R.; Cohen-Krausz, S.; Gosal, W.S.; Müller, S.; Orlova, E.V.; Radford, S.E.; Saibil, H.R. Globular Tetramers of Β2-Microglobulin Assemble into Elaborate Amyloid Fibrils. J. Mol. Biol. 2009, 389, 48–57. [Google Scholar] [CrossRef]
- Dutta, C.; Yang, M.; Long, F.; Shahbazian-Yassar, R.; Tiwari, A. Preformed Seeds Modulate Native Insulin Aggregation Kinetics. J. Phys. Chem. B 2015, 119, 15089–15099. [Google Scholar] [CrossRef]
- Li, S.; Leblanc, R.M. Aggregation of Insulin at the Interface. J. Phys. Chem. B 2014, 118, 1181–1188. [Google Scholar] [CrossRef]
- Kurouski, D.; Deckert-Gaudig, T.; Deckert, V.; Lednev, I.K. Structure and Composition of Insulin Fibril Surfaces Probed by TERS. J. Am. Chem. Soc. 2012, 134, 13323–13329. [Google Scholar] [CrossRef] [PubMed]
- Protein Data Bank. Available online: https://doi.org/10.2210/pdb1GUJ/pdb (accessed on 15 March 2025).
- Salehi, S.M.; Koner, D.; Meuwly, M. Dynamics and Infrared Spectroscopy of Monomeric and Dimeric Wild Type and Mutant Insulin. J. Phys. Chem. B 2020, 124, 11882–11894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Jones, K.C.; Fitzpatrick, A.; Peng, C.S.; Feng, C.-J.; Baiz, C.R.; Tokmakoff, A. Studying Protein–Protein Binding through T-Jump Induced Dissociation: Transient 2D IR Spectroscopy of Insulin Dimer. J. Phys. Chem. B 2016, 120, 5134–5145. [Google Scholar] [CrossRef] [PubMed]
- Dunkelberger, E.B.; Grechko, M.; Zanni, M.T. Transition Dipoles from 1D and 2D Infrared Spectroscopy Help Reveal the Secondary Structures of Proteins: Application to Amyloids. J. Phys. Chem. B 2015, 119, 14065–14075. [Google Scholar] [CrossRef]
- Ripanti, F.; Luchetti, N.; Nucara, A.; Minicozzi, V.; Venere, A.D.; Filabozzi, A.; Carbonaro, M. Normal Mode Calculation and Infrared Spectroscopy of Proteins in Water Solution: Relationship between Amide I Transition Dipole Strength and Secondary Structure. Int. J. Biol. Macromol. 2021, 185, 369–376. [Google Scholar] [CrossRef]
- Nucara, A.; Carbone, M.; Ripanti, F.; Manganiello, R.; Postorino, P.; Carbonaro, M. Achieving Cytochrome c Fibril/Aggregate Control towards Micro-Platelets and Micro-Fibers by Tuning PH and Protein Concentration: A Combined Morphological and Spectroscopic Analysis. Int. J. Biol. Macromol. 2019, 138, 106–115. [Google Scholar] [CrossRef]
- Caporaletti, F.; Carbonaro, M.; Maselli, P.; Nucara, A. Hydrogen–Deuterium Exchange Kinetics in β-Lactoglobulin (−)-Epicatechin Complexes Studied by FTIR Spectroscopy. Int. J. Biol. Macromol. 2017, 104, 521–526. [Google Scholar] [CrossRef]
- Nucara, A.; Ripanti, F.; Sennato, S.; Nisini, G.; De Santis, E.; Sefat, M.; Carbonaro, M.; Mango, D.; Minicozzi, V.; Carbone, M. Influence of Cortisol on the Fibril Formation Kinetics of Aβ42 Peptide: A Multi-Technical Approach. Int. J. Mol. Sci. 2022, 23, 6007. [Google Scholar] [CrossRef]
- Kaylor, J.; Bodner, N.; Edridge, S.; Yamin, G.; Hong, D.-P.; Fink, A.L. Characterization of Oligomeric Intermediates in Alpha-Synuclein Fibrillation: FRET Studies of Y125W/Y133F/Y136F Alpha-Synuclein. J. Mol. Biol. 2005, 353, 357–372. [Google Scholar] [CrossRef]
- Sadat, A.; Joye, I.J. Peak Fitting Applied to Fourier Transform Infrared and Raman Spectroscopic Analysis of Proteins. Appl. Sci. 2020, 10, 5918. [Google Scholar] [CrossRef]
- Lu, R.; Li, W.-W.; Katzir, A.; Raichlin, Y.; Yu, H.-Q.; Mizaikoff, B. Probing the Secondary Structure of Bovine Serum Albumin during Heat-Induced Denaturation Using Mid-Infrared Fiberoptic Sensors. Analyst 2015, 140, 765–770. [Google Scholar] [CrossRef]
- Cohen, S.I.A.; Linse, S.; Luheshi, L.M.; Hellstrand, E.; White, D.A.; Rajah, L.; Otzen, D.E.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P.J. Proliferation of Amyloid-Β42 Aggregates Occurs through a Secondary Nucleation Mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 9758–9763. [Google Scholar] [CrossRef] [PubMed]
- Kumar, E.K.; Haque, N.; Prabhu, N.P. Kinetics of Protein Fibril Formation: Methods and Mechanisms. Int. J. Biol. Macromol. 2017, 100, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Bruker. Available online: https://www.bruker.com/ (accessed on 15 March 2025).
- Fundamental of Statistics. Available online: http://www.statistics4u.info/fundstat_eng/dd_nipals_algo.html (accessed on 15 June 2025).
- Igor Pro Version: 9.0.5.1. Available online: https://www.wavemetrics.com/ (accessed on 15 March 2025).
- Jansen, R.; Dzwolak, W.; Winter, R. Amyloidogenic Self-Assembly of Insulin Aggregates Probed by High Resolution Atomic Force Microscopy. Biophys. J. 2005, 88, 1344–1353. [Google Scholar] [CrossRef]
- Manno, M.; Craparo, E.F.; Podestà, A.; Bulone, D.; Carrotta, R.; Martorana, V.; Tiana, G.; San Biagio, P.L. Kinetics of Different Processes in Human Insulin Amyloid Formation. J. Mol. Biol. 2007, 366, 258–274. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciufolini, G.; Filabozzi, A.; Capocefalo, A.; Ripanti, F.; Tavella, A.; Imparato, G.; Nucara, A.; Carbone, M. The Catalyzing Effect of Aggregates on the Fibrillation Pathway of Human Insulin: A Spectroscopic Investigation During the Lag Phase. Int. J. Mol. Sci. 2025, 26, 7599. https://doi.org/10.3390/ijms26157599
Ciufolini G, Filabozzi A, Capocefalo A, Ripanti F, Tavella A, Imparato G, Nucara A, Carbone M. The Catalyzing Effect of Aggregates on the Fibrillation Pathway of Human Insulin: A Spectroscopic Investigation During the Lag Phase. International Journal of Molecular Sciences. 2025; 26(15):7599. https://doi.org/10.3390/ijms26157599
Chicago/Turabian StyleCiufolini, Giorgia, Alessandra Filabozzi, Angela Capocefalo, Francesca Ripanti, Angelo Tavella, Giulia Imparato, Alessandro Nucara, and Marilena Carbone. 2025. "The Catalyzing Effect of Aggregates on the Fibrillation Pathway of Human Insulin: A Spectroscopic Investigation During the Lag Phase" International Journal of Molecular Sciences 26, no. 15: 7599. https://doi.org/10.3390/ijms26157599
APA StyleCiufolini, G., Filabozzi, A., Capocefalo, A., Ripanti, F., Tavella, A., Imparato, G., Nucara, A., & Carbone, M. (2025). The Catalyzing Effect of Aggregates on the Fibrillation Pathway of Human Insulin: A Spectroscopic Investigation During the Lag Phase. International Journal of Molecular Sciences, 26(15), 7599. https://doi.org/10.3390/ijms26157599






