Deciphering the Role of Functional Ion Channels in Cancer Stem Cells (CSCs) and Their Therapeutic Implications
Abstract
1. Introduction
2. Ion Channel Expression Profiles and Their Functions in CSCs
3. Calcium Ion Channels and Their Functions
3.1. SOCE Channels and Their Functions
3.2. TRP Channels and Their Functions
3.3. VGCCs and Their Functions
3.4. Ca2+ Release Channels (CRCs)
4. Mechanosensitive Channels and Their Functions
5. Chloride Channels and Their Functions
6. Potassium (K+) Channels and Their Functions
7. Sodium (Na+) Channels and Their Functions
8. ASICs and Their Functions
9. AQPs or Water Channels and Their Functions
Intracellular Organelles Ion Channels and Their Functions
10. Interplay Between Ion Channels and Epigenetic Control in CSCs
11. Ion Channel-Targeted Therapies in CSCs
12. Challenges and Strategies in Ion Channel Research for CSCs
13. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lobo, N.A.; Shimono, Y.; Qian, D.; Clarke, M.F. The biology of cancer stem cells. Annu. Rev. Cell Dev. Biol. 2007, 23, 675–699. [Google Scholar] [CrossRef]
- Fuchs, E. The tortoise and the hair: Slow-cycling cells in the stem cell race. Cell 2009, 137, 811–819. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, D.; Buchanan, P. Calcium channels and cancer stem cells. Cell Calcium. 2019, 81, 21–28. [Google Scholar] [CrossRef]
- Chen, K.; Huang, Y.H.; Chen, J.L. Understanding and targeting cancer stem cells: Therapeutic implications and challenges. Acta Pharmacol. Sin. 2013, 34, 732–740. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef]
- Clevers, H. The cancer stem cell premises, promises and challenges. Nat. Med. 2011, 17, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Li, G.R.; Deng, X.L. Functional ion channels in stem cells. World J. Stem Cells. 2011, 3, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Jardin, I.; Lopez, J.J.; Sanchez-Collado, J.; Gomez, L.J.; Salido, G.M.; Rosado, J.A. Store-operated Calcium entry and its implications in cancer stem cells. Cells 2022, 11, 1332. [Google Scholar] [CrossRef]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; CaceresCortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, A.; Du, Q.; Liao, Q.; Shuai, Z.; Chen, C.; Yang, X.; Hu, Y.; Zhao, J.; Liu, S.; et al. Novel insights into ion channels in cancer stem cells (Review). Int. J. Oncol. 2018, 53, 1435–1441. [Google Scholar] [CrossRef]
- Ertas, Y.N.; Abedi Dorcheh, K.; Akbari, A.; Jabbari, E. Nanoparticles for Targeted Drug Delivery to Cancer Stem Cells: A Review of Recent Advances. Nanomaterials 2021, 11, 1755. [Google Scholar] [CrossRef]
- Dalerba, P.; Cho, R.W.; Clarke, M.F. Cancer stem cells: Models and concepts. Annu. Rev. Med. 2007, 58, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Y.; Nie, B.; Pienta, K.J.; Morgan, T.M.; Taichman, R.S. Cancer stem cells and their role in metastasis. Pharmacol. Ther. 2013, 138, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Takebe, N.; Miele, L.R.; Jeong, W.; Bando, H.; Kahn, M.; Yang, S.X.; Ivy, S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nat. Rev. Clin. Oncol. 2015, 12, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, M.; Xu, J.; Cheng, W. The modulation of ion channels in cancer chemo-resistance. Front. Oncol. 2022, 12, 945896. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels in cancer: Are cancer hallmarks oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels and the hallmarks of cancer. Trends Mol. Med. 2010, 16, 107–121. [Google Scholar] [CrossRef]
- Zhuo, D.; Lei, Z.; Dong, L.; Chan, A.M.L.; Lin, J.; Jiang, L.; Qiu, B.; Jiang, X.; Tan, Y.; Yao, X. Orai1 and Orai3 act through distinct signalling axes to promote stemness and tumorigenicity of breast cancer stem cells. Stem Cell Res. Ther. 2024, 15, 256. [Google Scholar] [CrossRef]
- Lewuillon, C.; Guillemette, A.; Titah, S.; Shaik, F.A.; Jouy, N.; Labiad, O.; Farfariello, V.; Laguillaumie, M.O.; Idziorek, T.; Barthélémy, A.; et al. Involvement of ORAI1/SOCE in human AML cell lines and primary cells according to ABCB1 Activity, LSC Compartment and Potential Resistance to Ara-C Exposure. Int. J. Mol. Sci. 2022, 23, 5555. [Google Scholar] [CrossRef]
- Jardin, I.; Alvarado, S.; Jimenez-Velarde, V.; Nieto-Felipe, J.; Lopez, J.J.; Salido, G.M.; Smani, T.; Rosado, J.A. Orai1α and Orai1β support calcium entry and mammosphere formation in breast cancer stem cells. Sci. Rep. 2023, 13, 19471. [Google Scholar] [CrossRef]
- Kar, P.; Nelson, C.; Parekh, A.B. Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+ (CRAC) channels. J. Biol. Chem. 2011, 286, 14795–14803. [Google Scholar] [CrossRef]
- Samanta, K.; Kar, P.; Mirams, G.R.; Parekh, A.B. Cachannel re-localization to plasma-membrane microdomains strengthens activation of Ca2+-dependent nuclear gene expression. Cell Rep. 2015, 12, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Sung, Y.; Lee, S.H.; Martin, C.E.; Srikanth, S.; Chen, W.; Kang, M.K.; Kim, R.H.; Park, N.H.; Gwack, Y.; et al. Orai3 Calcium channel contributes to oral/oropharyngeal cancer stemness through the elevation of ID1 expression. Cells. 2023, 12, 2225. [Google Scholar] [CrossRef]
- Terrié, E.; Déliot, N.; Benzidane, Y.; Harnois, T.; Cousin, L.; Bois, P.; Oliver, L.; Arnault, P.; Vallette, F.; Constantin, B.; et al. Store-operated Calcium channels control proliferation and self-renewal of cancer stem cells from glioblastoma. Cancers 2021, 13, 3428. [Google Scholar] [CrossRef]
- Guo, J.; Shan, C.; Xu, J.; Li, M.; Zhao, J.; Cheng, W. New Insights into TRP Ion Channels in Stem Cells. Int. J. Mol. Sci. 2022, 23, 7766. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Nabissi, M.; Amantini, C.; Santoni, M.; Ricci-Vitiani, L.; Pallini, R.; Maggi, F.; Morelli, M.B. ERK phosphorylation regulates the Aml1/Runx1 splice variants and the TRP channels expression during the differentiation of glioma stem cell lines. Cells 2021, 10, 2052. [Google Scholar] [CrossRef] [PubMed]
- Luanpitpong, S.; Rodboon, N.; Samart, P.; Vinayanuwattikun, C.; Klamkhlai, S.; Chanvorachote, P.; Rojanasakul, Y.; Issaragrisil, S. A novel TRPM7/O-GlcNAc axis mediates tumour cell motility and metastasis by stabilising c-Myc and caveolin-1 in lung carcinoma. Br. J. Cancer 2020, 123, 1289–1301. [Google Scholar] [CrossRef]
- Liu, K.; Xu, S.H.; Chen, Z.; Zeng, Q.X.; Li, Z.J.; Chen, Z.M. TRPM7 overexpression enhances the cancer stem cell-like and metastatic phenotypes of lung cancer through modulation of the Hsp90α/uPA/MMP2 signaling pathway. BMC Cancer 2018, 18, 1167. [Google Scholar] [CrossRef]
- Xue, C.; Gao, Y.; Li, X.; Zhang, M.; Yang, Y.; Han, Q.; Sun, Z.; Bai, C.; Zhao, R.C. Mesenchymal stem cells derived from adipose accelerate the progression of colon cancer by inducing a MT-CAFs phenotype via TRPC3/NF-KB axis. Stem Cell Res. Ther. 2022, 13, 335. [Google Scholar] [CrossRef]
- So, C.L.; Milevskiy, M.J.G.; Monteith, G.R. Transient receptor potential cation channel subfamily V and breast cancer. Lab. Investig. 2020, 100, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Zhan, J.; Zhao, F.; Zhong, Z.; Chen, M.; Han, R.; Wang, Y. Oridonin Promotes Apoptosis and Restrains the Viability and Migration of Bladder Cancer by Impeding TRPM7 Expression via the ERK and AKT Signaling Pathways. Biomed. Res. Int. 2021, 2021, 4340950. [Google Scholar] [CrossRef]
- Liu, M.; Inoue, K.; Leng, T.; Guo, S.; Xiong, Z.G. TRPM7 channels regulate glioma stem cell through STAT3 and Notch signaling pathways. Cell Signal. 2014, 26, 2773–2781. [Google Scholar] [CrossRef]
- Middelbeek, J.; Visser, D.; Henneman, L.; Kamermans, A.; Kuipers, A.J.; Hoogerbrugge, P.M.; Jalink, K.; van Leeuwen, F.N. TRPM7 maintains progenitor-like features of neuroblastoma cells: Implications for metastasis formation. Oncotarget 2015, 6, 8760–8776. [Google Scholar] [CrossRef]
- Chen, T.M.; Huang, C.M.; Hsieh, M.S.; Lin, C.S.; Lee, W.H.; Yeh, C.T.; Liu, S.C. TRPM7 via calcineurin/NFAT pathway mediates metastasis and chemotherapeutic resistance in head and neck squamous cell carcinoma. Aging 2022, 14, 5250–5270. [Google Scholar] [CrossRef]
- Shiozaki, A.; Kurashima, K.; Kudou, M.; Shimizu, H.; Kosuga, T.; Takemoto, K.; Arita, T.; Konishi, H.; Yamamoto, Y.; Morimura, R.; et al. Cancer stem cells of hepatocellular carcinoma are suppressed by the voltage-gated Calcium channel inhibitor Amlodipine. Anticancer Res. 2023, 43, 4855–4864. [Google Scholar] [CrossRef]
- Shiozaki, A.; Katsurahara, K.; Kudou, M.; Shimizu, H.; Kosuga, T.; Ito, H.; Arita, T.; Konishi, H.; Komatsu, S.; Kubota, T.; et al. Amlodipine and Verapamil, voltage-gated Ca2+ channel inhibitors, suppressed the growth of gastric cancer stem cells. Ann. Surg. Oncol. 2021, 28, 5400–5411. [Google Scholar] [CrossRef]
- Lee, H.; Kwon, O.B.; Lee, J.E.; Jeon, Y.H.; Lee, D.S.; Min, S.H.; Kim, J.W. Repositioning trimebutine maleate as a cancer treatment targeting ovarian cancer stem cells. Cells 2021, 10, 918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wu, P.; Mu, M.; Niu, C.; Hu, S. Exosomal circZNF800 derived from glioma stem-like cells regulates glioblastoma tumorigenicity via the PIEZO1/Akt Axis. Mol. Neurobiol. 2024, 61, 6556–6571. [Google Scholar] [CrossRef] [PubMed]
- Lebon, D.; Collet, L.; Djordjevic, S.; Gomila, C.; Ouled-Haddou, H.; Platon, J.; Demont, Y.; Marolleau, J.P.; Caulier, A.; Garçon, L. PIEZO1 is essential for the survival and proliferation of acute myeloid leukemia cells. Cancer Med. 2024, 13, e6984. [Google Scholar] [CrossRef]
- Li, R.; Wang, D.; Li, H.; Lei, X.; Liao, W.; Liu, X.Y. Identification of Piezo1 as a potential target for therapy of colon cancer stem-like cells. Discov. Oncol. 2023, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Tijore, A.; Cheng, D.; Li, J.V.; Hariharan, A.; Martinac, B.; Tran Van Nhieu, G.; Cox, C.D.; Sheetz, M. Force- and cell state-dependent recruitment of Piezo1 drives focal adhesion dynamics and calcium entry. Sci. Adv. 2022, 8, eabo1461. [Google Scholar] [CrossRef]
- So, C.L.; Robitaille, M.; Sadras, F.; McCullough, M.H.; Milevskiy, M.J.G.; Goodhill, G.J.; Roberts-Thomson, S.J.; Monteith, G.R. Cellular geometry and epithelial-mesenchymal plasticity intersect with PIEZO1 in breast cancer cells. Commun. Biol. 2024, 7, 467. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, P.C.; Hong, J.H. Chloride channels and transporters: Roles beyond classical cellular homeostatic pH or ion balance in cancers. Cancers 2022, 14, 856. [Google Scholar] [CrossRef]
- Yan, Y.; Ding, X.; Han, C.; Gao, J.; Liu, Z.; Liu, Y.; Wang, K. Involvement of TMEM16A/ANO1 upregulation in the oncogenesis of colorectal cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166370. [Google Scholar] [CrossRef]
- Olsen, M.L.; Schade, S.; Lyons, S.A.; Amaral, M.D.; Sontheimer, H. Expression of voltage-gated chloride channels in human glioma cells. J. Neurosci. 2003, 23, 5572–5582. [Google Scholar] [CrossRef]
- Zhou, F.M.; Huang, Y.Y.; Tian, T.; Li, X.Y.; Tang, Y.B. Knockdown of Chloride channel-3 inhibits breast cancer growth In Vitro and In Vivo. J. Breast Cancer 2018, 21, 103–111. [Google Scholar] [CrossRef]
- Peretti, M.; Raciti, F.M.; Carlini, V.; Verduci, I.; Sertic, S.; Barozzi, S.; Garré, M.; Pattarozzi, A.; Daga, A.; Barbieri, F.; et al. Mutual influence of ROS, pH, and CLIC1 membrane protein in the regulation of G1-S phase progression in human glioblastoma stem cells. Mol. Cancer Ther. 2018, 17, 2451–2461. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.; Bosio, A.G.; Pattarozzi, A.; Tonelli, M.; Bajetto, A.; Verduci, I.; Cianci, F.; Cannavale, G.; Palloni, L.M.G.; Francesconi, V.; et al. Chloride intracellular channel 1 activity is not required for glioblastoma development but its inhibition dictates glioma stem cell responsivity to novel biguanide derivatives. J. Exp. Clin. Cancer Res. 2022, 41, 53. [Google Scholar] [CrossRef]
- Setti, M.; Savalli, N.; Osti, D.; Richichi, C.; Angelini, M.; Brescia, P.; Fornasari, L.; Carro, M.S.; Mazzanti, M.; Pelicci, G. Functional role of CLIC1 ion channel in glioblastoma-derived stem/progenitor cells. J. Natl. Cancer Inst. 2013, 105, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, L.; Cayo, A.; González, W.; Vilos, C.; Zúñiga, R. Potassium Channels as a Target for Cancer Therapy: Current Perspectives. OncoTargets Ther. 2022, 15, 783–797. [Google Scholar] [CrossRef]
- Ruggieri, P.; Mangino, G.; Fioretti, B.; Catacuzzeno, L.; Puca, R.; Ponti, D.; Miscusi, M.; Franciolini, F.; Ragona, G.; Calogero, A. The inhibition of KCa3.1 channels activity reduces cell motility in glioblastoma derived cancer stem cells. PLoS ONE 2012, 7, e47825. [Google Scholar] [CrossRef]
- Lallet-Daher, H.; Roudbaraki, M.; Bavencoffe, A.; Mariot, P.; Gackière, F.; Bidaux, G.; Urbain, R.; Gosset, P.; Delcourt, P.; Fleurisse, L.; et al. Intermediate-conductance Ca2+-activated K+ channels (IKCa1) regulate human prostate cancer cell proliferation through a close control of calcium entry. Oncogene 2009, 28, 1792–1806. [Google Scholar] [CrossRef]
- Guerriero, C.; Fanfarillo, R.; Mancini, P.; Sterbini, V.; Guarguaglini, G.; Sforna, L.; Michelucci, A.; Catacuzzeno, L.; Tata, A.M. M2 muscarinic receptors negatively modulate cell migration in human glioblastoma cells. Neurochem. Int. 2024, 174, 105673. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, A.; Konishi, T.; Kosuga, T.; Kudou, M.; Kurashima, K.; Inoue, H.; Shoda, K.; Arita, T.; Konishi, H.; Morimura, R.; et al. Roles of voltage-gated potassium channels in the maintenance of pancreatic cancer stem cells. Int. J. Oncol. 2021, 59, 76. [Google Scholar] [CrossRef] [PubMed]
- Lastraioli, E. Focus on triple-negative breast cancer: Potassium channel expression and clinical correlates. Front. Pharmacol. 2020, 11, 725. [Google Scholar] [CrossRef]
- Bian, Y.; Tuo, J.; He, L.; Li, W.; Li, S.; Chu, H.; Zhao, Y. Voltage-gated sodium channels in cancer and their specific inhibitors. Pathol. Res. Pract. 2023, 251, 154909. [Google Scholar] [CrossRef] [PubMed]
- Giammello, F.; Biella, C.; Priori, E.C.; Filippo, M.A.D.S.; Leone, R.; D’Ambrosio, F.; Paterno’, M.; Cassioli, G.; Minetti, A.; Macchi, F.; et al. Modulating voltage-gated sodium channels to enhance differentiation and sensitize glioblastoma cells to chemotherapy. Cell Commun. Signal. 2024, 22, 434. [Google Scholar] [CrossRef]
- Djamgoz, M.B.A. Stemness of Cancer: A study of triple-negative breast cancer from a neuroscience perspective. Stem Cell Rev. Rep. 2025, 21, 337–350. [Google Scholar] [CrossRef]
- Tian, Y.; Bresenitz, P.; Reska, A.; El Moussaoui, L.; Beier, C.P.; Gründer, S. Glioblastoma cancer stem cell lines express functional acid sensing ion channels ASIC1a and ASIC3. Sci. Rep. 2017, 7, 13674. [Google Scholar] [CrossRef]
- Clusmann, J.; Franco, K.C.; Suárez, D.A.C.; Katona, I.; Minguez, M.G.; Boersch, N.; Pissas, K.P.; Vanek, J.; Tian, Y.; Gründer, S. Acidosis induces RIPK1-dependent death of glioblastoma stem cells via acid-sensing ion channel 1a. Cell Death Dis. 2022, 13, 702. [Google Scholar] [CrossRef] [PubMed]
- King, P.; Wan, J.; Guo, A.A.; Guo, S.; Jiang, Y.; Liu, M. Regulation of gliomagenesis and stemness through acid sensor ASIC1a. Int. J. Oncol. 2021, 59, 82. [Google Scholar] [CrossRef]
- Guan, Y.; Han, J.; Chen, D.; Zhan, Y.; Chen, J. Aquaporin 1 overexpression may enhance glioma tumorigenesis by interacting with the transcriptional regulation networks of Foxo4, Maz, and E2F families. Chin. Neurosurg. J. 2023, 9, 34. [Google Scholar] [CrossRef]
- Huang, Y.T.; Zhou, J.; Shi, S.; Xu, H.Y.; Qu, F.; Zhang, D.; Chen, Y.D.; Yang, J.; Huang, H.F.; Sheng, J.Z. Identification of estrogen response element in Aquaporin-3 gene that mediates estrogen-induced cell migration and invasion in estrogen receptor-positive breast cancer. Sci. Rep. 2015, 5, 12484. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, G.; Fu, X.; Xu, S.; Wang, T.; Zhang, Q.; Yang, Y. Aquaporin 3 maintains the stemness of CD133+ hepatocellular carcinoma cells by activating STAT3. Cell Death Dis. 2019, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Yeh, C.T.; Lin, K.H. Cancer stem cell functions in hepatocellular carcinoma and comprehensive therapeutic strategies. Cells 2020, 9, 1331. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.; Zhang, Y.; Jing, H. Molecular mechanism of AQP3 in regulating differentiation and apoptosis of lung cancer stem cells through Wnt/GSK-3β/β-Catenin pathway. J BUON 2020, 25, 1714–1720. [Google Scholar] [PubMed]
- Simone, L.; Pisani, F.; Binda, E.; Frigeri, A.; Vescovi, A.L.; Svelto, M.; Nicchia, G.P. AQP4-dependent glioma cell features affect the phenotype of surrounding cells via extracellular vesicles. Cell Biosci. 2022, 12, 150. [Google Scholar] [CrossRef]
- Jang, S.J.; Moon, C. Aquaporin 5 (AQP5) expression in breast cancer and its clinicopathological characteristics. PLoS ONE 2023, 18, e0270752. [Google Scholar] [CrossRef]
- Zhao, R.; He, B.; Bie, Q.; Cao, J.; Lu, H.; Zhang, Z.; Liang, J.; Wei, L.; Xiong, H.; Zhang, B. AQP5 complements LGR5 to determine the fates of gastric cancer stem cells through regulating ULK1 ubiquitination. J. Exp. Clin. Cancer Res. 2022, 41, 322, Erratum in J. Exp. Clin. Cancer Res. 2022, 41, 342. https://doi.org/10.1186/s13046-022-02557-1. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Song, Y.; Pan, C.; Yu, J.; Zhang, J.; Zhu, X. Aquaporin-8 is a novel marker for progression of human cervical cancer cells. Cancer Biomark. 2021, 32, 391–400. [Google Scholar] [CrossRef]
- Zheng, X.; Li, C.; Yu, K.; Shi, S.; Chen, H.; Qian, Y.; Mei, Z. Aquaporin-9, mediated by IGF2, suppresses liver cancer stem cell properties via augmenting ROS/β-Catenin/FOXO3a signaling. Mol. Cancer Res. 2020, 18, 992–1003. [Google Scholar] [CrossRef]
- Balboni, A.; D’Angelo, C.; Collura, N.; Brusco, S.; Di Berardino, C.; Targa, A.; Massoti, B.; Mastrangelo, E.; Milani, M.; Seneci, P.; et al. Acid-sensing ion channel 3 is a new potential therapeutic target for the control of glioblastoma cancer stem cells growth. Sci. Rep. 2024, 14, 20421. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Jana, A.; Bhattacharjee, S.; Mitra, S.; De, S.; Alghamdi, B.S.; Alam, M.Z.; Mahmoud, A.B.; Al Shareef, Z.; Abdel-Rahman, W.M.; et al. The role of Aquaporins in tumorigenesis: Implications for therapeutic development. Cell Commun. Signal. 2024, 22, 106. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.T.; Gottschalk, B.; Sokolowski, A.A.; Malli, R.; Graier, W.F. Dynamic Control of Mitochondrial Ca2+ Levels as a Survival Strategy of Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 614668. [Google Scholar] [CrossRef]
- Wee, S.; Niklasson, M.; Marinescu, V.D.; Segerman, A.; Schmidt, L.; Hermansson, A.; Dirks, P.; Forsberg-Nilsson, K.; Westermark, B.; Uhrbom, L.; et al. Selective calcium sensitivity in immature glioma cancer stem cells. PLoS ONE 2014, 9, e115698. [Google Scholar] [CrossRef]
- Fernandez Garcia, E.; Paudel, U.; Noji, M.C.; Bowman, C.E.; Rustgi, A.K.; Pitarresi, J.R.; Wellen, K.E.; Arany, Z.; Weissenrieder, J.S.; Foskett, J.K. The mitochondrial Ca2+ channel MCU is critical for tumor growth by supporting cell cycle progression and proliferation. Front. Cell Dev. Biol. 2023, 11, 1082213. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, X.; Shen, Q.; Yang, X.; Yu, C.; Cai, C.; Cai, G.; Meng, X.; Zou, F. Mitochondrial Ca2+ uniporter is critical for store-operated Ca2+ entry-dependent breast cancer cell migration. Biochem. Biophys. Res. Commun. 2015, 458, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Bong, A.H.L.; Robitaille, M.; Lin, S.; McCart-Reed, A.; Milevskiy, M.; Angers, S.; Roberts-Thomson, S.J.; Monteith, G.R. TMCO1 is upregulated in breast cancer and regulates the response to pro-apoptotic agents in breast cancer cells. Cell Death Discov. 2024, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.F.; Dai, S.Y.; Wong, N.K.; Deng, S.; Wong, A.S.; Yang, D. Mediating K+/H+ transport on organelle membranes to selectively eradicate cancer stem cells with a small molecule. J. Am. Chem. Soc. 2020, 142, 10769–10779. [Google Scholar] [CrossRef]
- Zheng, J.; Feng, Y.; Yuan, C. Hsa_circ_0007905 as a modulator of miR-330-5p and VDAC1: Enhancing stemness and reducing apoptosis in cervical cancer stem cells. Gen. Physiol. Biophys. 2025, 44, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Seoane, J. Cancer: Division hierarchy leads to cell heterogeneity. Nature 2017, 549, 164–166. [Google Scholar] [CrossRef] [PubMed]
- O’Brien-Ball, C.; Biddle, A. Reprogramming to developmental plasticity in cancer stem cells. Dev. Biol. 2017, 430, 266–274. [Google Scholar] [CrossRef]
- Sun, X.X.; Yu, Q. Intra-tumor heterogeneity of cancer cells and its implications for cancer treatment. Acta Pharmacol. Sin. 2015, 36, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Polyak, K. Tumor heterogeneity: Causes and consequences. Biochim. Biophys. Acta 2010, 1805, 105–117. [Google Scholar] [CrossRef]
- Kang, Q.; Peng, X.; Li, X.; Hu, D.; Wen, G.; Wei, Z.; Yuan, B. Calcium Channel Protein ORAI1 Mediates TGF-β Induced Epithelial-to-Mesenchymal Transition in Colorectal Cancer Cells. Front. Oncol. 2021, 11, 649476. [Google Scholar] [CrossRef]
- Beck, B.; Lapouge, G.; Rorive, S.; Drogat, B.; Desaedelaere, K.; Delafaille, S.; Dubois, C.; Salmon, I.; Willekens, K.; Marine, J.C.; et al. Different levels of Twist1 regulate skin tumor initiation, stemness, and progression. Cell Stem Cell 2015, 16, 67–79. [Google Scholar] [CrossRef]
- He, T.; Wang, C.; Zhang, M.; Zhang, X.; Zheng, S.; Linghu, E.; Guo, M. Epigenetic regulation of voltage-gated potassium ion channel molecule Kv1.3 in mechanisms of colorectal cancer. Discov. Med. 2017, 23, 155–162. [Google Scholar]
- Herranz, N.; Pasini, D.; Díaz, V.M.; Francí, C.; Gutierrez, A.; Dave, N.; Escrivà, M.; Hernandez-Muñoz, I.; Di Croce, L.; Helin, K.; et al. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol. Cell Biol. 2008, 28, 4772–4781. [Google Scholar] [CrossRef]
- Luo, M.; Bao, L.; Xue, Y.; Zhu, M.; Kumar, A.; Xing, C.; Wang, J.E.; Wang, Y.; Luo, W. ZMYND8 protects breast cancer stem cells against oxidative stress and ferroptosis through activation of NRF2. J. Clin. Investig. 2024, 134, e171166. [Google Scholar] [CrossRef]
- Bao, L.; Festa, F.; Freet, C.S.; Lee, J.P.; Hirschler-Laszkiewicz, I.M.; Chen, S.J.; Keefer, K.A.; Wang, H.G.; Patterson, A.D.; Cheung, J.Y.; et al. The Human Transient Receptor Potential Melastatin 2 Ion Channel Modulates ROS Through Nrf2. Sci. Rep. 2019, 9, 14132. [Google Scholar] [CrossRef]
- Cheng, D.; Wu, R.; Guo, Y.; Kong, A.N. regulation of Keap1-Nrf2 signaling: The role of epigenetics. Curr. Opin. Toxicol. 2016, 1, 134–138. [Google Scholar] [CrossRef]
- Samanta, K.; Ahel, I.; Kar, P. Deciphering of the reactive oxygen species (ROS) induced calpain activation in cancer progression and its therapeutic potential. Adv. Redox Res. 2025, 15, 100124. [Google Scholar] [CrossRef]
- Arif, T.; Shteinfer-Kuzmine, A.; Shoshan-Barmatz, V. Decoding cancer through silencing the mitochondrial gatekeeper VDAC1. Biomolecules 2024, 14, 1304. [Google Scholar] [CrossRef] [PubMed]
- Shoshan-Barmatz, V.; Krelin, Y.; Shteinfer-Kuzmine, A.; Arif, T. Voltage-dependent anion channel 1 as an emerging drug target for novel anti-cancer therapeutics. Front. Oncol. 2017, 7, 154. [Google Scholar] [CrossRef]
- Alnasser, S.M. Advances and Challenges in Cancer Stem Cells for Onco-Therapeutics. Stem Cells Int. 2023, 2023, 8722803. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, T.J.; Stein, V.; Weinreich, F.; Zdebik, A.A. Molecular structure and physiological function of chloride channels. Physiol. Rev. 2002, 82, 503–568. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, J.W.; Kim, D.K.; Choi, D.K.; Lee, S.; Yu, J.H.; Kwon, O.B.; Lee, J.; Lee, D.S.; Kim, J.H.; et al. Calcium channels as novel therapeutic targets for ovarian cancer stem cells. Int. J. Mol. Sci. 2020, 21, 2327. [Google Scholar] [CrossRef]
- Zhang, Y.; Cruickshanks, N.; Yuan, F.; Wang, B.; Pahuski, M.; Wulfkuhle, J.; Gallagher, I.; Koeppel, A.F.; Hatef, S.; Papanicolas, C.; et al. Targetable T-type Calcium Channels Drive Glioblastoma. Cancer Res. 2017, 77, 3479–3490. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Y.; Schwarz, B.; Mysliwietz, J.; Hartig, R.; Camaj, P.; Bao, Q.; Jauch, K.W.; Guba, M.; Ellwart, J.W.; et al. Verapamil inhibits tumor progression of chemotherapy-resistant pancreatic cancer side population cells. Int. J. Oncol. 2016, 49, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.P.; Grimes, J.A.; Djamgoz, M.B. Effects of voltage-gated ion channel modulators on rat prostatic cancer cell proliferation: Comparison of strongly and weakly metastatic cell lines. Prostate 2000, 44, 61–76. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Grimaldi, A.; Chece, G.; Porzia, A.; Esposito, V.; Santoro, A.; Salvati, M.; Mainiero, F.; Ragozzino, D.; Di Angelantonio, S.; et al. KCa3.1 channel inhibition sensitizes malignant gliomas to temozolomide treatment. Oncotarget 2016, 7, 30781–30796. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Goel, H.L.; Xiong, C.; Goel, S.; Kumar, A.; Li, R.; Zhu, L.J.; Clark, J.L.; Brehm, M.A.; Mercurio, A.M. The calcium channel TRPC6 promotes chemotherapy-induced persistence by regulating integrin α6 mRNA splicing. Cell Rep. 2023, 42, 113347. [Google Scholar] [CrossRef]
- Comes, N.; Bielanska, J.; Vallejo-Gracia, A.; Serrano-Albarrás, A.; Marruecos, L.; Gómez, D.; Soler, C.; Condom, E.; Ramón, Y.; Cajal, S.; et al. The voltage-dependent K (+) channels Kv1.3 and Kv1.5 in human cancer. Front. Physiol. 2013, 4, 283. [Google Scholar] [CrossRef]
- Kischel, P.; Girault, A.; Rodat-Despoix, L.; Chamlali, M.; Radoslavova, S.; Abou Daya, H.; Lefebvre, T.; Foulon, A.; Rybarczyk, P.; Hague, F.; et al. Ion Channels: New Actors Playing in Chemotherapeutic Resistance. Cancers 2019, 11, 376. [Google Scholar] [CrossRef]
- Gründer, S.; Vanek, J.; Pissas, K.P. Acid-sensing ion channels and downstream signalling in cancer cells: Is there a mechanistic link? Pflug. Arch. 2024, 476, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Y.; Wen, J.; Zhao, H.; Dong, X.; Zhang, Z.; Wang, S.; Shen, L. Aquaporin 3 promotes the stem-like properties of gastric cancer cells via Wnt/GSK-3β/β-catenin pathway. Oncotarget 2016, 7, 16529–16541. [Google Scholar] [CrossRef]
- Gao, P.; Chen, A.; Tian, H.; Wang, F.; Wang, N.; Ge, K.; Lian, C.; Wang, F.; Zhang, Q. Investigating the mechanism and the effect of aquaporin 5 (AQP5) on the self-renewal capacity of gastric cancer stem cells. J. Cancer 2024, 15, 4313–4327. [Google Scholar] [CrossRef] [PubMed]
- Pollak, J.; Rai, K.G.; Funk, C.C.; Arora, S.; Lee, E.; Zhu, J.; Price, N.D.; Paddison, P.J.; Ramirez, J.M.; Rostomily, R.C. Ion channel expression patterns in glioblastoma stem cells with functional and therapeutic implications for malignancy. PLoS ONE 2017, 12, e0172884. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, H.; Gagnon, J.; Therrien, M. ERK signalling: A master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 2020, 21, 607–632. [Google Scholar] [CrossRef]
- Bortner, C.D.; Gomez-Angelats, M.; Cidlowski, J.A. Plasma membrane depolarization without repolarization is an early molecular event in anti-Fas-induced apoptosis. J. Biol. Chem. 2001, 276, 4304–4314. [Google Scholar] [CrossRef]
- Shi, Q.; Yang, Z.; Yang, H.; Xu, L.; Xia, J.; Gu, J.; Chen, M.; Wang, Y.; Zhao, X.; Liao, Z.; et al. Targeting ion channels: Innovative approaches to combat cancer drug resistance. Theranostics 2025, 15, 521–545. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yu, C.; Xu, M. Linking Tumor Microenvironment to Plasticity of Cancer Stem Cells: Mechanisms and Application in Cancer Therapy. Front. Oncol. 2021, 11, 678333. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.K.; Giam, C.S.; Leow, M.Y.; Chan, C.W.; Yim, E.K. Differential cell adhesion of breast cancer stem cells on biomaterial substrate with nanotopographical cues. J. Funct. Biomater. 2015, 6, 241–258. [Google Scholar] [CrossRef]
- Marjanovic, N.D.; Weinberg, R.A.; Chaffer, C.L. Cell plasticity and heterogeneity in cancer. Clin. Chem. 2013, 59, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.R.; Perez-Neut, M.; Kaja, S.; Gentile, S. Voltage-gated ion channels in cancer cell proliferation. Cancers 2015, 7, 849–875. [Google Scholar] [CrossRef]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef]
- Dallas, M.L.; Bell, D. Advances in ion channel high throughput screening: Where are we in 2023? Expert. Opin. Drug Discov. 2024, 19, 331–337. [Google Scholar] [CrossRef]
- Hjelmeland, A.B.; Wu, Q.; Heddleston, J.M.; Choudhary, G.S.; MacSwords, J.; Lathia, J.D.; McLendon, R.; Lindner, D.; Sloan, A.; Rich, J.N. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 2011, 18, 829–840. [Google Scholar] [CrossRef]
- Ganguly, K.; Krishn, S.R.; Rachagani, S.; Jahan, R.; Shah, A.; Nallasamy, P.; Rauth, S.; Atri, P.; Cox, J.L.; Pothuraju, R.; et al. Secretory Mucin 5AC Promotes Neoplastic Progression by Augmenting KLF4-Mediated Pancreatic Cancer Cell Stemness. Cancer Res. 2021, 81, 91–102. [Google Scholar] [CrossRef]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Satija, R.; Farrell, J.A.; Gennert, D.; Schier, A.F.; Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 2015, 33, 495–502. [Google Scholar] [CrossRef]
- Daniluk, U.; Świdnicka-Siergiejko, A.; Daniluk, J.; Rusak, M.; Dąbrowska, M.; Guzińska-Ustymowicz, K.; Pryczynicz, A.; Dąbrowski, A. The development of pancreatic cancer is accompanied by significant changes in the immune response in genetically predisposed mice. Front. Oncol. 2025, 15, 1603293. [Google Scholar] [CrossRef]
- Liu, J.; Gustafson, P.; Huo, D. Bayesian adjustment for the misclassification in both dependent and independent variables with application to a breast cancer study. Stat. Med. 2016, 35, 4252–4263. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; MacKinnon, R. Cryo-EM Structure of a KCNQ1/CaM Complex Reveals Insights into Congenital Long QT Syndrome. Cell 2017, 169, 1042–1050.e9. [Google Scholar] [CrossRef]
- Lees, J.A.; Dias, J.M.; Han, S. Applications of Cryo-EM in small molecule and biologics drug design. Biochem. Soc. Trans. 2021, 49, 2627–2638. [Google Scholar] [CrossRef]
- Li, C.; Gao, D.; Gao, Y.; Zhang, R.; Qu, X.; Li, S.; Xing, C. NIR-II Regulation of Mitochondrial Potassium Channel with Dual-Targeted Conjugated Oligomer Nanoparticles for Efficient Cancer Theranostics In Vivo. Adv. Healthc. Mater. 2023, 12, e2301954. [Google Scholar] [CrossRef]
- Rodríguez-Camacho, A.; Flores-Vázquez, J.G.; Moscardini-Martelli, J.; Torres-Ríos, J.A.; Olmos-Guzmán, A.; Ortiz-Arce, C.S.; Cid-Sánchez, D.R.; Pérez, S.R.; Macías-González, M.D.S.; Hernández-Sánchez, L.C.; et al. Glioblastoma Treatment: State-of-the-Art and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 7207. [Google Scholar] [CrossRef]
- Lastraioli, E.; Iorio, J.; Arcangeli, A. Ion channel expression as promising cancer biomarker. Biochim. Biophys. Acta 2015, 1848 Pt B, 2685–2702. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, A. The role of hERG1 ion channels in epithelial-mesenchymal transition and the capacity of riluzole to reduce cisplatin resistance in colorectal cancer cells. Cell. Oncol. 2017, 40, 367–378. [Google Scholar] [CrossRef]
- Wan, J.; Guo, A.A.; King, P.; Guo, S.; Saafir, T.; Jiang, Y.; Liu, M. TRPM7 Induces Tumorigenesis and Stemness Through Notch Activation in Glioma. Front. Pharmacol. 2020, 11, 590723. [Google Scholar] [CrossRef] [PubMed]
- Bill, A.; Gutierrez, A.; Kulkarni, S.; Kemp, C.; Bonenfant, D.; Voshol, H.; Duvvuri, U.; Gaither, L.A. ANO1/TMEM16A interacts with EGFR and correlates with sensitivity to EGFR-targeting therapy in head and neck cancer. Oncotarget 2015, 6, 9173–9188. [Google Scholar] [CrossRef]
- Jain, S.; Rick, J.W.; Joshi, R.S.; Beniwal, A.; Spatz, J.; Gill, S.; Chang, A.C.; Choudhary, N.; Nguyen, A.T.; Sudhir, S.; et al. Single-cell RNA sequencing and spatial transcriptomics reveal cancer-associated fibroblasts in glioblastoma with protumoral effects. J. Clin. Investig. 2023, 133, e147087. [Google Scholar] [CrossRef]
- Neftel, C.; Laffy, J.; Filbin, M.G.; Hara, T.; Shore, M.E.; Rahme, G.J.; Richman, A.R.; Silverbush, D.; Shaw, M.L.; Hebert, C.M.; et al. An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 2019, 178, 835–849.e21. [Google Scholar] [CrossRef]
- Cadwell, C.R.; Palasantza, A.; Jiang, X.; Berens, P.; Deng, Q.; Yilmaz, M.; Reimer, J.; Shen, S.; Bethge, M.; Tolias, K.F.; et al. Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat. Biotechnol. 2016, 34, 199–203. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhou, J.; Wang, W.; Wang, X.; Xu, B. An in silico investigation of Kv2.1 potassium channel: Model building and inhibitors binding sites analysis. Mol. Inform. 2023, 42, e202300072. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Chen, X.; Liu, F.; Cao, Y. Machine learning-based integration develops a neutrophil-derived signature for improving outcomes in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1216585. [Google Scholar] [CrossRef] [PubMed]
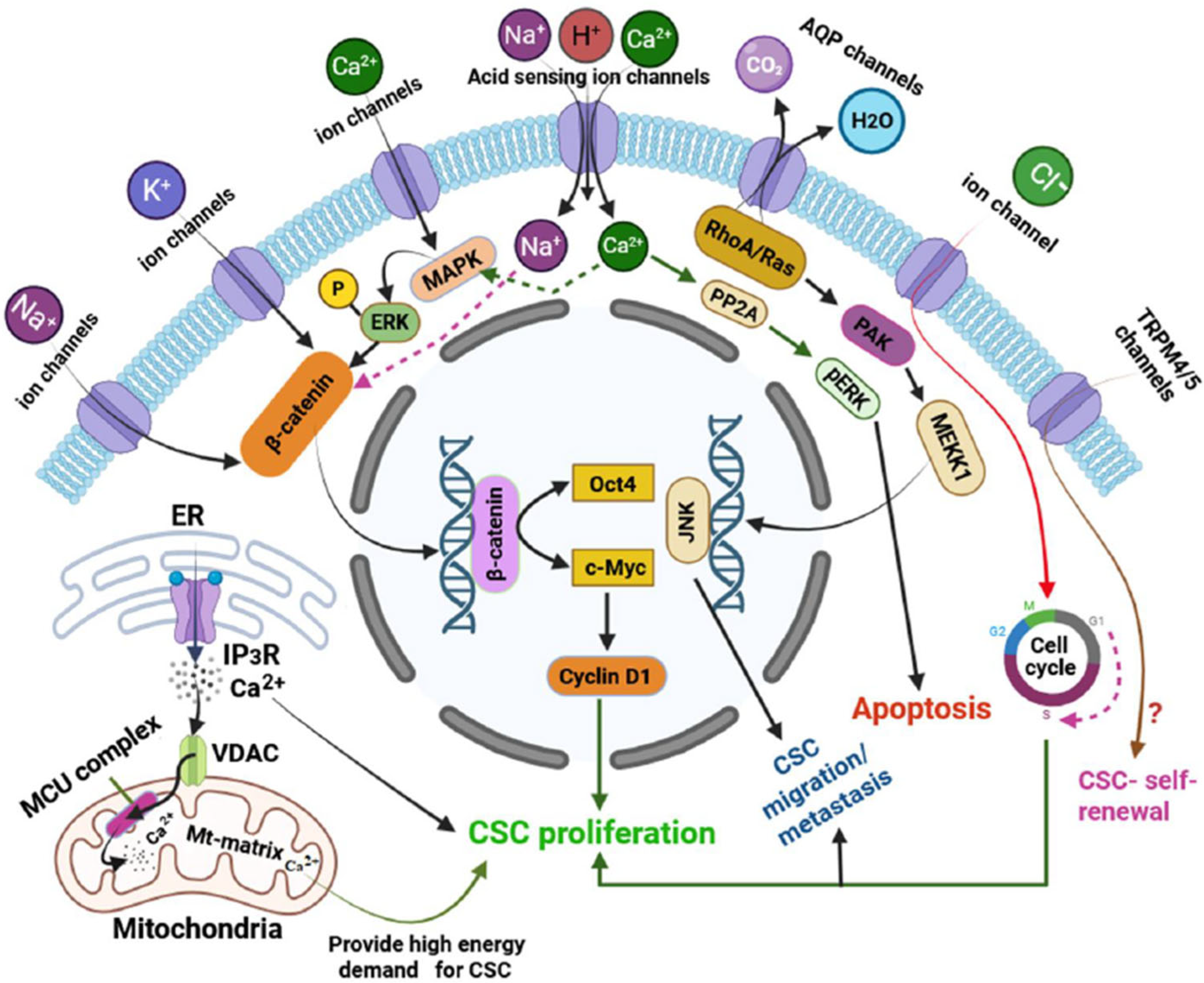
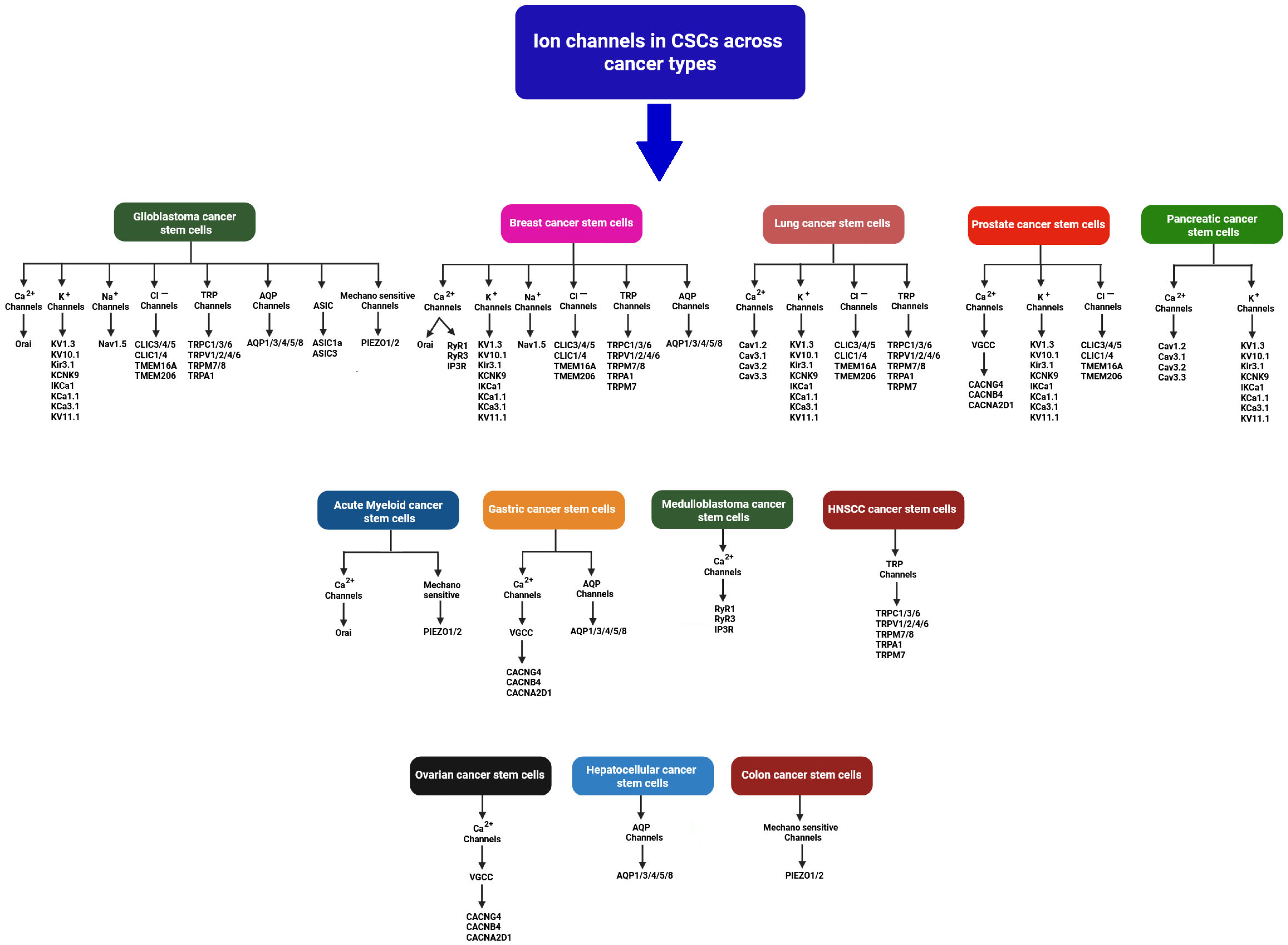
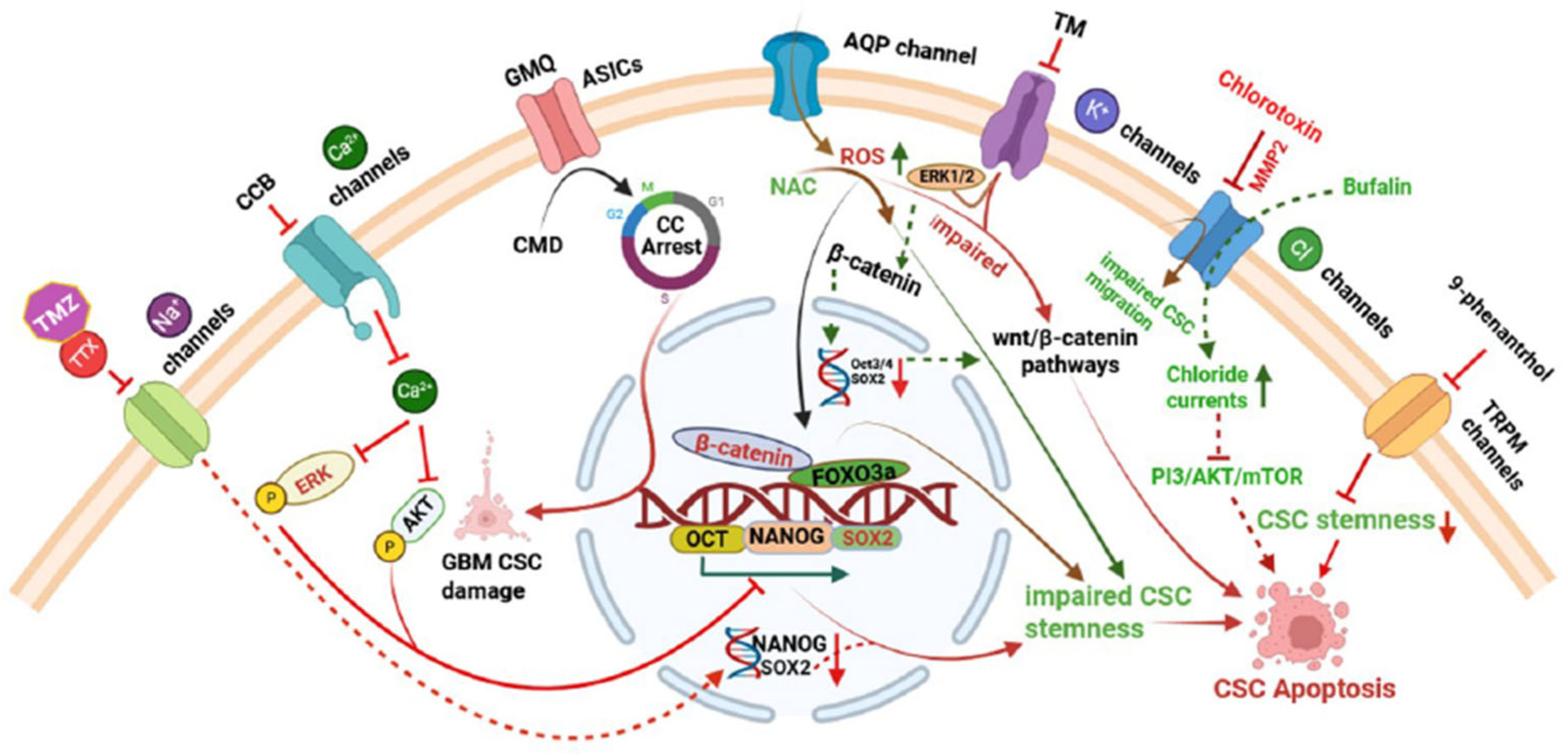
| Ion Channel Type | Specific Channels/Subtypes | Cancer Type/Model System | Transported Ion(s) | Functional Role in CSCs | Reference(s) |
|---|---|---|---|---|---|
| SOCE Channels | Orai channels | Breast (HER2+, TNBC), AML, OSCC, Glioblastoma | Ca2+ | Promote self-renewal, drug resistance, glycolysis, proliferation | [8,19,20,21,22,23,24,25] |
| SPCA2, SK3, Kv10.1 | Breast Cancer | Ca2+, K+ | Store-independent Ca2+ entry, stemness support | [8,19] | |
| TRP Channels | TRPC1/3/6, TRPV1/2/4/6, TRPM7/8, TRPA1 | Glioblastoma, TNBC, Breast, NSCLC, Bladder, HNSCC, Neuroblastoma | Ca2+, Na+, Mg2+ | Control proliferation, chemoresistance, migration, metastasis | [26,27,28,29,30,31,32,33,34,35] |
| TRPM7 | Lung, GBM, HNSCC | Ca2+, Mg2+ | Enhances stemness, EMT, invasion, survival | [28,29,33,34,35] | |
| VGCCs | CACNG4, CACNA2D1, CACNB4 | Gastric, HCC, Ovarian | Ca2+ | Mediate proliferation, drug resistance, survival | [36,37,38] |
| Cav1.2, Cav3.2, Cav3.1, Cav3.3 | Prostate, Breast, NSCLC, Pancreatic | Ca2+ | EMT, SNAIL, HIF-1α, RhoA, TGFβ, VEGF, PI3K/AKT/mTOR | [25,26,27,36,39] | |
| Calcium Release Channels (CRCs) | RyR1, RyR3, IP3Rs | Breast, Medulloblastoma, Melanoma | Ca2+ | ER Ca2+ release, CSC enrichment, stemness marker regulation (Nanog, Oct4, CD133, PROM1) | [3] |
| Mechanosensitive Channels | PIEZO1, PIEZO2 | Colon, AML, GBM, Breast | Ca2+ | Regulate stemness, adhesion dynamics, tumorigenesis, Ca2+ influx, EMT, immune evasion | [39,40,41,42,43] |
| Chloride Channels | TMEM16A, Bestrophin-1, ClC-2/3/4, TMEM206, CLIC1/4 | Lung, Breast, CRC, Glioma, HCC, Prostate | Cl− | EGFR, MAPK, PI3K/AKT, Wnt/β-catenin, oxidative stress, pH regulation, proliferation | [11,44,45,46,47,48,49,50] |
| Potassium Channels | Kv1.3, Kv10.1, Kv11.1, Kir3.1, KCNK9, IKCa1, KCa1.1, KCa3.1 | Prostate, Colon, Lung, Breast, Pancreatic, GBM | K+ | Regulate membrane potential, proliferation, EMT, ERK, JAK/STAT3, hypoxia | [51,52,53,54,55,56] |
| Sodium Channels (VGSCs) | Nav1.5 | GBM, TNBC | Na+ | Membrane potential, ERK activation, c-Myc, temozolomide resistance | [57,58,59] |
| ASICs | ASIC1a, ASIC3 | GBM | H+ (protons) | pH sensing, necroptosis, acidic tumour suppression | [60,61,62] |
| Aquaporins (AQPs) | AQP1, AQP3, AQP4, AQP5, AQP8 | Glioma, Breast, HCC, Cervical, Gastric | H2O, Glycerol, H2O2 | EMT, JAK/STAT3, CD133, autophagy, TRIM21, Wnt/β-catenin | [63,64,65,66,67,68,69,70,71,72] |
| Ion Channel Type | Channel Name/Subtype | Ion Transported | Inhibitor/Drug/Compound | CSC Type/Cancer | Effect on CSCs | Clinical Status | Reference |
|---|---|---|---|---|---|---|---|
| Voltage-Gated Ca2+ Channels (VGCCs) | L- and T-type (e.g., Cav1.2, Cav3.2) | Ca2+ | Manidipine, Lacidipine | Ovarian CSCs | ↓ Sphere formation, proliferation, stemness; apoptosis; inhibits AKT/ERK | FDA-approved (antihypertensives); preclinical for CSCs | [98] |
| Voltage-Gated Ca2+ Channel (T-type) | Cav3.2 | Ca2+ | Mibefradil | Glioblastoma stem-like cells (GSCs) | ↓ Proliferation, survival, stemness; sensitizes to temozolomide; suppresses AKT/mTOR, activates BAX | Withdrawn FDA drug; preclinical in GBM CSCs | [99] |
| Store-Operated Ca2+ Entry (SOCE) | Orai1/STIM1 | Ca2+ | SOCE inhibitors | Glioblastoma CSCs | ↓ Proliferation, self-renewal, SOX2 expression | Experimental compounds; preclinical | [26] |
| Ca2+ Channels (non-specific) | — | Ca2+ | Verapamil | Pancreatic CSCs | Targets MDR proteins; ↓ proliferation; ↑ apoptosis in gemcitabine-resistant CSCs | FDA-approved (cardiac); preclinical in CSCs | [100] |
| Ca2+-Activated K+ Channel | KCa3.1 | K+ | TRAM-34 + Temozolomide | GBM CSCs | ↓ DNA synthesis, CSC survival, tumour infiltration | TRAM-34 preclinical; Temozolomide FDA-approved | [101,102] |
| TRP Channel | TRPC6 | Ca2+/Na+ | TRPC6 inhibitors | Triple-negative breast CSCs | Disrupts integrin α6 splicing; sensitizes to chemotherapy | Preclinical | [103] |
| TRP Channel | TRPM7 | Ca2+/Mg2+ | Waixenicin A | Glioma CSCs | ↓ CSC maintenance; limited use due to non-specificity | Marine natural product; preclinical | [33] |
| VGCC Subunit | CACNG4 | Ca2+ | Amlodipine | Hepatocellular carcinoma CSCs | ↓ Stemness characteristics | FDA-approved (antihypertensive); preclinical in CSCs | [36] |
| Voltage-Gated K+ Channel | Kv1.3, others | K+ | Margatoxin (MgTX), 4-AP | Prostate and lung CSCs | ↓ Metastasis, ↑ apoptosis, G1-S arrest, ↓ lung CSC growth | MgTX preclinical; 4-AP FDA-approved (MS) | [104] |
| Chloride Channels | CLIC1, CLCN3 | Cl− | DIDS, Metformin, Q48, Q54 | Glioblastoma CSCs | ↑ Apoptosis, ↓ proliferation, invasion, self-renewal; overcome BCNU resistance | Metformin FDA-approved (T2D); others preclinical | [48,105] |
| Acid-Sensing Ion Channel | ASIC1a | H+ | PcTx1 | Glioblastoma CSCs | Induces necroptosis via RIPK1 | Preclinical peptide toxin | [60] |
| Acid-Sensing Ion Channel | ASIC3 | H+ | ASIC3 inhibitors | Glioblastoma CSCs | ↓ Proliferation, migration, tumour growth | Preclinical | [73] |
| Acid-Sensing Ion Channel | ASIC1a | H+ | — | Breast, prostate, pancreatic CSCs | Promotes ROS, EMT via RhoA; activates AKT/NF-κB | Mechanistic insight; no inhibitor used | [106] |
| Aquaporin Water Channel | AQP3 | H2O, Glycerol | AQP3 inhibition | Gastric CSCs | ↓ Self-renewal; blocks Wnt/GSK3β/β-catenin pathway | Preclinical | [107] |
| Aquaporin Water Channel | AQP5 | H2O | AQP5 inhibition | Gastric CSCs | ↓ Autophagy, ↓ stemness | Preclinical | [108] |
| Aquaporin Water Channel | AQP9 | H2O | AQP9 restoration | Liver CSCs | ↑ ROS, ↓ β-catenin activity; interacts with FOXO3a; ↓ stemness | Preclinical | [80] |
| Mixed Ion Channels | ENaC (Na+), GABA (Cl−), iGluRs (Na+, Ca2+) | Na+, Cl−, Ca2+ | TTX, TEA, 4-AP, CPP, CNQX, ω-Conotoxin MVIIC, CdCl2 | Glioblastoma CSCs | ↓ CSC viability; targets enriched ion channel families | TTX, CNQX, ω-CTX, CPP are preclinical; 4-AP FDA-a | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samanta, K.; Reddy, G.S.V.S.R.; Sharma, N.K.; Kar, P. Deciphering the Role of Functional Ion Channels in Cancer Stem Cells (CSCs) and Their Therapeutic Implications. Int. J. Mol. Sci. 2025, 26, 7595. https://doi.org/10.3390/ijms26157595
Samanta K, Reddy GSVSR, Sharma NK, Kar P. Deciphering the Role of Functional Ion Channels in Cancer Stem Cells (CSCs) and Their Therapeutic Implications. International Journal of Molecular Sciences. 2025; 26(15):7595. https://doi.org/10.3390/ijms26157595
Chicago/Turabian StyleSamanta, Krishna, Gali Sri Venkata Sai Rishma Reddy, Neeraj Kumar Sharma, and Pulak Kar. 2025. "Deciphering the Role of Functional Ion Channels in Cancer Stem Cells (CSCs) and Their Therapeutic Implications" International Journal of Molecular Sciences 26, no. 15: 7595. https://doi.org/10.3390/ijms26157595
APA StyleSamanta, K., Reddy, G. S. V. S. R., Sharma, N. K., & Kar, P. (2025). Deciphering the Role of Functional Ion Channels in Cancer Stem Cells (CSCs) and Their Therapeutic Implications. International Journal of Molecular Sciences, 26(15), 7595. https://doi.org/10.3390/ijms26157595






