Curcumin as a Dual Modulator of Pyroptosis: Mechanistic Insights and Therapeutic Potential
Abstract
1. Introduction
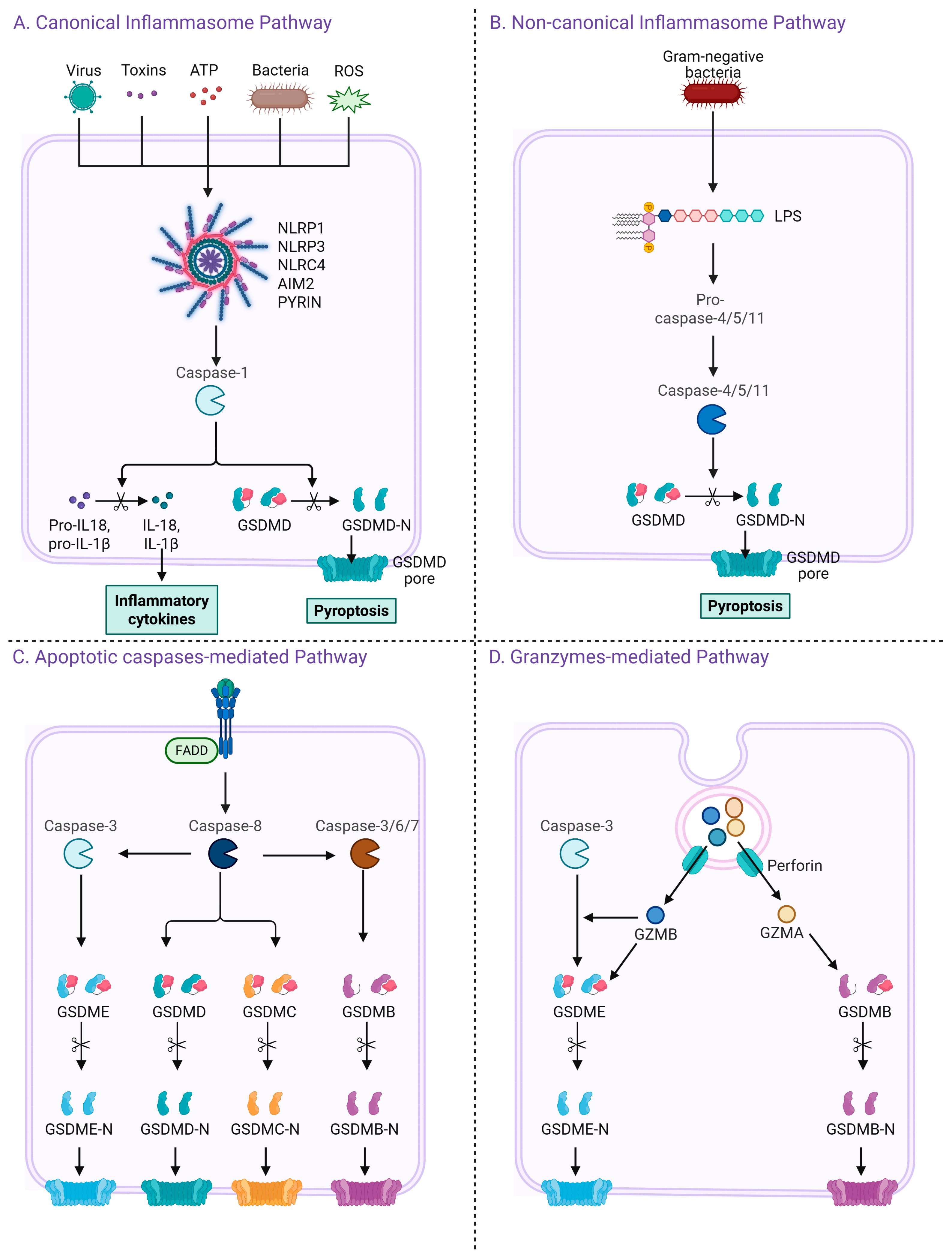
2. Pyroptosis Signaling Pathways
2.1. Canonical Inflammasome Pathway
2.2. Non-Canonical Inflammasome Pathway
2.3. Apoptotic Caspases-Mediated Pathway
2.4. Granzymes-Mediated Pathway
3. Pyroptosis as a Therapeutic Strategy in Cancer: Mechanisms, Targets, and Context-Dependent Roles Across Tumor Types
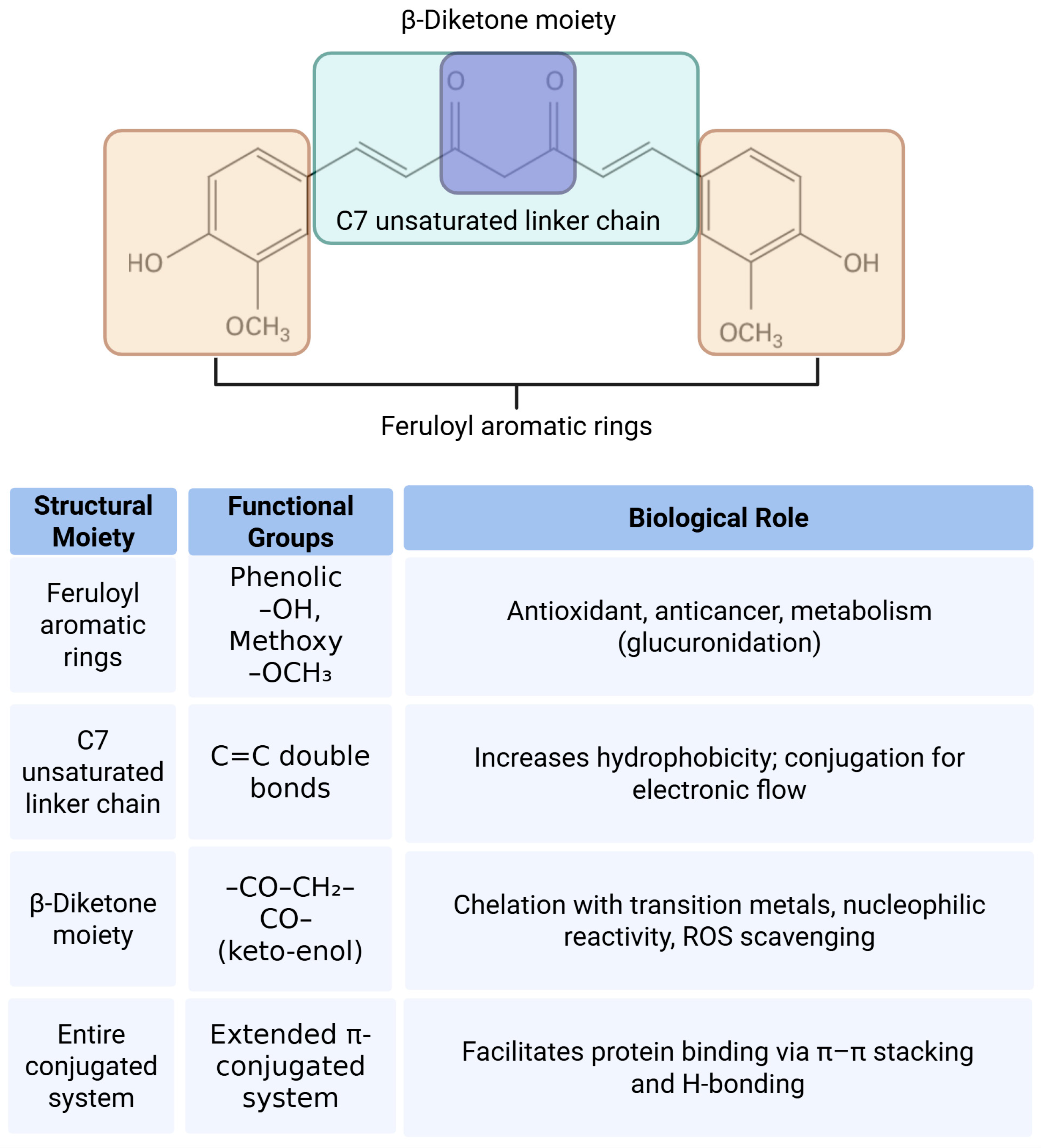
4. Pharmacological Potential and Structural Features of Curcumin
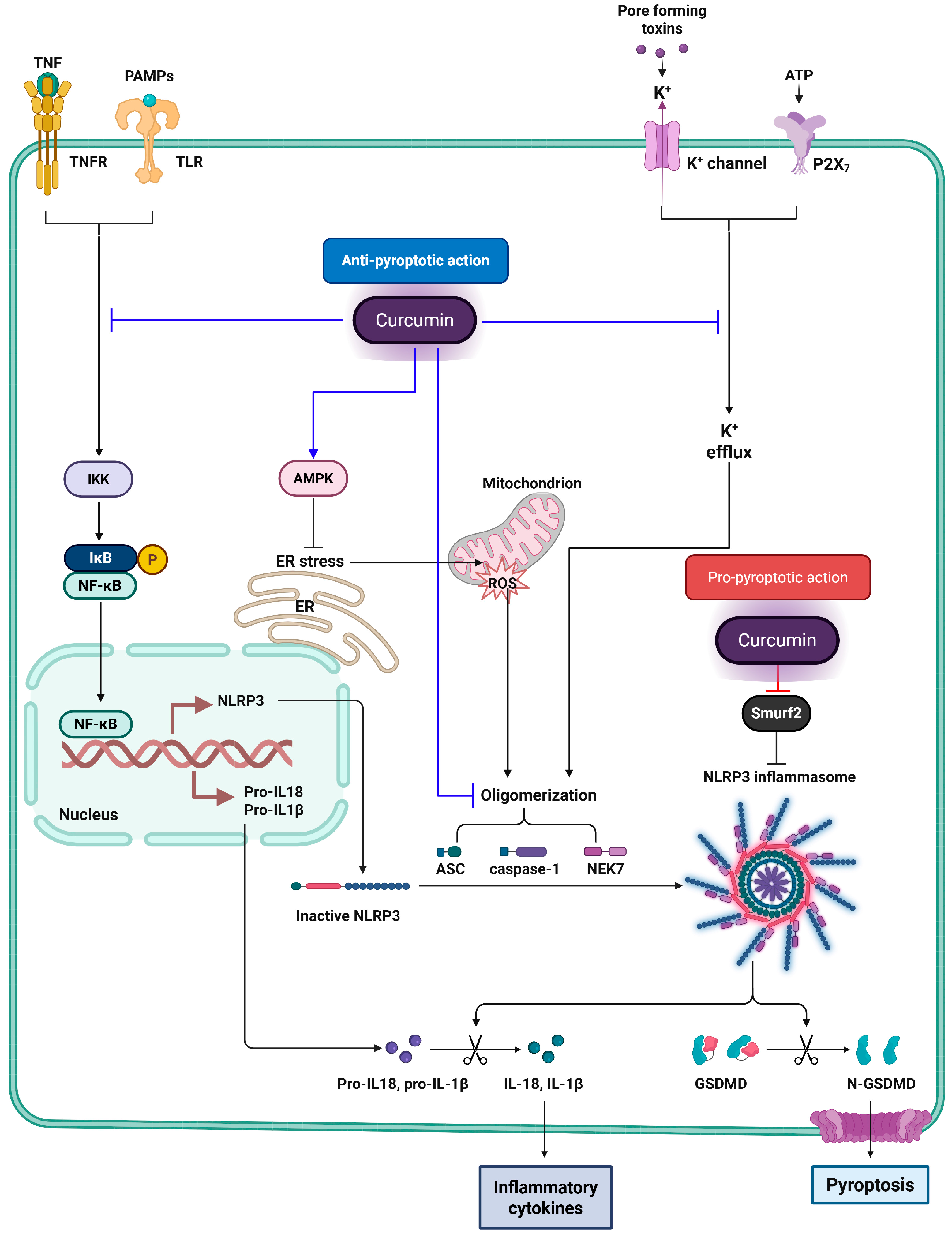
5. Inhibition of Inflammatory Pyroptosis by Curcumin: Molecular Mechanisms and Cellular Targets
6. Curcumin-Induced Pyroptosis: Molecular Mechanisms and Therapeutic Strategies in Cancer
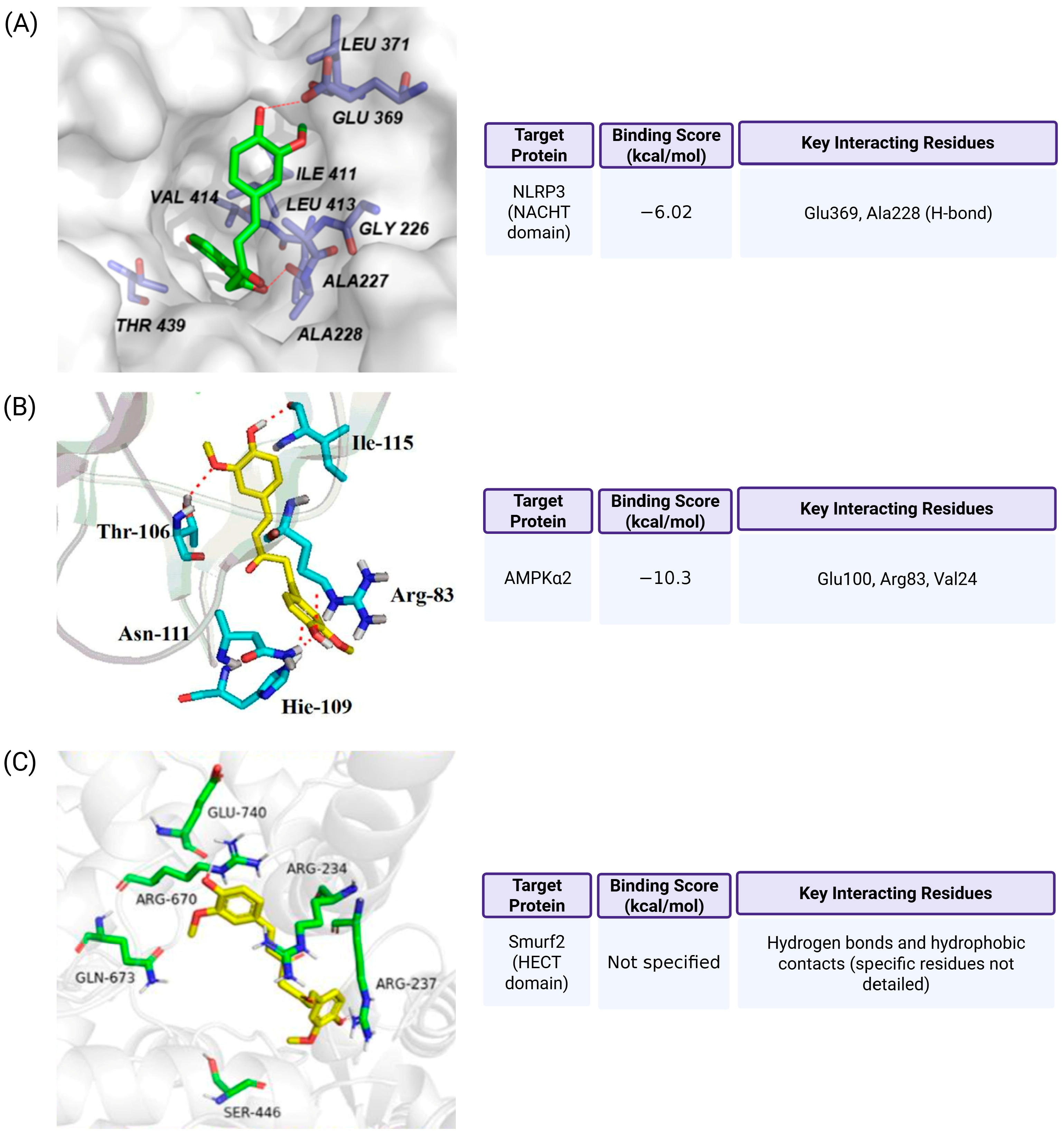
7. Molecular Docking Evidence for Curcumin Binding to Pyroptosis-Associated Targets
8. Dual Regulatory Roles of Curcumin in Pyroptosis: A Context-Dependent Mechanistic Perspective
9. Conclusions
Funding
Conflicts of Interest
References
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Wei, X.; Xie, F.; Zhou, X.; Wu, Y.; Yan, H.; Liu, T.; Huang, J.; Wang, F.; Zhou, F.; Zhang, L. Role of pyroptosis in inflammation and cancer. Cell. Mol. Immunol. 2022, 19, 971–992. [Google Scholar] [CrossRef] [PubMed]
- Burdette, B.E.; Esparza, A.N.; Zhu, H.; Wang, S. Gasdermin D in pyroptosis. Acta Pharm. Sin. B 2021, 11, 2768–2782. [Google Scholar] [CrossRef]
- Sharma, A.K.; Ismail, N. Non-Canonical Inflammasome Pathway: The Role of Cell Death and Inflammation in Ehrlichiosis. Cells 2023, 12, 2597. [Google Scholar] [CrossRef]
- Rao, Z.; Zhu, Y.; Yang, P.; Chen, Z.; Xia, Y.; Qiao, C.; Liu, W.; Deng, H.; Li, J.; Ning, P.; et al. Pyroptosis in inflammatory diseases and cancer. Theranostics 2022, 12, 4310–4329. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, J.W.; Huang, J.; Tang, L.; Xu, Y.H.; Sun, H.; Tang, J.; Wang, G. Pyroptosis, a target for cancer treatment? Apoptosis 2022, 27, 1–13. [Google Scholar] [CrossRef]
- Lu, X.; Guo, T.; Zhang, X. Pyroptosis in Cancer: Friend or Foe? Cancers 2021, 13, 3620. [Google Scholar] [CrossRef]
- Tan, G.; Huang, C.; Chen, J.; Zhi, F. HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway. J. Hematol. Oncol. 2020, 13, 149. [Google Scholar] [CrossRef]
- Zolondick, A.A.; Gaudino, G.; Xue, J.; Pass, H.I.; Carbone, M.; Yang, H. Asbestos-induced chronic inflammation in malignant pleural mesothelioma and related therapeutic approaches-a narrative review. Precis. Cancer Med. 2021, 4, 27. [Google Scholar] [CrossRef]
- Du, T.; Gao, J.; Li, P.; Wang, Y.; Qi, Q.; Liu, X.; Li, J.; Wang, C.; Du, L. Pyroptosis, metabolism, and tumor immune microenvironment. Clin. Transl. Med. 2021, 11, e492. [Google Scholar] [CrossRef]
- Ben-Sasson, S.Z.; Hogg, A.; Hu-Li, J.; Wingfield, P.; Chen, X.; Crank, M.; Caucheteux, S.; Ratner-Hurevich, M.; Berzofsky, J.A.; Nir-Paz, R.; et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J. Exp. Med. 2013, 210, 491–502. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Pancholi, V.; Smina, T.P.; Kunnumakkara, A.B.; Maliakel, B.; Krishnakumar, I.M. Safety assessment of a highly bioavailable curcumin-galactomannoside complex (CurQfen) in healthy volunteers, with a special reference to the recent hepatotoxic reports of curcumin supplements: A 90-days prospective study. Toxicol. Rep. 2021, 8, 1255–1264. [Google Scholar] [CrossRef]
- Zeng, L.; Yang, T.; Yang, K.; Yu, G.; Li, J.; Xiang, W.; Chen, H. Efficacy and Safety of Curcumin and Curcuma longa Extract in the Treatment of Arthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Front. Immunol. 2022, 13, 891822. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; He, L.; Liu, L.; Cheng, B.; Zhou, F.; Cao, D.; He, Y. A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health. Molecules 2022, 27, 4400. [Google Scholar] [CrossRef]
- Benameur, T.; Frota Gaban, S.V.; Giacomucci, G.; Filannino, F.M.; Trotta, T.; Polito, R.; Messina, G.; Porro, C.; Panaro, M.A. The Effects of Curcumin on Inflammasome: Latest Update. Molecules 2023, 28, 742. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhou, J.; Li, H.; Gao, Y.; Xu, C.; Zhao, S.; Chen, Y.; Cai, W.; Wu, J. Curcumin suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Mol. Nutr. Food Res. 2015, 59, 2132–2142. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xing, Z.; Wang, J.; Guo, Y.; Wu, X.; Ma, Y.; Xu, Z.; Kuang, Y.; Liao, T.; Li, C. Hyaluronic acid-mediated targeted nano-modulators for activation of pyroptosis for cancer therapy through multichannel regulation of Ca2+ overload. Int. J. Biol. Macromol. 2025, 299, 140116. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.F.; Gong, Y.X.; Li, H.F.; Sun, F.L.; Li, W.L.; Chen, D.Q.; Xie, D.P.; Ren, C.X.; Guo, X.Y.; Wang, Z.Y.; et al. Curcumin Activates ROS Signaling to Promote Pyroptosis in Hepatocellular Carcinoma HepG2 Cells. In Vivo 2021, 35, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef]
- Wu, J.; Fernandes-Alnemri, T.; Alnemri, E.S. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J. Clin. Immunol. 2010, 30, 693–702. [Google Scholar] [CrossRef]
- Xu, H.; Yang, J.; Gao, W.; Li, L.; Li, P.; Zhang, L.; Gong, Y.N.; Peng, X.; Xi, J.J.; Chen, S.; et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 2014, 513, 237–241. [Google Scholar] [CrossRef]
- Ting, J.P.; Lovering, R.C.; Alnemri, E.S.; Bertin, J.; Boss, J.M.; Davis, B.K.; Flavell, R.A.; Girardin, S.E.; Godzik, A.; Harton, J.A.; et al. The NLR gene family: A standard nomenclature. Immunity 2008, 28, 285–287. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Sandstrom, A.; Vance, R.E. The NLRP1 inflammasome: New mechanistic insights and unresolved mysteries. Curr. Opin. Immunol. 2019, 60, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.I.; Sutterwala, F.S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol. Rev. 2015, 265, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Kahlenberg, J.M.; Dubyak, G.R. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am. J. Physiol. Cell Physiol. 2004, 286, C1100–C1108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, J.; Shi, J.; Gong, Y.N.; Lu, Q.; Xu, H.; Liu, L.; Shao, F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 2011, 477, 596–600. [Google Scholar] [CrossRef]
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009, 458, 514–518. [Google Scholar] [CrossRef]
- Miao, E.A.; Leaf, I.A.; Treuting, P.M.; Mao, D.P.; Dors, M.; Sarkar, A.; Warren, S.E.; Wewers, M.D.; Aderem, A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010, 11, 1136–1142. [Google Scholar] [CrossRef]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Vande Walle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef]
- Man, S.M.; Kanneganti, T.D. Regulation of inflammasome activation. Immunol. Rev. 2015, 265, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Rühl, S.; Broz, P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur. J. Immunol. 2015, 45, 2927–2936. [Google Scholar] [CrossRef]
- Yang, D.; He, Y.; Muñoz-Planillo, R.; Liu, Q.; Núñez, G. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity 2015, 43, 923–932. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Sarhan, J.; Liu, B.C.; Muendlein, H.I.; Li, P.; Nilson, R.; Tang, A.Y.; Rongvaux, A.; Bunnell, S.C.; Shao, F.; Green, D.R.; et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl. Acad. Sci. USA 2018, 115, E10888–E10897. [Google Scholar] [CrossRef] [PubMed]
- Orning, P.; Weng, D.; Starheim, K.; Ratner, D.; Best, Z.; Lee, B.; Brooks, A.; Xia, S.; Wu, H.; Kelliher, M.A.; et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 2018, 362, 1064–1069. [Google Scholar] [CrossRef]
- Zheng, Z.; Deng, W.; Bai, Y.; Miao, R.; Mei, S.; Zhang, Z.; Pan, Y.; Wang, Y.; Min, R.; Deng, F.; et al. The Lysosomal Rag-Ragulator Complex Licenses RIPK1 and Caspase-8-mediated Pyroptosis by Yersinia. Science 2021, 372, eabg0269. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhao, R.; Xia, W.; Chang, C.W.; You, Y.; Hsu, J.M.; Nie, L.; Chen, Y.; Wang, Y.C.; Liu, C.; et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 2020, 22, 1264–1275. [Google Scholar] [CrossRef]
- Chao, K.L.; Kulakova, L.; Herzberg, O. Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proc. Natl. Acad. Sci. USA 2017, 114, E1128–E1137. [Google Scholar] [CrossRef]
- Chowdhury, D.; Lieberman, J. Death by a thousand cuts: Granzyme pathways of programmed cell death. Annu. Rev. Immunol. 2008, 26, 389–420. [Google Scholar] [CrossRef]
- Joeckel, L.T.; Bird, P.I. Are all granzymes cytotoxic in vivo? Biol. Chem. 2014, 395, 181–202. [Google Scholar] [CrossRef]
- Wensink, A.C.; Hack, C.E.; Bovenschen, N. Granzymes regulate proinflammatory cytokine responses. J. Immunol. 2015, 194, 491–497. [Google Scholar] [CrossRef]
- van Daalen, K.R.; Reijneveld, J.F.; Bovenschen, N. Modulation of Inflammation by Extracellular Granzyme A. Front. Immunol. 2020, 11, 931. [Google Scholar] [CrossRef]
- Zhou, Z.; He, H.; Wang, K.; Shi, X.; Wang, Y.; Su, Y.; Wang, Y.; Li, D.; Liu, W.; Zhang, Y.; et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 2020, 368, eaaz7548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Xia, S.; Kong, Q.; Li, S.; Liu, X.; Junqueira, C.; Meza-Sosa, K.F.; Mok, T.M.Y.; Ansara, J.; et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 2020, 579, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.W.M.; Zhang, X.; Wang, C.; Yang, Y.; Kang, W.Y.; Arnold, S.; Higashi, R.M.; Liu, J.; Lane, A.N. Exosomal lipids for classifying early and late stage non-small cell lung cancer. Anal. Chim. Acta 2018, 1037, 256–264. [Google Scholar] [CrossRef]
- Schwartz, A.G.; Cote, M.L. Epidemiology of Lung Cancer. Adv. Exp. Med. Biol. 2016, 893, 21–41. [Google Scholar] [PubMed]
- Teng, J.F.; Mei, Q.B.; Zhou, X.G.; Tang, Y.; Xiong, R.; Qiu, W.Q.; Pan, R.; Law, B.Y.; Wong, V.K.; Yu, C.L.; et al. Polyphyllin VI Induces Caspase-1-Mediated Pyroptosis via the Induction of ROS/NF-κB/NLRP3/GSDMD Signal Axis in Non-Small Cell Lung Cancer. Cancers 2020, 12, 193. [Google Scholar] [CrossRef]
- Wang, F.; Liu, W.; Ning, J.; Wang, J.; Lang, Y.; Jin, X.; Zhu, K.; Wang, X.; Li, X.; Yang, F.; et al. Simvastatin Suppresses Proliferation and Migration in Non-small Cell Lung Cancer via Pyroptosis. Int. J. Biol. Sci. 2018, 14, 406–417. [Google Scholar] [CrossRef]
- Yuan, R.; Zhao, W.; Wang, Q.Q.; He, J.; Han, S.; Gao, H.; Feng, Y.; Yang, S. Cucurbitacin B inhibits non-small cell lung cancer in vivo and in vitro by triggering TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharmacol. Res. 2021, 170, 105748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; He, Q. Distinct characteristics of dasatinib-induced pyroptosis in gasdermin E-expressing human lung cancer A549 cells and neuroblastoma SH-SY5Y cells. Oncol. Lett. 2020, 20, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Gao, J.; Wan, B.; Zhan, P.; Xu, W.; Lv, T.; Song, Y. GSDMD is required for effector CD8(+) T cell responses to lung cancer cells. Int. Immunopharmacol. 2019, 74, 105713. [Google Scholar] [CrossRef]
- Zheng, Z.; Bian, Y.; Zhang, Y.; Ren, G.; Li, G. Metformin activates AMPK/SIRT1/NF-κB pathway and induces mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer cell pyroptosis. Cell Cycle 2020, 19, 1089–1104. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Dong, Z.; Zhao, Y.; Deng, H.; Wu, J.; Wu, X.; Li, W. Piperlongumine analogue L50377 induces pyroptosis via ROS mediated NF-κB suppression in non-small-cell lung cancer. Chem. Biol. Interact. 2019, 313, 108820. [Google Scholar] [CrossRef]
- Chen, L.; Li, Q.; Zheng, Z.; Xie, J.; Lin, X.; Jiang, C.; Xu, H.; Wu, X.; Wu, J.; Zhang, H. Design and optimize N-substituted EF24 as effective and low toxicity NF-κB inhibitor for lung cancer therapy via apoptosis-to-pyroptosis switch. Chem. Biol. Drug Des. 2019, 94, 1368–1377. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, C.; Qing, Y.; Cheng, Y.; Jiang, X.; Li, M.; Yang, Z.; Wang, D. Genistein induces apoptosis by stabilizing intracellular p53 protein through an APE1-mediated pathway. Free Radic. Biol. Med. 2015, 86, 209–218. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.; Lin, Z.; Li, C.; Wang, Y.; Yang, L.; Liu, G. Reduced apurinic/apyrimidinic endonuclease activity enhances the antitumor activity of oxymatrine in lung cancer cells. Int. J. Oncol. 2016, 49, 2331–2340. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Li, W.; Zheng, X.; Ren, L.; Liu, J.; Li, S.; Wang, J.; Du, G. MELK is an oncogenic kinase essential for metastasis, mitotic progression, and programmed death in lung carcinoma. Signal Transduct. Target. Ther. 2020, 5, 279. [Google Scholar] [CrossRef]
- Inoue, H.; Kato, T.; Olugbile, S.; Tamura, K.; Chung, S.; Miyamoto, T.; Matsuo, Y.; Salgia, R.; Nakamura, Y.; Park, J.H. Effective growth-suppressive activity of maternal embryonic leucine-zipper kinase (MELK) inhibitor against small cell lung cancer. Oncotarget 2016, 7, 13621–13633. [Google Scholar] [CrossRef]
- Huang, J.; Fan, P.; Liu, M.; Weng, C.; Fan, G.; Zhang, T.; Duan, X.; Wu, Y.; Tang, L.; Yang, G.; et al. Famotidine promotes inflammation by triggering cell pyroptosis in gastric cancer cells. BMC Pharmacol. Toxicol. 2021, 22, 62. [Google Scholar] [CrossRef]
- Xia, Y.; Jin, Y.; Cui, D.; Wu, X.; Song, C.; Jin, W.; Huang, H. Antitumor Effect of Simvastatin in Combination With DNA Methyltransferase Inhibitor on Gastric Cancer via GSDME-Mediated Pyroptosis. Front. Pharmacol. 2022, 13, 860546. [Google Scholar] [CrossRef]
- Zhang, F.; Yin, Y.; Xu, W.; Song, Y.; Zhou, Z.; Sun, X.; Li, P. Icariin inhibits gastric cancer cell growth by regulating the hsa_circ_0003159/miR-223-3p/NLRP3 signaling axis. Hum. Exp. Toxicol. 2022, 41, 9603271221097363. [Google Scholar] [CrossRef]
- Li, C.; Qiu, J.; Xue, Y. Low-dose Diosbulbin-B (DB) activates tumor-intrinsic PD-L1/NLRP3 signaling pathway mediated pyroptotic cell death to increase cisplatin-sensitivity in gastric cancer (GC). Cell Biosci. 2021, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.Y.; Zhu, M.X.; Zhang, P.F.; Huang, X.Y.; Wan, J.K.; Yao, X.Z.; Hu, Z.T.; Chai, X.Q.; Peng, R.; Yang, X.; et al. PKCα/ZFP64/CSF1 axis resets the tumor microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma. J. Hepatol. 2022, 77, 163–176. [Google Scholar] [CrossRef]
- Hu, K.; Xu, Z.; Yao, L.; Yan, Y.; Zhou, L.; Li, J. Integrated analysis of expression, prognostic value and immune infiltration of GSDMs in hepatocellular carcinoma. Aging 2021, 13, 24117–24135. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Hu, Y.; Dong, S. Pan-cancer analysis reveals the expression, genetic alteration and prognosis of pyroptosis key gene GSDMD. Int. Immunopharmacol. 2021, 101 Pt A, 108270. [Google Scholar] [CrossRef]
- Yan, Z.; Da, Q.; Li, Z.; Lin, Q.; Yi, J.; Su, Y.; Yu, G.; Ren, Q.; Liu, X.; Lin, Z.; et al. Inhibition of NEK7 Suppressed Hepatocellular Carcinoma Progression by Mediating Cancer Cell Pyroptosis. Front. Oncol. 2022, 12, 812655. [Google Scholar] [CrossRef]
- Chen, Y.F.; Qi, H.Y.; Wu, F.L. Euxanthone exhibits anti-proliferative and anti-invasive activities in hepatocellular carcinoma by inducing pyroptosis: Preliminary results. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8186–8196. [Google Scholar]
- Zhang, Y.; Yang, H.; Sun, M.; He, T.; Liu, Y.; Yang, X.; Shi, X.; Liu, X. Alpinumisoflavone suppresses hepatocellular carcinoma cell growth and metastasis via NLRP3 inflammasome-mediated pyroptosis. Pharmacol. Rep. 2020, 72, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, P.; An, L.; Sun, N.; Peng, L.; Tang, W.; Ma, D.; Chen, J. Miltirone induces cell death in hepatocellular carcinoma cell through GSDME-dependent pyroptosis. Acta Pharm. Sin. B 2020, 10, 1397–1413. [Google Scholar] [CrossRef]
- Shangguan, F.; Zhou, H.; Ma, N.; Wu, S.; Huang, H.; Jin, G.; Wu, S.; Hong, W.; Zhuang, W.; Xia, H.; et al. A Novel Mechanism of Cannabidiol in Suppressing Hepatocellular Carcinoma by Inducing GSDME Dependent Pyroptosis. Front. Cell Dev. Biol. 2021, 9, 697832. [Google Scholar] [CrossRef]
- Wei, Q.; Zhu, R.; Zhu, J.; Zhao, R.; Li, M. E2-Induced Activation of the NLRP3 Inflammasome Triggers Pyroptosis and Inhibits Autophagy in HCC Cells. Oncol. Res. 2019, 27, 827–834. [Google Scholar] [CrossRef]
- Chu, Q.; Jiang, Y.; Zhang, W.; Xu, C.; Du, W.; Tuguzbaeva, G.; Qin, Y.; Li, A.; Zhang, L.; Sun, G.; et al. Pyroptosis is involved in the pathogenesis of human hepatocellular carcinoma. Oncotarget 2016, 7, 84658–84665. [Google Scholar] [CrossRef]
- Luo, J.; Lai, J. Pyroptosis-related molecular classification and immune microenvironment infiltration in breast cancer: A novel therapeutic target. J. Cell. Mol. Med. 2022, 26, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Y.; Kong, B.; Zhang, G.; Zhang, Q. Polydatin down-regulates the phosphorylation level of STAT3 and induces pyroptosis in triple-negative breast cancer mice with a high-fat diet. Ann. Transl. Med. 2022, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Luo, B.; Wu, X.; Guan, F.; Yu, X.; Zhao, L.; Ke, X.; Wu, J.; Yuan, J. Cisplatin Induces Pyroptosis via Activation of MEG3/NLRP3/caspase-1/GSDMD Pathway in Triple-Negative Breast Cancer. Int. J. Biol. Sci. 2021, 17, 2606–2621. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Li, A.; Huang, W.; Chen, S.; Han, F.; Wang, L. Dihydroartemisinin induces pyroptosis by promoting the AIM2/caspase-3/DFNA5 axis in breast cancer cells. Chem. Biol. Interact. 2021, 340, 109434. [Google Scholar] [CrossRef]
- Wang, J.G.; Jian, W.J.; Li, Y.; Zhang, J. Nobiletin promotes the pyroptosis of breast cancer via regulation of miR-200b/JAZF1 axis. Kaohsiung J. Med. Sci. 2021, 37, 572–582. [Google Scholar] [CrossRef]
- An, H.; Heo, J.S.; Kim, P.; Lian, Z.; Lee, S.; Park, J.; Hong, E.; Pang, K.; Park, Y.; Ooshima, A.; et al. Tetraarsenic hexoxide enhances generation of mitochondrial ROS to promote pyroptosis by inducing the activation of caspase-3/GSDME in triple-negative breast cancer cells. Cell Death Dis. 2021, 12, 159. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y.; Ma, X.F.; Hou, D.; Zhang, Y.H.; Sun, Y.; Shi, S.S.; Forouzanfar, T.; Lin, H.Y.; Fan, J.; et al. Triclabendazole Induces Pyroptosis by Activating Caspase-3 to Cleave GSDME in Breast Cancer Cells. Front. Pharmacol. 2021, 12, 670081. [Google Scholar] [CrossRef]
- Tian, W.; Wang, Z.; Tang, N.N.; Li, J.T.; Liu, Y.; Chu, W.F.; Yang, B.F. Ascorbic Acid Sensitizes Colorectal Carcinoma to the Cytotoxicity of Arsenic Trioxide via Promoting Reactive Oxygen Species-Dependent Apoptosis and Pyroptosis. Front. Pharmacol. 2020, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, B.; Sun, J.; Na, H.; Chen, Z.; Zhu, Z.; Yan, L.; Ren, S.; Zuo, Y. DAC can restore expression of NALP1 to suppress tumor growth in colon cancer. Cell Death Dis. 2015, 6, e1602. [Google Scholar] [CrossRef][Green Version]
- Lv, L.V.; Zhou, J.; Lin, C.; Hu, G.; Yi, L.U.; Du, J.; Gao, K.; Li, X. DNA methylation is involved in the aberrant expression of miR-133b in colorectal cancer cells. Oncol. Lett. 2015, 10, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Ji, X.; Zhang, Q.; Wei, Y. Curcumin combined with photodynamic therapy, promising therapies for the treatment of cancer. Biomed. Pharmacother. 2022, 146, 112567. [Google Scholar] [CrossRef]
- Sandur, S.K.; Ichikawa, H.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Sethi, G.; Aggarwal, B.B. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radic. Biol. Med. 2007, 43, 568–580. [Google Scholar] [CrossRef]
- Doello, K.; Ortiz, R.; Alvarez, P.J.; Melguizo, C.; Cabeza, L.; Prados, J. Latest in Vitro and in Vivo Assay, Clinical Trials and Patents in Cancer Treatment using Curcumin: A Literature Review. Nutr. Cancer 2018, 70, 569–578. [Google Scholar] [CrossRef]
- Xue, B.; Huang, J.; Zhang, H.; Li, B.; Xu, M.; Zhang, Y.; Xie, M.; Li, X. Micronized curcumin fabricated by supercritical CO2 to improve antibacterial activity against Pseudomonas aeruginosa. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1135–1143. [Google Scholar] [CrossRef]
- Kamat, A.M.; Sethi, G.; Aggarwal, B.B. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-kappaB and nuclear factor-kappaB-regulated gene products in IFN-alpha-sensitive and IFN-alpha-resistant human bladder cancer cells. Mol. Cancer Ther. 2007, 6, 1022–1030. [Google Scholar] [CrossRef]
- Zhu, G.H.; Dai, H.P.; Shen, Q.; Ji, O.; Zhang, Q.; Zhai, Y.L. Curcumin induces apoptosis and suppresses invasion through MAPK and MMP signaling in human monocytic leukemia SHI-1 cells. Pharm. Biol. 2016, 54, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, W.; Han, N.; Zou, Y.; Yin, D. Curcumin inhibits proliferation, migration, invasion and promotes apoptosis of retinoblastoma cell lines through modulation of miR-99a and JAK/STAT pathway. BMC Cancer 2018, 18, 1230. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, X.; Tan, J.; Tian, R.; Shen, P.; Cai, W.; Liao, H. Curcumin suppresses the stemness of non-small cell lung cancer cells via promoting the nuclear-cytoplasm translocation of TAZ. Environ. Toxicol. 2021, 36, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Li, J.; Wang, W.; Pan, L.; Cheng, J.; Li, L.; Zhao, H.; Lin, W. Curcumin induces G0/G1 arrest and apoptosis in hormone independent prostate cancer DU-145 cells by down regulating Notch signaling. Biomed. Pharmacother. 2016, 84, 177–184. [Google Scholar] [CrossRef]
- Borges, G.A.; Elias, S.T.; Amorim, B.; de Lima, C.L.; Coletta, R.D.; Castilho, R.M.; Squarize, C.H.; Guerra, E.N.S. Curcumin downregulates the PI3K-AKT-mTOR pathway and inhibits growth and progression in head and neck cancer cells. Phytother. Res. 2020, 34, 3311–3324. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Chemical and structural features influencing the biological activity of curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Chen, B.; Li, H.; Ou, G.; Ren, L.; Yang, X.; Zeng, M. Curcumin attenuates MSU crystal-induced inflammation by inhibiting the degradation of IκBα and blocking mitochondrial damage. Arthritis Res. Ther. 2019, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Su, W.; Gao, F.; Ding, Z.; Yang, S.; Ye, L.; Chen, X.; Tian, G.; Xi, J.; Liu, Z. Curcumin Ameliorates White Matter Injury after Ischemic Stroke by Inhibiting Microglia/Macrophage Pyroptosis through NF-κB Suppression and NLRP3 Inflammasome Inhibition. Oxidative Med. Cell. Longev. 2021, 2021, 1552127. [Google Scholar] [CrossRef]
- Yin, H.; Guo, Q.; Li, X.; Tang, T.; Li, C.; Wang, H.; Sun, Y.; Feng, Q.; Ma, C.; Gao, C.; et al. Curcumin Suppresses IL-1β Secretion and Prevents Inflammation through Inhibition of the NLRP3 Inflammasome. J. Immunol. 2018, 200, 2835–2846. [Google Scholar] [CrossRef]
- Fan, Z.; Jing, H.; Yao, J.; Li, Y.; Hu, X.; Shao, H.; Shen, G.; Pan, J.; Luo, F.; Tian, X. The protective effects of curcumin on experimental acute liver lesion induced by intestinal ischemia-reperfusion through inhibiting the pathway of NF-κB in a rat model. Oxidative Med. Cell. Longev. 2014, 2014, 191624. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Duan, S.; Yuan, X.; Liang, J.; Hou, S. Curcumin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Biomed. Pharmacother. 2019, 118, 109195. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhao, S.; Zhou, J.; Yan, J.; Wang, L.; Du, X.; Li, H.; Chen, Y.; Cai, W.; Wu, J. Curcumin alleviates DSS-induced colitis via inhibiting NLRP3 inflammsome activation and IL-1β production. Mol. Immunol. 2018, 104, 11–19. [Google Scholar] [CrossRef]
- Ding, X.Q.; Wu, W.Y.; Jiao, R.Q.; Gu, T.T.; Xu, Q.; Pan, Y.; Kong, L.D. Curcumin and allopurinol ameliorate fructose-induced hepatic inflammation in rats via miR-200a-mediated TXNIP/NLRP3 inflammasome inhibition. Pharmacol. Res. 2018, 137, 64–75. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Li, S.; Li, Y.; Wang, X.; Liu, B.; Fu, Q.; Ma, S. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol. Appl. Pharmacol. 2015, 286, 53–63. [Google Scholar] [CrossRef]
- Liu, S.; Li, Q.; Zhang, M.T.; Mao-Ying, Q.L.; Hu, L.Y.; Wu, G.C.; Mi, W.L.; Wang, Y.Q. Curcumin ameliorates neuropathic pain by down-regulating spinal IL-1β via suppressing astroglial NALP1 inflammasome and JAK2-STAT3 signalling. Sci. Rep. 2016, 6, 28956. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, Z.; Sun, Z.; Zhang, W.; Chen, X.; Nie, S. Curcumin relieves paraquat-induced lung injury through inhibiting the thioredoxin interacting protein/NLR pyrin domain containing 3-mediated inflammatory pathway. Mol. Med. Rep. 2019, 20, 5032–5040. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Zeng, S.; Tan, X.; Deng, X. Curcumin inhibits the activity of ubiquitin ligase Smurf2 to promote NLRP3-dependent pyroptosis in non-small cell lung cancer cells. Int. J. Oncol. 2025, 66, 21. [Google Scholar] [CrossRef]
- Zhou, Y.; Kong, Y.; Jiang, M.; Kuang, L.; Wan, J.; Liu, S.; Zhang, Q.; Yu, K.; Li, N.; Le, A.; et al. Curcumin activates NLRC4, AIM2, and IFI16 inflammasomes and induces pyroptosis by up-regulated ISG3 transcript factor in acute myeloid leukemia cell lines. Cancer Biol. Ther. 2022, 23, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, R.; Zhao, M.; Li, Y.; Sun, C.; Xie, J.; Chen, Y.; Jing, Q.; Mi, D.; Shi, S. PLGA confers upon conventional nonfluorescent molecules luminescent properties to trigger 1O2-induced pyroptosis and immune response in tumors. J. Nanobiotechnology 2025, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; He, L.; Wang, J.; Lai, L.; Ma, L.; Qu, K.; Yang, Z.; Wang, X.; Zhao, R.; Weng, L.; et al. Synergistic immunotherapy with a calcium-based nanoinducer: Evoking pyroptosis and remodeling tumor-associated macrophages for enhanced antitumor immune response. Nanoscale 2024, 16, 18570–18583. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Bi, J.; Zhang, Q.; Yang, Y.; Li, J.; Liang, Y. Mechanism of action of curcumin targeting TRPM2/NLRP3 signaling axis to mediate cell death in the treatment of knee osteoarthritis. Hum. Exp. Toxicol. 2024, 43, 9603271241308798. [Google Scholar] [CrossRef]
- Zheng, P.; Ding, B.; Zhu, G.; Li, C.; Lin, J. Biodegradable Ca2+ Nanomodulators Activate Pyroptosis through Mitochondrial Ca2+ Overload for Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2022, 61, e202204904. [Google Scholar] [CrossRef]
- Zhu, J.X.; Zhu, W.T.; Hu, J.H.; Yang, W.; Liu, P.; Liu, Q.H.; Bai, Y.X.; Xie, R. Curcumin-Loaded Poly(L-lactide-co-glycolide) Microbubble-Mediated Sono-photodynamic Therapy in Liver Cancer Cells. Ultrasound Med. Biol. 2020, 46, 2030–2043. [Google Scholar] [CrossRef]
- Dal, Z.; Aru, B. The role of curcumin on apoptosis and NLRP3 inflammasome-dependent pyroptosis on colorectal cancer in vitro. Turk. J. Med. Sci. 2023, 53, 883–893. [Google Scholar] [CrossRef]
- Wei, T.; Zheng, Z.; Wei, X.; Liu, Y.; Li, W.; Fang, B.; Yun, D.; Dong, Z.; Yi, B.; Li, W.; et al. Rational design, synthesis, and pharmacological characterisation of dicarbonyl curcuminoid analogues with improved stability against lung cancer via ROS and ER stress mediated cell apoptosis and pyroptosis. J. Enzym. Inhib. Med. Chem. 2022, 37, 2357–2369. [Google Scholar] [CrossRef]
- Nam, Y.J.; Choi, J.; Lee, J.S.; Seo, C.; Lee, G.; Lee, Y.; Kim, J.K.; Kim, P.; Lim, J.J.; Choi, H.S.; et al. Curcuma phaeocaulis Inhibits NLRP3 Inflammasome in Macrophages and Ameliorates Nanoparticle-Induced Airway Inflammation in Mice. Molecules 2022, 27, 2101. [Google Scholar] [CrossRef]
- Jena, A.B.; Dash, U.C.; Duttaroy, A.K. An in silico investigation on the interactions of curcumin and epigallocatechin-3-gallate with NLRP3 Inflammasome complex. Biomed. Pharmacother. 2022, 156, 113890. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cui, C.; Xu, P.; Dang, R.; Cai, H.; Liao, D.; Yang, M.; Feng, Q.; Yan, X.; Jiang, P. Curcumin Activates AMPK Pathway and Regulates Lipid Metabolism in Rats Following Prolonged Clozapine Exposure. Front. Neurosci. 2017, 11, 558. [Google Scholar] [CrossRef] [PubMed]
| Protein Name | Function |
|---|---|
| GSDMD (Gasdermin D) | Central executor of pyroptosis. Cleaved by caspase-1/4/5/11; the N-terminal fragment forms pores in the plasma membrane, leading to cell lysis. |
| GSDME (Gasdermin E) | Originally associated with apoptosis; cleaved by caspase-3 to switch cell death from apoptosis to pyroptosis in some contexts. |
| Caspase-1 | Key enzyme in the canonical inflammasome pathway. Activates pro-inflammatory cytokines IL-1β and IL-18 and cleaves GSDMD. |
| Caspase-4/Caspase-5 (Human) | Recognize intracellular LPS directly and activate the non-canonical inflammasome pathway by cleaving GSDMD. |
| Caspase-11 (Mouse) | Functional analog of human caspase-4/5 in mice. Activates non-canonical pyroptosis via GSDMD cleavage. |
| NLRP3 | A pattern recognition receptor (PRR) that forms the NLRP3 inflammasome upon activation, recruiting ASC and pro-caspase-1. |
| NLRC4 | Another PRR that forms an inflammasome in response to bacterial flagellin and type III secretion system proteins. |
| AIM2 | Recognizes double-stranded DNA (dsDNA) in the cytoplasm, forming the AIM2 inflammasome. |
| NLRP1 | Sensor that forms an inflammasome in response to various stress signals and pathogen-associated molecules. |
| IFI16 | DNA sensor that can trigger inflammasome formation, particularly in viral infections. |
| ASC (PYCARD) | Adaptor protein with a CARD domain; bridges inflammasome sensors (e.g., NLRP3) and pro-caspase-1 to facilitate activation. |
| IL-1β | Pro-inflammatory cytokine activated by caspase-1; promotes fever, inflammation, and immune cell recruitment. |
| IL-18 | Another cytokine activated by caspase-1; enhances NK cell activity and IFN-γ production. |
| HMGB1 | DAMP released during pyroptosis; amplifies inflammation. |
| NEK7 | Serine/threonine kinase that binds NLRP3 to facilitate its activation and inflammasome assembly. |
| Pannexin-1 | Channel protein that may facilitate ATP release during inflammasome activation; associated with pyroptosis initiation. |
| GBP (Guanylate-binding proteins) | Induced by IFNs; aid in LPS delivery to caspase-11 in the non-canonical pyroptosis pathway. |
| TLR4 | Toll-like receptor that primes inflammasome components via NF-κB pathway activation. |
| TRIF/MyD88 | Adaptor proteins for TLR signaling; regulate transcriptional priming of inflammasome components. |
| Cell/Animal Model | Key Findings | Mechanistic Highlights | Ref. |
|---|---|---|---|
| THP-1 and RAW264.7 macrophages; MSU-induced gout model in mice | Decreases mRNA and protein levels of IL-1β, IL-6, TNF-α, COX-2, and PGE2 (~2.0–2.5-fold reduction). | Blocks NF-κB priming; protects mitochondria to prevent NLRP3 assembly | [101] |
| Primary microglia and MCAO stroke mice | Decreases protein levels of IL-1β and IL-18 (~49–52% reduction) and suppresses NLRP3 inflammasome components (NLRP3, ASC, cleaved caspase-1) and GSDMD-N expression. | Suppresses NF-κB pathway; limits NLRP3-mediated microglial pyroptosis | [102] |
| J774A.1 macrophages (likely LPS + ATP) | Decreases IL-1β secretion and cleaved caspase-1 levels and suppresses NLRP3 inflammasome activation by inhibiting LPS priming, K+ efflux, mitochondrial clustering, and ASC speck formation. | Suggested direct inhibition of inflammasome machinery | [103] |
| Rat liver I/R injury model | Decreases serum and liver levels of TNF-α and IL-6 (by ~19–26% and ~26–36%, respectively), MPO (~32–33%), and NF-κB (~33–46%); increases SOD (~25–39%) and improves histopathological liver injury scores (~39–49%) in intestinal I/R-induced rats. | Prevents inflammasome priming via NF-κB suppression | [104] |
| Hyperuricemic mice and renal tubular epithelial cells | Decreases serum levels of IL-1β, IL-18, UA, CRE, and BUN and suppresses serum and liver XOD activity, MDA accumulation, and NLRP3 inflammasome activation in kidney; restores SOD and GSH-Px activities in potassium oxonate-induced hyperuricemic mice. | Inhibits priming (NF-κB) and NLRP3 assembly in kidney cells | [105] |
| LPS-primed macrophages + DSS colitis mice | Decreases IL-1β secretion, caspase-1 activation, IL-6, and MCP-1 levels, while suppressing NLRP3 inflammasome activation (via inhibition of K+ efflux, ROS, cathepsin B, and ASC speck formation). | Blocks ROS, K+ efflux, and cathepsin B release | [106] |
| HepG2 and BRL-3A cells; fructose-fed rats | Upregulates miR-200a and downregulates TXNIP and NLRP3 inflammasome activation in fructose-fed rat livers and fructose-exposed BRL-3A/HepG2 cell. | MicroRNA-mediated suppression of TXNIP-NLRP3 signaling | [107] |
| SH-SY5Y cells; rat hippocampal neurons | Decreases TXNIP expression, NLRP3 and cleaved caspase-1 levels, and IL-1β secretion and suppresses ER stress markers (p-IRE1α, p-PERK) and ROS production via AMPK activation. | Via AMPK-dependent inhibition of ER stress and inflammasome | [108] |
| Spinal astrocytes in SNI neuropathic pain mice | Decreases spinal mRNA and protein levels of IL-1β and NALP1 (~40–60% reduction) and suppresses cleaved caspase-1, GFAP, and phosphorylated JAK2/STAT3 (~30–55% reduction). | Targets astrocytic inflammasome and neuroinflammatory signaling | [109] |
| WI38VA13 lung epithelial cells and PQ lung injury rats | Decreases lung mRNA and protein levels of TXNIP, NLRP3, cleaved caspase-1, and IL-1β (~35–60% reduction) and suppresses inflammatory cell infiltration and histopathological lung damage in paraquat-induced acute lung injury model. | Protects epithelial cells by inhibiting TXNIP/NLRP3 activation | [110] |
| Cancer/Model | Curcumin’s Mechanism on Pyroptosis | Ref. |
|---|---|---|
| Non-small cell lung cancer | Inhibits Smurf2, stabilizes NLRP3, and promotes NLRP3-mediated pyroptosis via GSDMD; increases NLRP3, cleaved caspase-1, and GSDMD protein levels by ~1.6–2.3 fold (Western blot). | [111] |
| Acute myeloid leukemia | Increases ISG3 mRNA expression (qPCR, no fold-change reported), activates NLRC4, AIM2, and IFI16 inflammasomes, and increases cleaved caspase-1 and GSDMD-N protein levels and LDH release (Western blot and LDH assay). | [112] |
| Tumor (general) | Curcumin-loaded PLGA nanoparticles under 660 nm light irradiation generate singlet oxygen, activate caspase-3, cleave GSDME, and induce pyroptotic cell death in tumor cells. | [113] |
| Tumor (general) | Nano-curcumin disrupts Ca2+ homeostasis → activates caspase-1 → GSDMD-N → pyroptosis. | [20] |
| Colorectal cancer | CaZCH NP releases curcumin and Ca2+ → caspase-3 → GSDME-mediated pyroptosis + M2 to M1 TAM switch. | [114] |
| Hepatocellular carcinoma (HepG2) | Curcumin (30 μM, 12 h) increases ROS production, decreases pro-caspase-3 expression (~50%), upregulates GSDME-N (~2.5-fold), and elevates LDH release (~2.8-fold), leading to pyroptotic cell death. | [21] |
| Knee osteoarthritis | Modulates TRPM2/NLRP3 signaling (~40% reduction in TRPM2/NLRP3 expression) → suppresses ROS (~39% reduction) → inhibits caspase-1 and GSDMD cleavage → reduces pyroptosis. | [115] |
| Tumor (general) | Ca2+ nanomodulator with curcumin induces mitochondrial Ca2+ overload → pyroptosis. | [116] |
| Liver cancer (in vitro) | Curcumin-loaded microbubbles + SPDT → mitochondrial damage → pyroptosis + apoptosis. | [117] |
| Colorectal cancer | Upregulates NLRP3 inflammasome components (NLRP3, ASC, caspase-1) (~1.3–1.6-fold, qPCR); partially increases GSDMD expression (~1.2-fold), suggesting partial pyroptosis activation. | [118] |
| Lung cancer | Curcumin analogue B2 increases ROS levels (~1.8-fold), induces ER stress (↑ p-PERK and CHOP), leading to activation of both apoptosis (↑ cleaved caspase-3, Bax/Bcl-2 ratio) and pyroptosis (↑ GSDMD-N and IL-1β levels) in A549 and H1299 lung cancer cells. | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, D.O. Curcumin as a Dual Modulator of Pyroptosis: Mechanistic Insights and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 7590. https://doi.org/10.3390/ijms26157590
Moon DO. Curcumin as a Dual Modulator of Pyroptosis: Mechanistic Insights and Therapeutic Potential. International Journal of Molecular Sciences. 2025; 26(15):7590. https://doi.org/10.3390/ijms26157590
Chicago/Turabian StyleMoon, Dong Oh. 2025. "Curcumin as a Dual Modulator of Pyroptosis: Mechanistic Insights and Therapeutic Potential" International Journal of Molecular Sciences 26, no. 15: 7590. https://doi.org/10.3390/ijms26157590
APA StyleMoon, D. O. (2025). Curcumin as a Dual Modulator of Pyroptosis: Mechanistic Insights and Therapeutic Potential. International Journal of Molecular Sciences, 26(15), 7590. https://doi.org/10.3390/ijms26157590





