Fumiquinazolines F and G from the Fungus Penicillium thymicola Demonstrates Anticancer Efficacy Against Triple-Negative Breast Cancer MDA-MB-231 Cells by Inhibiting Epithelial–Mesenchymal Transition
Abstract
1. Introduction
2. Results
2.1. Fumiquinazolines Inhibit the Growth of Breast and Prostate Cancer Cells
2.2. Cytotoxic and Cytostatic Effects of Fumiquinazoline F on MDA-MB-231 Cells
2.3. Fumiquinazoline F Inhibits MDA-MB-231 Cell Migration
2.4. Fumiquinazolines F and G Increase E-Cadherin and Decrease Vimentin Protein Levels in MDA-MB-231 Cells
2.5. Fumiquinazoline F Reduces CD44 Protein Levels in MDA-MB-231 Mesenchymal Cells
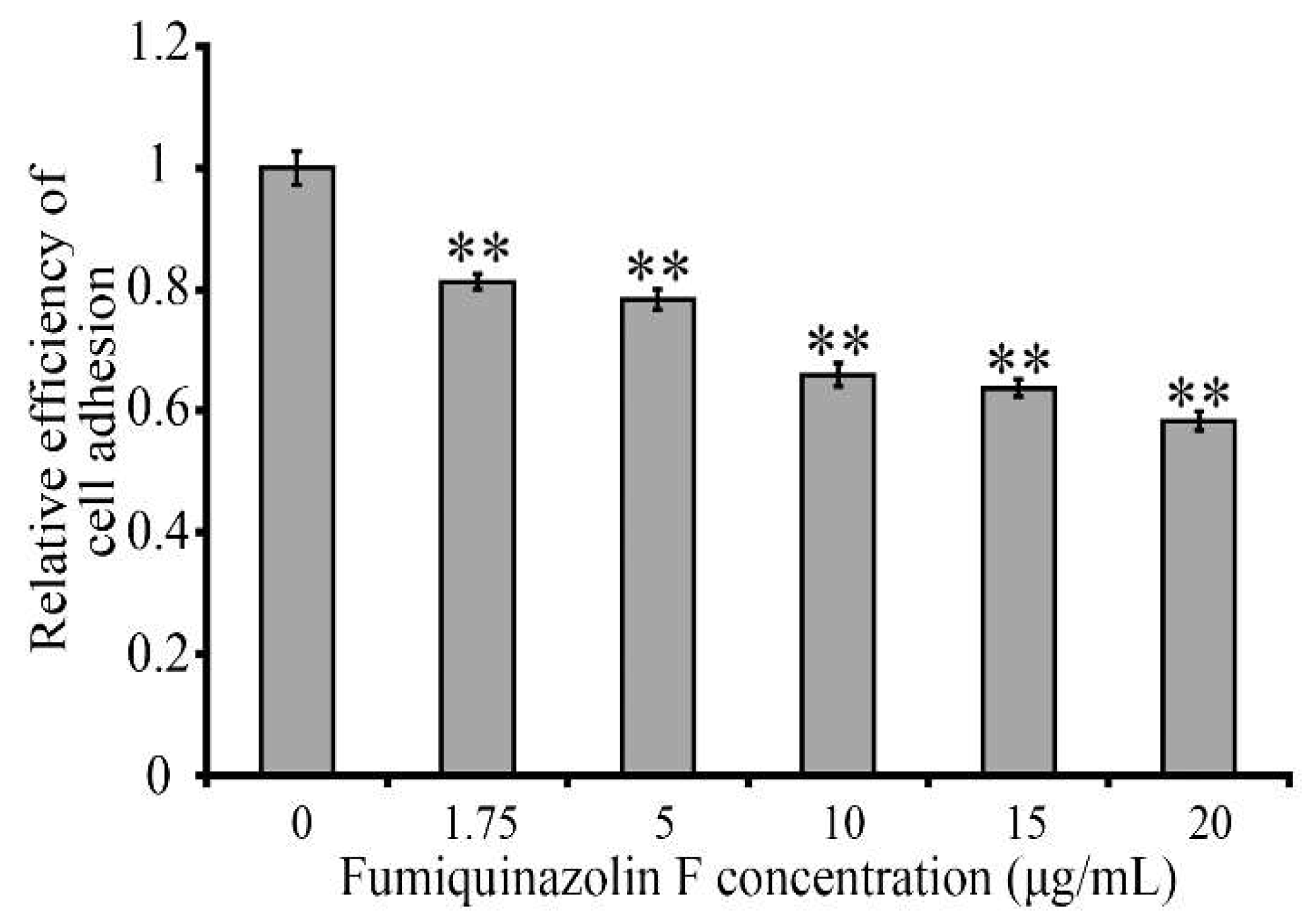
2.6. Fumiquinazoline F Inhibits MDA-MB-231 Cell Adhesion to a Hyaluronic Acid-Coated Scaffold
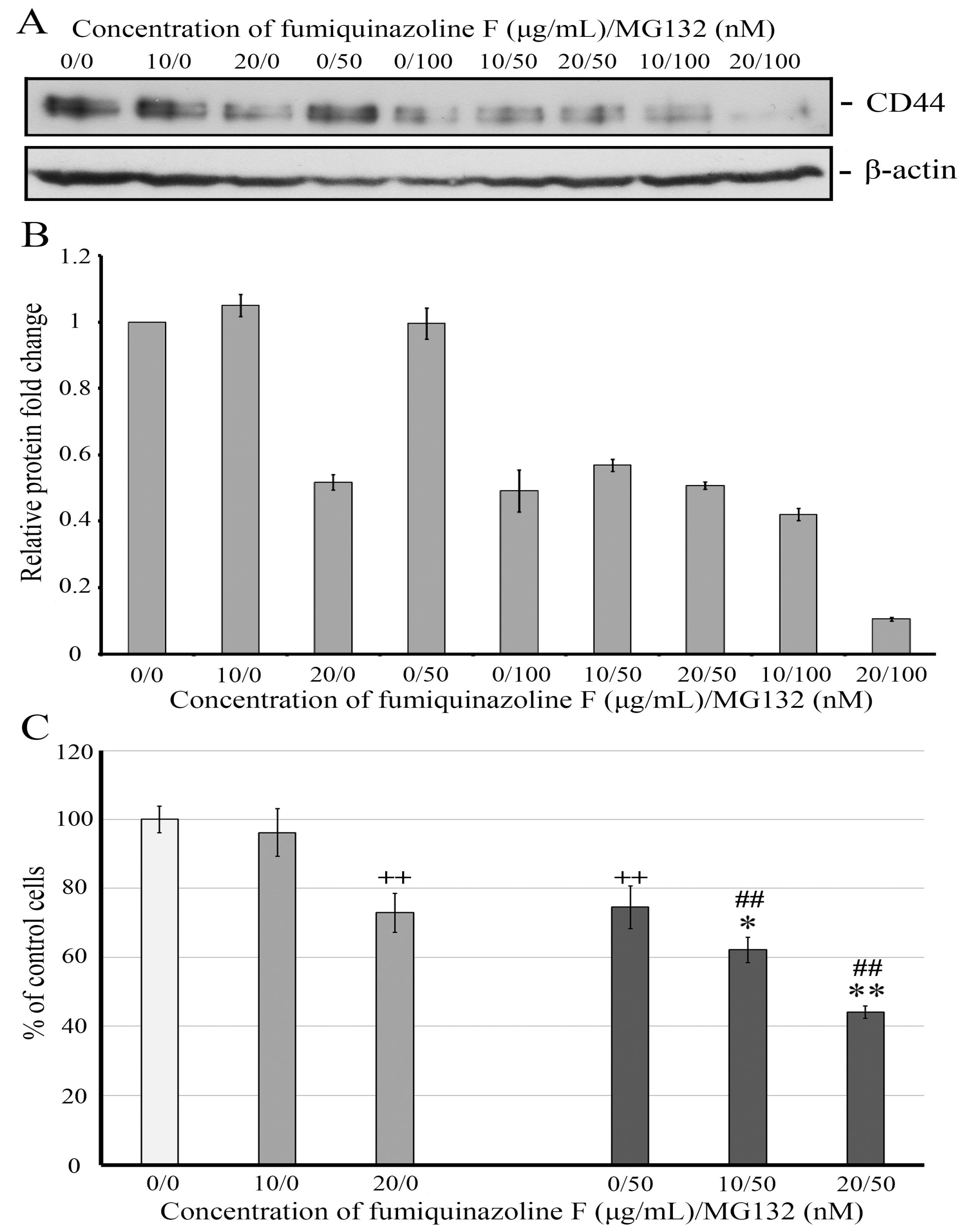
2.7. Fumiquinazoline F and Proteasome Inhibitor MG132 Combine Synergistically to Reduce CD44 Protein Level and Retard MDA-MB-231 Cell Growth
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Primers, Antibodies, and Reagents
4.3. Fungal Strain Cultivation and Isolation of Fumiquinazolines F and G
4.4. Cell Growth Assay
4.5. Cell Death Assay and Cell Cycle Analysis
4.6. Cell Migration Assay
4.7. ELISA
4.8. RT-qPCR
4.9. Western Blot Analysis
4.10. Cycloheximide Chase Assay
4.11. Hyaluronic Acid Adhesion Assay
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, R.; Zhu, Y.; Chen, J.; Wang, Y.; Song, X.; Wu, Y.; Jin, F.; Wang, Y. The Quinazoline Derivative, 04NB-03, Induces Cell Cycle Arrest and Apoptosis in Hepatocellular Carcinoma Cells in a Reactive Oxygen Species-Dependent Manner. Chem. Biol. Interact. 2021, 338, 109371. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Ozleyen, A.; Boyunegmez Tumer, T.; Oluwaseun Adetunji, C.; El Omari, N.; Balahbib, A.; Taheri, Y.; Bouyahya, A.; Martorell, M.; Martins, N.; et al. Natural Products and Synthetic Analogs as a Source of Antitumor Drugs. Biomolecules 2019, 9, 679. [Google Scholar] [CrossRef]
- Boulis, A.G.; Hamed, A.A.; El-Awady, M.E.; Mohamed, A.R.; Eliwa, E.M.; Asker, M.M.S.; Shaaban, M. Diverse Bioactive Metabolites from Penicillium sp. MMA Derived from the Red Sea: Structure Identification and Biological Activity Studies. Arch. Microbiol. 2020, 202, 1985–1996. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, Q.; Li, X.; Liu, X.; Lei, H.; Wu, B. Structures and Bioactivities of Secondary Metabolites from Penicillium Genus Since 2010. Fitoterapia 2022, 163, 105349. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.F.; Morris-Natschke, S.L.; Liu, Y.Q.; Guo, X.; Xu, X.-S.; Goto, M.; Li, J.-C.; Yang, G.-Z.; Lee, K.-H. Biologically Active Quinoline and Quinazoline Alkaloids Part I. Med. Res. Rev. 2018, 38, 775–828. [Google Scholar] [CrossRef]
- Kaur, T.; Bhandari, D.D. Annotated Review on Various Biological Activities of Quinoline Molecule. Biointerface Res. Appl. Chem. 2023, 13, 355. [Google Scholar] [CrossRef]
- Dai, L.H.; Zhang, G.R.; Ou, Y.H.; Liu, X.J.; Yao, H.L.; Hu, W.H.; Li, H.J.; Lan, W.J. Five New Indole Alkaloid Derivatives from Deep-Sea Fungus Aspergillus fumigatus AF1. Mar. Drugs 2025, 23, 4. [Google Scholar] [CrossRef]
- Almeida, M.C.; Szemerédi, N.; Durães, F.; Long, S.; Resende, D.I.S.P.; Martins da Costa, P.; Pinto, M.; Spengler, G.; Sousa, E. Effect of Indole-Containing Pyrazino [2,1-b]quinazoline-3,6-diones in the Virulence of Resistant Bacteria. Antibiotics 2023, 12, 922. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Boonpothong, P.; Sousa, E.; Kijjoa, A.; Pinto, M.M.M. Chemistry of the Fumiquinazolines and Structurally Related Alkaloids. Nat. Prod. Rep. 2019, 36, 7–34. [Google Scholar] [CrossRef]
- Han, X.; Xu, X.Y.; Cui, C.B.; Gu, Q. Alkaloidal Compounds Produced by a Marine-Derived Fungus, Aspergillus fumigatus H1-04, and Their Antitumor Activities. Chin. J. Med. Chem. 2007, 17, 232–237. [Google Scholar]
- Zhou, Y.M.; Debbab, A.; Mandi, A.; Wray, V.; Schulz, B.; Muller, W.E.G.; Kassack, M.; Lin, W.H.; Kurtan, T.; Proksch, P.; et al. Alkaloids from the Sponge-Associated Fungus Aspergillus sp. Appl. Environ. Microbiol. 2013, 2013, 894–906. [Google Scholar] [CrossRef]
- Zhelifonova, V.P.; Antipova, T.V.; Kozlovskii, A.G. Biosynthesis of Fumiquinazolines by the Fungus Penicillium thymicola. Appl. Biochem. Microbiol. 2012, 48, 302–306. [Google Scholar] [CrossRef]
- Rahimian, A.; Mahdavi, M.; Rahbarghazi, R.; Charoudeh, H.N. 4t-CHQ, a Spiro-Quinazolinone Benzenesulfonamide Derivative, Induces G0/G1 Cell Cycle Arrest and Triggers Apoptosis Through Down-Regulation of Survivin and Bcl2 in the Leukemia Stem-Like KG1-a Cells. Anticancer Agents Med. Chem. 2019, 19, 1340–1349. [Google Scholar] [CrossRef]
- Shang, X.F.; Morris-Natschke, S.L.; Liu, Y.Q.; Li, X.H.; Zhang, J.Y.; Lee, K.H. Biology of Quinoline and Quinazoline Alkaloids. Alkaloids Chem. Biol. 2022, 88, 1–47. [Google Scholar] [CrossRef]
- Lee, A.V.; Oesterreich, S.; Davidson, N.E. MCF-7 Cells—Changing the Course of Breast Cancer Research and Care for 45 Years. J. Natl. Cancer Inst. 2015, 107, djv073. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef]
- Zuo, T.; Wang, L.; Morrison, C.; Chang, X.; Zhang, H.; Li, W.; Liu, Y.; Wang, Y.; Liu, X.; Chan, M.W.; et al. FOXP3 Is an X-Linked Breast Cancer Suppressor Gene and an Important Repressor of the HER-2/ErbB2 Oncogene. Cell 2007, 129, 1275–1286, Erratum in Cell 2008, 134, 546. [Google Scholar] [CrossRef]
- Witt, B.L.; Tollefsbol, T.O. Molecular, Cellular, and Technical Aspects of Breast Cancer Cell Lines as a Foundational Tool in Cancer Research. Life 2023, 13, 2311. [Google Scholar] [CrossRef]
- Nadanaka, S.; Tamura, J.I.; Kitagawa, H. Chondroitin Sulfates Control Invasiveness of the Basal-Like Breast Cancer Cell Line MDA-MB-231 Through ROR1. Front. Oncol. 2022, 12, 914838. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-Negative Breast Cancer Molecular Subtyping and Treatment Progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Rubenstein, M.; Hollowell, C.M.; Guinan, P. In LNCaP Cells Enhanced Expression of Both Androgen Receptor and Costimulatory Protein p300 Compensate for Antisense Oligonucleotide Suppression of bcl-2. Ther. Adv. Urol. 2011, 3, 243–250. [Google Scholar] [CrossRef]
- Abate-Shen, C.; Nunes de Almeida, F. Establishment of the LNCaP Cell Line—The Dawn of an Era for Prostate Cancer Research. Cancer Res. 2022, 82, 1689–1691. [Google Scholar] [CrossRef]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.Z.; Huang, J. PC3 Is a Cell Line Characteristic of Prostatic Small Cell Carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef]
- Niu, Y.; Yeh, S.; Miyamoto, H.; Li, G.; Altuwaijri, S.; Yuan, J.; Han, R.; Ma, T.; Kuo, H.C.; Chang, C. Tissue Prostate-Specific Antigen Facilitates Refractory Prostate Tumor Progression via Enhancing ARA70-Regulated Androgen Receptor Transactivation. Cancer Res. 2008, 68, 7110–7119. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, L.; Krug, P.; Mazur, M.; Milczarek, M.; Chilmonczyk, Z.; Wiktorska, K. In the Triple-Negative Breast Cancer MDA-MB-231 Cell Line, Sulforaphane Enhances the Intracellular Accumulation and Anticancer Action of Doxorubicin Encapsulated in Liposomes. Int. J. Pharm. 2019, 558, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Lucantoni, F.; Lindner, A.U.; O’Donovan, N.; Prehn, J.H.M. Systems Modeling Accurately Predicts Responses to Genotoxic Agents and Their Synergism with BCL-2 Inhibitors in Triple Negative Breast Cancer Cells. Cell Death Dis. 2018, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yu, P.; Tang, J. Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model. Onco Targets Ther. 2020, 13, 5395–5405. [Google Scholar] [CrossRef]
- Isert, L.; Mehta, A.; Loiudice, G.; Oliva, A.; Roidl, A.; Merkel, O.M. An In Vitro Approach to Model EMT in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 7757. [Google Scholar] [CrossRef]
- Wei, Z.; Shan, Z.; Shaikh, Z.A. Epithelial-Mesenchymal Transition in Breast Epithelial Cells Treated with Cadmium and the Role of Snail. Toxicol. Appl. Pharmacol. 2018, 344, 46–55. [Google Scholar] [CrossRef]
- Hu, S.; Shi, X.; Liu, Y.; He, Y.; Du, Y.; Zhang, G.; Yang, C.; Gao, F. CD44 Cross-Linking Increases Malignancy of Breast Cancer via Upregulation of p-Moesin. Cancer Cell Int. 2020, 20, 563. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The Biology and Role of CD44 in Cancer Progression: Therapeutic Implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- Al-Othman, N.; Alhendi, A.; Ihbaisha, M.; Barahmeh, M.; Alqaraleh, M.; Al-Momany, B.Z. Role of CD44 in Breast Cancer. Breast Dis. 2019, 39, 1–13. [Google Scholar] [CrossRef]
- Olsson, E.; Honeth, G.; Bendahl, P.O.; Saal, L.H.; Gruvberger-Saal, S.; Ringnér, M.; Vallon-Christersson, J.; Jönsson, G.; Holm, K.; Lövgren, K.; et al. CD44 Isoforms Are Heterogeneously Expressed in Breast Cancer and Correlate with Tumor Subtypes and Cancer Stem Cell Markers. BMC Cancer 2011, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, I.G.; Choi, B.H.; Ku, S.K.; Kwak, M.K. High CD44 Expression Mediates p62-Associated NFE2L2/NRF2 Activation in Breast Cancer Stem Cell-Like Cells: Implications for Cancer Stem Cell Resistance. Redox Biol. 2018, 17, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Birzele, F.; Voss, E.; Nopora, A.; Honold, K.; Heil, F.; Lohmann, S.; Verheul, H.; Le Tourneau, C.; Delord, J.P.; van Herpen, C.; et al. CD44 Isoform Status Predicts Response to Treatment with Anti-CD44 Antibody in Cancer Patients. Clin. Cancer Res. 2015, 21, 2753–2762. [Google Scholar] [CrossRef]
- Luo, Y.; Tian, Z.; Hua, X.; Huang, M.; Xu, J.; Li, J.; Huang, H.; Cohen, M.; Huang, C. Isorhapontigenin (ISO) Inhibits Stem Cell-Like Properties and Invasion of Bladder Cancer Cell by Attenuating CD44 Expression. Cell. Mol. Life Sci. 2020, 77, 351–363. [Google Scholar] [CrossRef]
- McFarlane, S.; Coulter, J.A.; Tibbits, P.; O’Grady, A.; McFarlane, C.; Montgomery, N.; Hill, A.; McCarthy, H.O.; Young, L.S.; Kay, E.W.; et al. CD44 Increases the Efficiency of Distant Metastasis of Breast Cancer. Oncotarget 2015, 6, 11465–11476. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Wang, J.; Zhang, H.; Zhang, Y.; Wang, C.; Xu, H.; Zhang, H.; Lin, Y.; Ma, L.; Li, Q.; et al. CD44 Targets Na(+)/H(+) Exchanger 1 to Mediate MDA-MB-231 Cells’ Metastasis via the Regulation of ERK1/2. Br. J. Cancer 2014, 110, 916–927. [Google Scholar] [CrossRef]
- Liu, W.; Ji, Z.; Wu, B.; Huang, S.; Chen, Q.; Chen, X.; Wei, Y.; Jiang, J. Siglec-15 Promotes the Migration of Liver Cancer Cells by Repressing Lysosomal Degradation of CD44. FEBS Lett. 2021, 595, 2290–2302. [Google Scholar] [CrossRef]
- Haakenson, J.K.; Khokhlatchev, A.V.; Choi, Y.J.; Linton, S.S.; Zhang, P.; Zaki, P.M.; Fu, C.; Cooper, T.K.; Manni, A.; Zhu, J.; et al. Lysosomal Degradation of CD44 Mediates Ceramide Nanoliposome-Induced Anoikis and Diminished Extravasation in Metastatic Carcinoma Cells. J. Biol. Chem. 2015, 290, 8632–8643. [Google Scholar] [CrossRef]
- Nam, K.; Oh, S.; Lee, K.M.; Yoo, S.A.; Shin, I. CD44 Regulates Cell Proliferation, Migration, and Invasion via Modulation of c-Src Transcription in Human Breast Cancer Cells. Cell Signal. 2015, 27, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, A.K.; Kaur, S.; Wernyj, R.P.; Kumaran, M.N.; Miletti-Gonzalez, K.E.; Chan, R.; Lim, E.; Madura, K.; Rodriguez-Rodriguez, L. CD44 Promotes Multi-Drug Resistance by Protecting P-Glycoprotein from FBXO21-Mediated Ubiquitination. Oncotarget 2015, 6, 26308–26321. [Google Scholar] [CrossRef]
- Xu, H.; Niu, M.; Yuan, X.; Wu, K.; Liu, A. CD44 as a Tumor Biomarker and Therapeutic Target. Exp. Hematol. Oncol. 2020, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Vargas, C.; Aguirre-Ducler, A.; Cereceda, K.; Quijada, S.; Escobar-Gómez, N.; Castillo, R.L.; Escobar-Aguirre, M. CD44 Marks Dormant Tumor Cells After HER2 Inhibition in Breast Cancer Cells. Int. J. Mol. Sci. 2025, 26, 4907. [Google Scholar] [CrossRef]
- Chen, J.; Lu, L.; Feng, Y.; Wang, H.; Dai, L.; Yan, li; Zhang, P. PKD2 Mediates Multi-Drug Resistance in Breast Cancer Cells Through Modulation of P-Glycoprotein Expression. Cancer Lett. 2011, 300, 48–56. [Google Scholar] [CrossRef]
- Błaszczak, E.; Miziak, P.; Odrzywolski, A.; Baran, M.; Gumbarewicz, E.; Stepulak, A. Triple-Negative Breast Cancer Progression and Drug Resistance in the Context of Epithelial–Mesenchymal Transition. Cancers 2025, 17, 228. [Google Scholar] [CrossRef]
- Zwartsen, A.; Chottanapund, S.; Kittakoop, P.; Navasumrit, P.; Ruchirawat, M.; Van Duursen, M.B.M.; Van den Berg, M. Evaluation of Anti-Tumour Properties of Two Depsidones—Unguinol and Aspergillusidone D—In Triple-Negative MDA-MB-231 Breast Tumour Cells. Toxicol. Rep. 2019, 6, 1216–1222. [Google Scholar] [CrossRef]
- Simu, S.; Marcovici, I.; Dobrescu, A.; Malita, D.; Dehelean, C.A.; Coricovac, D.; Olaru, F.; Draghici, G.A.; Navolan, D. Insights into the Behavior of Triple-Negative MDA-MB-231 Breast Carcinoma Cells Following the Treatment with 17β-Ethinylestradiol and Levonorgestrel. Molecules 2021, 26, 2776. [Google Scholar] [CrossRef]
- Wong, C.C.; Cheng, K.W.; Rigas, B. Preclinical Predictors of Anticancer Drug Efficacy: Critical Assessment with Emphasis on Whether Nanomolar Potency Should Be Required of Candidate Agents. J. Pharmacol. Exp. Ther. 2012, 341, 572–578. [Google Scholar] [CrossRef]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of Early Drug Discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Hyland, R.; Jones, B.C.; Smith, D.A.; Hurst, S.; Goosen, T.C.; Peterkin, V.; Koup, J.R.; Ball, S.E. Drug-Drug Interactions for UDP-Glucuronosyltransferase Substrates: A Pharmacokinetic Explanation for Typically Observed Low Exposure (AUCi/AUC) Ratios. Drug Metab. Dispos. 2004, 32, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial-Mesenchymal Transitions in Tumour Progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Liang, L.; Kaufmann, A.M. The Significance of Cancer Stem Cells and Epithelial-Mesenchymal Transition in Metastasis and Anti-Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 2555. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.T.; Yang, M.H. Revisiting Epithelial-Mesenchymal Transition in Cancer Metastasis: The Connection Between Epithelial Plasticity and Stemness. Mol. Oncol. 2017, 11, 792–804. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The Molecular Mechanisms and Therapeutic Strategies of EMT in Tumor Progression and Metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Busch, E.L.; Don, P.K.; Chu, H.; Richardson, D.B.; Keku, T.O.; Eberhard, D.A.; Avery, C.L.; Sandler, R.S. Diagnostic Accuracy and Prediction Increment of Markers of Epithelial-Mesenchymal Transition to Assess Cancer Cell Detachment from Primary Tumors. BMC Cancer 2018, 18, 82. [Google Scholar] [CrossRef]
- Pérez-González, A.; Bévant, K.; Blanpain, C. Cancer Cell Plasticity During Tumor Progression, Metastasis and Response to Therapy. Nat. Cancer 2023, 4, 1063–1082. [Google Scholar] [CrossRef] [PubMed]
- Atiya, H.I.; Gorecki, G.; Garcia, G.L.; Frisbie, L.G.; Baruwal, R.; Coffman, L. Stromal-Modulated Epithelial-to-Mesenchymal Transition in Cancer Cells. Biomolecules 2023, 13, 1604. [Google Scholar] [CrossRef]
- Päll, T.; Pink, A.; Kasak, L.; Turkina, M.; Anderson, W.; Valkna, A.; Kogerman, P. Soluble CD44 Interacts with Intermediate Filament Protein Vimentin on Endothelial Cell Surface. PLoS ONE 2011, 6, e29305. [Google Scholar] [CrossRef]
- Steinmetz, N.F.; Maurer, J.; Sheng, H.; Bensussan, A.; Maricic, I.; Kumar, V.; Braciak, T.A. Two Domains of Vimentin Are Expressed on the Surface of Lymph Node, Bone and Brain Metastatic Prostate Cancer Lines along with the Putative Stem Cell Marker Proteins CD44 and CD133. Cancers 2011, 3, 2870–2885. [Google Scholar] [CrossRef]
- Le Bras, G.F.; Allison, G.L.; Richards, N.F.; Ansari, S.S.; Washington, M.K.; Andl, C.D. CD44 Upregulation in E-Cadherin-Negative Esophageal Cancers Results in Cell Invasion. PLoS ONE 2011, 6, e27063. [Google Scholar] [CrossRef][Green Version]
- Deep, G.; Jain, A.K.; Ramteke, A.; Ting, H.; Vijendra, K.C.; Gangar, S.C.; Agarwal, C.; Agarwal, R. SNAI1 Is Critical for the Aggressiveness of Prostate Cancer Cells with Low E-Cadherin. Mol. Cancer 2014, 13, 37. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, Q. E-Cadherin Negatively Regulates CD44-Hyaluronan Interaction and CD44-Mediated Tumor Invasion and Branching Morphogenesis. J. Biol. Chem. 2003, 278, 8661–8668. [Google Scholar] [CrossRef] [PubMed]
- Hatzfeld, M. The p120 Family of Cell Adhesion Molecules. Eur. J. Cell Biol. 2005, 84, 205–214. [Google Scholar] [CrossRef]
- Kourtidis, A.; Ngok, S.P.; Anastasiadis, P.Z. p120 Catenin: An Essential Regulator of Cadherin Stability, Adhesion-Induced Signaling, and Cancer Progression. Prog. Mol. Biol. Transl. Sci. 2013, 116, 409–432. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Yoon, S.; Sun, E.G.; Zhou, R.; Bae, J.A.; Seo, Y.W.; Chae, J.I.; Paik, M.J.; Ha, H.H.; Kim, H.; et al. Glycoprotein 90K Promotes E-Cadherin Degradation in a Cell Density-Dependent Manner via Dissociation of E-Cadherin-p120-Catenin Complex. Int. J. Mol. Sci. 2017, 18, 2601. [Google Scholar] [CrossRef] [PubMed]
- Korolchuk, V.I.; Mansilla, A.; Menzies, F.M.; Rubinsztein, D.C. Autophagy Inhibition Compromises Degradation of Ubiquitin-Proteasome Pathway Substrates. Mol. Cell 2009, 33, 517–527. [Google Scholar] [CrossRef]
- Pang, K.; Park, J.; Ahn, S.G.; Lee, J.; Park, Y.; Ooshima, A.; Mizuno, S.; Yamashita, S.; Park, K.-S.; Lee, S.-Y.; et al. RNF208, an Estrogen-Inducible E3 Ligase, Targets Soluble Vimentin to Suppress Metastasis in Triple-Negative Breast Cancers. Nat. Commun. 2019, 10, 5805. [Google Scholar] [CrossRef]
- Chen, W.; Patel, D.; Jia, Y.; Yu, Z.; Liu, X.; Shi, H.; Liu, H. MARCH8 Suppresses Tumor Metastasis and Mediates Degradation of STAT3 and CD44 in Breast Cancer Cells. Cancers 2021, 13, 2550. [Google Scholar] [CrossRef]
- Eyster, C.A.; Cole, N.B.; Petersen, S.; Viswanathan, K.; Früh, K.; Donaldson, J.G. MARCH Ubiquitin Ligases Alter the Itinerary of Clathrin-Independent Cargo from Recycling to Degradation. Mol. Biol. Cell 2011, 22, 3218–3230. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois-Daigneault, M.C.; Thibodeau, J. Autoregulation of MARCH1 Expression by Dimerization and Autoubiquitination. J. Immunol. 2012, 188, 4959–4970. [Google Scholar] [CrossRef]
- Larsson, P.; Pettersson, D.; Olsson, M.; Sarathchandra, S.; Abramsson, A.; Zetterberg, H.; Ittner, E.; Forssell-Aronsson, E.; Kovács, A.; Karlsson, P.; et al. Repurposing Proteasome Inhibitors for Improved Treatment of Triple-Negative Breast Cancer. Cell Death Discov. 2024, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Byers, H.A.; Brooks, A.N.; Vangala, J.R.; Grible, J.M.; Feygin, A.; Clevenger, C.V.; Harrell, J.C.; Radhakrishnan, S.K. Evaluation of the NRF1-Proteasome Axis as a Therapeutic Target in Breast Cancer. Sci. Rep. 2023, 13, 15843. [Google Scholar] [CrossRef]
- Chang, S.J.; Ou-Yang, F.; Tu, H.P.; Lin, C.H.; Huang, S.H.; Kostoro, J.; Hou, M.F.; Chai, C.Y.; Kwan, A.L. Decreased Expression of Autophagy Protein LC3 and Sternness (CD44+/CD24-/low) Indicate Poor Prognosis in Triple-Negative Breast Cancer. Hum. Pathol. 2016, 48, 48–55. [Google Scholar] [CrossRef]
- Vadhan, A.; Hou, M.F.; Vijayaraghavan, P.; Wu, Y.C.; Hu, S.C.; Wang, Y.M.; Cheng, T.L.; Wang, Y.Y.; Yuan, S.F. CD44 Promotes Breast Cancer Metastasis through AKT-Mediated Downregulation of Nuclear FOXA2. Biomedicines 2022, 10, 2488. [Google Scholar] [CrossRef]
- Vira, D.; Basak, S.K.; Veena, M.S.; Batra, R.K.; Srivatsan, E.S. Cancer Stem Cells, microRNAs, and Therapeutic Strategies Including Natural Products. Cancer Metastasis Rev. 2012, 31, 733–751. [Google Scholar] [CrossRef]
- Nakano, M.; Ito, M.; Tanaka, R.; Ariyama, H.; Mitsugi, K.; Makiyama, A.; Uchino, K.; Esaki, T.; Tsuruta, N.; Hanamura, F.; et al. Epithelial-Mesenchymal Transition Is Activated in CD44-Positive Malignant Ascites Tumor Cells of Gastrointestinal Cancer. Cancer Sci. 2018, 109, 3461–3470. [Google Scholar] [CrossRef]
- Zöller, M. CD44: Can a Cancer-Initiating Cell Profit from an Abundantly Expressed Molecule? Nat. Rev. Cancer 2011, 11, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.; Peyrollier, K.; Xia, W.; Gilad, E. Hyaluronan-CD44 Interaction Activates Stem Cell Marker Nanog, Stat-3-Mediated MDR1 Gene Expression, and Ankyrin-Regulated Multidrug Efflux in Breast and Ovarian Tumor Cells. J. Biol. Chem. 2008, 283, 17635–17651. [Google Scholar] [CrossRef] [PubMed]
- Chanmee, T.; Ontong, P.; Kimata, K.; Itano, N. Key Roles of Hyaluronan and Its CD44 Receptor in the Stemness and Survival of Cancer Stem Cells. Front. Oncol. 2015, 5, 180. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.V.; Phan, N.L.; Nguyen, N.T.; Truong, N.H.; Duong, T.T.; Le, D.V.; Truong, K.D.; Phan, N.K. Differentiation of Breast Cancer Stem Cells by Knockdown of CD44: Promising Differentiation Therapy. J. Transl. Med. 2011, 9, 209. [Google Scholar] [CrossRef]
- Chu, X.; Tian, W.; Ning, J.; Xiao, G.; Zhou, Y.; Wang, Z.; Zhai, Z.; Tanzhu, G.; Yang, J.; Zhou, R. Cancer Stem Cells: Advances in Knowledge and Implications for Cancer Therapy. Signal Transduct. Target. Ther. 2024, 9, 170. [Google Scholar] [CrossRef]
- Liu, S.; Li, L.; Ren, D. Anti-Cancer Potential of Phytochemicals: The Regulation of the Epithelial-Mesenchymal Transition. Molecules 2023, 28, 5069. [Google Scholar] [CrossRef]
- Taylor, W.F.; Jabbarzadeh, E. The Use of Natural Products to Target Cancer Stem Cells. Am. J. Cancer Res. 2017, 7, 1588–1605. [Google Scholar]
- Yu, J.; Wang, X.; Du, P.; Shi, H. The Therapeutic Potential and Application of Marine Alkaloids in Treating Breast Cancer. Front. Mar. Sci. 2024, 11, 1440928. [Google Scholar] [CrossRef]
- Kozlovskii, A.G.; Zhelifonova, V.P.; Antipova, T.V.; Baskunov, B.P.; Kochkina, G.A.; Ozerskaya, S.M. Secondary Metabolite Profiles of the Penicillium Fungi Isolated from the Arctic and Antarctic Permafrost as Elements of Polyphase Taxonomy. Microbiology 2012, 81, 306–311. [Google Scholar] [CrossRef]
- Limame, R.; Wouters, A.; Pauwels, B.; Fransen, E.; Peeters, M.; Lardon, F.; De Wever, O.; Pauwels, P. Comparative Analysis of Dynamic Cell Viability, Migration and Invasion Assessments by Novel Real-Time Technology and Classic Endpoint Assays. PLoS ONE 2012, 7, e46536. [Google Scholar] [CrossRef]
- Zemskova, M.; Lilly, M.B.; Lin, Y.W.; Song, J.H.; Kraft, A.S. p53-Dependent Induction of Prostate Cancer Cell Senescence by the PIM1 Protein Kinase. Mol. Cancer Res. 2010, 8, 1126–1141. [Google Scholar] [CrossRef] [PubMed]
- Krishan, A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J. Cell Biol. 1975, 66, 188–193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jonkman, J.E.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing as say using live-cell microscopy. Cell Adh Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Kucik, D.; Singh, R.K.; Listinsky, C.M.; Listinsky, J.J.; Siegal, G.P. Alterations in Human Breast Cancer Adhesion-Motility in Response to Changes in Cell Surface Glycoproteins Displaying α-L-Fucose Moieties. Int. J. Oncol. 2008, 32, 797–807. [Google Scholar] [CrossRef] [PubMed]
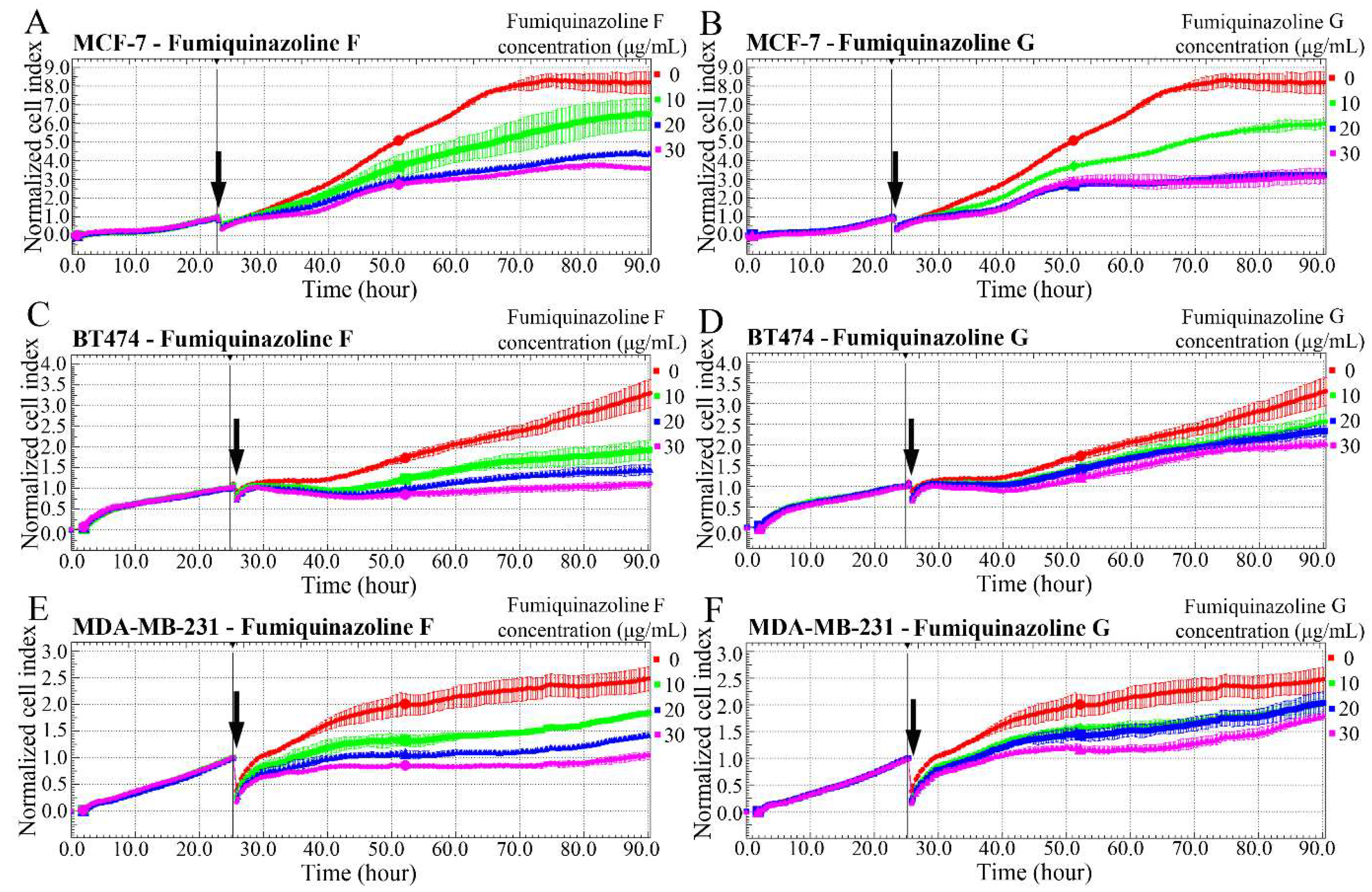


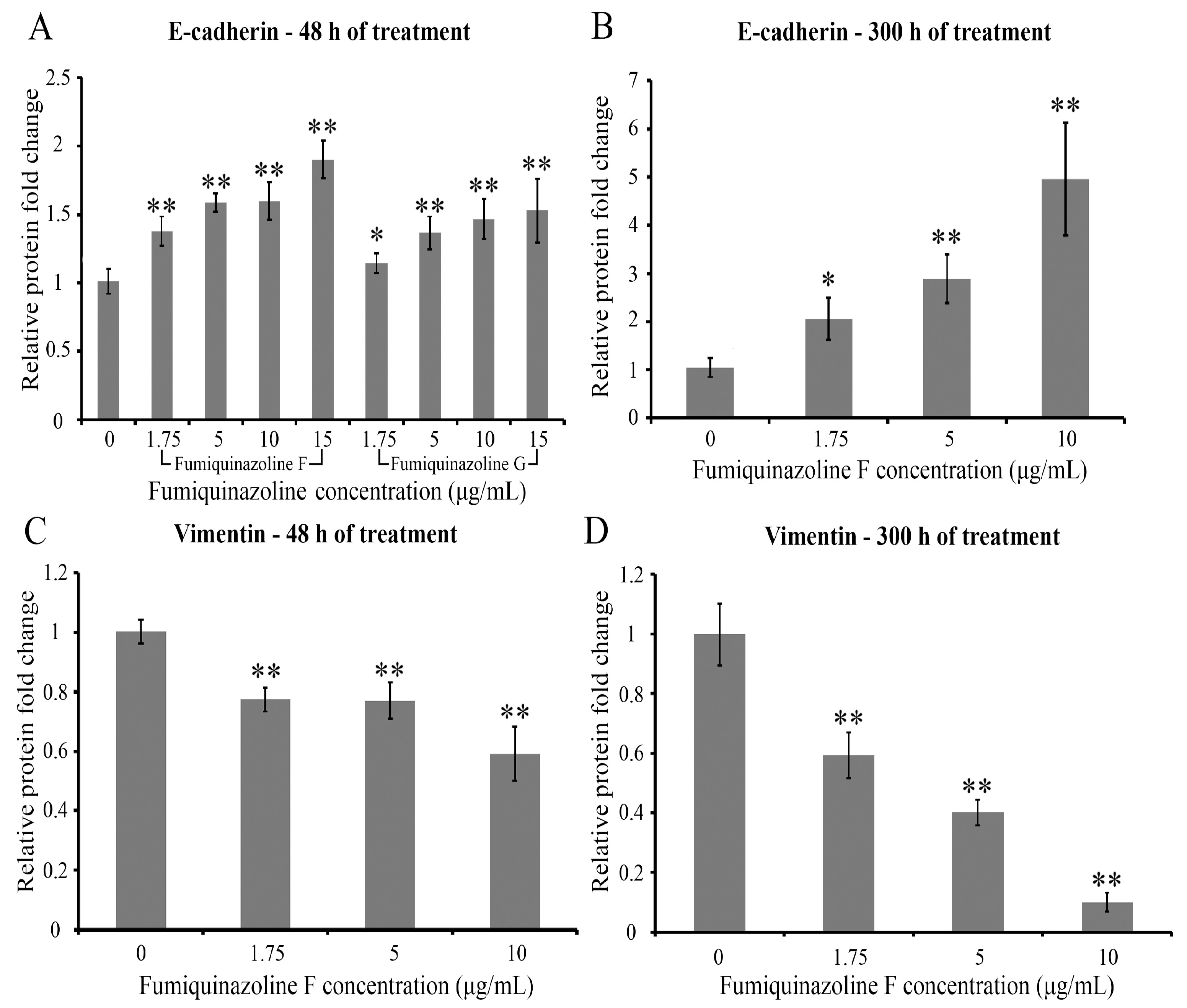
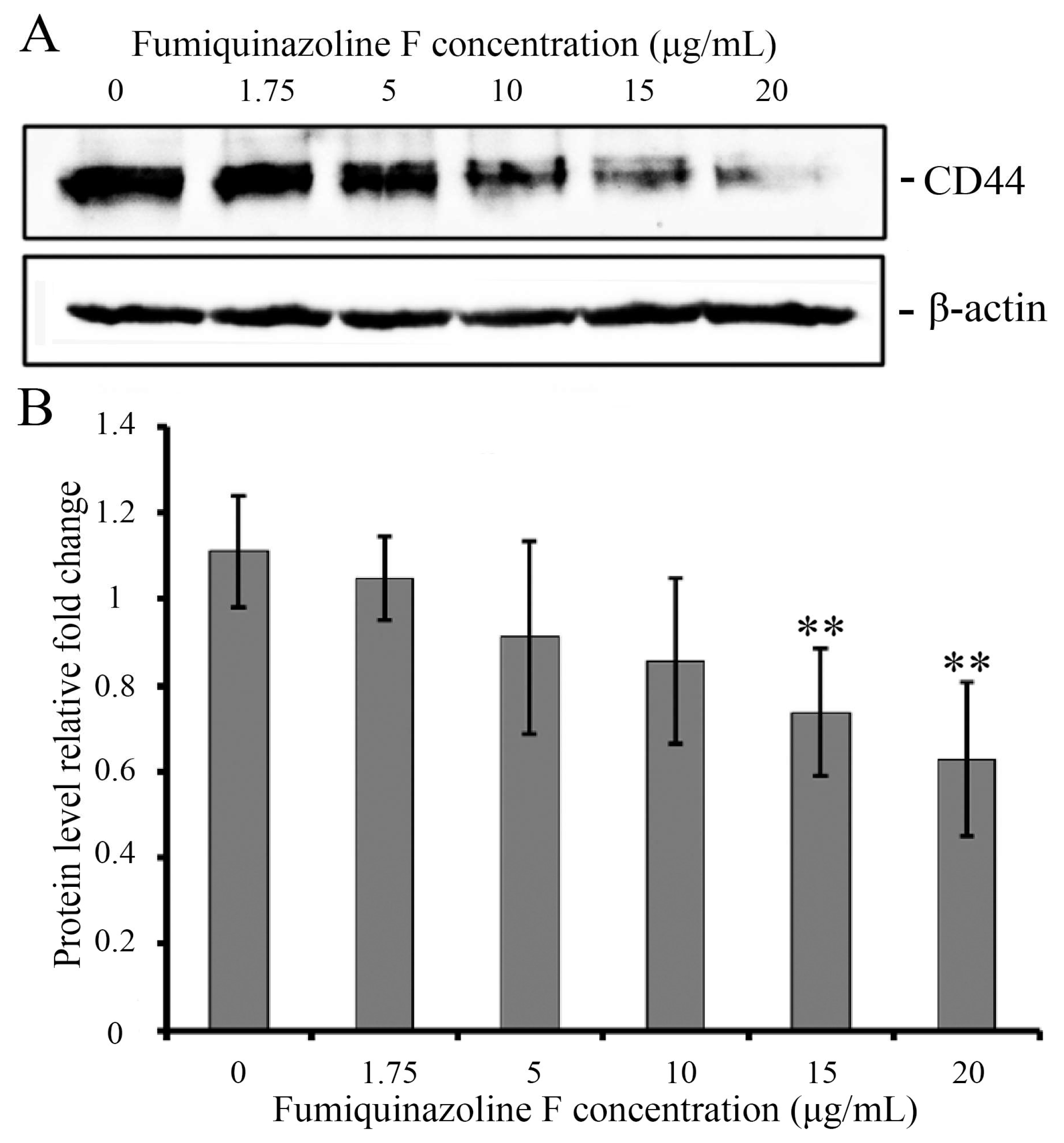
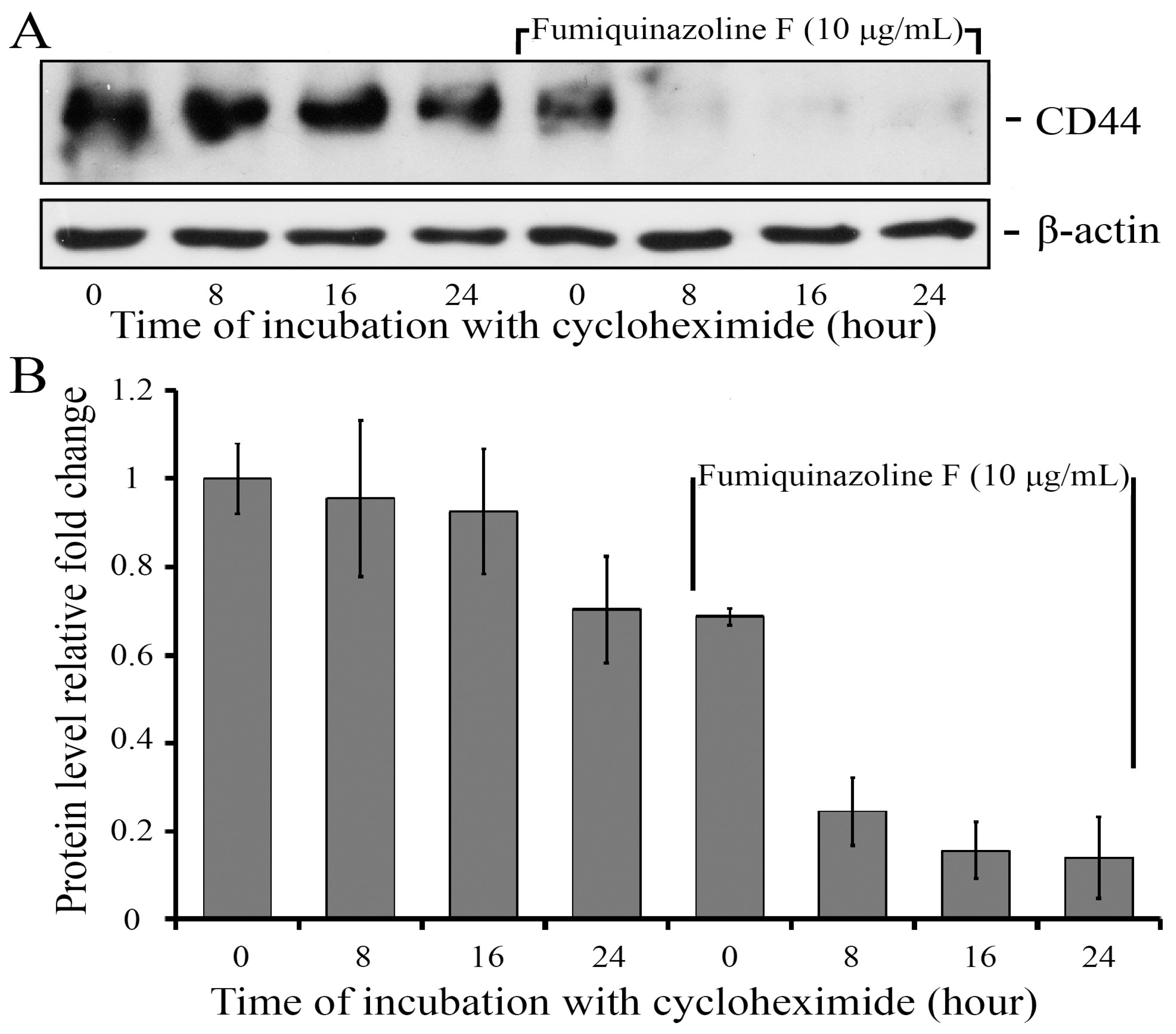
| Cell Lines and Compounds | IC50 Values—24 h | IC50 Values—48 h | IC50 Values—72 h |
|---|---|---|---|
| MCF-7 F | 21.2 μg/mL (59.1 μM) | 17.2 μg/mL (48 μM) | 18 μg/mL (50.3 μM) |
| MCF-7 G | 20.3 μg/mL (56.7 μM) | 14.5 μg/mL (40.4 μM) | 15.6 μg/mL (43.5 μM) |
| BT-474 F | 23.4 μg/mL (65.2 μM) | 19.9 μg/mL (55.5 μM) | 16.5 μg/mL (46 μM) |
| BT-474 G | >100 μM | >100 μM | 30.3 μg/mL (84.4 μM) |
| MDA-MB-231 F | 21.5 μg/mL (60.1 μM) | 19.4 μg/mL (54.1 μM) | 23.5 μg/mL (65.5 μM) |
| MDA-MB-231 G | 34.2 μg/mL (95.5 μM) | 31.6 μg/mL (88.2 μM) | >100 μM |
| LNCaP F | 21.6 μg/mL (60.2 μM) | 17 μg/mL (47.4 μM) | 22.8 μg/mL (63.7 μM) |
| LNCaP G | 29.2 μg/mL (81.5 μM) | 17.1 μg/mL (47.6 μM) | 21.4 μg/mL (59.8 μM) |
| PC-3 F | >100 μM | >100 μM | >100 μM |
| PC-3 G | >100 μM | >100 μM | >100 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rystsov, G.K.; Antipova, T.V.; Renfeld, Z.V.; Pilguy, L.S.; Shlyapnikov, M.G.; Vainshtein, M.B.; Granovsky, I.E.; Zemskova, M.Y. Fumiquinazolines F and G from the Fungus Penicillium thymicola Demonstrates Anticancer Efficacy Against Triple-Negative Breast Cancer MDA-MB-231 Cells by Inhibiting Epithelial–Mesenchymal Transition. Int. J. Mol. Sci. 2025, 26, 7582. https://doi.org/10.3390/ijms26157582
Rystsov GK, Antipova TV, Renfeld ZV, Pilguy LS, Shlyapnikov MG, Vainshtein MB, Granovsky IE, Zemskova MY. Fumiquinazolines F and G from the Fungus Penicillium thymicola Demonstrates Anticancer Efficacy Against Triple-Negative Breast Cancer MDA-MB-231 Cells by Inhibiting Epithelial–Mesenchymal Transition. International Journal of Molecular Sciences. 2025; 26(15):7582. https://doi.org/10.3390/ijms26157582
Chicago/Turabian StyleRystsov, Gleb K., Tatiana V. Antipova, Zhanna V. Renfeld, Lidiya S. Pilguy, Michael G. Shlyapnikov, Mikhail B. Vainshtein, Igor E. Granovsky, and Marina Y. Zemskova. 2025. "Fumiquinazolines F and G from the Fungus Penicillium thymicola Demonstrates Anticancer Efficacy Against Triple-Negative Breast Cancer MDA-MB-231 Cells by Inhibiting Epithelial–Mesenchymal Transition" International Journal of Molecular Sciences 26, no. 15: 7582. https://doi.org/10.3390/ijms26157582
APA StyleRystsov, G. K., Antipova, T. V., Renfeld, Z. V., Pilguy, L. S., Shlyapnikov, M. G., Vainshtein, M. B., Granovsky, I. E., & Zemskova, M. Y. (2025). Fumiquinazolines F and G from the Fungus Penicillium thymicola Demonstrates Anticancer Efficacy Against Triple-Negative Breast Cancer MDA-MB-231 Cells by Inhibiting Epithelial–Mesenchymal Transition. International Journal of Molecular Sciences, 26(15), 7582. https://doi.org/10.3390/ijms26157582







