Abstract
Zeugodacus cucuribitae (Coquillett) (Z. cucuribitae) is a global extremely invasive quarantine pest which has a wide host range of fruits and vegetables. At present, there are a few control measures for Z. cucuribitae, and deltamethrin and avermectin are commonly used. Among the hosts of Z. cucuribitae, Luffa acutangular, Luffa cylindrica, Sechium edule, Brassica oleracea var. botrytis, Musa nana, and Fragaria × ananassa are non-favored hosts. However, it is still not clear why these hosts are non-favored and whether there are any repellent components of Z. cucuribitae in these hosts. In this study, the components of these six hosts were collected from the literature, and the genes of odor and chemical sensation were determined from the genome of Z. cucuribitae. After the potential relationships between these components and genes were determined by molecular docking methods, the KEGG and GO enrichment analysis of these genes was conducted, and a complex network of genes vs. components vs. Kegg pathway vs. GO terms was constructed and used to select the key components for experiments. The results show that oleanolic acid (1 mg/mL, 0.1 mg/mL, and 0.01 mg/mL), rotenone (1 mg/mL, 0.1 mg/mL, and 0.01 mg/mL), and beta-caryophyllene oxide (1 mg/mL, 0.1 mg/mL, and 0.01 mg/mL) had a significant repellent effect on Z. cucuribitae, and three components, rotenone (1 mg/mL and 0.1 mg/mL), echinocystic acid (1 mg/mL, 0.1 mg/mL, and 0.01 mg/mL), and beta-caryophyllene oxide (1 mg/mL, and 0.1 mg/mL) had significant stomach toxicity in Z. cucuribitae. Furthermore, a complex signaling pathway was built and used to predict the effect of these components on Z. cucuribitae. These components probably play roles in the neuroactive ligand–receptor interaction (ko04080) and calcium signaling (ko04020) pathways. This study provides a reference for the prevention and control of Z. cucuribitae and a scientific reference for the rapid screening and development of new pest control drugs.
1. Introduction
Zeugodacus cucuribitae (Coquillett) (Z. cucuribitae) is a destructive agricultural pest and is distributed across a range of climatic regions, such as Central and East Asia and Oceania [1]. Due to its vast adaptability, high reproduction potential, and invasion capacity, it has been the subject of a worldwide pest management program [2]. It has widely invaded many crops and brought about serious losses to agricultural production. Z. cucuribitae can damage a wide variety of fruits and vegetables, including Luffa acutangular (L. acutangular), Luffa cylindrica (L. cylindrica), and Sechium edule (S. edule) [3,4]. At present, chemical insecticides, such as cypermethrin, fenvalerate, and abamectin, were commonly used in the field to reduce the damage caused to fruits and vegetables by Z. cucuribitae [5]. However, conventional methods in controlling Z. cucuribitae have some limitations, such as environmental pollution and a small number of physical trappings [5,6,7,8]. Therefore, there is an urgent need to find some effective and environmental protective components to control Z. cucuribitae.
As reported, many plants have evolved various methods to resist insects, such as secondary metabolites. Attacks by Bruchus pisorum would induce Pisum sativum to produce an isoflavone compound called pisatin, which is an insect resistance substance produced by plants and enables resistance against the invasion of B. pisorum [9,10]. The feeding of Spodoptera exigua would induce the production of momilactone A and momilactone B in Oryza sativa L. and could achieve a defense effect against Spodoptera exigua [11]. At the same time, natural compounds extracted from plants have been reported to have good application prospects in pest control. For example, volatile substances and secondary metabolites in some plant extracts have significant repellent effects on Bactrocera dorsalis (Hendel) (B. dorsalis) [12,13]. These metabolites usually have high safety and environmental protection and have little impact on non-target organisms. Secondly, plant-derived compounds have been proven not to easily cause pests to develop drug resistance and have effectively been used for pest control for a long time [14,15].
Plants of the Cucurbitaceae family are the main hosts of Z. cucuribitae. However, L. acutangular, L. cylindrica, and S. edule belong to Cucurbitaceae and are the relatively non-favored hosts of Z. cucuribitae. Compared with other Cucurbitaceae plants, the visit rate and oviposition rate of these species by Z. cucuribitae are lower [16,17,18]. In addition, some non-Cucurbitaceae plants, such as Brassica oleracea var. botrytis (B. oleracea), Musa nana (M. nana), and Fragaria × ananassa (F. ananassa), are relatively less attractive to Z. cucuribitae. The relative host performance and fecundity of Z. cucuribitae on these species is significantly lower than that on other species [16,19]. However, there are still no reports about whether there are some compounds that have a repellent effect on Z. cucuribitae. The main purpose of this study was to discover the compounds of host plants with repellent effects and a low damage rate against Z. cucuribitae.
Insects’ perception of chemical compounds is a diverse and complex process. According to previous reports, insects have some compound receptor proteins, including an olfactory receptor (OR), odor binding protein and general odorant-binding proteins (OBPGOBP), gustatory receptor (GR), pheromone-binding protein (PBP), ionotropic receptor (IR), and sensory neuron membrane protein (SNMP) [20]. For example, OR, a complex family of membrane protein receptors, was responsible for olfactory perception and communication in most insects [21,22]. These proteins were widely used in the screening of compounds to control pests [23,24].
The combination of network pharmacology and molecular docking has been widely used in the identification of effective drugs in the medical field [25,26,27] and is gradually being applied in the study of pest control [28,29,30]. These integrated approaches could accelerate the development of pesticides, disease-resistant breeding, and the screening of natural products. For example, molecular docking has been used to screen the attractive compounds of Z. cucuribitae and to screen the insecticidal metabolic compounds of Nephrolepis exaltata extract against Culicidae [31].
In this study, the potential repellent compounds of Z. cucuribitae in six non-favored hosts were screened out mainly based on network pharmacology, molecular docking technology, and behavior determinations. By screening the genes of Z. cucuribitae genome, a number of proteins related to the perception of chemical compounds of Z. cucuribitae were identified. These genes were combined with the compounds contained in the metabolic compounds of the six hosts, molecular virtual screening was carried out to obtain potential repellent compounds, and a genes vs. compounds vs. Kegg pathways vs. GO terms (CPPG) network was constructed. The effective repellent compounds were finally screened out with the degree value in this network. This study proposes an effective method in calculating and screening the compounds that have potential effects on Z. cucuribitae. Furthermore, oleanolic acid, rotenone, and beta-caryophyllene oxide were proven to have significant repellent effects on Z. cucuribitae with two-way selection tests. Rotenone and beta-caryophyllene oxide were proven to have the significant stomach toxicity of Z. cucuribitae with stomach toxicity tests. These results provide a new basis for the development of a novel insecticide to control Z. cucuribitae and provide a scientific reference in the prevention and management of pests.
2. Results
2.1. Identification and Enrichment of Olfactory Sensory Genes in Z. cucuribitae
2.1.1. Phylogenetic Analysis
The phylogenetic trees of Z. cucurbitae, including PBP, SNMP, OBP/GOBP, GR, OR, and IR genes, were constructed with R software (Version 4.3.1) after the identification of these genes from its genome collected from the NCBI database, an acknowledged database (Figure S1). The genes of Z. cucurbitae were clustered together with homologous genes from other Diptera insects, such as B. dorsalis and Musca domestica (M. domestica), demonstrating the accuracy of the odor-sensing genes of Z. cucurbitae. For example, in the phylogenetic analysis of IR genes (Figure 1), genes BcucIR25a and BcucIR21a from Z. cucurbitae and genes BdorIR25a and BdorIR21a from B. dorsalis formed a cluster with a short branching length, indicating high homology with the defined IR genes of B. dorsalis.

Figure 1.
Construction of phylogenetic analysis of IR genes based on gene sequences of Z. cucuribitae and several Diptera insects. Genes from Z. cucuribitae are marked in blue. Notes: Acer: Apis Cerana, Zcuc: Zeugodacus Cucurbitae, Bdor: Bactrocera Dorsalis, Bole: Bactrocera Oleae, Dmel: Drosophila Melanogaster, Mdom: Musca Domestica, Dana: Drosophila Ananassae, Dsim: Drosophila Simulans.
2.1.2. KEGG and GO Enrichment
In the KEGG and GO enrichment analysis of the olfactory sensory genes of Z. cucurbitae, 16 pathways and 20 GO terms were identified (Figure 2A,B). The results show that the odor recognition gene LOC105211704 was enriched in 16 Kegg pathways, including Systemic Lupus Erythematosus [Br: ko05322], Nicotine Addiction [Br: ko05033], Long-term Potentiation [Br: ko04720], Ion Channels [Br: ko04750], and Glutamatergic Synapse [Br: ko04724]. Previous studies have shown that Ion Channels [Br: ko04750] could affect the odor perception of the pea aphid Acyrthosiphon pisum to the host [32], and Glutamatergic Synapse [Br: ko04724] was related to neuronal development in Drosophila melanogaster and nerve sensation [33]. In addition, a total of 120 genes were enriched into 20 GO terms, including the sensory perception of chemical stimuli [GO:0007606] and the sensory perception of smell [GO:0007608]. According to studies, the sensory perception of smell [GO:0007608] has been shown to be closely related to OR and IR in Locusta migratoria [34].
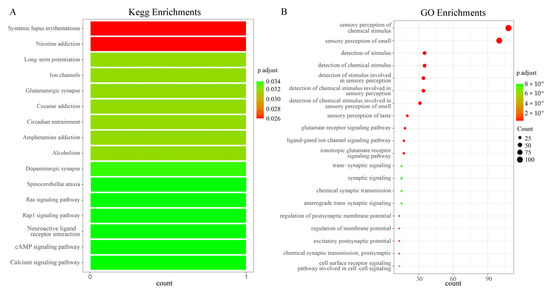
Figure 2.
KEGG and GO enrichment. (A) KEGG enrichment; (B) GO enrichment.
2.1.3. Metabolite Analysis of L. acutangular, L. cylindrica, S. edule, B. oleracea, M. nana, and F. ananassa
A total of 165 compounds of the six non-favored hosts, L. acutangular, L. cylindrica, S. edule, B. oleracea, M. nana, and F. ananassa, were collected from the literature. Among these 165 compounds, 55, 46, 10, 27, 57, and 24 compounds were from L. acutangular, L. cylindrical, S. edule, B. oleracea, M. nana, and F. ananassa, respectively (Table 1).

Table 1.
Analysis of L. acutangular, L. cylindrica, S. edule, B. oleracea, M. nana, and F. ananassa.
2.2. Molecular Docking
To identify whether the compounds of six non-favored hosts could effectively bind to the odorant proteins of Z. cucurbitae, molecular docking was performed on 166 compounds, and the proteins were translated by 196 olfactory sensory genes. A total of 239,475 relationships were obtained from the dockings.
The molecular docking information was evaluated with binding affinity, and the effective binding affinity ranged from −5.00 kcal/mol to −18.00 kcal/mol. The results show that A total of 160 compounds had affinity values equal to or lower than −5.00 kcal/mol, indicating that these compounds could successfully combine with the proteins of olfactory sensory genes of Z. cucurbitae. A total of 154 compounds had affinity values equal to or lower than −6.00 kcal/mol, and 40 compounds had affinity values equal to or lower than −10.00 kcal/mol. These 40 compounds had particularly strong binding capabilities and can be considered for further use.
Among the 196 genes encoding the proteins of olfactory sensory genes (Figure 3A), the average affinities of all genes were equal to or lower than −5.00 kcal/mol (Figure 3B), indicating that these compounds had strong affinity with the proteins of olfactory sensory genes of Z. cucurbitae.
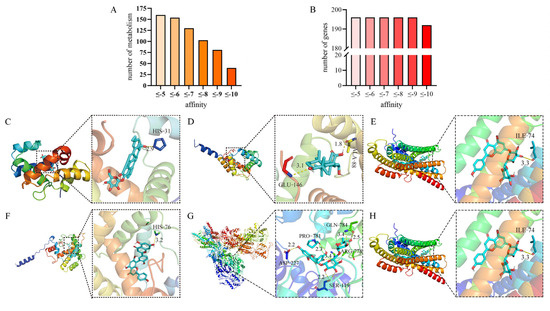
Figure 3.
A visualization of the docking of olfactory sensory genes with the compounds of hosts. (A) The numbers of compounds with different affinities between −5 kcal/mol and 18 kcal/mol were counted. (B) The numbers of genes with different affinities between −5 kcal/mol and −18 kcal/mol were counted. (C) The prediction results of molecular docking between maslinic acid 3-O-b-D-glucoside and the LOC105217972 gene of Luffa cylindrica. (D) The prediction of the combination of the metabolic compound echinocystic acid and the gene LOC105218953 of Luffa cylindrica. (E) The prediction of the combination of isorhoifolin and the gene LOC105215418. (F) The prediction of the combination of kaempferol, a metabolic compound of banana, and the gene LOC105209256. (G) The prediction of the combination of rutin, a metabolic compound of cauliflower, and the gene LOC105209182. (H) The prediction of the combination of damascenone, a metabolic compound of strawberry, and the gene LOC105217972. Notes: The molecular docking of Ligand X with Protein Receptor Y is shown. The figure illustrates the binding mode of Ligand X (shown in stick representation, colored by atom type, with carbon shown in blue and oxygen shown in red) within the active site of Protein Receptor Y (shown in cartoon representation, colored by secondary structure). Ligand X (stick representation) is shown interacting with key residues (labeled) of the receptor (cartoon representation). Hydrogen bonds are depicted as dashed lines.
Furthermore, according to the docking results, the affinity values of maslinic acid 3-O-β-D-glucoside, lucyoside H, and lucyoside G were the lowest, indicating that among all collected compounds, these three compounds had the strongest binding capabilities with the odor-sensing genes. Additionally, the genes LOC105218953, LOC105209256, and LOC105215418 had the lowest affinity in all docking results of olfactory sensory genes, suggesting that these genes might be more sensitive to the compounds of the six hosts. Z. cucurbitae was likely to recognize harmful metabolites produced by the hosts through these genes and produce repellent or stomach toxicity effects.
The most effective combinations in each host are shown in Figure 3C–H. The maslinic acid 3-O-b-D-glucoside existing in L. acutangular could effectively bind with the gene LOC105217972 with a binding energy of −9.28 kcal/mol (Figure 3C). The echinocystic acid existing in L. cylindrica could effectively bind with the gene LOC105218953 with a binding energy of −11.71 kcal/mol (Figure 3D). The isorhoifolin existing in S. edule could effectively bind with the gene LOC105215418 with a binding energy of −11.16 kcal/mol (Figure 3E). The kaempferol existing in M. nana could effectively bind with the gene LOC105209256 with a binding energy of −11.80 kcal/mol (Figure 3F). The rutin existing in B. oleracea could effectively bind with the gene LOC105209182 with a binding energy of −6.98 kcal/mol (Figure 3G). The damascenone existing in F. ananassa could effectively bind with the gene LOC105217972 with a binding energy of −7.73 kcal/mol (Figure 3H).
According to reports, maslinic acid 3-O-b-D-glucoside was transformed by maslinic acid, which had a potential effect on pest control [71]. Echinocystic acid was isolated from Eclipta prostrate and played an important role in insect control by inhibiting the activation of NF-κB [72]. Isorhoifolin is a flavonoid, which played an important role in insect prevention and control by affecting the OR and GR of insects [73]. Rutin significantly reduced the feeding behavior and reproductive ability of Bemisia tabaci by regulating the defense mechanism of plants [74]. Rutin and quercetin could also significantly change the feeding behavior of aphids and reduce the damage on plants [75]. Damascenone, a pheromone, was released through modified cellulose nanocrystals to attract and control Sogatella furcifera [76].
2.3. Construction of CPPG Networks and Screening of Core Functional Compounds
The CPPG network was constructed based on the results of molecular docking and the KEGG and GO enrichment of olfactory sensory genes. The CPPG network contained 356 nodes, including 160 compounds, 196 genes, 16 pathways, and 20 GO terms (Figure 4). To more clearly illustrate the interactions between nodes, the top 30 compound nodes, top 30 gene nodes by degree, KEGG pathway nodes, and GO term nodes were selected to construct the CPPG network (Figure 4, Supplementary Table S1). Through an analysis of the CPPG network, the ranking of the 165 compounds from the host plants after docking with the odor recognition genes of Z. cucurbitae could be obtained. Futhermore, five compounds were randomly selected from the top 20 compounds which had the lowest degrees in the network and were used for further experiments of the two-way selections, and the gastric toxicity of oleanolic acid (C90), rotenone (C10), echinocystic acid (C91), diosmin (C72), and beta-caryophyllene oxide (C165) was finally determined. These compounds might play the most important role in Z. cucurbitae. Additionally, the nodes representing genes LOC105218013, LOC105214272, and LOC105219487 had the highest degree, indicating the greatest interaction with each compound.
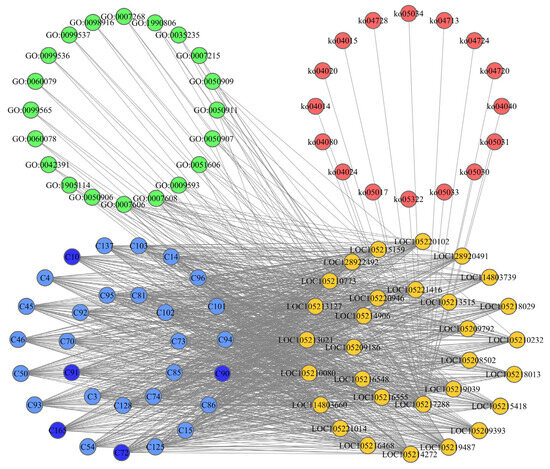
Figure 4.
A complex network node diagram of the compounds of L. acutangular, L. cylindrica, S. edule, B. oleracea, M. nana, and F. ananassa vs. Z. cucuribitae olfactory sensory genes vs. Kegg pathway vs. GO term. The core compounds are presented in blue, the Z. cucuribitae olfactory sensory genes are presented in yellow, the kegg pathway is presented in green, and the GO term is presented in red. The metabolites selected for the two-way selection test and gastric toxicity test are shown in dark blue.
2.4. Behavior Determination of Z. cucuribitae
2.4.1. Results of Two-Way Selection Experiments
The results of the two-way experiments show that oleanolic acid, rotenone, and beta-caryophyllene oxide had significant repellent effects on Z. cucurbitae at concentrations of 0.01 mg/mL, 0.1 mg/mL, and 1 mg/mL (Figure 5A,C,D). However, different concentrations of echinocystic acid and diosmin had no significant effect (Figure 5B,F). The repellent rate of 0.1 mg/mL rotenone could reach 86.67%, representing the most remarkable repellent effect on Z. cucurbitae.
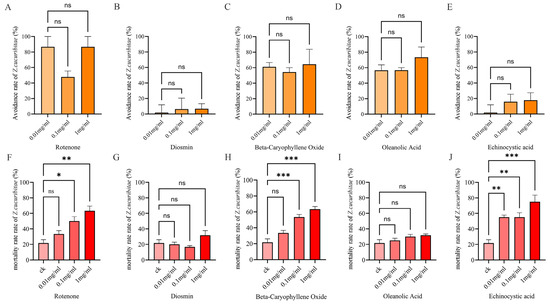
Figure 5.
Avoidance rate and mortality rate of Z. cucuribitae under different concentrations of five metabolic compounds. (A) Avoidance rate of rotenone. (B) Avoidance rate of diosmin. (C) Avoidance rate of beta-caryophyllene oxide. (D) Avoidance rate of oleanolic acid. (E) Avoidance rate of echinocystic acid. (F) Mortality rate of rotenone. (G) Mortality rate of diosmin. (H) Mortality rate of beta-caryophyllene oxide. (I) Mortality rate of oleanolic acid. (J) Mortality rate of echinocystic acid. Note: ck means control check; ns means no significance between two compared groups. * represents significant difference at p < 0.05; ** represents significant difference at p < 0.01; *** represents significant difference at p < 0.001 compared to model group.
2.4.2. Results of Gastric Toxicity Experiments
The results of the gastric toxicity experiments show that the mortality of Z. cucurbitae in the beta-caryophyllene oxide treatment reached 63.33% and 53.33% at concentrations of 1 mg/mL and 0.1 mg/mL, respectively (Figure 5F), which were significantly higher than the control (21.67%). The mortality rate of Z. cucurbitae in the rotenone treatment reached 63.33% and 50.00% at concentrations of 1 mg/mL and 0.1 mg/mL, respectively (Figure 5H), indicating a significant toxic effect on Z. cucurbitae. Echinocystic acid had significant insecticidal activity at concentrations of 1 mg/mL, 0.1 mg/mL, and 0.01 mg/mL, with mortality rates of 75.33%, 55.33%, and 55.00%, respectively (Figure 5J). Among these, the highest mortality was observed at a concentration of 1 mg/mL, and echinocystic acid also had a significant repellent effect at all concentrations. However, different concentrations of diosmin did not show significant effects in the experiment (Figure 5G,I).
These compounds also had certain control effects on other insects. According to reports, beta-caryophyllene oxide isolated from plants had obvious repellent activity against Tribolium castaneum and sitophilus granarius L. [77,78] and exhibited considerable fumigation toxicity and repellent characteristics toward Callosobruchus chinensis [79]. In addition, oleanolic acid had significant larvicidal activity against Aedes aegypti L. [80] and the significant antifeedant activity against spodoptera litura F. [81]. Rotenone is a broad-spectrum insecticidal active ingredient, which has a cytotoxic effect on SL-1 cells and significantly inhibits the larval growth of Spodoptera litura [82,83]. Furthermore, rotenone could disrupt the energy metabolism and mitochondrial dysfunction of Bombus terrestis glial cells and damage intestinal peristalsis [84].
2.5. Predicted Results of Molecular Mechanisms
The results of the two-way selection experiments and gastric toxicity experiments verified the effective effects of oleanolic acid, rotenone, and beta-caryophyllene oxide on Z. cucurbitae. The control effects of these compounds on Z. cucurbitae were achieved through the joint action of genes and pathways. According to the complex network, the targets of the successful actions of these compounds could be identified. Through the constructed CGGP network, we identified the gene LOC105217288. After the KEGG enrichment of LOC105217288, it was found that these compounds were mainly enriched in two pathways, the neuroactive ligand–receptor interaction (ko04080) and calcium signaling (ko04020) pathways. By marking the activated genes in these two pathways, we determined a molecular mechanism of the influence of the compounds apigenin, testosterone propionate, and biochanin A on the behavior of Z. cucurbitae (Figure 6). These compounds further activate proteins located on the cell membrane by activating the gene LOC105217288, including the glutamate receptor ionotropic NMDA1 (GRIN), glutamate receptor 1 (GRI), and nicotinic acetylcholine receptor alpha-7 (nAChRα7). These targets further affect downstream pathways by activating calcium ions and calmodulin (CALM), such as the MAPK signaling pathway (ko04010), apoptosis (ko04210), and long-term depression (ko04730). Eventually, Z. cucurbitae exhibits avoidance behavior and even death. Reports have shown that neonicotinoids, such as imidacloprid, could activate neuroactive ligand–receptor interaction (ko04080), leading to the dysfunction of insect nervous system and subsequently causing oxidative stress, mitochondrial dysfunction, inflammatory reaction, and apoptosis [85]. By combining RNA-Seq with isobaric ectopic tags for relative and absolute quantitative (iTRAQ) analysis, it was found that the calcium signaling pathway (ko04020) plays an important role in the adaptation and toxic reaction of Bursaphelenchus mucronatus to the host [86].
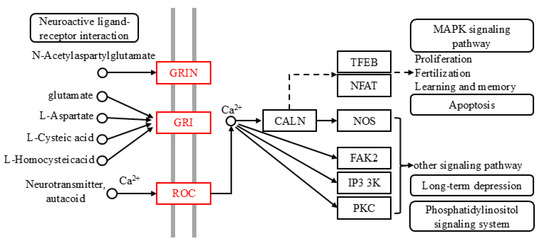
Figure 6.
Mechanism deduction of effect of effective compounds on odor recognition of Z. cucurbitae. Red nodes represent activated genes. Notes: GRIN: glutamate receptor ionotropic; GRI: glutamate receptor 1; ROC: nicotinic acetylcholine receptor alpha-7; CALN: calmodulin; TFEB: transcription factor EB; NFAT: nuclear factor of activated T-cells, cytoplasmic 1; NOS: nitric-oxide synthase, brain; FAK2: focal adhesion kinase 2; IP3 3K: 1D-myo-inositol-triphosphate 3-kinase; PKC: classical protein kinase C alpha type.
3. Discussions
Z. cucurbitae is a destructive pest which can damage a wide variety of fruits and vegetables, including L. acutangular, L. cylindrica, and S. edule. Currently, chemical pesticides are the main method in controlling Z. cucurbitae. However, this method has obvious limitations, such as the limited effectiveness of deltamethrin and abamectin [3,4,7]. Previous studies have shown that plant metabolites had good control effects and economic value in pest control. For instance, azadirachtin isolated from Azadirachta indica was proven to effectively control pests [87,88]. Host plants also contain insect-resistant metabolites to defend against insect damage [89,90,91]. Long-term observations indicate that although Z. cucurbitae can harm L. acutangular, L. cylindrica, S. edule, B. oleracea, M. nana, and F. ananassa, the damage is not severe [16,92]. It has been speculated that both cucurbitaceous hosts, such as L. acutangular, L. cylindrica, and S. edule, and non-cucurbitaceous hosts, such as B. oleracea, M. nana, and F. ananassa, contain metabolites that adversely affect Z. cucurbitae, thereby reducing the damage caused by this pest. These potential metabolites are worthy of further exploration and study for the prevention and control of Z. cucurbitae.
The compounds of L. acutangular, L. cylindrica, S. edule, B. oleracea, M. nana, and F. ananassa were collected from the literature. Meanwhile, the olfactory sensory genes of Z. cucurbitae were gathered based on its genome. Molecular docking was then conducted between these compounds and the olfactory sensory genes. Moreover, the genes were enriched via the KEGG pathway and GO term analyses, and a CPPG network was constructed. Through these steps, important compounds that may have potential effects on Z. cucurbitae were identified.
In the two-way selection experiment, oleanolic acid (0.01 mg/mL, 0.1 mg/mL, and 1 mg/mL), rotenone (0.01 mg/mL, 0.1 mg/mL, and 1 mg/mL), and beta-caryophyllene oxide (1 mg/mL and 0.1 mg/mL) were successfully screened. In the two-way selection experiment, echinocystic acid (0.01 mg/mL, 0.1 mg/mL, and 1 mg/mL), rotenone (0.1 mg/mL and 1 mg/mL), and beta-caryophyllene oxide (1 mg/mL and 0.1 mg/mL) have significant repellent effects against Z. cucurbitae. In the gastric toxicity experiment, echinocystic acid (0.01 mg/mL, 0.1 mg/mL, and 1 mg/mL), rotenone (0.1 mg/mL and 1 mg/mL), and beta-caryophyllene oxide (0.1 mg/mL and 1 mg/mL) had significant gastric toxicity against Z. cucurbitae. Among these compounds, rotenone has been reported as a widely used botanical insecticide, which has various insecticidal activities, including neurotoxicity against Spodoptera litura and Bombus terrestis [82,83]. Beta-caryophyllene oxide has been widely reported to have significant toxicity against Sitophilus Granarius L. and Callosobruchus chinensis [77,78,79]. Oleanolic acid has been found to have had significant antifeedant activity against Aedes aegypti L. and spodoptera litura F. [80,81]. However, there is still a lack of studies of echinocystic acid in controlling pests, indicating that this compound is worth being further studied.
After verifying the effects of oleanolic acid, rotenone, and beta-caryophyllene oxide on Z. cucurbitae, the gene LOC105217288 was identified through the KEGG enrichment of the gene set successfully docked with these compounds. Mechanism prediction was carried out after constructing a composite pathway, and it was found that LOC105217288 is mainly involved in the neuroactive ligand–receptor interaction (ko04080) and calcium signaling (ko04020) pathways. In these pathways, the proteins glutamate receptor ionotropic NMDA1 (GRIN), glutamate receptor 1 (GRI), and nicotinic acetylcholine receptor alpha-7 (nAChRα7) were activated, affecting downstream proteins and pathways. In the composite pathway, calmodulin (CALM) was activated, which influenced pathways such as the MAPK signaling pathway (ko04010), apoptosis (ko04210), and long-term depression (ko04730). Neuroactive ligand–receptor interaction (ko04080) and calcium signaling (ko04020) pathways have been widely reported in insect research and are closely related to insect avoidance and death [85,86]. Additionally, the activation of these pathways could indirectly affect downstream pathways, such as the MAPK signaling pathway (ko04010), apoptosis (ko04210), and long-term depression (ko04730). Transcriptome analysis showed that the expression of the MAPK signaling pathway (ko04010) changed after Bombyx mori was parasitized by Exorista japonica and played an important role in the response of insects to parasitic stress [93]. Transcriptome analysis and PCR-RFLP analysis showed that the differential genes of Aphis gossypii and Aedes aegypti, after being treated with pyrethroid insecticides, were closely related to the genes in the apoptosis pathway (ko04210) [94,95].
4. Materials and Methods
4.1. Insect Rearing
Z. cucuribitae was reared in the Invasive Pest Laboratory, Hainan University (Haikou, China). The larvae of the Z. cucuribitae colony were provided with an artificial larval diet mixture of 50 g of yeast extract, 250 g of wheat bran powder, 50 g of sugar, 1 g of sodium benzoate, 50 g of paper, and 400 mL of water. Adults were fed artificial diets of a 3:1 ratio of sucrose/yeast extract. All experimental adults were maintained in cages (60 × 60 × 60 cm) at 27 ± 1 °C under a 16 h/8 h light/dark cycle at a relative humidity of 70 ± 5% [96].
4.2. Identification and Functional Enrichment of Odor-Sensing Genes from Z. cucuribitae
Odor-sensing genes sets, including the OBP, PBP, OR, IR, GR, and SNMP genes of Z. cucuribitae, were collected based on the genome data (ID: GCF028554725.1) which was obtained from the NCBI database (https://www.ncbi.nlm.nih.gov/, accessed on 16 January 2025). In this process, phylogenetic analysis of the odor-sensing genes of Z. cucurbitae was conducted to verify the reliability of the gene function [20]. A phylogenetic tree was constructed and visually displayed using the APE package and the ggTree package of R software (Version 4.3.1) (R Foundation for Statistical Computing, Vienna, Austria) [97].
Among the genetic data, the GR data set contains 29 sequences from several species, such as Z. cucuribitae, Drosophila melanogaster (D. melanogaster), and B. dorsalis. The IR data set contains 8 sequences from several species, such as Z. cucuribitae, D. melanogaster, and M. domestica. The OBPGOBP data set contains 49 sequences from several species, such as Z. cucuribitae, Apis Cerana (A. Cerana), and Drosophila Simulans. The OR data set contains 95 sequences from several species, such as Z. cucuribitae, D. melanogaster, and A. cerana. The PBP data set contains 7 sequences from several species, such as Z. cucuribitae, B. dorsalis, and M. domestica. The SNMP data set contains 8 sequences from several species, such as Z. cucuribitae, B. dorsalis, and M. domestica. KEGG enrichment and GO enrichment were performed on odor-related protein targets (p < 0.05). The Cluster Profiler package of R software (Version 4.3.1) (R Foundation for Statistical Computing, Vienna, Austria) was used for enrichment analysis, and the ggplot2 package was used for visualization [98].
4.3. Acquisition of Compound Models of L. acutangular, L. cylindrica, S. edule, B. oleracea, M. nana, and F. ananassa and Protein Models of Z. cucuribitae
The compounds from six host plants were collected from published studies and integrated into the public database PubChem (https://pubchem.ncbi.nlm.nih.gov, accessed on 20 February 2025). Three-dimensional structures of compounds were collected from the PubChem database in the sdf format and converted into the mol2 format using OpenBABEL software (Version 3.1.1) (Open Babel development team, Cambridge, MA, USA) for molecular docking.
Three-dimensional protein structural models corresponding to all odor-sensing genes were predicted based on the d SWISS-MODEL (https://swissmodel.expasy.org/interactive, accessed on 5 March 2025) and saved in pdb format. Kegg pathways and GO terms related to odor-sensing genes were collected using KEGG and GO enrichment. The Cluster Profiler package of R software (Version 4.3.1) (R Foundation for Statistical Computing, Vienna, Austria) was used for enrichment analysis, and the ggplot2 package was used for visualization.
4.4. CPPG Networks and Screening of Core Functional Compounds
4.4.1. Molecular Docking of Olfactory Sensory Genes and Metabolic Compounds
All docking relationships between compounds of hosts and odor-sensing genes of Z. cucuribitae were obtained by molecular docking with AutoDock-VINA (Version 1.1.2) (The Scripps Research Institute, La Jolla, CA, USA). In order to evaluate the results of molecular docking, the affinity value from molecular docking was used to evaluate the effectiveness of protein binding with chemical compounds. A lower affinity value represents better binding energy. When the affinity value was equal to or less than −5 kcal/mol and greater than or equal to −18 kcal/mol, it was considered that the protein can effectively bind with chemical compounds [99,100]. Finally, PyMOL (Version 2.5.4) (Schrödinger, Inc., New York, NY, USA) software was used for visualization.
Data analysis was conducted using Poisson distribution for a non-normal distribution in a generalized linear model. Then, analysis of variance (ANOVA) and multiple comparisons were used for significance analysis. Finally, a histogram was created using the ggplot2 package. The data were analyzed using the R software (version 4.3.1) (R Foundation for Statistical Computing, Vienna, Austria) and multcomp, glm, emmeans, lsmeans, ggsignif, and ggplot2 packages.
4.4.2. Construction of CPPG Networks and Screening of Core Functional Compounds
Network visualization was carried out using Cytoscape (Version 3.9.1) (Cytoscape Consortium, Seattle, WA, USA). Based on the results of molecular docking, a CPPG network was constructed with metabolites from L. acutangular, L. cylindrica, S. edule, B. oleracea, M. nana, and F. ananassa, olfactory sensory genes of Z. cucurbitae, KEGG pathways, and GO terms as nodes. To clarify the relationships between different nodes, the degree of each node was used as an index to judge the importance of nodes. The degree was calculated as the product of the number of successful docking results and the average binding energy for that node. The higher the degree was, the more important the node was in the network, and the node played a more significant role in the comprehensive effect relationships among genes, compounds, KEGG pathways, and GO terms [29,30,101].
4.5. Effect of Core Compounds on Behavior of Z. cucuribitae
4.5.1. Two-Way Selection Experiment
A two-way selection experiment was conducted in an independent and ventilated laboratory. The behavior measurement laboratory was equipped with an exhaust fan for ventilation, maintained in a dark environment with a temperature of 25 ± 2 °C, a relative humidity of 70 ± 5%, and a photoperiod of 16 h:8 h. Each compound to be verified was dissolved in 5 µL of dimethyl sulfoxide (DMSO, RT, 99%), and distilled water was added. The concentration of DMSO used in the cell experiments needed to be less than 0.1% [29]. All Z. cucurbitae were starved for 24 h. Each compound was prepared into solutions of 0.01 mg/mL, 0.1 mg/mL, and 1 mg/mL, respectively. Screen cages (60 cm × 60 cm × 60 cm) were placed in the behavior measurement laboratory, and two thick slices of Cucurbita pepo (C. pepo) (radius ≈ 5 cm, thickness ≈ 2 cm) were placed in each cage. One slice was evenly coated with the treatment group liquid on the surface, and the other was coated with a DMSO solution of the same concentration as the control. Fifty adult Z. cucurbitae (male/female = 1:1) were placed in each cage, and the numbers on the C. pepo slices regarding the treatment and control were determined after 10 min. To reduce the possibility of interference between treatments as much as possible, the avoidance behavior test was carried out in an independent room, and each treatment was repeated 3 times.
4.5.2. Gastric Toxicity Experiment
Based on the methods of the two-way selection experiment described above, the compounds to be verified were prepared into solutions of 0.01 mg/mL, 0.1 mg/mL, and 1 mg/mL. The experiment was also conducted in an independent laboratory. An amount of 10 mL of each treatment group liquid of the solution was poured into a 250 mL conical flask (DaLong Experimental Instrument, Beijing, China), shaken well, and then slowly rotated for 1 min to form a uniform film on the flask wall. When the flask was dry, 30 adult Z. cucurbitae (male/female = 1:1) were placed in a conical flask coated with the drug film for 24 h and then transferred to a clean 250 mL conical flask (DaLong Experimental Instrument, Beijing, China) for observation. Each treatment was repeated three times.
4.6. Predicted Mechanism of Core Compounds
After verification using the two-way selection experiment and gastric toxicity experiment, genes of verified core components were collected in CPPG network. Furthermore, the KEGG enrichment results linked to genes were also found and collected. The maps of these pathways were obtained from the KEGG database, and the positions of these genes in the pathways were accurately marked. Combined with the genes and products of the upstream and downstream pathways of these genes, the action mechanism of composite pathways was predicted. Furthermore, in combination with the positions of marked genes, the influence of metabolic reactions or signal transduction events on the operation of the entire pathway was discussed.
5. Conclusions
In this study, the combination of network pharmacology and molecular docking technology was used to screen the components that have a repellent effect on Z. cucurbitae. This method comprehensively analyzes the relationship compounds of six non-favored hosts (L. acutangular, L. cylindrica, S. edule, B. oleracea, M. nana, and F. ananassa) and odor-sensing genes of Z. cucurbitae by constructing a CPPG network. Through this method, the compounds rotenone, beta-caryophyllene oxide, and echinocystic acid were successfully identified to exhibit significant repellent effects. These compounds were first found to have significant insecticidal activities against Z. cucurbitae. This study lays a solid foundation for further studies on the repellent mechanisms of Z. cucurbitae and the development of new and efficient control strategies. Future research will further explore the mechanisms, optimization, and applications of these metabolites to provide a scientific reference for the effective prevention and control of Z. cucurbitae.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms26146556/s1.
Author Contributions
F.C., Y.C., Z.Z. and S.J. created the concept and designed the study. Y.F. and Y.C. conducted the analyses and wrote the manuscript. Y.W. participated in data analysis, figure design, and revision. X.F., C.Y., X.B., Y.L. and W.M. participated in data analysis. X.G., X.L. and R.Y. contributed to the revision and proofreading of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the project of the Regional Collaborative Prevention and Control of Major Invasive Species: Technical System Design and Eco-Economic Evaluation (project leader: Fengqin Cao; Grant No. 2022YFC2601405).
Institutional Review Board Statement
Z. cucuribitae is not included in the “List of Endangered and Protected Animals in China” because it is a major pest in tropical and sub-tropical countries. All experiments were performed in compliance with the general ethical guidelines in order to minimize pain and discomfort to the insects.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| A. Cerana | Apis cerana |
| Acer | Apis cerana |
| Z. cucuribitae | Zeugodacus cucuribitae (Coquillett) |
| B. dorsalis | Bactrocera dorsalis |
| B. oleracea | Brassica oleracea var. botrytis |
| Bdor | Bactrocera dorsalis |
| Bole | Bactrocera oleae |
| C.pepo | Cucurbita pepo |
| CPPG | Genes vs. components vs. Kegg pathways vs. GO terms |
| D. melanogaster | Drosophila melanogaster |
| Dana | Drosophila ananassae |
| Dmel | Drosophila melanogaster |
| Dsim | Drosophila simulans |
| F. ananassa | Fragaria × ananassa |
| GR | Olfactory receptor |
| IR | Ionospheric receptor |
| L. cylindrica | Luffa cylindrica |
| L. acutangular | Luffa acutangular |
| M. domestica | Musca domestica |
| M. nana | Musa nana |
| Mdom | Musca domestica |
| OBPGOBP | Odor-binding protein and general odorant-binding proteins |
| OR | Olfactory receptor |
| PBP | Pheromone-binding protein |
| S. edule | Sechium edule |
| SNMP | Sensory neuron membrane protein |
| Bcuc | Zeugodacus cucuribitae (Coquillett) |
References
- Dhillon, M.K.; Singh, R.; Naresh, J.S.; Sharma, H.C. The Melon Fruit Fly, Bactrocera Cucurbitae: A Re-view of Its Biology and Management. J. Insect Sci. 2005, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Koyama, J.; Kakinohana, H.; Miyatake, T. Eradication of the Melon Fly, Bactrocera Cucurbitae, in Japan: Importance of Behavior, Ecology, Genetics, and Evolution. Annu. Rev. Entomol. 2004, 49, 331–349. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, M.; Delatte, H.; Mwatawala, M.; Quilici, S.; Vayssières, J.F.; Virgilio, M. A Review of the Current Knowledge on Zeugodacus Cucurbitae (Coquillett) (Diptera, Tephritidae) in Africa, with a List of Species Included in Zeugodacus. Zookeys 2015, 540, 539–557. [Google Scholar] [CrossRef]
- Dorla, E.; Gauvin-Bialecki, A.; Deuscher, Z.; Allibert, A.; Grondin, I.; Deguine, J.P.; Laurent, P. Insecticidal Activity of the Leaf Essential Oil of Peperomia Borbonensis Miq. (Piperaceae) and Its Major Components against the Melon Fly Bactrocera Cucurbitae (Diptera: Tephri-tidae). Chem. Biodivers. 2017, 14, e1600493. [Google Scholar] [CrossRef]
- Hsu, J.-C.; Haymer, D.S.; Chou, M.-Y.; Feng, H.-T.; Chen, H.-H.; Huang, Y.-B.; Mau, R.F.L. Monitoring Resistance to Spinosad in the Melon Fly (Bactrocera cucurbitae) in Hawaii and Taiwan. Sci. World J. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Vargas, R.I.; Piñero, J.C.; Leblanc, L. An Overview of Pest Species of Bactrocera Fruit Flies (Diptera: Tephritidae) and the Integration of Biopesticides with Other Biological Approaches for Their Management with a Focus on the Pacific Region. Insects 2015, 6, 297–318. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current Status of Pesticide Effects on Environ-ment, Human Health and It’s Eco-Friendly Management as Bioremediation: A Comprehensive Review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Wan, N.-F.; Fu, L.; Dainese, M.; Kiær, L.P.; Hu, Y.-Q.; Xin, F.; Goulson, D.; Woodcock, B.A.; Vanbergen, A.J.; Spurgeon, D.J.; et al. Pesticides Have Negative Effects on Non-Target Organisms. Nat. Commun. 2025, 16, 1360. [Google Scholar] [CrossRef]
- Nikolova, I. Pea weevil damage and chemical characteristics of pea cultivars determining their resistance to Bruchus pisorum L. Bull. Èntomol. Res. 2016, 106, 268–277. [Google Scholar] [CrossRef]
- Rubiales, D.; Barilli, E.; Rispail, N. Breeding for Biotic Stress Resistance in Pea. Agriculture 2023, 13, 1825. [Google Scholar] [CrossRef]
- Hisashi, K.-N. Defensive Molecules Momilactones a and B: Function, Biosynthesis, Induction and Occurrence. Toxins 2023, 15, 241. [Google Scholar] [CrossRef] [PubMed]
- Ramniwas, S.; Bilal, T.; Sharma, A. Repellent Activity of Salix Alba Bark Extract and Guava Oil-Based Formulation against the Oriental Fruit Fly, Bactrocera Dorsalis (Diptera: Tephritidae). Int. J. Trop. Insect Sci. 2024, 44, 831–842. [Google Scholar] [CrossRef]
- Akram, W.; Hussain, A.; Abbas, Q.; Abbas, A. Insecticidal Potential of Botanical Ex-tracts for Management of, the Oriental Fruit Fly, Bactrocera Dorsalis (Diptera: Tephritidae). J. Basic Appl. Zool. 2024, 85, 63. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Hussain, B.; Buhroo, A.A.; Ignacimuthu, S.; Sharma, H.C. Effect of Plant Secondary Metabolites on Legume Pod Borer, Helicoverpa Armigera. J. Pest Sci. 2013, 86, 399–408. [Google Scholar] [CrossRef]
- Li, L.; Han, D.Y.; Niu, L.M.; Zhang, F.P.; Chen, J.Y.; Fu, Y.G. Performance Evaluation of Melon Fruit Fly, Zeugodacus Cucurbitae on 39 Hosts. J. Environ. Entomol. 2019, 41, 1057–1064. [Google Scholar]
- Farooq, M.; Baig, S.; Honey, S.F.; Bajwa, B.E.; Fazlullah; Shah, I.H. Evaluation of host susceptibility, preference and offspring performance of Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae) on different hosts. Int. J. Trop. Insect Sci. 2019, 40, 93–99. [Google Scholar] [CrossRef]
- Zeng, B.; Lian, Y.; Jia, J.; Liu, Y.; Wang, A.; Yang, H.; Li, J.; Yang, S.; Peng, S.; Zhou, S. Multigenerational Effects of Short-Term High Temperature on the Development and Reproduction of the Zeugodacus cucurbitae (Coquillett, 1899). Agriculture 2022, 12, 954. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health (PLH); Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.; Miret, J.A.J.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; et al. Scientific opinion on the import of Musa fruits as a pathway for the entry of non-EU Tephritidae into the EU territory. EFSA J. 2021, 19, e06426. [Google Scholar] [CrossRef]
- Vieira, F.G.; Rozas, J. Comparative Genomics of the Odorant-Binding and Chemosensory Protein Gene Families across the Arthropoda: Origin and Evolutionary History of the Chemosensory System. Genome Biol. Evol. 2011, 3, 476–490. [Google Scholar] [CrossRef]
- Hua, Y. Insect Olfactory Neurons: Receptors, Development, and Function. Curr. Opin. Insect Sci. 2025, 67, 101288. [Google Scholar]
- Wicher, D.; Miazzi, F. Functional properties of insect olfactory receptors: Ionotropic receptors and odorant receptors. Cell Tissue Res. 2021, 383, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yang, M.; Dong, K.; Zhang, J.; Wang, H.; Xie, M.; Wu, W.; Zhang, Y.-J.; Chen, Z. Structural Insights into the Ligand-Binding and -Releasing Mechanism of Helicoverpa armigera Pheromone-Binding Protein PBP1. Int. J. Mol. Sci. 2022, 23, 1190. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, J.; Zhang, Z.; Yan, M.; Zhang, B.; Zhu, C.; Yan, W.; Shi, B.; Wang, Y.; Zhao, C.; et al. A plant virus enhances odorant-binding protein 5 (OBP5) in the vector whitefly for more actively olfactory orientation to the host plant. Pest Manag. Sci. 2022, 79, 1410–1419. [Google Scholar] [CrossRef]
- Guo, R.; Zheng, Q.; Zhang, L.; Rajasingh, J. Identification and validation of diagnostic markers and drugs for pediatric bronchopulmonary dysplasia based on integrating bioinformatics and molecular docking analysis. PLoS ONE 2025, 20, e0323006. [Google Scholar] [CrossRef]
- Wang, Z.; Li, R.; Chen, X.; Ren, H.; Wang, C.; Min, R.; Zhang, X. Network pharmacology, molecular docking and experimental validation to elucidate the anti-T2DM mechanism of Lanxangia tsaoko. Fitoterapia 2024, 178, 106117. [Google Scholar] [CrossRef]
- Dan, Y.; Jin, Y.; Wang, J.; Wang, L.; Zheng, D. Combination of RNA-sequencing data analysis, network pharmacology, molecular docking techniques to investigate the mechanism of Prunella vulgaris L. in the treatment of non-small cell lung cancer. Discov. Oncol. 2025, 16, 726. [Google Scholar] [CrossRef]
- Bisht, A.; Tewari, D.; Kumar, S.; Chandra, S. Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation to Elucidate the Mechanism of Anti-Aging Action of Tino-spora Cordifolia. Mol. Divers. 2024, 28, 1743–1763. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, Y.; Wang, D.; Xie, X.; Li, G.; Zheng, C.; Wen, J.; Su, H.; Liu, X.; Zeng, L.; et al. The Effects of Nine Compounds on Aldehyde-Oxidase-Related Genes in Bactrocera dorsalis (Hendel). Genes 2023, 15, 35. [Google Scholar] [CrossRef]
- Wang, J.J.; Ma, C.; Tian, Z.Y.; Zhou, Y.P.; Yang, J.F.; Gao, X.; Chen, H.S.; Ma, W.H.; Zhou, Z.S. Electroantennographic and Behavioral Responses of the Melon Fly, Zeugo-dacus Cucurbitae (Coquillett), to Volatile Compounds of Ridge Gourd, Luffa acutangular L. J. Chem. Ecol. 2024, 50, 1036–1045. [Google Scholar] [CrossRef]
- Ebeed, H.; Baz, M.; Habib, E.; Prabhu, S.; Ceasar, S.A. Integrated Metabolomic Analysis and Molecular Docking: Unveiling the Potential of Nephrolepis Exaltata (L.) Schott Phytocompounds for Mosquito Control Via Glutathione-S-Transferase Targeting. Int. J. Biol. Macromol. 2024, 273, 133072. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiu, L.; Wang, B.; Guan, Z.; Dong, Z.; Zhang, J.; Cao, S.; Yang, L.; Wang, B.; Gong, Z.; et al. Structural basis for odorant recognition of the insect odorant receptor OR-Orco heterocomplex. Science 2024, 384, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Newman, Z.L.; Bakshinskaya, D.; Schultz, R.; Kenny, S.J.; Moon, S.; Aghi, K.; Stanley, C.; Marnani, N.; Li, R.; Bleier, J.; et al. Determinants of synapse diversity revealed by super-resolution quantal transmission and active zone imaging. Nat. Commun. 2022, 13, 229. [Google Scholar] [CrossRef]
- Jiang, X.; Dimitriou, E.; Grabe, V.; Sun, R.; Chang, H.; Zhang, Y.; Gershenzon, J.; Rybak, J.; Hansson, B.S.; Sachse, S. Ring-shaped odor coding in the antennal lobe of migratory locusts. Cell 2024, 187, 3973–3991.e24. [Google Scholar] [CrossRef]
- Patel, S.B.; Ghane, S.G. Phyto-Constituents Profiling of Luffa Echinata and in Vitro As-sessment of Antioxidant, Anti-Diabetic, Anticancer and Anti-Acetylcholine Esterase Activities. Saudi J. Biol. Sci. 2021, 28, 3835–3846. [Google Scholar] [CrossRef]
- Chanda, J.; Mukherjee, P.K.; Biswas, R.; Biswas, S.; Tiwari, A.K.; Pargaonkar, A. Uplc-Qtof-Ms Analysis of a Carbonic Anhydrase-Inhibiting Extract and Fractions of (L.) Roxb (Ridge Gourd). Phytochem. Anal. 2019, 30, 148–155. [Google Scholar] [CrossRef]
- Ceci, C.; Lacal, P.M.; Tentori, L.; De Martino, M.G.; Miano, R.; Graziani, G. Experimental Evidence of the Antitumor, Antimetastatic and Antiangiogenic Activity of Ellagic Ac-id. Nutrients 2018, 10, 1756. [Google Scholar] [CrossRef]
- Ben Hlel, T.; Belhadj, F.; Gül, F.; Altun, M.; Yağlıoğlu, A.Ş.; Demirtaş, I.; Marzouki, M.N. Variations in the Bioactive Compounds Composition and Biological Activities of Loofah (Luffa cylindrica) Fruits in Relation to Maturation Stages. Chem. Biodivers. 2017, 14, e1700178. [Google Scholar] [CrossRef]
- Gedük, A.Ş.; Zengin, F. Lc–Ms/Ms Characterization, Antidiabetic, Antioxidative, and An-tibacterial Effects of Different Solvent Extracts of Anamur Banana (Musa Cavendishii). Food Sci. Biotechnol. 2021, 30, 1183–1193. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Kusznierewicz, B.; Wiczkowski, W.; Płatosz, N.; Bartoszek, A. Phytochemical Composition and Biological Activities of Differently Pigmented Cabbage (Bras-sica Oleracea Var. Capitata) and Cauliflower (Brassica Oleracea Var. Botrytis) Varieties. J. Sci. Food Agric. 2019, 99, 5499–5507. [Google Scholar] [CrossRef]
- Park, S.-Y.; Lim, S.-H.; Ha, S.-H.; Yeo, Y.; Park, W.T.; Kwon, D.Y.; Park, S.U.; Kim, J.K. Metabolite Profiling Approach Reveals the Interface of Primary and Secondary Metabolism in Colored Cauliflowers (Brassica oleracea L. ssp. botrytis). J. Agric. Food Chem. 2013, 61, 6999–7007. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Rosario, A.C.R.S.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.S.; El-Hefny, M.; Ghoneim, I.M.; El-Lahot, M.S.R.A.; Akrami, M.; Al-Huqail, A.A.; Ali, H.M.; Abd-Elkader, D.Y. Assessing the Use of Aloe vera Gel Alone and in Combination with Lemongrass Essential Oil as a Coating Material for Strawberry Fruits: HPLC and EDX Analyses. Coatings 2022, 12, 489. [Google Scholar] [CrossRef]
- Maamoun, A.A.; El-Akkad, R.H.; Farag, M.A. Mapping metabolome changes in Luffa aegyptiaca Mill fruits at different maturation stages via MS-based metabolomics and chemometrics. J. Adv. Res. 2021, 29, 179–189. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a Flavonoid, as an Anticancer Agent: A Review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Díaz-De-Cerio, E.; Verardo, V.; Fernández-Gutiérrez, A.; Gómez-Caravaca, A.M. New insight into phenolic composition of chayote (Sechium edule (Jacq.) Sw.). Food Chem. 2019, 295, 514–519. [Google Scholar] [CrossRef]
- Singh, A.S.; Vellapandian, C. Phytochemical Studies, Antioxidant Potential, and Identification of Bioactive Compounds Using Gc–Ms of the Ethanolic Extract of Luffa cylindrica (L.) Fruit. Appl. Biochem. Biotechnol. 2022, 194, 4018–4032. [Google Scholar]
- Hu, W.; Wang, X.; Wu, L.; Shen, T.; Ji, L.; Zhao, X.; Si, C.L.; Jiang, Y.; Wang, G. Apigenin-7-O-Β-D-Glucuronide Inhibits Lps-Induced Inflammation through the Inac-tivation of Ap-1 and Mapk Signaling Pathways in Raw 264.7 Macrophages and Protects Mice against En-dotoxin Shock. Food Funct. 2016, 7, 1002–1013. [Google Scholar] [CrossRef]
- Nath, L.R.; Gorantla, J.N.; Joseph, S.M.; Antony, J.; Thankachan, S.; Menon, D.B.; Sankar, S.; Lankalapalli, R.S.; Anto, R.J. Kaempferide, the most active among the four flavonoids isolated and characterized from Chromolaena odorata, induces apoptosis in cervical cancer cells while being pharmacologically safe. RSC Adv. 2015, 5, 100912–100922. [Google Scholar] [CrossRef]
- Arab, H.H.; Salama, S.A.; Omar, H.A.; Arafa, E.-S.A.; Maghrabi, I.A.; Sen, U. Diosmin Protects against Ethanol-Induced Gastric Injury in Rats: Novel Anti-Ulcer Actions. PLoS ONE 2015, 10, e0122417. [Google Scholar] [CrossRef]
- Siciliano, T.; De Tommasi, N.; Morelli, I.; Braca, A. Study of Flavonoids of Sechium edule (Jacq) Swartz (Cucurbitaceae) Different Edible Organs by Liquid Chromatography Photodiode Array Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 6510–6515. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Yang, H.; Son, G.W.; Park, H.R.; Park, C.S.; Jin, Y.H.; Park, Y.S. Eriodictyol Protects Endothelial Cells against Oxidative Stress-Induced Cell Death through Modu-lating Erk/Nrf2/Are-Dependent Heme Oxygenase-1 Expression. Int. J. Mol. Sci. 2015, 16, 14526–14539. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A Review of the Most Recent Research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Isemura, M. Catechin in Human Health and Disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, T.; Zhu, X.; Yang, C.; Wang, Y.; Zhou, N.; Ju, B.; Zhou, T.; Deng, G.; Qiu, C. Hyperoside Induces Breast Cancer Cells Apoptosis via ROS-Mediated NF-κB Signaling Pathway. Int. J. Mol. Sci. 2019, 21, 131. [Google Scholar] [CrossRef]
- Govindarasu, M.; Thangaraj, K.; Murugesan, V.; Vaiyapuri, M. Kaempferitrin Cause Cell Cycle Arrest at G2/M Phase and Reactive Oxygen Species Mediated Apoptosis in Human Colon Cancer Ht-29 Cells. Int. J. Recent Technol. Eng. Int. J. Recent Technol. Eng. 2020, 8, 2277–3878. [Google Scholar]
- Zhang, Y.; Guo, Y.; Wang, M.; Dong, H.; Zhang, J.; Zhang, L. Quercetrin from Toona sinensis leaves induces cell cycle arrest and apoptosis via enhancement of oxidative stress in human colorectal cancer SW620 cells. Oncol. Rep. 2017, 38, 3319–3326. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Locatelli, M.; Granica, S.; Cacciagrano, F.; Tomczyk, M. A Review on the Dietary Flavonoid Tiliroside. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1395–1421. [Google Scholar] [CrossRef]
- Kim, S.M.; Park, Y.J.; Shin, M.-S.; Kim, H.-R.; Kim, M.J.; Lee, S.H.; Yun, S.P.; Kwon, S.-H. Acacetin inhibits neuronal cell death induced by 6-hydroxydopamine in cellular Parkinson’s disease model. Bioorganic Med. Chem. Lett. 2017, 27, 5207–5212. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Liu, H.; Li, P.; Zhang, H.; Cheng, G. Fortunellin Pro-tects against High Fructose-Induced Diabetic Heart Injury in Mice by Suppressing Inflammation and Oxi-dative Stress Via Ampk/Nrf-2 Pathway Regulation. Biochem. Biophys. Res. Commun. 2017, 490, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fu, B.; Lu, F.; Hu, X.; Tang, J.; Huang, L. Inhibitory activity of linarin on osteoclastogenesis through receptor activator of nuclear factor κB ligand-induced NF-κB pathway. Biochem. Biophys. Res. Commun. 2018, 495, 2133–2138. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Hu, X.; Gong, D.; Zhang, G. Inhibitory mechanism of vitexin on α-glucosidase and its synergy with acarbose. Food Hydrocoll. 2020, 105, 105824. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Li, M.-F.; Yang, Y.-S.; Li, R.; Tan, J.; Tang, S.-H.; Jiang, Z.-T. Composition, cytotoxicity and antioxidant activities of polyphenols in the leaves of star anise (Illicium verum Hook. f.). ScienceAsia 2019, 45, 532–537. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic Acid and Its De-rivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef]
- Yu, Q.; Li, X.; Cao, X. Linarin Could Protect Myocardial Tissue from the Injury of Ische-mia-Reperfusion through Activating Nrf-2. Biomed. Pharmacother. 2017, 90, 1–7. [Google Scholar] [CrossRef]
- Karayildirim, T.; Ozmen, A.; Emirdag-Ozturk, S.; Capaci-Karagoez, A.; Poyrazoglu-Coban, E. Synthesis, Antimicrobial and Cytotoxic Activities, and Structure-Activity Relationships of Gyp-sogenin Derivatives against Human Cancer Cells. Eur. J. Med. Chem. Chim. 2014, 82, 565–573. [Google Scholar]
- Ren, Y.; Shen, L.; Zhang, D.W.; Dai, S.J. Two New Sesquiterpenoids from Solanum Lyratum with Cytotoxic Activities. Chem. Pharm. Bull. 2009, 57, 408–410. [Google Scholar] [CrossRef]
- Subagio, A.; Morita, N.; Sawada, S. Carotenoids and Their Fatty-Acid Esters in Bana-na Peel. J. Nutr. Sci. Vitaminol. 1996, 42, 553–566. [Google Scholar] [CrossRef]
- Cozzolino, R.; Pace, B.; Palumbo, M.; Laurino, C.; Picariello, G.; Siano, F.; De Giulio, B.; Pelosi, S.; Cefola, M. Profiles of Volatile and Phenolic Compounds as Markers of Ripening Stage in Candonga Strawberries. Foods 2021, 10, 3102. [Google Scholar] [CrossRef]
- Hu, F.; Chen, J.; Zhang, Y.; Sun, Y.; Liu, Y.; Yu, Y.; Xu, K.; Cai, H. Novel Bio-transformation of Maslinic Acid to Ma-2-O-Β-D-Glucoside by Udp-Glycosyltransferases from Bacillus Sub-tilis. Catalysts 2022, 12, 884. [Google Scholar] [CrossRef]
- Ryu, S.; Shin, J.S.; Jung, J.Y.; Cho, Y.W.; Kim, S.J.; Jang, D.S.; Lee, K.T. Echinocystic Acid Isolated from Eclipta Prostrata Suppresses Lipopolysaccharide-Induced Inos, Tnf-A, and Il-6 Expressions Via Nf-Κb Inac-tivation in Raw 264.7 Macrophages. Planta Med. 2013, 79, 1031–1037. [Google Scholar] [PubMed]
- Riddick, E.W. Evaluating the Effects of Flavonoids on Insects: Implications for Managing Pests Without Harming Beneficials. Insects 2024, 15, 956. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.; Wang, X.W.; Elkahky, M. Transitioning Towards Dynamic, Na-ture-Based Crop Defenses. J. Biosci. 2024, 49, 99. [Google Scholar] [CrossRef]
- Stec, K.; Kordan, B.; Gabryś, B. Quercetin and Rutin as Modifiers of Aphid Probing Behavior. Molecules 2021, 26, 3622. [Google Scholar] [CrossRef]
- Scott-Fordsmand, J.J.; Fraceto, L.F.; Amorim, M.J.B. Nano-pesticides: The lunch-box principle—Deadly goodies (semio-chemical functionalised nanoparticles that deliver pesticide only to target species). J. Nanobiotechnology 2022, 20, 13. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Campos, J.M.; da Silva Rolim, G.; Martínez, L.C.; Dos Santos, M.; Fernandes, F.L.; Serrão, J.E.; Zanuncio, J.C. Terpenoid constituents of cinnamon and clove essential oils cause toxic effects and behavior repellency response on granary weevil, Sitophilus granarius. Ecotoxicol. Environ. Saf. 2018, 156, 263–270. [Google Scholar] [CrossRef]
- You, C.-X.; Zhang, W.-J.; Guo, S.-S.; Wang, C.-F.; Yang, K.; Liang, J.-Y.; Wang, Y.; Geng, Z.-F.; Du, S.-S.; Deng, Z.-W. Chemical composition of essential oils extracted from six Murraya species and their repellent activity against Tribolium castaneum. Ind. Crop. Prod. 2015, 76, 681–687. [Google Scholar] [CrossRef]
- Hazarika, M.; Nath, P.; Begum, T.; Lal, M.; Kalita, J.; Dutta, P. In-sights into Insecticidal Efficacy of Cymbopogon Essential Oils against Callosobruchus Chinensis: An Inte-grated Approach through Bioassays and in-Silico Molecular Docking for Sustainable Pest Management. J. Stored Prod. Res. 2025, 112, 102655. [Google Scholar] [CrossRef]
- de Oliveira, P.M.C.; Sousa, J.P.B.; Albernaz, L.C.; Coelho-Ferreira, M.; Espindola, L.S. Bioprospection for new larvicides against Aedes aegypti based on ethnoknowledge from the Amazonian São Sebastião de Marinaú riverside community. J. Ethnopharmacol. 2022, 293, 115284. [Google Scholar] [CrossRef]
- Mallavadhani, U.V.; Mahapatra, A.; Raja, S.S.; Manjula, C. Antifeedant Activity of Some Pentacyclic Triterpene Acids and Their Fatty Acid Ester Analogues. J. Agric. Food Chem. 2003, 51, 1952–1955. [Google Scholar] [CrossRef] [PubMed]
- Cen, Y.; Zou, X.; Li, L.; Chen, S.; Lin, Y.; Liu, L.; Zheng, S. Inhibition of the glutathione biosynthetic pathway increases phytochemical toxicity to Spodoptera litura and Nilaparvata lugens. Pestic. Biochem. Physiol. 2020, 168, 104632. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xue, L.; Li, Y.; Cui, G.; Sun, R.; Hu, M.; Zhong, G. Rote-none-Induced Necrosis in Insect Cells Via the Cytoplasmic Membrane Damage and Mitochondrial Dys-function. Pestic. Biochem. Physiol. 2021, 173, 104801. [Google Scholar] [CrossRef]
- Chen, J.; Mu, X.; Liu, H.; Yong, Q.; Ouyang, X.; Liu, Y.; Zheng, L.; Chen, H.; Zhai, Y.; Ma, J.; et al. Rotenone impairs brain glial energetics and locomotor behavior in bumblebees. Sci. Total. Environ. 2023, 907, 167870. [Google Scholar] [CrossRef]
- Wei, F.; Wang, D.; Li, H.; Xia, P.; Ran, Y.; You, J. Toxicogenomics Provides Insights to Toxicity Pathways of Neonicotinoids to Aquatic Insect, Chironomus Dilutus. Environ. Pollut. 2020, 260, 114011. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, F.; Pan, H.; Ye, J.; Dong, X.; Li, C.; Lin, F. Identifying Virulence-Associated Genes Using Transcriptomic and Proteomic Association Analyses of the Plant Parasitic Nematode Bursaphelenchus mucronatus. Int. J. Mol. Sci. 2016, 17, 1492. [Google Scholar] [CrossRef]
- Dua, V.K.; Pandey, A.C.; Raghavendra, K.; Gupta, A.; Sharma, T.; Dash, A.P. Larvicidal activity of neem oil (Azadirachta indica) formulation against mosquitoes. Malar. J. 2009, 8, 124. [Google Scholar] [CrossRef]
- Ayinde, A.; Morakinyo, O.; Sridhar, M. Repellency and larvicidal activities of Azadirachta indica seed oil on Anopheles gambiae in Nigeria. Heliyon 2020, 6, e03920. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, J.; Huang, Y.; Liu, Y.; Hu, G.; Yan, W.; Yan, G.; Guo, Q.; Shi, J.; Han, R.; et al. Sustainable pest management using plant secondary metabolites regulated azadirachtin nano-assemblies. Nat. Commun. 2025, 16, 1721. [Google Scholar] [CrossRef]
- Bae, M.; Lewis, A.; Liu, S.; Arcot, Y.; Lin, Y.-T.; Bernal, J.S.; Cisneros-Zevallos, L.; Akbulut, M. Novel Biopesticides Based on Nanoencapsulation of Azadirachtin with Whey Protein to Control Fall Armyworm. J. Agric. Food Chem. 2022, 70, 7900–7910. [Google Scholar] [CrossRef]
- Iqbal, N.; Hazra, D.K.; Purkait, A.; Agrawal, A.; Kumar, J. Bioengineering of neem nano-formulation with adjuvant for better adhesion over applied surface to give long term insect control. Colloids Surf. B Biointerfaces 2022, 209, 112176. [Google Scholar] [CrossRef] [PubMed]
- Nazir, N.; Fatima, S.; Aasim, M.; Yaqoob, F.; Mahmood, K.; Ali, S.A.; Awan, S.I.; Haq, I.U. Zeugodacus fruit flies (Diptera: Tephritidae) host preference analysis by machine learning-based approaches. Comput. Electron. Agric. 2024, 222, 109095. [Google Scholar] [CrossRef]
- Dai, M.; Yang, J.; Liu, X.; Gu, H.; Li, F.; Li, B.; Wei, J. Parasitism by the Tachinid Parasitoid Exorista japonica Leads to Suppression of Basal Metabolism and Activation of Immune Response in the Host Bombyx mori. Insects 2022, 13, 792. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, H.; Adelman, Z.N. Transcriptomic analyses of Aedes aegypti cultured cells and ex vivo midguts in response to an excess or deficiency of heme: A quest for transcriptionally-regulated heme transporters. BMC Genom. 2020, 21, 604. [Google Scholar] [CrossRef]
- Marshall, K.L.; Moran, C.; Chen, Y.; Herron, G.A. Detection of Kdr Pyre-throid Resistance in the Cotton Aphid, Aphis Gossypii (Hemiptera: Aphididae), Using a Pcr-Rflp Assay. J. Pestic. Sci. 2012, 37, 169–172. [Google Scholar] [CrossRef]
- Lixiang, C.; Zhenya, T.; Weihua, M.; Jingjing, W.; Qiaofen, H.; Yongping, Z.; Xuyuan, G.; Hongsong, C.; Zhongshi, Z. Comparison of bacterial diversity in Bactrocera cucurbitae (Coquillett) ovaries and eggs based on 16S rRNA sequencing. Sci. Rep. 2023, 13, 1–15. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. Clusterprofiler: An R Package for Comparing Biological Themes among Gene Clusters. OMICS A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y. KEGG Mapper for inferring cellular functions from protein sequences. Protein Sci. 2020, 29, 28–35. [Google Scholar] [CrossRef]
- Li, J.; Fu, A.; Zhang, L. An Overview of Scoring Functions Used for Protein–Ligand Interactions in Molecular Docking. Interdiscip. Sci. Comput. Life Sci. 2019, 11, 320–328. [Google Scholar] [CrossRef]
- Elhenawy, A.A.; Al-Harbi, L.M.; El-Gazzar, M.A.; Khowdiary, M.M.; Alosaimi, A.M.; elhamid Salim, A. Naproxenylamino Acid Derivatives: Design, Synthesis, Docking, Qsar and Anti-Inflammatory and Analgesic Activity. Biomed. Pharmacother. 2019, 116, 109024. [Google Scholar] [CrossRef]
- Wu, B.; Lan, X.; Chen, X.; Wu, Q.; Yang, Y.; Wang, Y. Researching the molecular mechanisms of Taohong Siwu Decoction in the treatment of varicocele-associated male infertility using network pharmacology and molecular docking: A review. Medicine 2023, 102, e34476. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).