Biomarkers of Metabolism and Inflammation in Individuals with Obesity and Normal Weight: A Comparative Analysis Exploring Sex Differences
Abstract
1. Introduction
2. Results
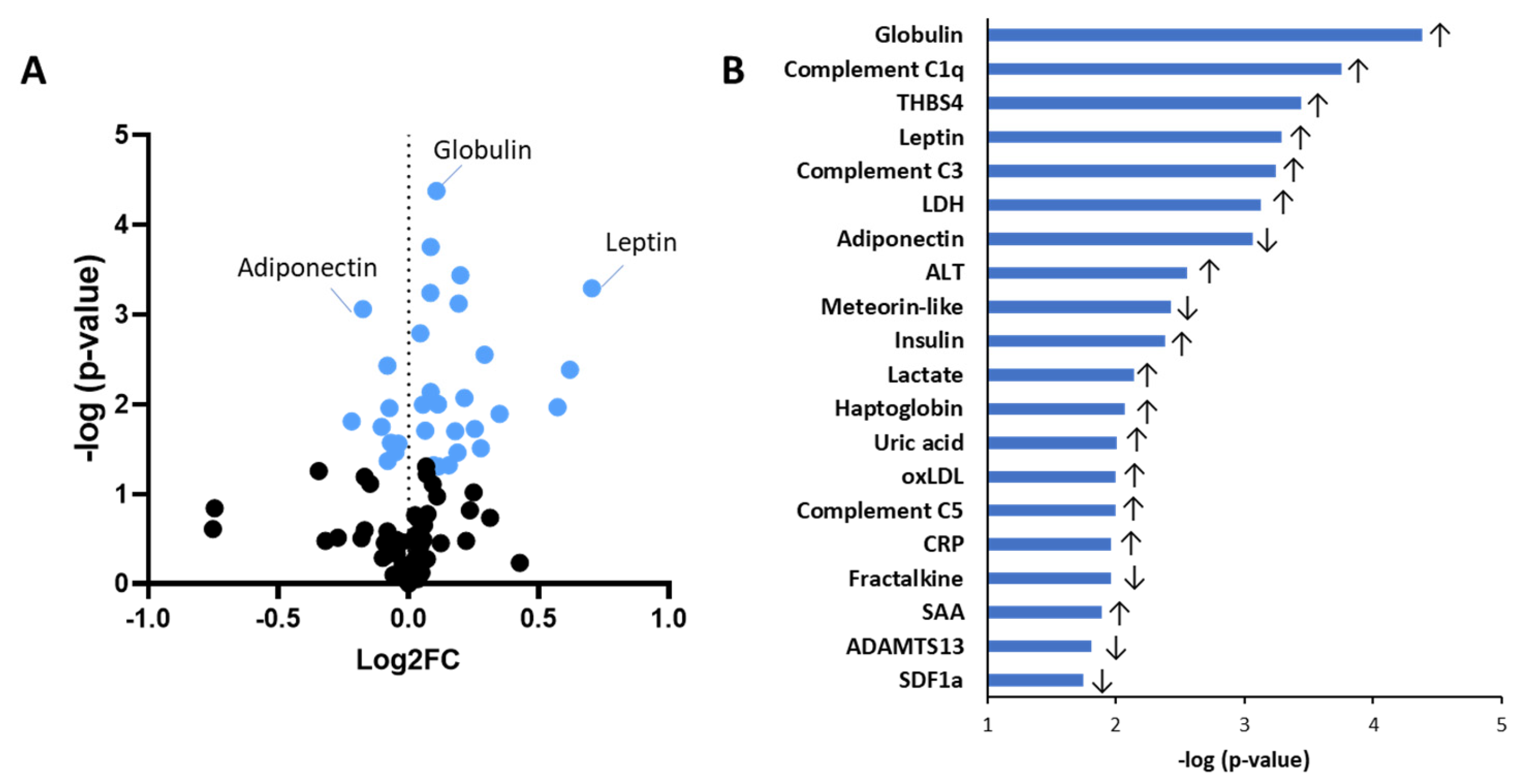
2.1. Obesity-Associated Differences in Circulating Biomarkers Independent of Sex
2.2. Sex Differences in Biomarker Expression in the Lean Group
2.3. Sex Differences in Biomarker Expression in the Obese Group
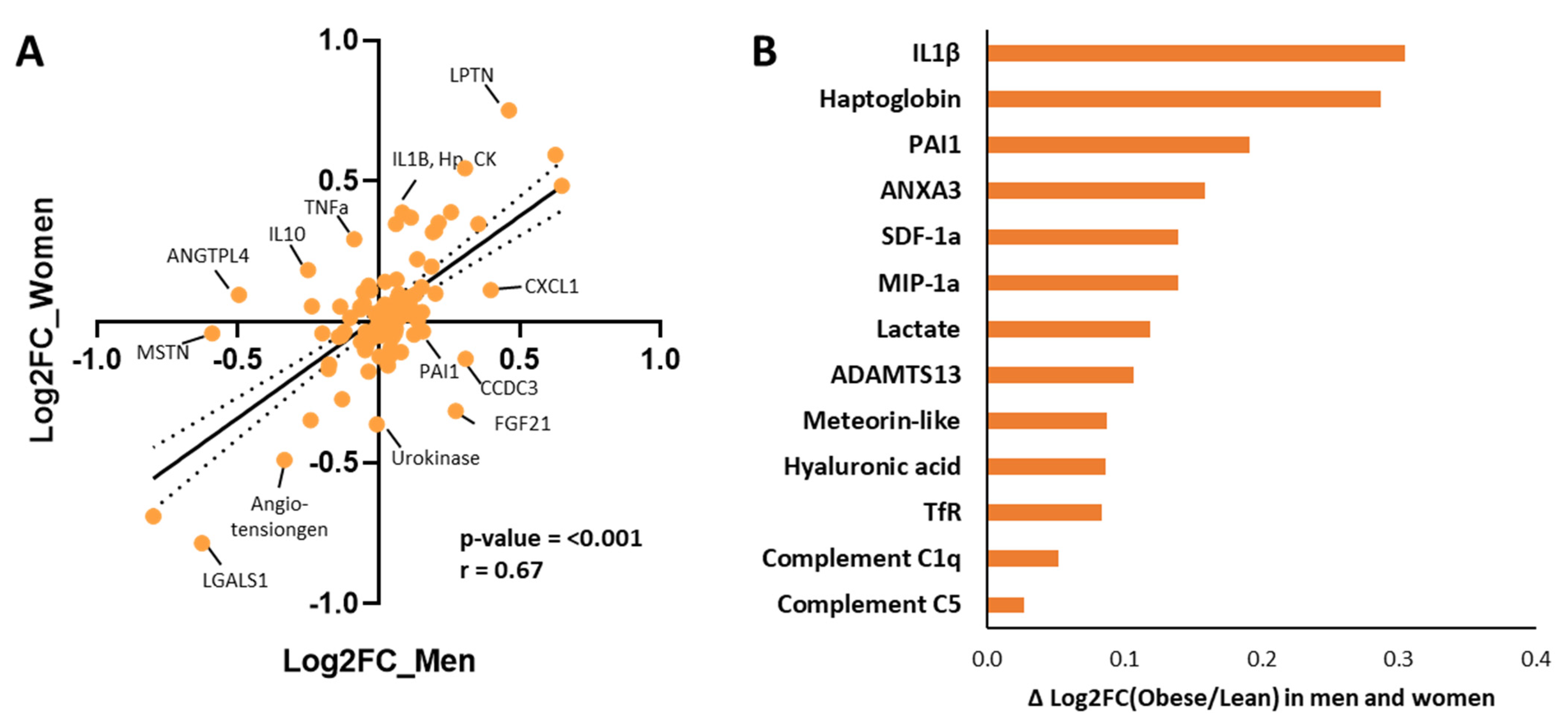
2.4. Sex-Specific Biomarker Direction Regulation
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Blood Samples of Normal-Weight and Obese Women and Men
5.2. Biomarker Assays
5.3. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, A.; Sun, H.; Yan, G.; Wang, P.; Wang, X. Metabolomics for Biomarker Discovery: Moving to the Clinic. Biomed Res. Int. 2015, 2015, 354671. [Google Scholar] [CrossRef]
- Vreeken, D.; Seidel, F.; Custers, E.M.; Olsthoorn, L.; Cools, S.; Aarts, E.O.; Kleemann, R.; Kessels, R.P.C.; Wiesmann, M.; Hazebroek, E.J.; et al. Factors Associated With Cognitive Improvement After Bariatric Surgery Among Patients With Severe Obesity in the Netherlands. JAMA Netw. Open 2023, 6, e2315936. [Google Scholar] [CrossRef] [PubMed]
- Vreeken, D.; Seidel, F.; de La Roij, G.; Vening, W.; den Hengst, W.A.; Verschuren, L.; Özsezen, S.; Kessels, R.P.C.; Duering, M.; Mutsaerts, H.J.M.M.; et al. Impact of White Adipose Tissue on Brain Structure, Perfusion, and Cognitive Function in Patients with Severe Obesity. Neurology 2023, 100, E703–E718. [Google Scholar] [CrossRef] [PubMed]
- Schutte, M.H.; Kleemann, R.; Nota, N.M.; Wiepjes, C.M.; Snabel, J.M.; T’Sjoen, G.; Thijs, A.; Heijer, M. Den The Effect of Transdermal Gender-Affirming Hormone Therapy on Markers of Inflammation and Hemostasis. PLoS ONE 2022, 17, e0261312. [Google Scholar] [CrossRef]
- Tyagi, T.; Jain, K.; Gu, S.X.; Qiu, M.; Gu, V.W.; Melchinger, H.; Rinder, H.; Martin, K.A.; Gardiner, E.E.; Lee, A.I.; et al. A Guide to Molecular and Functional Investigations of Platelets to Bridge Basic and Clinical Sciences. Nat. Cardiovasc. Res. 2022, 1, 223–237. [Google Scholar] [CrossRef]
- Stroncek, D.; Slezak, S.; Khuu, H.; Basil, C.; Tisdale, J.; Leitman, S.F.; Marincola, F.M.; Panelli, M.C. Proteomic Signature of Myeloproliferation and Neutrophilia: Analysis of Serum and Plasma from Healthy Subjects given Granulocyte Colony-Stimulating Factor. Exp. Hematol. 2005, 33, 1109–1117. [Google Scholar] [CrossRef]
- Weisman, C.S.; Cassard, S.D. Health Consequences of Exclusion or Underrepresentation of Women in Clinical Studies (I). In Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies; National Academies Press: Washington, DC, USA, 1994; Volume 2. [Google Scholar]
- Mauvais-Jarvis, F.; Bairey Merz, N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and Gender: Modifiers of Health, Disease, and Medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Willemars, M.M.A.; Nabben, M.; Verdonschot, J.A.J.; Hoes, M.F. Evaluation of the Interaction of Sex Hormones and Cardiovascular Function and Health. Curr. Heart Fail. Rep. 2022, 19, 200–212. [Google Scholar] [CrossRef]
- Pinquart, M.; Sörensen, S. Gender Differences in Caregiver Stressors, Social Resources, and Health: An Updated Meta-Analysis. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2006, 61, P33–P45. [Google Scholar] [CrossRef]
- Costanzo, M.; Caterino, M.; Sotgiu, G.; Ruoppolo, M.; Franconi, F.; Campesi, I. Sex Differences in the Human Metabolome. Biol. Sex Differ. 2022, 13, 30. [Google Scholar] [CrossRef]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Cooper, A.J.; Gupta, S.R.; Moustafa, A.F.; Chao, A.M. Sex/Gender Differences in Obesity Prevalence, Comorbidities, and Treatment. Curr. Obes. Rep. 2021, 10, 458–466. [Google Scholar] [CrossRef]
- Gervois, P.; Kleemann, R.; Pilon, A.; Percevault, F.; Koenig, W.; Staels, B.; Kooistra, T. Global Suppression of IL-6-Induced Acute Phase Response Gene Expression after Chronic in Vivo Treatment with the Peroxisome Proliferator-Activated Receptor-α Activator Fenofibrate. J. Biol. Chem. 2004, 279, 16154–16160. [Google Scholar] [CrossRef]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial Dysfunction: Cardiovascular Risk Factors, Therapy, and Outcome. Vasc. Health Risk Manag. 2005, 1, 183. [Google Scholar]
- Schrover, I.M.; van der Graaf, Y.; Spiering, W.; Visseren, F.L.J. The Relation between Body Fat Distribution, Plasma Concentrations ofadipokines and the Metabolic Syndrome in Patients with Clinically Manifest Disease. Eur. J. Prev. Cardiol. 2018, 25, 1548. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2021, 11, 2248. [Google Scholar] [CrossRef] [PubMed]
- Knutson, M.D. Iron Transport Proteins: Gateways of Cellular and Systemic Iron Homeostasis. J. Biol. Chem. 2017, 292, 12735. [Google Scholar] [CrossRef]
- Ludwiczek, S.; Aigner, E.; Theurl, I.; Weiss, G. Cytokine-Mediated Regulation of Iron Transport in Human Monocytic Cells. Blood 2003, 101, 4148–4154. [Google Scholar] [CrossRef]
- Qiu, F.; Wu, L.; Yang, G.; Zhang, C.; Liu, X.; Sun, X.; Chen, X.; Wang, N. The Role of Iron Metabolism in Chronic Diseases Related to Obesity. Mol. Med. 2022, 28, 130. [Google Scholar] [CrossRef]
- Cate, V.T.; Koeck, T.; Prochaska, J.; Schulz, A.; Panova-Noeva, M.; Rapp, S.; Eggebrecht, L.; Lenz, M.; Glunz, J.; Sauer, M.; et al. A Targeted Proteomics Investigation of the Obesity Paradox in Venous Thromboembolism. Blood Adv. 2021, 5, 2909. [Google Scholar] [CrossRef]
- Alizadeh, H. Meteorin-like Protein (Metrnl): A Metabolic Syndrome Biomarker and an Exercise Mediator. Cytokine 2022, 157, 155952. [Google Scholar] [CrossRef]
- Muennig, P.; Lubetkin, E.; Jia, H.; Franks, P. Gender and the Burden of Disease Attributable to Obesity. Am. J. Public Health 2006, 96, 1662. [Google Scholar] [CrossRef]
- Kapoor, N.; Arora, S.; Kalra, S. Gender Disparities in People Living with Obesity-An Unchartered Territory. J. Mid-Life Health 2021, 12, 103–107. [Google Scholar] [CrossRef]
- Bays, H.E.; Bindlish, S.; Clayton, T.L. Obesity, Diabetes Mellitus, and Cardiometabolic Risk: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2023. Obes. Pillars 2023, 5, 100056. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-K.; Ahima, R.S. Physiology of Leptin: Energy Homeostasis, Neuroendocrine Function and Metabolism. Metabolism 2014, 64, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, M.G.; Poppelaars, F.; Van Kooten, C.; Mollnes, T.E.; Tedesco, F.; Würzner, R.; Trouw, L.A.; Truedsson, L.; Daha, M.R.; Roos, A.; et al. Age and Sex-Associated Changes of Complement Activity and Complement Levels in a Healthy Caucasian Population. Front. Immunol. 2018, 9, 2664. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Ballantyne, C.M.; Sharrett, A.R.; Smith, L.C.; Davis, C.E.; Gotto, A.M.; Boerwinkle, E. Circulating Adhesion Molecules VCAM-1, ICAM-1, and E-Selectin in Carotid Atherosclerosis and Incident Coronary Heart Disease Cases. Circulation 1997, 96, 4219–4225. [Google Scholar] [CrossRef]
- Nieto-Lima, B.; Cano-Martínez, A.; Rubio-Ruiz, M.E.; Pérez-Torres, I.; Guarner-Lans, V. Age-, Gender-, and in Vivo Different Doses of Isoproterenol Modify in Vitro Aortic Vasoreactivity and Circulating VCAM-1. Front. Physiol. 2018, 9, 20. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef]
- Schumann, R.R.; Kirschning, C.J.; Unbehaun, A.; Aberle, H.P.; Knope, H.P.; Lamping, N.; Ulevitch, R.J.; Herrmann, F. The Lipopolysaccharide-Binding Protein Is a Secretory Class 1 Acute-Phase Protein Whose Gene Is Transcriptionally Activated by APRF/STAT/3 and Other Cytokine-Inducible Nuclear Proteins. Mol. Cell. Biol. 1996, 16, 3490. [Google Scholar] [CrossRef]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between Adipocyte Size and Adipokine Expression and Secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef]
- Morrison, M.C.; Kleemann, R. Role of Macrophage Migration Inhibitory Factor in Obesity, Insulin Resistance, Type 2 Diabetes, and Associated Hepatic Co-Morbidities: A Comprehensive Review of Human and Rodent Studies. Front. Immunol. 2015, 6, 308. [Google Scholar] [CrossRef]
- Vatier, C.; Kadiri, S.; Muscat, A.; Chapron, C.; Capeau, J.; Antoine, B. Visceral and Subcutaneous Adipose Tissue from Lean Women Respond Differently to Lipopolysaccharide-Induced Alteration of Inflammation and Glyceroneogenesis. Nutr. Diabetes 2012, 2, e51. [Google Scholar] [CrossRef] [PubMed]
- Lopes, H.F.; Corrêa-Giannella, M.L.; Consolim-Colombo, F.M.; Egan, B.M. Visceral Adiposity Syndrome. Diabetol. Metab. Syndr. 2016, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Thiele, N.D.; Wirth, J.W.; Steins, D.; Koop, A.C.; Ittrich, H.; Lohse, A.W.; Kluwe, J. TIMP-1 Is Upregulated, but Not Essential in Hepatic Fibrogenesis and Carcinogenesis in Mice. Sci. Rep. 2017, 7, 714. [Google Scholar] [CrossRef] [PubMed]
- de Jong, J.C.B.C.; Caspers, M.P.M.; Dulos, R.; Snabel, J.; van der Hoek, M.D.; van der Leij, F.R.; Kleemann, R.; Keijer, J.; Nieuwenhuizen, A.G.; van den Hoek, A.M.; et al. Blood-Based Biomarkers for Early Frailty Are Sex-Specific: Validation of a Combined in Silico Prediction and Data-Driven Approach. GeroScience 2025, 47, 3741–3758. [Google Scholar] [CrossRef]
- de Jong, J.C.B.C.; Verschuren, L.; Caspers, M.P.M.; van der Hoek, M.D.; van der Leij, F.R.; Kleemann, R.; van den Hoek, A.M.; Nieuwenhuizen, A.G.; Keijer, J. Evidence for Sex-Specific Intramuscular Changes Associated to Physical Weakness in Adults Older than 75 Years. Biol. Sex Differ. 2023, 14, 45. [Google Scholar] [CrossRef]
- O’caoimh, R.; Sezgin, D.; O’donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of Frailty in 62 Countries across the World: A Systematic Review and Meta-Analysis of Population-Level Studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef]
- de Jong, J.C.B.C.; Attema, B.J.; van der Hoek, M.D.; Verschuren, L.; Caspers, M.P.M.; Kleemann, R.; van der Leij, F.R.; van den Hoek, A.M.; Nieuwenhuizen, A.G.; Keijer, J. Sex Differences in Skeletal Muscle-Aging Trajectory: Same Processes, but with a Different Ranking. GeroScience 2023, 45, 2367–2386. [Google Scholar] [CrossRef]
- Lorenz, T.I.; Schreuders, E.; Stuldreher, I.V.; Thammasan, N.; Brouwer, A.M.; Giletta, M. The Interplay of Peer Victimization and Parasympathetic Nervous System Activity on Acute Inflammatory Stress Responses in Adolescence. Res. Child Adolesc. Psychopathol. 2024, 52, 757–771. [Google Scholar] [CrossRef]
- Bottenheft, C.; Hogenelst, K.; Stuldreher, I.; Kleemann, R.; Groen, E.; van Erp, J.; Brouwer, A.M. Understanding the Combined Effects of Sleep Deprivation and Acute Social Stress on Cognitive Performance Using a Comprehensive Approach. Brain Behav. Immun. Health 2023, 34, 100706. [Google Scholar] [CrossRef]
- Hogenelst, K.; Özsezen, S.; Kleemann, R.; Verschuren, L.; Stuldreher, I.; Bottenheft, C.; van Erp, J.; Brouwer, A.M. Seven Robust and Easy to Obtain Biomarkers to Measure Acute Stress. Brain Behav. Immun. Health 2024, 38, 100789. [Google Scholar] [CrossRef]
- Seidel, F.; Vreeken, D.; Custers, E.; Wiesmann, M.; Özsezen, S.; van Duyvenvoorde, W.; Caspers, M.; Menke, A.; Morrison, M.C.; Verschuren, L.; et al. Metabolic Dysfunction-Associated Steatotic Liver Disease Is Associated with Effects on Cerebral Perfusion and White Matter Integrity. Heliyon 2024, 10, e38516. [Google Scholar] [CrossRef]
- Kaufmann, L.K.; Custers, E.; Vreeken, D.; Snabel, J.; Morrison, M.C.; Kleemann, R.; Wiesmann, M.; Hazebroek, E.J.; Aarts, E.; Kiliaan, A.J. Additive Effects of Depression and Obesity on Neural Correlates of Inhibitory Control. J. Affect. Disord. 2024, 362, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, E.; Pronk, A.; Kleemann, R.; Vlaanderen, J.; Lan, Q.; Rothman, N.; Silverman, D.; Hoet, P.; Godderis, L.; Vermeulen, R. Cardiovascular Effects among Workers Exposed to Multiwalled Carbon Nanotubes. Occup. Environ. Med. 2018, 75, 351–358. [Google Scholar] [CrossRef]

| Normal-Weight Men | Normal-Weight Women | Mean Normal Weight | Obese Men | Obese Women | Mean Obese | |
|---|---|---|---|---|---|---|
| Adipokines | ||||||
| Adiponectin [µg/mL] | 3.74 ± 1.17 | 4.80 ± 1.46 | 4.27 ± 1.40 | 2.49 ± 0.82 | 3.38 ± 1.22 | 2.85 ± 1.07 # |
| Angiopoietin-like 4 [µg/mL] | 1.02 ± 1.83 | 0.34 ± 0.25 | 0.68 ± 1.32 | 0.33 ± 0.14 | 0.42 ± 0.53 | 0.36 ± 0.34 |
| Leptin [ng/mL] | 7.91 ± 2.50 | 12.20 ± 9.88 * | 10.97 ± 8.55 | 22.88 ± 18.96 | 68.74 ± 46.52 * | 41.23 ± 39.20 # |
| Resistin [ng/mL] | 16.61 ± 8.40 | 17.18 ± 4.23 | 16.90 ± 6.48 | 18.82 ± 8.29 | 18.62 ± 3.85 | 18.74 ± 6.72 |
| Visfatin [ng/mL] | 11.12 ± 6.02 | 9.63 ± 6.34 | 10.37 ± 6.06 | 7.36 ± 5.89 | 6.54 ± 3.53 | 7.03 ± 4.98 |
| Onset and amplification of inflammation | ||||||
| Calprotectin S100A8 [ng/mL] | 34.24 ± 47.84 | 18.77 ± 19.73 | 24.19 ± 32.03 | 31.99 ± 32.64 | 16.74 ± 20.61 | 26.66 ± 29.37 |
| Calprotectin S100A8/S100A9 [µg/mL] | 0.86 ± 1.01 | 0.56 ± 0.47 | 0.66 ± 0.70 | 1.04 ± 0.85 | 0.60 ± 0.55 | 0.88 ± 0.77 |
| CD14 [µg/mL] | 1.40 ± 0.20 | 1.55 ± 0.31 | 1.50 ± 0.28 | 1.33 ± 0.19 | 1.35 ± 0.15 | 1.34 ± 0.17 # |
| IFN-g [pg/mL] | 0.15 ± 0.18 | 0.14 ± 0.21 | 0.15 ± 0.19 | 0.09 ± 0.06 | 0.16 ± 0.09 | 0.12 ± 0.08 |
| IL-1β [pg/mL] | 0.37 ± 0.22 | 0.27 ± 0.20 | 0.32 ± 0.21 | 0.45 ± 0.39 | 0.65 ± 0.46 | 0.53 ± 0.42 |
| IL-1Ra [ng/mL] | 0.82 ± 1.55 | 0.49 ± 1.22 | 0.65 ± 1.36 | 0.61 ± 0.54 | 0.78 ± 0.75 | 0.68 ± 0.62 |
| IL-6 [pg/mL] | 0.89 ± 0.47 | 0.97 ± 0.39 | 0.93 ± 0.42 | 1.60 ± 0.97 | 2.39 ± 1.21 | 1.92 ± 1.11 # |
| IL-8 [pg/mL] | 9.05 ± 4.58 | 7.41 ± 4.12 | 8.23 ± 4.33 | 8.00 ± 4.41 | 9.45 ± 8.84 | 8.58 ± 6.37 |
| IL-10 [pg/mL] | 1.05 ± 1.02 | 0.39 ± 0.15 | 0.72 ± 0.79 | 0.59 ± 0.18 | 0.59 ± 0.25 | 0.59 ± 0.20 |
| IL-13 [pg/mL] | 1.54 ± 1.55 | 1.28 ± 1.55 | 1.41 ± 1.51 | 1.14 ± 0.92 | 0.68 ± 0.75 | 0.95 ± 0.87 |
| IL-18 [ng/mL] | 0.52 ± 0.31 | 0.39 ± 0.12 | 0.45 ± 0.24 | 0.74 ± 0.39 | 0.51 ± 0.27 | 0.65 ± 0.35 # |
| MIF [ng/mL] | 2.39 ± 2.95 | 1.09 ± 1.42 | 1.55 ± 2.10 | 1.72 ± 0.72 | 0.97 ± 0.52 * | 1.46 ± 0.74 |
| MPO [ng/mL] | 43.47 ± 14.66 | 35.61 ± 27.88 | 39.54 ± 22.05 | 42.10 ± 20.90 | 38.17 ± 10.21 | 40.53 ± 17.18 |
| TGF-β [ng/mL] | 27.41 ± 6.44 | 24.67 ± 8.72 | 26.04 ± 7.59 | 25.04 ± 11.64 | 21.22 ± 7.49 | 23.51 ± 10.14 |
| TNF-α [pg/mL] | 3.11 ± 1.70 | 2.57 ± 1.23 | 2.84 ± 1.47 | 2.54 ± 0.53 | 5.05 ± 3.60 * | 3.54 ± 2.55 |
| TNF-RII [ng/mL] | 2.89 ± 0.73 | 3.53 ± 0.96 | 3.21 ± 0.90 | 2.58 ± 0.86 | 2.80 ± 0.50 | 2.67 ± 0.73 # |
| CCL2/MCP-1 [pg/mL] | 120.16 ± 27.31 | 87.16 ± 18.78 * | 98,71 ± 26.82 | 128.90 ± 22.29 | 91.59 ± 24.81 * | 115.84 ± 29.01 |
| CCL3/MIP-1a [pg/mL] | 10.64 ± 4.87 | 12.65 ± 4.41 | 11.94 ± 4.55 | 17.34 ± 3.63 | 38.43 ± 46.18 | 24.72 ± 28.02 |
| CCL5 [ng/mL] | 74.79 ± 23.40 | 65.47 ± 31.46 | 70.13 ± 27.41 | 88.84 ± 70.07 | 72.72 ± 102.42 | 82.39 ± 82.30 |
| CXCL1/GROa [pg/mL] | 153.69 ± 64.34 | 189.70 ± 121.93 | 177.10 ± 104.92 | 383.88 ± 356.74 | 246.05 ± 111.53 | 335.64 ± 298.08 # |
| Fractalkine [ng/mL] | 6.82 ± 1.74 | 7.56 ± 1.52 | 7.31 ± 1.60 | 6.15 ± 0.98 | 6.20 ± 1.19 | 6.17 ±1.03 # |
| CXCL4/PF-4 [µg/mL] | 5.34 ± 3.05 | 4.48 ± 1.88 | 4.91 ± 2.51 | 4.61 ± 2.35 | 3.80 ± 1.81 | 4.28 ± 2.14 |
| CXCL7/NAP-2 [µg/mL] | 2.77 ± 1.09 | 2.25 ± 0.76 | 2.51 ± 0.95 | 2.70 ± 1.21 | 2.14 ± 0.77 | 2.47 ± 1.07 |
| CXCL10/IP10 [pg/mL] | 278.15 ± 192.44 | 260.32 ± 80.94 | 266.56 ± 126.13 | 361.36 ± 144.09 | 319.68 ± 74.20 | 346.77 ± 123.56 |
| CXCL11/I-TAC [pg/mL] | 42.40 ± 17.37 | 61.75 ± 23.90 | 54.98 ± 23.36 | 60.33 ± 24.11 | 66.93 ± 28.91 | 62.64 ± 25.33 |
| CXCL12/SDF-1a [pg/mL] | 1652.16 ± 403.53 | 1899.55 ± 347.81 | 1812.96 ± 377.47 | 1518.75 ± 663.21 | 1265.63 ± 363.44 | 1430.16 ± 578.66 # |
| Osteopontin [ng/mL] | 40.33 ± 16.13 | 25.49 ± 14.53 * | 32.91 ± 16.77 | 31.84 ± 11.82 | 26.36 ± 8.81 | 29.65 ± 10.82 |
| Complement system | ||||||
| C1q [µg/mL] | 26.62 ± 3.85 | 29.63 ± 3.34 | 28.12 ± 3.84 | 34.34 ± 5.16 | 33.89 ± 5.72 | 34.16 ± 5.24 # |
| C3 [mg/mL] | 0.56 ± 0.06 | 0.53 ± 0.08 | 0.54 ± 0.07 | 0.65 ± 0.13 | 0.66 ± 0.10 | 0.66 ± 0.12 # |
| C5 [µg/mL] | 72.61 ± 4.80 | 81.51 ± 7.78 * | 77.06 ± 7.77 | 85.54 ± 12.73 | 90.25 ± 18.29 | 87.42 ± 14.92 # |
| Acute-phase response | ||||||
| hs-CRP [µg/mL] | 0.73 ± 0.47 | 0.84 ± 0.84 | 0.80 ± 0.72 | 1.81 ± 1.40 | 2.30 ± 1.95 | 2.02 ± 1.62 # |
| Haptoglobin [mg/mL] | 0.89 ± 0.70 | 0.55 ± 0.35 | 0.67 ± 0.51 | 1.03 ± 0.49 | 1.21 ± 0.36 | 1.09 ± 0.45 # |
| SAA [µg/mL] | 2.35 ± 0.90 | 2.46 ± 1.27 | 2.41 ± 2.15 | 5.31 ± 3.88 | 5.48 ± 5.59 | 5.38 ± 4.50 # |
| LPS-binding protein [µg/mL] | 3.65 ± 1.29 | 3.87 ± 1.03 | 3.76 ± 1.14 | 4.00 ± 1.19 | 2.95 ± 0.79 * | 3.58 ± 1.15 |
| α1-antichymotrypsin [µg/mL] | 157.50 ± 44.72 | 193.24 ± 73.22 | 175.37 ± 61.83 | 209.72 ± 81.66 | 174.08 ± 25.21 | 195.46 ± 66.45 |
| Fetuin-A [mg/mL] | 0.65 ± 0.11 | 0.74 ± 0.27 | 0.69 ± 0.20 | 0.73 ± 0.13 | 0.75 ± 0.31 | 0.74 ± 0.21 |
| Fetuin-B [µg/mL] | 3.92 ± 0.52 | 3.96 ± 1.05 | 3.94 ± 0.80 | 4.17 ± 0.53 | 4.20 ± 0.77 | 4.18 ± 0.62 |
| Oxidative stress | ||||||
| Oxidized LDL [U/L] | 40.23 ± 9.00 | 41.84 ± 14.64 | 41.27 ± 12.71 | 54.17 ± 16.75 | 52.30 ± 14.67 | 52.52 ± 15.68 # |
| Liver health | ||||||
| Albumin [g/L] | 44.81 ± 2.64 | 41.95 ± 4.96 | 42.95 ± 4.59 | 44.53 ± 2.03 | 43.07 ± 1.75 | 44.02 ± 2.02 |
| Globulin [g/L] | 22.61 ± 3.35 | 20.35 ± 5.08 | 21.14 ± 4.59 | 26.12 ± 3.40 | 28.71 ± 2.75 | 27.03 ± 3.36 # |
| ALT [U/L] | 18.18 ± 17.52 | 11.73 ± 4.59 | 13.99 ± 10.97 | 28.78 ± 12.06 | 24.70 ± 20.56 | 27.35 ± 15.14 # |
| AST [U/L] | 23.23 ± 5.62 | 23.91 ± 5.81 | 23.67 ± 5.61 | 29.44 ± 11.74 | 29.00 ± 15.29 | 29.29 ± 12.69 |
| Fibrosis | ||||||
| Galectin-3 [ng/mL] | 6.18 ± 2.94 | 3.97 ± 2.31 | 5.07 ± 2.81 | 5.31 ± 2.76 | 4.48 ± 2.29 | 4.98 ± 2.55 |
| Hyaluronic acid [ng/mL] | 33.01 ± 19.52 | 24.52 ± 10.67 | 28.77 ± 15.92 | 78.89 ± 119.27 | 40.93 ± 16.30 | 63.71 ± 93.26 # |
| TIMP-1 [ng/mL] | 135.43 ± 16.37 | 122.91 ± 26.45 | 129.17 ± 22.35 | 151.57 ± 33.67 | 108.34 ± 14.20 * | 134.28 ± 34.68 |
| Iron metabolism | ||||||
| TfR [µg/mL] | 1.16 ± 0.14 | 1.31 ± 0.29 | 1.26 ± 0.26 | 1.50 ± 0.25 | 1.40 ± 0.30 | 1.46 ± 0.26 # |
| Transferrin (mg/mL) | 4.56 ± 1.10 | 4.78 ± 1.22 | 4.70 ± 1.15 | 4.00 ± 0.58 | 4.07 ± 0.69 | 4.03 ± 0.60 # |
| Hepcidin [ng/mL] | 6.62 ± 7.36 | 6.98 ± 10.20 | 6.86 ± 9.10 | 10.30 ± 7.26 | 14.54 ± 18.30 | 11.78 ± 11.97 |
| EPO [mIU/mL] | 107.55 ± 265.27 | 46.70 ± 117.93 | 67.99 ± 178.58 | 9.69 ± 10.10 | 8.14 ± 11.38 | 9.15 ± 10.29 |
| Vascular and endothelial activation | ||||||
| sICAM-1 [ng/mL] | 219.18 ± 43.11 | 207.70 ± 41.01 | 213.44 ± 41.37 | 239.95 ± 50.34 | 220.41 ± 40.39 | 232.14 ± 46.52 |
| E-selectin [ng/mL] | 6.02 ± 2.60 | 6.43 ± 1.72 | 6.24 ± 2.12 | 7.60 ± 2.72 | 5.02 ± 1.44 * | 6.57 ± 2.59 |
| p-selectin [ng/mL] | 79.98 ± 20.38 | 73.67 ± 26.47 | 76.82 ± 23.22 | 81.24 ± 32.42 | 65.71 ± 19.58 | 75.02 ± 28.47 |
| sVCAM-1 [ng/mL] | 487.98 ± 86.00 | 395.22 ± 68.86 * | 441.60 ± 89.52 | 418.92 ± 75.02 | 437.28 ± 170.26 | 426.26 ± 118.42 |
| Coagulation: thrombosis and fibrinolysis | ||||||
| Alpha-1 antitrypsin [mg/mL] | 2.72 ± 0.52 | 3.02 ± 1.13 | 2.92 ± 0.95 | 2.77 ± 0.50 | 2.95 ± 0.67 | 2.84 ± 0.55 |
| Fibrinogen [mg/mL] | 2.68 ± 0.86 | 2.73 ± 0.94 | 2.70 ± 0.88 | 3.02 ± 1.41 | 3.02 ± 0.92 | 3.02 ± 1.21 |
| PAI-1 [ng/mL] | 34.55 ± 12.21 | 27.33 ± 9.62 | 30.94 ± 11.32 | 49.54 ± 19.65 | 25.24 ± 14.33 * | 39.82 ± 21.18 |
| vWF-A2 [ng/mL] | 1.39 ± 1.35 | 1.04 ± 0.56 | 1.21 ± 1.03 | 1.02 ± 0.39 | 0.92 ± 0.34 | 0.98 ± 0.36 |
| ADAMTS13 [ng/mL] | 323.95 ± 187.45 | 260.14 ± 108.36 | 290.37 ± 150.20 | 185.26 ± 86.58 | 116.42 ± 80.53 | 175.43 ± 86.41 # |
| ANXA3 [ng/mL] | 3.87 ± 1.75 | 2.10 ± 1.00 | 2.72 ± 1.54 | 2.44 ± 1.04 | 1.91 ± 0.81 | 2.25 ± 0.98 |
| Blood pressure modulators | ||||||
| Angiotensinogen [ng/mL] | 465.16 ± 530.28 | 215.21 ± 201.32 | 400.44 ± 464.80 | 365.59 ± 444.63 | 117.98 ± 84.44 | 181.18 ± 173.53 |
| ACE [ng/mL] | 146.94 ± 118.06 | 113.39 ± 69.41 | 130.17 ± 95.82 | 129.77 ± 58.35 | 131.16 ± 77.15 | 130.36 ± 64.86 |
| Neurological factors | ||||||
| BDNF [ng/mL] | 13.98 ± 4.16 | 14.84 ± 4.77 | 14.41 ± 4.38 | 14.04 ± 5.89 | 11.15 ± 3.70 | 12.88 ± 5.22 |
| NF-light [pg/mL] | 9.21 ± 4.33 | 7.17 ± 1.76 | 7.88 ± 2.98 | 8.60 ± 4.70 | 9.25 ± 7.63 | 8.83 ± 5.69 |
| IGF2 [ng/mL] | 59.49 ± 9.96 | 69.22 ± 14.24 | 64.35 ± 12.96 | 59.14 ± 15.53 | 61.91 ± 12.25 | 60.25 ± 14.03 |
| IGFBP7 [ng/mL] | 143.60 ± 15.76 | 167.26 ± 101.46 | 155.43 ± 71.70 | 153.49 ± 29.18 | 154.51 ± 27.59 | 153.90 ± 27.82 |
| S100B [pg/mL] | 108.61 ± 50.63 | 143.08 ± 88.34 | 131.01 ± 77.61 | 116.82 ± 81.56 | 100.16 ± 50.41 | 110.99 ± 71.21 |
| Muscle health | ||||||
| Myostatin (pg/mL) | 519.29 ± 887.59 | 89.45 ± 150.28 | 239.89 ± 554.34 | 132.96 ± 75.89 | 81.42 ± 189.04 | 114.92 ± 124.73 |
| Cathepsin B [ng/mL] | 39.58 ± 5.36 | 38.67 ± 12.47 | 38.99 ± 10.37 | 38.71 ± 13.96 | 35.70 ± 15.25 | 37.66 ± 14.10 |
| Galectin-1 [ng/mL] | 40.76 ± 23.95 | 25.68 ± 10.02 | 30.39 ± 16.49 | 32.55 ± 8.12 | 34.08 ± 18.03 | 33.08 ± 12.04 |
| Creatine kinase [U/L] | 158.71 ± 70.18 | 99.31 ± 48.59 * | 120.10 ± 62.38 | 206.15 ± 124.64 | 232.57 ± 216.90 | 215.40 ± 157.60 # |
| Irisin [pg/mL] | 2296.07 ± 647.72 | 2060.82 ± 681.41 | 2135.1 ± 662.3 | 2575.71 ± 890.23 | 2331.79 ± 1106.02 | 2490.34 ± 949.25 |
| Titin [pmol/L] | 104.42 ± 100.91 | 77.21 ± 62.26 | 86.74 ± 76.42 | 211.03 ± 195.96 | 270.70 ± 379.75 | 231.91 ± 265.79 # |
| Kidney health | ||||||
| Urea nitrogen [mmol/L] | 5.04 ± 1.46 | 3.65 ± 1.06 * | 4.14 ± 1.36 | 5.05 ± 1.0 | 3.71 ± 0.58 * | 4.58 ± 1.14 |
| Others | ||||||
| LDH [U/L] | 223.71 ± 42.89 | 209.23 ± 37.86 | 214.30 ± 39.19 | 364.54 ± 150.24 | 276.71 ± 60.15 | 333.80 ± 131.32 # |
| Uric acid [umol/L] | 279.87 ± 41.89 | 203.93 ± 48.66 * | 230.51 ± 58.57 | 324.98 ± 87.57 | 246.57 ± 87.97 | 297.53 ± 93.59 # |
| Cystatin C [µg/mL] | 1.07 ± 0.16 | 1.31 ± 0.68 | 1.19 ± 0.49 | 1.18 ± 0.21 | 1.05 ± 0.20 | 1.13 ± 0.21 |
| THBS4 [ng/mL] | 393.94 ± 100.91 | 368.22 ± 111.26 | 377.22 ± 105.79 | 606.08 ± 231.67 | 578.28 ± 202.54 | 596.35 ± 216.88 # |
| Meteorin-like [ng/mL] | 1.95 ± 0.42 | 1.66 ± 0.20 | 1.80 ± 0.36 | 1.47 ± 0.33 | 1.53 ± 0.18 | 1.49 ± 0.27 # |
| PAM [ug/mL] | 0.74 ± 0.07 | 0.73 ± 0.12 | 0.73 ± 0.11 | 0.67 ± 0.07 | 0.66 ± 0.06 | 0.67 ± 0.07 |
| Normal Weight Men (n = 17) | Normal Weight Women | Mean Normal Weight (n = 40) | Obese Men (n = 25) | Obese Women (n = 15) | Mean Obese (n = 40) | |
|---|---|---|---|---|---|---|
| Age | 28.9 ± 12.5 | 30.4 ± 12.1 | 29.8 ± 12.1 | 37.3 ± 12.9 | 31.5 ± 10.1 | 35.1 ± 12.1 |
| BMI | 22.3 ± 2.1 | 22.1 ± 1.6 | 22.2 ± 1.8 | 34.8 ± 3.1 | 36.7 ±6.1 | 35.5 ± 4.5 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gart, E.; Snabel, J.; de Jong, J.C.B.C.; Verschuren, L.; van den Hoek, A.M.; Morrison, M.C.; Kleemann, R. Biomarkers of Metabolism and Inflammation in Individuals with Obesity and Normal Weight: A Comparative Analysis Exploring Sex Differences. Int. J. Mol. Sci. 2025, 26, 7576. https://doi.org/10.3390/ijms26157576
Gart E, Snabel J, de Jong JCBC, Verschuren L, van den Hoek AM, Morrison MC, Kleemann R. Biomarkers of Metabolism and Inflammation in Individuals with Obesity and Normal Weight: A Comparative Analysis Exploring Sex Differences. International Journal of Molecular Sciences. 2025; 26(15):7576. https://doi.org/10.3390/ijms26157576
Chicago/Turabian StyleGart, Eveline, Jessica Snabel, Jelle C. B. C. de Jong, Lars Verschuren, Anita M. van den Hoek, Martine C. Morrison, and Robert Kleemann. 2025. "Biomarkers of Metabolism and Inflammation in Individuals with Obesity and Normal Weight: A Comparative Analysis Exploring Sex Differences" International Journal of Molecular Sciences 26, no. 15: 7576. https://doi.org/10.3390/ijms26157576
APA StyleGart, E., Snabel, J., de Jong, J. C. B. C., Verschuren, L., van den Hoek, A. M., Morrison, M. C., & Kleemann, R. (2025). Biomarkers of Metabolism and Inflammation in Individuals with Obesity and Normal Weight: A Comparative Analysis Exploring Sex Differences. International Journal of Molecular Sciences, 26(15), 7576. https://doi.org/10.3390/ijms26157576






