GLUT4 Trafficking and Storage Vesicles: Molecular Architecture, Regulatory Networks, and Their Disruption in Insulin Resistance
Abstract
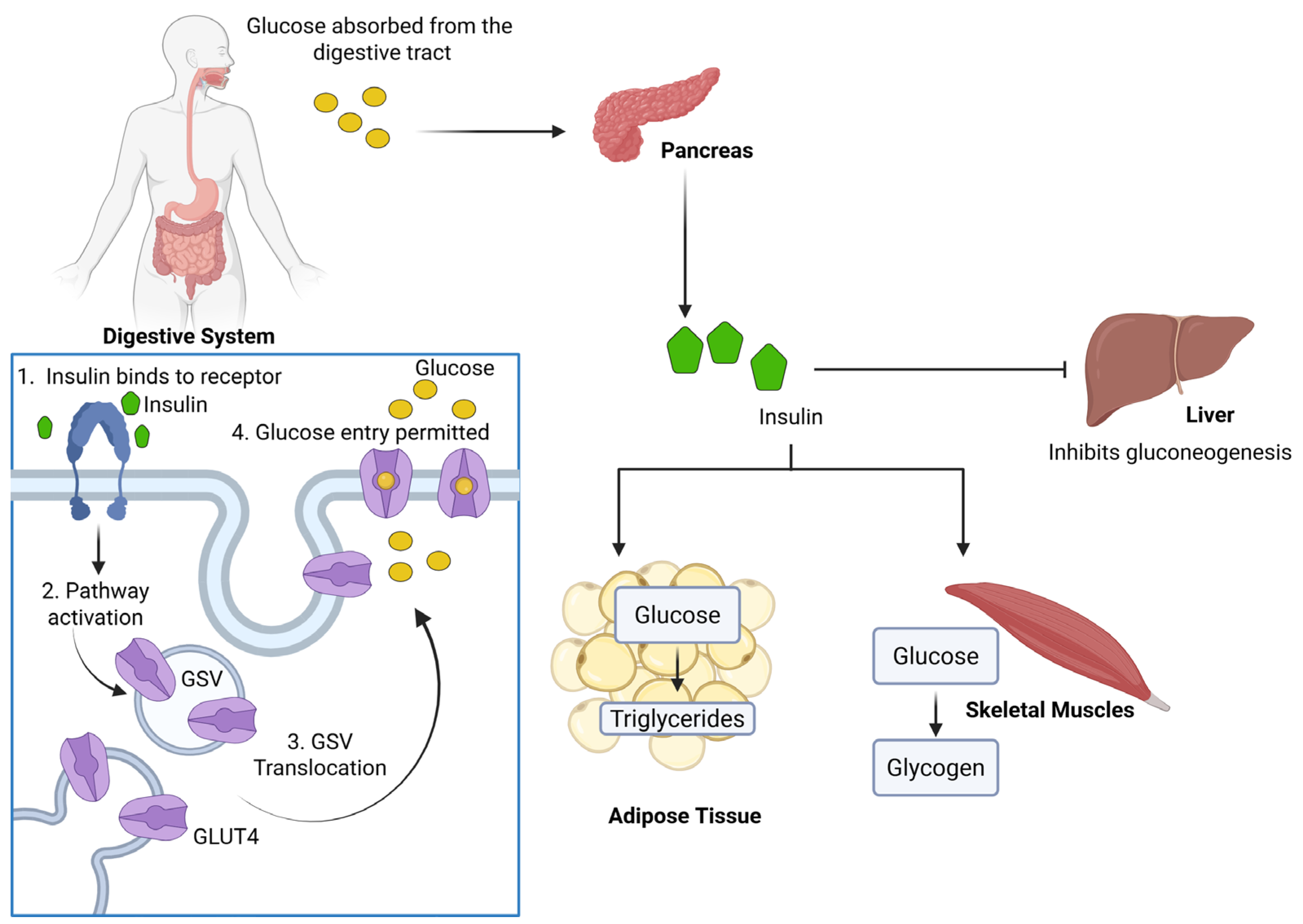
1. Introduction
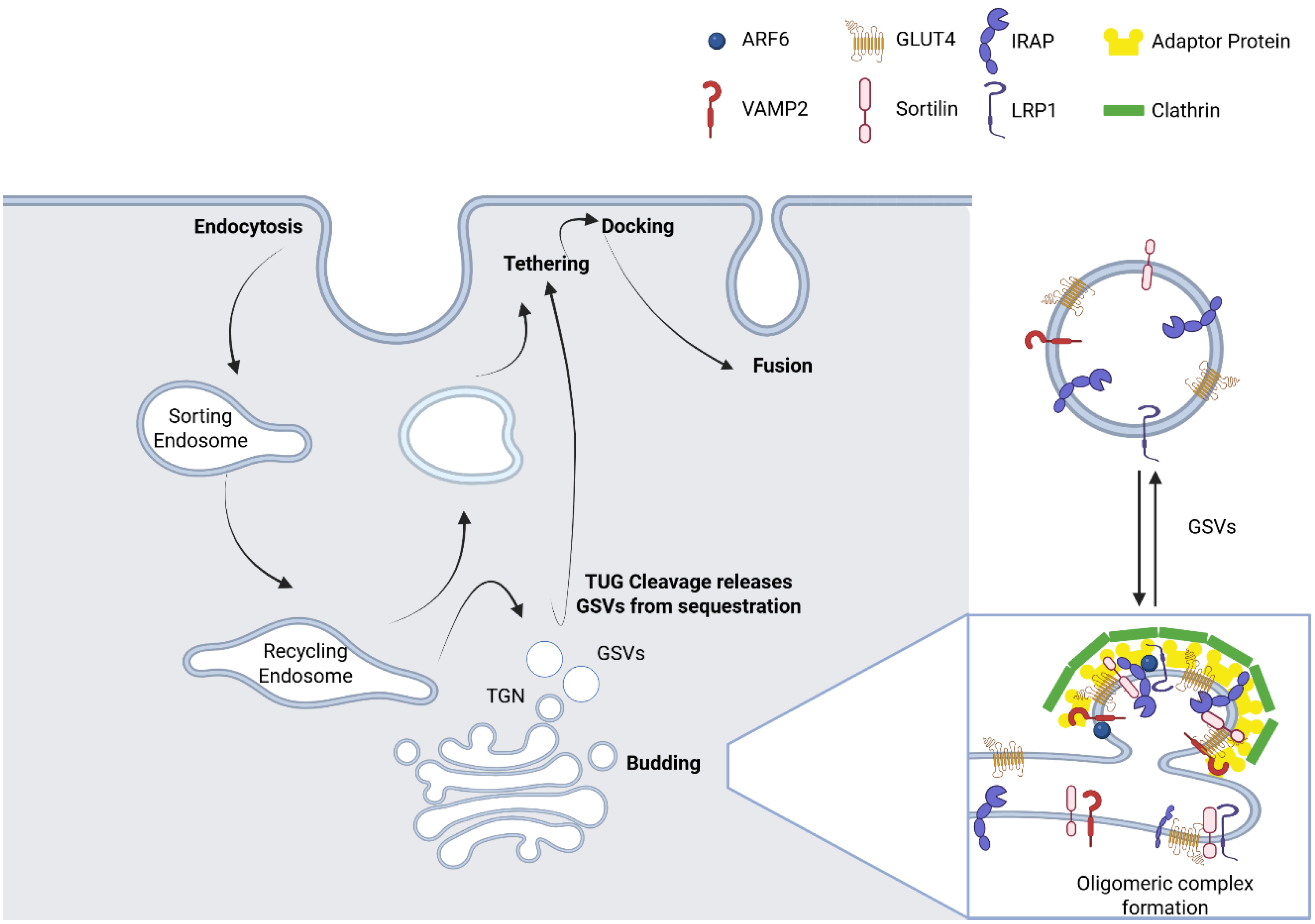
2. Biogenesis and Molecular Composition of GSVs
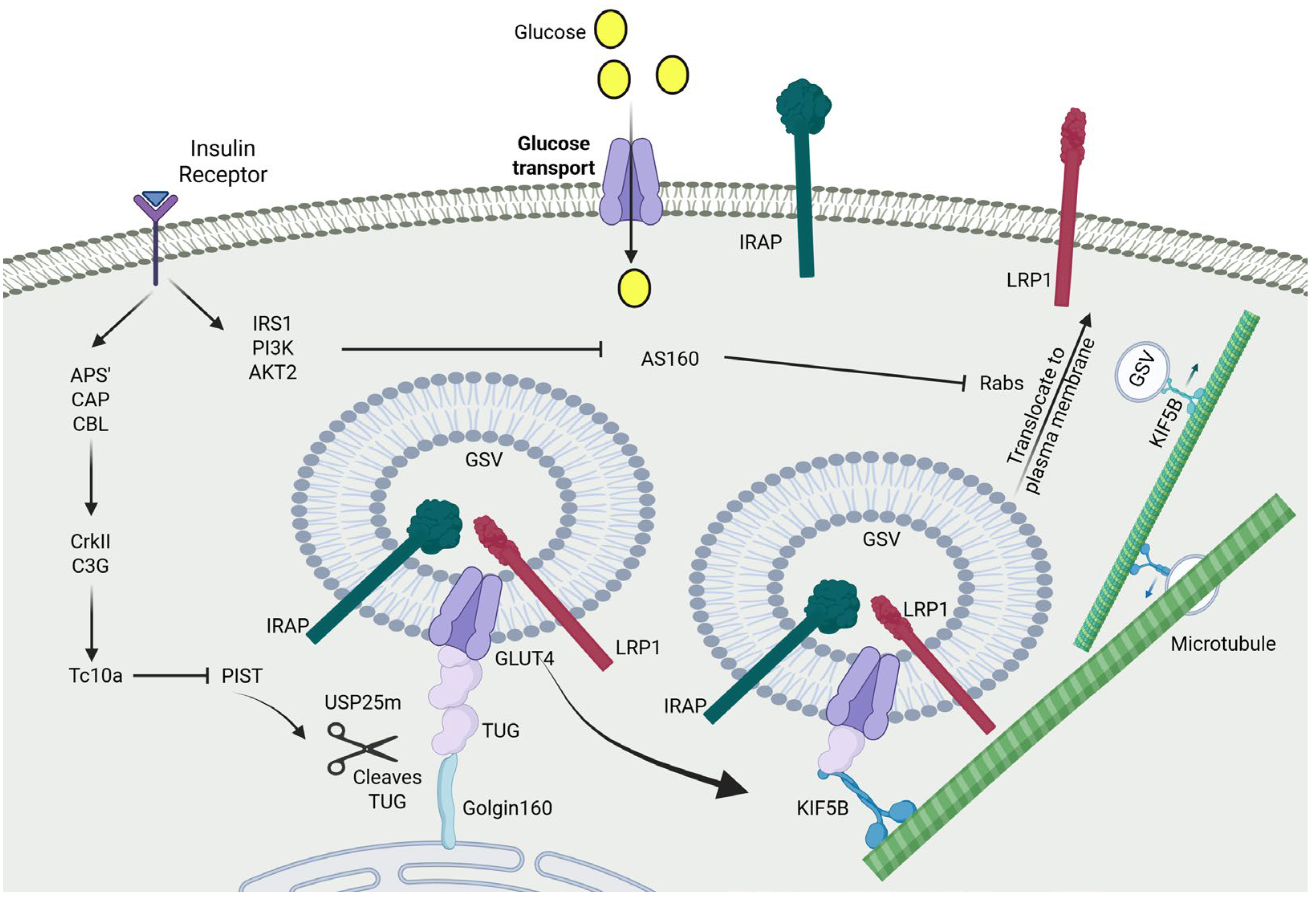
3. Regulation of GSV Translocation by Insulin
4. Functional Role of TUG in GSV Retention and Insulin Response
5. Kinetic Models of IRV Trafficking: Retention vs. Repulsion
6. The Lifecycle of IRVs After Insulin Stimulation
6.1. Approach
6.2. Tethering: Bridging GSVs to the Plasma Membrane
6.3. Docking and Fusion
6.4. Reconstitution of IRVs After Insulin Stimulation
7. Involvement of Actin Cytoskeleton Remodeling in GSV Trafficking
8. Mechanistic Links Between GSV Dysfunction and the Development of Insulin Resistance
8.1. Proximal Insulin Signalling: Cause or Consequence
8.2. Mis-Sorting of GLUT4
8.3. Impaired GSV Translocation to the Plasma Membrane
8.4. Cytoskeletal Impairment in GLUT4 Trafficking
9. Outstanding Questions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. Diabetes Atlas. 2025. Available online: https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/ (accessed on 1 February 2025).
- Rothman, D.L.; Magnusson, I.; Cline, G.; Gerard, D.; Kahn, C.R.; Shulman, R.G.; Shulman, G.I. Decreased muscle glucose transport/phosphorylation is an early defect in the pathogenesis of non-insulin-dependent diabetes mellitus. Proc. Natl. Acad. Sci. USA 1995, 92, 983–987. [Google Scholar] [CrossRef]
- Saltiel, A.R. Insulin resistance in the defense against obesity. Cell Metab. 2012, 15, 798–804. [Google Scholar] [CrossRef]
- Saltiel, A.R. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell 2001, 104, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kyrou, I.; Randeva, H.S.; Weickert, M.O. Mechanisms of Insulin Resistance at the Crossroad of Obesity with Associated Metabolic Abnormalities and Cognitive Dysfunction. Int. J. Mol. Sci. 2021, 22, 546. [Google Scholar] [CrossRef]
- van Gerwen, J.; Shun-Shion, A.S.; Fazakerley, D.J. Insulin signalling and GLUT4 trafficking in insulin resistance. Biochem. Soc. Trans. 2023, 51, 1057–1069. [Google Scholar] [CrossRef]
- James, D.E.; Lederman, L.; Pilch, P.F. Purification of insulin-dependent exocytic vesicles containing the glucose transporter. J. Biol. Chem. 1987, 262, 11817–11824. [Google Scholar] [CrossRef]
- Vilaró, S.; Palacín, M.; Pilch, P.F.; Testar, X.; Zorzano, A. Expression of an insulin-regulatable glucose carrier in muscle and fat endothelial cells. Nature 1989, 342, 798–800. [Google Scholar] [CrossRef]
- Lauritzen, H.P. Insulin- and contraction-induced glucose transporter 4 traffic in muscle: Insights from a novel imaging approach. Exerc. Sport. Sci. Rev. 2013, 41, 77–86. [Google Scholar] [CrossRef]
- Nandi, A.; Kitamura, Y.; Kahn, C.R.; Accili, D. Mouse models of insulin resistance. Physiol. Rev. 2004, 84, 623–647. [Google Scholar] [CrossRef]
- Shulman, G.I. Cellular mechanisms of insulin resistance. J. Clin. Investig. 2000, 106, 171–176. [Google Scholar] [CrossRef]
- Martin, L.B.; Shewan, A.; Millar, C.A.; Gould, G.W.; James, D.E. Vesicle-associated membrane protein 2 plays a specific role in the insulin-dependent trafficking of the facilitative glucose transporter GLUT4 in 3T3-L1 adipocytes. J. Biol. Chem. 1998, 273, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Kupriyanova, T.A.; Kandror, V.; Kandror, K.V. Isolation and characterization of the two major intracellular Glut4 storage compartments. J. Biol. Chem. 2002, 277, 9133–9138. [Google Scholar] [CrossRef]
- Holman, G.D.; Lo Leggio, L.; Cushman, S.W. Insulin-stimulated GLUT4 glucose transporter recycling. A problem in membrane protein subcellular trafficking through multiple pools. J. Biol. Chem. 1994, 269, 17516–17524. [Google Scholar] [CrossRef] [PubMed]
- Habtemichael, E.N.; Brewer, P.D.; Romenskaia, I.; Mastick, C.C. Kinetic evidence that Glut4 follows different endocytic pathways than the receptors for transferrin and alpha2-macroglobulin. J. Biol. Chem. 2011, 286, 10115–10125. [Google Scholar] [CrossRef] [PubMed]
- Blot, V.; McGraw, T.E. GLUT4 is internalized by a cholesterol-dependent nystatin-sensitive mechanism inhibited by insulin. Embo J. 2006, 25, 5648–5658. [Google Scholar] [CrossRef]
- Fazakerley, D.J.; Holman, G.D.; Marley, A.; James, D.E.; Stöckli, J.; Coster, A.C. Kinetic evidence for unique regulation of GLUT4 trafficking by insulin and AMP-activated protein kinase activators in L6 myotubes. J. Biol. Chem. 2010, 285, 1653–1660. [Google Scholar] [CrossRef]
- Govers, R.; Coster, A.C.; James, D.E. Insulin increases cell surface GLUT4 levels by dose dependently discharging GLUT4 into a cell surface recycling pathway. Mol. Cell. Biol. 2004, 24, 6456–6466. [Google Scholar] [CrossRef]
- Muretta, J.M.; Romenskaia, I.; Mastick, C.C. Insulin releases Glut4 from static storage compartments into cycling endosomes and increases the rate constant for Glut4 exocytosis. J. Biol. Chem. 2008, 283, 311–323. [Google Scholar] [CrossRef]
- Stöckli, J.; Fazakerley, D.J.; Coster, A.C.; Holman, G.D.; James, D.E. Muscling in on GLUT4 kinetics. Commun. Integr. Biol. 2010, 3, 260–262. [Google Scholar] [CrossRef]
- Karylowski, O.; Zeigerer, A.; Cohen, A.; McGraw, T.E. GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes. Mol. Biol. Cell 2004, 15, 870–882. [Google Scholar] [CrossRef]
- Martin, O.J.; Lee, A.; McGraw, T.E. GLUT4 distribution between the plasma membrane and the intracellular compartments is maintained by an insulin-modulated bipartite dynamic mechanism. J. Biol. Chem. 2006, 281, 484–490. [Google Scholar] [CrossRef]
- Xu, Y.; Rubin, B.R.; Orme, C.M.; Karpikov, A.; Yu, C.; Bogan, J.S.; Toomre, D.K. Dual-mode of insulin action controls GLUT4 vesicle exocytosis. J. Cell Biol. 2011, 193, 643–653. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Zhang, J.; Deng, Y.; Jiang, L.; Song, E.; Wu, X.S.; Hammer, J.A.; Xu, T.; Lippincott-Schwartz, J. Rab10 and myosin-Va mediate insulin-stimulated GLUT4 storage vesicle translocation in adipocytes. J. Cell Biol. 2012, 198, 545–560. [Google Scholar] [CrossRef]
- Lauritzen, H.P.; Galbo, H.; Brandauer, J.; Goodyear, L.J.; Ploug, T. Large GLUT4 vesicles are stationary while locally and reversibly depleted during transient insulin stimulation of skeletal muscle of living mice: Imaging analysis of GLUT4-enhanced green fluorescent protein vesicle dynamics. Diabetes 2008, 57, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Kandror, K.V.; Coderre, L.; Pushkin, A.V.; Pilch, P.F. Comparison of glucose-transporter-containing vesicles from rat fat and muscle tissues: Evidence for a unique endosomal compartment. Biochem. J. 1995, 307 Pt 2, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Millar, C.A.; Lyttle, C.T.; Meerloo, T.; Marsh, B.J.; Gould, G.W.; James, D.E. Effects of insulin on intracellular GLUT4 vesicles in adipocytes: Evidence for a secretory mode of regulation. J. Cell Sci. 2000, 113 Pt 19, 3427–3438. [Google Scholar] [CrossRef] [PubMed]
- Hashiramoto, M.; James, D.E. Characterization of insulin-responsive GLUT4 storage vesicles isolated from 3T3-L1 adipocytes. Mol. Cell. Biol. 2000, 20, 416–427. [Google Scholar] [CrossRef]
- Kupriyanova, T.A.; Kandror, K.V. Cellugyrin is a marker for a distinct population of intracellular Glut4-containing vesicles. J. Biol. Chem. 2000, 275, 36263–36268. [Google Scholar] [CrossRef]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef]
- Bryant, N.J.; Govers, R.; James, D.E. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell Biol. 2002, 3, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Larance, M.; Ramm, G.; Stöckli, J.; van Dam, E.M.; Winata, S.; Wasinger, V.; Simpson, F.; Graham, M.; Junutula, J.R.; Guilhaus, M.; et al. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J. Biol. Chem. 2005, 280, 37803–37813. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, M.P.; Gartner, C.A.; Gygi, S.P.; Zhou, L.; Herz, J.; Kandror, K.V.; Pilch, P.F. Proteomic analysis of GLUT4 storage vesicles reveals LRP1 to be an important vesicle component and target of insulin signaling. J. Biol. Chem. 2010, 285, 104–114. [Google Scholar] [CrossRef]
- Xu, Z.; Kandror, K.V. Translocation of small preformed vesicles is responsible for the insulin activation of glucose transport in adipose cells. Evidence from the in vitro reconstitution assay. J. Biol. Chem. 2002, 277, 47972–47975. [Google Scholar] [CrossRef]
- Bogan, J.S.; Kandror, K.V. Biogenesis and regulation of insulin-responsive vesicles containing GLUT4. Curr. Opin. Cell Biol. 2010, 22, 506–512. [Google Scholar] [CrossRef]
- Li, D.T.; Habtemichael, E.N.; Julca, O.; Sales, C.I.; Westergaard, X.O.; DeVries, S.G.; Ruiz, D.; Sayal, B.; Bogan, J.S. GLUT4 Storage Vesicles: Specialized Organelles for Regulated Trafficking. Yale J. Biol. Med. 2019, 92, 453–470. [Google Scholar]
- Kandror, K.V.; Pilch, P.F. The sugar is sIRVed: Sorting Glut4 and its fellow travelers. Traffic 2011, 12, 665–671. [Google Scholar] [CrossRef]
- Grusovin, J.; Macaulay, S.L. Snares for GLUT4—Mechanisms directing vesicular trafficking of GLUT4. Front. Biosci. 2003, 8, 620–641. [Google Scholar] [CrossRef]
- Kandror, K.V.; Pilch, P.F. Compartmentalization of protein traffic in insulin-sensitive cells. Am. J. Physiol. 1996, 271 Pt 1, E1–E14. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, L.; Lopez, J.A.; Fan, J.; Burchfield, J.G.; Bai, L.; Hong, W.; Xu, T.; James, D.E. Variations in the requirement for v-SNAREs in GLUT4 trafficking in adipocytes. J. Cell Sci. 2009, 122 Pt 19, 3472–3480. [Google Scholar] [CrossRef]
- Martin, S.; Rice, J.E.; Gould, G.W.; Keller, S.R.; Slot, J.W.; James, D.E. The glucose transporter GLUT4 and the aminopeptidase vp165 colocalise in tubulo-vesicular elements in adipocytes and cardiomyocytes. J. Cell Sci. 1997, 110 Pt 18, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; Scott, H.M.; Morris, N.J.; Leung, W.Y.; Mao, F.; Lienhard, G.E.; Keller, S.R. Characterization of the insulin-regulated membrane aminopeptidase in 3T3-L1 adipocytes. J. Biol. Chem. 1996, 271, 3328–3332. [Google Scholar] [CrossRef] [PubMed]
- Peck, G.R.; Ye, S.; Pham, V.; Fernando, R.N.; Macaulay, S.L.; Chai, S.Y.; Albiston, A.L. Interaction of the Akt substrate, AS160, with the glucose transporter 4 vesicle marker protein, insulin-regulated aminopeptidase. Mol. Endocrinol. 2006, 20, 2576–2583. [Google Scholar] [CrossRef]
- Park, S.; Kim, K.Y.; Kim, S.; Yu, Y.S. Affinity between TBC1D4 (AS160) phosphotyrosine-binding domain and insulin-regulated aminopeptidase cytoplasmic domain measured by isothermal titration calorimetry. BMB Rep. 2012, 45, 360–364. [Google Scholar] [CrossRef][Green Version]
- Jordens, I.; Molle, D.; Xiong, W.; Keller, S.R.; McGraw, T.E. Insulin-regulated aminopeptidase is a key regulator of GLUT4 trafficking by controlling the sorting of GLUT4 from endosomes to specialized insulin-regulated vesicles. Mol. Biol. Cell 2010, 21, 2034–2044. [Google Scholar] [CrossRef]
- Beaton, N.; Rudigier, C.; Moest, H.; Müller, S.; Mrosek, N.; Röder, E.; Rudofsky, G.; Rülicke, T.; Ukropec, J.; Ukropcova, B.; et al. TUSC5 regulates insulin-mediated adipose tissue glucose uptake by modulation of GLUT4 recycling. Mol. Metab. 2015, 4, 795–810. [Google Scholar] [CrossRef]
- Fazakerley, D.J.; Naghiloo, S.; Chaudhuri, R.; Koumanov, F.; Burchfield, J.G.; Thomas, K.C.; Krycer, J.R.; Prior, M.J.; Parker, B.L.; Murrow, B.A.; et al. Proteomic Analysis of GLUT4 Storage Vesicles Reveals Tumor Suppressor Candidate 5 (TUSC5) as a Novel Regulator of Insulin Action in Adipocytes. J. Biol. Chem. 2015, 290, 23528–23542. [Google Scholar] [CrossRef]
- Jaldin-Fincati, J.R.; Pavarotti, M.; Frendo-Cumbo, S.; Bilan, P.J.; Klip, A. Update on GLUT4 Vesicle Traffic: A Cornerstone of Insulin Action. Trends Endocrinol. Metab. 2017, 28, 597–611. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef]
- Li, L.V.; Kandror, K.V. Golgi-localized, gamma-ear-containing, Arf-binding protein adaptors mediate insulin-responsive trafficking of glucose transporter 4 in 3T3-L1 adipocytes. Mol. Endocrinol. 2005, 19, 2145–2153. [Google Scholar] [CrossRef]
- Watson, R.T.; Khan, A.H.; Furukawa, M.; Hou, J.C.; Li, L.; Kanzaki, M.; Okada, S.; Kandror, K.V.; Pessin, J.E. Entry of newly synthesized GLUT4 into the insulin-responsive storage compartment is GGA dependent. Embo J. 2004, 23, 2059–2070. [Google Scholar] [CrossRef]
- Nielsen, M.S.; Madsen, P.; Christensen, E.I.; Nykjaer, A.; Gliemann, J.; Kasper, D.; Pohlmann, R.; Petersen, C.M. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. Embo J. 2001, 20, 2180–2190. [Google Scholar] [CrossRef]
- Shi, J.; Kandror, K.V. Sortilin is essential and sufficient for the formation of Glut4 storage vesicles in 3T3-L1 adipocytes. Dev. Cell 2005, 9, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peters, P.J.; Bai, M.; Dai, J.; Bos, E.; Kirchhausen, T.; Kandror, K.V.; Hsu, V.W. An ACAP1-containing clathrin coat complex for endocytic recycling. J. Cell Biol. 2007, 178, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, H.Q.; Macia, E.; Kirchhausen, T.; Watson, H.; Bonifacino, J.S.; Yin, H.L. PI4P promotes the recruitment of the GGA adaptor proteins to the trans-Golgi network and regulates their recognition of the ubiquitin sorting signal. Mol. Biol. Cell 2007, 18, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Demmel, L.; Gravert, M.; Ercan, E.; Habermann, B.; Müller-Reichert, T.; Kukhtina, V.; Haucke, V.; Baust, T.; Sohrmann, M.; Kalaidzidis, Y.; et al. The clathrin adaptor Gga2p is a phosphatidylinositol 4-phosphate effector at the Golgi exit. Mol. Biol. Cell 2008, 19, 1991–2002. [Google Scholar] [CrossRef]
- Hou, J.C.; Suzuki, N.; Pessin, J.E.; Watson, R.T. A specific dileucine motif is required for the GGA-dependent entry of newly synthesized insulin-responsive aminopeptidase into the insulin-responsive compartment. J. Biol. Chem. 2006, 281, 33457–33466. [Google Scholar] [CrossRef]
- Williams, D.; Pessin, J.E. Mapping of R-SNARE function at distinct intracellular GLUT4 trafficking steps in adipocytes. J. Cell Biol. 2008, 180, 375–387. [Google Scholar] [CrossRef]
- Khan, A.H.; Capilla, E.; Hou, J.C.; Watson, R.T.; Smith, J.R.; Pessin, J.E. Entry of newly synthesized GLUT4 into the insulin-responsive storage compartment is dependent upon both the amino terminus and the large cytoplasmic loop. J. Biol. Chem. 2004, 279, 37505–37511. [Google Scholar] [CrossRef]
- Watson, R.T.; Hou, J.C.; Pessin, J.E. Recycling of IRAP from the plasma membrane back to the insulin-responsive compartment requires the Q-SNARE syntaxin 6 but not the GGA clathrin adaptors. J. Cell Sci. 2008, 121 Pt 8, 1243–1251. [Google Scholar] [CrossRef]
- Koumanov, F.; Jin, B.; Yang, J.; Holman, G.D. Insulin signaling meets vesicle traffic of GLUT4 at a plasma-membrane-activated fusion step. Cell Metab. 2005, 2, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Stockli, J.; Fazakerley, D.J.; James, D.E. GLUT4 exocytosis. J. Cell Sci. 2011, 124 Pt 24, 4147–4159. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R. Insulin signaling in health and disease. J. Clin. Investig. 2021, 131, e142241. [Google Scholar] [CrossRef] [PubMed]
- Aslamy, A.; Thurmond, D.C. Exocytosis proteins as novel targets for diabetes prevention and/or remediation? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R739–R752. [Google Scholar] [CrossRef]
- Bogan, J.S. Regulation of glucose transporter translocation in health and diabetes. Annu. Rev. Biochem. 2012, 81, 507–532. [Google Scholar] [CrossRef]
- Beg, M.; Abdullah, N.; Thowfeik, F.S.; Altorki, N.K.; McGraw, T.E. Distinct Akt phosphorylation states are required for insulin regulated Glut4 and Glut1-mediated glucose uptake. Elife 2017, 6, e26896. [Google Scholar] [CrossRef]
- Sakamoto, K.; Holman, G.D. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E29–E37. [Google Scholar] [CrossRef]
- Sano, H.; Eguez, L.; Teruel, M.N.; Fukuda, M.; Chuang, T.D.; Chavez, J.A.; Lienhard, G.E.; McGraw, T.E. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007, 5, 293–303. [Google Scholar] [CrossRef]
- Bruno, J.; Brumfield, A.; Chaudhary, N.; Iaea, D.; McGraw, T.E. SEC16A is a RAB10 effector required for insulin-stimulated GLUT4 trafficking in adipocytes. J. Cell Biol. 2016, 214, 61–76. [Google Scholar] [CrossRef]
- Xu, X.H.; Deng, C.Y.; Liu, Y.; He, M.; Peng, J.; Wang, T.; Yuan, L.; Zheng, Z.S.; Blackshear, P.J.; Luo, Z.G. MARCKS regulates membrane targeting of Rab10 vesicles to promote axon development. Cell Res. 2014, 24, 576–594. [Google Scholar] [CrossRef]
- Encarnação, M.; Espada, L.; Escrevente, C.; Mateus, D.; Ramalho, J.; Michelet, X.; Santarino, I.; Hsu, V.W.; Brenner, M.B.; Barral, D.C.; et al. A Rab3a-dependent complex essential for lysosome positioning and plasma membrane repair. J. Cell Biol. 2016, 213, 631–640. [Google Scholar] [CrossRef]
- Sun, Y.; Chiu, T.T.; Foley, K.P.; Bilan, P.J.; Klip, A. Myosin Va mediates Rab8A-regulated GLUT4 vesicle exocytosis in insulin-stimulated muscle cells. Mol. Biol. Cell 2014, 25, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, S.; Takeda, T.; Kitaura, T.; Takenaka, N.; Kataoka, T.; Satoh, T. Akt2 regulates Rac1 activity in the insulin-dependent signaling pathway leading to GLUT4 translocation to the plasma membrane in skeletal muscle cells. Cell. Signal. 2013, 25, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, N.; Nakao, M.; Matsui, S.; Satoh, T. A Crucial Role for the Small GTPase Rac1 Downstream of the Protein Kinase Akt2 in Insulin Signaling that Regulates Glucose Uptake in Mouse Adipocytes. Int. J. Mol. Sci. 2019, 20, 5443. [Google Scholar] [CrossRef]
- Chang, L.; Chiang, S.H.; Saltiel, A.R. TC10alpha is required for insulin-stimulated glucose uptake in adipocytes. Endocrinology 2007, 148, 27–33. [Google Scholar] [CrossRef]
- Leto, D.; Saltiel, A.R. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 2012, 13, 383–396. [Google Scholar] [CrossRef]
- JeBailey, L.; Rudich, A.; Huang, X.; Di Ciano-Oliveira, C.; Kapus, A.; Klip, A. Skeletal muscle cells and adipocytes differ in their reliance on TC10 and Rac for insulin-induced actin remodeling. Mol. Endocrinol. 2004, 18, 359–372. [Google Scholar] [CrossRef]
- Kanzaki, M.; Watson, R.T.; Hou, J.C.; Stamnes, M.; Saltiel, A.R.; Pessin, J.E. Small GTP-binding protein TC10 differentially regulates two distinct populations of filamentous actin in 3T3L1 adipocytes. Mol. Biol. Cell 2002, 13, 2334–2346. [Google Scholar] [CrossRef]
- Löffler, M.G.; Birkenfeld, A.L.; Philbrick, K.M.; Belman, J.P.; Habtemichael, E.N.; Booth, C.J.; Castorena, C.M.; Choi, C.S.; Jornayvaz, F.R.; Gassaway, B.M.; et al. Enhanced fasting glucose turnover in mice with disrupted action of TUG protein in skeletal muscle. J. Biol. Chem. 2013, 288, 20135–20150. [Google Scholar] [CrossRef]
- Bogan, J.S.; Rubin, B.R.; Yu, C.; Loffler, M.G.; Orme, C.M.; Belman, J.P.; McNally, L.J.; Hao, M.; Cresswell, J.A. Endoproteolytic cleavage of TUG protein regulates GLUT4 glucose transporter translocation. J. Biol. Chem. 2012, 287, 23932–23947. [Google Scholar] [CrossRef] [PubMed]
- Kohn, A.D.; Barthel, A.; Kovacina, K.S.; Boge, A.; Wallach, B.; Summers, S.A.; Birnbaum, M.J.; Scott, P.H.; Lawrence, J.C., Jr.; Roth, R.A. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J. Biol. Chem. 1998, 273, 11937–11943. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.; Ramm, G.; Lopez, J.A.; James, D.E. Rapid activation of Akt2 is sufficient to stimulate GLUT4 translocation in 3T3-L1 adipocytes. Cell Metab. 2008, 7, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.R.; Bogan, J.S. Chapter 7 Intracellular Retention and Insulin-Stimulated Mobilization of GLUT4 Glucose Transporters. In Insulin and IGFs; Vitamins & Hormones: New York, NY, USA, 2009; pp. 155–192. [Google Scholar]
- Bogan, J.S.; Hendon, N.; McKee, A.E.; Tsao, T.S.; Lodish, H.F. Functional cloning of TUG as a regulator of GLUT4 glucose transporter trafficking. Nature 2003, 425, 727–733. [Google Scholar] [CrossRef]
- Yu, C.; Cresswell, J.; Löffler, M.G.; Bogan, J.S. The glucose transporter 4-regulating protein TUG is essential for highly insulin-responsive glucose uptake in 3T3-L1 adipocytes. J. Biol. Chem. 2007, 282, 7710–7722. [Google Scholar] [CrossRef]
- Belman, J.P.; Habtemichael, E.N.; Bogan, J.S. A proteolytic pathway that controls glucose uptake in fat and muscle. Rev. Endocr. Metab. Disord. 2014, 15, 55–66. [Google Scholar] [CrossRef]
- Neudauer, C.L.; Joberty, G.; Macara, I.G. PIST: A novel PDZ/coiled-coil domain binding partner for the rho-family GTPase TC10. Biochem. Biophys. Res. Commun. 2001, 280, 541–547. [Google Scholar] [CrossRef]
- Belman, J.P.; Bian, R.R.; Habtemichael, E.N.; Li, D.T.; Jurczak, M.J.; Alcazar-Roman, A.; McNally, L.J.; Shulman, G.I.; Bogan, J.S. Acetylation of TUG protein promotes the accumulation of GLUT4 glucose transporters in an insulin-responsive intracellular compartment. J. Biol. Chem. 2015, 290, 4447–4463. [Google Scholar] [CrossRef]
- Feldman, J.L.; Dittenhafer-Reed, K.E.; Kudo, N.; Thelen, J.N.; Ito, A.; Yoshida, M.; Denu, J.M. Kinetic and Structural Basis for Acyl-Group Selectivity and NAD(+) Dependence in Sirtuin-Catalyzed Deacylation. Biochemistry 2015, 54, 3037–3050. [Google Scholar] [CrossRef]
- Emoto, M.; Langille, S.E.; Czech, M.P. A role for kinesin in insulin-stimulated GLUT4 glucose transporter translocation in 3T3-L1 adipocytes. J. Biol. Chem. 2001, 276, 10677–10682. [Google Scholar] [CrossRef]
- Habtemichael, E.N.; Li, D.T.; Alcazar-Roman, A.; Westergaard, X.O.; Li, M.; Petersen, M.C.; Li, H.; DeVries, S.G.; Li, E.; Julca-Zevallos, O.; et al. Usp25m protease regulates ubiquitin-like processing of TUG proteins to control GLUT4 glucose transporter translocation in adipocytes. J. Biol. Chem. 2018, 293, 10466–10486. [Google Scholar] [CrossRef]
- Bogan, J.S. Ubiquitin-like processing of TUG proteins as a mechanism to regulate glucose uptake and energy metabolism in fat and muscle. Front. Endocrinol. 2022, 13, 1019405. [Google Scholar] [CrossRef]
- Semiz, S.; Park, J.G.; Nicoloro, S.M.; Furcinitti, P.; Zhang, C.; Chawla, A.; Leszyk, J.; Czech, M.P. Conventional kinesin KIF5B mediates insulin-stimulated GLUT4 movements on microtubules. Embo J. 2003, 22, 2387–2399. [Google Scholar] [CrossRef]
- Cui, J.; Pang, J.; Lin, Y.J.; Gong, H.; Wang, Z.H.; Li, Y.X.; Li, J.; Wang, Z.; Jiang, P.; Dai, D.P.; et al. Adipose-specific deletion of Kif5b exacerbates obesity and insulin resistance in a mouse model of diet-induced obesity. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 2533–2547. [Google Scholar] [CrossRef]
- Blot, V.; McGraw, T.E. Molecular mechanisms controlling GLUT4 intracellular retention. Mol. Biol. Cell 2008, 19, 3477–3487. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Wang, Y.; Fan, J.; Chen, Y.; Ji, W.; Qu, A.; Xu, P.; James, D.E.; Xu, T. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 2007, 5, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lifshitz, L.M.; Jones, C.; Bellve, K.D.; Standley, C.; Fonseca, S.; Corvera, S.; Fogarty, K.E.; Czech, M.P. Insulin stimulates membrane fusion and GLUT4 accumulation in clathrin coats on adipocyte plasma membranes. Mol. Cell. Biol. 2007, 27, 3456–3469. [Google Scholar] [CrossRef] [PubMed]
- Lizunov, V.A.; Matsumoto, H.; Zimmerberg, J.; Cushman, S.W.; Frolov, V.A. Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J. Cell Biol. 2005, 169, 481–489. [Google Scholar] [CrossRef]
- Ng, Y.; Ramm, G.; Burchfield, J.G.; Coster, A.C.; Stöckli, J.; James, D.E. Cluster analysis of insulin action in adipocytes reveals a key role for Akt at the plasma membrane. J. Biol. Chem. 2010, 285, 2245–2257. [Google Scholar] [CrossRef]
- Gonzalez, E.; McGraw, T.E. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol. Biol. Cell 2006, 17, 4484–4493. [Google Scholar] [CrossRef]
- Randhawa, V.K.; Ishikura, S.; Talior-Volodarsky, I.; Cheng, A.W.; Patel, N.; Hartwig, J.H.; Klip, A. GLUT4 vesicle recruitment and fusion are differentially regulated by Rac, AS160, and Rab8A in muscle cells. J. Biol. Chem. 2008, 283, 27208–27219. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, K.L.; Hohnen-Behrens, C.; Cederberg, A.; Wu, L.E.; Turner, N.; Yuasa, T.; Ebina, Y.; James, D.E. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 2008, 7, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Jordens, I.; Gonzalez, E.; McGraw, T.E. GLUT4 is sorted to vesicles whose accumulation beneath and insertion into the plasma membrane are differentially regulated by insulin and selectively affected by insulin resistance. Mol. Biol. Cell 2010, 21, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- van Dam, E.M.; Govers, R.; James, D.E. Akt activation is required at a late stage of insulin-induced GLUT4 translocation to the plasma membrane. Mol. Endocrinol. 2005, 19, 1067–1077. [Google Scholar] [CrossRef]
- Perera, H.K.; Clarke, M.; Morris, N.J.; Hong, W.; Chamberlain, L.H.; Gould, G.W. Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes. Mol. Biol. Cell 2003, 14, 2946–2958. [Google Scholar] [CrossRef]
- Shepherd, P.R.; Kahn, B.B. Glucose transporters and insulin action—implications for insulin resistance and diabetes mellitus. N. Engl. J. Med. 1999, 341, 248–257. [Google Scholar] [CrossRef]
- Lodhi, I.J.; Chiang, S.H.; Chang, L.; Vollenweider, D.; Watson, R.T.; Inoue, M.; Pessin, J.E.; Saltiel, A.R. Gapex-5, a Rab31 guanine nucleotide exchange factor that regulates Glut4 trafficking in adipocytes. Cell Metab. 2007, 5, 59–72. [Google Scholar] [CrossRef]
- Boguslavsky, S.; Chiu, T.; Foley, K.P.; Osorio-Fuentealba, C.; Antonescu, C.N.; Bayer, K.U.; Bilan, P.J.; Klip, A. Myo1c binding to submembrane actin mediates insulin-induced tethering of GLUT4 vesicles. Mol. Biol. Cell 2012, 23, 4065–4078. [Google Scholar] [CrossRef]
- Tunduguru, R.; Thurmond, D.C. Promoting Glucose Transporter-4 Vesicle Trafficking along Cytoskeletal Tracks: PAK-Ing Them Out. Front. Endocrinol. 2017, 8, 329. [Google Scholar] [CrossRef]
- Toyoda, T.; An, D.; Witczak, C.A.; Koh, H.J.; Hirshman, M.F.; Fujii, N.; Goodyear, L.J. Myo1c regulates glucose uptake in mouse skeletal muscle. J. Biol. Chem. 2011, 286, 4133–4140. [Google Scholar] [CrossRef]
- Li, F.; Pincet, F.; Perez, E.; Eng, W.S.; Melia, T.J.; Rothman, J.E.; Tareste, D. Energetics and dynamics of SNAREpin folding across lipid bilayers. Nat. Struct. Mol. Biol. 2007, 14, 890–896. [Google Scholar] [CrossRef]
- Brown, F.C.; Pfeffer, S.R. An update on transport vesicle tethering. Mol. Membr. Biol. 2010, 27, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Lopez, J.A.; James, D.E.; Hunziker, W. Snapin interacts with the Exo70 subunit of the exocyst and modulates GLUT4 trafficking. J. Biol. Chem. 2008, 283, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Guo, W. Rabs and the exocyst in ciliogenesis, tubulogenesis and beyond. Trends Cell Biol. 2011, 21, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, M.; Chavrier, P. Cell polarity during motile processes: Keeping on track with the exocyst complex. Biochem. J. 2011, 433, 403–409. [Google Scholar] [CrossRef]
- Munson, M.; Novick, P. The exocyst defrocked, a framework of rods revealed. Nat. Struct. Mol. Biol. 2006, 13, 577–581. [Google Scholar] [CrossRef]
- Hsu, S.C.; Hazuka, C.D.; Roth, R.; Foletti, D.L.; Heuser, J.; Scheller, R.H. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron 1998, 20, 1111–1122. [Google Scholar] [CrossRef]
- Lopez, J.A.; Burchfield, J.G.; Blair, D.H.; Mele, K.; Ng, Y.; Vallotton, P.; James, D.E.; Hughes, W.E. Identification of a distal GLUT4 trafficking event controlled by actin polymerization. Mol. Biol. Cell 2009, 20, 3918–3929. [Google Scholar] [CrossRef]
- Omata, W.; Shibata, H.; Li, L.; Takata, K.; Kojima, I. Actin filaments play a critical role in insulin-induced exocytotic recruitment but not in endocytosis of GLUT4 in isolated rat adipocytes. Biochem. J. 2000, 346 Pt 2, 321–328. [Google Scholar] [CrossRef]
- Tong, P.; Khayat, Z.A.; Huang, C.; Patel, N.; Ueyama, A.; Klip, A. Insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles. J. Clin. Investig. 2001, 108, 371–381. [Google Scholar] [CrossRef]
- Tsakiridis, T.; Vranic, M.; Klip, A. Disassembly of the actin network inhibits insulin-dependent stimulation of glucose transport and prevents recruitment of glucose transporters to the plasma membrane. J. Biol. Chem. 1994, 269, 29934–29942. [Google Scholar] [CrossRef]
- Bose, A.; Guilherme, A.; Robida, S.I.; Nicoloro, S.M.; Zhou, Q.L.; Jiang, Z.Y.; Pomerleau, D.P.; Czech, M.P. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature 2002, 420, 821–824. [Google Scholar] [CrossRef]
- Yip, M.F.; Ramm, G.; Larance, M.; Hoehn, K.L.; Wagner, M.C.; Guilhaus, M.; James, D.E. CaMKII-mediated phosphorylation of the myosin motor Myo1c is required for insulin-stimulated GLUT4 translocation in adipocytes. Cell Metab. 2008, 8, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, T.; Imamura, T.; Babendure, J.L.; Lu, J.C.; Sonoda, N.; Olefsky, J.M. Myosin 5a is an insulin-stimulated Akt2 (protein kinase Bbeta) substrate modulating GLUT4 vesicle translocation. Mol. Cell. Biol. 2007, 27, 5172–5183. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Sano, H.; Rochford, J.J.; Semple, R.K.; Yeo, G.; Hyden, C.S.; Soos, M.A.; Clark, J.; Rodin, A.; Langenberg, C.; et al. A truncation mutation in TBC1D4 in a family with acanthosis nigricans and postprandial hyperinsulinemia. Proc. Natl. Acad. Sci. USA 2009, 106, 9350–9355. [Google Scholar] [CrossRef] [PubMed]
- Mîinea, C.P.; Sano, H.; Kane, S.; Sano, E.; Fukuda, M.; Peränen, J.; Lane, W.S.; Lienhard, G.E. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem. J. 2005, 391, 87–93. [Google Scholar] [CrossRef]
- Babbey, C.M.; Bacallao, R.L.; Dunn, K.W. Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. Am. J. Physiol. Renal Physiol. 2010, 299, F495–F506. [Google Scholar] [CrossRef]
- Roland, J.T.; Bryant, D.M.; Datta, A.; Itzen, A.; Mostov, K.E.; Goldenring, J.R. Rab GTPase-Myo5B complexes control membrane recycling and epithelial polarization. Proc. Natl. Acad. Sci. USA 2011, 108, 2789–2794. [Google Scholar] [CrossRef]
- Südhof, T.C.; Rothman, J.E. Membrane fusion: Grappling with SNARE and SM proteins. Science 2009, 323, 474–477. [Google Scholar] [CrossRef]
- Ramalingam, L.; Yoder, S.M.; Oh, E.; Thurmond, D.C. Munc18c: A controversial regulator of peripheral insulin action. Trends Endocrinol. Metab. 2014, 25, 601–608. [Google Scholar] [CrossRef]
- Melia, T.J.; Weber, T.; McNew, J.A.; Fisher, L.E.; Johnston, R.J.; Parlati, F.; Mahal, L.K.; Sollner, T.H.; Rothman, J.E. Regulation of membrane fusion by the membrane-proximal coil of the t-SNARE during zippering of SNAREpins. J. Cell Biol. 2002, 158, 929–940. [Google Scholar] [CrossRef]
- Fukuda, N.; Emoto, M.; Nakamori, Y.; Taguchi, A.; Miyamoto, S.; Uraki, S.; Oka, Y.; Tanizawa, Y. DOC2B: A novel syntaxin-4 binding protein mediating insulin-regulated GLUT4 vesicle fusion in adipocytes. Diabetes 2009, 58, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Jewell, J.L.; Oh, E.; Bennett, S.M.; Meroueh, S.O.; Thurmond, D.C. The tyrosine phosphorylation of Munc18c induces a switch in binding specificity from syntaxin 4 to Doc2beta. J. Biol. Chem. 2008, 283, 21734–21746. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Rathore, S.S.; Davis, E.M.; Ouyang, Y.; Shen, J. Doc2b promotes GLUT4 exocytosis by activating the SNARE-mediated fusion reaction in a calcium- and membrane bending-dependent manner. Mol. Biol. Cell 2013, 24, 1176–1184. [Google Scholar] [CrossRef]
- Ramalingam, L.; Oh, E.; Thurmond, D.C. Doc2b enrichment enhances glucose homeostasis in mice via potentiation of insulin secretion and peripheral insulin sensitivity. Diabetologia 2014, 57, 1476–1484. [Google Scholar] [CrossRef]
- Ramalingam, L.; Oh, E.; Yoder, S.M.; Brozinick, J.T.; Kalwat, M.A.; Groffen, A.J.; Verhage, M.; Thurmond, D.C. Doc2b is a key effector of insulin secretion and skeletal muscle insulin sensitivity. Diabetes 2012, 61, 2424–2432. [Google Scholar] [CrossRef]
- Min, J.; Okada, S.; Kanzaki, M.; Elmendorf, J.S.; Coker, K.J.; Ceresa, B.P.; Syu, L.J.; Noda, Y.; Saltiel, A.R.; Pessin, J.E. Synip: A novel insulin-regulated syntaxin 4-binding protein mediating GLUT4 translocation in adipocytes. Mol. Cell 1999, 3, 751–760. [Google Scholar] [CrossRef]
- Yu, H.; Rathore, S.S.; Shen, J. Synip arrests soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-dependent membrane fusion as a selective target membrane SNARE-binding inhibitor. J. Biol. Chem. 2013, 288, 18885–18893. [Google Scholar] [CrossRef]
- Tamori, Y.; Kawanishi, M.; Niki, T.; Shinoda, H.; Araki, S.; Okazawa, H.; Kasuga, M. Inhibition of insulin-induced GLUT4 translocation by Munc18c through interaction with syntaxin4 in 3T3-L1 adipocytes. J. Biol. Chem. 1998, 273, 19740–19746. [Google Scholar] [CrossRef]
- Tellam, J.T.; Macaulay, S.L.; McIntosh, S.; Hewish, D.R.; Ward, C.W.; James, D.E. Characterization of Munc-18c and syntaxin-4 in 3T3-L1 adipocytes. Putative role in insulin-dependent movement of GLUT-4. J. Biol. Chem. 1997, 272, 6179–6186. [Google Scholar] [CrossRef]
- Jewell, J.L.; Oh, E.; Ramalingam, L.; Kalwat, M.A.; Tagliabracci, V.S.; Tackett, L.; Elmendorf, J.S.; Thurmond, D.C. Munc18c phosphorylation by the insulin receptor links cell signaling directly to SNARE exocytosis. J. Cell Biol. 2011, 193, 185–199. [Google Scholar] [CrossRef]
- Oh, E.; Spurlin, B.A.; Pessin, J.E.; Thurmond, D.C. Munc18c heterozygous knockout mice display increased susceptibility for severe glucose intolerance. Diabetes 2005, 54, 638–647. [Google Scholar] [CrossRef]
- Spurlin, B.A.; Thomas, R.M.; Nevins, A.K.; Kim, H.J.; Kim, Y.J.; Noh, H.L.; Shulman, G.I.; Kim, J.K.; Thurmond, D.C. Insulin resistance in tetracycline-repressible Munc18c transgenic mice. Diabetes 2003, 52, 1910–1917. [Google Scholar] [CrossRef]
- Thurmond, D.C.; Kanzaki, M.; Khan, A.H.; Pessin, J.E. Munc18c function is required for insulin-stimulated plasma membrane fusion of GLUT4 and insulin-responsive amino peptidase storage vesicles. Mol. Cell. Biol. 2000, 20, 379–388. [Google Scholar] [CrossRef]
- Hu, S.H.; Latham, C.F.; Gee, C.L.; James, D.E.; Martin, J.L. Structure of the Munc18c/Syntaxin4 N-peptide complex defines universal features of the N-peptide binding mode of Sec1/Munc18 proteins. Proc. Natl. Acad. Sci. USA 2007, 104, 8773–8778. [Google Scholar] [CrossRef] [PubMed]
- Latham, C.F.; Lopez, J.A.; Hu, S.H.; Gee, C.L.; Westbury, E.; Blair, D.H.; Armishaw, C.J.; Alewood, P.F.; Bryant, N.J.; James, D.E.; et al. Molecular dissection of the Munc18c/syntaxin4 interaction: Implications for regulation of membrane trafficking. Traffic 2006, 7, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, D.C.; Ceresa, B.P.; Okada, S.; Elmendorf, J.S.; Coker, K.; Pessin, J.E. Regulation of insulin-stimulated GLUT4 translocation by Munc18c in 3T3L1 adipocytes. J. Biol. Chem. 1998, 273, 33876–33883. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Tamori, Y.; Shinoda, H.; Yoshikawa, M.; Sakaue, M.; Udagawa, J.; Otani, H.; Tashiro, F.; Miyazaki, J.; Kasuga, M. Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J. Clin. Investig. 2005, 115, 291–301. [Google Scholar] [CrossRef]
- Aran, V.; Bryant, N.J.; Gould, G.W. Tyrosine phosphorylation of Munc18c on residue 521 abrogates binding to Syntaxin 4. BMC Biochem. 2011, 12, 19. [Google Scholar] [CrossRef]
- Bruton, J.D.; Katz, A.; Westerblad, H. Insulin increases near-membrane but not global Ca2+ in isolated skeletal muscle. Proc. Natl. Acad. Sci. USA 1999, 96, 3281–3286. [Google Scholar] [CrossRef]
- Whitehead, J.P.; Molero, J.C.; Clark, S.; Martin, S.; Meneilly, G.; James, D.E. The role of Ca2+ in insulin-stimulated glucose transport in 3T3-L1 cells. J. Biol. Chem. 2001, 276, 27816–27824. [Google Scholar] [CrossRef]
- Lalioti, V.; Muruais, G.; Dinarina, A.; van Damme, J.; Vandekerckhove, J.; Sandoval, I.V. The atypical kinase Cdk5 is activated by insulin, regulates the association between GLUT4 and E-Syt1, and modulates glucose transport in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. USA 2009, 106, 4249–4253. [Google Scholar] [CrossRef]
- Ramalingam, L.; Lu, J.; Hudmon, A.; Thurmond, D.C. Doc2b serves as a scaffolding platform for concurrent binding of multiple Munc18 isoforms in pancreatic islet β-cells. Biochem. J. 2014, 464, 251–258. [Google Scholar] [CrossRef]
- McMahon, H.T.; Kozlov, M.M.; Martens, S. Membrane curvature in synaptic vesicle fusion and beyond. Cell 2010, 140, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Nagano, F.; Orita, S.; Sasaki, T.; Naito, A.; Sakaguchi, G.; Maeda, M.; Watanabe, T.; Kominami, E.; Uchiyama, Y.; Takai, Y. Interaction of Doc2 with tctex-1, a light chain of cytoplasmic dynein. Implication in dynein-dependent vesicle transport. J. Biol. Chem. 1998, 273, 30065–30068. [Google Scholar] [CrossRef] [PubMed]
- Jewell, J.L.; Luo, W.; Oh, E.; Wang, Z.; Thurmond, D.C. Filamentous actin regulates insulin exocytosis through direct interaction with Syntaxin 4. J. Biol. Chem. 2008, 283, 10716–10726. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jedrychowski, M.P.; Gygi, S.P.; Pilch, P.F. Role of insulin-dependent cortical fodrin/spectrin remodeling in glucose transporter 4 translocation in rat adipocytes. Mol. Biol. Cell 2006, 17, 4249–4256. [Google Scholar] [CrossRef]
- Kalwat, M.A.; Wiseman, D.A.; Luo, W.; Wang, Z.; Thurmond, D.C. Gelsolin associates with the N terminus of syntaxin 4 to regulate insulin granule exocytosis. Mol. Endocrinol. 2012, 26, 128–141. [Google Scholar] [CrossRef]
- Geiser, A.; Foylan, S.; Tinning, P.W.; Bryant, N.J.; Gould, G.W. GLUT4 dispersal at the plasma membrane of adipocytes: A super-resolved journey. Biosci. Rep. 2023, 43, BSR20230946. [Google Scholar] [CrossRef]
- Jiang, L.; Fan, J.; Bai, L.; Wang, Y.; Chen, Y.; Yang, L.; Chen, L.; Xu, T. Direct quantification of fusion rate reveals a distal role for AS160 in insulin-stimulated fusion of GLUT4 storage vesicles. J. Biol. Chem. 2008, 283, 8508–8516. [Google Scholar] [CrossRef]
- Antonescu, C.N.; Díaz, M.; Femia, G.; Planas, J.V.; Klip, A. Clathrin-dependent and independent endocytosis of glucose transporter 4 (GLUT4) in myoblasts: Regulation by mitochondrial uncoupling. Traffic 2008, 9, 1173–1190. [Google Scholar] [CrossRef]
- Volchuk, A.; Narine, S.; Foster, L.J.; Grabs, D.; De Camilli, P.; Klip, A. Perturbation of dynamin II with an amphiphysin SH3 domain increases GLUT4 glucose transporters at the plasma membrane in 3T3-L1 adipocytes. Dynamin II participates in GLUT4 endocytosis. J. Biol. Chem. 1998, 273, 8169–8176. [Google Scholar] [CrossRef]
- Huang, J.; Imamura, T.; Olefsky, J.M. Insulin can regulate GLUT4 internalization by signaling to Rab5 and the motor protein dynein. Proc. Natl. Acad. Sci. USA 2001, 98, 13084–13089. [Google Scholar] [CrossRef] [PubMed]
- Esk, C.; Chen, C.Y.; Johannes, L.; Brodsky, F.M. The clathrin heavy chain isoform CHC22 functions in a novel endosomal sorting step. J. Cell. Biol. 2010, 188, 131–144. [Google Scholar] [CrossRef]
- Vassilopoulos, S.; Esk, C.; Hoshino, S.; Funke, B.H.; Chen, C.Y.; Plocik, A.M.; Wright, W.E.; Kucherlapati, R.; Brodsky, F.M. A role for the CHC22 clathrin heavy-chain isoform in human glucose metabolism. Science 2009, 324, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Huang, G.; Kandror, K.V. Self-assembly of Glut4 storage vesicles during differentiation of 3T3-L1 adipocytes. J. Biol. Chem. 2008, 283, 30311–30321. [Google Scholar] [CrossRef] [PubMed]
- Eyster, C.A.; Duggins, Q.S.; Gorbsky, G.J.; Olson, A.L. Microtubule network is required for insulin signaling through activation of Akt/protein kinase B: Evidence that insulin stimulates vesicle docking/fusion but not intracellular mobility. J. Biol. Chem. 2006, 281, 39719–39727. [Google Scholar] [CrossRef]
- Li, L.V.; Bakirtzi, K.; Watson, R.T.; Pessin, J.E.; Kandror, K.V. The C-terminus of GLUT4 targets the transporter to the perinuclear compartment but not to the insulin-responsive vesicles. Biochem. J. 2009, 419, 105–112. [Google Scholar] [CrossRef]
- Shi, J.; Kandror, K.V. The luminal Vps10p domain of sortilin plays the predominant role in targeting to insulin-responsive Glut4-containing vesicles. J. Biol. Chem. 2007, 282, 9008–9016. [Google Scholar] [CrossRef]
- Capilla, E.; Suzuki, N.; Pessin, J.E.; Hou, J.C. The glucose transporter 4 FQQI motif is necessary for Akt substrate of 160-kilodalton-dependent plasma membrane translocation but not Golgi-localized (gamma)-ear-containing Arf-binding protein-dependent entry into the insulin-responsive storage compartment. Mol. Endocrinol. 2007, 21, 3087–3099. [Google Scholar] [CrossRef]
- Lamb, C.A.; McCann, R.K.; Stöckli, J.; James, D.E.; Bryant, N.J. Insulin-regulated trafficking of GLUT4 requires ubiquitination. Traffic 2010, 11, 1445–1454. [Google Scholar] [CrossRef]
- Dannhauser, P.N.; Camus, S.M.; Sakamoto, K.; Sadacca, L.A.; Torres, J.A.; Camus, M.D.; Briant, K.; Vassilopoulos, S.; Rothnie, A.; Smith, C.J.; et al. CHC22 and CHC17 clathrins have distinct biochemical properties and display differential regulation and function. J. Biol. Chem. 2017, 292, 20834–20844. [Google Scholar] [CrossRef]
- Habtemichael, E.N.; Alcázar-Román, A.; Rubin, B.R.; Grossi, L.R.; Belman, J.P.; Julca, O.; Löffler, M.G.; Li, H.; Chi, N.W.; Samuel, V.T.; et al. Coordinated Regulation of Vasopressin Inactivation and Glucose Uptake by Action of TUG Protein in Muscle. J. Biol. Chem. 2015, 290, 14454–14461. [Google Scholar] [CrossRef]
- Shi, J.; Kandror, K.V. Study of glucose uptake in adipose cells. Methods Mol. Biol. 2008, 456, 307–315. [Google Scholar]
- Pilch, P.F. The mass action hypothesis: Formation of Glut4 storage vesicles, a tissue-specific, regulated exocytic compartment. Acta Physiol. 2008, 192, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Buckler-Pena, D.; Nauta, T.; Singh, M.; Asmar, A.; Shi, J.; Kim, J.Y.; Kandror, K.V. Insulin responsiveness of glucose transporter 4 in 3T3-L1 cells depends on the presence of sortilin. Mol. Biol. Cell 2013, 24, 3115–3122. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.D.; Graveleau, C.; Betuing, S.; Pham, M.; Reay, P.A.; Kandror, V.; Kupriyanova, T.; Xu, Z.; Kandror, K.V. Regulation of insulin-responsive aminopeptidase expression and targeting in the insulin-responsive vesicle compartment of glucose transporter isoform 4-deficient cardiomyocytes. Mol. Endocrinol. 2004, 18, 2491–2501. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, J.; Katz, E.B.; Charron, M.J. GLUT4 ablation in mice results in redistribution of IRAP to the plasma membrane. Biochem. Biophys. Res. Commun. 2001, 284, 519–525. [Google Scholar] [CrossRef]
- Keller, S.R.; Davis, A.C.; Clairmont, K.B. Mice deficient in the insulin-regulated membrane aminopeptidase show substantial decreases in glucose transporter GLUT4 levels but maintain normal glucose homeostasis. J. Biol. Chem. 2002, 277, 17677–17686. [Google Scholar] [CrossRef]
- Carvalho, E.; Schellhorn, S.E.; Zabolotny, J.M.; Martin, S.; Tozzo, E.; Peroni, O.D.; Houseknecht, K.L.; Mundt, A.; James, D.E.; Kahn, B.B. GLUT4 overexpression or deficiency in adipocytes of transgenic mice alters the composition of GLUT4 vesicles and the subcellular localization of GLUT4 and insulin-responsive aminopeptidase. J. Biol. Chem. 2004, 279, 21598–21605. [Google Scholar] [CrossRef]
- Yeh, T.Y.; Sbodio, J.I.; Tsun, Z.Y.; Luo, B.; Chi, N.W. Insulin-stimulated exocytosis of GLUT4 is enhanced by IRAP and its partner tankyrase. Biochem. J. 2007, 402, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Gross, D.N.; Farmer, S.R.; Pilch, P.F. Glut4 storage vesicles without Glut4: Transcriptional regulation of insulin-dependent vesicular traffic. Mol. Cell. Biol. 2004, 24, 7151–7162. [Google Scholar] [CrossRef] [PubMed]
- Olson, A.L.; Trumbly, A.R.; Gibson, G.V. Insulin-mediated GLUT4 translocation is dependent on the microtubule network. J. Biol. Chem. 2001, 276, 10706–10714. [Google Scholar] [CrossRef] [PubMed]
- Ai, H.; Ralston, E.; Lauritzen, H.P.; Galbo, H.; Ploug, T. Disruption of microtubules in rat skeletal muscle does not inhibit insulin- or contraction-stimulated glucose transport. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E836–E844. [Google Scholar] [CrossRef]
- Brozinick, J.T., Jr.; Hawkins, E.D.; Strawbridge, A.B.; Elmendorf, J.S. Disruption of cortical actin in skeletal muscle demonstrates an essential role of the cytoskeleton in glucose transporter 4 translocation in insulin-sensitive tissues. J. Biol. Chem. 2004, 279, 40699–40706. [Google Scholar] [CrossRef]
- Zaid, H.; Antonescu, C.N.; Randhawa, V.K.; Klip, A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem. J. 2008, 413, 201–215. [Google Scholar] [CrossRef]
- Tunduguru, R.; Chiu, T.T.; Ramalingam, L.; Elmendorf, J.S.; Klip, A.; Thurmond, D.C. Signaling of the p21-activated kinase (PAK1) coordinates insulin-stimulated actin remodeling and glucose uptake in skeletal muscle cells. Biochem. Pharmacol. 2014, 92, 380–388. [Google Scholar] [CrossRef]
- Tunduguru, R.; Zhang, J.; Aslamy, A.; Salunkhe, V.A.; Brozinick, J.T.; Elmendorf, J.S.; Thurmond, D.C. The actin-related p41ARC subunit contributes to p21-activated kinase-1 (PAK1)-mediated glucose uptake into skeletal muscle cells. J. Biol. Chem. 2017, 292, 19034–19043. [Google Scholar] [CrossRef]
- Chiu, T.T.; Patel, N.; Shaw, A.E.; Bamburg, J.R.; Klip, A. Arp2/3- and cofilin-coordinated actin dynamics is required for insulin-mediated GLUT4 translocation to the surface of muscle cells. Mol. Biol. Cell 2010, 21, 3529–3539. [Google Scholar] [CrossRef]
- Vadlamudi, R.K.; Li, F.; Barnes, C.J.; Bagheri-Yarmand, R.; Kumar, R. p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep. 2004, 5, 154–160. [Google Scholar] [CrossRef]
- Andrade, L.G.; Albarnaz, J.D.; Mügge, F.L.; David, B.A.; Abrahão, J.S.; da Fonseca, F.G.; Kroon, E.G.; Menezes, G.B.; McFadden, G.; Bonjardim, C.A. Vaccinia virus dissemination requires p21-activated kinase 1. Arch. Virol. 2016, 161, 2991–3002. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Chawla, A.; Bose, A.; Way, M.; Czech, M.P. A phosphatidylinositol 3-kinase-independent insulin signaling pathway to N-WASP/Arp2/3/F-actin required for GLUT4 glucose transporter recycling. J. Biol. Chem. 2002, 277, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Takamure, M.; Murata, K.Y.; Tamada, Y.; Azuma, M.; Ueno, S. Calpain-dependent alpha-fodrin cleavage at the sarcolemma in muscle diseases. Muscle Nerve 2005, 32, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Gurlo, T.; Haataja, L.; Costes, S.; Daval, M.; Ryazantsev, S.; Wu, X.; Butler, A.E.; Butler, P.C. Calcium-activated calpain-2 is a mediator of beta cell dysfunction and apoptosis in type 2 diabetes. J. Biol. Chem. 2010, 285, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.Y.; Boyd, A.E., 3rd. Gelsolin, a Ca2+-dependent actin-binding protein in a hamster insulin-secreting cell line. J. Clin. Investig. 1985, 75, 1015–1022. [Google Scholar] [CrossRef]
- Li, G.H.; Arora, P.D.; Chen, Y.; McCulloch, C.A.; Liu, P. Multifunctional roles of gelsolin in health and diseases. Med. Res. Rev. 2012, 32, 999–1025. [Google Scholar] [CrossRef]
- Choe, H.; Burtnick, L.D.; Mejillano, M.; Yin, H.L.; Robinson, R.C.; Choe, S. The calcium activation of gelsolin: Insights from the 3A structure of the G4-G6/actin complex. J. Mol. Biol. 2002, 324, 691–702. [Google Scholar] [CrossRef]
- James, D.E.; Stöckli, J.; Birnbaum, M.J. The aetiology and molecular landscape of insulin resistance. Nat. Rev. Mol. Cell Biol. 2021, 22, 751–771. [Google Scholar] [CrossRef]
- Himsworth, H.P. Diabetes mellitus: Its differentiation into insulin-sensitive and insulin-insensitive types. Diabet. Med. A J. Br. Diabet. Assoc. 2011, 28, 1440–1444. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 2015, 1, 15019. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martin, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Chen, M.Z.; Hudson, C.A.; Vincent, E.E.; de Berker, D.A.; May, M.T.; Hers, I.; Dayan, C.M.; Andrews, R.C.; Tavaré, J.M. Bariatric surgery in morbidly obese insulin resistant humans normalises insulin signalling but not insulin-stimulated glucose disposal. PLoS ONE 2015, 10, e0120084. [Google Scholar] [CrossRef] [PubMed]
- Burchfield, J.G.; Kebede, M.A.; Meoli, C.C.; Stöckli, J.; Whitworth, P.T.; Wright, A.L.; Hoffman, N.J.; Minard, A.Y.; Ma, X.; Krycer, J.R.; et al. High dietary fat and sucrose results in an extensive and time-dependent deterioration in health of multiple physiological systems in mice. J. Biol. Chem. 2018, 293, 5731–5745. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R.; Flier, J.S.; Bar, R.S.; Archer, J.A.; Gorden, P.; Martin, M.M.; Roth, J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N. Engl. J. Med. 1976, 294, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Le Marchand-Brustel, Y.; Grémeaux, T.; Ballotti, R.; Van Obberghen, E. Insulin receptor tyrosine kinase is defective in skeletal muscle of insulin-resistant obese mice. Nature 1985, 315, 676–679. [Google Scholar] [CrossRef]
- Tan, S.X.; Fisher-Wellman, K.H.; Fazakerley, D.J.; Ng, Y.; Pant, H.; Li, J.; Meoli, C.C.; Coster, A.C.; Stöckli, J.; James, D.E. Selective insulin resistance in adipocytes. J. Biol. Chem. 2015, 290, 11337–11348. [Google Scholar] [CrossRef]
- Yu, C.; Chen, Y.; Cline, G.W.; Zhang, D.; Zong, H.; Wang, Y.; Bergeron, R.; Kim, J.K.; Cushman, S.W.; Cooney, G.J.; et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 2002, 277, 50230–50236. [Google Scholar] [CrossRef]
- Fazakerley, D.J.; Krycer, J.R.; Kearney, A.L.; Hocking, S.L.; James, D.E. Muscle and adipose tissue insulin resistance: Malady without mechanism? J. Lipid Res. 2019, 60, 1720–1732. [Google Scholar] [CrossRef]
- Copps, K.D.; White, M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef]
- Erion, D.M.; Shulman, G.I. Diacylglycerol-mediated insulin resistance. Nat. Med. 2010, 16, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Gehart, H.; Kumpf, S.; Ittner, A.; Ricci, R. MAPK signalling in cellular metabolism: Stress or wellness? EMBO Rep. 2010, 11, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Barham, F.W. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J. Biol. Chem. 1971, 246, 6210–6216. [Google Scholar] [CrossRef]
- Kahn, C.R. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: A necessary distinction. Metabolism 1978, 27 (Suppl. S2), 1893–1902. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Kolterman, O.G.; Scarlett, J.A. Insulin action and resistance in obesity and noninsulin-dependent type II diabetes mellitus. Am. J. Physiol. 1982, 243, E15–E30. [Google Scholar] [CrossRef]
- Czech, M.P. Cellular basis of insulin insensitivity in large rat adipocytes. J. Clin. Investig. 1976, 57, 1523–1532. [Google Scholar] [CrossRef]
- Melvin, A.; O’Rahilly, S.; Savage, D.B. Genetic syndromes of severe insulin resistance. Curr. Opin. Genet. Dev. 2018, 50, 60–67. [Google Scholar] [CrossRef]
- Crouthamel, M.C.; Kahana, J.A.; Korenchuk, S.; Zhang, S.Y.; Sundaresan, G.; Eberwein, D.J.; Brown, K.K.; Kumar, R. Mechanism and management of AKT inhibitor-induced hyperglycemia. Clin. Cancer Res. 2009, 15, 217–225. [Google Scholar] [CrossRef]
- Tonks, K.T.; Ng, Y.; Miller, S.; Coster, A.C.; Samocha-Bonet, D.; Iseli, T.J.; Xu, A.; Patrick, E.; Yang, J.Y.; Junutula, J.R.; et al. Impaired Akt phosphorylation in insulin-resistant human muscle is accompanied by selective and heterogeneous downstream defects. Diabetologia 2013, 56, 875–885. [Google Scholar] [CrossRef]
- Jaiswal, N.; Gavin, M.G.; Quinn, W.J., 3rd; Luongo, T.S.; Gelfer, R.G.; Baur, J.A.; Titchenell, P.M. The role of skeletal muscle Akt in the regulation of muscle mass and glucose homeostasis. Mol. Metab. 2019, 28, 1–13. [Google Scholar] [CrossRef]
- Lu, M.; Wan, M.; Leavens, K.F.; Chu, Q.; Monks, B.R.; Fernandez, S.; Ahima, R.S.; Ueki, K.; Kahn, C.R.; Birnbaum, M.J. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat. Med. 2012, 18, 388–395. [Google Scholar] [CrossRef]
- Tan, S.X.; Ng, Y.; Meoli, C.C.; Kumar, A.; Khoo, P.S.; Fazakerley, D.J.; Junutula, J.R.; Vali, S.; James, D.E.; Stöckli, J. Amplification and demultiplexing in insulin-regulated Akt protein kinase pathway in adipocytes. J. Biol. Chem. 2012, 287, 6128–6138. [Google Scholar] [CrossRef] [PubMed]
- Larance, M.; Rowland, A.F.; Hoehn, K.L.; Humphreys, D.T.; Preiss, T.; Guilhaus, M.; James, D.E. Global phosphoproteomics identifies a major role for AKT and 14-3-3 in regulating EDC3. Mol. Cell Proteom. 2010, 9, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.K.; Ahlsén, M.; Zierath, J.R.; Wallberg-Henriksson, H.; Koistinen, H.A. Insulin signaling and glucose transport in skeletal muscle from first-degree relatives of type 2 diabetic patients. Diabetes 2006, 55, 1283–1288. [Google Scholar] [CrossRef]
- Ramos, P.A.; Lytle, K.A.; Delivanis, D.; Nielsen, S.; LeBrasseur, N.K.; Jensen, M.D. Insulin-Stimulated Muscle Glucose Uptake and Insulin Signaling in Lean and Obese Humans. J. Clin. Endocrinol. Metab. 2021, 106, e1631–e1646. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.M.; Levin, K.; Grimmsmann, T.; Beck-Nielsen, H.; Klein, H.H. Insulin signalling in skeletal muscle of subjects with or without Type II-diabetes and first degree relatives of patients with the disease. Diabetologia 2002, 45, 813–822. [Google Scholar] [CrossRef]
- Kim, Y.B.; Nikoulina, S.E.; Ciaraldi, T.P.; Henry, R.R.; Kahn, B.B. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J. Clin. Investig. 1999, 104, 733–741. [Google Scholar] [CrossRef]
- Fazakerley, D.J.; Minard, A.Y.; Krycer, J.R.; Thomas, K.C.; Stöckli, J.; Harney, D.J.; Burchfield, J.G.; Maghzal, G.J.; Caldwell, S.T.; Hartley, R.C.; et al. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J. Biol. Chem. 2018, 293, 7315–7328. [Google Scholar] [CrossRef]
- Fazakerley, D.J.; Chaudhuri, R.; Yang, P.; Maghzal, G.J.; Thomas, K.C.; Krycer, J.R.; Humphrey, S.J.; Parker, B.L.; Fisher-Wellman, K.H.; Meoli, C.C.; et al. Mitochondrial CoQ deficiency is a common driver of mitochondrial oxidants and insulin resistance. Elife 2018, 7, e32111. [Google Scholar] [CrossRef]
- Kolterman, O.G.; Insel, J.; Saekow, M.; Olefsky, J.M. Mechanisms of insulin resistance in human obesity: Evidence for receptor and postreceptor defects. J. Clin. Investig. 1980, 65, 1272–1284. [Google Scholar] [CrossRef]
- Friedman, J.E.; Caro, J.F.; Pories, W.J.; Azevedo, J.L., Jr.; Dohm, G.L. Glucose metabolism in incubated human muscle: Effect of obesity and non-insulin-dependent diabetes mellitus. Metabolism 1994, 43, 1047–1054. [Google Scholar] [CrossRef]
- Shulman, G.I.; Rothman, D.L.; Jue, T.; Stein, P.; DeFronzo, R.A.; Shulman, R.G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N. Engl. J. Med. 1990, 322, 223–228. [Google Scholar] [CrossRef]
- Baron, A.D.; Laakso, M.; Brechtel, G.; Edelman, S.V. Reduced capacity and affinity of skeletal muscle for insulin-mediated glucose uptake in noninsulin-dependent diabetic subjects. Effects of insulin therapy. J. Clin. Investig. 1991, 87, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.E.; Dohm, G.L.; Leggett-Frazier, N.; Elton, C.W.; Tapscott, E.B.; Pories, W.P.; Caro, J.F. Restoration of insulin responsiveness in skeletal muscle of morbidly obese patients after weight loss. Effect on muscle glucose transport and glucose transporter GLUT4. J. Clin. Investig. 1992, 89, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Batista, T.M.; Jayavelu, A.K.; Wewer Albrechtsen, N.J.; Iovino, S.; Lebastchi, J.; Pan, H.; Dreyfuss, J.M.; Krook, A.; Zierath, J.R.; Mann, M.; et al. A Cell-Autonomous Signature of Dysregulated Protein Phosphorylation Underlies Muscle Insulin Resistance in Type 2 Diabetes. Cell Metab. 2020, 32, 844–859.e845. [Google Scholar] [CrossRef]
- Haider, N.; Lebastchi, J.; Jayavelu, A.K.; Batista, T.M.; Pan, H.; Dreyfuss, J.M.; Carcamo-Orive, I.; Knowles, J.W.; Mann, M.; Kahn, C.R. Signaling defects associated with insulin resistance in nondiabetic and diabetic individuals and modification by sex. J. Clin. Investig. 2021, 131, e151818. [Google Scholar] [CrossRef]
- Fazakerley, D.J.; van Gerwen, J.; Cooke, K.C.; Duan, X.; Needham, E.J.; Díaz-Vegas, A.; Madsen, S.; Norris, D.M.; Shun-Shion, A.S.; Krycer, J.R.; et al. Phosphoproteomics reveals rewiring of the insulin signaling network and multi-nodal defects in insulin resistance. Nat. Commun. 2023, 14, 923. [Google Scholar] [CrossRef]
- Needham, E.J.; Hingst, J.R.; Parker, B.L.; Morrison, K.R.; Yang, G.; Onslev, J.; Kristensen, J.M.; Højlund, K.; Ling, N.X.Y.; Oakhill, J.S.; et al. Personalized phosphoproteomics identifies functional signaling. Nat. Biotechnol. 2022, 40, 576–584. [Google Scholar] [CrossRef]
- Martin, S.; Tellam, J.; Livingstone, C.; Slot, J.W.; Gould, G.W.; James, D.E. The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulin-sensitive cells. J. Cell Biol. 1996, 134, 625–635. [Google Scholar] [CrossRef]
- Garvey, W.T.; Maianu, L.; Zhu, J.H.; Hancock, J.A.; Golichowski, A.M. Multiple defects in the adipocyte glucose transport system cause cellular insulin resistance in gestational diabetes. Heterogeneity in the number and a novel abnormality in subcellular localization of GLUT4 glucose transporters. Diabetes 1993, 42, 1773–1785. [Google Scholar] [CrossRef]
- Garvey, W.T.; Maianu, L.; Zhu, J.H.; Brechtel-Hook, G.; Wallace, P.; Baron, A.D. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J. Clin. Investig. 1998, 101, 2377–2386. [Google Scholar] [CrossRef]
- Maianu, L.; Keller, S.R.; Garvey, W.T. Adipocytes exhibit abnormal subcellular distribution and translocation of vesicles containing glucose transporter 4 and insulin-regulated aminopeptidase in type 2 diabetes mellitus: Implications regarding defects in vesicle trafficking. J. Clin. Endocrinol. Metab. 2001, 86, 5450–5456. [Google Scholar] [CrossRef]
- Chavez, J.A.; Summers, S.A. A ceramide-centric view of insulin resistance. Cell Metab. 2012, 15, 585–594. [Google Scholar] [CrossRef]
- Hu, W.; Xu, R.; Zhang, G.; Jin, J.; Szulc, Z.M.; Bielawski, J.; Hannun, Y.A.; Obeid, L.M.; Mao, C. Golgi fragmentation is associated with ceramide-induced cellular effects. Mol. Biol. Cell 2005, 16, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.P.; Klip, A. Dynamic GLUT4 sorting through a syntaxin-6 compartment in muscle cells is derailed by insulin resistance-causing ceramide. Biol. Open 2014, 3, 314–325. [Google Scholar] [CrossRef]
- JeBailey, L.; Wanono, O.; Niu, W.; Roessler, J.; Rudich, A.; Klip, A. Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent Rac-activation and actin remodeling in muscle cells. Diabetes 2007, 56, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Lizunov, V.A.; Lee, J.P.; Skarulis, M.C.; Zimmerberg, J.; Cushman, S.W.; Stenkula, K.G. Impaired tethering and fusion of GLUT4 vesicles in insulin-resistant human adipose cells. Diabetes 2013, 62, 3114–3119. [Google Scholar] [CrossRef] [PubMed]
- Koester, A.M.; Geiser, A.; Bowman, P.R.T.; van de Linde, S.; Gadegaard, N.; Bryant, N.J.; Gould, G.W. GLUT4 translocation and dispersal operate in multiple cell types and are negatively correlated with cell size in adipocytes. Sci. Rep. 2022, 12, 20535. [Google Scholar] [CrossRef]
- Gao, L.; Chen, J.; Gao, J.; Wang, H.; Xiong, W. Super-resolution microscopy reveals the insulin-resistance-regulated reorganization of GLUT4 on plasma membranes. J. Cell Sci. 2017, 130, 396–405. [Google Scholar] [CrossRef]
- Maier, V.H.; Melvin, D.R.; Lister, C.A.; Chapman, H.; Gould, G.W.; Murphy, G.J. v- and t-SNARE protein expression in models of insulin resistance: Normalization of glycemia by rosiglitazone treatment corrects overexpression of cellubrevin, vesicle-associated membrane protein-2, and syntaxin 4 in skeletal muscle of Zucker diabetic fatty rats. Diabetes 2000, 49, 618–625. [Google Scholar] [CrossRef]
- Bowman, P.R.T.; Smith, G.L.; Gould, G.W. Cardiac SNARE Expression in Health and Disease. Front. Endocrinol. 2019, 10, 881. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, I.R.; Pulawa, L.K.; Ferreira, L.D.; James, D.E.; Capell, W.H.; Eckel, R.H. Increased expression of the SNARE accessory protein Munc18c in lipid-mediated insulin resistance. J. Lipid Res. 2003, 44, 1174–1181. [Google Scholar] [CrossRef]
- Patki, V.; Buxton, J.; Chawla, A.; Lifshitz, L.; Fogarty, K.; Carrington, W.; Tuft, R.; Corvera, S. Insulin action on GLUT4 traffic visualized in single 3T3-l1 adipocytes by using ultra-fast microscopy. Mol. Biol. Cell 2001, 12, 129–141. [Google Scholar] [CrossRef]
- Kee, A.J.; Yang, L.; Lucas, C.A.; Greenberg, M.J.; Martel, N.; Leong, G.M.; Hughes, W.E.; Cooney, G.J.; James, D.E.; Ostap, E.M.; et al. An actin filament population defined by the tropomyosin Tpm3.1 regulates glucose uptake. Traffic 2015, 16, 691–711. [Google Scholar] [CrossRef]
- Lim, C.Y.; Bi, X.; Wu, D.; Kim, J.B.; Gunning, P.W.; Hong, W.; Han, W. Tropomodulin3 is a novel Akt2 effector regulating insulin-stimulated GLUT4 exocytosis through cortical actin remodeling. Nat. Commun. 2015, 6, 5951. [Google Scholar] [CrossRef]
- Kadowaki, T.; Fujita-Yamaguchi, Y.; Nishida, E.; Takaku, F.; Akiyama, T.; Kathuria, S.; Akanuma, Y.; Kasuga, M. Phosphorylation of tubulin and microtubule-associated proteins by the purified insulin receptor kinase. J. Biol. Chem. 1985, 260, 4016–4020. [Google Scholar] [CrossRef]
- Olson, A.L.; Eyster, C.A.; Duggins, Q.S.; Knight, J.B. Insulin promotes formation of polymerized microtubules by a phosphatidylinositol 3-kinase-independent, actin-dependent pathway in 3T3-L1 adipocytes. Endocrinology 2003, 144, 5030–5039. [Google Scholar] [CrossRef]
- Parker, S.S.; Krantz, J.; Kwak, E.A.; Barker, N.K.; Deer, C.G.; Lee, N.Y.; Mouneimne, G.; Langlais, P.R. Insulin Induces Microtubule Stabilization and Regulates the Microtubule Plus-end Tracking Protein Network in Adipocytes. Mol. Cell Proteom. 2019, 18, 1363–1381. [Google Scholar] [CrossRef]
- Chen, G.; Raman, P.; Bhonagiri, P.; Strawbridge, A.B.; Pattar, G.R.; Elmendorf, J.S. Protective effect of phosphatidylinositol 4,5-bisphosphate against cortical filamentous actin loss and insulin resistance induced by sustained exposure of 3T3-L1 adipocytes to insulin. J. Biol. Chem. 2004, 279, 39705–39709. [Google Scholar] [CrossRef]
- McCarthy, A.M.; Spisak, K.O.; Brozinick, J.T.; Elmendorf, J.S. Loss of cortical actin filaments in insulin-resistant skeletal muscle cells impairs GLUT4 vesicle trafficking and glucose transport. Am. J. Physiol. Cell Physiol. 2006, 291, C860–C868. [Google Scholar] [CrossRef]
- Grice, B.A.; Barton, K.J.; Covert, J.D.; Kreilach, A.M.; Tackett, L.; Brozinick, J.T.; Elmendorf, J.S. Excess membrane cholesterol is an early contributing reversible aspect of skeletal muscle insulin resistance in C57BL/6NJ mice fed a Western-style high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E362–E373. [Google Scholar] [CrossRef]
- Habegger, K.M.; Penque, B.A.; Sealls, W.; Tackett, L.; Bell, L.N.; Blue, E.K.; Gallagher, P.J.; Sturek, M.; Alloosh, M.A.; Steinberg, H.O.; et al. Fat-induced membrane cholesterol accrual provokes cortical filamentous actin destabilisation and glucose transport dysfunction in skeletal muscle. Diabetologia 2012, 55, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.B.; Knudsen, J.R.; Henriquez-Olguin, C.; Angin, Y.; Zaal, K.J.; Sylow, L.; Schjerling, P.; Ralston, E.; Jensen, T.E. β-Actin shows limited mobility and is required only for supraphysiological insulin-stimulated glucose transport in young adult soleus muscle. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E110–E125. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.R.; Madsen, A.B.; Li, Z.; Andersen, N.R.; Schjerling, P.; Jensen, T.E. Gene deletion of γ-actin impairs insulin-stimulated skeletal muscle glucose uptake in growing mice but not in mature adult mice. Physiol. Rep. 2022, 10, e15183. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.R.; Persson, K.W.; Henriquez-Olguin, C.; Li, Z.; Di Leo, N.; Hesselager, S.A.; Raun, S.H.; Hingst, J.R.; Trouillon, R.; Wohlwend, M.; et al. Microtubule-mediated GLUT4 trafficking is disrupted in insulin-resistant skeletal muscle. Elife 2023, 12, e83338. [Google Scholar] [CrossRef]
- Renguet, E.; De Loof, M.; Fourny, N.; Ginion, A.; Bouzin, C.; Poüs, C.; Horman, S.; Beauloye, C.; Bultot, L.; Bertrand, L. α-Tubulin acetylation on lysine 40 controls cardiac glucose uptake. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H1032–H1043. [Google Scholar] [CrossRef]
| Process | Proteins Involved |
|---|---|
| GSV cargo proteins | GLUT4, IRAP, Sortilin, LRP1 |
| GSV sequestration | TUG, PIST, Golgin-160 |
| GSV release | TC10α, USP25m |
| GSV translocation | AS160, RAB, TUGUL, Kinesin motor, KIF5B |
| GSV tethering, docking, and fusion with plasma membrane | v-SNARE VAMP2, t-SNARE syntaxin-3/4 and SNAP23, Munc18C, Mun13, DOC2, Synip |
| Retrograde traffic | Clathrin, Caveolin, CHC22 |
| GSV budding | GGA, ACAP1, AP1, AP2, AP3, ARF6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drobiova, H.; Alhamar, G.; Ahmad, R.; Al-Mulla, F.; Al Madhoun, A. GLUT4 Trafficking and Storage Vesicles: Molecular Architecture, Regulatory Networks, and Their Disruption in Insulin Resistance. Int. J. Mol. Sci. 2025, 26, 7568. https://doi.org/10.3390/ijms26157568
Drobiova H, Alhamar G, Ahmad R, Al-Mulla F, Al Madhoun A. GLUT4 Trafficking and Storage Vesicles: Molecular Architecture, Regulatory Networks, and Their Disruption in Insulin Resistance. International Journal of Molecular Sciences. 2025; 26(15):7568. https://doi.org/10.3390/ijms26157568
Chicago/Turabian StyleDrobiova, Hana, Ghadeer Alhamar, Rasheed Ahmad, Fahd Al-Mulla, and Ashraf Al Madhoun. 2025. "GLUT4 Trafficking and Storage Vesicles: Molecular Architecture, Regulatory Networks, and Their Disruption in Insulin Resistance" International Journal of Molecular Sciences 26, no. 15: 7568. https://doi.org/10.3390/ijms26157568
APA StyleDrobiova, H., Alhamar, G., Ahmad, R., Al-Mulla, F., & Al Madhoun, A. (2025). GLUT4 Trafficking and Storage Vesicles: Molecular Architecture, Regulatory Networks, and Their Disruption in Insulin Resistance. International Journal of Molecular Sciences, 26(15), 7568. https://doi.org/10.3390/ijms26157568









