Lycopene Inhibits PRRSV Replication by Suppressing ROS Production
Abstract
1. Introduction
2. Results
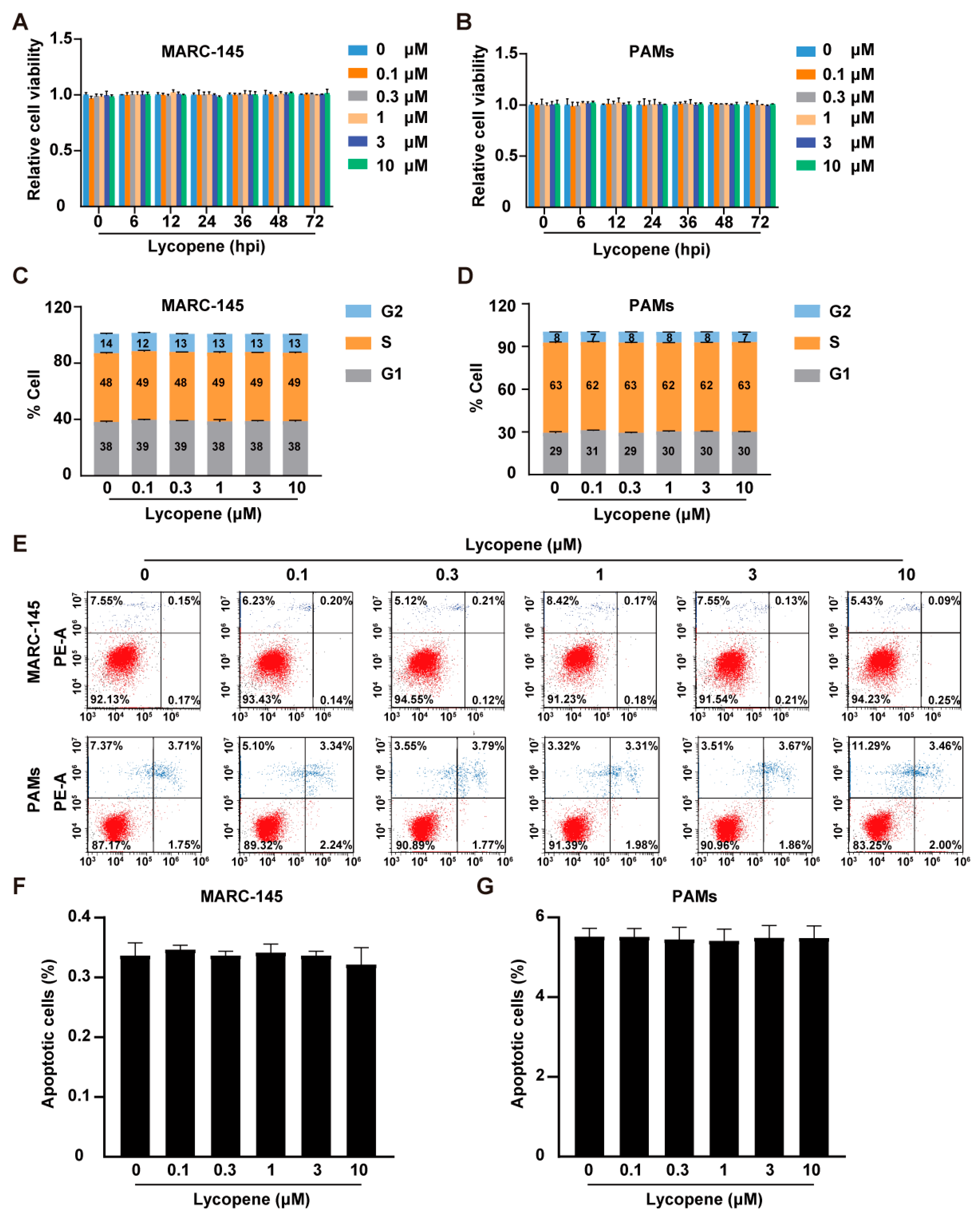
2.1. Effects of Lycopene on Cell Viability, Cell Cycle, and Apoptosis
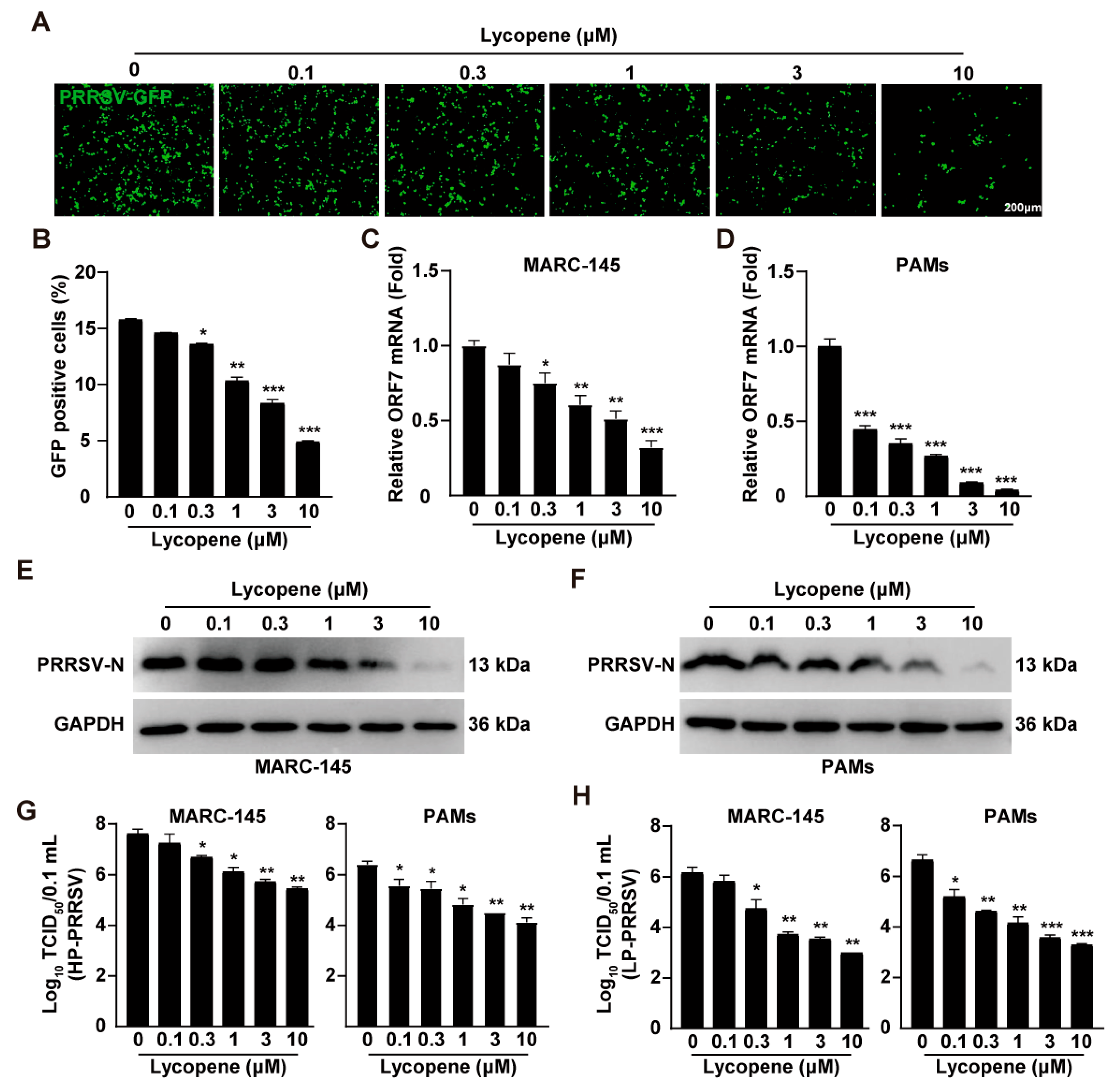
2.2. Lycopene Inhibits PRRSV Infection In Vitro
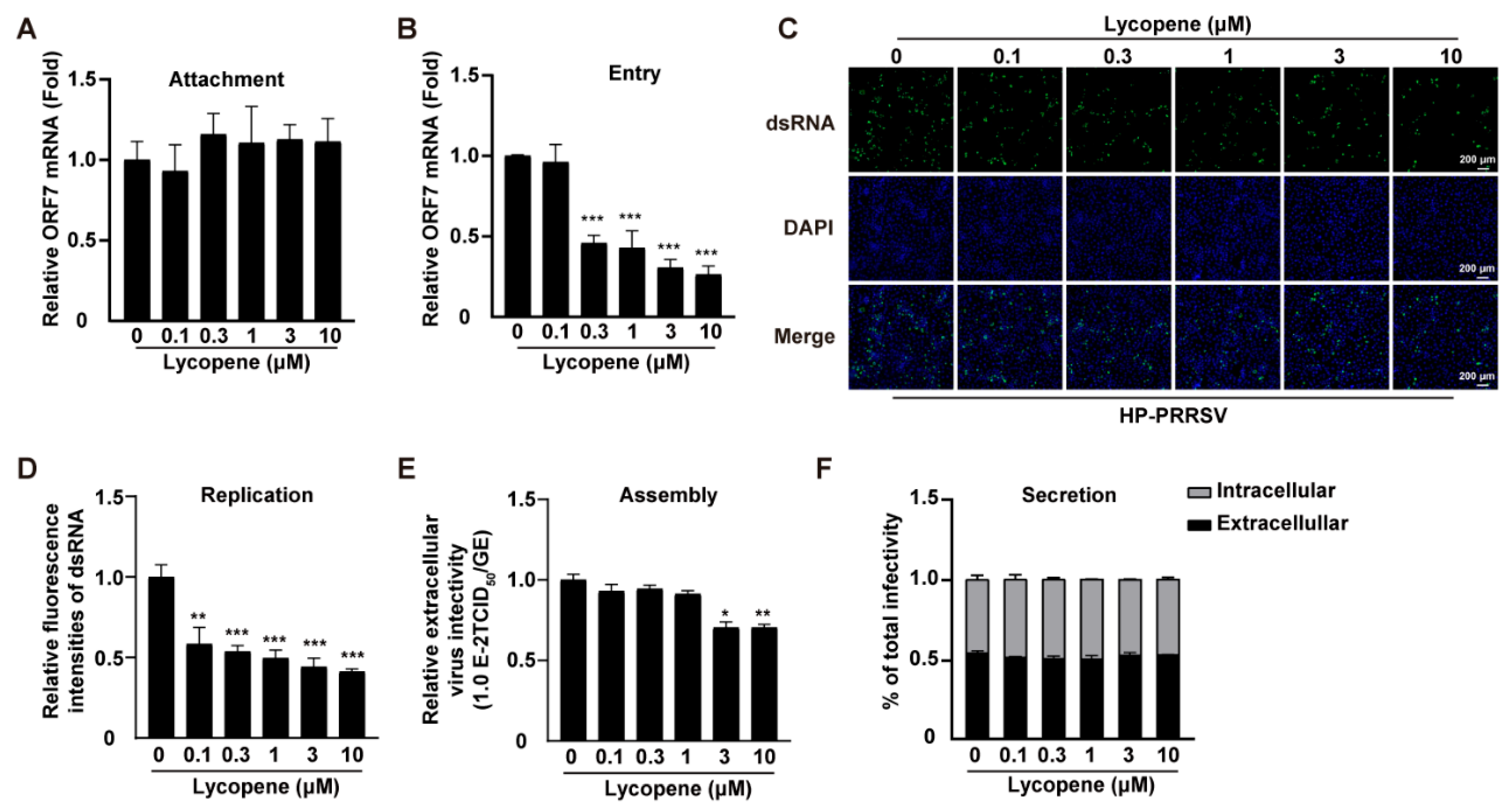
2.3. Lycopene Restricts PRRSV Entry, Replication, and Assembly
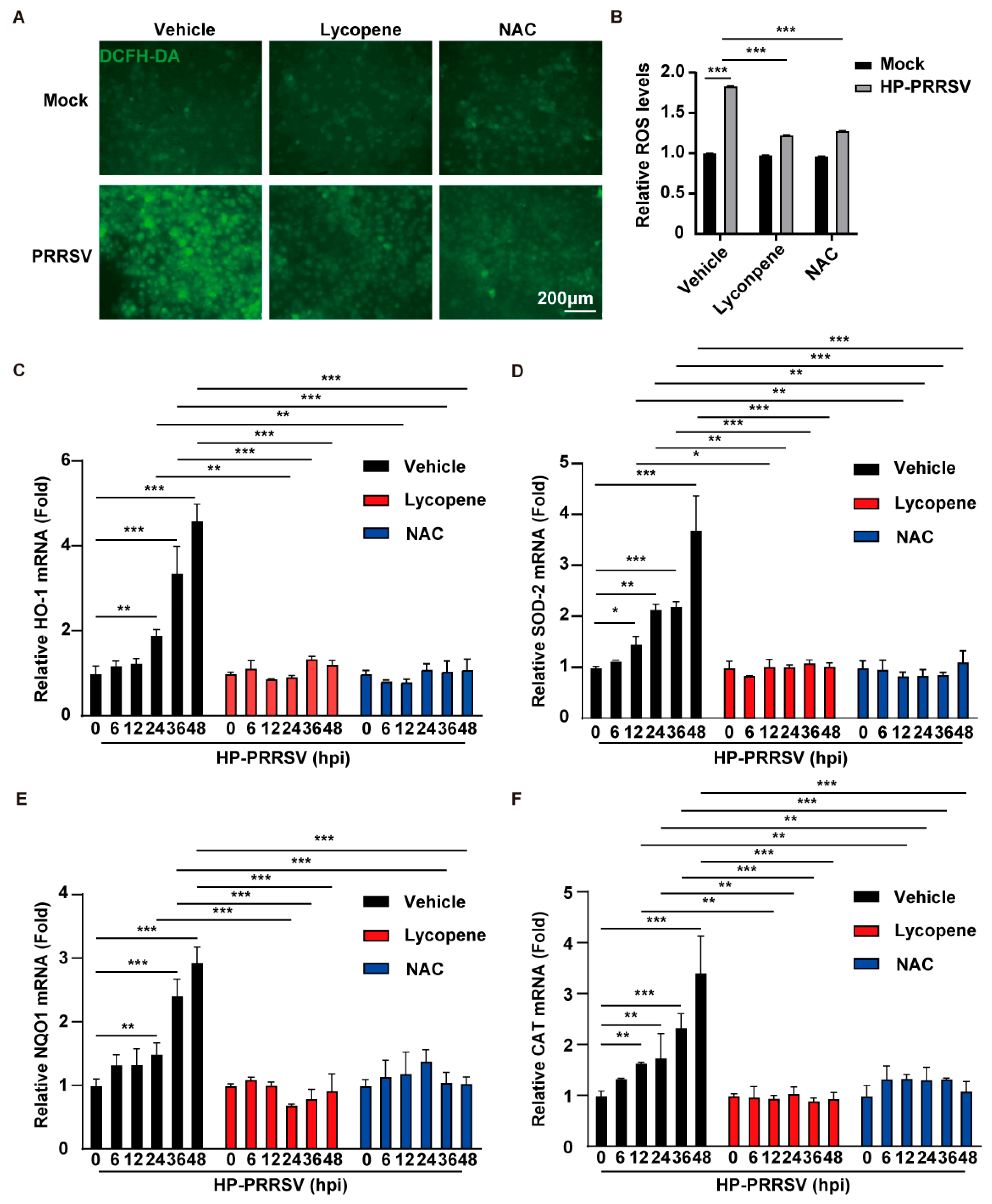
2.4. Lycopene Inhibits PRRSV Proliferation by Suppressing ROS
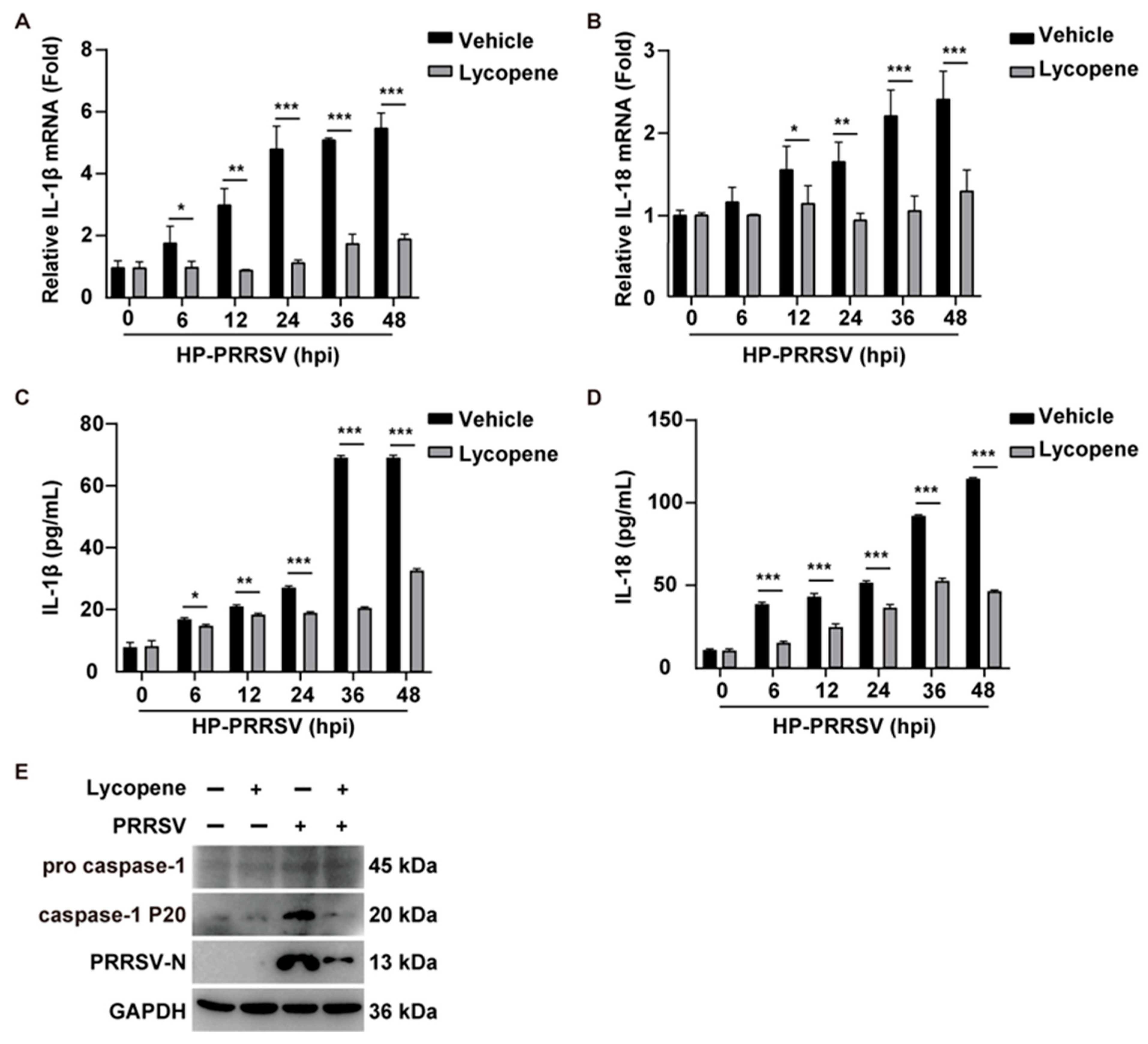
2.5. Lycopene Inhibits PRRSV-Induced Inflammatory Response
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cells and Viruses
4.3. Cell Viability Analysis
4.4. Cell Cycle Analysis
4.5. Apoptosis Analysis
4.6. Fluorescence Observation and Flow Cytometry Detection
4.7. qRT-PCR Analysis
4.8. Immunoblotting Analysis
4.9. 50% Tissue Culture Infectious Dose (TCID50) Assay
4.10. Viral Attachment Assay
4.11. Viral Entry Assay
4.12. Viral Replication Assay
4.13. Viral Assembly Assay
4.14. Viral Secretion Assay
4.15. 2′,7′-Dichlorodihydrofluorescein Diacetate (DCFH-DA) Assay
4.16. ELISA
4.17. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef]
- Collins, J.E.; Benfield, D.A.; Christianson, W.T.; Harris, L.; Hennings, J.C.; Shaw, D.P.; Goyal, S.M.; McCullough, S.; Morrison, R.B.; Joo, H.S.; et al. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Invest. 1992, 4, 117–126. [Google Scholar] [CrossRef]
- Baron, T.; Albina, E.; Leforban, Y.; Madec, F.; Guilmoto, H.; Plana Duran, J.; Vannier, P. Report on the first outbreaks of the porcine reproductive and respiratory syndrome (PRRS) in France. Diagnosis and viral isolation. Ann. Rech. Vet. 1992, 23, 161–166. [Google Scholar] [PubMed]
- Conzelmann, K.K.; Visser, N.; Van Woensel, P.; Thiel, H.J. Molecular characterization of porcine reproductive and respiratory syndrome virus, a member of the arterivirus group. Virology 1993, 193, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Snijder, E.J.; Meulenberg, J.J. The molecular biology of arteriviruses. J. Gen. Virol. 1998, 79 Pt 5, 961–979. [Google Scholar] [CrossRef]
- Kim, H.S.; Kwang, J.; Yoon, I.J.; Joo, H.S.; Frey, M.L. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch. Virol. 1993, 133, 477–483. [Google Scholar] [CrossRef]
- Duan, X.; Nauwynck, H.J.; Pensaert, M.B. Virus quantification and identification of cellular targets in the lungs and lymphoid tissues of pigs at different time intervals after inoculation with porcine reproductive and respiratory syndrome virus (PRRSV). Vet. Microbiol. 1997, 56, 9–19. [Google Scholar] [CrossRef]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxid. Med. Cell Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef]
- Bandeira, A.C.B.; da Silva, T.P.; de Araujo, G.R.; Araujo, C.M.; da Silva, R.C.; Lima, W.G.; Bezerra, F.S.; Costa, D.C. Lycopene inhibits reactive oxygen species production in SK-Hep-1 cells and attenuates acetaminophen-induced liver injury in C57BL/6 mice. Chem. Biol. Interact. 2017, 263, 7–17. [Google Scholar] [CrossRef]
- Choi, S.; Kim, H. The Remedial Potential of Lycopene in Pancreatitis through Regulation of Autophagy. Int. J. Mol. Sci. 2020, 21, 5775. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharmacother. 2020, 129, 110459. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.D.; et al. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020, 159, 104966. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; He, W.; Jia, Z.; Hao, S. Lycopene Improves Insulin Sensitivity through Inhibition of STAT3/Srebp-1c-Mediated Lipid Accumulation and Inflammation in Mice fed a High-Fat Diet. Exp. Clin. Endocrinol. Diabetes 2017, 125, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, J.; Zhang, S.; Zhang, C.; Shen, C.; Song, S.; Wang, Y.; Peng, Y.; Gong, X.; Dai, J.; et al. A novel nanobody broadly neutralizes SARS-CoV-2 via induction of spike trimer dimers conformation. Exploration 2024, 4, 20230086. [Google Scholar] [CrossRef] [PubMed]
- Khongthaw, B.; Dulta, K.; Chauhan, P.K.; Kumar, V.; Ighalo, J.O. Lycopene: A therapeutic strategy against coronavirus disease 19 (COVID-19). Inflammopharmacology 2022, 30, 1955–1976. [Google Scholar] [CrossRef]
- Foo, J.; Bellot, G.; Pervaiz, S.; Alonso, S. Mitochondria-mediated oxidative stress during viral infection. Trends Microbiol. 2022, 30, 679–692. [Google Scholar] [CrossRef] [PubMed]
- de Zoete, M.R.; Palm, N.W.; Zhu, S.; Flavell, R.A. Inflammasomes. Cold Spring Harb. Perspect. Biol. 2014, 6, a016287. [Google Scholar] [CrossRef]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef]
- Kuriakose, T.; Man, S.M.; Malireddi, R.K.; Karki, R.; Kesavardhana, S.; Place, D.E.; Neale, G.; Vogel, P.; Kanneganti, T.D. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 2016, 1, aag2045. [Google Scholar] [CrossRef]
- Wang, W.; Hu, D.; Wu, C.; Feng, Y.; Li, A.; Liu, W.; Wang, Y.; Chen, K.; Tian, M.; Xiao, F.; et al. STING promotes NLRP3 localization in ER and facilitates NLRP3 deubiquitination to activate the inflammasome upon HSV-1 infection. PLoS Pathog. 2020, 16, e1008335. [Google Scholar] [CrossRef]
- Shen, C.; Zhang, Z.; Xie, T.; Ji, J.; Xu, J.; Lin, L.; Yan, J.; Kang, A.; Dai, Q.; Dong, Y.; et al. Rhein Suppresses Lung Inflammatory Injury Induced by Human Respiratory Syncytial Virus Through Inhibiting NLRP3 Inflammasome Activation via NF-κB Pathway in Mice. Front. Pharmacol. 2019, 10, 1600. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.Y.; Shao, K.Y.; Han, Y.Q.; Li, G.L.; Ming, S.L.; Su, B.Q.; Du, Y.K.; Liu, Z.H.; Zhang, G.P.; et al. Porcine Reproductive and Respiratory Syndrome Virus Activates Lipophagy To Facilitate Viral Replication through Downregulation of NDRG1 Expression. J. Virol. 2019, 93, e00526-19. [Google Scholar] [CrossRef]
- Pedre, B.; Barayeu, U.; Ezeriņa, D.; Dick, T.P. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H2S and sulfane sulfur species. Pharmacol. Ther. 2021, 228, 107916. [Google Scholar] [CrossRef]
- Yu, F.; Yan, Y.; Shi, M.; Liu, H.Z.; Zhang, H.L.; Yang, Y.B.; Huang, X.Y.; Gauger, P.C.; Zhang, J.; Zhang, Y.H.; et al. Phylogenetics, Genomic Recombination, and NSP2 Polymorphic Patterns of Porcine Reproductive and Respiratory Syndrome Virus in China and the United States in 2014–2018. J. Virol. 2020, 94, e01813-19. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Meng, X.J. Novel strategies and approaches to develop the next generation of vaccines against porcine reproductive and respiratory syndrome virus (PRRSV). Virus Res. 2010, 154, 141–149. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C. Porcine reproductive and respiratory syndrome virus vaccines: Current status and strategies to a universal vaccine. Transbound. Emerg. Dis. 2014, 61, 109–120. [Google Scholar] [CrossRef]
- Xia, T.; Yang, M.; Marabella, I.; Lee, E.M.; Olson, B.; Zarling, D.; Torremorell, M.; Clack, H.L. Inactivation of airborne porcine reproductive and respiratory syndrome virus (PRRSv) by a packed bed dielectric barrier discharge non-thermal plasma. J. Hazard. Mater. 2020, 393, 122266. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, J.; Xie, C.; Wang, T.; Sun, P.; Wang, J.; Li, J.; Li, G.; Qiu, J.; Zhang, Y.; et al. High-throughput screening unveils nitazoxanide as a potent PRRSV inhibitor by targeting NMRAL1. Nat. Commun. 2024, 15, 4813. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhang, J.; Wang, J.; Liu, J.; Sun, P.; Li, J.; Li, G.; Sun, Y.; Ying, J.; Li, K.; et al. Caffeic acid phenethyl ester: An effective antiviral agent against porcine reproductive and Respiratory Syndrome Virus. Antivir. Res. 2024, 225, 105868. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, Y.; Sun, H.; Bai, J.; Zhang, J.; Zhang, L.; Liu, X.; Sun, Y.; Jiang, P. Ursonic acid from medicinal herbs inhibits PRRSV replication through activation of the innate immune response by targeting the phosphatase PTPN1. Vet. Res. 2024, 55, 67. [Google Scholar] [CrossRef]
- Zhang, P.; Tang, Y.; Li, N.G.; Zhu, Y.; Duan, J.A. Bioactivity and chemical synthesis of caffeic acid phenethyl ester and its derivatives. Molecules 2014, 19, 16458–16476. [Google Scholar] [CrossRef]
- Borková, L.; Frydrych, I.; Jakubcová, N.; Adámek, R.; Lišková, B.; Gurská, S.; Medvedíková, M.; Hajdúch, M.; Urban, M. Synthesis and biological evaluation of triterpenoid thiazoles derived from betulonic acid, dihydrobetulonic acid, and ursonic acid. Eur. J. Med. Chem. 2020, 185, 111806. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and Its Antioxidant Role in the Prevention of Cardiovascular Diseases-A Critical Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1868–1879. [Google Scholar] [CrossRef]
- Leh, H.E.; Lee, L.K. Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus. Molecules 2022, 27, 2335. [Google Scholar] [CrossRef] [PubMed]
- Przybylska, S.; Tokarczyk, G. Lycopene in the Prevention of Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 1957. [Google Scholar] [CrossRef]
- Paul, R.; Mazumder, M.K.; Nath, J.; Deb, S.; Paul, S.; Bhattacharya, P.; Borah, A. Lycopene—A pleiotropic neuroprotective nutraceutical: Deciphering its therapeutic potentials in broad spectrum neurological disorders. Neurochem. Int. 2020, 140, 104823. [Google Scholar] [CrossRef]
- Zou, Y.; Gao, B.; Lu, J.; Zhang, K.; Zhai, M.; Yuan, Z.; Aschner, M.; Chen, J.; Luo, W.; Wang, L.; et al. Long non-coding RNA CASC15 enhances learning and memory in mice by promoting synaptic plasticity in hippocampal neurons. Exploration 2024, 4, 20230154. [Google Scholar] [CrossRef] [PubMed]
- El-Kazaz, S.E.; Hafez, M.H.; Noreldin, A.E.; Khafaga, A.F. Lycopene alleviates cognitive dysfunctions in an Alzheimer’s disease rat model via suppressing the oxidative and neuroinflammatory signaling. Tissue Cell 2025, 96, 102975. [Google Scholar] [CrossRef]
- Yu, P.W.; Fu, P.F.; Zeng, L.; Qi, Y.L.; Li, X.Q.; Wang, Q.; Yang, G.Y.; Li, H.W.; Wang, J.; Chu, B.B.; et al. EGCG Restricts PRRSV Proliferation by Disturbing Lipid Metabolism. Microbiol. Spectr. 2022, 10, e0227621. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, J.; Ding, Y.; Luo, M.; Tong, Y.; Hu, T.; Wei, Y. A Novel Polysaccharide from Sargassum weizhouense: Extraction Optimization, Structural Characterization, Antiviral and Antioxidant Effects. Antioxidants 2023, 12, 1832. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Q.; Tang, J.; Feng, W.H. Lipid rafts both in cellular membrane and viral envelope are critical for PRRSV efficient infection. Virology 2015, 484, 170–180. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Y.P.; Yu, Y.L.; Sun, M.X.; Li, C.; Chen, P.Y.; Mao, X. Role of lipid rafts in porcine reproductive and respiratory syndrome virus infection in MARC-145 cells. Biochem. Biophys. Res. Commun. 2011, 414, 545–550. [Google Scholar] [CrossRef]
- Li, G.; Su, B.; Fu, P.; Bai, Y.; Ding, G.; Li, D.; Wang, J.; Yang, G.; Chu, B. NPC1-regulated dynamic of clathrin-coated pits is essential for viral entry. Sci. China Life Sci. 2022, 65, 341–361. [Google Scholar] [CrossRef]
- Long, S.; Zhou, Y.; Bai, D.; Hao, W.; Zheng, B.; Xiao, S.; Fang, L. Fatty Acids Regulate Porcine Reproductive and Respiratory Syndrome Virus Infection via the AMPK-ACC1 Signaling Pathway. Viruses 2019, 11, 1145. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, L.; Su, B.Q.; Yang, G.Y.; Wang, J.; Ming, S.L.; Chu, B.B. The glycoprotein 5 of porcine reproductive and respiratory syndrome virus stimulates mitochondrial ROS to facilitate viral replication. mBio 2023, 14, e0265123. [Google Scholar] [CrossRef]
- Ma, Y.X.; Han, Y.Q.; Wang, P.Z.; Wang, M.Y.; Yang, G.Y.; Li, J.L.; Wang, J.; Chu, B.B. Porcine reproductive and respiratory syndrome virus activates lipid synthesis through a ROS-dependent AKT/PCK1/INSIG/SREBPs axis. Int. J. Biol. Macromol. 2024, 282, 136720. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, B.; Kong, N.; Li, Q.; Ma, Y.; Li, Z.; Gao, J.; Zhang, C.; Wang, X.; Liang, C.; et al. A novel porcine reproductive and respiratory syndrome virus vector system that stably expresses enhanced green fluorescent protein as a separate transcription unit. Vet. Res. 2013, 44, 104. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, X.X.; Qiao, S.; Li, R.; Xie, S.; Zhou, X.; Deng, R.; Zhou, E.M.; Zhang, G. Porcine Reproductive and Respiratory Syndrome Virus Enhances Self-Replication via AP-1-Dependent Induction of SOCS1. J. Immunol. 2020, 204, 394–407. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.-X.; Han, Y.-Q.; Wang, P.-Z.; Chu, B.-B.; Ming, S.-L.; Zeng, L. Lycopene Inhibits PRRSV Replication by Suppressing ROS Production. Int. J. Mol. Sci. 2025, 26, 7560. https://doi.org/10.3390/ijms26157560
Ma Y-X, Han Y-Q, Wang P-Z, Chu B-B, Ming S-L, Zeng L. Lycopene Inhibits PRRSV Replication by Suppressing ROS Production. International Journal of Molecular Sciences. 2025; 26(15):7560. https://doi.org/10.3390/ijms26157560
Chicago/Turabian StyleMa, Ying-Xian, Ya-Qi Han, Pei-Zhu Wang, Bei-Bei Chu, Sheng-Li Ming, and Lei Zeng. 2025. "Lycopene Inhibits PRRSV Replication by Suppressing ROS Production" International Journal of Molecular Sciences 26, no. 15: 7560. https://doi.org/10.3390/ijms26157560
APA StyleMa, Y.-X., Han, Y.-Q., Wang, P.-Z., Chu, B.-B., Ming, S.-L., & Zeng, L. (2025). Lycopene Inhibits PRRSV Replication by Suppressing ROS Production. International Journal of Molecular Sciences, 26(15), 7560. https://doi.org/10.3390/ijms26157560






